Abstract
Maresin 1 (MaR1) is an immunoresolvent that governs resolution of acute inflammation and its local metabolism in the context infectious-inflammation is of interest. Here, we investigated the MaR1 metabolome in infectious exudates and its bioactions in regulating leukocyte responses in the context of bacterial infection. In Escherichia coli infectious exudates, MaR1 was temporally regulated with maximal levels at 4 h (2.2 ± 0.4 pg/lavage). In these exudates we also identified two novel products and their structure elucidation gave 22-hydroxy-MaR1 and 14-oxo-MaR1. Using human primary leukocytes we found that neutrophils primarily produced 22-OH-MaR1 whereas the main macrophage product was 14-oxo-MaR1. Both 22-OH-MaR1 and 14-oxo-MaR1 incubated with human primary macrophages gave dose dependent increases in macrophage phagocytosis of ~75% at 1 pM 22-OH-MaR1 and ~25% at 1 pM 14-oxo-MaR1, while 14-oxo-MaR1 was less active than MaR1 at higher concentrations. Together these findings establish the temporal regulation of MaR1 during infectious inflammation as well as elucidate the structures and actions of two novel MaR1 further metabolites that carry bioactivities.
Introduction
Acute inflammation is a natural host protective mechanism mounted by the body in response to injury or invading pathogens that when self-limited leads to homeostasis (1). If uncontrolled, inflammation may become chronic and lead to tissue damage (2). It is now recognized that the resolution of inflammation is an active process orchestrated by a new genus of potent molecules known as specialized pro-resolving mediators (SPM) (3). SPM are bioactive autacoids having both anti-inflammatory and pro-resolving properties. They are enzymatically produced from essential fatty acids (EFA), with unique stereochemistries. Recently, a new family of macrophage-derived mediators from docosahexaenoic acid (DHA) was identified and coined as maresins for macrophage mediator resolving inflammation (MaR), (4).
The biosynthesis of MaR1 (7R,14S-dihydroxydocosa-4Z,8E,10E,12Z,16Z,19Z-hexaenoic acid), the first mediator identified in this family, is initiated by human macrophage 12-lipoxygenase to produce 14S-hydroperoxy-4Z,7Z,10Z,12E,16Z,19Z DHA that is then converted to 13S,14S-epoxy-docosa-4Z,7Z,9E,11E,16Z,19Z-hexaenoic acid (5, 6) and subsequently to MaR1. MaR1 displays potent leukocytes directed actions, decreasing PMN infiltration during murine peritonitis and increasing human macrophage efferocytosis of apoptotic PMN. In addition, MaR1 stimulates tissue regeneration in planaria and reduces chemotherapy-induced neuropathic pain (5). It exerts protective actions in murine model of colitis where it reduces colonic damages, body weight loss and pro-inflammatory mediators (7). MaR1 is also organ-protective in murine acute respiratory distress syndrome (8).
Given that MaR1 carries potent leukocyte directed actions, we questioned whether MaR1 is produced during self-limited infections. We also investigated the further conversion of MaR1 in self-limited infectious exudates, identifying two novel MaR1 further metabolic products and established their bioactions with human macrophages
Materials and Methods
Animals
Male FVB mice (6 to 8 weeks old) purchased from Charles River Laboratories were fed ad libitum Laboratory Rodent Diet 20-5058 (Lab Diet, Purina Mills). All animal experimental procedures were approved by the Standing Committee on Animals of Harvard Medical School (protocol no. 02570) and complied with institutional and US National Institutes of Health (NIH) guidelines.
E. coli peritonitis
Mice were infected with live E. coli (serotype O6:K2:H1; 105 CFU). Mice were euthanized and peritoneal lavages were collected at 0h, 4h, 12h, 24h and 48 h after E. coli administration. Leukocyte numbers and differential counts were assessed using Turks solution and flow cytometry analysis. Rest of lavages were placed in two volumes of methanol and subjected to lipid mediator metabololipidomics.
Flow Cytometry
Murine peritoneal lavages cells were suspended in fluorescence-activated cell sorting (FACS) buffer (5% BSA in DPBS) and incubated with Fc block (15 min, 4°C; BD PharMingen) and then rat anti-mouse (from eBioscience) CD11b-PerCP/Cy5.5 (Clone:M1/70) and Ly6G-fluorescein iso-thiocyanate (FITC) (Clone 1A8) (30 min, 4°C) or appropriate isotype controls. Staining was assessed using FACSDiva CantoII (BD Biosciences) and analyzed using FlowJo (Tree Star).
PMN isolation
Peripheral blood human PMN were purified using density-based Ficoll-Histopaque 1077-1 (Sigma) as previously described.(9). PMN were isolated from human whole blood from healthy human volunteers giving informed consent under protocol # 1999-P- 001297 approved by the Partners Human Research Committee. Red blood cells were lysed by hypotonic lysis.
Further metabolites preparation. 14-oxo-MaR1
MaR1 (10 nM) suspended in PBS+/+ was added to isolated human macrophages (1×106 cells/mL) and conversion initiated by E coli (50:1). Macrophages were incubated for 1 h at 37 °C. Samples were extracted with C18 solid phase extraction. Also, 14-oxoMaR1 was produced by incubating MaR1 (10 μg) with Dess-Martin periodinane (10 mg/mL in CH2Cl2) for 1 h at room temperature. Reaction was stop with NaHCO3 (saturated) and Na2S2O3 (10%) and 14-oxo-MaR1 was extracted with MeOH.
22-OH-MaR1
MaR1 (10 μg) was dried down under N2 stream and suspended in PBS+/+. Isolated human PMN (50×106 cells/mL) were added and conversion initiated by zymosan (0.1 mg). PMN were incubated for 1 h at 37 °C. Samples were extracted with C18 solid phase extraction.
