Abstract
Recent studies have highlighted a myriad of ways in which the activity and composition of the gut microbiota can affect the host organism. A primary way in which the gut microbiota affect host physiology is by the production of metabolites, such as short-chain fatty acids (SCFAs), which are subsequently absorbed into the bloodstream of the host. Although recent studies have begun to unravel the ways in which gut microbial SCFAs affect host physiology, less is understood regarding the underlying cell biological mechanisms. In this review, we will outline the known receptors and transporters for SCFAs, and review what is known about the cell biological effects of microbial SCFAs.
Keywords: Gpr41, Gpr43, microbiota, Olfr78, SCFAs
in recent years, studies have demonstrated a variety of ways in which microbiota, particularly in the gut, interact with and influence host physiology. For instance, it is now well established that changes in the composition and diversity of the gut microbial community are associated with obesity and/or with changes in diet (14–16, 41, 50, 76–78). In addition, gut microbiota have been linked to a wide range of changes in other physiological parameters and pathophysiological conditions, including renal function (59, 80, 81), cardiovascular function (58, 59), irritable bowel syndrome (13, 19), atherosclerosis (83), and immune disorders (2, 38, 43, 54). However, it is important to note that although it seems clear that there is a correlation between changes in gut microbiota and changes in host status (i.e., obesity), the mechanism by which these changes are linked is largely unknown. In addition, although it is well appreciated that host-microbe interactions play important roles in host physiology, in many cases we do not yet understand how these interactions occur at the cell signaling level. To truly understand the nature of these interactions, with the hope of potentially manipulating them therapeutically, we must understand the cell signaling pathways underlying these communications.
One way that commensal microbiota communicate with the host is through the generation of metabolites, which are then absorbed into the bloodstream and sensed by host G protein-coupled receptors (GPCRs). The most well-studied microbial metabolites to date are short chain fatty acids (SCFAs), the most abundant of which are acetate, propionate, and butyrate. SCFAs are produced by microbial fermentation of complex polysaccharides (starches and fiber) in the colon, and as a result the concentration of SCFAs in the colon is ∼100 mM (8). Acetate, propionate, and butyrate are absorbed into the colonic epithelium, where some butyrate is utilized by colonocytes as an energy source before the remaining SCFAs are absorbed into the portal circulation of the host (34). SCFAs in the bloodstream of the host have been reported at concentrations ranging from 0.1 mM to 10 mM (49, 54, 61). Although there are other minor nonmicrobial sources of plasma SCFAs, the primary source of SCFAs in the bloodstream is gut microbial metabolism (8). Interest in SCFAs as microbe-to-host signals has steadily increased in recent years, as several studies linking gut microbes to host physiology have shown that SCFAs appear to be the “link” by which microbes communicate with the host (50, 54, 58, 62, 87, 91). For example, it was reported that gut microbiota-mediated effects on host adiposity are mediated by a host receptor for SCFAs (62). Therefore, it appears that SCFAs and SCFA-mediated cell signaling are key to further our understanding of host-microbe interactions. In this review, we will consider what is currently known regarding the proteins responsible for SCFA detection and transport on the cellular level, and then will discuss known effects of SCFAs on the cell physiology (Fig. 1). In the future, we hope that by combining our knowledge of whole animal physiology studies with an understanding of SCFA-mediated cell signaling, we will be able to obtain a fuller understanding of host-microbe interactions, and ultimately a better understanding of host physiology.
Fig. 1.
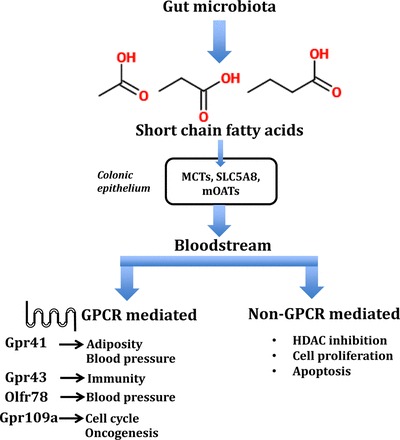
Overview of the role of short-chain fatty acids (SCFAs) in physiology: production, absorption, and systemic effects. Short-chain fatty acids, produced by the breakdown of complex dietary fiber by microbiota in the gut, are absorbed in the bloodstream to be delivered to target tissues. At the target site, they act via G protein coupled receptors (GPCR)—Gpr41, Gpr43, Gpr109a, and Olfr78—to modulate a wide range of cellular responses. MCTs, monocarboxylate transporters; mOATs, multispecific organic anion transporters; HDAC, histone deacetylase.
RECEPTORS AND TRANSPORTERS FOR SCFAs
To understand the physiological actions of SCFAs, we must consider the cellular mechanisms underlying the detection and transport of these compounds. In this portion of the review, we will introduce the proteins that are thought to play a role in sensing or transporting SCFAs.
SCFA Transporters
Initial reports of SCFA transport in the colon found that SCFA absorption was associated with increased Na+ uptake (4, 5); this Na+ dependency was begun to be understood when it was discovered that SLC5A8 functions as an apical transporter in the colon. SLC5A8 is a sodium-coupled monocarboxylate transporter (10, 28, 56), which is well-expressed in the apical membrane of the colon (40) where it transports SCFAs and Na+ with a 1:3 stoichiometry (10). Interestingly, SLC5A8 is also a known tumor suppressor, which is downregulated in colon cancers and colon cancer cell lines owing to the loss of polarity following oncogenesis (56, 57, 74). However, it should be noted that although the evidence for SLC5A8 in SCFA transport is quite strong, it is clear that we cannot explain intestinal SCFA transport by SLC5A8 alone: Ussing chamber experiments examining butyrate and propionate transport in colons from wild-type and SLC5A8-null mice revealed that “no effect was detected that could be attributed to SLC5A8 transport” (22); in contrast, lactate transport was significantly altered in the colons of SLC5A8-null animals. Therefore, there must be other apical transporters in the colon capable of transporting SCFAs, although it is unclear whether they play important roles under basal conditions or are upregulated only when SLC5A8 function is compromised.
