Abstract
Recent population-based studies of expecting mothers identified a unique profile of immune markers that are associated with an increased risk of having a child diagnosed with autism spectrum disorder (ASD). This immune profile, including increased levels of maternal and placental interleukin (IL)-4 and IL-5, is consistent with an immune response found in an allergic-asthma phenotype. Allergies and asthma reflect an imbalance in immune responses including polarization towards T-helper type 2 (TH2) responses, with both genetic susceptibility and environmental factors affecting this T-cell polarization. Mouse strains provide a known and controlled source of genetic diversity to explore the role of genetic predisposition on environmental factors. In particular, the FVB background exhibits a skew towards TH2-mediated allergic-asthma response in traditional models of asthma whereas the C57 strain exhibits a more blunted TH2 polarized phenotype resulting in an attenuated allergic-asthma response. C57BL/6J (C57) and the sighted FVB.129P2-Pde6b(+) Tyr(c-ch)/Ant (FVB/Ant) lines were selected based on their characteristic high sociability and differing sensitivity to TH2-mediated stimuli. Based on the distinct allergy-sensitive immune responses of these two strains we hypothesized that unique developmental consequences would occur in offspring following maternal allergy-asthma exposure. Female C57 and FVB/Ant dams were primed/sensitized with an exposure to ovalbumin (OVA) before pregnancy, then exposed to either aerosolized OVA or PBS-vehicle throughout gestation. Sera from pregnant dams were analyzed for changes in cytokine profiles using multiplex-arrays and offspring were assessed for changes in autism-like behavioral responses. Analysis of maternal sera revealed elevated IL-4 and IL-5 in OVA-treated dams of both strains but only C57 mice expressed increased levels of IL-1β, IL-6, TNFα, and IL-17. Behavioral assessments revealed strain-dependent changes in juvenile reciprocal social interaction in offspring of maternal allergic asthma dams. Moreover, mice of both strains showed decreased repetitive grooming and increased marble burying behavior when born to OVA-exposed dams. Together, these findings support the important role genetic predisposition plays in the effects of maternal immune activation and underscore differences in ASD-like behavioral outcomes across mouse strains.
Keywords: Allergies, Asthma, Animal Model, Autism, ASD, Translational, Maternal Immune Activation
1.0 INTRODUCTION
Autism spectrum disorders (ASD) are a phenotypically diverse class of neurodevelopmental disorders characterized by social-communication deficits and restrictive/repetitive behaviors (Association, 2013). Twin studies indicate that ASD is highly heritable (Hallmayer et al., 2011) and research suggests that familial history of autoimmune diseases like rheumatoid arthritis, celiac disease, and type-1 diabetes increase the risk for an ASD diagnosis (Atladottir et al., 2009). However, the hundreds of genes linked to predisposition and the wide variation in the genomic pattern from individual to individual has made it difficult to identify a distinct genetic marker (Vijayakumar and Judy, 2016). Consequently, research is looking beyond exclusively genetic origins of ASD and considering environmental influences, particularly the connection to immune system perturbations. Epidemiological studies have found that gestational exposure to air pollutants associated with freeways can increase the risk for an ASD diagnoses in offspring (Raz et al., 2015; Volk et al., 2011), and maternal inflammation, most notably by viral or bacterial pathogen, during gestation can also affect neurodevelopment (Jiang et al., 2016).
Most models of immune system dysfunction and ASD evaluate the effects of an immune responses using polyinosinic:polycytidylic acid (Poly[I:C]) to mimic the double stranded RNA in viruses or lipopolysaccharide (LPS), a bacterial membrane protein, to activate pathways of innate immunity. A less studied pathway of maternal immune activation postulates that allergic asthma and T-Helper 2 (TH2) immune responses may contribute to an ASD diagnosis in offspring. Epidemiological studies have shown that mothers with allergies or asthma during pregnancy were at increased risk for having a child with ASD (Croen et al., 2005; Lyall et al., 2014). Furthermore, a case-control study conducted by Goines and colleagues revealed elevated gestational levels of interleukin (IL)-4 and IL-5 in mothers who had children with autism, describing a 50% increased risk of ASD diagnosis in offspring (Goines et al., 2011). These cytokines are consistent with the profiles associated with allergy/asthma immune responses, highlighting the correlations between maternal TH2 immune responses and increased incidence of ASD in the child. Interestingly, elevated levels of IL-4 have been detected in the amniotic fluid of children who were later diagnosed with ASD (Abdallah et al., 2013). More recently, an analysis of cytokine levels in newborn blood spots linked elevations in IL-4 at birth with more severe ASD symptoms and cognitive deficits (Krakowiak et al., 2015). Together, these findings implicate TH2-associated maternal-fetal signaling as a mediating factor in the etiology of ASD and underscore the need to investigate causal links between maternal allergic-asthma and ASD-like behavioral outcomes.