Further metabolites isolation
Biogenic 14-oxo-MaR1 and 22-OH-MaR1 were isolated by Reverse Phase-High-Pressure Liquid Chromatography (HPLC). Liquid chromatographic analyses were performed using a Hewlett-Packard Series 1100 HPLC system equipped with a Poroshell column (100 mm × 4.6 mm × 2.7 μm, Agilent) and a UV diode array detector. 14-oxo-MaR1 was eluted with a mobile phase consisting of methanol-water-acetic acid of 55:45:0.01 (vol/vol/vol) that was ramped to 65:35:0.01 (vol/vol/vol) over 2 min then to 75:25:0.01 (vol/vol/vol) over 18 min and finally to 98:2:0.01 (vol/vol/vol) over 0.1 min. 22-OH-MaR1 was eluted with a mobile phase consisting of methanol-water-acetic acid of 50:50:0.01 (vol/vol/vol) isocratic for 2 min, ramped to 57:43:0.01 (vol/vol/vol) over 12.5 min, isocratic for 3 min and then ramped to 98:2:0.01 (vol/vol/vol) over 5.5 min. Flow rate was maintained at 0.4 mL/min for both compound.
Sample extraction and LM-metabololipidomics
Samples were processed as in (10). Internal labeled standards d4-PGE2, d5-LXA4, d8-5HETE, d4-LTB4, and d5-RvD2 (500 pg each) in 2 volume of ice-cold methanol were added to each sample to facilitate quantification and sample recovery. Next, samples were held at −20°C for 45 min to allow protein precipitation and then centrifuged (1,200 g, 4°C, 10 min). Supernatants were collected and brought to less than 1 ml of methanol content with nitrogen gas. Samples were then placed into an automated extraction system (RapidTrace) and products extracted. Briefly solid-phase C18 cartridges were equilibrated with 3 ml of methanol and 6 ml of H2O. Acidified samples (pH 3.5, HCl) were rapidly loaded onto the conditioned C18 columns and were washed with 4 ml of H2O. Next, 5 ml of hexane were added and products eluted with 9 ml of methyl formate. Products were brought to dryness and immediately suspended in methanol-water (50:50 vol/vol) for LC-MS-MS automated injections.
The LC-MS-MS system, a Shimadzu LC-20AD HPLC and a Shimadzu SIL-20AC autoinjector (Shimadzu), paired with a QTrap 5500 (ABSciex), was routinely employed. A Poroshell column (100 mm × 4.6 mm × 2.7 μm, Agilent) was kept in a column oven maintained at 50°C (ThermaSphere), and LM were eluted with a mobile phase consisting of methanol-water-acetic acid of 50:50:0.01 (vol/vol/vol) isocratic for 2 min that was ramped to 80:20:0.01 (vol/vol/vol) over 9 min, kept isocratic for 3.5 min and then ramped to 98:2:0.01 (vol/vol/vol) for the next 0.1 min. This was subsequently maintained at 98:2:0.01 (vol/vol/vol) for 5.4 min, and the flow rate was maintained at 0.5 ml/min. The QTrap 5500 was operated in negative ionization mode using scheduled multiple reaction monitoring (MRM) coupled with information-dependent acquisition (IDA) and an enhanced product ion scan (EPI). The scheduled MRM window was 90 s.
GC-MS analysis
Isolated 22-OH-MaR1 was taken to dryness using a stream of N2, suspended in MeOH (50 μl), and treated with excess ethereal diazomethane (45 min at room temperature), followed by N,O-bis(trimethylsilyl)trifluoroacetamide) (BSTFA) treatment (10 min at room temperature, obtained from supelco). GC-MS analysis was performed with a Hewlett-Packard 593 mass-selective quadrupole detector and HP6890 GC system (Agilent column HP-5MS 30m × 0.25mm × 0.25μm). Samples were injected with hexane as the solvent and the temperature program was initiated at 150 °C and held for 2 min and reached 230 °C at 10 min (10 °C/min) and then 280 °C at 20 min (5 °C/ min). Reference saturated fatty acid methyl esters carbons C16-C24 gave the following retention times (min): C16, 9.1; C18, 11.1; C20, 13.2; C22, 15.6; and C24, 18.1; these were used to calculate respective C values of 22-OH-MaR1.
Macrophage phagocytosis
Experiments were conducted as previously described (9). Macrophages, 7 days differentiated PBMC with GM-CSF (10 ng/ml), were adhered (24 hours) on a 96-well plate (5 × 104 cells/well) in culture medium deprived of GM-CSF. Macrophages were incubated with vehicle (PBS+/+) alone or compounds (MaR1, 14-oxo-MaR1 or 22-OH-MaR1) for 15 min at 37°C, followed by fluorescent-labeled E. coli (BacLight, Molecular Probes, Carlsbad, CA) at a 50:1 ratio (E. coli:macrophage) for 60 min. Plates were gently washed, extracellular fluorescence quenched by trypan blue, and phagocytosis determined by measuring total fluorescence (Ex 495 nm/Ex 535 nm) using a fluorescent plate reader (Molecular Probes).
Real-time imaging
Macrophages were plated onto 8-well chamber slides (1 ×105 cells/well in PBS++). Chamber slides were kept in a Stage Top Incubation system for microscopes equipped with a built-in digital gas mixer and temperature regulator (TOKAI HIT model INUF-K14). MaR1 was added to macrophages (10 nM, 15 min at 37°C) followed by BacLight Green-labeled E. coli (5 × 106 CFU). Images were then acquired every 5 min for 60 min (37°C) with Keyence BZ-9000 (BIOREVO) inverted fluorescence phase-contrast microscope (20X objective) equipped with a monochrome/color switching camera using BZ-II Viewer software (Keyence, Itasca, IL, USA). Green fluorescence intensity was quantified using BZ-II Analyzer.
Electric Cell-substrate Impedance Sensing
Ligand-receptor interactions were monitored by measuring impedance across cultured CHO-hBLT1 cell monolayers using an ECIS (Applied Biophysics)(11) and essentially performed as in (12). Briefly, CHO-hBLT1 cells were plated in 8W10E+ ECIS chambers (1×105/well) and incubated with MaR1 and 22-OH-MaR1 (0-100nM) in HAM serum free culture media for 10 minutes, followed by addition of 1nM LTB4 for 10 minutes.