There are several reports in the literature of other potential candidates to mediate colonic SCFA transport: for example, one recent report described a basolateral maxi-anion channel in the bovine GI tract that is permeable to SCFAs (26), and an organic anion transporter (mOat2) found in the kidney and liver has also been identified as an SCFA transporter (39). The most well-described candidate aside from SLC5A8 is likely monocarboxylate transporter 1 (MCT1). MCT1 was found in the colon where it was reported to play a role in both lactate and butyrate transport (60); subsequently, it was reported to facilitate Na+ independent butyrate transport in Caco-2 cells (33). More recently, it was reported that Gpr109a sensing of butyrate increased MCT1 surface expression and MCT1-mediated butyrate uptake (6). However, there is conflicting evidence in the literature as to the role of MCT1, as it has been reported to be localized both apically (27, 60) and basolaterally (25, 40). In sum, there are a variety of players in the cell biology of SCFA sensing and transport, and future research is required to more fully elucidate the roles of these proteins in this important process.
SCFA Receptors
Although a total of four SCFA receptors have been described (Gpr41, Gpr43, Gpr109a, and Olfr78), Gpr41 and Gpr43 are the most well-studied SCFA receptors (7, 42, 47–49, 54, 62, 73, 87). Here, we will review what is known about each of these four SCFA receptors.
Gpr41 (free fatty acid receptor 3, Ffar3).
The initial reports describing Gpr41 and Gpr43 were published in 2003, when two groups reported that these previously orphaned receptors are, in fact, both receptors for SCFAs (7, 49). Gpr41 was found to couple to Gi (7, 49) and to be most responsive to propionate [EC50: Gpr41 = 12 μM; (49)], although a variety of other short-chain fatty acids including formate, acetate, butyrate, and isobutyrate elicit varying degrees of activation. Importantly, the human and rat orthologs of Gpr41 displayed similar SCFA response profiles (7), indicating that the signaling of this receptor is likely to be evolutionarily conserved. An additional receptor present in humans, Gpr42, has a very high level of homology to Gpr41, but it is unclear whether Gpr42 is a functional gene or a pseudogene (7, 52).
In the initial 2003 paper, Le Poul et al. (49) astutely pointed out that a subset of the stronger ligands for Gpr41 and Gpr43 (acetate, propionate, and butyrate) are “produced in considerable amounts by microbial fermentation in the hindgut.” Indeed, subsequent studies demonstrated that Gpr41 is expressed in a variety of tissues and cell types including the colon, kidneys, sympathetic nervous system, and blood vessels, (42, 47, 58, 73, 87) where they respond to microbiota-generated SCFAs to mediate physiological responses of the host (62). For example, Gpr41−/− mice weigh less and gain weight at a slower rate compared with their wild-type littermates; furthermore, the differences in weight gain are dependent on gut microbiota (as illustrated by the lack of weight differences between genotypes in germ-free animals) (71). On the cellular level, other studies have shown that Gpr41 inhibits cell proliferation and induces apoptosis via the activation of p53 and MAPK (48, 88).
Gpr43 (free fatty acid receptor 2, Ffar2).
Like Gpr41, Gpr43 was also found to be most responsive to propionate [EC50: 300 μM; (49)], although it can also be activated a number of other SCFAs, with acetate, propionate, and butyrate being the strongest three ligands (49). Whereas Gpr41 couples to Gi, Gpr43 was found to couple to both Gi and Gq (7, 49). Gpr43 is expressed mainly in vasculature and immune cells including lymphocytes, neutrophils, monocytes, and peripheral blood mononuclear cells (PBMCs) (42, 42, 47, 73, 87). Functionally, Gpr43 has been shown to regulate inflammatory responses of the host in response to SCFAs produced by the gut microbiota (49, 54). SCFAs activate cytokines and chemokines both in cultured intestinal epithelial cells and in mice via the activation of Gpr43, as the response was absent in Gpr43−/− mice (46). In addition, Gpr43−/− mice have extensive dysregulation of inflammatory responses, showing excessive inflammation in models of colitis, arthritis, and asthma (54).
SCFA Receptor: Gpr109a
Gpr109a was initially identified as a receptor for niacin (75, 85) and subsequently was also found to respond to β-d-hydroxybutyrate as well as butyrate (70). Interestingly, this receptor does not respond to acetate or propionate, but it has an EC50 for butyrate of ∼1 mM (70). Gpr109a has been localized to epithelial cells in the colon (12), where its level of expression is suppressed in the absence of gut microbiota. Intriguingly, two studies published in early 2014 (20, 67) reported that activation of Gpr109a can suppress carcinogenesis.
SCFA Receptor: Olfr78
Recently, a number of “sensory” receptors (olfactory and taste receptors) have been shown to play important roles in a variety of tissues and physiological processes (17, 21, 29, 31, 32, 36, 64, 69). One such receptor is olfactory receptor 78 (Olfr78), which was an orphan receptor before a ligand screen identified it as a receptor for SCFAs (58). Olfr78 responds to acetate and propionate (EC50 2.35 mM and 920 μM, respectively), but it does not respond to butyrate (in contrast, Gpr109a responds only to butyrate). Importantly, the human ortholog of this receptor was independently found to also respond to SCFAs (92), and this finding has since been confirmed (58). These data, then, suggest that the function of this receptor is likely to be conserved by evolution.
The same study that deorphanized Olfr78 also reported that Olfr78, Gpr41, and Gpr43 localize to blood vessels, and that Olfr78 localizes to a specialized renal vessel (afferent arteriole) where renin is stored and secreted (58). Subsequently, it was demonstrated that both Olfr78 and Gpr41 play roles in sensing gut microbiota-derived SCFAs to modulate blood pressure.
