Abstract
Fitness and education may protect against cognitive impairments in aging. They may also counteract age-related structural changes within the brain. Here we analyzed volumetric differences in cerebrospinal fluid and gray and white matter, along with neuropsychological data, in adults differing in age, fitness, and education. Cognitive performance was correlated with fitness and education. Voxel-based morphometry was used for a whole-brain analysis of structural magnetic resonance images. We found age-related losses in gray and white matter in medial-temporal, parietal, and frontal areas. As in previous work, fitness within the old correlated with preserved gray matter in the same areas. In contrast, higher education predicted preserved white matter in inferior frontal areas. These data suggest that fitness and education may both be predictive of preserved cognitive function in aging through separable effects on brain structure.
Descriptors: Aging, Fitness, Education, Voxel-based morphometry (VBM), Grey matter, Cerebrospinal fluid (CSF), White matter, Structural magnetic resonance imaging (MRI)
Normal aging is often accompanied by a reduction in cognitive function. Over the life span, speed of processing, working memory, and short- and long-term memory appear to decline (Park et al., 2002; Sliwinski & Buschke, 1999) along with corresponding changes in brain function. These age-related changes are not limited to functional outcomes, as physical degradation of gray and white matter also occurs (Davatzikos & Resnick, 2002; Raz, 2002).
Although some age-related loss of mental abilities may be inevitable, the decline is not uniform for all individuals, as variability in cognitive function also increases with age (Fleischman, Wilson, Gabrieli, Bienias, & Bennett, 2004; Morse, 1993). This suggests that it is possible to undergo successful aging with many of one’s cognitive faculties intact. Proposed mediators of this differential decline include physical fitness (Colcombe & Kramer, 2003; Spirduso, 1975) and education (Richards & Deary, 2005; Stern, 2002), as well as other factors. Only in the last decade have studies begun to investigate these relationships by examining the links between volumetric changes in brain anatomy and physical fitness (Colcombe et. al., 2003, 2006), whereas fewer studies have investigated the relationship between education and brain anatomy (e.g., relationship between education and white matter hyperintensities; Dufouil, Alperovitch, & Tzourio, 2003; Nebes et al., 2006). In addition, it may be very important to examine the effects of fitness and education within the same sample, because these two variables are likely to be correlated. Thus, in this study, we sought to explore the inter-relationship between these factors, by examining the effects of age, fitness, and education on brain anatomy and cognitive function.
Research in the late 1970s and early 1980s laid the ground-work for the study of the relationship between fitness and aging (Dustman et al., 1984; Spirduso, 1975; Spirduso & Clifford, 1978). Although there is a vast literature on the cognitive and emotional correlates of exercise and fitness, there is a dearth of knowledge of how exercise affects the biological properties of the human brain. Much of this knowledge is gained through work with animal models. These studies have shown that exercise may lead to both angiogenesis (Cotman & Berchtold, 2002) and neurogenesis (van Pragg, Kemperman, & Gage, 1999; see also Kramer, Bherer, Colcombe, Dong, & Greenough, 2004). Interestingly, exercise also leads to increases in a number of nerve growth factors, such as brain-derived neurotrophic factor (Neeper, Gomez-Pinilla, Choi, & Cotman, 1995) and insulin-like growth factor I (Carro, Trejo, Busiguina, & Torres-Aleman, 2001), which may in some way contribute to its effects on brain structures. These and other alterations due to exercise are thought to contribute to improved learning and memory (Anderson et al., 2000; Greenough, Madden, & Fleischmann, 1972), as tested with enriched environment manipulations.
Structural changes in animals, such as increases in dendritic length and branching (Greenough & Volkmar, 1973) and hippocampal neurogenesis (Brown et al., 2003; Rossi et al., 2006) have also resulted from environmental enrichment. This manipulation typically includes an exercise component, although it may potentially be considered in some way an analog to increased education in humans, due to the greater cognitive demand on the animals.
From these animal studies, it is plausible to expect that cardiovascular exercise will also have an impact on human brain structure. In humans, age-related gray matter atrophy appears to reflect primarily cell shrinkage rather than cell death (Terry, DeTeresa & Hansen, 1987). Longitudinal interventions involving aerobic training in humans have led to increases not only in gray matter, but also in frontal white matter (Colcombe et al., 2006). Because changes in gray matter are more extensive, they may be more likely to be detected with noninvasive imaging methods than those in white matter tracts.
In recent years, a number of experiments have provided further support for the role of physical fitness in human aging by showing that structural changes are also associated with improved cognitive function (Colcombe & Kramer, 2003, 2006; Hillman, Belopolsky, Snook, Kramer, & McAuley, 2004; Kramer et al., 1999; McAuley, Kramer, & Colcombe, 2004). For example, Colcombe and colleagues (2004) examined brain activations during an Eriksen flanker task (Botvinick, Nystrom, Fissel, Carter, & Cohen, 1999; Eriksen & Eriksen, 1974). In both cross-sectional and clinical studies, higher levels of fitness were implicated in improved attentional control.
Similarly to physical fitness, education has been suggested as a possible factor in successful aging (Vaillant & Mukamal, 2001). For example, higher levels of education may contribute to preserved memory performance through increased brain activity at frontal sites in old age (Czernochowski et al., in press). Education serves to improve cognitive function and may also delay mental decline in later years by creating a reserve capacity (Stern, 2002). The “cognitive reserve” hypothesis suggests that individuals can cope with advancing brain pathology through either a set of acquired skills or inherent abilities. This theoretical standpoint has been operationally translated into an examination of specific lifestyle factors, including level of education, IQ, and occupational status (Habeck et al., 2003; Richards & Deary, 2005; Scarmeas et al., 2003). In fact, the concept of cognitive reserve was initially invoked to account for the imperfect coupling between the degree of brain pathology and loss of cognitive capacity. For example, one study reported that a substantial number of individuals without behavioral symptoms of Alzheimer’s disease during life were found to have histological signs of the disease upon postmortem examination (Ince, 2001). This behavioral sparing may be mediated by high levels of education or IQ (Snowdon, Greiner, & Markesbery, 2000). Several neuroimaging studies have recently been conducted to examine the brain substrates supporting cognitive reserve (Habeck et al., 2003; Scarmeas et al., 2003). However the possible role played by education is still not determined. Due to its suggested role in creating a cognitive reserve, it is possible that years of education may affect the brain structurally. However, as mentioned, the examination of structural effects of education concurrently with those due to aerobic fitness has not yet been carried out. Education could have a direct effect due to “use,” as seen with other factors such as psychomotor activity (Draganski et al., 2004), or an indirect effect through its influence on life habits such as nutrition, exercise, and health. Although we predict potential structural changes in older adults to be moderated by both aerobic fitness and years of education, these effects could manifest in different ways and affect different brain structures.
In contrast to the effect of aerobic fitness, it is not yet established how an increased brain use due to education may lead to structural effects. However, there is some evidence that education may influence white matter integrity (Dufouil et al., 2003; Nebes et al., 2006; however, see also Christensen et al., 2007). A possible mechanism is that axonal function (through use) may influence myelination (Colello, Devey, Imperato, & Pot, 1995), but other mechanisms are also possible. Coffey, Saxton, Ratcliff, Bryan, and Lucke (1999) found reduced cortical atrophy (indexed by sulcal CSF volume) to be correlated with education in a large sample of older adults. However, these data were not replicated by Christensen et al. (2007), who, however, used a younger and more restricted group of subjects (60–64 years of age).
In summary, although this latter group of studies suggests effects of education on white and possibly gray matter, two issues remain unresolved. First, all of these studies used global volumetric assessments, whereas it is well known that aging affects frontal and temporal areas more than sensory or motor areas (for reviews, see Colcombe et al., 2003; Kemper, 1994; see also Esiri, 1994). Second, none of these studies controlled for physical fitness, and, as we have already mentioned, it is likely that these two variables could be confounded.
Within the field of human aging research, there are comparatively few studies that have assessed the presence of systematic volumetric changes in the whole brain. Until recently, quantitative measurements of the brain in vivo would have been impossible. However, the advent of high-resolution structural magnetic resonance imaging (MRI) has made in vivo brain volumetric assessment possible. Volumetric measures have been used, for example, in studies of Alzheimer’s disease (Jack, Petersen, O’Brien, & Tangalos, 1992; Kohler et al., 1998) and schizophrenia (Sachdev, Brodaty, Cheang, & Cathcart, 2000;Wright et al., 1995) as well as in aging (Gunning-Dixon & Raz, 2003; Raz, Gunning-Dixon, Head, Dupuis, & Acker, 1998). The gold standard used in volumetric studies is the manual tracing of specific areas on high-resolution images by trained neuroanatomists. However, with manual tracing, the number of subjects and/or brain regions investigated is limited, because the work is time intensive and requires extensive neuroanatomical knowledge. Furthermore, although interrater reliability within a laboratory can be quite high, reproducibility is not guaranteed, which may limit the generalizability of results.