Statistical analysis
Data are expressed as mean ± SEM. Difference between groups were compared using Student's t test. Criterion for statistical significance was p < 0.05. Partial least squares-discriminant analysis (PLS-DA) was performed using SIMCA 13.0.3 software (Umetrics) following mean centering and unit variance scaling of LM amount.
Results
Endogenous production of novel metabolites derived from MaR1 during E. coli self-limited peritonitis
Since SPM are temporally regulated during the time course of sterile inflammation (13) and during infection (14), we first investigated if during self-resolving E. coli infections, MaR1 was temporally regulated (Figure 1). Mice were inoculated with E. coli at 105 CFU by intraperitoneal injection that gave a self-limited inflammatory response with PMN reaching maximum at 12 h, followed by decline until 48 h (Figure 1A, inset). Peritoneal lavages were collected and LM were investigated using LM metabololipidomics. MaR1 was identified in these exudates in accordance to published criteria including retention time (RT) and MS-MS fragmentation (4). MaR1 levels peaked 4h after inoculation (2.2±0.4 pg/lavage) and gradually decreased to 24h (Figure 1A). In these exudates, we also identified two further metabolites of MaR1. The first gave a RT of 13.7 min and MS-MS spectrum consistent with 14-oxo-MaR1 with a characteristic parent ion of m/z 357 and daughter ion of m/z 248 (Supplemental Table I). The second gave a RT of 10.0 min and a MS-MS fragmentation consistent with 22-OH-MaR1 with a characteristic parent ion of m/z 375 and daughter ion of m/z 221 (Figure 1B). Levels for 14-oxo-MaR1 peaked at 4h (1.4±0.6 pg/lavage) while those of 22-OH-MaR1 gradually increased during the initiation phase of the inflammatory response reaching a maximum at 24h (3.4±0.5 pg/lavage). To assess the temporal regulation of MaR1 and its further metabolites in comparison with other SPM and LM, we assessed the LM profile during the course of the self-limited inflammatory response (Supplemental Figure 1). Using PLS-DA (Figure 1C), we interrogated the LM profile. Data-driven modelling such as partial least squares-discriminant analysis (PLS-DA) is useful to interrogate large biological dataset such as metabolomics. Principal components were calculated from the systematic variation in the data matrix consisting of the overall bioactive LM identified in each sample (15). Each of the intervals gave distinct clusters with the 4h cluster giving a leftward shift within respect to the 0h cluster. The 12h-48h clusters gave an upward and rightward shift within respect to the 4h cluster with the 24h and 48h clusters giving similar coordinates on the score plot. Assessment of the loading plot (Figure 1D) demonstrated that the 4h group (initiation) was characterized by the presence of MaR1, 14-oxo-MaR1, Lipoxin (LX) B4 and RvD1 while the 12h (peak of inflammation) had higher levels of Aspirin Triggered-LXA4, RvD5, Prostaglandin (PG) E2 and PGD2. These results establish the MaR1 temporal profile during self-resolving E. coli infections in relation to its further metabolites, SPM and eicosanoids.
Figure 1. Endogenous production of MaR1 and its biotransformation during E. coli self-limited peritonitis.
Mice were inoculated with E. coli at 105 CFU by intraperitoneal injection and euthanized at indicated interval. Peritoneal lavages were collected and lipid mediators (LM) were investigated by LM metabololipidomics. (A) Time course of MaR1, 14-oxo-MaR1 and 22-OH-MaR1 levels at indicated interval. Results are expressed as pg/lavage and are mean ± SEM, n = 3 for 4h, 12h, 24h and 48h and n = 4 for 0h. Inset: Peritoneal leukocytes were enumerated. (B) MS-MS spectrum used for the identification of 22-OH-MaR1. (C) PLD-DA 2D score plot, grey ellipse denotes 95% confidence regions. (D) PLS-DA 2D loading plot, LM colored in grey displayed a variable importance in projection coefficient ≥ 1.
Human leukocytes convert MaR1 to 14-oxo-MaR1 and 22-OH-MaR1
Having identified MaR1 metabolites in vivo during self-resolving inflammation, we next questioned whether human leukocytes also converted this SPM to these novel products (Figure 2). Given that MaR1 is a macrophage product, we first assessed whether these cells converted MaR1. Incubation of human macrophages with MaR1 (Figure 2A and B) gave 14-oxo-MaR1 with RT of 13.7 min and characteristic MS-MS fragmentation that matched those of endogenous 14-oxo-MaR1 produced in vivo in E. coli infectious exudates (Fig. 1 and Supplemental Table I). These included the following characteristic ions m/z 357 (M-H), 339 (M-H-H2O), 313 (M-H-CO2), 295 (M-H-CO2-H2O), 248, 245, 221, 217 and 177 (221-CO2) (Figure 2A,C). This product also gave a UV chromophore with λmaxMeOH/Water at 338nm (Figure 2E) that is characteristic of a triene double bound system conjugated to a ketone (16). The presence of a ketone at carbon 14 was confirmed using a selective reaction described by Dess and Martin (17) to convert secondary alcohol groups into ketones (n = 3 preparations, data not shown). Levels of 22-OH-MaR1 in these incubations were below limits in these macrophage incubations. We next investigated whether human neutrophils converted MaR1 to these novel products. Incubation of human PMN with MaR1 gave a peak at RT 10.0 min and the MS-MS spectrum for the product under this peak gave ions with m/z 375 (M-H), 357 (M-H-H2O), 339 (M-H-2H2O), 331 (M-H-CO2), 311 (M-H-CO2-H2O), 295 (M-H-CO2-2H2O), 244 (262-H2O), 250, 244 (262-H2O), 226 (262-2H2O), 221, 177 (221-CO2), 159 (221-CO2-H2O), 141 135 (153-H2O) and 113 that is consistent with 22-OH-MaR1 (Figure 2A and 2D). This product also gave a UV chromophore with λmaxMeOH/Water of 271 nm and 2 shoulders at 261 nm and 282 nm (Figure 2E), characteristic of a conjugated triene double bound system. In order to obtain further evidence for each of the proposed structure, we assessed the MS-MS spectrum of methyl ester and trimethyl silyl ether derivatives of 14-oxo-MaR1 and 22-OH-MaR1 (Supplemental Figure 2).
