Abstract
The last decade has seen a substantial increase in research focused on the identification of blood-based biomarkers that have utility in Alzheimer’s disease (AD). Blood-based biomarkers have significant advantages of being time- and cost-efficient as well as reduced invasiveness and increased patient acceptance. Despite these advantages and increased research efforts, the field has been hampered by lack of reproducibility as well as an unclear path for moving basic discovery towards clinical utilization. Here we reviewed the recent literature on blood-based biomarkers in AD to provide a current state-of-the-art. Additionally, a collaborative model is proposed that leverages academic and industry strengths to facilitate the field in moving past discovery only work and towards clinical use. Key resources are provided. This new public-private partnership model is intended to circumvent the traditional hand-off model and provide a clear and useful paradigm for the advancement of biomarker science in AD and other neurodegenerative diseases.
Keywords: Alzheimer’s disease, biomarker, blood, diagnosis, cerebrospinal fluid, imaging, diagnosis, context of use
1. Current State of the Science
There has been a significant amount of research focused on the identification of blood-based biomarkers that have utility in Alzheimer’s disease (AD) or other neurological disoders[1–4]. Blood-based biomarkers have important advantages of being cost- and time-effective, compared to the collection of cerebrospinal fluid (CSF) or neuroimaging, while simultaneously being feasible at the population level[4, 5]. Therefore, blood-based biomarkers can serve as the first-step in a multi-stage process[2, 5, 6] similar to the procedures utilized in other disease states (e.g. cancer, cardiovascular disease, infectious disease). Given the insidious nature of AD, this multi-step approach can aid in the detection of disease as early as possible. Acknowledging that peripheral biomarkers (blood or otherwise) of brain disorders are more difficult to identify and lock-down, there are many potential contexts of use (COU) for blood-based AD biomarkers, including, but not limited to, primary care screening, diagnostics, predictive risk (i.e. risk for incident AD, risk for progression from MCI to AD), disease monitoring, stratification into clinical trials and pharmacodynamic or treatment response monitoring (positive or adverse). Multiple international working groups have provided overviews of the landscape, potential uses and challenges for blood-based AD biomarkers[1, 2, 7]. Since those reviews/perspectives were published, there has been significant movement in the field, including a recent special issue of Alzheimer’s & Dementia: Diagnosis, Assessment & Disease Monitoring focused specifically on advances in blood-based biomarkers of AD[3]. Here, we discuss additional recent advances in the field.
1.1. Methodological Considerations
One key advancement produced by the international Professional Interest Area (PIA) on Blood Based Biomarkers was the generation of the first-ever guidelines for pre-analytic processing of specimens[8]. This initial effort was the result of a tremendous work spanning industry and academic investigators from across the globe. It provided a basic set of pre-analytic processing variables to be followed (and refined) and a minimum set of information that should be provided within publications to allow for appropriately designed cross-validation efforts. More recently, this workgroup published data comparing biomarkers from the same blood draw (person, date and time) across assay platforms and blood fraction (serum and plasma)[9]. Results indicated that individual markers, while often statistically significantly correlated, may share minimal variance across platform or tissue indicating that direct comparisons are regularly not possible. Differences in concentration for specific analytes on different technological platforms can be due to a number of things including (1) calibrators, (2) neat biological samples or different dilutions may not have the same immunoreactivity with the antibodies included, (3) differences in antibodies as well as (4) differences in overall sensitivity and reliability of the instrument. Additionally, the use of different assay design can impact findings[10]. Together, this work clearly demonstrated methodological factors that must be considered when comparing across studies, cohorts, and biorepositories. Andreasson, Blennow and Zetterberg[11] provided an update and overview of ultrasensitive technologies to measure AD-related biomarkers in blood as well as CSF. While still early in the process, these novel assay technologies have the capacity to detect very low-levels of markers that may be of significant advantage when seeking to move from research-grade to “pharmaceutical-grade” kits in future attempts to take research use only (RUO) methods towards laboratory developed tests (LDTs) and in vitro diagnostics (IVDs)[12, 13]. As evident from the continued progress of the Global Biomarkers Standardization Consortium of CSF biomarkers (GBSC), the blood-based biomarker field will need to address additional methodological barriers in order to produce clinically useful and applicable biomarkers.
1.2 Blood Biomarkers of AD Risk
An important potential COU for blood-based AD biomarker science is the identification of individuals at greatest risk, which can take several forms: (1) risk of incident cognitive impairment and AD, (2) risk of progressing from mild cognitive impairment (MCI) to AD, and (3) risk for rapid progression within AD. Biomarkers related to these specific COUs have tremendous potential for clinical intervention trials aimed at preventing AD, halting progression from MCI, as well as slowing progression among patients with manifest AD. Enrichment of these specific subjects into trials has the benefit of reducing the diluting effect of enrolling those subjects not likely to progress. Indeed, an important potential of AD blood biomarkers could be to increase the likelihood of subjects being positive on more expensive (e.g. PET imaging) or invasive (lumbar puncture for CSF sampling) biomarkers used later to determine trial eligibility.
A substantial amount of work has been conducted examining blood-based markers within the COU of predicting progression of AD[14], conversion from MCI to AD[15] as well as risk for future AD[16]. Mapstone et al[17] recently examined plasma lipidomic and metabolomic markers from 525 community-dwelling older adults in an effort to identify a signature of risk for incident aMCI/AD. The authors identified a signature of 10 metabolites that yielded approximately 80% accuracy in discriminating controls from MCI/AD and 90% or greater accuracy in detecting those normal controls who converted to aMCI/AD over time. However, cross-validation attempts have been unsuccessful. Casanova et al[18] examined these same 10 metabolites in the Baltimore Longitudinal Study of Aging (BLSA) and the Age, Gene/Environment Susceptibility-Reykjavik Study (AGES-RS). In that work, these metabolites yielded an AUC=0.64 (BLSA) and an AUC=0.40 (AGES-RS) in these independent cohorts. Additionally, examining data from the Atherosclerosis Risk in Communities (ARIC) study, Li and colleagues[19] were unable to cross-validate the cross-sectional discrimination capacity of the 10 metabolites in discriminating normal controls from MCI/AD. This work and cross-validation attempts, is important to propel the field forward. Hye et al[20] analyzed plasma proteomics from 452 cognitively normal elders, 169 MCI non-converters, 51 MCI converters and 476 AD cases from across three independent cohorts, AddNeuromed (ANM), Kings Health Partners-Dementia Case Register (KHP-DCR) and Genetics AD Association (GenAD). A set of 10 proteins predicted progression from MCI to AD (average time of conversion approximately one year)(AUC=0.78).
There has also recently been a surge in research devoted towards the potential utility of exosome markers in predicting and detecting AD and other neurodegenerative diseases[21–23]. Recently, Rissman[24] examined the utility of neuronally-derived exosomes (NDEs) in predicting conversion from MCI to dementia. Alterations in plasma NDE levels of P-tau, Aβ1–42, neurogranin (NRGN) and repressor element 1-silencing transcription factor (REST) were found among AD and MCI cases that converted to AD within 36 months compared to stable MCI cases and normal controls. Additionally, when injected into the right hippocampus of wild-type (C57/BL6) mice, the NDEs from MCI cases that converted to AD caused increased P-tau when compared to NDEs from normal controls and stable MCI cases. This work significantly advances the utility of exosome biomarkers in AD and, critically, back-translates these findings into animal models for additional study, which is rarely done. A significant amount of work remains to standardize methods to effectively understand and work with exosome biomarkers; however, strong signals have been identified and confirm the need for this effort.
An example of a blood-based biomarker that has received a great deal of attention for predicting future risk is plasma clusterin (aka ApoJ). Levy[25] examine plasma clusterin from 1,532 non-demented subjects of the Framingham Study Offspring cohort to determine whether this putative biomarker could predict incident dementia and stroke. Among older adults (age>80), plasma clusterin was associated with increased risk for dementia; however, plasma clusterin was related to a reduced risk of dementia (age 60–69) and stroke (age < 80) among younger participants. These results suggest the importance of considering age when interpreting the predictive utility of this putative biomarker.
