Abstract
Inflammation occurs after disruption of tissue homeostasis by cell stress, injury or infection and ultimately involves the recruitment and retention of cells of hematopoietic origin, which arrive at the affected sites to resolve damage and initiate repair. Interleukin 1α (IL-1α) and IL-1β are equally potent inflammatory cytokines that activate the inflammatory process, and their deregulated signaling causes devastating diseases manifested by severe acute or chronic inflammation. Although much attention has been given to understanding the biogenesis of IL-1β, the biogenesis of IL-1α and its distinctive role in the inflammatory process remain poorly defined. In this review we examine key aspects of IL-1α biology and regulation and discuss its emerging importance in the initiation and maintenance of inflammation that underlie the pathology of many human diseases.
In 1974, Dinarello et al.1 described acidic and neutral human pyrogens, which could be purified from monocytes and neutrophils, respectively, and showed that they have similar potencies in increasing body temperature in rabbits1. It was another ten years before the acidic and neutral pyrogens were identified as proteins that are distinct at the amino acid level, and the first described mouse acidic pyrogen and human neutral pyrogen were both called interleukine 1 (first reported in 1984)2,3. In 1985, amino acid sequences for both the acidic and neutral human pyrogens were reported, and these proteins were called interleukins IL-1α and IL-1β, respectively4, a designation that is accepted today. Early research into the molecular properties of IL-1α and IL-1β revealed numerous similarities between these cytokines that appear to justify naming them as two forms of IL-1, a family of cytokines that has grown to 11 members5. Specifically, both IL-1α and IL-1β are synthesized as precursor (proform) proteins with molecular weights of about 31 kDa, can be cleaved to smaller mature forms of 17 kDa and bind the cell surface receptor IL-1R1, and they trigger identical biological responses. Despite these similarities, IL-1α and IL-1β have different amino acid sequences, and the factors that control their functional maturation and bioavailability are highly dissimilar. First, whereas only the cleaved mature form of IL-1β is a functional pyrogen and a ligand for IL-1R1, both the proform (pro-IL-1α) and the cleaved form of IL-1α are biologically active IL-1R1 ligands6. Second, although mature IL-1β is a released protein, IL-1α functions both as a secreted and as a membrane-bound cytokine7. Third, during the functional maturation process, pro-IL-1β is cleaved by an aspartic protease caspase-1 downstream of a multi-protein complex called the inflammasome8–10, whereas capsase-1 and the inflammasome have no direct role in cleaving pro-IL-1α. Fourth, although IL-1α has higher affinity than IL-1β for IL-1R1, IL-1β has higher affinity for the decoy soluble receptor IL-1R2 (ref. 11). And fifth, although IL-1β is absent in cells at homeostasis and is expressed upon activation only in cells of hematopoietic origin, IL-1α is present in health in a wide variety of cells and expressed in hematopoietic and nonhematopoietic cells alike in response to appropriate stimuli12. These differences translate directly into the biological contexts in which IL-1α and IL-1β exert their functions. Remarkably, although IL-1β expression regulation, cleavage and release are relatively well understood, most aspects of IL-1α biogenesis and function and its role in the inflammatory process remain areas of active debate. The literature on IL-1β biology is abundant and has been reviewed in depth elsewhere13,14; here we will review the current understanding of the roles of IL-1α in initiating and sustaining the inflammatory processes that stem from the unique biochemical and functional properties of this pleiotropic cytokine and that may point to new opportunities for therapies of numerous human diseases and pathologies mechanistically linked to IL-1-driven inflammation.
Control of IL-1α expression
IL-1α is constitutively expressed in many cell types in healthy tissues at steady state, and its expression can be increased in response to growth factors and proinflammatory or stress-associated stimuli. Absolute amounts of IL-1α protein vary among cell types, but barrier cells—such as endothelial and epithelial cells—express substantial amounts of this cytokine at steady state5,12,15. The Il1a promoter lacks canonical TATA and CAAT box regulatory regions, containing instead a binding site for the Sp1 transcription factor16 that is known to mediate expression of housekeeping genes at homeostasis17. The inducible expression of IL-1α depends on the presence of binding sites for AP1 and NF-κB transcription factors18–20, which can upregulate IL-1α expression in a cell-type-specific manner. It has also been shown that the proximal Il1a promoter region contains a transcriptional-repressor-binding site that reduces its transcriptional activity16, thus dissociation of a transcriptional repressor can be an additional mechanism to increase IL-1α expression upon stimulation. In human CD4+ T cells, IL-1α expression is monoallelic and regulated via hyper- or hypomethylation of CpG nucleotides located in promoter regions proximal to the transcription initiation site21. Monocytes have a unique mechanism of inducible Il1a expression that involves upregulation of the long noncoding RNA AS-IL-1a, a natural antisense transcript that is partially complementary to IL-1α mRNA22. Although constitutive IL-1α expression is likely to be regulated by Sp1-family transcription factors in terminally differentiated cells, inducible IL-1α expression occurs rapidly in response to a variety of physiological stimuli, including oxidative stress15,23,24, lipid overload25,26, hormonal stimulation27, exposure to cytokines (including IL-1β and IL-1α itself)28–30 and canonical proinflammatory mediators of microbial origin with Toll-like receptor (TLR) agonistic activities29. The responsiveness of the Il1a promoter to such a broad spectrum of stimuli, which trigger inducible expression of IL-1α in addition to its constitutive expression in both hematopoietic and nonhematopoietic cells, has important implications for IL-1α’s ability to drive sterile and pathogen-induced inflammation.
IL-1α biogenesis
IL-1α is translated as pro-IL-1α, and a number of studies have described post-translational modifications of this precursor form. Specifically, pro-IL-1α was shown to be phosphorylated at Ser90 (refs. 31,32), myristoylated on Lys82 (ref. 33) and acetylated on Lys82 (refs. 33,34). The functional significance of these modifications has not been formally established. Furthermore, it remains a matter of debate whether proteolytic cleavage of pro-IL-1α into the N-terminal IL-1α propiece (IL-1α-NTP) and a C-terminal mature IL-1α is a genuine functional maturation step that is necessary for efficient IL-1α-dependent biological responses. In early studies of human pyrogens it was established that human acidic pyrogen (corresponding to pro-IL-1α) is as potent at causing fever as human neutral pyrogen (corresponding to mature IL-1β)1. Subsequent analyses of the receptor binding kinetics of pro-IL-1α and mature IL-1α showed that the forms have similar receptor dissociation constants (Kd = 4.0 nM and 4.5 nM for pro-IL-1α and mature IL-1α, respectively)35. More recently, it was confirmed that recombinant pro-IL-1α and mature IL-1α have identical biological activities as measured by their ability to trigger secretion of the inflammatory cytokines IL-6 and TNF from epithelial and hematopoietic cells6. The mature IL-1α used in these studies was comprised of the C-terminal portion of IL-1α starting at Ser115. Cleavage of mouse pro-IL-1α at this site and of human pro-IL-1α at Phe118 (ref. 36) is mediated by the calcium-dependent neutral protease calpain. Calpain can cleave pro-IL-1α inside the cell or under cell-free conditions36–38. However, in the extracellular space, pro-IL-1α can also be cleaved at the evolutionary conserved Asp103 by granzyme B39, and the C-terminal mature IL-1α generated via granzyme B cleavage is more biologically active than the pro-IL-1α form. This same study demonstrated that pro-IL-1α cleavage with elastase or chymase, as well as calpain, produces a mature IL-1α C-terminal piece that is more biologically active than the pro-IL-1α form39. Although it is plausible that pro-IL-1α cleavage at different sites may generate mature IL-1α forms that exhibit different biological activities, the reasons for different biological activity of pro-IL-1α form observed in different studies6,35,39,40 require further clarification.