Figure 2. Activated human leukocytes convert MaR1: physical properties of these new metabolites.
Synthetic MaR1 was incubated with E. coli activated human macrophages, Dess-Martin periodinane for 14-oxo-MaR1, or with zymosan activated PMN for 22-OH-MaR1 synthesis. (A) Representative chromatogram obtained by multiple reaction monitoring of a parent ion (Q1) and a diagnostic daughter ion (Q3) (Q1/Q3 for MaR1=359/250, 14-oxo-MaR1=357/248 and 22-OH-MaR1=375/221). Representative MS-MS spectra used for identification of (B) MaR1, (C) 14-oxo-MaR1 and (D) 22-OH-MaR1 along with (E) characteristic in phase UV chromophore for each compound. A-E are representative of 3 different incubations.
Real-time recording of human macrophage phagocytosis of E. coli: enhancement by MaR1 while it is biotransformed in vitro
Given that MaR1 was identified in infectious exudates, we next question if MaR1 regulated human macrophages phagocytosis of E. coli by and whether MaR1 was further converted during this process (Figure 3A-C). Human macrophages were incubated with vehicle or MaR1 followed by the addition of fluorescently labeled E. coli and fluorescence recorded every 5 min. MaR1 at 10 nM gave 38% increase in fluorescence, representing increased phagocytosis of E. coli, compared to macrophages incubated with vehicle at the 60 min interval (Figure 3A and 3B). Using LM-metabololipidomics, we investigated the kinetics of conversion of MaR1 to its further metabolites in these incubations. Macrophages were incubated with 10 nM of MaR1. At 5 min after E. coli addition, MaR1 levels started to decrease with a 36% reduction at the 60 min interval (Figure 3C). 14-oxo-MaR1 was present at the initial timepoint t=0 with levels equivalent to 6.7 pg/2×106 cells and its level increased to 53.0 pg/2×106 cells after 60 min. Levels of 22-OH-MaR1 were below the limit of detection in these incubations. Of note, E. coli incubated with MaR1 did not yield either 14-oxo-MaR1 or 22-OH-MaR1, pointing to leukocytes as the main source of these products in these ex vivo conditions. These results demonstrate that MaR1 potently regulates the clearance of bacteria by up-regulating macrophage phagocytosis and that these macrophages actively convert MaR1 to 14-oxo-MaR1 during this process.
Figure 3. MaR1 enhances human macrophage phagocytosis of E. coli and biotransformed to new metabolites.
Human macrophages were plated (1 ×105 cells/well in a 8-well chamber slide) and incubated with MaR1 (10 nM, black) or vehicle control (white), followed by addition of labeled E. coli to initiate phagocytosis. Fluorescent images were recorded every 5 min for 60 min. (A) Increase in phagocytosis (0-60 min, n=3). In each experiment, 4 fields (20X) per condition (per well) were recorded. Results are mean fluorescence intensity (MFI) of phagocytized E. coli of 4 fields/well from one representative experiment. (inset) MFI of phagocytized E. coli at 60 min, mean ± SEM. * p < 0.05 MaR1 vs. vehicle. (B) Representative fluorescent images from one experiment. Scale bars: 100 μm. (C) Human macrophages were plated (2 ×106 cells/well in a 6-well plate) stimulated with E. coli and incubated with MaR1 (10 nM) (60 min, 37°C). At the indicated time points, samples were subjected to LM metabololipidomics. Results are expressed as % above time 0 for MaR1 and pg/2×106 cells for its further metabolites; mean ± SEM, n = 5 for 0, 10, 15, 30 and 60 min, and n = 4 for 5 min. (inset) E. coli incubated with MaR1 (10 nM) (60 min, 37°C), Results are expressed as % above time 0 for MaR1 and pg/2×106 cells for its further metabolites (A=14-oxoMaR1 and B=22-OH-MaR1); mean ± SEM, n = 3
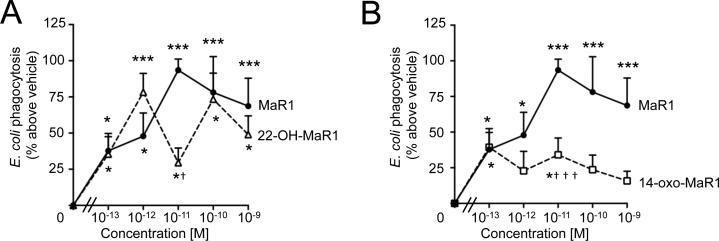
14-oxo-MaR1 and 22-OH-MaR1 retain biological activity with human macrophages
Having identified these novel products in infectious exudates and with human leukocytes, we next tested the biological activities of the MaR1 further metabolites. Here, we investigated whether these products stimulated macrophage phagocytosis of E. coli and their relative actions compared to MaR1 (Figure 4A and 4B). At a concentration as low as 0.1 pM, MaR1 and its 2 further metabolites had similar potency and increased E. coli phagocytosis by ~ 37% compared to macrophages incubated with vehicle. At higher concentration, 14-oxo-MaR1 was significantly less potent, increasing phagocytosis by only ~30% at 10 pM whereas MaR1 increased E. coli phagocytosis ~ 93% at 10 pM (Figure 4B). These results indicate that both further metabolites retain biological actions associated with the parent molecule.
Figure 4. MaR1 new metabolites retained biological activity with human macrophages.
Human macrophages were plated (5 ×104 cells/well in a 96-well plate) and incubated with vehicle, MaR1, (Panel A) 22-OH-MaR1 or (Panel B) 14-oxo-MaR1 (0.1 pM–1 nM) (15 min, 37°C) followed by addition of fluorescent-labeled E. coli (1h, 37°C)(see methods). Results are mean ± SEM of n = 7 cell preparation, d = 3. * p <0.05 and *** p<0.001 vs. vehicle; † p <0.05 and ††† p<0.001 vs. MaR1.