Mouse models afford researchers a powerful tool to directly evaluate the causative effects of maternal immune activation. Though ASD is a strictly human disorder, several indicative behaviors like repetitive stereotypies or social avoidance can be modeled in mice (Silverman et al., 2010). One novel model precipitates ASD-like behavioral deficits in offspring exposed to maternal allergic asthma (MAA) (Schwartzer et al., 2015); this model sensitizes female mice to ovalbumin (OVA) protein followed by induction of airway hypersensitivity through aerosolized OVA exposure at gestational days (G)9.5, 12.5, and 17.5 to mimic early, middle, and late gestation in humans. Importantly, variations in genetic background between mouse strains result in varying severity of immune responses to allergic asthma induction. For example, the FVB/NJ strain displays heightened TH2-responses compared to C57Bl/6J (C57) mice in OVA-induced allergy models (Zhu and Gilmour, 2009). Additionally, all FVB lines carry the Fv-1b allele (van Wyk et al., 2015), leading to increased histamine sensitivity and a significantly greater airway hyperreactivity in OVA-induced asthma (Whitehead et al., 2003). These phenotypic differences in immune responses predispose the FVB line to heightened immune activation. However, it remains unknown whether this heightened immune response in FVB strains will result in greater behavioral deficits in offspring following MAA.
To this end, we explored whether genetic predisposition in FVB strains results in greater offspring behavioral deficits following MAA exposure. Two highly social strains of mice, C57 and a sighted hybrid FVB.129P2-Pde6b(+) Tyr(c-ch)/Ant (FVB/Ant), were selected for their distinct genetic backgrounds and immune profiles. C57 and FVB lines are widely studied inbred strains and known to display typical social and repetitive behaviors (Moy et al., 2007). FVB/Ant mice are a sighted hybrid of the FVB/NJ, characterized by high sociability and low self-grooming, comparable to the C57 strain, and this strain has previously been used as a highly social control strain (Yang et al., 2013). Offspring of OVA- and PBS-treated dams were assessed for changes in sociability at a juvenile age using the reciprocal social interaction task. This behavioral paradigm has the benefit of measuring complex and intricate social behaviors, providing new insights into underlying features of social interaction. While this task often requires a trained observer, demanding significant human resources and increased sensitivity to human error (Silverman et al., 2010), we employed automated tracking technology to produce unbiased measures of juvenile play behavior in offspring. Then, mice were measured for changes in restricted/repetitive behaviors using the self-grooming and marble burying tasks. In addition, differences in cytokine responses were assessed in pregnant dams using multiplex bead-based arrays.