It is possible to avoid these problems through the use of voxel-based morphometry (VBM; Ashburner & Friston, 2000, 2001). VBM analysis is based on the computation of the probability that each voxel in a subject’s structural image is classified as a particular tissue type: cerebrospinal fluid, gray matter, or white matter. These classifications can then be statistically compared between groups of subjects or regressed on a continuous variable within a group. VBM allows for a whole-brain analysis in a semi-automated fashion. Thus, it is easily reproducible and does not require expert-level anatomical knowledge.
The use of VBM as a tool for studying structural differences between groups presents some controversial issues. In particular, there is concern that potential misalignment during the registration step could negatively impact results (Bookstein, 2001). However, when used appropriately, VBM is a robust and accurate tool (Ashburner & Friston, 2000). The method has proved to be reliable in the study of a variety of populations, including, but not limited to, schizophrenic patients (Wright et al., 1995), gender differences (Good et al., 2001a), obsessive-compulsive disorder (Pujol et al., 2004), diabetes (Musen et al., 2006), and aging (Colcombe et al., 2003).
In the present study, we used VBM to test a series of hypotheses on a sample of younger and older adults. VBM allows for a whole-brain comparison between younger and older adults as well as for analyses of fitness and education effects within the older adults. The initial comparison based on age should lead to robust differences between the two groups as previously reported (Good et al., 2001a; Raz, 2002). We predicted that, in older adults, fitness and education may result in somewhat different profiles of tissue sparing (with possible overlap). The mitigating effects of aerobic fitness have shown their greatest effects on frontal and medio-temporal gray matter as well as anterior white matter (Colcombe et al., 2006). Although there is no previous work indicating where the effects of education should manifest, studies of general aging have indicated greater anterior decline in white matter while showing relative preservation in other areas (for a review, see Kemper, 1994). To measure potential functional correlates of structural changes, a battery of neuropsychological tests was administered to all subjects. Analyses examining the effects of age, education, and fitness were performed on these data. These behavioral measures provide a parallel to the structural data.
In summary, although these analyses draw upon previous research, they provide a new and important examination of the differential contributions of age, cardiovascular fitness, and years of formal education on brain structure. In particular, because both fitness and education have been suggested to serve as protective factors against cognitive aging, it is important to compare their relative contributions within the same sample with a method that allows for the independent and parallel evaluation of gray and white matter effects.
Methods
Participants
Participants were drawn from a study that investigated the effects of differences in physical fitness on neurovascular coupling (Fabiani et al., 2004).1 The final sample comprised 20 younger adults (age 20–28, 10 women, recruited from the University of Illinois student population through postings in university buildings and by word of mouth) and 40 older adults (age 65–81, 23 women, recruited through ads in the local newspaper, campus-wide e-mailings, and postings at area gyms, retirement homes, and community centers). Participants were all right-handed and fluent in English. Prior to graded maximal exercise testing, all participants completed a personal health history form and obtained medical clearance from their personal physician. The demographic characteristics of the participants are summarized in Table 1.
Table 1.
Mean (SD) Demographic Characteristics of the Younger and Older Adults, and Separately for High- and Low-Fit Older Adults
| Measure | Young (n = 20) | Old (n = 40) | Old low-fit (n = 20) | Old high-fit (n = 20) | Old-low vs. old-high:t(38), p |
|---|---|---|---|---|---|
| Age (years) | 22.5(2.1) | 71.5(4.7) | 72.7(4.9) | 70.4(4.4) | 1.53, n.s. |
| Range | 20–28 | 65–81 | 65–81 | 65–79 | |
| Education (years) | 16.5(1.4) | 16.7(2.8) | 15.1(2.4) | 18.3(2.2) | 4.31, <.001 |
| Range | 15–20 | 12–20 | 12–20 | 13–20 | |
| VO2max (mL kg −1 min) | — | 25.1(7.8) | 18.7(2.4) | 31.4(5.9) | 8.87, <.001 |
| Range | 10.3–44.7 | 10.3–21.3 | 24.3–44.7 | ||
| BP systolic | — | 141.2(16.2) | 143.5(21.4) | 139.8 (12.5) | 0.59, n.s. |
| Range | 120–190 | 124–190 | 120–172 | ||
| BP diastolic | — | 83.2(7.6) | 86.9(9.3) | 80.9(5.5) | 2.20, <.05 |
| Range | 70–104 | 78–104 | 70–92 | ||
| HR resting | — | 68.4(15.0) | 68.8(12.3) | 68.2(16.7) | 0.11, n.s. |
| Range | 42–109 | 55–95 | 42–109 | ||
| HR peak | — | 158.4(14.9) | 152.6(19.2) | 162.0(10.6) | 1.70, n.s. |
| Range | 104–180 | 104–179 | 136–180 | ||
| BDI score | 1.3(1.2) | 2.0(2.4) | 2.3(2.4) | 1.6(2.3) | 0.94, n.s. |
| Range | 0–3 | 0–9 | 0–7 | 0–9 |
Notes. Ranges are also provided, as well as the results of a comparison of high- and low-fir older adults. The blood pressure measurements (BP) were taken at the time of VO2 max assessment. BDI: Beck’s Depression Inventory; HR: heart rate.
Screening Procedures
Potential participants were screened with a phone interview prior to their entering the study. During this phone assessment, potential participants were excluded if they regularly took medications known to directly affect the central nervous system or the cardiovascular system (e.g., beta blockers, CNS stimulants, antidepressants, anti-psychotics, sedating antihistamines, or migraine medication) or had other serious or chronic medical conditions. Screenings for these medications would eliminate participants being treated for common health problems such as hypertension. Participants who passed the phone screening then underwent a session of screening and neuropsychological testing. To continue in the experiment, individuals had to score at least 51 on the modified Mini-Mental Status exam (mMMS; Mayeux, Stern, Rosen, & Leventhal, 1981) and show no signs of depression on Beck’s Depression Scale (Beck, Steer, & Brown, 1996; a score of 12 or greater was exclusionary).
Neuropsychological Assessment
Participants who were within the limits of our screening procedures also received an extensive battery of neuropsychological tests, designed to assess the integrity of their cognitive functions. In addition to the mMMS test, which was used to examine global cognitive competency, and the forward and backward digit span, used to assess short-term memory, participants were administered the Vocabulary subtest of the Wechsler Adult Intelligence Scale–Revised (WAIS-R; Wechsler, 1981) to assess verbal skills, the full version of the Wisconsin Card Sorting Test (WCST; Heaton, 1981), the verbal fluency (CFL version) of the Controlled Oral Word Association Test (Benton & Hamsher, 1976), and the Alternate Category Test (Benton & Hamsher, 1976) to assess flexibility and frontal lobe function, the short version of the Raven’s Progressive Matrices (Engle, Tuholski, Laughlin, & Conway, 1999; Raven, Court, & Raven, 1977) to evaluate fluid intelligence, the operation word span task (OSPAN; Engle et al., 1999) to evaluate working memory and attention control, the digit copying test (Kendrick &Watts, 1999), the timed box completion of the WAIS-R (Wechsler, 1981), and the Trail Making Tests A and B (Corrigan & Hinkeldey, 1987) to assess motor speed, the digit-symbol copying test, and the three-, six-, and nine-letter comparison tests (Salthouse, 1992) to assess processing speed.
Fitness Assessment
Cardiovascular fitness for the older adults was assessed by a maximal graded exercise test on a motor-driven treadmill using a modified Balke protocol (American College of Sports Medicine, 1991). The goal of the test is to find a participant’s maximal rate of oxygen uptake (VO2max). This measure is accepted as the criterion measure of cardiorespiratory fitness (American College of Sports Medicine, 1991). The protocol involves walking at a speed of 3 mph with increasing grade increments of 2% every 2 min and was supervised by a physician, exercise test technologist, and a registered nurse certified in Advanced Cardiac Life Support. Expired gases were sampled at 30-s intervals and analyzed using a Parvomedics Inc. True Max 2400 metabolic system. The test termination criteria included any of the following: (a) objective evidence that VO2max had been attained, (b) volitional exhaustion on the part of the participant, or (c) cardiovascular abnormalities or other symptoms indicating ischemia or abnormal test responses. Individuals with abnormal responses were excluded from the study. Heart rate was assessed at each work stage via continuous 12-lead electrocardiographic monitoring. Blood pressure was monitored by auscultation and sphygmomanometer. As is common practice within fitness literature, when serving as controls, young participants were considered as being relatively high fit as compared to the older adults.