MaR1 and 22-OH-MaR1 interact with recombinant human BLT1 receptor
Given that the leukotriene B4 receptor (BLT1) is critical in the propagation of inflammation, we next investigated whether MaR1 could regulate the response of this receptor to LTB4. We used an electric cell-substrate impedance sensing (ECIS) system to monitor impedance change upon ligand activation of the receptor. Electric Cell-substrate Impedance Sensing (ECIS) allows for real time monitoring of adherent cell shape change by measuring impedance across the electrodes. When cells are added to the ECIS Arrays and attach to the electrodes, they act as insulators increasing the impedance. The current is impeded in a manner related to the number of cells covering the electrode, the morphology of the cells and the nature of the cell attachment. When cells are stimulated to change their function, the accompanying changes in cell morphology alter the impedance. The data generated is impedance versus time. Given that ligand engagement to cognate GPCR lead to characteristic shape change, this provides an ideal system for measuring ligand-GPCR interaction in vitro. In CHO cells transfected with hBLT1, LTB4 (0-100 nM) initiated impedance change with EC50 ~ 0.23 nM (Figure 5A). LTB4 activation of CHO-hBLT1 cells was abolished when cells were incubated with a BLT1 inhibitor U75302 (18) (Figure 5B). We next investigated the actions of MaR1 on CHO-hBLT1 cells. MaR1 dose dependently increased impedance to ~ 30 Ω (Figure 5C). To confirm that these responses were hBLT1-mediated, we incubated cells with U75302 (0 nM-1 μM) for 15 min prior to the addition of 100 nM of MaR1 (Figure 5D). U75302 dose-dependently inhibited the change in impedance elicited by MaR1. We next questioned whether MaR1 regulated hBLT1 response to LTB4. Addition of MaR1 (1-100 nM, 15 min) prior LTB4 (1 nM) decreased LTB4 elicited response from ~ 40 Ω to ~10 Ω, an action that was found to be dose dependent. Since 22-OH-MaR retained the bioaction in phagocytosis, we tested this metabolite with CHO-hBLT1 cells and found that 22-OH-MaR1 (1-100 nM) gave dose-dependent impedance changes, with ~ 40 Ω increase at 100 nM (Figure 5F). Incubation of the 22-OH-MaR1 with CHO-hBLT1 cells also led to a dose-dependent inhibition of LTB4 elicited impedance change (Figure 5G). Of note, in CHO cell incubation with MaR1 (10 nM, 30 min), we did not identify the MaR1 further metabolites nor a decrease in MaR1 levels suggesting that the responses observed in the ECIS incubation were in response to the products added and not the local metabolism of MaR1 by the CHO cells (n=3). These results demonstrated that both MaR1 and 22-OH-MaR1 are partial agonists to hBLT1 as well as antagonize the activation of hBLT1 by the potent pro-inflammatory mediator LTB4.
Figure 5. MaR1 and 22-OH-MaR1 are partial agonist of human recombinant BLT1 receptor in vitro.
Ligand-receptor interactions were monitored by measuring impedance changes in cultured CHO-hBLT1 cells using an ECIS system (Applied Biophysics). Cells were plated (1×105/well in a 8-well chamber slide) and (A) incubated 10 min with LTB4 (0-100 nM). (B) Cells were incubated with U75302 (0-1 μM), a BLT1 inhibitor, for 10 min prior the addition of 1 nM LTB4 for 10 min. (C) Cells were incubated 10 min with MaR1 (0-100 nM). Cells were incubated with (D) U75302 (0-1 μM) or (E) MaR1 (0-100 nM) for 10 min prior the addition of (D) 100 nM MaR1 or (E) 1 nM of LTB4 for 10 min. (F) Cells were incubated 10 min with 22-OH-MaR1 (0-100 nM). Cells were incubated with (G) 22-OH-MaR1 (0-100 nM) for 10 min followed by addition of 1 nM of LTB4 for 10 min. Results are mean of 6 separate tracings from n = 3 independent experiments.
Discussion
In the present report, we investigated the metabolism of MaR1, a potent pro-resolving and anti-nociceptive bioactive mediator, in the context of infectious inflammation. MaR1 was temporally regulated in exudates from self-resolving E. coli peritonitis. In these exudates we also identified and elucidated structures of two new molecules derived from MaR1, namely 14-oxo-MaR1 and 22-OH-MaR1. The production of these MaR1-derived molecules was also temporally regulated, with 14-oxo-MaR1 levels reaching a maximum at 4h and 22-OH-MaR1 levels reaching a maximum at 24h. Incubation of human macrophages with MaR1 gave 14-oxo-MaR1 while the main product with human neutrophils was 22-OH-MaR1. Each of these novel molecules retained at low concentrations the bioactions of their parent SPM, i.e. MaR1, with human macrophages. Of note, 22-OH-MaR1 gave a biphasic dose-response at upregulating E. coli phagocytosis by human macrophages. This response is suggestive of the engagement of a high and a low affinity receptor by 22-OH-MaR1 as observed for other pro-resolving mediators including RvD1 (19). In addition, both MaR1 and its metabolite 22-OH-MaR1 act at the hBLT1 receptor and impact LTB4 signaling. These results identify novel molecules and establish novel functions for the MaR1 bioactive metabolome.
Since SPM orchestrate the resolution of inflammation, it is essential to gain a detailed understanding of their temporal regulation during disease progression and their relationship with the classical pro-inflammatory mediators (i.e. leukotrienes and prostaglandins). MaR1 is a mediator from the 12-lipoxygenase pathway originally identified in macrophages (4, 20). MaR1 biosynthesis at early time points could result from activation of resident macrophages (21) as well as involvement of transcellular mechanisms with leukocytes and platelets as reported in (8). The MaR1 pathway is temporally regulated during self-limited sterile inflammation, with levels of its pathway marker 14-HDHA reaching a maximum during the resolution phase (4). MaR1 also enhances macrophage phagocytosis of Porphyromonas gingivalis and Aggregatibacter actinomycetemcomitans (22). MaR1 may also be produced by eosinophils given that these cells express the murine homologue of initiating enzyme in the MaR1 metabolome; in addition these leukocytes are also known to produce SPM including PD1, RvD5 and LXA4 during sterile peritonitis (23). Future studies will need to establish their trafficking during self-limited bacterial infections as well as their contributions to the MaR1 metabolome.