While still early in development, the above-described studies provide sufficient evidence for ongoing research investigating the potential use of blood-based biomarkers when considering the specific COU of predicting future risk. However, a great deal of additional work is required including, but not limited to, independent cross-validation, rigorous standardization of methods and assay technologies, and prospective studies designed to explicitly test the COU (with direct application of specific cut-scores). This COU may, in fact, be the “Holy Grail” of AD biomarkers and blood-based biomarkers provide an optimal first step in a multi-stage approach to addressing this COU. It is also possible that blood-based biomarkers may serve as the first-line in a multi-stage approach where the biomarker-specific COU is to rule out those least likely to progress, thereby screening out those who are not in need of more costly and invasive procedures, not only in clinical trial contexts but also in general medical practice. If this is the most valuable COU and market strategy, the design of the studies should be appropriately tailored.
1.3 Biomarkers of AD Diagnosis
The most studied potential COU for blood-based biomarkers in AD are diagnostic biomarkers. Some of this work seeks to identify screening tools for primary care clinics as part of a multi-stage approach[5] while others seek to identify diagnostic tools[26, 27].
One biomarker investigated in this potential COU is plasma total tau (T-tau) concentration. One study suggested that plasma tau was higher in the dementia stage of AD but the data were less clear in the MCI stage of the disease[28]. The Mayo Clinic Study of Aging recently reported that higher levels of plasma tau were cross-sectionally associated with worse memory performance and lower cortical thickness in an AD-signature region among non-demented individuals[29]. However, in analyses comparing eight groups defined by cognitive status and amyloid and neurodegeneration imaging markers there was high overlap between groups, suggesting that plasma tau may not be a good diagnostic AD biomarker. Unfortunately, there is no clear correlation between plasma and CSF T-tau concentrations[30] and this low correlation between CSF and blood biomarkers is quite common. In fact, different isoforms are present for almost every protein, in addition to the fact that the assay design can have an impact on the equilibrium between bound and free analyte in the sample. Another biomarker receiving a significant amount of attention in this COU is neurofilament light (NF-L). In contrast to tau, there is excellent correlation between CSF and plasma concentrations of NF-L[31]. CSF NF-L concentration is increased in both dementia and MCI stages of AD[28]. Additionally, these findings were recently replicated on serum and plasma samples[32].
Martins et al[33] examined baseline and 18-month follow-up plasma ApoJ (aka clusterin) concentrations in the AIBL cohort. The authors found that ApoJ levels were significantly higher among MCI and AD cases at both time-points and were also correlated with standardized uptake value ratio (SUVR) PET amyloid levels and hippocampal volume. Recently, specific glycosylated forms of ApoJ have been found to be more robust markers within this group. Nagele et al have conducted a series of studies examining the potential utility of autoantibodies in detecting AD and other neurodegenerative diseases[34, 35]. Recently from this lab, DeMarshall[36] examined serum autoantibodies from 236 participants (50 MCI with low CSF Aβ42 levels, 25 early stage Parkinson’s disease [PD], 25 mild-to moderate PD, 50 mild-moderate AD, 25 multiple sclerosis, 11 breast cancer, 50 controls). The top 50 differentially expressed autoantibodies were utilized for the classification analyses. The authors found >95% (96–100%) sensitivity and specificity for discriminating MCI from all other diagnostic categories within this cross-sectional cohort. Using the top 10 markers, excellent accuracy was retained for discriminating MCI from all categories. Mielke et al[37] recently analyzed plasma sphingolipid changes among autopsy-confirmed AD, Lewy Body Dementia (DLB) and control subjects. The authors found significant plasma ceramide alterations and monohexosylceramide alterations between dementia cases (AD and DLB) and controls suggesting that these biomarkers may have utility in identifying possible AD and/or DLB pathology. O’Bryant and colleagues cross-validated a serum-based algorithm for discriminating AD from controls across an independent platform, animal model and brain tissue and demonstrated preliminary data for the algorithm in discriminating AD from PD[38]. More recently, that group[5] created the locked-down referent cohort for an AD blood screen intended for primary care use and demonstrated excellent positive and negative predictive values when compared to screening tests. In the long-term, it is likely that the most viable and applicable COU for blood-based biomarkers within the “diagnostic” realm is to serve as the first-step in a multi-stage diagnostic process where CSF and PET amyloid and tau imaging will serve as the final diagnostics of presence of AD pathology[5]. Given the cost of PET and CSF methods relative to blood-based methods, the availability of a blood-based tool in primary care settings that is utilized to determine who does and does not undergo PET and CSF exams has a viable cost and patient acceptability strategies, which are also the strategies followed in the cancer arena (i.e., PET scans are not first-line diagnostics[39]).
1.4 Blood Biomarkers of Amyloid Pathology
Another COU with high potential to aid in clinical trials is the identification of blood-based biomarkers that can identify those individuals with high (or low) likelihood of being amyloid positive[40–42]. Westwood and colleagues[40] recently examined proteomic markers among longitudinal plasma samples collected over a 12-year period among non-demented individuals with [11C]PiB PET scans available. In this study, seven plasma proteins (including A2M, Apo-A1, and multiple complement proteins) were significantly associated with amyloid burden. In a small-scale pilot study, Kaneko[42] examined 40 PiB positive individuals (controls, MCI, AD) along with 22 PiB negative individuals (controls) and found that plasma amyloid proteins (Aβ40, Aβ42) and Aβapproximate peptides (AβAPs; APP669-71) were significantly correlated with amyloid PET positivity with a sensitivity and specificity of 0.93 and 0.96, respectively. In a larger analysis of 273 participants of the AIBL study, Burnham et al[43] identified a plasma-based nine-analyte signature that yielded a sensitivity and specificity of 0.80 and 0.82, respectively. Saykin and colleagues conducted a pilot study among 96 participants of the ADNI study and found a significant relationship between plasma amyloid and [11C]PiB uptake among APOE non-carriers[44]. None of these studies reflect the population that would be needed to support this COU. Though still very early in discovery phases, this COU has tremendous potential for influencing the design of clinical trials targeting amyloid.
The vast majority of the blood-based biomarker work described above (across potential COUs) remains in early stage discovery with only a few instances where multiple replication steps have been undertaken. If these discovery findings are to become clinically meaningful, a great deal of work must be completed. Besides the requisite cross-validation and longitudinal understanding, there is the significant need to fully comprehend the impact of other factors (including pre-analytical) on disorders and diseases and associated interventions on the levels of these blood-based biomarkers. For instance"hallmark” AD biomarkers have been shown to change in association with factors such as depression, cardiac arrest, head injury as well as hematological and cancer interventions[45, 46]. Additionally, the stratification of populations by genetics (ApoE4 genotype) or concomitant/comorbid diseases must be considered. These important considerations need to be addressed prior to considering moving from initial discovery towards the clinic. This process of going from discovery to clinic is best undertaken as a partnership between academia and industry/biotech in order to most effectively leverage the differing skillsets of those within each organizational structure and to appropriately consider the long-term plan and study designs. In the next section, we provide an updated model for advancing biomarker discovery through the stages of development towards clinical implementation.