2.0 MATERIALS AND METHODS
2.1 Animals
Male and Female C57Bl/67 (Jackson Laboratory, Bar Harbor, Maine USA & Sacramento, CA) and FVB/Ant (Jackson Laboratory, Bar Harbor, Maine) mice were bred and maintained at Mount Holyoke College and the Center for Laboratory Animal Research, at the University of California, Davis. Mice were maintained at ambient room temperature on a 12 h light/dark cycle (lights on at 0800 hours). Each strain was housed separately in standard plastic cages with same-sex littermates, and food and water were provided ad libitum. Mice were group-housed 2–4 per cage and pregnant female mice were single housed following the start of pregnancy. All mice were provided nestlets for enrichment. Behavioral procedures were performed during the first 4 h of the light cycle and all procedures were approved by Mount Holyoke College, Institutional Animal Care and Use Committee and the University of California, Davis Institutional Animal Care and Use Committee in accordance with the guidelines provided by the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
2.2 Maternal allergy/asthma induction
Sexually naïve female C57 and FVB/Ant mice were randomly assigned to either the allergic asthma (OVA) or the control group (PBS) and sensitized with 10 µg ovalbumin (OVA, Sigma, St Louis, MO, USA) in 1 mg (Al)OH3 (InvivoGen, San Diego, CA, USA) dissolved in 200 µl phosphate-buffered saline (PBS) or vehicle alone on postnatal day (P)42 and again 1 week later at P49. One week following the second sensitization period, mice were mated overnight and females were checked daily for the presence of seminal plugs, noted as gestational day 0.5 (G0.5). Pregnant mice were exposed to either an aerosolized solution of 1% (wt/vl) OVA in PBS (OVA group) or PBS control for three 45-minute induction sessions throughout gestation. Specifically, these induction sessions occurred at gestational days 9.5, 12.5, and 17.5, to correspond with early, middle, and late gestation as previously described (Schwartzer et al., 2015). Four hours after the final induction, 100µl of blood was drawn and collected from the saphenous vein for cytokine analysis and mice were returned to their home cages, single housed, and left undisturbed until the birth of their litters. Pups remained with their mother until weaning on P21, at which time the offspring were group housed with same-sex littermates. A total of 34 C57 dams (17 PBS and 17 OVA) were used to generate 152 offspring (67 PBS, 85 OVA) and a total of 28 FVB/Ant dams (10 PBS and 12 OVA) were used to generate 63 offspring (25 PBS, 38 OVA) (Table 1).
Table 1.
Sample size of litters and offspring
| PBS | OVA | |||||
|---|---|---|---|---|---|---|
| Offspring | Offspring | |||||
| Strain | Dams | Dams | ||||
| Male | Female | Male | Female | |||
| C57 | 17 | 41 | 26 | 17 | 49 | 36 |
| FVB/Ant | 10 | 15 | 10 | 12 | 32 | 6 |
2.3 Behavioral Assessments - Experimental Design
Experimental offspring were generated from two laboratories (Schwatzer – Mount Holyoke College; Ashwood – University of California, Davis). Juvenile reciprocal social interaction (Mount Holyoke College) was assessed at P21 following weaning. Then, adult offspring (Mount Holyoke and University of California Davis) were evaluated for repetitive, anxiety and motor behaviors beginning at 8 weeks of age. All procedures were run in parallel at both institutions using both strains and the same experimental procedures.
2.3.1 Juvenile Reciprocal Social Interaction
On P21, experimental offspring and stimulus mice were weaned, ear notched, and evaluated for changes in interactive social behavior between offspring of OVA and PBS-exposed dams using the reciprocal social interaction (RSI) task. Mice were acclimated to the experimental room in their home cage for 30 min prior to the RSI task (McFarlane et al., 2008). Then, experimental mice were placed into clean plastic cages (40 × 50 × 20cm) and allowed to habituate for 20 minutes. Following habituation, experimental mice were quickly returned to their home cage and marked with blue (experimental) or pink (stimulus C57 mice) hair chalk for automated detection. Experimental mice were placed back into the arena along with a novel C57 stimulus mouse and the dyads were video recorded while freely interacting for a 30-minute period. Mice were analyzed for discrete social interactions using EthoVision XT 11.5 with the social interaction and three-point body modules. Behaviors assessed included nose-to-nose, body sniff, nose-to-tail, and following behaviors.
2.3.2 Grooming
Mice were placed inside an empty, clear plastic cage and left undisturbed to habituate for 10 min in a dimly lit room as previously described (McFarlane et al., 2008). Following the habituation period, mice were video recorded for an additional 10 min and later hand scored for self-grooming behavior by two individuals blind to the treatment conditions. Grooming was defined as time spent licking paws, washing the nose and face, or scratching fur with any foot. Inter-rater reliability was confirmed using an interclass-correlation coefficient to be greater than 95%
2.3.3 Marble burying
Repetitive marble burying behavior was assessed using procedures modified from Deacon (2006). Mice were habituated for 10 min to a clean Plexiglas cage (37 × 14 × 12.5 cm) filled with a 4-cm thick layer of clean corncob bedding. Following habituation, animals were returned to their home cage and 15 glass marbles were laid out in five rows of three marbles placed equidistantly apart. Mice were then returned to their cages and allowed to explore under dim illumination for 10 min. At the end of the 10-min period, animals were gently removed from the testing cages and the number of marbles buried was recorded. Only marbles covered by 75% or more bedding were counted as buried, as previously described.