SCFA EFFECTS ON CELL BIOLOGY
SCFAs have been shown to have effects on several aspects of cell biology, including histone acetylation, cell proliferation, and apoptosis. In this portion of the review, we will cover what is currently understood regarding these effects and the mechanisms underlying them.
Modulation of Histone Acetylation by SCFAs
Gene expression is regulated by modulation of histone acetylation by histone acetyl transferases (HATs) and histone deacetylases (HDACs) (53, 63). Addition of acetyl side chains to lysine residues on histones allows activation of transcription from specific regions of the genome. SCFA metabolites produced by microbiota inhibit histone deacetylase activity in cells thereby modulating gene expression in target cells (16, 35, 55, 72, 79). Butyrate is the most potent SCFA inhibitor of histone deacetylases, achieving ∼80% in vitro inhibition of calf thymus HDAC1/2; propionate and pentanoate are the next most efficacious with a ∼60% in vitro inhibition (11). Hyperacetylation of histones, stemming from SCFA-mediated inhibition of HDACs, changes chromatin structure. Thus, histone hyperacetylation alters the accessibility of transcription factors to specific genes, thereby causing SCFA-mediated alterations in gene expression (30).
Mechanistically, SCFAs function as noncompetitive inhibitors of HDACs, functioning effectively in vitro as well as in vivo with a Ki ∼60 μM for butyrate in vitro (11). Moreover, it has been shown that butyrate, propionate, and pyruvate (a 3 carbon intermediate of glycolysis, not a SCFA metabolite) specifically inhibit HDAC1 and HDAC3 (37, 74). Butyrate induces histone hyperacetylation in a wide spectrum of cell lines, both normal and cancerous (1, 44, 45, 51, 86). In cell-free extracts, addition of butyrate increases the half-life of acetyl groups added to histones by 150 times (11). However, butyrate does not affect the process of histone acetylation itself; therefore, the increase in half-life is due to inhibition of HDACs (11). HDAC inhibition is believed to be mediated directly by SCFAs, independent of GPCRs, based on the requirement for SLC5A8 for SCFA entry into colonocytes and subsequent HDAC inhibition (68); however, there is also evidence from another group that Gpr41 may help mediate SCFA effects on HDAC (86). 3-Hydroxy butyrate (β-hydroxybutyrate), a structurally similar ketone intermediate endogenously produced during starvation and diabetes, inhibits class I HDAC in HEK293T cells (65).
In addition to the gut microbiome, constituents of the oral microbiome produce SCFA metabolites that have similar effects on HDAC1/2 (90). In contrast to the gut where commensal symbiotic microbiota produce SCFAs, in the oral cavity, periodontal pathogens produce SCFAs that downregulate silent information regulator-1 (SIRT1) and two histone N-lysine methyltransferases (HLMTs), EZH1 and SUV39H1, enabling Kaposi sarcoma herpesvirus lytic replication in cell lines and in patients with periodontal disease (90). Saliva of patients with periodontal disease has significantly higher concentrations of butyrate, isobutyrate, and propionate compared with healthy individuals (90).
Cell Proliferation and Apoptosis
SCFAs, especially butyrate, exhibit strong antitumorigenic properties, inhibit cell proliferation, and induce differentiation and apoptosis in a variety of cell lines, including human colorectal cancer cell lines HCT-116 and HT-29 (1, 3, 18, 24, 44, 48, 55, 72, 74, 86). In fact, SCFAs have been shown to confer protection from the development of colorectal cancer (3, 18, 23, 55, 72, 79). Propionate and butyrate have been shown to significantly inhibit cell proliferation, whereas acetate does not significantly affect cell proliferation (3, 44, 66). In addition, both propionate and butyrate have been shown to induce apoptosis in vitro (1, 9, 23). It has been hypothesized that inhibition of cell proliferation by butyrate is a direct consequence of HDAC inhibition (86).
Arrest of cell cycle following butyrate treatment occurs by both p53-dependent and -independent mechanisms, whereas apoptosis is initiated by a change in chromosomal structure leading to mitochondrial activation of caspase-3 (55). p53-dependent apoptosis occurs via Gpr41 signaling, as expression of Gpr41 in H9c2 cells increases accumulation of phosphorylated p53 in the nucleus, which in turn induces Bax expression, initiating apoptosis following hypoxia (48). Butyrate also enhances osteogenic differentiation while inhibiting adipogenic differentiation from model adult multipotent mesenchymal stem cells via HDAC inhibition and upregulation of ERK phosphorylation (9).
Mechanism of Butyrate Action
Comparative analysis of a butyrate-treated human colorectal carcinoma cell line, HCT-116, and its butyrate-insensitive derivative, HCT-116BR, revealed differential expression of genes involved in a wide variety of cellular functions, nearly half of the genes being involved in transcription, translation, and protein folding (23). It was found that butyrate downregulates key proteins associated with the actin cytoskeleton in both HT-29 and HCT-116 cells (23, 24). One of the genes downregulated by butyrate in HT-29 cells was cortactin, which is a key player in actin remodeling. Induction of apoptosis in butyrate-treated HT-29 cells is hypothesized to be caused by cortactin downregulation, which causes actin cytoskeletal remodeling (23). Furthermore, genes associated with inflammation, angiogenesis, immunoregulation, and structural proteins were differentially regulated by butyrate in HT-29 and HCT-116 cells. In addition, butyrate induces a cellular stress response in addition to endoplasmic reticulum (ER) and mitochondrial stress. ER chaperones heat shock protein A5 (HSPA5), endoplasmic reticulum protein 29 (ERP29), and protein disulfide isomerase family A member 3 (PDIA3) were differentially regulated upon butyrate treatment in HCT-116 cells (23, 24). Butyrate does not have any effect on total p38 levels in a cell; it rather alters phosphorylation of p38. Treatment of cells with butyrate downregulates HSP27 expression in addition to phosphorylation of HSP27 at Ser-15, -78, and -82 independent of p38 signaling (23, 88, 89). It is important to note that this study (23) was focused on the effect of butyrate on colorectal carcinoma progression, but did not carry out any analysis of the effects of other SCFAs (i.e., propionate or acetate). Hence, it is unknown whether propionate or acetate would have any similar effects on the genes differentially regulated by butyrate.