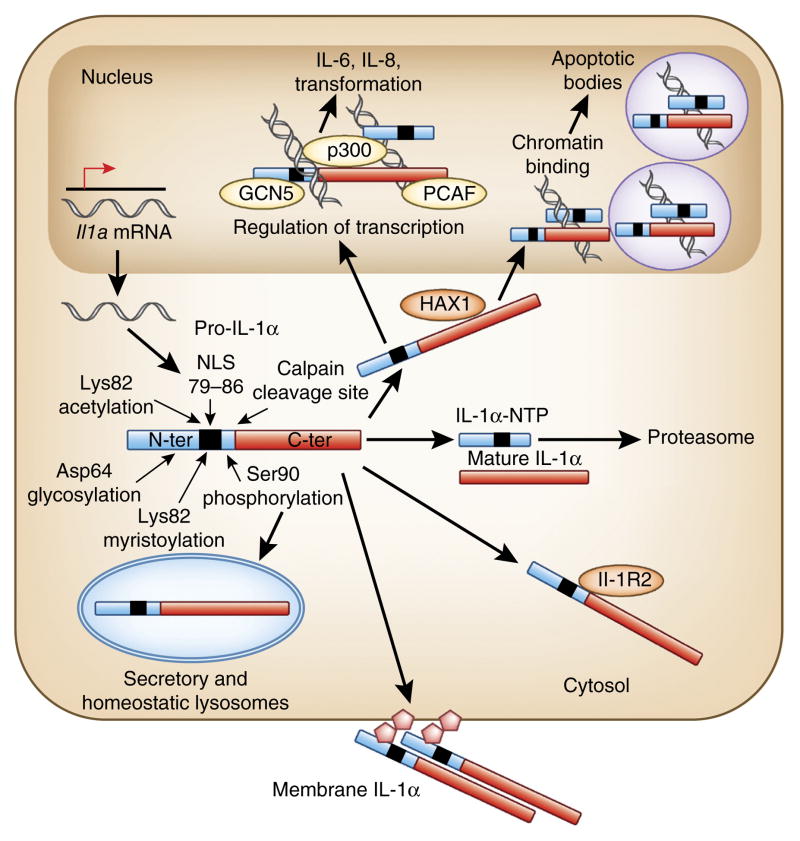
Pro-IL-1α has a functional nucleus localization signal (NLS) LKKRRL41–43, which is retained in the IL-1α-NTP after pro-IL-1α cleavage with calpain or other proteases (Fig. 1). Pro-IL-1α and IL-1α-NTP can translocate into the nucleus; however, whether there is a single mechanism that regulates nuclear localization of pro-IL-1α and IL-1α-NTP in all cell types remain unknown. Genotoxic stress increases nuclear localization of pro-IL-1α, which in part depends on acetylation of Lys82 (ref. 34). Whereas macrophage pro-IL-1α is localized to the nucleus upon lipopolysaccharide (LPS) stimulation, in keratinocytes and fibroblasts, pro-IL-1α is localized to the nucleus at steady state without any cell stimulation43. One of the mechanisms that may control nuclear localization of pro-IL-1α is its association with a repressor in the cytosol. In vascular smooth muscle cells, pro-IL-1α is bound to an intracellular form of inhibitory IL-1R2 (ref. 40) that may mask the NLS, leading to cytosolic retention of pro-IL-1α. Association of pro-IL-1α with IL-1R2 has also been shown in fibroblasts from systemic sclerosis patients44; however, in these cells, pro-IL-1α exhibits nuclear localization at steady state, which is regulated by HAX1, a ubiquitously expressed protein localized in mitochondria, endoplasmic reticulum and at the nuclear envelope45. HAX1 is able to bind both pro-IL-1α and IL-1α-NTP44, and suppression of HAX1 abolishes nuclear localization of pro-IL-1α. Nuclear localization of pro-IL-1α and IL-1α-NTP is functionally important, as they are able to interact directly with histone acetyltransferases p300, PCAF and GCN5 (refs. 46,47) and stimulate transcription of genes, including those encoding proinflammatory chemokines, independently of IL-1R1 signaling48,49. Furthermore, pro-IL-1α can bind chromatin50, and IL-1α-NTP localization to spliceosomes triggers apoptosis of numerous malignant cell types but not primary nontransformed cells51.
Figure 1.
Molecular modifications of IL-1α that regulate its intracellular distribution and bioavailability. Constitutive or inducible expression of IL-1α produces full-length pro-IL-1α. After synthesis, pro-IL-1α is localized into the nucleus, cytosol or lysosomal compartment or is displayed on the outer leaflet of the plasma membrane. Intracellular localization of pro-IL-1α can change in response to specific stimuli and pro-IL-1α can also be cleaved by calpain to generate IL-1α-NTP and a mature C-terminal IL-1α form. Both IL-1α-NTP and pro-IL-1α possess a functional NLS signal and bind HAX1 to allow translocation into the nucleus. In the nucleus, IL-1α-NTP binds transcription regulation factors and activates expression of proinflammatory cytokines and chemokines independently of IL-1R1 signaling. Pro-IL-1α can bind chromatin, which allows sequestration of pro-IL-1α from the cytosol into the nucleus, thereby limiting aberrant IL-1α-dependent inflammation during apoptosis. In the cytosol, glycosylation of pro-IL-1α may allow pro-IL-1α translocation onto the outer surface of the cell. Plasma-membrane-bound pro-IL-1α can be released by mannose, suggesting a lectin-like mechanism of anchorage of pro-IL-1α to the outer leaflet of the plasma membrane. In the cytosol, pro-IL-1α can form a complex with inhibitory IL-1R2. Cytosolic pro-IL-1α can be polyubiquitinated, leading to its proteosomal degradation. Although several post-translational modifications of IL-1α-NTP and pro-IL-1α have been reported, formal confirmation of their functional importance is currently lacking.
IL-1α can function as a membrane-bound cytokine. A study using primary macrophage cultures stimulated with heat-killed Listeria monocytogenes found that IL-1 has biological activity at the plasma membrane of intact cells as well as in isolated plasma membrane preparations7. This finding was confirmed in many subsequent studies52–55, which also demonstrated that the plasma membrane-associated IL-1α represents a full-length pro-IL-1α form that is fully biologically active. Plasma-membrane-bound IL-1α can be eluted from intact cells with D-mannose56. On the basis of this observation, it was proposed that pro-IL-1α is glycosylated and anchoring onto the membrane is mediated by a lectin-like interaction. Upon stimulation of cells with proinflammatory stimuli, the appearance of IL-1α on the plasma membrane occurs within hours on both hematopoietic and nonhematopoietic cells, such as fibroblasts and endothelial cells57. This implies the existence of a specialized molecular machinery that regulates translocation of pro-IL-1α from the cytosol to the outer side of the plasma membrane, thus allowing initiation of IL-1α– IL-1R1-mediated signaling in a paracrine manner. Over time, plasma-membrane-bound pro-IL-1α can be released via cleavage with extracellular proteases39,54,57. The excess of intracellular pro-IL-1α is subjected to proteosomal degradation through ubiquitin-dependent mechanisms58.