2.3.4 Elevated Plus-Maze
Experimental offspring were evaluated for changes in general anxiety using an elevated plus-maze as previously described (Walf and Frye, 2007). Mice were placed in the central platform and allowed to freely explore the maze for 5 min. Behaviors were video recorded and later analyzed using EthoVision XT 11.5 for the number of entries into each arm and the percentage of time spent in open arms. Reductions in open arm exploration were interpreted as increased anxiety.
2.3.5 Open Field Exploration and Locomotor Activity
Mice were individually placed in an empty Plexiglas arena (30 cm × 30 cm × 38 cm) and allowed to freely explore the environment for 20 min. Horizontal locomotor activity was assessed using Ethovision XT 11.5 to measure the time in the center, total distance traveled, and average velocity during the 20-min exploration task.
2.4 Serum Cytokine Analysis
In order to minimize the effects of maternal stress on pup development, serum was collected from a separate cohort of pregnant dams whose offspring were not assessed behaviorally. 100µl of blood was collected from pregnant dams four hours after the last induction of OVA or PBS via lateral saphenous vein. Whole blood was centrifuged at 1000g for 10 min and plasma was collected and stored at −80°C. Quantification of IL-1β, IL-2, IL-4, IL-5, IL-6, IL-10, IL-13, IL-17, IFNγ, and TNFα was carried out using a custom murine multiplexing bead immunoassay (Millipore, Billerica, MA, USA) per manufacturer’s specifications. 25µl of plasma was incubated with antibody-coupled beads. After a series of washes, a biotinylated detection antibody was added to the beads, and the reaction mixture was detected by the addition of streptavidin–phycoerythrin. The bead sets were analyzed using a flow-based Luminex 100 suspension array system (Bio-Plex 200; Bio-Rad Laboratories, Hercules, CA, USA). Unknown sample cytokine concentrations were calculated by Bio-Plex Manager software using a standard curve derived from the known reference cytokine concentrations supplied by the manufacturer. A five-parameter model was used to calculate final concentrations and values were expressed in pg/ml. The sensitivity of this assay allowed the detection of cytokine concentrations with the following limit of detection: IL-1β (5.4 pg/ml), IL-2 (1.0pg/ml), IL-4 (0.4 pg/ml), IL-5 (1.0 pg/ml), IL-6 (1.1), IL-10 (2.0 pg/ml), IL-13 (7.8 pg/ml), IL-17 (0.5 pg/ml), IFNγ (1.1 pg/ml), and TNFα (2.3 pg/ml). Concentrations obtained below the limit of detection were assigned a value of one-half the limit of detection for statistical comparisons. Plasma aliquots had not undergone any previous freeze/thaw cycle.
2.5 Statistical analysis
Data were analyzed with R statistical software version 3.2.1 (2015) with the “nlme” package. Behavioral data were analyzed using linear mixed-effects models with maximum likelihood estimates, Type III sums of squares, Kenward-Roger degrees of freedom approximations. Treatment, strain, and sex were set as fixed factors and litter and location (University of California, Davis or Mount Holyoke) as random effects. Each mouse was nested within litter and litters were nested in location. Post hoc analyses were conducted using pairwise comparisons with bonferroni corrections. Cytokine data were log transformed to allow for parametric analysis and analyzed by two-way analysis of variance (strain by treatment) followed by Tukey post hoc analysis when applicable.
3.0 RESULTS
3.1 Juvenile Reciprocal Social Interaction
Juvenile social interaction parameters were assessed using generalized least squares models followed by adjustments for random effects. There was a significant treatment by strain interaction in the total time mice spent engaged in all social behaviors, F(1, 141) = 27.16, p < 0.0001. The addition of sex did not further improve the model, χ2(9) = 6.66, p = 0.16, and no sex differences were detected between groups F(1, 138) = 2.33, p = 0.13. C57 offspring of OVA-treated dams spent significantly less time engaged in social interactions with a novel stimulus mouse compared to C57 offspring from control dams, p < 0.01 (Figure 1A). Conversely in FVB/Ant offspring, OVA treatment resulted in significantly elevated time engaged in social interactions compared to FVB/Ant offspring from PBS-treated dams, p < 0.001 (Figure 1B). Importantly, when adjusting for litter effects our overall model was improved, χ2(6) = 45.69, p < 0.001, but the adjusted treatment by strain interaction effect no longer reached statistical significance, F(1, 18), = 3.48, p = 0.07.