2. A Public-Private Partnership Paradigm for Advancing Biomarker Discovery Towards Clinical Use
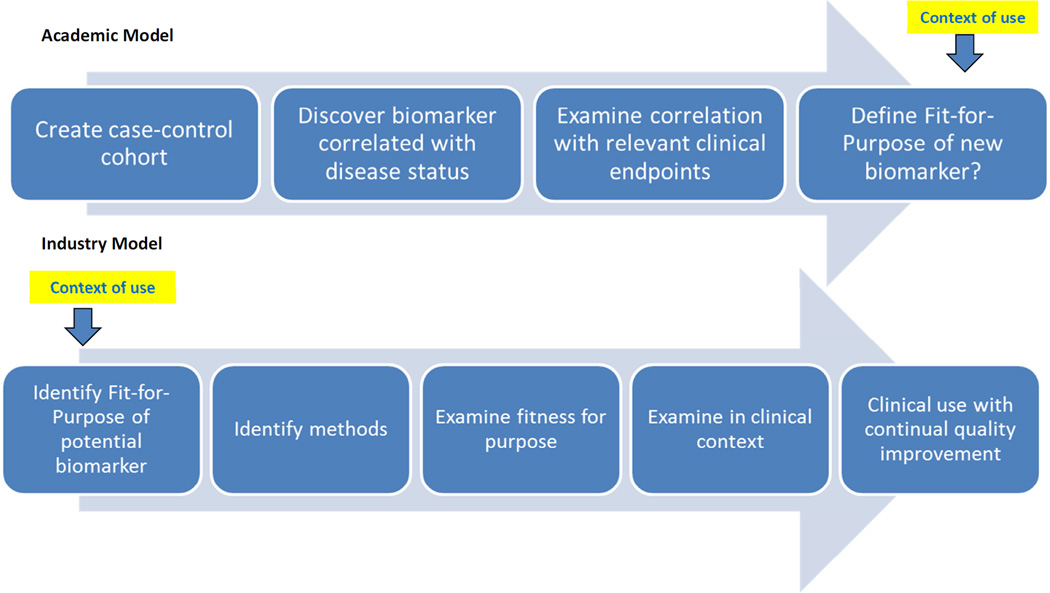
While both academia and industry (industry is used to reflect pharmaceutical, biotechnology, diagnostic and other companies working in the space) have the common goal of identifying biomarkers relevant to AD, there are drastically different perspectives between academia and industry[47]. Further, due to increasing cost structures, industry has been putting less funds and effort into “front end” research and discovery (R&D)[48]. While academia seeks the novel and best solution to a problem or answer a question, industry focuses on the intended use of a safe and effective product with an identified market value[47]. While there are several notable exceptions in the drug discovery space (particularly cancer)[48], academia and industry currently largely work independently with regards to biomarker R&D and continue to inherently follow the traditional “hand-off” approach such that academic discoveries are “handed-off” to industry for further development towards the clinic. There is a large concern regarding the lack of reproducibility of research findings across independent laboratories, within laboratory settings, and particularly from academic laboratory settings to industry settings[49–52]. Indeed, the “unspoken rule” among venture capital firms is that 50% (higher if speaking with industry personnel) of published studies will not replicate in industrial labs[52]. While this lack of validation from academia to industry is likely largely due to fundamentally different approaches to the problem being addressed (see Figure 1) rather than flawed research designs, this “reproducibility crisis” remains a significant problem[53]. The NIH recently outlined a plan to address this problem[50]; however, this is not an issue that can be resolved by academics or industry alone and an updated collaborative model is required.
Figure 1.
Current Model of Biomarker Development
The traditional hand-off model of academic biomarker discovery to industry validation is outlined in Figure 1. The academic model broadly falls into four stages: (1) a case-control cohort is established to examine a wide-range of possible “biomarkers”, (2) a “biomarker” or “biomarkers” are statistically shown to be differentially related to disease status (e.g. significant mean group differences, significant fold-change scores), (3) the “biomarker(s)” are then correlated with relevant clinical disease endpoints (e.g. memory scores, disease severity, age of onset, rate/risk of progression, amyloid positivity) and finally (4) the context of use (COU) is proposed (e.g. biomarker of disease presence, biomarker of disease risk, biomarker of disease subgroups). Few academic studies validate discovery findings across cohorts[54], much less across technological platforms[38]. Those that do attempt to cross-validate oftentimes fail[55]. To date, one can convincingly argue that no prior work has explicitly validated a blood-based biomarker within a specific COU, which may require a prospective clinical trial[5]. In fact, when reviewing the literature outlined above, few studies were validations of previously identified biomarkers. Most were discovery studies following the initial steps (1–3) outlined above. This approach starkly contrasts the product-driven model of industry that begins with defining the COU and validating the fit-for-purpose of this COU with a constant eye towards regulatory pathways and market strategy. While several novel public-private models have been developed for the advancement of drug development[48], less attention has been focused specifically on the biomarker discovery to clinical use pathway. Here we provide a novel integrated partnership model for advancing AD biomarkers from discovery to clinic. While much of the examples and discussion focus on blood-based biomarkers, this model is applicable to biomarker development more broadly.
2.1 Biomarker Development Concepts of Relevance and Available Resources
There are several relevant resources that can assist in the process of establishing a biomarker discovery program that has the goal of translating these discoveries to clinic.
BEST (Biomarkers, EndpointS, and other Tools) Resource
“Effective, unambiguous communication is essential for efficient translation of promising scientific discoveries into approved medical products”[56]. If there is to be a bridge to not only foster, but to also expedite the process of advancing discovery findings towards clinical implementation, there must be a common nomenclature and working definitions for key terms. To that end, the Food and Drug Administration (FDA) / National Institutes of Health (NIH) Biomarker Working Group (FDA-NIH Biomarker Working Group) released the BEST (Biomarkers, EndpointS, and other Tools) Resource to provide such a common working vernacular. The BEST Resource provides definitions for a broad range of relevant terms and concepts, including analytical validation, candidate surrogate endpoint, clinical benefit, as well as the term biomarker itself. A “biomarker” is defined as a “characteristic that is measured as an indicator of normal biological processes, pathogenic processes, or response to an exposure or intervention, including therapeutic interventions. A biomarker is not an assessment of how an individual feels, functions or survives.” The proposed categories of biomarkers included susceptibility/risk biomarker, diagnostic biomarker, monitoring biomarker, prognostic biomarker, pharmacodynamic/response biomarker and predictive biomarker[56], while enrichment biomarkers (e.g. context used in clinical intervention trials) are not defined. Another important definition with relevance for biomarker development is the notion of context of use (COU), which is defined as “a statement that fully and clearly describes the way the medical product development tool is to be used and the medical product development-related purpose of the use” (discussed more below).
U.S. Food and Drug Administration – Biomarker Qualification Program
The FDA Biomarker Qualification Program was created to work with the Center for Drug Evaluation and Research and others to aid in the identification of biomarkers for use in the drug development process. Through this program, one can seek regulatory qualification of a biomarker with a clearly defined COU in drug development (http://www.fda.gov/Drugs/DevelopmentApprovalProcess/DrugDevelopmentToolsQualificationProgram/ucm284076.htm)
Institute of Medicine
The IOM “Evolution of Translational Omics: Lessons Learned and the Path Forward”[57] provides a model for considering the process for biomarker (focused on ‘omics’) development process. This model is broken down into two broad categories: “Discovery and Test Validation Stage” and the “Evaluation for Clinical Utility and Use Stage”. This model can be applied not only to ‘omics’ methods, but other biomarker discovery technologies. In the blood-based AD biomarker space, the vast majority of work has remained within the “Discovery Phase” without the additional work required for the “Test Validation Phase”, which has traditionally been the “hand-off” to industry.
Fit-for-Purpose Biomarker Validation[58]
The fit-for-purpose biomarker validation methods were proposed to assist in the development and validation of clinically useful biomarkers. These methods were developed on the basis that biomarkers would have the capacity to identify the most promising drug candidates within the drug development pipeline[58]. First, the intended use or COU is determined, which guides the remaining steps. These guidelines place the steps within the equivalent steps for pharmacokinetic (PK) assay, biomarker assay for drug development and biomarker assay for diagnostic development. Once the COU is defined, these methods can be placed within the IOM model using the BEST terminology (see new model proposed below). In the AD blood-based biomarker space, the recently published preanalytic guidelines can assist with the design of the fit-for-purpose steps in biomarker discovery and development[8]. If the ultimate goal is to generate a laboratory developed test (LDT), clinical trial assay (CTA) or in vitro diagnostic (IVD), the CLSI and CLIA guidelines must also be reviewed and incorporated into the program development from the beginning.
In addition to the resources outlined above, there are three important commonalities found across biomarker development programs that have progressed from discovery to clinical implementation and should be considered from program inception[47]: (1) predefined vision of the commercialization path, (2) straightforward and controllable manufacturing process and, (3) focus on applied research[47].
2.2 Defining the Context of Use (COU)
While the definition of the COU is outlined above, this point warrants additional consideration. The COU sets the entire stage of science for any new putative biomarker and the importance of this step cannot be overstated as it is largely ignored in academic discovery science. While Industry-lead work has less room for basic biology discovery, this is one of the primary objectives of academic work. However, there are many interesting, novel and potentially useful discoveries that have little chance of reaching the clinic or impact patient care. Within the development of the COU, conceptualization and inception phases should include the following considerations: scalability, manufacturability, compatibility with traditional large-scale methods, intellectual property (IP), and regulatory pathway[47]. When using a blood-based biomarker for detecting AD in primary care clinics, each of these points will be considered individually:
Scalability – If the COU for the blood based biomarker is defined as “a detection tool for primary care clinics to determine which patients should or should not be referred for additional cost-intensive and invasive procedures”, how does scalability become a consideration? First, there are currently over 40 million Americans age 65 and above and this segment of the population will grow dramatically in the near future[59]. If a novel biomarker is to fit the CMS-regulated annual wellness visit (AWV), which is a current need based on the 2015 report of the Gerontological Society of America[60], it must be available to all primary care clinics. This translates to a biomarker platform that can potentially be performed on over 10,000 patients daily. Therefore, is this biomarker (or biomarker assay) sufficiently scalable to be offered to everyone in need? If the biomarker requires specialized equipment, cumbersome pre-analytic procedures or even a single reference lab, this biomarker will not meet the scalability needs of primary care providers.