Physiological Consequences of SCFAs in the Whole Animal
Although this review has concentrated on the cell biological effects of SCFAs, it is important to remember that SCFA signaling has important physiological effects as well. For example, in whole animal studies, butyrate and valproate inhibit histone deacetylase activity, resulting in reduced cerebral infarction and inflammation following ischemia in the brain (45). In addition, microbiota in the gut block dendritic cell development via HDAC inhibition elicited by SCFA metabolites. This suppresses a host immune response against the resident microbial population (9). In yet another example, SCFAs propionate and butyrate inhibit TNF-α production and decrease NF-κB production in neutrophils, thereby having an anti-inflammatory effect (72, 82).
Recent studies have also tied SCFAs to the regulation of metabolism: it has been shown that administration of butyrate decreases plasma glucose and increases insulin levels in diabetic rats as a result of β-cell proliferation in the pancreatic islets (44). Similarly, inclusion of dietary fiber and fructo-oligosaccharides (precursors of SCFAs) improves glucose handling and decreases body weight gain in healthy rats, presumably acting via Gpr41 (16).
CONCLUSION
Metabolites produced by gut microbiota modulate a variety of physiological pathways in the host (13, 19, 38, 49, 54, 58, 62, 80, 81, 84). One of the most well-studied modes of microbe-to-host communication is via SCFAs, which are produced by the breakdown of dietary fiber in the colon by microbiota. The highest concentrations of SCFAs are in the colon following a meal; from there, they are transported into the bloodstream where they can act via GPCRs—Gpr41, Gpr43, Olfr78, and Gpr109a. However, SCFAs can also act independent of GPCRs to modulate histone acetylation and cell proliferation. In the future, it will be necessary to understand the interaction between the physiological effects of SCFAs, and the cell biological changes that SCFAs induce. This is an extremely promising area of research; for example, a better understanding of the role of SCFAs in cell cycle inhibition may be therapeutically useful, as SCFAs are known to be antitumorogenic. In the future, it is especially exciting to consider that modulating metabolite production by the gut microbiota may be a useful tool to obtain beneficial effects on host physiology.
GRANTS
This work was supported by funding from the American Heart Association (Predoctoral Fellowship, to N. Natarajan) and the Hopkins Digestive Diseases Basic & Translational Research Core Center (to J. L. Pluznick).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
N.N. and J.L.P. drafted manuscript; N.N. and J.L.P. edited and revised manuscript; J.L.P. approved final version of manuscript.
REFERENCES
- 1.Aoyama M, Kotani J, Usami M. Butyrate and propionate induced activated or non-activated neutrophil apoptosis via HDAC inhibitor activity but without activating GPR-41/GPR-43 pathways. Nutrition 26: 653–661, 2010. [DOI] [PubMed] [Google Scholar]
- 2.Berer K, Mues M, Koutrolos M, Rasbi ZA, Boziki M, Johner C, Wekerle H, Krishnamoorthy G. Commensal microbiota and myelin autoantigen cooperate to trigger autoimmune demyelination. Nature 479: 538–541, 2011. [DOI] [PubMed] [Google Scholar]
- 3.Bindels LB, Porporato P, Dewulf EM, Verrax J, Neyrinck AM, Martin JC, Scott KP, Buc CP, Feron O, Muccioli GG, Sonveaux P, Cani PD, Delzenne NM. Gut microbiota-derived propionate reduces cancer cell proliferation in the liver. Br J Cancer 107: 1337–1344, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Binder HJ. Role of colonic short-chain fatty acid transport in diarrhea. Annu Rev Physiol 72: 297–313, 2010. [DOI] [PubMed] [Google Scholar]
- 5.Binder HJ, Mehta P. Short-chain fatty acids stimulate active sodium and chloride absorption in vitro in the rat distal colon. Gastroenterology 96: 989–996, 1989. [DOI] [PubMed] [Google Scholar]
- 6.Borthakur A, Priyamvada S, Kumar A, Natarajan AA, Gill RK, Alrefai WA, Dudeja PK. A novel nutrient sensing mechanism underlies substrate-induced regulation of monocarboxylate transporter-1. Am J Physiol Gastrointest Liver Physiol 303: G1126–G1133, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown AJ, Goldsworthy SM, Barnes AA, Eilert MM, Tcheang L, Daniels D, Muir AI, Wigglesworth MJ, Kinghorn I, Fraser NJ, Pike NB, Strum JC, Steplewski KM, Murdock PR, Holder JC, Marshall FH, Szekeres PG, Wilson S, Ignar DM, Foord SM, Wise A, Dowell SJ. The orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J Biol Chem 278: 11312–11319, 2003. [DOI] [PubMed] [Google Scholar]
- 8.Bugaut M. Occurrence, absorption and metabolism of short chain fatty acids in the digestive tract of mammals. Comp Biochem Physiol B 86: 439–472, 1987. [DOI] [PubMed] [Google Scholar]
- 9.Chen TH, Chen WM, Hsu KH, Kuo CD, Hung SC. Sodium butyrate activates ERK to regulate differentiation of mesenchymal stem cells. Biochem Biophys Res Commun 355: 913–918, 2007. [DOI] [PubMed] [Google Scholar]