Biological contexts of IL-1α signaling
Because pro-IL-1α is fully biologically active and because of its constitutive and induced expression in a wide variety of cell types, cell death due to injury or infection may result in a passive leakage of the cytosolic pro-IL-1α into the surrounding milieu and activation of inflammation in an IL-1R1-dependent manner. This assumption has been confirmed experimentally in several studies, where administration of necrotic cells to mice triggered neutrophilic inflammation that was completely dependent on the presence of IL-1α in necrotic cells and IL-1R1 signaling on stromal, nonhematopoietic cells59,60. These and subsequent studies50,61 confirming the idea that the cytosolic pro-IL-1α is a principal inflammation-triggering moiety in necrotic cells have led to the designation of IL-1α as a key ‘alarmin’ in the cell5,15,43,62 that alerts the host to injury or damage. Using the same experimental approach59, it was also found that pro-IL-1α association with an inhibitory IL-1R2 in the cytosol44 is functionally important, as it can prevent or reduce the magnitude of inflammatory response to necrotic cells owing to sequestration of pro-IL-1α into cytosolic complexes with IL-1R2 (ref. 40) (Fig. 2). The importance of pro-IL-1α as a principal intracellular alarmin is underscored by findings that point to the existence of a machinery to sequester cytosolic pro-IL-1α during a physiologically regulated noninflammatory cell death routine, such as apoptosis. Upon induction of apoptosis, cytosolic pro-IL-1α is sequestered into the nucleus, preventing its inadvertent release and inflammation48. However, if not promptly phagocytosed, IL-1α-containing apoptotic bodies can trigger inflammation, as has been shown for apoptotic bodies produced by endothelial cells63.
Figure 2.
Biological contexts of IL-1α-mediated signaling. (a) If a cell experiences supra-physiological stress that leads to necrosis, cytosolic pro-IL-1α is passively released through the ruptured plasma membrane and triggers the ‘alarm’ call, a functional IL-1R1-dependent proinflammatory response. Pro-IL-1α association with inhibitory IL-1R2 in the cytosol interferes with pro-IL-1 alarmin function. Inhibitory IL-1R2 association with pro-IL-1α can be relieved by capsase-1-dependent proteolysis of IL-1R2. In stimulated cells, pro-IL-1α is displayed on the cell surface. Plasma-membrane-bound pro-IL-1α is fully biologically active and can trigger local inflammatory responses from living cells. Membrane-bound pro-IL-1α can be cleaved extracellularly by granzyme B, chymase or elastase to produce a C-terminal mature IL-1α fragment that is also biologically active. (b) In hematopoietic cells, the proinflammatory stimuli that induce pro-IL-1β expression also activate expression of pro-IL-1α. However, pro-IL-1β is not biologically active and requires cleavage by capsase-1 in an inflammasome-dependent manner. This cleavage produces mature IL-1β and stimulates its secretion from the cell. Many pathogens express virulence factors that block caspase-1 activation and, therefore, maturation of pro-IL-1β and pyroptotic cell death. In these settings, pro-IL-1α can still be displayed at the cell surface and activate local IL-1R1-dependent inflammatory responses, thus bypassing blockade of IL-1β processing and release. Under the conditions of pyroptotic cell death, both pro-IL-1α and mature IL-1β are released to activate local and systemic inflammation.
To execute its function as an extracellular alarmin, pro-IL-1α requires the loss of plasma membrane integrity, which is indicative of necrotic-type cell death64, for passive release and activation of the inflammatory cascade. However, during normal responses to physiological stimuli, necrotic cell death is an unusual event; therefore, great effort has been put toward analyzing the biological contexts and molecular mechanisms that may control IL-1α-mediated signaling from living non-necrotic cells. These efforts have produced numerous findings65–69, but their physiological relevance requires further clarification. Because pro-IL-1α lacks a signaling peptide to mediate its secretion from the cell, the most debated issue is whether caspase-1 and the inflammasome facilitate or are needed for pro-IL-1α proteolytic processing and release70. A study using LPS-stimulated monocytes from caspase-1-deficient mice showed that cells from these mice release significantly lower amounts of IL-1α than cells from wild-type mice71. The conclusion that caspase-1 and the inflammasome control release of IL-1α from hematopoietic cells such as macrophages and dendritic cells after their stimulation with LPS and ATP65 can be explained by the induction of pyroptosis, a form of cell death that leads to plasma-membrane permeabilization72, allowing release of intracellular pro-IL-1α and mature IL-1α forms without involvement of any specific secretory mechanism. A detailed analysis of IL-1α and IL-1β release from dendritic cells showed that LPS-stimulated cells treated with clostridium toxin B or alum, urea crystals or silica release mature IL-1α in a capsase-1- and NLRP3 inflammasome–independent manner, whereas the release of mature IL-1β in response to the same stimuli was completely dependent on caspase-1 and NLRP3 inflammasome components73. Because pro-IL-1α and pro-IL-1β are expressed in hematopoietic cells in response to the same stimuli, and because caspase-1 is targeted by many pathogens to suppress cell death and inflammation74–76, release of IL-1α in an inflammasome-independent manner may provide an alternative pathway to trigger IL-1R1-dependent defense mechanisms and alert the host of an ongoing infection.
The ability of IL-1α to function as a plasma-membrane-bound cytokine is unique within the IL-1 family5,77. Indeed, exposure of hematopoietic cells to LPS or heat-killed bacteria such as L. monocytogenes7,55 or Mycobacterium tuberculosis78 stimulates intracellular expression of IL-1α as well as the rapid appearance of membrane-bound IL-1α that is fully biologically active, as shown by stimulation of T cell proliferation and production of chemokines. Similarly, exposure of endothelial cells to LPS or TNF also results in membrane IL-1α expression52, providing important insight into biological contexts where IL-1α–IL-1R1 signaling can be triggered from living cells under stress or during infection without necrotic cell death. The capacity of membrane-bound IL-1α to activate IL-1R1 signaling in an intracrine and paracrine manner on surrounding cells has important implications for the induction and maintenance of local inflammatory responses.
The ‘inflammatory loop’ model of IL-1α-driven inflammation
The diversity of stimuli that activate IL-1α expression in hematopoietic and nonhematopoietic cells and its capacity to activate IL-1R1 signaling from both living and necrotic cells provide the conceptual framework for understanding the role of IL-1α in inflammation and as a principal driver of pathologies that mechanistically depend on aberrant IL-1R1 signaling. Because IL-1α and IL-1β are equally potent activators of IL-1R1 signaling, the availability of mice deficient in either IL-1α or IL-1β provides an opportunity to analyze the contribution of each in the development of specific pathologies. Although the development of atherosclerotic lesions has an important IL-1R1 signaling component79, evidence indicates that macrophage-derived IL-1α is a principal cytokine controlling the development of atherosclerotic plaques26. Mechanistically, the expression of IL-1α is induced in macrophages by fatty acids that accumulate in atherosclerotic plaques and leads to IL-1α-driven vasculitis under conditions where IL-1β activation is blocked via mitochondrial uncoupling26. IL-1α-deficient mice are resistant to ischemic injury in models of myocardial infarction80 and ischemic brain injury81. Importantly, during ischemic brain injury, IL-1α expression in microglia precedes expression of IL-1β, providing conceptual insight into the role of IL-1α in the initiation of inflammation. In the model of spontaneous foot-pad inflammation in mice deficient in PTPN6 phosphatase, the development of neutrophilic inflammation in the foot pad is completely abrogated by the deletion of IL-1α in stromal, nonhematopoietic cells82. IL-1α produced by intestinal epithelial cells was found to be the principal driver of inflammation in a mouse model of colitis12, where IL-1α-deficient mice showed improved survival after intestinal epithelial damage.