Manufacturability – Academic investigators excel in creating new and novel procedures that surpass currently available methods. However, it is important to consider whether the product components can be produced at a level that will meet the 10,000 patients per day scale? If this is a new and novel platform, how easy is it utilized and produced? If this is a new biomarker (or set of biomarkers) that leverages existing platforms (e.g. ELISA)? Can the antibodies and reagents meet CLIA and good manufacturing practices (cGMP) and be produced large-scale reliably? What is the long-term availability of antibodies given, for instance, the inherent difficulties with long-term availability with polyclonal antibodies? If not, a potentially scalable biomarker without available manufacturing components will have to meet that latter need prior to being considered for movement towards clinic.
Compatibility with traditional large-scale methods – A blood-based biomarker serving the first-step in the AD assessment process for primary care providers offers advantages to increasing appropriate access to invasive and costly methods for confirmatory diagnostics (as well as therapies); however, how does this biomarker make it to primary care providers? There is an existing large-scale (global) network of companies specifically designed to collect and analyze blood samples at a scale far beyond 10,000 patients per day. However, can this biomarker fit into that infrastructure? Can this biomarker work with the existing global network of companies already providing primary care clinics with daily blood work results? If not, this barrier must be considered before the path to clinic can be realized.
IP Considerations – One phrase commonly heard in public-private-partnership meetings is “academics discover things and industry brings things to patients.” A new and novel biomarker that meets all of the outlined needs above that does not have an adequately structured IP strategy has a significantly reduced chance of reaching patients because there will be no financial incentive to capture an industry partner.
Regulatory Considerations - Considerations regarding regulatory issues early in the process also help appropriately design the studies, without which much of the data produced in the academic laboratory will likely be rendered useless when the regulatory path is realized.
Together, these points provide a contextual pathway to advance academic discovery toward clinical utilization. As can be seen in Figure 1, the standard inherent approach in academic research does not consider these points, which explains a tremendous amount of the failure to replicate academic findings in industry settings. In fact, the “reproducibility crisis” likely has little to do with the soundness of the academic research, but more to do with the context within which the work was conducted. Rather than the traditional “hand-off” model of scientific discovery findings to industry laboratories, a partnership that leverages the strengths of academic centers, pharmaceutical, diagnostic and biotechnology partners at the outset can greatly expedite moving new and better tools into the clinic in a manageable timeframe. Figure 2 proposes a new collaborative public-private partnership model that begins at the conceptualization of the biomarker itself. While industry’s primary expertise is not basic discovery, academic research aiming at discovery that is, from the outset, put into one of the above frameworks toward clinical implementation, will have a far greater chance of success. Additionally, this model allows for the “fail fast, fail forward” industry mindset. That is, one can identify flaws early in the process, learn from those issues and continue moving forward either with this asset or a new one. The new model combines the strengths of both academia and industry partners from the very outset and incorporates the IOM framework. From Figure 2, one can see the gradual shift in leadership from full academic lead on the left to full industry lead on the right. The “hand-off”, as traditionally conceptualized, would best be considered at the shift from Level 2 to Level 3 work. The four levels of the research are as follows:
Figure 2.
Public-Private Partnership Model for Moving from Biomarker Discovery to Clinical Use
2.3 STAGE 1 – Define Context of Use; Academic Lead
Defining the COU sets the stage for the entire program of research. Without a clearly defined COU, the research risks being unfocused without a clear path toward patient care. Clearly define the optimum and minimum performance criteria (e.g. sensitivity, specificity, positive and negative predictive value) and standards within the specific COU should be established. Bidirectional discussions from the outset between all partners is essential to fully describe and characterize the COU in order to ensure that the team is on the same page and remains focused towards a common goal. Additionally, early stage discussions should clearly articulate the considerations for the funding pathway based on existing models (e.g. Industry/State funded centers, Sponsored Research, corporate mini-labs[48]). Academic Roles: Innovation here is crucial. Academic partners are responsible for identifying and discovering new biomarkers that may have potential for marketability. The current model incorporating industry and other partners from the outset allows for (a) rapid communication and consideration of novel ideas and findings through a lense of their marketability, which also leads to (b) discussions of new and novel COUs for the biomarkers, (c) potential generation of improved methods and technologies that offer significant advantages over available biomarkers with similar COUs (keeping in mind that it is exceedingly difficult to “beat good enough” in the marketplace), and (d) identification of the infrastructure upon which to build the collaborative program of research. Industry Roles: Evaluate the COU within the competitive landscape, market value and opportunity, and focus on relevant endpoints and understanding of the regulatory pathway for assay and clinical validation as well as the potential for approval. Go-no-Go: Failure to identify a novel and useful COU that has a readily identifiable market potential.
Discovery and Test Validation Phase – Academic Lead
STAGE 2 – Discovery Studies
Academic Roles: In the second stage, the academic group continues to lead the program with the primary contributions including the design of the study protocol, recruitment of the case-control study population, generation of the methods/technology for biomarker discovery, and capture and analysis of biomarker data relative to the “gold standard” or clinical outcome(s). Detailed documentation of methods used across all aspects is critical, from sample collection and processing, to assay technological aspects and analytic/post-processing. These methods will require deep-level qualification and lock-down at later stages. If academic investigators utilize discovery platforms within the discovery science phase, this further complicates the methodological standardization needs further down. Therefore, any biomarkers identified or discovered utilizing a discovery-based assay technology should immediately be cross-validated on an established technology or the discovery technology must be fully validated prior to additional studies. Industry Roles: Independent analysis of the data, generate the strategy for regulatory approval, consider the market entry point and strategy to market, consider payer issues (e.g. considerations for reimbursement strategy), consider scalability of the discovered biomarker technology, and discuss IP strategy and technology startup needs (e.g. new company [NewCo], fold into existing biotech). Additionally, industry scientists must work with academic scientists to examine the performance parameters of the assay technology. If discovery-based technologies were utilized, academic and industry/biotech scientists must outline the plan to either (1) validate findings on an independent technological platform with known assay validity (including bridging studies) or (2) outline the process for validation of the discovery platform. Go-no-Go: Failure to identify a priori hypothesized or discovery-based biomarker for the intended COU results in no-go and flow back to the initial discovery samples/cohort for additional discovery work. Identification of a biomarker that has no scalability results in no-go. Inability to identify a validated assay technology or ability to validate discovery-based technology results in no-go. Success in discovery and potential scalability moves to Stage 3.
STAGE 3 – Confirmation of Biomarker(s) & Lock-Down of Methods
Academic Roles: Recruit an independent validation case-control study population, replace methods/technology from biomarker discovery, capture and analyze biomarker data relative to the “gold standard” or clinical outcome(s). Industry Roles – Independent analysis of data, review of potential production capacity in fine-tuned scalability analysis, review the methods and determine the ability to transfer technology to existing platforms/infrastructure to meet scalability and provider needs (e.g. assimilation of new radiotracer into PET scan capacity of existing cancers, transition of proteomic marker to FDA-approved existing platforms versus seeking approval of new technologies), determination of LDT versus IVD strategy, initial discussion with regulatory bodies. At this stage it is critical to review and finalize the methods for generation of standard operating procedures, generation of critical raw material (e.g. monoclonal antibodies) needed for long-term supply of assay and confirmation of results, characterization of the markers of interest (including isoforms and/or secondary modifications). Go-no-Go: Failure to replicate results is no-go and shift back to Stage 1 or 2 (“fail fast, fail forward”). Validation in independent sample serves as initial proof-of-principle for industry transition.