- 10.Coady MJ, Chang MH, Charron FM, Plata C, Wallendorff B, Sah JF, Markowitz SD, Romero MF, Lapointe JY. The human tumour suppressor gene SLC5A8 expresses a Na+-monocarboxylate cotransporter. J Physiol 557: 719–731, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cousens LS, Gallwitz D, Alberts BM. Different accessibilities in chromatin to histone acetylase. J Biol Chem 254: 1716–1723, 1979. [PubMed] [Google Scholar]
- 12.Cresci GA, Thangaraju M, Mellinger JD, Liu K, Ganapathy V. Colonic gene expression in conventional and germ-free mice with a focus on the butyrate receptor GPR109A and the butyrate transporter SLC5A8. J Gastrointest Surg 14: 449–461, 2010. [DOI] [PubMed] [Google Scholar]
- 13.Dahlqvist G, Piessevaux H. Irritable bowel syndrome: the role of the intestinal microbiota, pathogenesis and therapeutic targets. Acta Gastroenterol Belg 74: 375–380, 2011. [PubMed] [Google Scholar]
- 14.David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, Ling AV, Devlin AS, Varma Y, Fischbach MA, Biddinger SB, Dutton RJ, Turnbaugh PJ. Diet rapidly and reproducibly alters the human gut microbiome. Nature 505: 559–563, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de La Serre CB, Ellis CL, Lee J, Hartman AL, Rutledge JC, Raybould HE. Propensity to high-fat diet-induced obesity in rats is associated with changes in the gut microbiota and gut inflammation. Am J Physiol Gastrointest Liver Physiol 299: G440–G448, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De VF, Kovatcheva-Datchary P, Goncalves D, Vinera J, Zitoun C, Duchampt A, Backhed F, Mithieux G. Microbiota-generated metabolites promote metabolic benefits via gut-brain neural circuits. Cell 156: 84–96, 2014. [DOI] [PubMed] [Google Scholar]
- 17.Deshpande DA, Wang WC, McIlmoyle EL, Robinett KS, Schillinger RM, An SS, Sham JS, Liggett SB. Bitter taste receptors on airway smooth muscle bronchodilate by localized calcium signaling and reverse obstruction. Nat Med 16: 1299–1304, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Drummond DC, Noble CO, Kirpotin DB, Guo Z, Scott GK, Benz CC. Clinical development of histone deacetylase inhibitors as anticancer agents. Annu Rev Pharmacol Toxicol 45: 495–528, 2005. [DOI] [PubMed] [Google Scholar]
- 19.DuPont AW, DuPont HL. The intestinal microbiota and chronic disorders of the gut. Nat Rev Gastroenterol Hepatol 8: 523–531, 2011. [DOI] [PubMed] [Google Scholar]
- 20.Elangovan S, Pathania R, Ramachandran S, Ananth S, Padia RN, Lan L, Singh N, Martin PM, Hawthorn L, Prasad PD, Ganapathy V, Thangaraju M. The niacin/butyrate receptor GPR109A suppresses mammary tumorigenesis by inhibiting cell survival. Cancer Res 74: 1166–1178, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elliott RA, Kapoor S, Tincello DG. Expression and distribution of the sweet taste receptor isoforms T1R2 and T1R3 in human and rat bladders. J Urol 186: 2455–2462, 2011. [DOI] [PubMed] [Google Scholar]
- 22.Frank H, Groger N, Diener M, Becker C, Braun T, Boettger T. Lactaturia and loss of sodium-dependent lactate uptake in the colon of SLC5A8-deficient mice. J Biol Chem 283: 24729–24737, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fung KY, Brierley GV, Henderson S, Hoffmann P, McColl SR, Lockett T, Head R, Cosgrove L. Butyrate-induced apoptosis in HCT116 colorectal cancer cells includes induction of a cell stress response. J Proteome Res 10: 1860–1869, 2011. [DOI] [PubMed] [Google Scholar]
- 24.Fung KY, Lewanowitsch T, Henderson ST, Priebe I, Hoffmann P, McColl SR, Lockett T, Head R, Cosgrove LJ. Proteomic analysis of butyrate effects and loss of butyrate sensitivity in HT29 colorectal cancer cells. J Proteome Res 8: 1220–1227, 2009. [DOI] [PubMed] [Google Scholar]
- 25.Garcia CK, Goldstein JL, Pathak RK, Anderson RG, Brown MS. Molecular characterization of a membrane transporter for lactate, pyruvate, and other monocarboxylates: implications for the Cori cycle. Cell 76: 865–873, 1994. [DOI] [PubMed] [Google Scholar]
- 26.Georgi MI, Rosendahl J, Ernst F, Gunzel D, Aschenbach JR, Martens H, Stumpff F. Epithelia of the ovine and bovine forestomach express basolateral maxi-anion channels permeable to the anions of short-chain fatty acids. Pflügers Arch 466: 1689–1712, 2014. [DOI] [PubMed] [Google Scholar]
- 27.Gill RK, Saksena S, Alrefai WA, Sarwar Z, Goldstein JL, Carroll RE, Ramaswamy K, Dudeja PK. Expression and membrane localization of MCT isoforms along the length of the human intestine. Am J Physiol Cell Physiol 289: C846–C852, 2005. [DOI] [PubMed] [Google Scholar]
- 28.Gopal E, Fei YJ, Sugawara M, Miyauchi S, Zhuang L, Martin P, Smith SB, Prasad PD, Ganapathy V. Expression of slc5a8 in kidney and its role in Na+-coupled transport of lactate. J Biol Chem 279: 44522–44532, 2004. [DOI] [PubMed] [Google Scholar]
- 29.Griffin CA, Kafadar KA, Pavlath GK. MOR23 promotes muscle regeneration and regulates cell adhesion and migration. Dev Cell 17: 649–661, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grunstein M. Histone acetylation in chromatin structure and transcription. Nature 389: 349–352, 1997. [DOI] [PubMed] [Google Scholar]
- 31.Gu X, Ben-Shahar Y. Olfactory receptors in human airway epithelia. Methods Mol Biol 1003: 161–169, 2013. [DOI] [PubMed] [Google Scholar]