These findings can be conceptualized into an ‘inflammatory loop’ model, in which inflammation is initiated by stressed or damaged cells via IL-1α-dependent activation of chemokines that recruit inflammatory hematopoietic cells to the site of damage or stress (Fig. 3). These hematopoietic cells respond to the IL-1α-containing milieu, where pro-IL-1α can be either released from damaged cells or exposed as membrane-bound IL-1α on the surface of cells undergoing oxidative or metabolic stress, and in turn activate their own IL-1α and IL-1β production downstream of IL-1R1. The initial IL-1α–IL-1R1 signaling therefore initiates a loop of sustained and self-perpetuating inflammation that results in extensive tissue damage that occurs until IL-1R1 signaling is either exhausted or suppressed. This model is also supported by the role of IL-1α in host defense against viral and bacterial pathogens. IL-1α has a nonredundant role in initiating inflammatory responses to Legionella pneumophila83 and Yersinia enterocolitica84. Splenic marginal zone macrophages sequester adenovirus from the blood and activate IL-1α-dependent chemokine production85 and recruitment of polymorphonuclear cells to the spleen86. This IL-1α-dependent inflammation is critical for elimination of virus-containing cells from the host.
Figure 3.
IL-1α-driven inflammatory loop model. (a) IL-1α expression is induced in response to oxidative, genotoxic and metabolic stressors; hormonal stimulation; proinflammatory mediators that activate TNF-R1, IL-1R1 or TLR signaling; or infection. Without cell death, these stimuli trigger translocation of pro-IL-1α onto a plasma membrane and the appearance of membrane-bound pro-IL-1α, which activates IL-1R1-dependent chemokine and cytokine production from neighboring nonhematopoietic cells or tissue-resident macrophages. This initial IL-1α-dependent chemokine production leads to a recruitment of myeloid cells to the site of stress. Upon arrival, myeloid cells receive IL-1α-dependent IL-1R1 stimulation, which leads to the expression of pro-IL-1α and pro-IL-1β. Therefore, in the context of alive nonhematopoietic or residential hematopoietic cells under stress, IL-1α-initiated signaling triggers the recruitment of cells from hematopoietic compartment, which can amplify and sustain IL-1R1-dependent inflammation through the new round of IL-1α and IL-1β production, thus closing an inflammatory loop of IL-1-IL-1RI-signaling-dependent chemokine production and recruitment of inflammatory cells to the site of stress that now can be sustained only by cells of hematopoietic origin. (b) Upon necrotic cell death due to damage, stress or infection, the IL-1α-driven induction of the inflammatory loop is triggered by the passive release of pro-IL-1α into the surrounding milieu, where IL-1α functions as an alarmin. The pro-IL-1α released from necrotic cells activates IL-1R1 signaling on neighboring cells, leading to the recruitment of hematopoietic cells that can further sustain inflammation as in a.
Because IL-1β is not known to be produced in a biologically active form in nonhematopoietic cells, the IL-1α-driven inflammatory loop model addresses the conundrum of apparent functional excess and redundancy of IL-1α and IL-1β production at sites of inflammation. It is also consistent with abundant clinical data demonstrating the efficacy of IL-1β-targeted therapies at controlling autoinflammatory diseases87, as the perpetuation of IL-1α-initiated inflammation into a clinically significant pathology is accomplished by the exuberant production of both IL-1α and IL-1β, despite their redundant functions or when pathology development depends largely on subsequent IL-1β production from activated hematopoietic cells.
IL-1α and autoinflammatory disease
IL-1α-mediated signaling initiates processes beyond recruitment of inflammatory hematopoietic cells. Although IL-1α–IL-1R1-dependent chemokine production leading to hematopoietic cell recruitment is a major component of numerous pathologies, autocrine and paracrine IL-1α signaling can also mediate activation of nonhematopoietic cells.
Fibroblasts purified from lesional skin of patients with systemic sclerosis (SSc) express abundant amounts of IL-1α, which is localized on the plasma membrane, in the cytosol and in the nucleus88. Compared to fibroblasts from normal skin, SSc fibroblasts express high amounts of IL-6, the growth factor PDGF-α89, IL-1R1 (ref. 90) and collagen. Suppression of IL-1α expression in SSc fibroblasts reduces amounts of secreted IL-6 and pro-collagen, and overexpression of pro-IL-1α in normal fibroblasts increases IL-6 and pro-collagen production88. It has been demonstrated that IL-1-dependent PDGF-α production has direct mitogenic effects on fibroblasts and smooth muscle cells91, mechanistically linking excessive IL-1α expression and signaling with a pathological tissue response that manifests in excessive deposition of collagen and fibrosis.
IL-1α and cancer
Aging cells are known to acquire a ‘senescence-associated secretory phenotype’ characterized by production of IL-6 and other proinflammatory mediators that sustain the low-grade chronic inflammation underlying many age-related pathologies and cancer92. Normal human fibroblasts triggered to senesce by ionizing radiation show an increase in NF-κB activity, which stimulates production of proinflammatory mediators such as IL-6 and IL-8 (ref. 93). This NF-κB activity is due to an increase in IL-1α translation, leading to production of membrane-bound IL-1α that stimulates cells in an autocrine manner. Rapamycin treatment was found to repress IL-1α translation, resulting in suppression of production of senescence-associated factors. Senescent fibroblasts have also been shown to be pro-tumorigenic, as they promote growth of malignant epithelial cells and tumor formation in mice93. Therefore, this study92 implicates IL-1α as a principal component in a feed-forward signaling amplification loop that leads to production of pro-tumorigenic factors by aged or senesced cells. There are many examples implicating an IL-1α-driven feed-forward amplification loop and even IL-1α itself in malignant transformation, tumor formation and support of tumor growth through stimulation of cell growth and production of vasculogenic factors94–100. Confirmation of the critical contribution of IL-1α to tumor development in humans comes from the field of clinical oncology. In patients with head and neck squamous cell carcinoma, IL-1α expression has been evaluated as a prognostic marker of distant metastases101. IL-1α mRNA and protein expression were found to be higher in tumor samples from patients who later developed distant metastases than in tumors from patients who did not. Patients who had high IL-1α expression in tumors had significantly lower 5-year survival rates than patients with low IL-1α expression101. Analysis of genetic risk factors for the development of ovarian cancer in a large patient cohort (15,604 cases) showed a significant association between the Il1a single nucleotide polymorphism (SNP) rs17561 and reduced susceptibility to clear cell ovarian cancer type. rs17561 is a missense SNP that results in an alanine-to-serine substitution at residue 114 (A114S)102. The A114S substitution produces a pro-IL-1α form that is more readily cleaved by calpain103. Because the plasma-membrane-bound IL-1α form represents a full-length pro-IL-1α protein, enhanced cleavage by calpain may lead to reduced expression of membrane-bound IL-1α and, thus, tempering of IL-1α-driven autocrine pro-tumorigenic IL-1R1 signaling. Clinical trials with an IL-1α-blocking monoclonal antibody targeting this IL-1α-driven feed-forward signaling amplification loop as a cancer therapeutic have shown promising outcomes104,105.
