Abstract
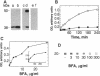
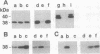
Brefeldin A (BFA) is a fungal metabolite that exerts profound and generally inhibitory actions on membrane transport. At least some of the BFA effects are due to inhibition of the GDP-GTP exchange on the ADP-ribosylation factor (ARF) catalyzed by membrane protein(s). ARF activation is likely to be a key event in the association of non-clathrin coat components, including ARF itself, onto transport organelles. ARF, in addition to participating in membrane transport, is known to function as a cofactor in the enzymatic activity of cholera toxin, a bacterial ADP-ribosyltransferase. In this study we have examined whether BFA, in addition to inhibiting membrane transport, might affect endogenous ADP-ribosylation in eukaryotic cells. Two cytosolic proteins of 38 and 50 kDa were enzymatically ADP-ribosylated in the presence of BFA in cellular extracts. The 38-kDa substrate was tentatively identified as the glycolytic enzyme glyceraldehyde-3-phosphate dehydrogenase. The BFA-binding components mediating inhibition of membrane traffic and stimulation of ADP-ribosylation appear to have the same ligand specificity. These data demonstrate the existence of a BFA-sensitive mono(ADP-ribosyl)transferase that may play a role in membrane movements.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aktories K., Just I., Rosenthal W. Different types of ADP-ribose protein bonds formed by botulinum C2 toxin, botulinum ADP-ribosyltransferase C3 and pertussis toxin. Biochem Biophys Res Commun. 1988 Oct 14;156(1):361–367. doi: 10.1016/s0006-291x(88)80849-0. [DOI] [PubMed] [Google Scholar]
- Brüning A., Ishikawa T., Kneusel R. E., Matern U., Lottspeich F., Wieland F. T. Brefeldin A binds to glutathione S-transferase and is secreted as glutathione and cysteine conjugates by Chinese hamster ovary cells. J Biol Chem. 1992 Apr 15;267(11):7726–7732. [PubMed] [Google Scholar]
- Chen C. L., Feigelson P. Cycloheximide inhibition of hormonal induction of alpha 2u-globulin mRNA. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2669–2673. doi: 10.1073/pnas.76.6.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Matteis M. A., Di Tullio G., Buccione R., Luini A. Characterization of calcium-triggered secretion in permeabilized rat basophilic leukemia cells. Possible role of vectorially acting G proteins. J Biol Chem. 1991 Jun 5;266(16):10452–10460. [PubMed] [Google Scholar]
- De Matteis M. A., Santini G., Kahn R. A., Di Tullio G., Luini A. Receptor and protein kinase C-mediated regulation of ARF binding to the Golgi complex. Nature. 1993 Aug 26;364(6440):818–821. doi: 10.1038/364818a0. [DOI] [PubMed] [Google Scholar]
- Di Girolamo M., D'Arcangelo D., Cacciamani T., Gierschik P., Corda D. K-ras transformation greatly increases the toxin-dependent ADP-ribosylation of GTP binding proteins in thyroid cells. Involvement of an inhibitor of the ADP-ribosylation reaction. J Biol Chem. 1992 Aug 25;267(24):17397–17403. [PubMed] [Google Scholar]
- Dimmeler S., Brüne B. Characterization of a nitric-oxide-catalysed ADP-ribosylation of glyceraldehyde-3-phosphate dehydrogenase. Eur J Biochem. 1992 Nov 15;210(1):305–310. doi: 10.1111/j.1432-1033.1992.tb17422.x. [DOI] [PubMed] [Google Scholar]
- Donaldson J. G., Finazzi D., Klausner R. D. Brefeldin A inhibits Golgi membrane-catalysed exchange of guanine nucleotide onto ARF protein. Nature. 1992 Nov 26;360(6402):350–352. doi: 10.1038/360350a0. [DOI] [PubMed] [Google Scholar]
