Figure 6.
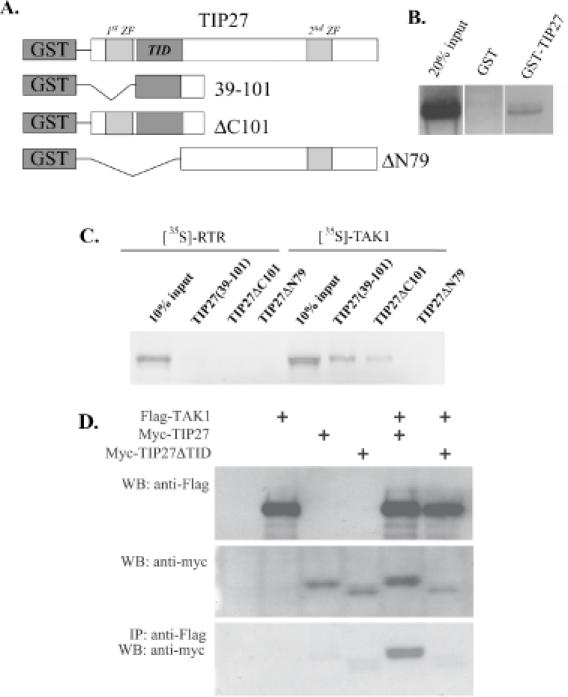
TIP27 interacts directly with TAK1. (A) Schematic representation of GST-TIP27 fusion proteins used in pull-down assays. (B) and (C) GST and different GST-TIP27 fusion proteins were bound to glutathione–Sepharose 4B beads and then incubated with [35S]methionine TAK1 or the nuclear receptor RTR. After 1 h incubation, beads were washed extensively and bound proteins solubilized and analyzed by western-blot analysis. Radiolabeled proteins were visualized by autoradiography. (D) TAK1 pulls down TIP27, but not TIP27(ΔTID), from cellular extracts prepared from COS-1 cells co-transfected with p3XFlagCMV-TAK1 and pCMV-myc-TIP27 or pCMV-myc-TIP27(ΔTID). Protein lysates were prepared 48 h after transfection as described in Materials and Methods. One part was used in western-blot (WB) analysis using anti-Flag or anti-Myc antibodies, the remaining was used in IP assay using anti-Flag M2 agarose resin. Bound proteins were then examined by western-blot analysis using an anti-Myc antibody.