- 32.Gu X, Karp PH, Brody SL, Pierce RA, Welsh MJ, Holtzman MJ, Ben-Shahar Y. Chemosensory functions for pulmonary neuroendocrine cells. Am J Respir Cell Mol Biol 50: 637–646, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hadjiagapiou C, Schmidt L, Dudeja PK, Layden TJ, Ramaswamy K. Mechanism(s) of butyrate transport in Caco-2 cells: role of monocarboxylate transporter 1. Am J Physiol Gastrointest Liver Physiol 279: G775–G780, 2000. [DOI] [PubMed] [Google Scholar]
- 34.Harris K, Kassis A, Major G, Chou CJ. Is the gut microbiota a new factor contributing to obesity and its metabolic disorders? J Obes 2012: 879151, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hinnebusch BF, Meng S, Wu JT, Archer SY, Hodin RA. The effects of short-chain fatty acids on human colon cancer cell phenotype are associated with histone hyperacetylation. J Nutr 132: 1012–1017, 2002. [DOI] [PubMed] [Google Scholar]
- 36.Huang AL, Chen X, Hoon MA, Chandrashekar J, Guo W, Trankner D, Ryba NJ, Zuker CS. The cells and logic for mammalian sour taste detection. Nature 442: 934–938, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huber K, Doyon G, Plaks J, Fyne E, Mellors JW, Sluis-Cremer N. Inhibitors of histone deacetylases: correlation between isoform specificity and reactivation of HIV type 1 (HIV-1) from latently infected cells. J Biol Chem 286: 22211–22218, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hwang JS, Im CR, Im SH. Immune disorders and its correlation with gut microbiome. Immune Netw 12: 129–138, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Islam R, Anzai N, Ahmed N, Ellapan B, Jin CJ, Srivastava S, Miura D, Fukutomi T, Kanai Y, Endou H. Mouse organic anion transporter 2 (mOat2) mediates the transport of short chain fatty acid propionate. J Pharmacol Sci 106: 525–528, 2008. [DOI] [PubMed] [Google Scholar]
- 40.Iwanaga T, Takebe K, Kato I, Karaki S, Kuwahara A. Cellular expression of monocarboxylate transporters (MCT) in the digestive tract of the mouse, rat, and humans, with special reference to slc5a8. Biomed Res 27: 243–254, 2006. [DOI] [PubMed] [Google Scholar]
- 41.Jumpertz R, Le DS, Turnbaugh PJ, Trinidad C, Bogardus C, Gordon JI, Krakoff J. Energy-balance studies reveal associations between gut microbes, caloric load, and nutrient absorption in humans. Am J Clin Nutr 94: 58–65, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Karaki S, Mitsui R, Hayashi H, Kato I, Sugiya H, Iwanaga T, Furness JB, Kuwahara A. Short-chain fatty acid receptor, GPR43, is expressed by enteroendocrine cells and mucosal mast cells in rat intestine. Cell Tissue Res 324: 353–360, 2006. [DOI] [PubMed] [Google Scholar]
- 43.Kau AL, Ahern PP, Griffin NW, Goodman AL, Gordon JI. Human nutrition, the gut microbiome and the immune system. Nature 474: 327–336, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Khan S, Jena GB. Protective role of sodium butyrate, a HDAC inhibitor on beta-cell proliferation, function and glucose homeostasis through modulation of p38/ERK MAPK and apoptotic pathways: study in juvenile diabetic rat. Chem Biol Interact 213: 1–12, 2014. [DOI] [PubMed] [Google Scholar]
- 45.Kim HJ, Rowe M, Ren M, Hong JS, Chen PS, Chuang DM. Histone deacetylase inhibitors exhibit anti-inflammatory and neuroprotective effects in a rat permanent ischemic model of stroke: multiple mechanisms of action. J Pharmacol Exp Ther 321: 892–901, 2007. [DOI] [PubMed] [Google Scholar]
- 46.Kim MH, Kang SG, Park JH, Yanagisawa M, Kim CH. Short-chain fatty acids activate GPR41 and GPR43 on intestinal epithelial cells to promote inflammatory responses in mice. Gastroenterology 145: 396–406, 2013. [DOI] [PubMed] [Google Scholar]
- 47.Kimura I, Inoue D, Maeda T, Hara T, Ichimura A, Miyauchi S, Kobayashi M, Hirasawa A, Tsujimoto G. Short-chain fatty acids and ketones directly regulate sympathetic nervous system via G protein-coupled receptor 41 (GPR41). Proc Natl Acad Sci USA 108: 8030–8035, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kimura M, Mizukami Y, Miura T, Fujimoto K, Kobayashi S, Matsuzaki M. Orphan G protein-coupled receptor, GPR41, induces apoptosis via a p53/Bax pathway during ischemic hypoxia and reoxygenation. J Biol Chem 276: 26453–26460, 2001. [DOI] [PubMed] [Google Scholar]
- 49.Le Poul E, Loison C, Struyf S, Springael JY, Lannoy V, Decobecq ME, Brezillon S, Dupriez V, Vassart G, Van DJ, Parmentier M, Detheux M. Functional characterization of human receptors for short chain fatty acids and their role in polymorphonuclear cell activation. J Biol Chem 278: 25481–25489, 2003. [DOI] [PubMed] [Google Scholar]
- 50.Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature 444: 1022–1023, 2006. [DOI] [PubMed] [Google Scholar]
- 51.Li RW, Li C. Butyrate induces profound changes in gene expression related to multiple signal pathways in bovine kidney epithelial cells. BMC Genomics 7: 234, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liaw CW, Connolly DT. Sequence polymorphisms provide a common consensus sequence for GPR41 and GPR42. DNA Cell Biol 28: 555–560, 2009. [DOI] [PubMed] [Google Scholar]