Structural Imaging
All participants included in this study underwent a high-resolution structural MRI scan in a Siemens 3-Tesla Magnetom Allegra MR Headscanner. Using an MPRAGE sequence, a 144-slice scan with a 1.2-mm slice thickness was obtained either in the sagittal or axial plane.2 The pulse parameters used in MR recording included a repetition time of 1800 ms, an echo time of 4.38 ms, and a flip angle of 8°. The field of view was 240 × 240 × 172.8 mm with matrix dimensions of 192 × 256 × 144 and voxel size of 1.25 × 0.938 × 1.2 mm.
Data Analyses
MR data were processed using tools in the FMRIB Software Library (Image Analysis Group, FMRIB, Oxford, UK; Smith et al., 2004). A VBM method was used to determine the impact of age, fitness level, and education on brain tissues. Before applying this technique, the MR images underwent a series of automated preprocessing steps. The purposes of these steps were to (1) remove all non brain matter from the MR images (Smith, 2002); (2) align different individuals to a common template in MNI space, which is achieved through a series of affine transformations (Jenkinson, Bannister, Brady, & Smith, 2002; Jenkinson & Smith, 2001); (3) for each participant, assign to each voxel partial volume estimates describing the probabilities that that voxel contains cerebrospinal fluid, white matter, or gray matter (“segmentation”; Zhang, Brady, & Smith, 2001)— separate maps for each participant and tissue type are thus derived; and (4) smooth the tissue maps with an 8-mm full-width at half-maximum Gaussian kernel. (For further descriptions of VBM methodologies and template see Ashburner & Firston, 2000; Good et al., 2001b; Pujol et al., 2004).
In all the analyses presented here we used a template (20 young, 10 female, 20 old, 10 female), selected from a pool of participants acquired from other studies in our laboratory. The template was composed of an equal numbers of older and younger participants and gender balanced to make it as representative as possible of the participants examined. By balancing the template on both age and gender, we removed any potential systematic registration bias that could have occurred.
Within the segmentation processes, the tissue maps were multiplied by their Jacobian determinants obtained from the registration step to account for the changes in voxel size due to the spatial normalization. This partial volume correction procedure makes it possible to describe the results of the analysis in terms of actual volumes rather than tissue densities. Within the tissue segmentation step of preprocessing, there was also a B1 inhomogeneity correction.
The final outputs of the processing were three parametric maps representing partial volume estimates for each of the tissue types—gray matter, white matter, and CSF—for each participant. For the comparison between younger and older adults, a group comparison was then carried out on each of the three sets of parametric maps. The effect of age within the older population was estimated by means of linear regression analyses in a voxel-wise fashion for each map type. The regression analyses using log-transformed VO2max scores as predictors were conducted solely on the parametric maps of partial volume estimates of the older adults. A third set of regressions was run on the older adults to examine if there were structural changes associated solely with education. Within each of these analyses, the statistics were adjusted for multiple comparisons. Each comparison generated two t-statistic maps, corresponding to the opposite directional contrasts—a possible increase or decrease for each tissue type with age, fitness, or education. Because education and fitness were correlated, we used each of them as a covariate in the analysis of the other. Thus, the results presented here indicate only their independent contributions. Similarly, gender was used as a covariate in all analyses to avoid possible biases and eliminate its effects. Finally, age was also used as a covariate to evaluate its effects within the older adult group.
Results
Neuropsychological Tests
The results of the neuropsychological tests are summarized in Tables 2 through 5. Tables 2 and 3 report mean data separately for the younger and older adults, whereas Tables 4 and 5 report results on these same tests for the older adults only, grouped by fitness level using a median split. The majority of neuropsychological measures showed significant differences as a function of age. A similar pattern was present when older adults were divided into high- and low-fit groups (Tables 4 and 5).
Table 2.
Mean (SD) Scores for Each Neuropsychological Test for Participants Grouped by Age
| Test | Young | Old | p value | Directiona |
|---|---|---|---|---|
| mMMS | 55.8(1.5) | 54.8(1.7) | * | + |
| WAIS-R Vocabulary scaled | 13.4(1.8) | 13.4(2.7) | + | |
| Forward Digit Span | 7.2(1.4) | 6.9(1.1) | + | |
| Backwards Digit Span | 6.0(1.4) | 5.1(1.6) | * | + |
| Boxes | 52.4(11.5) | 43.7(12.7) | * | + |
| Trail Making A | 12.7(4.2) | 20.8(11.0) | * | – |
| Trail Making B | 20.0(6.5) | 35.5(18.3) | ** | – |
| Digit Copying | 79.5(5.9) | 65.7(13.3) | ** | + |
| Digit Symbol | 49.3(5.3) | 34.4(5.8) | ** | + |
| Letter Completion 3 | 23.6(2.7) | 15.6(3.1) | ** | + |
| Letter Completion 6 | 11.1(2.9) | 8.4(1.9) | ** | + |
| Letter Completion 9 | 7.3(1.6) | 6.2(1.5) | * | + |
| Raven’s Prog. Matrices | 11.1(2.5) | 5.3(2.6) | ** | + |
| OSPAN | 21.6(0.8) | 12.7(6.5) | * | + |
| Verbal Fluency (CFL)% | 80.5(20.4) | 60.2(29.6) | * | + |
| Alternate Category | 10.4(2.0) | 7.7(2.4) | * | + |
A plus sign means a higher score indicates higher performance on the test; a minus sign means a lower score indicates higher performance on the test.
<.05;
<.001.
Table 5.
Mean (SD) for the Wisconsin Card Sorting Test Scores for Old Adults Grouped by Fitness Level
| Subtest | High-fit old |
Low-fit old |
p value | Directiona |
|---|---|---|---|---|
| Categories completed | 5.3 (1.2) | 3.4 (2.0) | ** | + |
| Number of trials | 88.5 (16.3) | 113.4 (20.5) | ** | − |
| Correct | 68.8 (12.8) | 67.5 (16.1) | + | |
| Errors | 19.7 (11.2) | 45.9 (20.1) | ** | − |
| Perseverative responses | 10.9 (6.7) | 24.7 (15.1) | ** | − |
| Perseverative errors | 10.1 (5.7) | 22.1 (13.0) | ** | − |
| Nonperseverative errors | 9.7 (7.0) | 23.8 (12.7) | ** | − |
| % perseverative errors | 11.3 (6.6) | 18.5 (9.0) | * | − |
| Completion first category | 12.3 (2.8) | 20.8 (14.2) | * | − |
| % conceptual level resp. | 73.9 (14.4) | 49.0 (19.6) | ** | + |
| Failure to maintain set | 0.8 (0.9) | 1.4 (1.4) | * | − |
| Learning to learn | −3.6 (6.9) | −10.5 (11.3) | * | + |
A plus sign means a higher score indicates higher performance on the test; a minus sign means a lower score indicates higher performance on the test.
<.05;
<.001.
Table 3.
Mean (SD) for the Wisconsin Card Sorting Test Subscores for Participants Grouped by Age
| Young | Old | p value | Directiona | |
|---|---|---|---|---|
| Categories completed | 5.7 (0.8) | 4.3 (1.90) | * | + |
| Number of trials | 76.9 (7.0) | 101.3 (22.3) | ** | − |
| Correct | 64.6 (4.9) | 68.1 (14.4) | + | |
| Errors | 12.3 (5.8) | 33.2 (20.9) | ** | − |
| Perseverative responses | 7.2 (4.3) | 18.0 (13.6) | ** | − |
| Perseverative errors | 6.8 (3.5) | 16.2 (11.7) | ** | − |
| Nonperseverative errors | 5.6 (3.3) | 16.9 (12.4) | ** | − |
| % perseverative errors | 8.7 (4.2) | 15.0 (8.6) | * | − |
| Completion first category | 17.4 (22.8) | 16.6 (11.1) | − | |
| % conceptual level resp. | 81.6 (8.5) | 61.1 (21.2) | ** | + |
| Failure to maintain set | 0.3 (0.6) | 1.1 (1.2) | * | − |
| Learning to learn | −1.5 (5.2) | −6.7 (9.9) | * | + |
A plus sign means a higher score indicates higher performance on the test; a minus sign means a lower score indicates higher performance on the test.
<.05;
<.001.
Table 4.