MaR1 also potently regulates host responses to bacteria, stimulating phagocytosis and killing of oral pathogens by human leukocytes (22). In the present report we investigated the temporal regulation of MaR1 during self-limited infections. In self-resolving E. coli peritonitis, we identified MaR1 at biologically active concentrations (2.2±0.4 pg/lavage) that peaked at 4h and subsequently decreased (Figure 1).
Autacoids are by definition locally produced, act locally and are rapidly enzymatically inactivated (24). LTB4 is metabolized by 12-hydroxydehydrogenase to 12-oxo-LTB4 (25) or by P450 to 20-hydroxy-LTB4 (26). PGE2 is converted by prostaglandin dehydrogenase (PGDH) to 15-oxo-PGE2 (27), which is further converted by prostaglandin reductase (PGR) to 13,14-dihydro-PGE2 (28)._RvE1, LXA4, RvD1, AT-RvD1 and RvD2 (29-31) are also substrates for PGDH, the eicosanoid oxidoreductase EOR (32). SPM further conversion may yield products that retain the pro-resolving actions of the parent SPM as for example 22-OH-PD1 that also potently regulates both human and murine leukocytes (33). In other instances, SPM conversion may result in a partial or complete loss of bioactivity, as observed for 16-oxo-RvD2 (30), 10,11-dihydro-RvE1, 18-oxo-RvE1 and 20-carboxy-RvE1 (34), 8-oxo-RvD1 and 17-oxo-RvD1 (29). In the present work, we investigated further conversion of MaR1 during bacterial infections. In self-resolving exudates we identified two new MaR1 derived molecules. The production of 14-oxo-MaR1 was also found to be temporally regulated with human macrophages during E. coli phagocytosis. Assessment of the biological actions of this molecule with human macrophages demonstrated that 14-oxo-MaR1 was less potent then MaR1at promoting macrophage responses to E. coli thereby suggesting that the conversion of MaR1 to 14-oxo-MaR1 in macrophages is an inactivation route for this potent SPM. Conversely, 22-OH-MaR1, which reached a maximum during the resolution phase of the inflammatory response and was the main product with human neutrophils, was found to display similar potencies at regulating human macrophages responses to E. coli (Figure 3). These suggest that conversion to 14-oxo-MaR1 is an inactivation in the MaR1 metabolome whereas conversion to 22-OH-MaR1 does not lead to inactivation. The latter product may in turn undergo further conversion to yield a dicarboxylate, in line with the LTB4 metabolome where 20-OH-LTB4 is further converted to 22-COOH-LTB4, which is inactive (35). Further studies will need to determine whether 22-OH-MaR1 is also converted to this product and whether this indeed is an inactivation route.
SPM are by definition potent in resolving inflammatory processes. Their actions are mediated by G protein-coupled receptors (GPCR); for example, ALX receptor mediates the actions of LXA4 and RvD1 (12), GPR18 the actions of RvD2 (36). In addition to activating their cognate receptors, SPM are also partial agonists or antagonists to receptors for pro-inflammatory LM. For example RvE1 is a partial agonist to hBLT1, one of the LTB4 receptors, thereby regulating the actions of this potent inflammation-initiating LM (37). In the present study we found that MaR1 and its further product 22-OH-MaR1 are both partial agonists to hBLT1 and regulate the activation of this receptor by its ligand LTB4. Thus, in addition to increasing the clearance of bacteria (Figure 4), MaR1 also regulates BLT1 activation by its cognate ligand, the potent chemo-attractant LTB4, a biological action retained by the MaR1 further metabolite 22-OH-MaR1. Thus these experiments identify novel mechanisms for the regulation of inflammatory responses by the MaR1 metabolome in governing the responses within the resolution of acute-inflammation.
In conclusion, in the present study using lipid mediator profiling we established the temporal regulation of MaR1 in self-resolving infectious exudates. In these exudates we also identified two novel MaR1 derived products, 14-oxo-MaR1 and 22-hydroxy-MaR1, and conducted their structure elucidation with the proposed structure being 7R-hydroxy,14-oxo docosa-4Z,8E,10E,12Z,16Z,19Z-hexaenoic acid and 7R,14S,22-trihydroxydocosa-4Z,8E,10E,12Z,16Z,19Z-hexaenoic acid respectively. The production of these molecules was also established with human phagocytes, where 14-oxo-MaR1 was the main product produced by human macrophages, whereas 22-hydroxy-MaR1 was primarily produced by human neutrophils. Assessment of the bioactions of these products demonstrated that while 22-hydroxy-MaR1 retained the bioactions on human macrophages of its parent SPM, 14-oxo-MaR1 was less potent. MaR1 and 22-hydroxy-MaR1 were partial agonists of the recombinant hBLT1. Together these findings provide novel structures for developing therapeutics in bacterial infection focusing on further conversion pathways and cross talk between inflammatory and resolution pathways. They afford novel leads to understanding local regulation of distinct SPM metabolomes furnishing new insights into mechanisms leading to disease as well as establish the basis for design of MaR1 mimetics that could resist further enzymatic conversion to give prolonged MaR1 bioactions.
Supplementary Material
Figure 6.
Maresin 1 further metabolome formation and biological actions.
Acknowledgments
We thank Mary Halm Small for expert assistance in manuscript preparation and Chien-Yee C. Cheng for technical assistance.