STAGE 4 – Finalize COU, Validation, Regulatory
Here the lead shifts to industry partners with extensive input from academic scientists. Industry Roles: Finalize COU statement in regulatory aligned format that is clearly articulated (in terms that fit with regulatory needs), validate proof-of-principle findings in STAGE 3 on a blinded set or initial small-scale (possibly prospective) study utilizing standardized locked-down methods. If the technology requires transition to a different platform (to meet production and scalability needs), additional bridging study work will be required to refine the locked-down methods and compare findings on the new platform or technology to that from the discovery platform utilizing the initial study banked samples and new study. Lastly, regulatory consultation (e.g. FDA, EMEA) to obtain guidance for the needs and requirements to move into regulated trials and approval procedures are mandatory (e.g. LDT versus IVD regulatory considerations, a companion diagnostic biomarker for a drug is approved with the drug whereas a new device may require a 510k exemption or clearance through a pre-market approval (PMA) mechanism). Academic Roles: Recruit new clinical subjects for industry study per locked-down methods, possibly conduct the biomarker studies (e.g. if biomarker is assay-based and academic lab has, as will be required within the Regulatory framework, CLIA lab or 510k approved platforms in-house), work with industry partners to transfer technological methods to widely-available and Regulatory-approved platforms and partner on appropriate bridge-studies, work with the industry partner to the refine locked-down methods and referent cohort, if applicable. The academic role in Stage 4 is of key importance as this public-private partnership model avoids the “hand-off” and allows for the scientists that discovered the technology to explicitly partner with the industry scientists for transfer of the methods. It is possible, and likely, that additional work will be needed to successfully transfer the methods. This stage is likely the most critical juncture to avoid failure of the technology in clinical trials. Go-no-Go: Failure to replicate within internal industry partner hands results in no-go and a re-evaluation of the locked-down methods and data from Stages 2–3. Validation with blinded data or cohorts within industry laboratory setting and standards results in movement to STAGE 5.
STAGE 5: Validation Study in Intended Use Population
Industry Roles - Obtain specific input from the regulatory agencies (FDA, PMDA,EMA or others if applicable) regarding the procedures required for regulatory approval (e.g. within FDA 510k exempt, clinical trial, LDT versus IVD), conduct a study to explicitly test the COU (e.g. primary care patients are screened if the COU is primary care). A prospective study may be required depending on the availability of an existing cohort matching the specific COU. Academic Roles – Participation in study design and in subject recruitment. Go-no-Go: Success in prospective study.
STAGE 6: Registration Trials
Industry Roles – Design and carry out regulated trials (including partnerships with CROs, contracting with e.g. CLIA approved labs, etc.), work with Regulators for appropriate regulatory classification of approval. Academic Roles – Participation in study design, site participation in subject recruitment. Go-no-Go: Determined by meeting or not meeting clinical trial endpoints.
STAGE 7: Clinical Use
Industry Roles – Market deployment, Phase 4 evaluations, provides access to buyers, marketing strategies. Academic Roles – provision of early adopters, engagement in Phase 4 studies.
3. Placing Blood-Based Biomarkers into a Broader Context
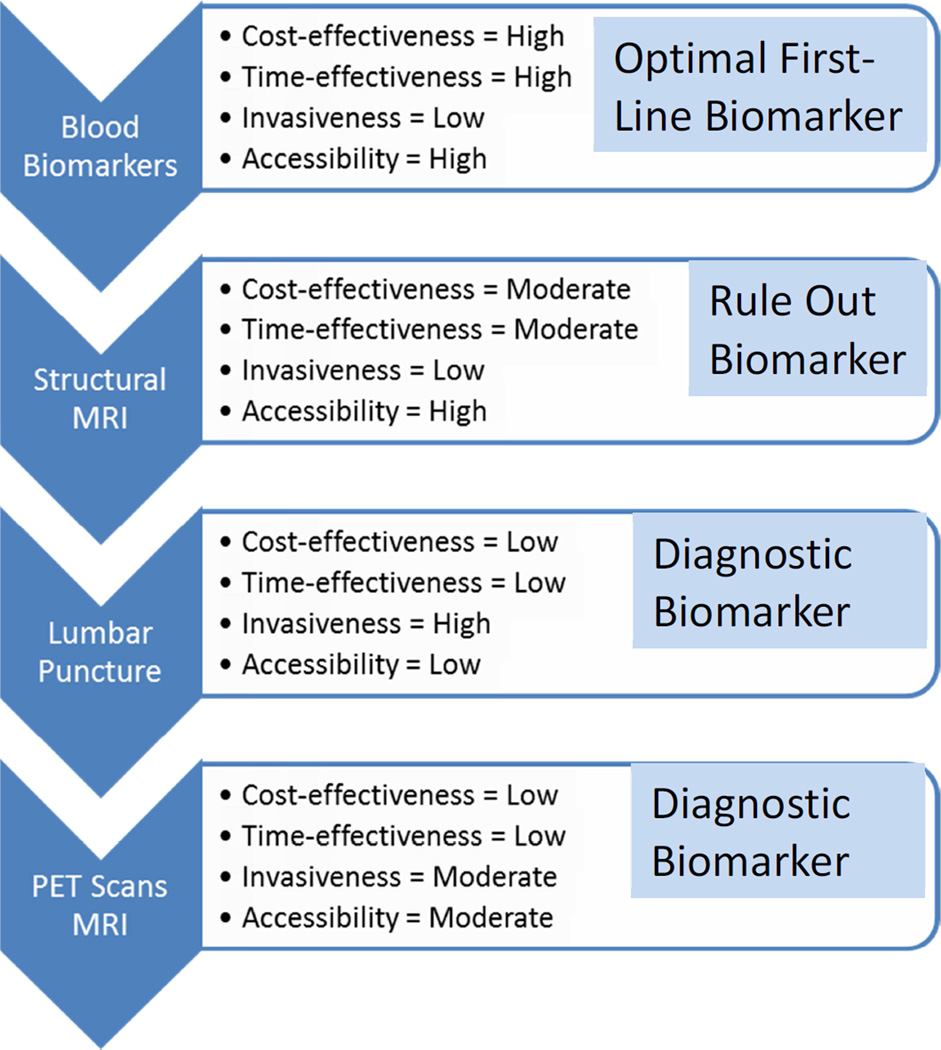
It is important to keep in mind where blood-based biomarkers potentially fit within the bigger picture for specific COUs. With regards to AD diagnostics, the majority of work in the AD space has focused on CSF and imaging biomarker modalities, which will likely be the confirmatory diagnostic procedures. However, first-line biomarkers are needed to fit the needs of the rapidly growing aging segment of the population. As was the case with breast cancer screening 30-years ago, primary care screening tools are needed for AD though significant issues related to fear, stigma and misinformation remain[61]. Additionally, when considering the historical context of the emergence of diagnostic imaging technologies for breast cancer along with the regulatory and reimbursement approval patterns of those technologies[39], the availability of cost- and resource-effective strategies for staging the allocation of diagnostic resources in AD that fit within the existing medical infrastructure will increase the likelihood of regulatory approval for additional imaging modalities, as well as result in more rapid speed-to-market. It is important to be clear that, at this point, blood-based biomarkers are not viewed as “diagnostic”, but rather as the potential first-line in a multi-staged diagnostic process, because they are potentially more cost- and time-effective than other biomarker technologies, and may yield excellent accuracy when compared to primary care screening tools with similar COU[5]. Therefore, once available in primary care settings successful blood-based biomarkers should enhance appropriate access to confirmatory diagnostic testing (e.g. CSF, PET). When considering therapeutics, blood-based biomarkers can serve important roles in increasing access to disease modifying and other Regulatory approved AD therapeutics. When put within the COU of an AD multi-stage neuro-diagnostic process, Figure 3 provides a landscape for immediate biomarker opportunities. Clear Regulatory pathways and fit-for-purpose biomarker validation studies could be immediately generated with these goals. Blood-based biomarkers have clear advantages over PET technologies for front-line testing, but PET and CSF biomarkers can provide final confirmatory (and differential) diagnosis. There is a non-overlapping, complementary COU landscape that fits within the current medical infrastructure where each technology can be scalable to meet the needs of the population; however, neither biomarker is capable of fitting the COU of the other.
Figure 3.