Figure 1.
Strain-specific changes in juvenile social interactions in MAA offspring. (A) C57 offspring of OVA-treated dams displayed significant reductions in the total time engaged with a novel stimulus mouse compared to offspring of PBS-treated dams. (B) Conversely, FVB/Ant offspring born to MAA dams engaged in significantly more social interactions with a novel C57 juvenile compared to strain-matched offspring of control dams. MAA exposure resulted in (C) reduced sniffing in C57 offspring and (D) increased body sniffing in FVB/Ant offspring during the juvenile reciprocal social interaction task. No difference in following behavior was observed between treatment conditions in either (E) C57 or (F) FVB/Ant strains. * p < 0.05
Differences in social interaction were most apparent in the time MAA offspring spent sniffing the body, F(1, 141) = 32.74, p < 0.001, and anogenital sniffing, F(1, 141) = 31.44, p < 0.001, with treatment by strain interactions observed in both C57 (less sniffing after OVA exposure) and FVB/Ant (more sniffing after OVA exposure) strains (p < 0.001 for all comparisons) (Figure 1C–D). These differences remained significant when adjusted for variations in litter, p = 0.05. Importantly, not all social behaviors differed between treatment conditions. The total time juvenile mice engaged in following behaviors was similar across both strains and treatment conditions, F(1, 18) = 1.44, p > 0.05 (Figure 1E–F).
3.2 Repetitive Grooming and Marble Burying Behaviors
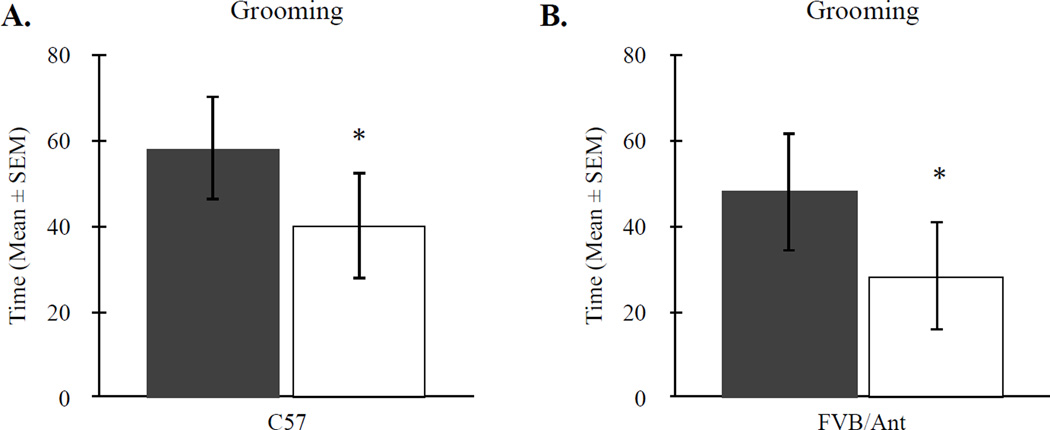
To assess the presence of strain-specific differences in repetitive behavior between offspring of OVA and PBS-exposed dams, male and female mice were measured for the amount of time spent grooming during a ten-minute period. Linear mixed-effects model revealed a significant main effect for strain, F(1, 103) = 7.143, p < 0.01, with C57 mice spending more time grooming (48.15 ± 4.629) compared to FVB/Ant (37.13 ± 5.235) mice. Moreover, there was a significant effect of treatment, F(1, 103) = 4.6511, p < 0.05. Both C57 and FVB/Ant offspring of OVA-treated dams engaged in significantly reduced self-grooming compared to offspring of PBS-treated mice (Figure 2). These differences were independent of strain as indicated by a non-significant strain by treatment interaction, F(1,12) = 0.006, p > 0.05. Moreover, there were no differences between sex, F(1,12) = 0.213, p > 0.05, and no sex by treatment interactions, F(1,12) = 0.120, p > 0.05.
Figure 2.