Mean (SD) for the Neuropsychological Test Scores of Old Adults Grouped by Fitness Level
| Test | High-fit old |
Low-fit old |
p value | Directiona |
|---|---|---|---|---|
| mMMS | 55.6 (1.2) | 54.1 (1.9) | ** | + |
| WAIS-R vocabulary scaled |
14.7 (2.9) | 12.1 (1.7) | * | + |
| Forward Digit Span | 7.1 (1.1) | 6.8 (1.1) | + | |
| Backwards Digit Span | 5.4 (1.6) | 4.9 (1.6) | + | |
| Boxes | 46.7 (12.6) | 40.8 (12.5) | + | |
| Trail Making A | 19.2 (9.6) | 22.5 (12.2) | − | |
| Trail Making B | 30.5 (9.6) | 40.5 (23.3) | * | − |
| Digit Copying | 71.3 (14.8) | 60.2 (8.9) | * | + |
| Digit Symbol | 36.1 (6.2) | 32.7 (5.0) | * | + |
| Letter Completion 3 | 16.6 (3.5) | 14.6 (2.4) | * | + |
| Letter Completion 6 | 8.9 (1.9) | 7.9 (1.7) | * | + |
| Letter Completion 9 | 6.6 (1.4) | 5.9 (1.6) | + | |
| OSPAN | 14.2 (7.0) | 10.9 (6.6) | + | |
| Raven’s Prog. Matrices | 6.2 (2.2) | 4.5 (2.7) | * | + |
| Verbal Fluency (CFL)% | 57.5 (29.1) | 62.9 (30.6) | + | |
| Alternate Category | 8.9 (2.1) | 6.6 (2.2) | ** | + |
A plus sign means a higher score indicates higher performance on the test; a minus sign means a lower score indicates higher performance on the test.
<.05;
<.001.
Correlations between the neuropsychological test scores and either aerobic fitness or education within the older adult group also yielded multiple significant effects (see Table 6, first two columns). Both years of education and aerobic fitness were correlated with higher performance on a range of neuropsychological tests. However, some of these effects may be correlated or confounded with other factors, such as age and gender. Therefore, to evaluate their independent contributions we ran a multiple regression analysis for each neuropsychological variables using education, VO2max, age, and gender as predictors. The results of this analysis (beta weights and multiple correlations) are also reported in Table 6.
Table 6.
Correlations and Beta Weights of Neuropsychological Measures with Fitness and Years of Education for the Older Adults
| Raw correlations |
|||||
|---|---|---|---|---|---|
| Variable | VO2max | Education | VO2max β Weight | Education β Weight | Multiple Correl. |
| Age | −.25 | −.16 | — | — | — |
| Gender | −.32* | .02 | — | — | — |
| VO2 | 1.00 | .56* | — | — | — |
| Education | .56* | 1.00 | — | — | — |
| mMMS | .42* | .04 | .58* | −.31+ | .50* |
| WAIS-R Vocab. | .44* | .52* | .21 | .38* | .55* |
| Forward Digit | .10 | −.01 | .13 | −.08 | .13 |
| Backward Digit | .06 | .18 | −.04 | .23 | .24 |
| Boxes | .15 | .29+ | .02 | .29 | .31 |
| Trail-A | −.13 | −.05 | −.13 | .01 | .15 |
| Trail-B | −.28 | −.32+ | −.07 | −.29 | .37 |
| Digit Copying | .30+ | .29+ | .26 | .10 | .43 |
| Digit Symbol | .26 | .28 | .33 | .08 | .48+ |
| Letter Comp. (3, 6, 9) | .28+ | .29+ | .18 | .16 | .36 |
| OSPAN | .19 | .19 | .28 | .01 | .36 |
| Raven’s | .32* | .18 | .19 | .11 | .49* |
| Verbal Fluency (CFL) % | −.11 | .12 | −.14 | .21 | .32 |
| Alternate | .39* | .33+ | .29 | .17 | .41 |
| WCST | |||||
| Categories completed | .34* | .23 | .15 | .13 | .44 |
| Number of trials | −.40* | −.46* | −.20 | −.34+ | .50* |
| Correct | .02 | −.06 | −.16 | .00 | .48+ |
| Errors | −.44* | −.45* | −.10 | −.37* | .61* |
| Perseverative resp. | −.32* | −.40* | −.01 | −.39* | .48+ |
| Perseverative errors | −.33* | −.43* | .03 | −.44* | .53* |
| Nonpers. errors | −.43* | −.36* | −.19 | −.21 | .56* |
| % perseverative errors | −.25 | −.37* | .12 | −.44* | .50* |
| Completion first category | −.30+ | −.29+ | −.18 | −.19 | .34 |
| % conceptual level resp. | .40* | .38* | .07 | .30+ | .58* |
| Failure to maintain set | −.22 | .00 | −.46* | .24 | .38 |
| Learning to learn | .20 | .06 | −.13 | −.02 | .20 |
Notes. Age and gender are included in the model as predictors. Alternate: Alternate Category test. Letter Comp (3, 6, 9): Letter completion (combined score for 3,6,9). WCST: Wisconsin Card Sorting Test.
p < .05;
p < .10 (note that these correlations would all be significant if one-tailed tests were used, which would be justified here given that all our a priori hypotheses were unidirectional).
A number of significant beta weights were present for education and a smaller number for VO2max. To avoid redundancy and improve our understanding of the relationships between education, VO2max, and neuropsychological variables, we also ran a factor analysis (based on a principal component analysis followed by a varimax rotation) to group the latter. Both the Kaiser’s criterion (eigenvalue > 1) and a scree test converged into selecting eight factors (accounting for 83.9% of the variance) for the rotation. The factor analysis grouped the variables in a way that was consistent with our expectations: most factors (seven out of eight) appeared to correspond to the constructs we expected to be assessed by the neuropsychological tests. Another multiple regression was then run for each of these factors, using age, gender, VO2max, and education as predictors and the factor scores as dependent variables. The factor loadings and the results of this multiple regression analysis are reported in Table 7. They indicate that education was a significant predictor of Factor 1 (mainly related to several of the WCST scales, which could be tentatively interpreted as representing cognitive flexibility) and that VO2max was a significant predictor of Factor 7 (mainly related to mMMS and WAIS-R vocabulary score and tentatively interpreted as an index of global cognitive competence). These data suggest that the effects of VO2max and education, although largely overlapping, also have some unique contributions.
Table 7.
Summary of the Factor Analysis of the Neuropsychological Tests and of the Related Multiple Regression Analyses
| Loadings > .50 | Factor |
|||||||
|---|---|---|---|---|---|---|---|---|
| Variable | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
| mMMS | 0.87 | |||||||
| WAIS-R Vocab. | 0.56 | |||||||
| Forward Digit | 0.80 | |||||||
| Backward Digit | 0.83 | |||||||
| Boxes | 0.82 | |||||||
| Trail-A | −0.63 | |||||||
| Trail-B | 0.63 | 0.55 | ||||||
| Digit Copying | 0.83 | |||||||
| Digit Symbol | 0.81 | |||||||
| Letter Comp 3 | 0.67 | |||||||
| Letter Comp 6 | 0.75 | |||||||
| Letter Comp 9 | 0.73 | |||||||
| OSPAN | 0.68 | |||||||
| Raven’s | −0.87 | |||||||
| Verbal Fluency (CFL) % | ||||||||
| Alternate | ||||||||
| WCST | ||||||||
| Categories completed | 0.84 | |||||||
| Number of trials | −0.63 | |||||||
| Correct | 0.77 | |||||||
| Errors | −0.91 | |||||||
| Perverative resp. | −0.93 | |||||||
| Perseverative errors | −0.94 | |||||||
| Nonperseverative errors | −0.68 | −0.51 | ||||||
| % perseverative errors | −0.94 | |||||||
| Completion first category | −0.53 | |||||||
| % conceptual level resp. | 0.89 | |||||||
| Failure to maintain set | 0.78 | |||||||
| Learning to learn | 0.89 | |||||||
| Beta weights | ||||||||
| Age | −0.12 | −0.19 | 0.10 | 0.10 | −0.32 | −0.04 | −0.10 | −0.24 |
| Gender | −0.30 | 0.20 | 0.14 | −0.02 | −0.44 | 0.20 | 0.12 | 0.43 |
| VO2 | −0.16 | 0.22 | −0.03 | −0.17 | −0.32 | 0.19 | 0.58 | 0.02 |
| Education | 0.48 | −0.01 | 0.01 | 0.23 | −0.03 | −0.02 | −0.22 | −0.32 |
| Multiple R | 0.52 | 0.33 | 0.20 | 0.20 | 0.54 | 0.23 | 0.48 | 0.52 |
Notes. Beta weights in bold indicate p <.05, and in italics p <.10.
Age Effects on Brain Volumes
The tissue density maps for the young and old adults are presented in Figure 1A (with Figure 1B showing 3D renderings of the same effects). Statistical comparisons showed a number of significant areas of change for all three tissue types (Z > 1.96, p<.05). The analysis of cerebrospinal fluid showed an increase with age along the midline and in tissue bordering the lateral ventricles and cerebral aqueduct (first row). There were significant decreases in gray matter density in extensive temporal, parietal, and prefrontal regions as a function of age (second row). A significant age-related decline in white matter volume was evident in anterior medial areas near the lateral and third ventricles (third row). The white matter showed relative preservation in posterior regions, especially parietal and occipital areas. These results are in line with previous findings of age-related volumetric declines (Colcombe et al., 2003; Raz et al., 1998). No areas showed a reduction of CSF or an increase in gray matter as a function of age. However, older adults showed increases in white matter in one section posterior and one superior to the corpus callosum (fourth row). This suggests that some of the apparent reduction of periventricular white matter may not be due solely to atrophy, but also to white matter tissue displacement in older adults into regions normally occupied by gray matter in younger adults.