IL-1α and granulomatous diseases
Chronic granulomatous diseases manifest through the formation of focal inflammatory lesions, or granulomas, that consist of both hematopoietic and nonhematopoietic cells. Granulomas arise in response to poorly degradable particulate matter or can be induced by microbial, viral, fungal, protozoan or helminthic infections. Because granulomas are fundamentally a local inflammatory response, it is not surprising that IL-1α, with its capacity to produce proinflammatory signaling from the plasma membrane, is involved in granuloma formation. In a mouse model of pulmonary M. tuberculosis infection, IL-1α-deficient mice failed to establish protective granuloma structures and succumbed to infection sooner than did wild-type mice, even in the presence of functional M. tuberculosis–specific adaptive immunity78. Exposure of macrophages to heat-killed M. tuberculosis resulted in rapid expression of biologically active plasma-membrane-bound IL-1α, and lung epithelial cells treated with heat-killed M. tuberculosis and TNF upregulated IL-1α expression. Bone marrow transplantation studies further showed that an absence of IL-1R1 and TNF receptor TNF-R1 on stromal or hematopoietic cells compromised host resistance to M. tuberculosis, suggesting that TNF- and IL-1-driven cross-talk between monocytes and stromal cells is necessary for optimal control of this pathogen78. Cryptococcus neoformans infection causes severe meningoencephalitis in susceptible BALB/c mice. Cytokine profiling in the brain after systemic C. neoformans infection showed constitutive and induced IL-1α expression that preceded detectible expression of IL-1β by 7 d106. Although there is no consensus on whether granulomas arise as a host-protective response or are induced by pathogens to evade immunity, the potential pro-pathogenic role of IL-1α expression in response to pathogens that induce granuloma formation has also been noted. Specifically, IL-1α was found to promote pathogenesis during Leishmania major infection in susceptible BALB/c mice, and IL-1α-deficient mice are more resistant than wild-type mice to L. major infection107. Similarly, given that histologically confined granulomas do not form in the lungs of IL-1α-deficient mice after M. tuberculosis infection78 and that granulomas are necessary for M. tuberculosis survival and spread, it is plausible that IL-1α induction in the context of tuberculosis may serve a pro-pathogenic function.
Conceptual perspective and remaining questions
IL-1α has a number of unique features, including its transcription in response to a wide range of stimuli, its widespread expression and its ability to activate IL-1R1 signaling from the plasma membrane or as an alarmin. Given these features, IL-1α emerges as a molecular decision-making nexus of the cell that gauges the magnitude of stress or damage or severity of infection to launch either the tissue or the whole body into action through initiation of inflammation or reparative fibrosis. Aberration of these sequelae can produce devastating disruption of tissue homeostasis and underlies the pathology of numerous human diseases. Because pro-IL-1α is present in healthy cells, it is likely that a number of specific mechanisms exist to tightly control and suppress aberrant IL-1α activity.
Even after more than 30 years of research, many critical questions related to IL-1α biology remain unanswered. Specifically, it is unclear what factors control pro-IL-1α translocation from the cytosol to the outer surface of the plasma membrane to allow it to signal as a membrane-bound cytokine. The functional significance of calpain-dependent cleavage of pro-IL-1α in the cell also remains unclear. Does calpain cleavage facilitate release of pro-IL-1α and mature IL-1 from living cells, or is it necessary only to induce IL-1α-NTP translocation into the nucleus? Which factors control the sequestration of IL-1α in the nucleus during apoptosis? The identity of factors that allow for IL-1α expression in aged and senescent cells also requires further investigation. As methodologies for quantitative genomics, proteomics and metabolomics continue to advance, it is likely that IL-1α will be implicated as a key driver in many human pathologies and diseases. The numerous unresolved questions related to this powerful yet understudied cytokine certainly warrant further investigation.
Acknowledgments
Supported by the US National Institutes of Health (NIH) grants AI065429, AI126816 and AI107960 (D.M.S.), AI123126 (N.C.D.P.) and the Children’s Healthcare of Atlanta Research Trust (D.M.S.).
Footnotes
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
References
- 1.Dinarello CA, Goldin NP, Wolff SM. Demonstration and characterization of two distinct human leukocytic pyrogens. J Exp Med. 1974;139:1369–1381. doi: 10.1084/jem.139.6.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Auron PE, et al. Nucleotide sequence of human monocyte interleukin 1 precursor cDNA. Proc Natl Acad Sci USA. 1984;81:7907–7911. [PubMed] [Google Scholar]
- 3.Lomedico PT, et al. Cloning and expression of murine interleukin-1 cDNA in Escherichia coli. Nature. 1984;312:458–462. doi: 10.1038/312458a0. [DOI] [PubMed] [Google Scholar]
- 4.March CJ, et al. Cloning, sequence and expression of two distinct human interleukin-1 complementary DNAs. Nature. 1985;315:641–647. doi: 10.1038/315641a0. [DOI] [PubMed] [Google Scholar]
- 5.Garlanda C, Dinarello CA, Mantovani A. The interleukin-1 family: back to the future. Immunity. 2013;39:1003–1018. doi: 10.1016/j.immuni.2013.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim B, et al. The interleukin-1α precursor is biologically active and is likely a key alarmin in the IL-1 family of cytokines. Front Immunol. 2013;4:391. doi: 10.3389/fimmu.2013.00391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kurt-Jones EA, Beller DI, Mizel SB, Unanue ER. Identification of a membrane-associated interleukin 1 in macrophages. Proc Natl Acad Sci USA. 1985;82:1204–1208. doi: 10.1073/pnas.82.4.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martinon F, Mayor A, Tschopp J. The inflammasomes: guardians of the body. Annu Rev Immunol. 2009;27:229–265. doi: 10.1146/annurev.immunol.021908.132715. [DOI] [PubMed] [Google Scholar]
- 9.van de Veerdonk FL, Netea MG, Dinarello CA, Joosten LA. Inflammasome activation and IL-1β and IL-18 processing during infection. Trends Immunol. 2011;32:110–116. doi: 10.1016/j.it.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 10.Lamkanfi M, Dixit VM. Mechanisms and functions of inflammasomes. Cell. 2014;157:1013–1022. doi: 10.1016/j.cell.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 11.Dinarello CA. Biologic basis for interleukin-1 in disease. Blood. 1996;87:2095–2147. [PubMed] [Google Scholar]
- 12.Bersudsky M, et al. Non-redundant properties of IL-1α and IL-1β during acute colon inflammation in mice. Gut. 2014;63:598–609. doi: 10.1136/gutjnl-2012-303329. [DOI] [PubMed] [Google Scholar]
- 13.Netea MG, van de Veerdonk FL, van der Meer JW, Dinarello CA, Joosten LA. Inflammasome-independent regulation of IL-1-family cytokines. Annu Rev Immunol. 2015;33:49–77. doi: 10.1146/annurev-immunol-032414-112306. [DOI] [PubMed] [Google Scholar]