Potential Landscape of Diagnostic Process Biomarkers in Alzheimer’s Disease
Blood-based biomarkers also offer significant advancements to the clinical trial structure, for patient selection as well as potentially monitoring treatment response. For selection into trials, blood-based biomarkers can be utilized as part of the initial screening process to (1) increase access to clinical trials beyond specialty clinic settings, while simultaneously (2) reducing the cost and resource burden in the screening process. PET and CSF biomarkers can then serve as the differential diagnostic step. Overall, this two-step process would significantly reduce time to randomization and reduce overall resources needed for trial start-up. With regards to monitoring treatment response, the traditional outcome in AD clinical trials are change in cognitive test scores (i.e. decreased decline within a period of time – typically 12–24 months). Given the slow nature of cognitive change, this outcome by default requires lengthy trial designs thereby increasing cost, reducing patent life and providing an overall unfavorable cost landscape. Therefore, there has been a significant interest in predictive and response biomarkers. Blood-based biomarkers may have utility to provide a cost-effective means for the identification of predictive biomarkers that identify specific subsets of patients most likely to respond to a given therapy[2, 62], which is a key focus of blood-based (genetic, proteomic and other) markers in the precision medicine approach to cancer therapy (e.g. EGFR in predicting response to non-small cell lung cancer, BRCA1/2 mutations in predicting response among women with ovarian cancer). It is also possible that blood-based biomarkers have the potential to exclude those who have increased risk of responding unfavorably to specific therapies or interventions. CSF and imaging biomarkers may have roles in the generation of predictive biomarkers, which are being examined as secondary outcomes in many ongoing trials. Response biomarkers have tremendous potential to change the landscape of AD clinical trials. Specifically, if a change in a biomarker is an early sign of treatment response (i.e. likelihood of stabilized or improved cognition or lack of worsening in cognition in that specific individual) such a marker could conceivably be introduced as a surrogate biomarker for the primary outcome rather than change in cognition after appropriate validation work and ongoing discussions with regulators. Recent work suggests that early change in plasma S100β and neuron specific enolase (NSE) may predict six month clinical outcomes in stroke patients[63] and this area has been studied extensively in cancer[64–66]. The ideal situation would be the identification of such a response biomarker that changes within six months (given a specific profile of study subjects included in the cohort at the beginning), thereby significantly decreasing the time of the clinical trials. If blood-based biomarkers can be utilized for the sub-stratification of specific patient populations most likely to respond to a given therapy, change in that biomarker over time can be evaluated as a potential response biomarker and such investigations are ongoing. Overall, the evidence and focus on utilization of any biomarkers as outcomes in clinical trials targeting AD has been weak, which is in part related to the regulatory requirements for Phase 3 trials. However, if the COU of the biomarkers are outlined from the inception of the drug development program and built into all stages of development via fit-for-purpose steps, this process can significantly improve the trial process[67]. Given their use for other diseases, it is likely that blood-based biomarkers can significantly improve the clinical trial design and precision medicine model for AD and other neurodegenerative diseases[68] (Figure 4).
Figure 4.
Potential Biomarker COUs in Alzheimer’s Disease Clinical Trial Designs
4. Conclusions
Overall, there has been substantial progress in the area of blood-based biomarkers in AD[3]. Recent discovery-based work has identified potential biomarkers that predict future risk of AD among cognitively normal older adults, risk of progression from MCI to AD, and that discriminate between AD, MCI and cognitively normal elders. While these advancements are significant, the lack of cross-validation across academic labs, methodologies, cohorts, and industry laboratories remains an ongoing limitation. Academic laboratories excel in basic scientific discovery and this strength should be leveraged in the biomarker sciences, as should the industry expertise of taking novel biomarkers to clinic. In this article, we have outlined several important concepts that must be taken into account early in the biomarker discovery program and have provided several resources of importance to discovery laboratories. Herein we have provided a detailed structure of how one can advance from discovery science to clinical implementation via close collaboration between academic and industry laboratories. The public-private partnership arrangement has produced tremendous success in the cancer arena and that model can be leveraged for advancement of biomarker work in AD. Finally, while validation of a biomarker for clinical use (e.g. LDT, approval or clearance of device) may be seen as the final goal, discussions with clinicians to foster adoption and understanding of the COU should be initiated as early as possible to avoid delays in clinical utilization. The clinical use, regulatory and reimbursement landscapes should not be viewed as stagnant, but rather as fluid and changing and should be regularly monitored. These topics will be considered in future articles.
Acknowledgments
Sid E. O’Bryant has multiple patients pending, submitted by the University of North Texas Health Science Center wherein he is an inventor and receives research grants from the National Institutes of Health, National Institute on Aging, award number R01AG051848 and R56AG054073. Kim Henriksen has research supported by the Danish Research Foundation and reports no conflicts of interests. Andreas Jeromin holds affiliations with Quanterix. Harald Hampel is supported by the AXA Research Fund, the Fondation Université Pierre et Marie Curie and the Fondation pour la Recherche sur Alzheimer, Paris, France. The research leading to these results has received funding from the program “Investissements d’avenir” ANR-10-IAIHU-06. Robert A. Rissman is supported by National Institutes of Health, National Institute on Aging, award number R01AG051848 and reports no conflicts of interests. Holly Posner holds affiliations with Pfizer Inc. and reports no conflicts of interests. Richard Batrla-Utermann holds affiliation with Roche and reports no conflicts of interest. Henrik Zetterberg reports no conflicts of interest. Michelle Mielke, Leigh Johnson, James Hall, Simone Lista, Robert Rissman, Peter Snyder, Alcibiades Villarreal, Gabrielle Britton, Paula Grammas, Veer Gupta and Ralph Martins report no conflicts of interest
Abbreviations
- AD
Alzheimer’s disease
- MCI
mild cognitive impairment
- SUVR
standard uptake value ratio
- NDEs
neuronally-derived exosomes
- Aβ
amyloid beta
- BLSA
Baltimore Longitudinal Study of Aging
- AGES-RS
Age, Gene/Environment Susceptibility-Reykjavik Study
- COU
context of use
- IVD
in vitro diagnostic
- LDT
laboratory developed test
- RUO
research use only
- IP
intellectual property
- LBD
Lewy Body Dementia
- PD
Parkinson’s disease
- CSF
cerebrospinal fluid
- PET
positron emission tomgraphy
- PiB
Pittsburg compound B
- R&D
research and development
- NIH
National Institutes of Health
- FDA
Federal Drug Administration
- EMA
European Medicines Agency
- IOM
Institute of Medicine
- BEST
Biomarkers, EndpointS, and other Tools resource
- CTA
Clinical trial assay
- AWV
annual wellness visit
- CMS
Centers for Medicare and Medicaid Services
- CLSI
Clinical and Laboratory Standards Institute
- CLIA
Clinical Laboratory Improvement Amendments