Changes in restricted/repetitive behaviors in offspring of OVA-treated dams. (A) MAA exposure significantly reduced the total time mice spent grooming during a 10-minute period regardless of strain. (B) There was a significant increase in the percentage of marbles buried for both C57 and FVB/Ant offspring of OVA-treated dams compared to PBS-treated controls. * p < 0.05
In addition to grooming, mice were assessed for repetitive digging behavior in the marble burying task. There was a significant difference in percent of marbles buried between C57 and FVB/Ant mice, F(1,12) = 20.65, p < 0.01, with C57 mice burying fewer marbles (10.9% ± 3.38) compared to FVB/Ant mice (47.07% ± 4.43). There was also a significant main effect for treatment, F(1, 12) = 5.32, p < 0.05. Offspring of OVA-treated dams buried more marbles (39.38% ± 5.48) compared to offspring of PBS-exposed dams (23.19% ± 4.43). These treatment differences were similar between the two strains, F(1,12) = 2.87, p > 0.05
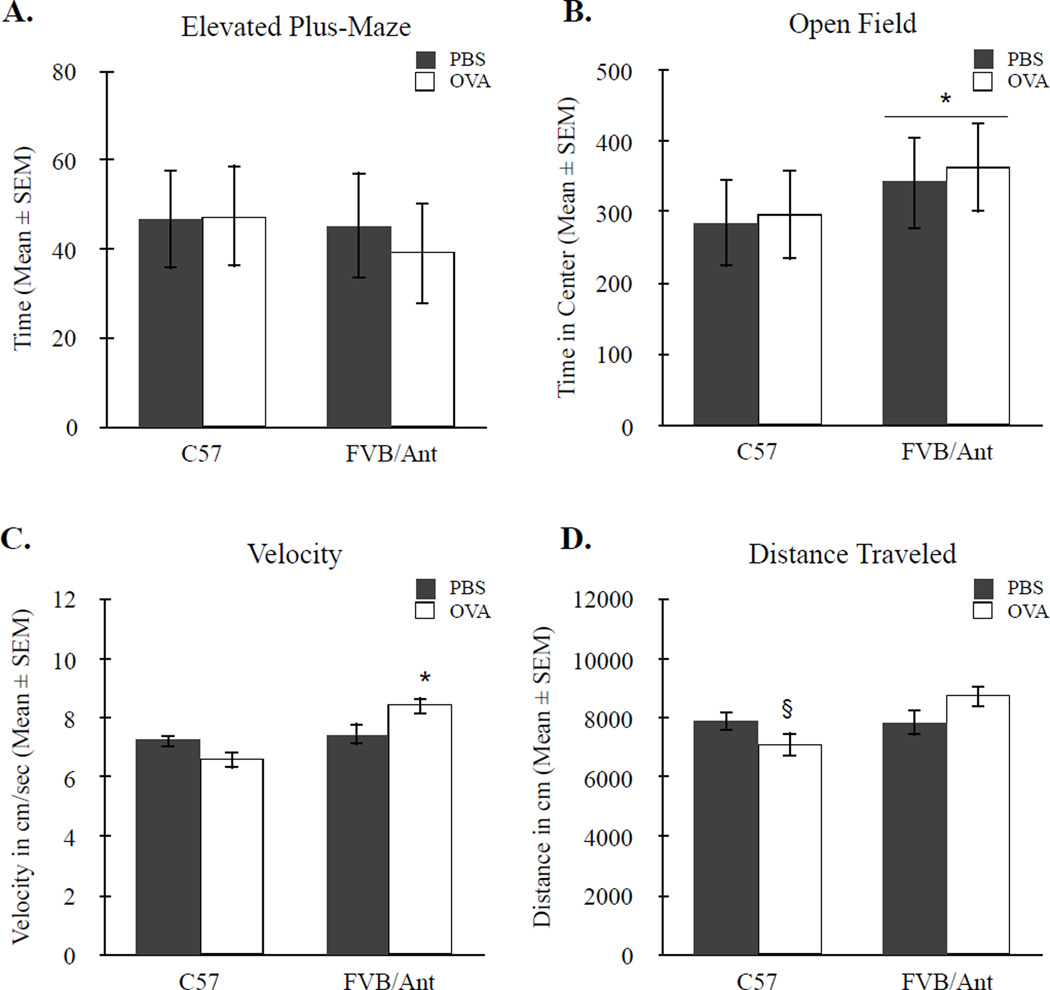
3.3 Anxiety-like behaviors
Mice were assessed for changes in anxiety-like behaviors in the elevated-plus maze and open field tasks. There were no differences between strains, F(1, 20) = 0.156, p > 0.05, and between treatment conditions, F(1, 20) = 0.956, p > 0.05, in the percent of time spent exploring the open arms of the elevated plus-maze (Figure 3A). There was also no treatment by strain interaction, F(1, 20) = 0.627, p > 0.05. Interestingly, in the open field task, FVB/Ant mice spent more time exploring the center arena compared to C57 mice, F(1,18) = 5.013, p < 0.05 (Figure 3B). These differences in open field exploration were independent of treatment as noted by a non-significant main effect for treatment, F(1, 18) = 0.266, p > 0.05, and a non-significant treatment by strain interaction, F(1, 18) = 0.036, p > 0.05. Together, these data indicate that changes in social and grooming behavior observed in offspring of OVA-exposed dams are not driven by increases in anxiety.