- 53.MacDonald VE, Howe LJ. Histone acetylation: where to go and how to get there. Epigenetics 4: 139–143, 2009. [DOI] [PubMed] [Google Scholar]
- 54.Maslowski KM, Vieira AT, Ng A, Kranich J, Sierro F, Yu D, Schilter HC, Rolph MS, Mackay F, Artis D, Xavier RJ, Teixeira MM, Mackay CR. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature 461: 1282–1286, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Medina V, Edmonds B, Young GP, James R, Appleton S, Zalewski PD. Induction of caspase-3 protease activity and apoptosis by butyrate and trichostatin A (inhibitors of histone deacetylase): dependence on protein synthesis and synergy with a mitochondrial/cytochrome c-dependent pathway. Cancer Res 57: 3697–3707, 1997. [PubMed] [Google Scholar]
- 56.Miyauchi S, Gopal E, Fei YJ, Ganapathy V. Functional identification of SLC5A8, a tumor suppressor down-regulated in colon cancer, as a Na+-coupled transporter for short-chain fatty acids. J Biol Chem 279: 13293–13296, 2004. [DOI] [PubMed] [Google Scholar]
- 57.Paroder V, Spencer SR, Paroder M, Arango D, Schwartz S, Jr., Mariadason JM, Augenlicht LH, Eskandari S, Carrasco N. Na+/monocarboxylate transport (SMCT) protein expression correlates with survival in colon cancer: molecular characterization of SMCT. Proc Natl Acad Sci USA 103: 7270–7275, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pluznick JL, Protzko RJ, Gevorgyan H, Peterlin Z, Sipos A, Han J, Brunet I, Wan LX, Rey F, Wang T, Firestein SJ, Yanagisawa M, Gordon JI, Eichmann A, Peti-Peterdi J, Caplan MJ. Olfactory receptor responding to gut microbiota-derived signals plays a role in renin secretion and blood pressure regulation. Proc Natl Acad Sci USA 110: 4410–4415, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pluznick JL, Zou DJ, Zhang X, Yan Q, Rodriguez-Gil DJ, Eisner C, Wells E, Greer CA, Wang T, Firestein S, Schnermann J, Caplan MJ. Functional expression of the olfactory signaling system in the kidney. Proc Natl Acad Sci USA 106: 2059–2064, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ritzhaupt A, Wood IS, Ellis A, Hosie KB, Shirazi-Beechey SP. Identification and characterization of a monocarboxylate transporter (MCT1) in pig and human colon: its potential to transport l-lactate as well as butyrate. J Physiol 513: 719–732, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Samuel BS, Gordon JI. A humanized gnotobiotic mouse model of host-archaeal-bacterial mutualism. Proc Natl Acad Sci USA 103: 10011–10016, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Samuel BS, Shaito A, Motoike T, Rey FE, Backhed F, Manchester JK, Hammer RE, Williams SC, Crowley J, Yanagisawa M, Gordon JI. Effects of the gut microbiota on host adiposity are modulated by the short-chain fatty-acid binding G protein-coupled receptor, Gpr41. Proc Natl Acad Sci USA 105: 16767–16772, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Seto E, Yoshida M. Erasers of histone acetylation: the histone deacetylase enzymes. Cold Spring Harb Perspect Biol 6: a018713, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shah AS, Ben-Shahar Y, Moninger TO, Kline JN, Welsh MJ. Motile cilia of human airway epithelia are chemosensory. Science 325: 1131–1134, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shimazu T, Hirschey MD, Newman J, He W, Shirakawa K, Le Moan N, Grueter CA, Lim H, Saunders LR, Stevens RD, Newgard CB, Farese RV, Jr., de Cabo R, Ulrich S, Akassoglou K, Verdin E. Suppression of oxidative stress by β-hydroxybutyrate, an endogenous histone deacetylase inhibitor. Science 339: 211–214, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Siavoshian S, Segain JP, Kornprobst M, Bonnet C, Cherbut C, Galmiche JP, Blottiere HM. Butyrate and trichostatin A effects on the proliferation/differentiation of human intestinal epithelial cells: induction of cyclin D3 and p21 expression. Gut 46: 507–514, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Singh N, Gurav A, Sivaprakasam S, Brady E, Padia R, Shi H, Thangaraju M, Prasad PD, Manicassamy S, Munn DH, Lee JR, Offermanns S, Ganapathy V. Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity 40: 128–139, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Singh N, Thangaraju M, Prasad PD, Martin PM, Lambert NA, Boettger T, Offermanns S, Ganapathy V. Blockade of dendritic cell development by bacterial fermentation products butyrate and propionate through a transporter (Slc5a8)-dependent inhibition of histone deacetylases. J Biol Chem 285: 27601–27608, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Spehr M, Gisselmann G, Poplawski A, Riffell JA, Wetzel CH, Zimmer RK, Hatt H. Identification of a testicular odorant receptor mediating human sperm chemotaxis. Science 299: 2054–2058, 2003. [DOI] [PubMed] [Google Scholar]
- 70.Taggart AK, Kero J, Gan X, Cai TQ, Cheng K, Ippolito M, Ren N, Kaplan R, Wu K, Wu TJ, Jin L, Liaw C, Chen R, Richman J, Connolly D, Offermanns S, Wright SD, Waters MG. (D)-beta-Hydroxybutyrate inhibits adipocyte lipolysis via the nicotinic acid receptor PUMA-G. J Biol Chem 280: 26649–26652, 2005. [DOI] [PubMed] [Google Scholar]