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Snyder HM, et al. Developing novel blood-based biomarkers for Alzheimer's disease. Alzheimer's & dementia : the journal of the Alzheimer's Association. 2014;10(1):109–114. doi: 10.1016/j.jalz.2013.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Henriksen K, et al. The future of blood-based biomarkers for Alzheimer's disease. Alzheimer's and Dementia. 2014;10(1):115–131. doi: 10.1016/j.jalz.2013.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O'Bryant SE. Introduction to special issue on advances in blood-based biomarkers of Alzheimer's disease. Alzheimer's & Dementia: Diagnosis, Assessment & Disease Monitoring. 2016;3:1–3. doi: 10.1016/j.dadm.2016.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lista S, F F, Prvulovic D, Hampel H. Blood and plasma-based proteomic biomarker research in Alzheimer's disease. Prog Neurobiol. 2013;101–102:1–17. doi: 10.1016/j.pneurobio.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 5.O'Bryant SE, E M, Johnson LA, Hall JA, Villarreal AE, Britton GB, Quiceno M, Cullum CM, Graff-Radford NR. A Blood Screening Test for Alzheimer's Disease. Alzheimer's & Dementia: Diagnosis, Assessment & Disease Monitoring. 2016;3:83–90. doi: 10.1016/j.dadm.2016.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schneider P, Hampel H, Buerger K. Biological marker candidates of alzheimer's disease in blood, plasma, and serum. CNS Neuroscience and Therapeutics. 2009;15(4):358–374. doi: 10.1111/j.1755-5949.2009.00104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Laske C, et al. Innovative diagnostic tools for early detection of Alzheimer's disease. Alzheimer's and Dementia. 2015;11(5):561–578. doi: 10.1016/j.jalz.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 8.O'Bryant SE, G V, Henriksen K, Edwards M, Jeromin A, Lista S, Bazenet C, Soares H, Lovestone S, Hampel H, Montine T, Blennow K, Foroud T, Carrillo M, Graff-Radford N, Laske C, Breteler M, Shaw L, Trojanowski JQ, Schupf N, Rissman R, Fagan AM, Oberoi P, Umek R, Weiner MW, Grammas P, Posner H, Martins R. Guidelines for the standardization fo preanalytic variables for blood-based biomarker studies in Alzheimer's disease. Alzheimer's & Dementia. 2015;11:549–560. doi: 10.1016/j.jalz.2014.08.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O'Bryant SE, et al. Comparing biological markers of Alzheimer's disease across blood fraction and platforms: Comparing apples to oranges. Alzheimer's and Dementia: Diagnosis, Assessment and Disease Monitoring. 2016;3:27–34. doi: 10.1016/j.dadm.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vanderstichele HM, et al. Alzheimer disease biomarker testing in cerebrospinal fluid: A method to harmonize assay platforms in the absence of an absolute reference standard. Clinical Chemistry. 2013;59(4):710–712. doi: 10.1373/clinchem.2012.201830. [DOI] [PubMed] [Google Scholar]
- 11.Andreasson U, B K, Zetterberg H. Update on ultrasensitive technologies to facilitate research on blood biomarkers for central nervous system disorders. Alzheimer's & Dementia: Diagnosis, Assessment & Disease Monitoring. 2016;3 doi: 10.1016/j.dadm.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bowsher RR, et al. Application of commercial research-grade biomarker assays in drug development: Is it time to create 'pharmaceutical-grade kits? Bioanalysis. 2012;4(20):2427–2430. doi: 10.4155/bio.12.238. [DOI] [PubMed] [Google Scholar]
- 13.Bowsher RR, Sailstad JM. Insights in the application of research-grade diagnostic kits for biomarker assessments in support of clinical drug development: Bioanalysis of circulating concentrations of soluble receptor activator of nuclear factor κB ligand. Journal of Pharmaceutical and Biomedical Analysis. 2008;48(5):1282–1289. doi: 10.1016/j.jpba.2008.09.026. [DOI] [PubMed] [Google Scholar]
- 14.Blennow K, et al. Evolution of Aβ42 and Aβ40 levels and Aβ42/Aβ40 ratio in plasma during progression of Alzheimer's disease: A multicenter assessment. Journal of Nutrition, Health and Aging. 2009;13(3):205–208. doi: 10.1007/s12603-009-0059-0. [DOI] [PubMed] [Google Scholar]
- 15.Hansson O, et al. Evaluation of plasma Aβ<inf>40</inf> and Aβ<inf>42</inf> as predictors of conversion to Alzheimer's disease in patients with mild cognitive impairment. Neurobiology of Aging. 2010;31(3):357–367. doi: 10.1016/j.neurobiolaging.2008.03.027. [DOI] [PubMed] [Google Scholar]
- 16.Chouraki V, et al. Plasma amyloid-β and risk of Alzheimer's disease in the Framingham Heart Study. Alzheimer's and Dementia. 2015;11(3):249–257.e1. doi: 10.1016/j.jalz.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mapstone M, et al. Plasma phospholipids identify antecedent memory impairment in older adults. Nature Medicine. 2014;20(4):415–418. doi: 10.1038/nm.3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Casanova R, et al. Blood metabolite markers of preclinical Alzheimer's disease in two longitudinally followed cohorts of older individuals. Alzheimer's and Dementia. 2016 doi: 10.1016/j.jalz.2015.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li D, M J, Boerwinkle E, Gottesman RF, Sharrett AR, Mosley TH, Coresh J, Wruck LM, Knopman DS, Alonso A. Plasma phospholipids and prevalence of mild cognitive impairment/dementia in the ARIC Neurocognitive Study (ARIC-NCS) Alzheimer's & Dementia: Diagnosis, Assessment & Disease Monitoring. 2016;3 doi: 10.1016/j.dadm.2016.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hye A, et al. Plasma proteins predict conversion to dementia from prodromal disease. Alzheimer's and Dementia. 2014 doi: 10.1016/j.jalz.2014.05.1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Malm T, Loppi S, Kanninen KM. Exosomes in Alzheimer's disease. Neurochemistry International. 2016;97:193–199. doi: 10.1016/j.neuint.2016.04.011. [DOI] [PubMed] [Google Scholar]
- 22.Kanninen KM, et al. Exosomes as new diagnostic tools in CNS diseases. Biochimica et Biophysica Acta - Molecular Basis of Disease. 2016;1862(3):403–410. doi: 10.1016/j.bbadis.2015.09.020. [DOI] [PubMed] [Google Scholar]
- 23.Stern RA, et al. Preliminary study of plasma exosomal tau as a potential biomarker for chronic traumatic encephalopathy. Journal of Alzheimer's Disease. 2016;51(4):1099–1109. doi: 10.3233/JAD-151028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Winston CN, G E, Akers JC, Carter BS, Rockenstein EM, Galasko D, Masliah E, Rissman R. Prediction of conversion from mild cognitive impairment to dementia with neuronally-derived blood exosome protein profile. Alzheimer's & Dementia: Diagnosis, Assessment & Disease Monitoring. 2016;3 doi: 10.1016/j.dadm.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weinstein G, B A, Preis SR, Courchesne P, Chouraki V, Levy D, Seshadri S. Plasma clusterin levels and risk of demenita, Alzheimer's disease and stroke. Alzheimer's & Dementia: Diagnosis, Assessment & Disease Monitoring. 2016;3 doi: 10.1016/j.dadm.2016.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chang KA, et al. Plasma soluble neuregulin-1 as a diagnostic biomarker for Alzheimer's disease. Neurochemistry International. 2016;97:1–7. doi: 10.1016/j.neuint.2016.04.012. [DOI] [PubMed] [Google Scholar]
- 27.Blasko I, et al. Plasma amyloid beta-42 independently predicts both late-onset depression and Alzheimer disease. American Journal of Geriatric Psychiatry. 2010;18(11):973–982. doi: 10.1097/JGP.0b013e3181df48be. [DOI] [PubMed] [Google Scholar]
- 28.Olsson B, et al. CSF and blood biomarkers for the diagnosis of Alzheimer's disease: A systematic review and meta-analysis. The Lancet Neurology. 2016 doi: 10.1016/S1474-4422(16)00070-3. [DOI] [PubMed] [Google Scholar]
- 29.Dage JL, W A, Airey DC, Hagen CE, Knopman DS, Machulda MM, Roberts RO, Jack CR, Petersen R, Mielke MM. Levels of tau protein in plasma are associated with neurodegeneration and cognitive function in a population-based elderly cohort. Alzheimer's & Dementia. 2016 doi: 10.1016/j.jalz.2016.06.001. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zetterberg H, et al. Plasma tau levels in Alzheimer's disease. Alzheimer's Research and Therapy. 2013;5(2) doi: 10.1186/alzrt163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gisslen M, P R, Andreasson U, Norgren N, Nilsson S, Hagberg L, Fuchs D, Spudich S, et al. Plasma concentration of the neurofilament light protein (NFL) as a biomarker of CNS injury in HIV infection: A cross-sectional study. EbioMedicine. 2016;3:135–140. doi: 10.1016/j.ebiom.2015.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bacioglu M, M L, Preische O, Schelle J, Apel A, Kaeser SA, Schweighauser M, Eninger T, et al. Neurofilament light chain in blood and CSF as marker of disease progression in mouse models and in neurodegenerative diseases. Neuron. 2016;91(2):494–496. doi: 10.1016/j.neuron.2016.07.007. [DOI] [PubMed] [Google Scholar]
