Fig 4.
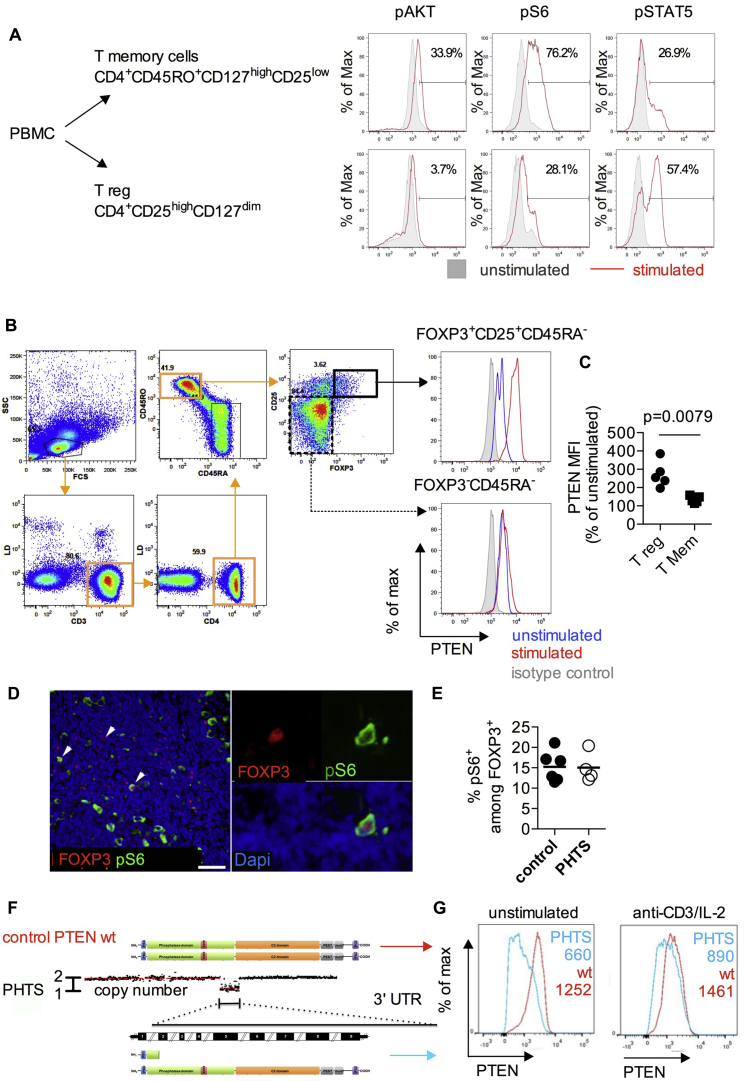
PTEN activity and PI3K signaling in natural Treg cells. A, FACS-sorted Treg cells (CD4+CD25highCD127low) and Tmem cells (CD4+CD45RO+CD127highCD25low) were left unstimulated or subjected to anti-CD3/CD28 and IL-2 stimulation for 10 minutes. Cellular levels of pAKT, pS6, and phosphorylated signal transducer and activator of transcription 5 (pSTAT5) in each cell type with or without stimulation were analyzed by using Phosflow. B and C, PBMCs from healthy donors were rested or stimulated for 24 hours with anti-CD3 and anti-CD28 T-cell activator beads in the presence of IL-2. PTEN expression in FOXP3− nonregulatory and FOXP3+ Treg cell populations among total PBMCs was measured by using FACS to detect changes in PTEN protein levels after TCR and IL-2 receptor stimulation. D, MALT sections were stained for FOXP3+ cells, as well as phosphorylation of the S6 ribosomal protein (p[Ser235/236]S6). Scale bar = 50 μm. E, Percentage of pS6+ expressing cells among FOXP3+ cells in patients with PHTS versus control subjects determined by using immunohistochemical staining. Symbols represent individual patients, and lines represent means. Differences were analyzed by using the Mann-Whitney U test. F, Multiplex ligation-dependent probe amplification copy number analysis revealing a microdeletion spanning from exon 2 to exon 9 and a 3′ untranslated region (UTR) of PTEN. Protein representation of a wild-type (wt) subject and a patient with PHTS with the microdeletion (del E2-9) are shown for comparison. G, PTEN expression in CD3+ T cells from a control donor or a patient with PHTS (del E2-9, PTEN microdeletion) was determined by using FACS with an mAb that recognizes the C-terminal region of PTEN, allowing selective detection of the nonmutated copy of PTEN protein. PTEN levels were measured in cells with or without anti-CD3/CD28 and IL-2 stimulation for 24 hours.