Figure 1.
A: Partial-volume maps depicting the comparisons between younger and older adults. Row 1: CSF; colors identify regions in which CSF partial volume increases as a function of age. Row 2: Grey matter; colors identify regions in which gray matter partial volume decreases as a function of age. Rows 3 and 4: White matter; colors identify regions in which white matter partial volume decreases (row 3) or increases (row 4) as a function of age. All images are presented in radiological convention. Y: young; O: old. Saggital slices: MNI coordinates x: 20 (right), 0 (medial), −20 (left); Coronal slices: y: −38 (posterior), −18 (middle), 2 (anterior); Horizontal slices: z: 2 (inferior), 18 (middle), 38 (superior). B: Three-dimensional renderings of the effects in A, also shown in radiological convention.
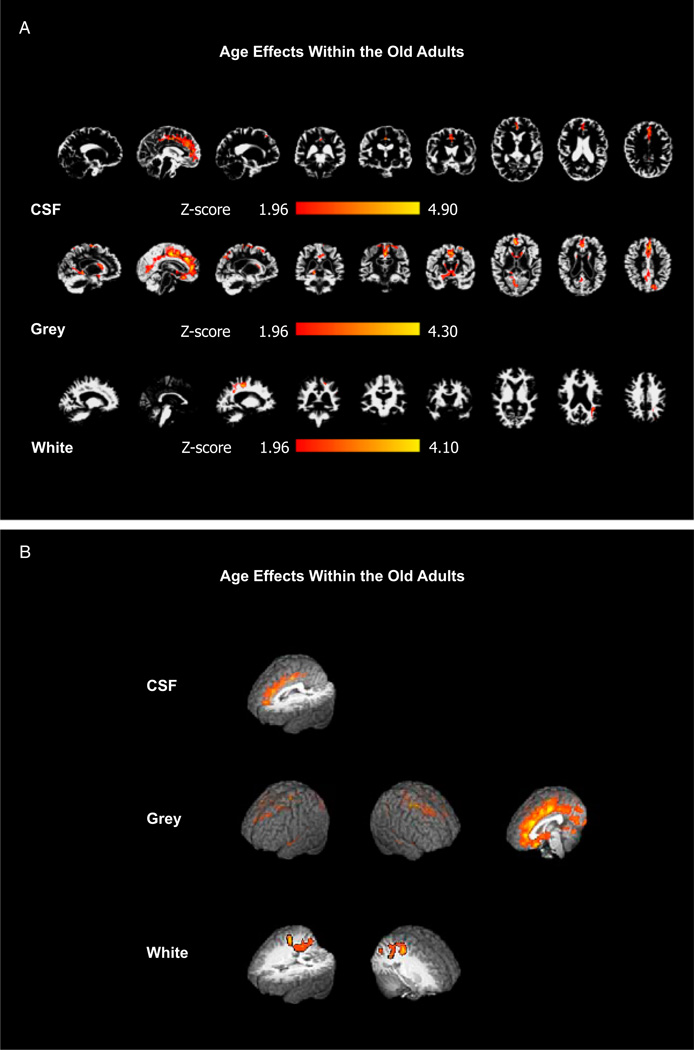
A second analysis, based on a regression with age, was performed within the older adult group. The rationale for this approach was that, although the range of ages within the old group was somewhat limited (65–81 years), if tissue deterioration continues over time, then differences may become detectable even within this age group. This prediction was confirmed by the results presented in Figure 2A (with 3D renderings of the same effects in Figure 2B). Smaller clusters of voxels showed significant effects in midline cerebrospinal fluid (first row) and posterior white matter (third row) (Z > 1.96, p<.05). Significant tissue loss with increasing age was found for gray matter in the medial temporal lobe (Z > 1.96, p<.05) (second row). This type of effect has been previously found along with effects in frontal cortex (Colcombe et al., 2003).
Figure 2.
A: Partial-volume maps depicting the analysis of age-effects within the older population. Row 1: CSF; colors identify regions in which CSF partial volume increases as a function of age. Row 2: Grey matter; colors identify regions in which gray matter partial volume decreases as a function of age. Row 3: White matter;: colors identify regions in which white matter partial volume decreases as a function of age. All images are presented in radiological convention. Saggital slices: MNI coordinates x: 20 (right), 0 (medial), −20 (left); Coronal slices: y: −38 (posterior), −18 (middle), 2 (anterior); Horizontal slices: z: 2 (inferior), 18 (middle), 38 (superior). B: Three-dimensaional renderings of the effects in A, also shown in radiological convention.
Fitness Effects on Brain Volumes
The comparisons based on the VO2max scores within the older adults (with gender, age, and education as covariates) yielded significant results for gray matter (Figure 3A, first row; 3D renderings in Figure 3B). In particular, there were significant positive associations between gray matter volume and aerobic fitness in medial-temporal, anterior parietal, and inferior frontal areas. No significant relationships were found in the white matter (but see Colcombe et al., 2003, 2006, for white matter changes and differences with fitness), whereas a marginally negative association was found with CSF, with more CSF for low-fit older adults.
Figure 3.
A: Gray matter partial-volume maps depicting the unique contributions of fitness (row 1)—colors identify regions in which gray matter increases as a function of fitness across all older adults, with education, gender, and age as variables in the model—and education (row 2)—colors identify regions in which white matter increases as a function of education across the older adults, with fitness, gender, and age as variables in the model. All images are presented in radiological convention. Saggital slices: MNI coordinates x: 20 (right), 0 (medial), −20 (left); Coronal slices: y: −38 (posterior), −18 (middle), 2 (anterior); Horizontal slices: z: 2 (inferior), 18 (middle), 38 (superior). B: Three-dimensional renderings of the effects in A, also shown in radiological convention.
Education Effects on Brain Volume
An analysis of the three tissue types based upon years of education was conducted within the older population, with age, gender, and fitness as covariates. This comparison yielded a significant positive effect in anterior white matter. Specifically, significant effects were found in the rostrum of the corpus callosum as well as in inferior frontal cortex. No other significant effects were found for education. These results are shown in Figure 3A (second row; 3D renderings in Figure 3B).
Discussion
The data presented here showed structural and behavioral changes associated with age, fitness level, and education. The comparison between the independent influence of fitness and education shows different patterns of effects: Fitness effects were mainly found in gray matter whereas education effects were found for frontal white matter connections.
Our analysis of tissue density as a function of age yielded results that are in accord with those previously observed (Colcombe et al., 2003; Raz et al., 1998). Decreases of gray and white matter in the frontal and medial temporal lobes are also consistent with extensive behavioral evidence showing impairments in tasks of executive control and memory for which these areas are considered to be important (Park et al., 2002; West, 1996). Cerebrospinal fluid increase in the parietal lobe is probably due to decrease in the density or volume of the white matter surrounding the ventricles, whereas increased amounts of cerebrospinal fluid in the longitudinal fissure and around the outer brain surface can be attributed to a general reduction of the brain volume.
The fitness data presented here support the idea that the behavioral effects observed in both the exercise and aging literature may have a counterpart in brain anatomy. We also showed that, within the older adults, higher fitness levels are associated with relative sparing of tissue in brain areas typically vulnerable to the effects of aging. This suggests that higher levels of fitness may stave off some of the deleterious effects of aging on the brain and possibly prevent or delay ensuing brain pathologies. This is consistent with research in animal models as well as in humans (Colcombe et al., 2003, 2004; Neeper et al., 1995; van Pragg et al., 1999).
According to the cognitive reserve hypothesis, higher education could potentially lead to, or be associated with, a surplus of tissue. Specifically, our results are consistent with those indicating decreased occurrence of white matter hyperintensities in people with higher education (Dufouil et al., 2003; Nebes et al., 2006) and do suggest preferential sparing of white matter in this group. The spatial localization of the education effect is very similar to that observed in previous work after exercise intervention (Colcombe et al., 2006). It is known that anterior white matter is selectively sensitive to age-related declines, so any effect of education on white matter was expected to show the greatest effect in this area. Previous work on exercise interventions indicates that it is possible to partially reverse this decline, whereas the current study shows that education may be related to the preservation of this area.