- 14.Man SM, Kanneganti TD. Converging roles of caspases in inflammasome activation, cell death and innate immunity. Nat Rev Immunol. 2016;16:7–21. doi: 10.1038/nri.2015.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rider P, et al. The transcription of the alarmin cytokine interleukin-1 alpha is controlled by hypoxia inducible factors 1 and 2 alpha in hypoxic cells. Front Immunol. 2012;3:290. doi: 10.3389/fimmu.2012.00290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McDowell TL, Symons JA, Duff GW. Human interleukin-1α gene expression is regulated by Sp1 and a transcriptional repressor. Cytokine. 2005;30:141–153. doi: 10.1016/j.cyto.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 17.Wierstra I. Sp1: emerging roles—beyond constitutive activation of TATA-less housekeeping genes. Biochem Biophys Res Commun. 2008;372:1–13. doi: 10.1016/j.bbrc.2008.03.074. [DOI] [PubMed] [Google Scholar]
- 18.Alheim K, McDowell TL, Symons JA, Duff GW, Bartfai T. An AP-1 site is involved in the NGF induction of IL-1α in PC12 cells. Neurochem Int. 1996;29:487–496. doi: 10.1016/0197-0186(96)00017-4. [DOI] [PubMed] [Google Scholar]
- 19.Bailly S, Fay M, Israël N, Gougerot-Pocidalo MA. The transcription factor AP-1 binds to the human interleukin 1α promoter. Eur Cytokine Netw. 1996;7:125–128. [PubMed] [Google Scholar]
- 20.Mori N, Prager D. Transactivation of the interleukin-1α promoter by human T-cell leukemia virus type I and type II Tax proteins. Blood. 1996;87:3410–3417. [PubMed] [Google Scholar]
- 21.van Rietschoten JG, et al. Differentially methylated alleles in a distinct region of the human interleukin-1α promoter are associated with allele-specific expression of IL-1α in CD4+ T cells. Blood. 2006;108:2143–2149. doi: 10.1182/blood-2006-01-021147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chan J, et al. Cutting edge: a natural antisense transcript, AS-IL1α, controls inducible transcription of the proinflammatory cytokine IL-1α. J Immunol. 2015;195:1359–1363. doi: 10.4049/jimmunol.1500264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McCarthy DA, et al. Redox-control of the alarmin, interleukin-1α. Redox Biol. 2013;1:218–225. doi: 10.1016/j.redox.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McCarthy DA, Clark RR, Bartling TR, Trebak M, Melendez JA. Redox control of the senescence regulator interleukin-1α and the secretory phenotype. J Biol Chem. 2013;288:32149–32159. doi: 10.1074/jbc.M113.493841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tynan GA, et al. Endogenous oils derived from human adipocytes are potent adjuvants that promote IL-1α-dependent inflammation. Diabetes. 2014;63:2037–2050. doi: 10.2337/db13-1476. [DOI] [PubMed] [Google Scholar]
- 26.Freigang S, et al. Fatty acid-induced mitochondrial uncoupling elicits inflammasome-independent IL-1α and sterile vascular inflammation in atherosclerosis. Nat Immunol. 2013;14:1045–1053. doi: 10.1038/ni.2704. [DOI] [PubMed] [Google Scholar]
- 27.Itoh Y, et al. 17β-estradiol induces IL-1α gene expression in rheumatoid fibroblast-like synovial cells through estrogen receptor α (ERα) and augmentation of transcriptional activity of Sp1 by dissociating histone deacetylase 2 from ERα. J Immunol. 2007;178:3059–3066. doi: 10.4049/jimmunol.178.5.3059. [DOI] [PubMed] [Google Scholar]
- 28.Kimura H, et al. Molecular analysis of constitutive IL-1α gene expression in human melanoma cells: autocrine stimulation through NF-κB activation by endogenous IL-1α. Cytokine. 1998;10:872–879. doi: 10.1006/cyto.1998.0369. [DOI] [PubMed] [Google Scholar]
- 29.Weber A, Wasiliew P, Kracht M. Interleukin-1 (IL-1) pathway. Sci Signal. 2010;3:cm1. doi: 10.1126/scisignal.3105cm1. [DOI] [PubMed] [Google Scholar]
- 30.Bandman O, Coleman RT, Loring JF, Seilhamer JJ, Cocks BG. Complexity of inflammatory responses in endothelial cells and vascular smooth muscle cells determined by microarray analysis. Ann NY Acad Sci. 2002;975:77–90. doi: 10.1111/j.1749-6632.2002.tb05943.x. [DOI] [PubMed] [Google Scholar]
- 31.Beuscher HU, Nickells MW, Colten HR. The precursor of interleukin-1 alpha is phosphorylated at residue serine 90. J Biol Chem. 1988;263:4023–4028. [PubMed] [Google Scholar]
- 32.Kobayashi Y, et al. Phosphorylation of intracellular precursors of human IL-1. J Immunol. 1988;140:2279–2287. [PubMed] [Google Scholar]
- 33.Stevenson FT, Bursten SL, Fanton C, Locksley RM, Lovett DH. The 31-kDa precursor of interleukin 1α is myristoylated on specific lysines within the 16-kDa N-terminal propiece. Proc Natl Acad Sci USA. 1993;90:7245–7249. doi: 10.1073/pnas.90.15.7245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Idan C, et al. IL-1α is a DNA damage sensor linking genotoxic stress signaling to sterile inflammation and innate immunity. Sci Rep. 2015;5:14756. doi: 10.1038/srep14756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mosley B, et al. The interleukin-1 receptor binds the human interleukin-1α precursor but not the interleukin-1β precursor. J Biol Chem. 1987;262:2941–2944. [PubMed] [Google Scholar]
- 36.Kobayashi Y, et al. Identification of calcium-activated neutral protease as a processing enzyme of human interleukin 1α. Proc Natl Acad Sci USA. 1990;87:5548–5552. doi: 10.1073/pnas.87.14.5548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carruth LM, Demczuk S, Mizel SB. Involvement of a calpain-like protease in the processing of the murine interleukin 1α precursor. J Biol Chem. 1991;266:12162–12167. [PubMed] [Google Scholar]
- 38.Kavita U, Mizel SB. Differential sensitivity of interleukin-1α and -β precursor proteins to cleavage by calpain, a calcium-dependent protease. J Biol Chem. 1995;270:27758–27765. doi: 10.1074/jbc.270.46.27758. [DOI] [PubMed] [Google Scholar]
- 39.Afonina IS, et al. Granzyme B-dependent proteolysis acts as a switch to enhance the proinflammatory activity of IL-1α. Mol Cell. 2011;44:265–278. doi: 10.1016/j.molcel.2011.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zheng Y, Humphry M, Maguire JJ, Bennett MR, Clarke MC. Intracellular interleukin-1 receptor 2 binding prevents cleavage and activity of interleukin-1α, controlling necrosis-induced sterile inflammation. Immunity. 2013;38:285–295. doi: 10.1016/j.immuni.2013.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wessendorf JH, Garfinkel S, Zhan X, Brown S, Maciag T. Identification of a nuclear localization sequence within the structure of the human interleukin-1α precursor. J Biol Chem. 1993;268:22100–22104. [PubMed] [Google Scholar]
- 42.Luheshi NM, Rothwell NJ, Brough D. The dynamics and mechanisms of interleukin-1α and β nuclear import. Traffic. 2009;10:16–25. doi: 10.1111/j.1600-0854.2008.00840.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rider P, Carmi Y, Voronov E, Apte RN. Interleukin-1α. Semin Immunol. 2013;25:430–438. doi: 10.1016/j.smim.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 44.Kawaguchi Y, et al. Intracellular IL-1α-binding proteins contribute to biological functions of endogenous IL-1α in systemic sclerosis fibroblasts. Proc Natl Acad Sci USA. 2006;103:14501–14506. doi: 10.1073/pnas.0603545103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Suzuki Y, et al. HAX-1, a novel intracellular protein, localized on mitochondria, directly associates with HS1, a substrate of Src family tyrosine kinases. J Immunol. 1997;158:2736–2744. [PubMed] [Google Scholar]