Grant Support:
This work was supported in part by NIH Grants PO1 GM095467 and R01 GM38765 (Prof. Charles N. Serhan)
Abbreviations
- EFA
Essential Fatty Acid
- DHA
Docosahexaenoic acid
- HPLC
High-Pressure Liquid Chromatography
- LC-MS-MS
Liquid chromatography tandem mass spectrometry
- LM
Lipid Mediator
- LX
Lipoxin
- MaR1
Maresin 1 (7R,14S-dihydroxydocosa-4Z,8E,10E,12Z,16Z,19Z-hexaenoic acid)
- MRM
Multiple Reaction Monitoring
- PD
Protectin
- PG
Prostaglandin
- PMN
Neutrophil
- SPM
Specialized Proresolving Mediators
- RT
Retention Time
- Rv
Resolvin
- 14-oxo-MaR1
7R-hydroxy,14-oxo docosa-4Z,8E,10E,12Z,16Z,19Z-hexaenoic acid
- 22-hydroxy-MaR1
7R,14S,22-trihydroxydocosa-4Z,8E,10E,12Z,16Z,19Z-hexaenoic acid
Footnotes
Author contributions: R.A.C., J.D., N.C and C.N.S. conception and design of research; R.A.C., J.D., N.C., I.V., J.M.S. and I.R.R. performed experiments; R.A.C., J.D., N.C., I.V., J.M.S. and I.R.R. analyzed data; R.A.C., J.D., N.C., I.V., J.M.S. and C.N.S. contributed to manuscript preparation, edited and revised manuscript. C.N.S. conceived the overall research plan.
References
- 1.Cotran RS, Kumar V, Collins T, editors. Robbins Pathologic Basis of Disease. W.B. Saunders Co.; Philadelphia: 1999. [Google Scholar]
- 2.Tabas I, Glass CK. Anti-inflammatory therapy in chronic disease: challenges and opportunities. Science. 2013;339:166–172. doi: 10.1126/science.1230720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Serhan CN, Chiang N, Dalli J. The resolution code of acute inflammation: Novel pro-resolving lipid mediators in resolution. Semin. Immunol. 2015;27:200–215. doi: 10.1016/j.smim.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Serhan CN, Yang R, Martinod K, Kasuga K, Pillai PS, Porter TF, Oh SF, Spite M. Maresins: novel macrophage mediators with potent anti-inflammatory and pro-resolving actions. J. Exp. Med. 2009;206:15–23. doi: 10.1084/jem.20081880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Serhan CN, Dalli J, Karamnov S, Choi A, Park CK, Xu ZZ, Ji RR, Zhu M, Petasis NA. Macrophage pro-resolving mediator maresin 1 stimulates tissue regeneration and controls pain. FASEB J. 2012;26:1755–1765. doi: 10.1096/fj.11-201442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dalli J, Zhu M, Vlasenko NA, Deng B, Haeggstrom JZ, Petasis NA, Serhan CN. The novel 13S,14S-epoxy-maresin is converted by human macrophages to maresin 1 (MaR1), inhibits leukotriene A4 hydrolase (LTA4H), and shifts macrophage phenotype. FASEB J. 2013;27:2573–2583. doi: 10.1096/fj.13-227728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marcon R, Bento AF, Dutra RC, Bicca MA, Leite DF, Calixto JB. Maresin 1, a proresolving lipid mediator derived from omega-3 polyunsaturated fatty acids, exerts protective actions in murine models of colitis. J. Immunol. 2013;191:4288–4298. doi: 10.4049/jimmunol.1202743. [DOI] [PubMed] [Google Scholar]
- 8.Abdulnour RE, Dalli J, Colby JK, Krishnamoorthy N, Timmons JY, Tan SH, Colas RA, Petasis NA, Serhan CN, Levy BD. Maresin 1 biosynthesis during platelet-neutrophil interactions is organ-protective. Proc. Natl. Acad. Sci. USA. 2014;111:16526–16531. doi: 10.1073/pnas.1407123111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dalli J, Serhan CN. Specific lipid mediator signatures of human phagocytes: microparticles stimulate macrophage efferocytosis and pro-resolving mediators. Blood. 2012;120:e60–72. doi: 10.1182/blood-2012-04-423525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Colas RA, Shinohara M, Dalli J, Chiang N, Serhan CN. Identification and signature profiles for pro-resolving and inflammatory lipid mediators in human tissue. Am. J. Physiol. Cell Physiol. 2014;307:C39–54. doi: 10.1152/ajpcell.00024.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peters MF, Scott CW. Evaluating cellular impedance assays for detection of GPCR pleiotropic signaling and functional selectivity. J. Biomol. Screen. 2009;14:246–255. doi: 10.1177/1087057108330115. [DOI] [PubMed] [Google Scholar]
- 12.Krishnamoorthy S, Recchiuti A, Chiang N, Yacoubian S, Lee CH, Yang R, Petasis NA, Serhan CN. Resolvin D1 binds human phagocytes with evidence for proresolving receptors. Proc. Natl. Acad. Sci. USA. 2010;107:1660–1665. doi: 10.1073/pnas.0907342107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dalli J, Winkler JW, Colas RA, Arnardottir H, Cheng CYC, Chiang N, Petasis NA, Serhan CN. Resolvin D3 and aspirin-triggered resolvin D3 are potent immunoresolvents. Chem. Biol. 2013;20:188–201. doi: 10.1016/j.chembiol.2012.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chiang N, Fredman G, Backhed F, Oh SF, Vickery T, Schmidt BA, Serhan CN. Infection regulates pro-resolving mediators that lower antibiotic requirements. Nature. 2012;484:524–528. doi: 10.1038/nature11042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Janes KA, Yaffe MB. Data-driven modelling of signal-transduction networks. Nat. Rev. Mol. Cell Biol. 2006;7:820–828. doi: 10.1038/nrm2041. [DOI] [PubMed] [Google Scholar]
- 16.Wainwright SL, Powell WS. Mechanism for the formation of dihydro metabolites of 12-hydroxyeicosanoids. Conversion of leukotriene B4 and 12-hydroxy-5,8,10,14-eicosatetraenoic acid to 12-oxo intermediates. J. Biol. Chem. 1991;266:20899–20906. [PubMed] [Google Scholar]
- 17.Dess DB, Martin JC. Readily accessible 12-I-5 oxidant for the conversion of primary and secondary alcohols to aldehydes and ketones. J. Org. Chem. 1983;48:4155–4156. [Google Scholar]