- Donaldson J. G., Kahn R. A., Lippincott-Schwartz J., Klausner R. D. Binding of ARF and beta-COP to Golgi membranes: possible regulation by a trimeric G protein. Science. 1991 Nov 22;254(5035):1197–1199. doi: 10.1126/science.1957170. [DOI] [PubMed] [Google Scholar]
- Donaldson J. G., Lippincott-Schwartz J., Bloom G. S., Kreis T. E., Klausner R. D. Dissociation of a 110-kD peripheral membrane protein from the Golgi apparatus is an early event in brefeldin A action. J Cell Biol. 1990 Dec;111(6 Pt 1):2295–2306. doi: 10.1083/jcb.111.6.2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson J. G., Lippincott-Schwartz J., Klausner R. D. Guanine nucleotides modulate the effects of brefeldin A in semipermeable cells: regulation of the association of a 110-kD peripheral membrane protein with the Golgi apparatus. J Cell Biol. 1991 Feb;112(4):579–588. doi: 10.1083/jcb.112.4.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doucet J. P., Tuana B. S. Identification of low molecular weight GTP-binding proteins and their sites of interaction in subcellular fractions from skeletal muscle. J Biol Chem. 1991 Sep 15;266(26):17613–17620. [PubMed] [Google Scholar]
- Füchtbauer A., Jockusch B. M., Leberer E., Pette D. Actin-severing activity copurifies with phosphofructokinase. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9502–9506. doi: 10.1073/pnas.83.24.9502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill D. M., Coburn J. ADP-ribosylation of membrane proteins by bacterial toxins in the presence of NAD glycohydrolase. Biochim Biophys Acta. 1988 Apr 28;954(1):65–72. doi: 10.1016/0167-4838(88)90056-8. [DOI] [PubMed] [Google Scholar]
- Hara N., Mishima K., Tsuchiya M., Tanigawa Y., Shimoyama M. Mono(ADP-ribosyl)ation of Ca2+-dependent ATPase in rabbit skeletal muscle sarcoplasmic reticulum and the effect of poly L-lysine. Biochem Biophys Res Commun. 1987 Apr 29;144(2):856–862. doi: 10.1016/s0006-291x(87)80043-8. [DOI] [PubMed] [Google Scholar]
- Helms J. B., Rothman J. E. Inhibition by brefeldin A of a Golgi membrane enzyme that catalyses exchange of guanine nucleotide bound to ARF. Nature. 1992 Nov 26;360(6402):352–354. doi: 10.1038/360352a0. [DOI] [PubMed] [Google Scholar]
- Hendricks L. C., McClanahan S. L., McCaffery M., Palade G. E., Farquhar M. G. Golgi proteins persist in the tubulovesicular remnants found in brefeldin A-treated pancreatic acinar cells. Eur J Cell Biol. 1992 Aug;58(2):202–213. [PubMed] [Google Scholar]
- Hidalgo J., Garcia-Navarro R., Gracia-Navarro F., Perez-Vilar J., Velasco A. Presence of Golgi remnant membranes in the cytoplasm of brefeldin A-treated cells. Eur J Cell Biol. 1992 Aug;58(2):214–227. [PubMed] [Google Scholar]
- Hilz H., Fanick W., Klapproth K. 2'-Phosphoadenylylation of eukaryotic proteins: a type of covalent modification. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6267–6271. doi: 10.1073/pnas.83.17.6267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilz H., Koch R., Fanick W., Klapproth K., Adamietz P. Nonenzymic ADP-ribosylation of specific mitochondrial polypeptides. Proc Natl Acad Sci U S A. 1984 Jul;81(13):3929–3933. doi: 10.1073/pnas.81.13.3929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huitorel P., Pantaloni D. Bundling of microtubules by glyceraldehyde-3-phosphate dehydrogenase and its modulation by ATP. Eur J Biochem. 1985 Jul 15;150(2):265–269. doi: 10.1111/j.1432-1033.1985.tb09016.x. [DOI] [PubMed] [Google Scholar]