- 71.Takeyasu K, Tamkun MM, Renaud KJ, Fambrough DM. Ouabain-sensitive (Na+ + K+)-ATPase activity expressed in mouse L cells by transfection with DNA encoding the alpha-subunit of an avian sodium pump. J Biol Chem 263: 4347–4354, 1988. [PubMed] [Google Scholar]
- 72.Tan J, McKenzie C, Potamitis M, Thorburn AN, Mackay CR, Macia L. The role of short-chain fatty acids in health and disease. Adv Immunol 121: 91–119, 2014. [DOI] [PubMed] [Google Scholar]
- 73.Tazoe H, Otomo Y, Karaki S, Kato I, Fukami Y, Terasaki M, Kuwahara A. Expression of short-chain fatty acid receptor GPR41 in the human colon. Biomed Res 30: 149–156, 2009. [DOI] [PubMed] [Google Scholar]
- 74.Thangaraju M, Carswell KN, Prasad PD, Ganapathy V. Colon cancer cells maintain low levels of pyruvate to avoid cell death caused by inhibition of HDAC1/HDAC3. Biochem J 417: 379–389, 2009. [DOI] [PubMed] [Google Scholar]
- 75.Tunaru S, Kero J, Schaub A, Wufka C, Blaukat A, Pfeffer K, Offermanns S. PUMA-G and HM74 are receptors for nicotinic acid and mediate its anti-lipolytic effect. Nat Med 9: 352–355, 2003. [DOI] [PubMed] [Google Scholar]
- 76.Turnbaugh PJ, Backhed F, Fulton L, Gordon JI. Diet-induced obesity is linked to marked but reversible alterations in the mouse distal gut microbiome. Cell Host Microbe 3: 213–223, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Turnbaugh PJ, Hamady M, Yatsunenko T, Cantarel BL, Duncan A, Ley RE, Sogin ML, Jones WJ, Roe BA, Affourtit JP, Egholm M, Henrissat B, Heath AC, Knight R, Gordon JI. A core gut microbiome in obese and lean twins. Nature 457: 480–484, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 444: 1027–1031, 2006. [DOI] [PubMed] [Google Scholar]
- 79.Ulven T. Short-chain free fatty acid receptors FFA2/GPR43 and FFA3/GPR41 as new potential therapeutic targets. Front Endocrinol (Lausanne) 3: 111, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Vaziri ND. CKD impairs barrier function and alters microbial flora of the intestine: a major link to inflammation and uremic toxicity. Curr Opin Nephrol Hypertens 21: 587–592, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Vaziri ND, Yuan J, Norris K. Role of urea in intestinal barrier dysfunction and disruption of epithelial tight junction in chronic kidney disease. Am J Nephrol 37: 1–6, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Vinolo MA, Rodrigues HG, Hatanaka E, Sato FT, Sampaio SC, Curi R. Suppressive effect of short-chain fatty acids on production of proinflammatory mediators by neutrophils. J Nutr Biochem 22: 849–855, 2011. [DOI] [PubMed] [Google Scholar]
- 83.Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar B, Feldstein AE, Britt EB, Fu X, Chung YM, Wu Y, Schauer P, Smith JD, Allayee H, Tang WH, DiDonato JA, Lusis AJ, Hazen SL. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 472: 57–63, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar B, Feldstein AE, Britt EB, Fu X, Chung YM, Wu Y, Schauer P, Smith JD, Allayee H, Tang WH, DiDonato JA, Lusis AJ, Hazen SL. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 472: 57–63, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wise A, Foord SM, Fraser NJ, Barnes AA, Elshourbagy N, Eilert M, Ignar DM, Murdock PR, Steplewski K, Green A, Brown AJ, Dowell SJ, Szekeres PG, Hassall DG, Marshall FH, Wilson S, Pike NB. Molecular identification of high and low affinity receptors for nicotinic acid. J Biol Chem 278: 9869–9874, 2003. [DOI] [PubMed] [Google Scholar]
- 86.Wu J, Zhou Z, Hu Y, Dong S. Butyrate-induced GPR41 activation inhibits histone acetylation and cell growth. J Genet Genomics 39: 375–384, 2012. [DOI] [PubMed] [Google Scholar]
- 87.Xiong Y, Miyamoto N, Shibata K, Valasek MA, Motoike T, Kedzierski RM, Yanagisawa M. Short-chain fatty acids stimulate leptin production in adipocytes through the G protein-coupled receptor GPR41. Proc Natl Acad Sci USA 101: 1045–1050, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yonezawa T, Haga S, Kobayashi Y, Katoh K, Obara Y. Short-chain fatty acid signaling pathways in bovine mammary epithelial cells. Regul Pept 153: 30–36, 2009. [DOI] [PubMed] [Google Scholar]
- 89.Yonezawa T, Kobayashi Y, Obara Y. Short-chain fatty acids induce acute phosphorylation of the p38 mitogen-activated protein kinase/heat shock protein 27 pathway via GPR43 in the MCF-7 human breast cancer cell line. Cell Signal 19: 185–193, 2007. [DOI] [PubMed] [Google Scholar]
- 90.Yu X, Shahir AM, Sha J, Feng Z, Eapen B, Nithianantham S, Das B, Karn J, Weinberg A, Bissada NF, Ye F. Short-chain fatty acids from periodontal pathogens suppress histone deacetylases, EZH2, and SUV39H1 to promote Kaposi's sarcoma-associated herpesvirus replication. J Virol 88: 4466–4479, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zaibi MS, Stocker CJ, O'Dowd J, Davies A, Bellahcene M, Cawthorne MA, Brown AJ, Smith DM, Arch JR. Roles of GPR41 and GPR43 in leptin secretory responses of murine adipocytes to short chain fatty acids. FEBS Lett 584: 2381–2386, 2010. [DOI] [PubMed] [Google Scholar]
- 92.Zhang X, Firestein S. The olfactory receptor gene superfamily of the mouse. Nat Neurosci 5: 124–133, 2002. [DOI] [PubMed] [Google Scholar]