- 33.Gupta VB, et al. Plasma apolipoprotein J as a potential biomarker for Alzheimer's disease: Australian Imaging, Biomarkers and Lifestyle study of aging. Alzheimer's and Dementia: Diagnosis, Assessment and Disease Monitoring. 2016;3:18–26. doi: 10.1016/j.dadm.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nagele E, et al. Diagnosis of Alzheimer's disease based on disease-specific autoantibody profiles in human sera. PLoS ONE. 2011;6(8) doi: 10.1371/journal.pone.0023112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.DeMarshall CA, et al. Potential utility of autoantibodies as blood-based biomarkers for early detection and diagnosis of Parkinson's disease. Immunology Letters. 2015;168(1):80–88. doi: 10.1016/j.imlet.2015.09.010. [DOI] [PubMed] [Google Scholar]
- 36.DeMarshall CA, et al. Detection of Alzheimer's disease at mild cognitive impairment and disease progression using autoantibodies as blood-based biomarkers. Alzheimer's and Dementia: Diagnosis, Assessment and Disease Monitoring. 2016;3:51–62. doi: 10.1016/j.dadm.2016.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Savica R, et al. Plasma sphingolipid changes with autopsy-confirmed Lewy body or Alzheimer's pathology. Alzheimer's and Dementia: Diagnosis, Assessment and Disease Monitoring. 2016;3:43–50. doi: 10.1016/j.dadm.2016.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.O'Bryant SE, et al. Validation of a serum screen for alzheimer's disease across assay platforms, species, and tissues. Journal of Alzheimer's Disease. 2014;42(4):1325–1335. doi: 10.3233/JAD-141041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gold LS, et al. The emergence of diagnostic imaging technologies in breast cancer: Discovery, regulatory approval, reimbursement, and adoption in clinical guidelines. Cancer Imaging. 2012;12(1):13–24. doi: 10.1102/1470-7330.2012.0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Westwood S, et al. Blood-Based Biomarker Candidates of Cerebral Amyloid Using PiB PET in Non-Demented Elderly. Journal of Alzheimer's Disease. 2016;52(2):561–572. doi: 10.3233/JAD-151155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tzen KY, et al. Plasma Aβ but not tau is related to brain PiB retention in early Alzheimer's disease. ACS Chemical Neuroscience. 2014;5(9):830–836. doi: 10.1021/cn500101j. [DOI] [PubMed] [Google Scholar]
- 42.Kaneko N, et al. Novel plasma biomarker surrogating cerebral amyloid deposition. Proceedings of the Japan Academy Series B: Physical and Biological Sciences. 2014;90(9):353–364. doi: 10.2183/pjab.90.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Burnham SC, et al. A blood-based predictor for neocortical Aβ burden in Alzheimer's disease: Results from the AIBL study. Molecular Psychiatry. 2014;19(4):519–526. doi: 10.1038/mp.2013.40. [DOI] [PubMed] [Google Scholar]
- 44.Swaminathan S, et al. Association of plasma and cortical amyloid beta is modulated by APOE ε4 status. Alzheimer's and Dementia. 2014;10(1):e9–e18. doi: 10.1016/j.jalz.2013.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Van Gool SW, et al. Neurotoxicity marker profiles in the CSF are not age-dependent but show variation in children treated for acute lymphoblastic leukemia. NeuroToxicology. 2004;25(3):471–480. doi: 10.1016/j.neuro.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 46.Van Gool SW, et al. Disease- and treatment-related elevation of the neurodegenerative marker tau in children with hematological malignancies. Leukemia. 2000;14(12):2076–2084. doi: 10.1038/sj.leu.2401934. [DOI] [PubMed] [Google Scholar]
- 47.Serban MA. Translational biomaterials - the journey from the bench to the market - think 'product'. Current Opinion in Biotechnology. 2016;40:31–34. doi: 10.1016/j.copbio.2016.02.009. [DOI] [PubMed] [Google Scholar]
- 48.Tralau-Stewart CJ, et al. Drug discovery: new models for industry–academic partnerships. Drug Discovery Today. 2009;14(1–2):95–101. doi: 10.1016/j.drudis.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 49.Ioannidis JPA. Why Most Published Research Findings Are False. PLoS Med. 2005;2(8):e124. doi: 10.1371/journal.pmed.0020124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Collins FS, T L. NIH plans to enhance reproducibility. Nature. 2014;505:612–613. doi: 10.1038/505612a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.The Economist. 2013. Trouble at the lab. [Google Scholar]
- 52.Prinz F, Schlange T, Asadullah K. Believe it or not: How much can we rely on published data on potential drug targets? Nature Reviews Drug Discovery. 2011;10(9):712–713. doi: 10.1038/nrd3439-c1. [DOI] [PubMed] [Google Scholar]
- 53.Regelado A. Merk Wants Its Money Back if University Research is Wrong. MIT Technology Review. 2016 [Google Scholar]
- 54.Kiddle SJ, et al. Candidate blood proteome markers of Alzheimer's disease onset and progression: A systematic review and replication study. Journal of Alzheimer's Disease. 2014;38(3):515–531. doi: 10.3233/JAD-130380. [DOI] [PubMed] [Google Scholar]
- 55.Soares HD, et al. Identifying early markers of alzheimer's disease using quantitative multiplex proteomic immunoassay panels. 2009:56–67. doi: 10.1111/j.1749-6632.2009.05066.x. [DOI] [PubMed] [Google Scholar]
- 56.Group F-NBW. BEST (Biomarkers, EndpointS, and other Tools) Resource. Food and Drug Administration / National Institutes of Health; 2016. [PubMed] [Google Scholar]
- 57.Biomarker Development Working Group. Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Ther. 69:89–95. doi: 10.1067/mcp.2001.113989. [DOI] [PubMed] [Google Scholar]
- 58.Lee JW, et al. Fit-for-purpose method development and validation for successful biomarker measurement. Pharmaceutical Research. 2006;23(2):312–328. doi: 10.1007/s11095-005-9045-3. [DOI] [PubMed] [Google Scholar]
- 59.Alzheimer's Association. Alzheimer's Disease Facts and Figures. Alzheimer's & Dementia. 2012;8(2):1–72. doi: 10.1016/j.jalz.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 60.The Gerontological Socieity of American Workgroup on Cognitive Impairment Detection: Report and Recommendations. 2015 [Google Scholar]
- 61.Lundquist TS, Ready RE. Screening for Alzheimer's disease: Inspiration and ideas from breast cancer strategies. Journal of Applied Gerontology. 2015;34(3):317–328. doi: 10.1177/0733464813500711. [DOI] [PubMed] [Google Scholar]
- 62.Mattsson N, et al. Revolutionizing Alzheimer's disease and clinical trials through biomarkers. Alzheimer's and Dementia: Diagnosis, Assessment and Disease Monitoring. 2015;1(4):412–419. doi: 10.1016/j.dadm.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Quintard H, et al. Early and persistent high level of PS 100β is associated with increased poor neurological outcome in patients with SAH: Is there a PS 100β threshold for SAH prognosis? Critical Care. 2016;20(1) doi: 10.1186/s13054-016-1200-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Winterhoff B, et al. PG545 enhances anti-cancer activity of chemotherapy in ovarian models and increases surrogate biomarkers such as VEGF in preclinical and clinical plasma samples. European Journal of Cancer. 2015;51(7):879–892. doi: 10.1016/j.ejca.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Clemons MJ, et al. Randomised, phase II, placebo-controlled, trial of fulvestrant plus vandetanib in postmenopausal women with bone only or bone predominant, hormone-receptor-positive metastatic breast cancer (MBC): The OCOG ZAMBONEY study. Breast Cancer Research and Treatment. 2014;146(1):153–162. doi: 10.1007/s10549-014-3015-6. [DOI] [PubMed] [Google Scholar]
- 66.Winter MC, et al. Neoadjuvant chemotherapy with or without zoledronic acid in early breast cancer-a randomized biomarker pilot study. Clinical Cancer Research. 2013;19(10):2755–2765. doi: 10.1158/1078-0432.CCR-12-3235. [DOI] [PubMed] [Google Scholar]
- 67.Cohen AF, et al. The use of biomarkers in human pharmacology (Phase I) studies. Annual Review of Pharmacology and Toxicology. 2015:55–74. doi: 10.1146/annurev-pharmtox-011613-135918. [DOI] [PubMed] [Google Scholar]
- 68.Hampel H, O BS, Castrillo JI, Ritchie C, Rojkova K, Benda N, Nistico R, Frank RA, Dubois B, Escott-Price V, Lista S. Precision Medicine: The Golden Gate for detection, treatment and prevention of Alzheimer's disease. Journal of Prevention of Alzheimer's Disease. 2016 doi: 10.14283/jpad.2016.112. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]