- 46.Buryskova M, Pospisek M, Grothey A, Simmet T, Burysek L. Intracellular interleukin-1α functionally interacts with histone acetyltransferase complexes. J Biol Chem. 2004;279:4017–4026. doi: 10.1074/jbc.M306342200. [DOI] [PubMed] [Google Scholar]
- 47.Zamostna B, et al. N-terminal domain of nuclear IL-1α shows structural similarity to the C-terminal domain of Snf1 and binds to the HAT/core module of the SAGA complex. PLoS One. 2012;7:e41801. doi: 10.1371/journal.pone.0041801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cohen I, et al. Differential release of chromatin-bound IL-1α discriminates between necrotic and apoptotic cell death by the ability to induce sterile inflammation. Proc Natl Acad Sci USA. 2010;107:2574–2579. doi: 10.1073/pnas.0915018107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Werman A, et al. The precursor form of IL-1α is an intracrine proinflammatory activator of transcription. Proc Natl Acad Sci USA. 2004;101:2434–2439. doi: 10.1073/pnas.0308705101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lamacchia C, Rodriguez E, Palmer G, Gabay C. Endogenous IL-1α is a chromatin-associated protein in mouse macrophages. Cytokine. 2013;63:135–144. doi: 10.1016/j.cyto.2013.04.010. [DOI] [PubMed] [Google Scholar]
- 51.Pollock AS, Turck J, Lovett DH. The prodomain of interleukin 1α interacts with elements of the RNA processing apparatus and induces apoptosis in malignant cells. FASEB J. 2003;17:203–213. doi: 10.1096/fj.02-0602com. [DOI] [PubMed] [Google Scholar]
- 52.Kurt-Jones EA, Fiers W, Pober JS. Membrane interleukin 1 induction on human endothelial cells and dermal fibroblasts. J Immunol. 1987;139:2317–2324. [PubMed] [Google Scholar]
- 53.Kurt-Jones EA, Virgin HW, IV, Unanue ER. In vivo and in vitro expression of macrophage membrane interleukin 1 in response to soluble and particulate stimuli. J Immunol. 1986;137:10–14. [PubMed] [Google Scholar]
- 54.Bakouche O, Brown DC, Lachman LB. Subcellular localization of human monocyte interleukin 1: evidence for an inactive precursor molecule and a possible mechanism for IL 1 release. J Immunol. 1987;138:4249–4255. [PubMed] [Google Scholar]
- 55.Kurt-Jones EA, Kiely JM, Unanue ER. Conditions required for expression of membrane IL 1 on B cells. J Immunol. 1985;135:1548–1550. [PubMed] [Google Scholar]
- 56.Brody DT, Durum SK. Membrane IL-1: IL-1α precursor binds to the plasma membrane via a lectin-like interaction. J Immunol. 1989;143:1183–1187. [PubMed] [Google Scholar]
- 57.Giri JG, Lomedico PT, Mizel SB. Studies on the synthesis and secretion of interleukin 1. I A 33,000 molecular weight precursor for interleukin 1. J Immunol. 1985;134:343–349. [PubMed] [Google Scholar]
- 58.Ainscough JS, et al. Dendritic cell IL-1α and IL-1β are polyubiquitinated and degraded by the proteasome. J Biol Chem. 2014;289:35582–35592. doi: 10.1074/jbc.M114.595686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen CJ, et al. Identification of a key pathway required for the sterile inflammatory response triggered by dying cells. Nat Med. 2007;13:851–856. doi: 10.1038/nm1603. [DOI] [PubMed] [Google Scholar]
- 60.Eigenbrod T, Park JH, Harder J, Iwakura Y, Núñez G. Cutting edge: critical role for mesothelial cells in necrosis-induced inflammation through the recognition of IL-1α released from dying cells. J Immunol. 2008;181:8194–8198. doi: 10.4049/jimmunol.181.12.8194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kono H, Karmarkar D, Iwakura Y, Rock KL. Identification of the cellular sensor that stimulates the inflammatory response to sterile cell death. J Immunol. 2010;184:4470–4478. doi: 10.4049/jimmunol.0902485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rock KL, Latz E, Ontiveros F, Kono H. The sterile inflammatory response. Annu Rev Immunol. 2010;28:321–342. doi: 10.1146/annurev-immunol-030409-101311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Berda-Haddad Y, et al. Sterile inflammation of endothelial cell-derived apoptotic bodies is mediated by interleukin-1α. Proc Natl Acad Sci USA. 2011;108:20684–20689. doi: 10.1073/pnas.1116848108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Galluzzi L, et al. Essential versus accessory aspects of cell death: recommendations of the NCCD 2015. Cell Death Differ. 2015;22:58–73. doi: 10.1038/cdd.2014.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fettelschoss A, et al. Inflammasome activation and IL-1β target IL-1α for secretion as opposed to surface expression. Proc Natl Acad Sci USA. 2011;108:18055–18060. doi: 10.1073/pnas.1109176108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Perregaux DG, Gabel CA. Post-translational processing of murine IL-1: evidence that ATP-induced release of IL-1α and IL-1β occurs via a similar mechanism. J Immunol. 1998;160:2469–2477. [PubMed] [Google Scholar]
- 67.Yazdi AS, Drexler SK. Regulation of interleukin 1α secretion by inflammasomes. Ann Rheum Dis. 2013;72(suppl 2):ii96–ii99. doi: 10.1136/annrheumdis-2012-202252. [DOI] [PubMed] [Google Scholar]
- 68.Mandinova A, et al. S100A13 mediates the copper-dependent stress-induced release of IL-1α from both human U937 and murine NIH 3T3 cells. J Cell Sci. 2003;116:2687–2696. doi: 10.1242/jcs.00471. [DOI] [PubMed] [Google Scholar]
- 69.England H, Summersgill HR, Edye ME, Rothwell NJ, Brough D. Release of interleukin-1α or interleukin-1β depends on mechanism of cell death. J Biol Chem. 2014;289:15942–15950. doi: 10.1074/jbc.M114.557561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Keller M, Rüegg A, Werner S, Beer HD. Active caspase-1 is a regulator of unconventional protein secretion. Cell. 2008;132:818–831. doi: 10.1016/j.cell.2007.12.040. [DOI] [PubMed] [Google Scholar]
- 71.Kuida K, et al. Altered cytokine export and apoptosis in mice deficient in interleukin-1β converting enzyme. Science. 1995;267:2000–2003. doi: 10.1126/science.7535475. [DOI] [PubMed] [Google Scholar]
- 72.Bergsbaken T, Fink SL, Cookson BT. Pyroptosis: host cell death and inflammation. Nat Rev Microbiol. 2009;7:99–109. doi: 10.1038/nrmicro2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gross O, et al. Inflammasome activators induce interleukin-1α secretion via distinct pathways with differential requirement for the protease function of caspase-1. Immunity. 2012;36:388–400. doi: 10.1016/j.immuni.2012.01.018. [DOI] [PubMed] [Google Scholar]