- Jacobson M. K., Loflin P. T., Aboul-Ela N., Mingmuang M., Moss J., Jobson E. L. Modification of plasma membrane protein cysteine residues by ADP-ribose in vivo. J Biol Chem. 1990 Jul 5;265(19):10825–10828. [PubMed] [Google Scholar]
- Kahn R. A., Gilman A. G. Purification of a protein cofactor required for ADP-ribosylation of the stimulatory regulatory component of adenylate cyclase by cholera toxin. J Biol Chem. 1984 May 25;259(10):6228–6234. [PubMed] [Google Scholar]
- Kawamoto R. M., Caswell A. H. Autophosphorylation of glyceraldehydephosphate dehydrogenase and phosphorylation of protein from skeletal muscle microsomes. Biochemistry. 1986 Feb 11;25(3):657–661. doi: 10.1021/bi00351a022. [DOI] [PubMed] [Google Scholar]
- Kiley S., Schaap D., Parker P., Hsieh L. L., Jaken S. Protein kinase C heterogeneity in GH4C1 rat pituitary cells. Characterization of a Ca2(+)-independent phorbol ester receptor. J Biol Chem. 1990 Sep 15;265(26):15704–15712. [PubMed] [Google Scholar]
- Kim K. C., Caswell A. H., Talvenheimo J. A., Brandt N. R. Isolation of a terminal cisterna protein which may link the dihydropyridine receptor to the junctional foot protein in skeletal muscle. Biochemistry. 1990 Oct 2;29(39):9281–9289. doi: 10.1021/bi00491a025. [DOI] [PubMed] [Google Scholar]
- Klausner R. D., Donaldson J. G., Lippincott-Schwartz J. Brefeldin A: insights into the control of membrane traffic and organelle structure. J Cell Biol. 1992 Mar;116(5):1071–1080. doi: 10.1083/jcb.116.5.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kots AYa, Skurat A. V., Sergienko E. A., Bulargina T. V., Severin E. S. Nitroprusside stimulates the cysteine-specific mono(ADP-ribosylation) of glyceraldehyde-3-phosphate dehydrogenase from human erythrocytes. FEBS Lett. 1992 Mar 23;300(1):9–12. doi: 10.1016/0014-5793(92)80153-8. [DOI] [PubMed] [Google Scholar]
- López Vinals A. E., Farías R. N., Morero R. D. Characterization of the fusogenic properties of glyceraldehyde-3-phosphate dehydrogenase: fusion of phospholipid vesicles. Biochem Biophys Res Commun. 1987 Feb 27;143(1):403–409. doi: 10.1016/0006-291x(87)90680-2. [DOI] [PubMed] [Google Scholar]
- Maehama T., Takahashi K., Ohoka Y., Ohtsuka T., Ui M., Katada T. Identification of a botulinum C3-like enzyme in bovine brain that catalyzes ADP-ribosylation of GTP-binding proteins. J Biol Chem. 1991 Jun 5;266(16):10062–10065. [PubMed] [Google Scholar]
- Matsuyama S., Tsuyama S. Mono-ADP-ribosylation in brain: purification and characterization of ADP-ribosyltransferases affecting actin from rat brain. J Neurochem. 1991 Oct;57(4):1380–1387. doi: 10.1111/j.1471-4159.1991.tb08304.x. [DOI] [PubMed] [Google Scholar]
- McDonald L. J., Moss J. Nitric oxide-independent, thiol-associated ADP-ribosylation inactivates aldehyde dehydrogenase. J Biol Chem. 1993 Aug 25;268(24):17878–17882. [PubMed] [Google Scholar]
- McDonald L. J., Moss J. Stimulation by nitric oxide of an NAD linkage to glyceraldehyde-3-phosphate dehydrogenase. Proc Natl Acad Sci U S A. 1993 Jul 1;90(13):6238–6241. doi: 10.1073/pnas.90.13.6238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Siegler K., Mauro D. J., Seal G., Wurzer J., deRiel J. K., Sirover M. A. A human nuclear uracil DNA glycosylase is the 37-kDa subunit of glyceraldehyde-3-phosphate dehydrogenase. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8460–8464. doi: 10.1073/pnas.88.19.8460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss J., Stanley S. J., Watkins P. A. Isolation and properties of an NAD- and guanidine-dependent ADP-ribosyltransferase from turkey erythrocytes. J Biol Chem. 1980 Jun 25;255(12):5838–5840. [PubMed] [Google Scholar]