- 18.Falcone RC, Aharony D. Modulation of ligand binding to leukotriene B4 receptors on guinea pig lung membranes by sulfhydryl modifying reagents. J. Pharmacol. Exp. Ther. 1990;255:565–571. [PubMed] [Google Scholar]
- 19.Orr SK, Colas RA, Dalli J, Chiang N, Serhan CN. Proresolving actions of a new resolvin D1 analog mimetic qualifies as an immunoresolvent. Am. J. Physiol. Lung Cell. Mol. Physiol. 2015;308:L904–911. doi: 10.1152/ajplung.00370.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deng B, Wang CW, Arnardottir HH, Li Y, Cheng CY, Dalli J, Serhan CN. Maresin biosynthesis and identification of maresin 2, a new anti-inflammatory and pro-resolving mediator from human macrophages. PLoS One. 2014;9:e102362. doi: 10.1371/journal.pone.0102362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davies LC, Rosas M, Jenkins SJ, Liao CT, Scurr MJ, Brombacher F, Fraser DJ, Allen JE, Jones SA, Taylor PR. Distinct bone marrow-derived and tissue-resident macrophage lineages proliferate at key stages during inflammation. Nat. Commun. 2013;4:1886. doi: 10.1038/ncomms2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang CW, Colas RA, Dalli J, Arnardottir HH, Nguyen D, Hasturk H, Chiang N, Van Dyke TE, Serhan CN. Maresin 1 Biosynthesis and Proresolving Anti-infective Functions with Human-Localized Aggressive Periodontitis Leukocytes. Infect. Immun. 2015;84:658–665. doi: 10.1128/IAI.01131-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamada T, Tani Y, Nakanishi H, Taguchi R, Arita M, Arai H. Eosinophils promote resolution of acute peritonitis by producing proresolving mediators in mice. FASEB J. 2011;25:561–568. doi: 10.1096/fj.10-170027. [DOI] [PubMed] [Google Scholar]
- 24.Majno G, Joris I. Cells, Tissues, and Disease: Principles of General Pathology. Oxford University Press; New York: 2004. [Google Scholar]
- 25.Yokomizo T, Izumi T, Takahashi T, Kasama T, Kobayashi Y, Sato F, Taketani Y, Shimizu T. Enzymatic inactivation of leukotriene B4 by a novel enzyme found in the porcine kidney. Purification and properties of leukotriene B4 12-hydroxydehydrogenase. J. Biol. Chem. 1993;268:18128–18135. [PubMed] [Google Scholar]
- 26.Soberman RJ, Harper TW, Murphy RC, Austen KF. Identification and functional characterization of leukotriene B4 20-hydroxylase of human polymorphonuclear leukocytes. Proc. Natl. Acad. Sci. USA. 1985;82:2292–2295. doi: 10.1073/pnas.82.8.2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ensor CM, Yang JY, Okita RT, Tai HH. Cloning and sequence analysis of the cDNA for human placental NAD(+)-dependent 15-hydroxyprostaglandin dehydrogenase. J. Biol. Chem. 1990;265:14888–14891. [PubMed] [Google Scholar]
- 28.Ensor CM, Zhang H, Tai HH. Purification, cDNA cloning and expression of 15-oxoprostaglandin 13-reductase from pig lung. Biochem. J. 1998;330(Pt 1):103–108. doi: 10.1042/bj3300103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun YP, Oh SF, Uddin J, Yang R, Gotlinger K, Campbell E, Colgan SP, Petasis NA, Serhan CN. Resolvin D1 and its aspirin-triggered 17R epimer. Stereochemical assignments, anti-inflammatory properties, and enzymatic inactivation. J. Biol. Chem. 2007;282:9323–9334. doi: 10.1074/jbc.M609212200. [DOI] [PubMed] [Google Scholar]
- 30.Claria J, Dalli J, Yacoubian S, Gao F, Serhan CN. Resolvin D1 and resolvin D2 govern local inflammatory tone in obese fat. J. Immunol. 2012;189:2597–2605. doi: 10.4049/jimmunol.1201272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arita M, Oh SF, Chonan T, Hong S, Elangovan S, Sun YP, Uddin J, Petasis NA, Serhan CN. Metabolic inactivation of resolvin E1 and stabilization of its anti-inflammatory actions. J. Biol. Chem. 2006;281:22847–22854. doi: 10.1074/jbc.M603766200. [DOI] [PubMed] [Google Scholar]
- 32.Clish CB, Levy BD, Chiang N, Tai H-H, Serhan CN. Oxidoreductases in lipoxin A4 metabolic inactivation. J. Biol. Chem. 2000;275:25372–25380. doi: 10.1074/jbc.M002863200. [DOI] [PubMed] [Google Scholar]
- 33.Tungen JE, Aursnes M, Vik A, Ramon S, Colas RA, Dalli J, Serhan CN, Hansen TV. Synthesis and anti-inflammatory and pro-resolving activities of 22-OH-PD1, a monohydroxylated metabolite of protectin D1. J. Nat. Prod. 2014;77:2241–2247. doi: 10.1021/np500498j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hong S, Porter TF, Lu Y, Oh SF, Pillai PS, Serhan CN. Resolvin E1 metabolome in local inactivation during inflammation-resolution. J. Immunol. 2008;180:3512–3519. doi: 10.4049/jimmunol.180.5.3512. [DOI] [PubMed] [Google Scholar]
- 35.Hansson G, Lindgren JA, Dahlen SE, Hedqvist P, Samuelsson B. Identification and biological activity of novel omega-oxidized metabolites of leukotriene B4 from human leukocytes. FEBS Lett. 1981;130:107–112. doi: 10.1016/0014-5793(81)80676-x. [DOI] [PubMed] [Google Scholar]
- 36.Chiang N, Dalli J, Colas RA, Serhan CN. Identification of resolvin D2 receptor mediating resolution of infections and organ protection. J. Exp. Med. 2015;212:1203–1217. doi: 10.1084/jem.20150225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arita M, Ohira T, Sun YP, Elangovan S, Chiang N, Serhan CN. Resolvin E1 selectively interacts with leukotriene B4 receptor BLT1 and ChemR23 to regulate inflammation. J. Immunol. 2007;178:3912–3917. doi: 10.4049/jimmunol.178.6.3912. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.