Due to the fact that the formal education used here as a predictor is completed long before adults reach an old age, longitudinal studies are difficult to perform. Therefore, it is not easy to determine whether education may be responsible for the preserved white matter integrity we reported or whether the propensity for more established frontal connections provides for greater scholastic aptitude. Current work on cognitive intervention on at-risk, low-socioeconomic status older adults (Carlson et al., in press) suggests that improvements in neuropsychological performance can be gained longitudinally. It would be useful to expand this work to include structural brain measures to assess whether white matter sparing and/or recovery accompanies these functional changes.
It is also important to consider that higher levels of education could be a proxy for a host of other influences that may affect brain function and structure, such as improved access to health care, better diet, and less stressful living environments. Irrespective of the possible origins of these effects, the identification of structural correlates of higher education is an intriguing result.
The neuropsychological tests provide a behavioral parallel to the structural analyses. Our data indicate that high fitness scores predicted improved performance on a wide range of cognitive tasks (Tables 4–6, raw correlations). This evidence supports previously published human behavioral data indicating that higher cognitive performance is associated with higher aerobic fitness (Churchill et al., 2002; Colcombe & Kramer, 2003; Hall, Smith, & Keele, 2001). These data are also consistent with work done within the animal literature providing evidence for improved learning and memory with enriched environments, which lead animals to engage in increased motor and exploratory behaviors (Anderson et al., 2000; Churchill et al., 2002; Greenough et al., 1972). In a manner similar to aerobic fitness, higher levels of education were associated with better performance on several neuropsychological tests (Tables 4–6).
Even though fitness and years of education were significantly correlated, the volumetric analyses controlled for the other variable, as well as for age and gender. Therefore, it was possible to investigate the independent structural contributions of the two factors. In the same way, the neuropsychological test results also supported the existence of dissociations between the effects of fitness and education, when the other factors were controlled for (Tables 6 and 7, regression analyses). Specifically, the behavioral data indicate that the independent effects of education are most evident in tests highlighting flexibility (the WCST scales) and semantic knowledge (WAIS-R vocabulary). These scales may be closely related to the concept of cognitive reserve, which is thought to be increased as a function of education (Stern, 2002). In contrast, the greatest influence of fitness appears to be on the mMMS scale, a measure of overall cognitive performance in older adults. This suggests that, whereas education may boost potential resources, fitness may in fact influence the way in which these cognitive resources are actually deployed in everyday tasks. This would suggest that these two factors may exert a complementary role in the preservation of cognitive function with aging—and that intervention strategies emphasizing both may be particularly beneficial.
This study does have a number of potential limitations that should be taken into account when interpreting and/or generalizing our findings. For example, the cross-sectional nature of this study may help to explain differences in results between this and previous reports (Colcombe et al., 2006) that have shown aerobic effects on both white and gray matter. The within-subject design used by Colcombe and colleagues provides greater power to detect relatively small anatomical changes. Thus a longitudinal study would be able to detect relatively localized but small changes in white matter whereas a cross-sectional study would have more difficulty.
In the examination of the effects of fitness, secondary medical conditions such as mild hypertension could influence the results. However, our stringent screening process and the medical release requirements should have limited some of these potential confounds. In fact, these screening and recruiting procedures are likely to reduce the sizes of possible fitness or education effects, as both variables have reduced ranges in our samples compared to the overall population. Notwithstanding these reduced ranges, however, significant contributions of both factors were found.
The relatively small sample size in this study also poses another limitation, weakening the power of statistical inference. The number of individuals included is not as large as other studies (Good et al., 2001b). However, a sample size smaller than 40 subjects is not uncommon in the literature (Buskova, Vaneckova, Sonka, Seidl, & Nevsimalova, 2006; Holzapfel, Barnea-Goraly, Eckert, Kesler, & Reiss, 2006). Although the current sample size is more than sufficient to detect robust differences, it may lead to a small reduction in statistical power compared to some previous work (Colcombe et al., 2003, 2006). In the current study, the effect of fitness on gray matter found by Colcombe and colleagues (2003, 2006) was replicated, but the fitness effects on white matter may have remained subthreshold. Further investigation and generalization of the effects of fitness and education will benefit from a larger sample size. Notwithstanding these possible limitations, the results presented in this article advance our understanding of the aging brain. This work reaffirms the behavioral and volumetric effects associated with aerobic fitness. It expands upon this work to also show that education impacts not only cognitive measures, but also white matter integrity.
Although it has much support in the literature (Habeck et al., 2003; Richards & Deary, 2005; Scarmeas et al., 2003) the “cognitive reserve” hypothesis is still debated (Christensen et al., 2007). These results add support to this idea that higher education may be predictive of preserved brain tissue. In the future we hope to directly examine the interaction of such factors as socioeconomic status, verbal IQ, as well as years of education. A composite score based upon these three would potentially allow us to directly examine the cognitive reserve hypothesis in a volumetric manner.
This work goes beyond purely structural implications. For example, it may be possible to directly correlate age-related structural changes to changes in patterns of blood flow in fMRI and other functional imaging studies (Buckner et al., 2004). The anatomical differences between the higher- and lower-fit older adults, as well as the effects of education, suggest that the changes in functional activation observed in older adults may be related, at least in part, to specific structural changes in the brain. Research has shown that, compared to less fit older adults, aerobically fit older adults exhibit greater activation in brain areas related to attention and reduced activation in brain areas related to conflict (Colcombe et al., 2004). Similarly, Czernochowski and colleagues (in press) showed that increased brain activity in highly educated older adults, compared to older adults with lower education, was associated with preserved memory performance.
In conclusion, this article takes a novel approach to examining the effects of both education and fitness in aging. Simultaneous comparison of the effects of these two factors allowed us to differentiate their influence on brain anatomy and cognitive performance. Further research efforts may be directed toward establishing cause–effect relationships between cardiovascular fitness, brain anatomy, and function through fitness intervention studies and to further clarify the potential effects of education through cognitive intervention studies.
Acknowledgments
Our thanks to the Image Analysis Group, FMRIB, Oxford, UK, and their programmers and scientists who created the tools we used to do the analysis. Thanks to Kirk Erickson for helpful suggestions on data analyses. Finally we wish to show appreciation to Brad Sutton and Andrew Webb, who answered numerous questions of a technical nature related to MRI acquisition and protocol setup. This research was supported by NIA grant #AG21887 to Monica Fabiani.
Footnotes
Note that this sample was newly assessed and independent from those previously reported by Kramer and colleagues.
A small number of scans (<4) were acquired in the axial rather than the saggital plane. This was due to wrapping issues on the scans. There was no effect of this difference.