- Northup J. K., Sternweis P. C., Smigel M. D., Schleifer L. S., Ross E. M., Gilman A. G. Purification of the regulatory component of adenylate cyclase. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6516–6520. doi: 10.1073/pnas.77.11.6516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Orci L., Tagaya M., Amherdt M., Perrelet A., Donaldson J. G., Lippincott-Schwartz J., Klausner R. D., Rothman J. E. Brefeldin A, a drug that blocks secretion, prevents the assembly of non-clathrin-coated buds on Golgi cisternae. Cell. 1991 Mar 22;64(6):1183–1195. doi: 10.1016/0092-8674(91)90273-2. [DOI] [PubMed] [Google Scholar]
- Pelham H. R. Multiple targets for brefeldin A. Cell. 1991 Nov 1;67(3):449–451. doi: 10.1016/0092-8674(91)90517-3. [DOI] [PubMed] [Google Scholar]
- Price S. R., Nightingale M., Tsai S. C., Williamson K. C., Adamik R., Chen H. C., Moss J., Vaughan M. Guanine nucleotide-binding proteins that enhance choleragen ADP-ribosyltransferase activity: nucleotide and deduced amino acid sequence of an ADP-ribosylation factor cDNA. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5488–5491. doi: 10.1073/pnas.85.15.5488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaife R. M., Wilson L., Purich D. L. Microtubule protein ADP-ribosylation in vitro leads to assembly inhibition and rapid depolymerization. Biochemistry. 1992 Jan 14;31(1):310–316. doi: 10.1021/bi00116a042. [DOI] [PubMed] [Google Scholar]
- Singh R., Green M. R. Sequence-specific binding of transfer RNA by glyceraldehyde-3-phosphate dehydrogenase. Science. 1993 Jan 15;259(5093):365–368. doi: 10.1126/science.8420004. [DOI] [PubMed] [Google Scholar]
- Soman G., Graves D. J. Endogenous ADP-ribosylation in skeletal muscle membranes. Arch Biochem Biophys. 1988 Jan;260(1):56–66. doi: 10.1016/0003-9861(88)90424-9. [DOI] [PubMed] [Google Scholar]
- Tanaka Y., Yoshihara K., Kamiya T. Enzymic and nonenzymic mono ADP-ribosylation of proteins in skeletal muscle. Biochem Biophys Res Commun. 1989 Sep 15;163(2):1063–1070. doi: 10.1016/0006-291x(89)92329-2. [DOI] [PubMed] [Google Scholar]
- Tsai S. C., Adamik R., Haun R. S., Moss J., Vaughan M. Effects of brefeldin A and accessory proteins on association of ADP-ribosylation factors 1, 3, and 5 with Golgi. J Biol Chem. 1993 May 25;268(15):10820–10825. [PubMed] [Google Scholar]
- Tsai S. C., Noda M., Adamik R., Moss J., Vaughan M. Enhancement of choleragen ADP-ribosyltransferase activities by guanyl nucleotides and a 19-kDa membrane protein. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5139–5142. doi: 10.1073/pnas.84.15.5139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda K., Hayaishi O. ADP-ribosylation. Annu Rev Biochem. 1985;54:73–100. doi: 10.1146/annurev.bi.54.070185.000445. [DOI] [PubMed] [Google Scholar]
- Zhang J., Snyder S. H. Nitric oxide stimulates auto-ADP-ribosylation of glyceraldehyde-3-phosphate dehydrogenase. Proc Natl Acad Sci U S A. 1992 Oct 15;89(20):9382–9385. doi: 10.1073/pnas.89.20.9382. [DOI] [PMC free article] [PubMed] [Google Scholar]