Figure 3.
Assessment of anxiety and locomotor behaviors. (A) No differences were observed across strains or treatment conditions in the percent of time spent in the open arms of the elevated plus-maze. (B) FVB/Ant mice spent significantly more time in the center of the open field arena compared to C57 mice, suggesting that FVB/Ant mice may have reduced baseline anxiety levels. (C) There was a significant increase in velocity for MAA FVB/Ant offspring and (D) a trend towards significant reduction in total distance traveled among C57 offspring of MAA dams. * p < 0.05, § p < 0.06.
3.4 Locomotor
To determine whether maternal allergic asthma alters motor activity in either strain, mice were assessed for locomotor activity in the open field arena. There was a main effect in average velocity between strains F(1,18) = 23.67 p < 0.001 with FVB/Ant mice moving at a greater velocity than C57 mice. There was also a significant strain by treatment interaction F(1,18) = 7.879, p < 0.05. Specifically, there was a significant increase in average velocity of FVB/Ant offspring of OVA dams compared to FVB/Ant PBS-controls, t(18) = 2.136, p < 0.05 (Figure 3C).
Similarly, for total distance traveled in a 20-minute period, there was a significant main effect for strain F(1,18) = 14.3, p < 0.01. These strain differences are likely a result of changes in motor activity observed between treatment conditions as reflected in a significant strain by treatment interaction F(1, 18) = 7.0029, p < 0.05. Post hoc analysis revealed a trend towards significant treatment effect in C57 mice, t(18) = −1.989, p = 0.06, with OVA-exposed offspring moving less (7084cm ± 388) than PBS mice (7878cm ± 328) (Figure 3D). Importantly, when including velocity as a covariate, these differences were no longer present, indicating that changes in distance traveled are a result of overall difference in velocity.
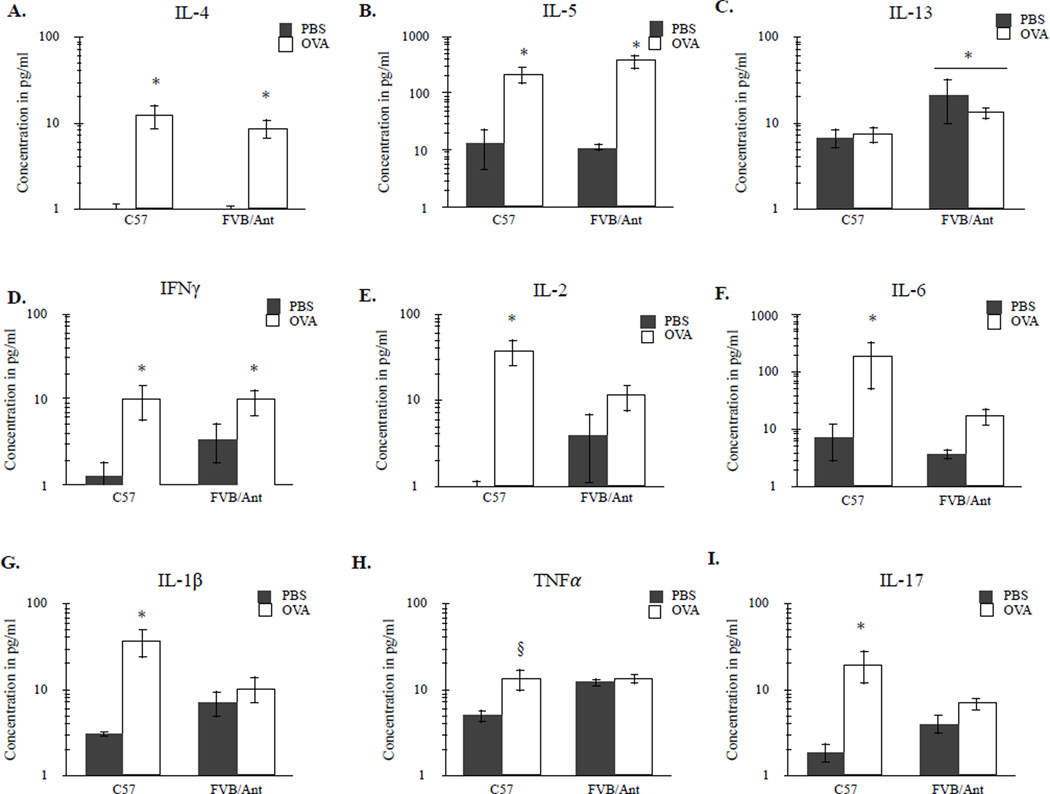
3.5 Maternal Cytokine Analysis