- 74.Taxman DJ, Huang MT, Ting JP. Inflammasome inhibition as a pathogenic stealth mechanism. Cell Host Microbe. 2010;8:7–11. doi: 10.1016/j.chom.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shah S, et al. Cutting edge: Mycobacterium tuberculosis but not nonvirulent mycobacteria inhibits IFN-β and AIM2 inflammasome-dependent IL-1β production via its ESX-1 secretion system. J Immunol. 2013;191:3514–3518. doi: 10.4049/jimmunol.1301331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.LaRock CN, Cookson BT. The Yersinia virulence effector YopM binds caspase-1 to arrest inflammasome assembly and processing. Cell Host Microbe. 2012;12:799–805. doi: 10.1016/j.chom.2012.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dinarello CA. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood. 2011;117:3720–3732. doi: 10.1182/blood-2010-07-273417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Di Paolo NC, et al. Interdependence between interleukin-1 and tumor necrosis factor regulates TNF-dependent control of Mycobacterium tuberculosis infection. Immunity. 2015;43:1125–1136. doi: 10.1016/j.immuni.2015.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chamberlain J, et al. Interleukin-1 regulates multiple atherogenic mechanisms in response to fat feeding. PLoS One. 2009;4:e5073. doi: 10.1371/journal.pone.0005073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lugrin J, et al. Cutting edge: IL-1α is a crucial danger signal triggering acute myocardial inflammation during myocardial infarction. J Immunol. 2015;194:499–503. doi: 10.4049/jimmunol.1401948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Luheshi NM, Kovács KJ, Lopez-Castejon G, Brough D, Denes A. Interleukin-1α expression precedes IL-1β after ischemic brain injury and is localised to areas of focal neuronal loss and penumbral tissues. J Neuroinflammation. 2011;8:186. doi: 10.1186/1742-2094-8-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lukens JR, et al. RIP1-driven autoinflammation targets IL-1α independently of inflammasomes and RIP3. Nature. 2013;498:224–227. doi: 10.1038/nature12174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Barry KC, Fontana MF, Portman JL, Dugan AS, Vance RE. IL-1α signaling initiates the inflammatory response to virulent Legionella pneumophila in vivo. J Immunol. 2013;190:6329–6339. doi: 10.4049/jimmunol.1300100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dube PH, Revell PA, Chaplin DD, Lorenz RG, Miller VL. A role for IL-1α in inducing pathologic inflammation during bacterial infection. Proc Natl Acad Sci USA. 2001;98:10880–10885. doi: 10.1073/pnas.191214498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Di Paolo NC, et al. Virus binding to a plasma membrane receptor triggers interleukin-1α-mediated proinflammatory macrophage response in vivo. Immunity. 2009;31:110–121. doi: 10.1016/j.immuni.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Di Paolo NC, et al. IL-1α and complement cooperate in triggering local neutrophilic inflammation in response to adenovirus and eliminating virus-containing cells. PLoS Pathog. 2014;10:e1004035. doi: 10.1371/journal.ppat.1004035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dinarello CA. An expanding role for interleukin-1 blockade from gout to cancer. Mol Med. 2014;20(suppl 1):S43–S58. doi: 10.2119/molmed.2014.00232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kawaguchi Y, McCarthy SA, Watkins SC, Wright TM. Autocrine activation by interleukin 1α induces the fibrogenic phenotype of systemic sclerosis fibroblasts. J Rheumatol. 2004;31:1946–1954. [PubMed] [Google Scholar]
- 89.Kawaguchi Y, Hara M, Wright TM. Endogenous IL-1α from systemic sclerosis fibroblasts induces IL-6 and PDGF-A. J Clin Invest. 1999;103:1253–1260. doi: 10.1172/JCI4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kawaguchi Y, et al. Increased interleukin 1 receptor, type I, at messenger RNA and protein level in skin fibroblasts from patients with systemic sclerosis. Biochem Biophys Res Commun. 1992;184:1504–1510. doi: 10.1016/s0006-291x(05)80053-1. [DOI] [PubMed] [Google Scholar]
- 91.Raines EW, Dower SK, Ross R. Interleukin-1 mitogenic activity for fibroblasts and smooth muscle cells is due to PDGF-AA. Science. 1989;243:393–396. doi: 10.1126/science.2783498. [DOI] [PubMed] [Google Scholar]
- 92.Kuilman T, et al. Oncogene-induced senescence relayed by an interleukin-dependent inflammatory network. Cell. 2008;133:1019–1031. doi: 10.1016/j.cell.2008.03.039. [DOI] [PubMed] [Google Scholar]
- 93.Laberge RM, et al. MTOR regulates the pro-tumorigenic senescence-associated secretory phenotype by promoting IL1A translation. Nat Cell Biol. 2015;17:1049–1061. doi: 10.1038/ncb3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Stevenson FT, Turck J, Locksley RM, Lovett DH. The N-terminal propiece of interleukin 1α is a transforming nuclear oncoprotein. Proc Natl Acad Sci USA. 1997;94:508–513. doi: 10.1073/pnas.94.2.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Niu J, Li Z, Peng B, Chiao PJ. Identification of an autoregulatory feedback pathway involving interleukin-1α in induction of constitutive NF-κB activation in pancreatic cancer cells. J Biol Chem. 2004;279:16452–16462. doi: 10.1074/jbc.M309789200. [DOI] [PubMed] [Google Scholar]
- 96.Murakami Y, et al. N-myc downstream-regulated gene 1 promotes tumor inflammatory angiogenesis through JNK activation and autocrine loop of interleukin-1α by human gastric cancer cells. J Biol Chem. 2013;288:25025–25037. doi: 10.1074/jbc.M113.472068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sakurai T, et al. Hepatocyte necrosis induced by oxidative stress and IL-1α release mediate carcinogen-induced compensatory proliferation and liver tumorigenesis. Cancer Cell. 2008;14:156–165. doi: 10.1016/j.ccr.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tjomsland V, et al. IL-1α expression in pancreatic ductal adenocarcinoma affects the tumor cell migration and is regulated by the p38MAPK signaling pathway. PLoS One. 2013;8:e70874. doi: 10.1371/journal.pone.0070874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Löffek S, et al. High invasive melanoma cells induce matrix metalloproteinase-1 synthesis in fibroblasts by interleukin-1α and basic fibroblast growth factor-mediated mechanisms. J Invest Dermatol. 2005;124:638–643. doi: 10.1111/j.0022-202X.2005.23629.x. [DOI] [PubMed] [Google Scholar]
- 100.Xu D, et al. Cancer cell-derived IL-1α promotes HGF secretion by stromal cells and enhances metastatic potential in pancreatic cancer cells. J Surg Oncol. 2010;102:469–477. doi: 10.1002/jso.21530. [DOI] [PubMed] [Google Scholar]
- 101.León X, et al. Expression of IL-1α correlates with distant metastasis in patients with head and neck squamous cell carcinoma. Oncotarget. 2015;6:37398–37409. doi: 10.18632/oncotarget.6054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Charbonneau B, et al. Risk of ovarian cancer and the NF-κB pathway: genetic association with IL1A and TNFSF10. Cancer Res. 2014;74:852–861. doi: 10.1158/0008-5472.CAN-13-1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kawaguchi Y, et al. Contribution of single nucleotide polymorphisms of the IL1A gene to the cleavage of precursor IL-1α and its transcription activity. Immunogenetics. 2007;59:441–448. doi: 10.1007/s00251-007-0213-y. [DOI] [PubMed] [Google Scholar]
- 104.Hong DS, et al. MABp1, a first-in-class true human antibody targeting interleukin-1α in refractory cancers: an open-label, phase 1 dose-escalation and expansion study. Lancet Oncol. 2014;15:656–666. doi: 10.1016/S1470-2045(14)70155-X. [DOI] [PubMed] [Google Scholar]
- 105.Dinarello CA. Interleukin-1α neutralisation in patients with cancer. Lancet Oncol. 2014;15:552–553. doi: 10.1016/S1470-2045(14)70164-0. [DOI] [PubMed] [Google Scholar]
- 106.Maffei CM, Mirels LF, Sobel RA, Clemons KV, Stevens DA. Cytokine and inducible nitric oxide synthase mRNA expression during experimental murine cryptococcal meningoencephalitis. Infect Immun. 2004;72:2338–2349. doi: 10.1128/IAI.72.4.2338-2349.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Voronov E, et al. IL-1-induced inflammation promotes development of leishmaniasis in susceptible BALB/c mice. Int Immunol. 2010;22:245–257. doi: 10.1093/intimm/dxq006. [DOI] [PubMed] [Google Scholar]