REFERENCES
- American College of Sports Medicine. Guidelines for Exercise Testing and Prescription. Philadelphia: Lea & Febiger; 1991. [Google Scholar]
- Anderson BJ, Rapp DN, Baek DH, McCloskey DP, Coburn-Litvak PS, Robinson JK. Exercise influences spatial learning in the radial arm maze. Physiology and Behavior. 2000;70:425–429. doi: 10.1016/s0031-9384(00)00282-1. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Voxel-based morphometry: The methods. NeuroImage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Why voxel-based morphometry should be used. NeuroImage. 2001;14:1454–1462. doi: 10.1006/nimg.2001.0961. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory. 2nd. San Antonio: The Psychological Corporation; 1996. [Google Scholar]
- Benton A, Hamsher K. Multilingual aphasia exam. Iowa City: University of Iowa Press; 1976. [Google Scholar]
- Bookstein FL. Voxel-based morphometry should not be used with imperfectly registered images. NeuroImage. 2001;14:1238–1243. doi: 10.1006/nimg.2001.0770. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Nystrom LE, Fissel K, Carter C, Cohen JD. Conflict monitoring versus selection-for-action in anterior cingulate cortex. Nature. 1999;402:179–181. doi: 10.1038/46035. [DOI] [PubMed] [Google Scholar]
- Brown J, Cooper-Kuhn CM, Kempermann G, Van Praag H, Winkler J, Gage FM, et al. Enriched environment and physical activity stimulate hippocampal but not olfactory bulb neurogenesis. European Journal of Neuroscience. 2003;17:2042–2046. doi: 10.1046/j.1460-9568.2003.02647.x. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Head D, Parker J, Fotenos AF, Marcus D, Morris JC, et al. A unified approach for morphometric and functional data analysis in young, old, and demented adults using automated atlas-based head size normalization: Reliability and validation against manual measurement of total intracranial volume. NeuroImage. 2004;23:724–738. doi: 10.1016/j.neuroimage.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Buskova J, Vaneckova M, Sonka K, Seidl Z, Nevsimalova S. Reduced hypothalamic gray matter in narcolepsy with cataplexy. Neuro Endocrinology Letters. 2006;27:769–772. [PubMed] [Google Scholar]
- Carlson MC, Saczynski JS, Rebok GW, McGill S, Tielsch J, Seeman T, et al. Experience corps: Effects of a pilot trial of a senior service program on executive and memory functions in older adults. Journal of Gerontology. (in press) [Google Scholar]
- Carro E, Trejo JL, Busiguina S, Torres-Aleman I. Circulating insulin-like growth factor I mediates the protective effects of physical exercise against brain insults of different etiology and anatomy. Journal of Neuroscience. 2001;21:5678–5684. doi: 10.1523/JNEUROSCI.21-15-05678.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen H, Anstey KJ, Parslow RA, Maller J, Mackinnon A, Sachdev P. The brain reserve hypothesis, brain atrophy and aging. Gerontology. 2007;53:82–95. doi: 10.1159/000096482. [DOI] [PubMed] [Google Scholar]
- Churchill JD, Galvez R, Colcombe S, Swain RA, Kramer AF, Greenough WT. Exercise, experience and the aging brain. Neurobiology of Aging. 2002;23:941–955. doi: 10.1016/s0197-4580(02)00028-3. [DOI] [PubMed] [Google Scholar]
- Coffey CE, Saxton JA, Ratcliff G, Bryan RN, Lucke JF. Relation of education to brain size in normal aging: Implications for the reserve hypothesis. Neurology. 1999;53:189. doi: 10.1212/wnl.53.1.189. [DOI] [PubMed] [Google Scholar]
- Colcombe SJ, Erickson KI, Raz N, Webb AG, Cohen NJ, McAuley E, et al. Aerobic fitness reduces brain tissue loss in aging humans. Journal of Gerontology: Biological Sciences, Medical Sciences. 2003;58A:176–180. doi: 10.1093/gerona/58.2.m176. [DOI] [PubMed] [Google Scholar]
- Colcombe SJ, Erickson KI, Scalf PE, Kim JS, Prakash R, McAuley E, et al. Aerobic exercise training increases brain volume in aging humans. Journal of Gerontology: Medical Sciences. 2006;61:1166–1170. doi: 10.1093/gerona/61.11.1166. [DOI] [PubMed] [Google Scholar]
- Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults: A meta-analytic study. Psychological Science. 2003;14:125–130. doi: 10.1111/1467-9280.t01-1-01430. [DOI] [PubMed] [Google Scholar]
- Colcombe SJ, Kramer AF, Erickson KI, Scalf P, McAuley E, Cohen NJ, et al. Cardiovascular fitness, cortical plasticity, and aging. Proceedings of the National Academy of Sciences, USA. 2004;101:3316–3321. doi: 10.1073/pnas.0400266101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colello RJ, Devey LR, Imperato E, Pott U. The chronology of oligodendrocyte differentiation in the rat optic nerve: Evidence for a signaling step initiating myelination in the CNS. Journal of Neuroscience. 1995;15:7665–7672. doi: 10.1523/JNEUROSCI.15-11-07665.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrigan JD, Hinkeldey NS. Relationships between parts A and B of the Trail Making Test. Journal of Clinical Psychology. 1987;43:402–409. doi: 10.1002/1097-4679(198707)43:4<402::aid-jclp2270430411>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Cotman CW, Berchtold NC. Exercise: A behavioral intervention to enhance brain health and plasticity. Trends in Neuroscience. 2002;25:295–301. doi: 10.1016/s0166-2236(02)02143-4. [DOI] [PubMed] [Google Scholar]
- Czernochowski D, Fabiani M, Friedman D. Use it or lose it? SES mitigates age-related decline in a recency/recognition task. Neurobiology of Aging. doi: 10.1016/j.neurobiolaging.2006.12.017. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davatzikos C, Resnick SM. Degenerative age changes in white matter connectivity visualized in vivo using magnetic resonance imaging. Cerebral Cortex. 2002;12:767–771. doi: 10.1093/cercor/12.7.767. [DOI] [PubMed] [Google Scholar]
- Draganski B, Gaser P, Busch V, Schuierer G, Bogdahn U, May A. Neuroplasticity: Changes in grey matter induced by training. Nature. 2004;427:311–312. doi: 10.1038/427311a. [DOI] [PubMed] [Google Scholar]
- Dufouil C, Alperovitch A, Tzourio C. Influence of education on the relationship between white matter lesions and cognition. Neurology. 2003;60:831–836. doi: 10.1212/01.wnl.0000049456.33231.96. [DOI] [PubMed] [Google Scholar]
- Dustman RE, Ruhling RO, Russell EM, Shearer DE, Bonekat HW, Shigeoka JW, et al. Aerobic exercise training and improved neuropsychological function of older individuals. Neurobiology of Aging. 1984;5:35–42. doi: 10.1016/0197-4580(84)90083-6. [DOI] [PubMed] [Google Scholar]
- Engle RW, Tuholski SW, Laughlin JE, Conway ARA. Working memory, short-term memory, and general fluid intelligence: A latent-variable approach. Journal of Experimental Psychology: General. 1999;128:309–331. doi: 10.1037//0096-3445.128.3.309. [DOI] [PubMed] [Google Scholar]
- Eriksen BA, Eriksen CW. Effects of noise letters upon the identification of target letter in a non-search task. Perception and Psychophysics. 1974;16:143–149. [Google Scholar]
- Esiri M. Dementia and normal aging: Neuropathology. In: Huppert FA, Brayne C, O’Connor DW, editors. Dementia and normal aging. Cambridge, England: Cambridge University Press; 1994. pp. 385–436. [Google Scholar]
- Fabiani M, Brumback C, Gordon B, Pearson M, Lee Y, Kramer A, et al. Effects of cardiopulmonary fitness on neurovascular coupling in visual cortex in younger and older adults. Psychophysiology. 2004;41:S19. [Google Scholar]
- Fleischman DA, Wilson RS, Gabrieli JDE, Bienias JL, Bennett DA. A longitudinal study of implicit and explicit memory in old persons. Psychology and Aging. 2004;19:617–625. doi: 10.1037/0882-7974.19.4.617. [DOI] [PubMed] [Google Scholar]
- Good CD, Johnsrude I, Ashburner J, Henson RNA, Friston K, Frackowiak RSJ. Cerebral asymmetry and the effects of sex and handedness on brain structure: A voxel-based morphometric analysis of 465 normal adult human brains. NeuroImage. 2001a;14:685–700. doi: 10.1006/nimg.2001.0857. [DOI] [PubMed] [Google Scholar]
- Good CD, Johnsrude IS, Ashburner J, Henson RNA, Friston KJ, Frackowiak RSJ. Avoxel-based morphometric study of ageing in 465 normal adult human brains. NeuroImage. 2001b;14:21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- Greenough WT, Madden TC, Fleischmann TB. Effects of isolation, daily handling, and enriched rearing on maze learning. Psychonomic Sciences. 1972;1:279–280. [Google Scholar]
- Greenough WT, Volkmar FR. Pattern of dendritic branching in occipital cortex of rats reared in complex environments. Experimental Neurology. 1973;40:491–504. doi: 10.1016/0014-4886(73)90090-3. [DOI] [PubMed] [Google Scholar]
- Gunning-Dixon FM, Raz N. Neuroanatomical correlates of selected executive function in middle-aged and older adults: A prospective MRI study. Neuropsychologia. 2003;41:1929–1941. doi: 10.1016/s0028-3932(03)00129-5. [DOI] [PubMed] [Google Scholar]
- Habeck C, Hilton HJ, Zarahn E, Flynn J, Moeller J, Stern Y. Relation of cognitive reserve and task performance to expression of regional covariance networks in an event-related study of non-verbal memory. NeuroImage. 2003;20:1723–1733. doi: 10.1016/j.neuroimage.2003.07.032. [DOI] [PubMed] [Google Scholar]
- Hall CD, Smith AL, Keele SW. The impact of aerobic activity on cognitive function in older adults: A new synthesis based on the concept of executive control. European Journal of Cognitive Psychology. 2001;13:279–300. [Google Scholar]
- Heaton RK. Wisconsin card sorting test manual. Odessa, FL: Psychological Assessment Resources; 1981. [Google Scholar]
- Hillman CH, Belopolsky AV, Snook EM, Kramer AF, McAuley E. Physical activity and executive control: Implications for increased cognitive health during older adulthood. Research Quarterly of Exercise and Sport. 2004;75:176–185. doi: 10.1080/02701367.2004.10609149. [DOI] [PubMed] [Google Scholar]
- Holzapfel M, Barnea-Goraly N, Eckert MA, Kesler SR, Reiss AL. Selective alterations of white matter associated with visuospatial and sensorimotor dysfunction in Turner syndrome. The Journal of Neuroscience. 2006;26:7007–7013. doi: 10.1523/JNEUROSCI.1764-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ince P. Pathological correlates of late-onset dementia in a multicenter community-based population in England and Wales. Lancet. 2001;357:169–175. doi: 10.1016/s0140-6736(00)03589-3. [DOI] [PubMed] [Google Scholar]
- Jack CR, Jr, Petersen RC, O’Brien PC, Tangalos EG. MR-based hippocampal volumetry in the diagnosis of Alzheimer’s disease. Neurology. 1992;42:183–188. doi: 10.1212/wnl.42.1.183. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister PR, Brady JM, Smith SM. Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith S. A global optimization method for robust affine registration of brain images. Medical Image Analysis. 2001;5:143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Kemper TL. Neuroanatomial and neuropathological changes during aging and in dementia. In: Albert MLE, Knoepfel EJ, editors. Clinical neurology of aging. 2nd. New York: Oxford University Press; 1994. pp. 3–67. [Google Scholar]
- Kendrick DC, Watts GD. The Kendrick Assessment Scales of Cognitive Ageing. Windsor: NFER-Nelson; 1999. [Google Scholar]
- Kohler S, Black SE, Sinden M, Szekely C, Kidron D, Parker JL, et al. Memory impairments associated with hippocampal versus parahippocampal-gyrus atrophy: An MR volumetry study in Alzheimer’s disease. Neuropsychologia. 1998;36:901–914. doi: 10.1016/s0028-3932(98)00017-7. [DOI] [PubMed] [Google Scholar]
- Kramer AF, Bherer L, Colcombe S, Dong W, Greenough WT. Environmental influences on cognitive and brain plasticity during aging. Journal of Gerontology: Medical Sciences. 2004;59A:940–957. doi: 10.1093/gerona/59.9.m940. [DOI] [PubMed] [Google Scholar]
- Kramer AF, Hahn S, Cohen NJ, Banich MT, McAuley E, Harrison CR, et al. Aging, fitness, and neurocognitive function. Nature. 1999;400:418–419. doi: 10.1038/22682. [DOI] [PubMed] [Google Scholar]
- Mayeux R, Stern Y, Rosen J, Leventhal J. Depression, intellectual impairment and Parkinson’s disease. Neurology. 1981;31:645–650. doi: 10.1212/wnl.31.6.645. [DOI] [PubMed] [Google Scholar]
- McAuley E, Kramer AF, Colcombe SJ. Cardiovascular fitness and neurocognitive function in older adults: A brief review. Brain Behavioral Immunology. 2004;18:214–220. doi: 10.1016/j.bbi.2003.12.007. [DOI] [PubMed] [Google Scholar]
- Morse CK. Does variability increase with age? An archival study of cognitive measures. Psychology and Aging. 1993;8:156–164. doi: 10.1037//0882-7974.8.2.156. [DOI] [PubMed] [Google Scholar]
- Musen G, Lyoo IK, Sparks CR, Weinger K, Hwang J, Ryan CM, et al. Effects of type 1 diabetes on gray matter density as measured by voxel-based morphometry. Diabetes. 2006;55:326–333. doi: 10.2337/diabetes.55.02.06.db05-0520. [DOI] [PubMed] [Google Scholar]
- Nebes RD, Meltzer CC, Whyte EM, Scanlon JM, Halligan EM, Saxton JA, et al. The relation of white matter hyper intensities to cognitive performance in the normal old: Education matters. Aging, Neuropsychology, and Cognition. 2006;13:326–340. doi: 10.1080/138255890969294. [DOI] [PubMed] [Google Scholar]
- Neeper SA, Gomez-Pinilla F, Choi J, Cotman C. Exercise and brain neurotrophins. Nature. 1995;373:109. doi: 10.1038/373109a0. [DOI] [PubMed] [Google Scholar]
- Park DC, Davidson N, Lautenschlager G, Smith AD, Smith P, Hedden T. Models of visuospatial and verbal memory across the adult life span. Psychology and Aging. 2002;17:299–320. [PubMed] [Google Scholar]
- Pujol J, Soriano-Mas C, Alonso P, Cardoner N, Menchon JM, Deus J, et al. Mapping structural brain alterations in obsessive-compulsive disorder. Archives of General Psychiatry. 2004;61:720–730. doi: 10.1001/archpsyc.61.7.720. [DOI] [PubMed] [Google Scholar]
- Raven JC, Court JH, Raven J. Standard progressive matrices. London: HK Lewis; 1977. [Google Scholar]
- Raz N. Aging of the brain and its impact on cognitive performance. In: Craik FIM, Salthouse TA, editors. The handbook of aging and cognition. Mahwah, NJ: Erlbaum; 2002. pp. 1–90. [Google Scholar]
- Raz N, Gunning-Dixon FM, Head D, Dupuis JH, Acker JD. Neuroanatomical correlates of cognitive aging: Evidence from structural magnetic resonance imaging. Neuropsychology. 1998;12:95–114. doi: 10.1037//0894-4105.12.1.95. [DOI] [PubMed] [Google Scholar]
- Richards M, Deary IJ. A life course approach to cognitive reserve: A model for cognitive aging and development? Annals of Neurology. 2005;58:617–622. doi: 10.1002/ana.20637. [DOI] [PubMed] [Google Scholar]
- Rossi C, Angelucci A, Costantin L, Braschi C, Mazzantini M, Babbini F, et al. Brain-derived neurotrophic factor (BDNF) is required for the enhancement of hippocampal neurogenesis following environmental enrichment. European Journal of Neuroscience. 2006;24:1850–1856. doi: 10.1111/j.1460-9568.2006.05059.x. [DOI] [PubMed] [Google Scholar]
- Sachdev P, Brodaty H, Cheang D, Cathcart S. Hippocampus and amygdala volumes in elderly schizophrenic patients as assessed by magnetic resonance imaging. Psychiatry and Clinical Neurosciences. 2000;54:105. doi: 10.1046/j.1440-1819.2000.00644.x. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. Influence of processing speed on adult age differences in working memory. Acta Psychologica. 1992;79:155–170. doi: 10.1016/0001-6918(92)90030-h. [DOI] [PubMed] [Google Scholar]
- Scarmeas N, Zarahn E, Anderson KE, Hilton J, Flynn J, Van Heertum RL, et al. Cognitive reserve modulates functional brain responses during memory tasks: A PET study in health young and elderly subjects. NeuroImage. 2003;19:1215–1227. doi: 10.1016/s1053-8119(03)00074-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sliwinski M, Buschke H. Cross-sectional and longitudinal relationships among age, cognition and processing speed. Psychology and Aging. 1999;14:18–33. doi: 10.1037//0882-7974.14.1.18. [DOI] [PubMed] [Google Scholar]
- Smith S. Fast robust automated brain extraction. Human Brain Mapping. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen-Berg H, et al. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage. 2004;23:208–219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Snowdon DA, Greiner LH, Markesbery WR. Linguistic ability in early life and the neuropathology of Alzheimer’s disease and cerebrovascular disease: Findings from the nun study. Annals of the New York Academy of Sciences. 2000;903:34–38. doi: 10.1111/j.1749-6632.2000.tb06347.x. [DOI] [PubMed] [Google Scholar]
- Spirduso WW. Reaction and movement time as a function of age and physical activity level. Journal of Gerontology. 1975;30:18–23. doi: 10.1093/geronj/30.4.435. [DOI] [PubMed] [Google Scholar]
- Spirduso WW, Clifford P. Replication of age and physical activity effects on reaction and movement time. Journal of Gerontology. 1978;1:26–30. doi: 10.1093/geronj/33.1.26. [DOI] [PubMed] [Google Scholar]
- Stern Y. What is cognitive reserve? Theory and research application of the reserve concept. Journal of the International Neuropsychological Society. 2002;8:448–460. [PubMed] [Google Scholar]
- Terry RD, DeTeresa R, Hansen LA. Neocortical cell counts in normal human adult aging. Annals of Neurology. 1987;21:530–539. doi: 10.1002/ana.410210603. [DOI] [PubMed] [Google Scholar]
- Vaillant GE, Mukamal K. Successful aging. American Journal of Psychiatry. 2001;158:839–847. doi: 10.1176/appi.ajp.158.6.839. [DOI] [PubMed] [Google Scholar]
- van Pragg H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nature Neuroscience. 1999;2:266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale-Revised. San Antonio: The Psychological Corporation; 1981. [Google Scholar]
- West RL. An application of prefrontal cortex function theory to cognitive aging. Psychological Bulletin. 1996;120:272–292. doi: 10.1037/0033-2909.120.2.272. [DOI] [PubMed] [Google Scholar]
- Wright IC, McGuirec PK, Poline JB, Travere JM, Murray RM, Frith CD, et al. Avoxel-based method for the statistical analysis of gray and white matter density applied to schizophrenia. NeuroImage. 1995;170:406–410. doi: 10.1006/nimg.1995.1032. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation maximization algorithm. IEEE Transactions on Medical Imaging. 2001;20:45–57. doi: 10.1109/42.906424. [DOI] [PubMed] [Google Scholar]