Following OVA-induction, C57 and FVB/Ant dams expressed elevated levels of key TH2-mediated cytokines, including IL-4, F(1, 34) = 53.96 p < 0.001, and IL-5, F(1, 34) = 57.78, p < 0.001, but not IL-13, F(1, 34) = 0.17, p = 0.68. These increases in signaling factors were conserved between strains (Figure 4A, 4B) as indicated by a null treatment by strain interaction, IL-4, F(1, 34) = 1.51, p = 0.40; IL-5, F(1, 34) = 0.31, p = 0.58. Interestingly, FVB/Ant mice showed elevated baseline levels of IL-13 compared to C57 mice, F(1, 34) = 12.44, p < 0.01 (Figure 4C) which was apparent regardless of treatment groups. Increased levels of the TH1-associated cytokine IFNγ was also observed in OVA-treated dams, F(1, 34) = 4.97, p < 0.05, regardless of strain, F(1, 34) = 0.92, p = 0.34 (Figure 4D). There was a significant strain by treatment interaction for IL-2, F(1, 34) = 8.82, p < 0.01; IL-6, F(1, 34) = 4.74, p < 0.05; and IL-1β, F(1, 34) = 4.94, p < 0.05, along with a marginally significant strain by treatment interaction for TNFα, F(1, 34) = 3.92, p = 0.056. These strain by treatment interactions were reflected in elevated signaling in C57 dams (p = 0.06 for TNFα, p < 0.001 for all other cytokines measured) but not FVB/Ant mice (Figure 4E–4H). Similarly, a strain by treatment interaction was detected for IL-17, F(1, 34) = 6.48, p < 0.05, with only OVA-induced C57 dams producing significantly elevated IL-17 levels (p < 0.001) (Figure 4I). Finally, there was a significant main effect for treatment in the levels of anti-inflammatory IL-10, F(1, 34) = 14.47, p < 0.001, with OVA-induced dams expressing elevated responses compared to PBS-treated controls (p < 0.05).
Figure 4.
Cytokine signaling in serum of dams following OVA or PBS induction on gestational day 17. Maternal serum of OVA-treated dams in both C57 and FVB/Ant strains contained elevated levels of (A) IL-4, and (B) IL-5 compared to strain-matched PBS controls. (C) FVB/Ant mice had higher levels of circulating IL-13 compared to C57 mice and these differences were independent of treatment condition. (D) Both C57 and FVB/Ant mice exposed to aerosolized-OVA expressed higher levels of IFNγ compared to PBS-treated control dams. C57 dams treated with OVA, but not FVB/Ant dams, showed increased levels of (E) IL-2, (F) IL-6, (G) IL-1β, (H) TNFα and (I) IL-17 compared to PBS-treated dams. * p < 0.05, § p = 0.06
4.0 DISCUSSION
Activation of the mother’s immune system during pregnancy is a well-established risk factor for neurodevelopmental disorders (for review see (Knuesel et al., 2014). Numerous epidemiological findings and animal model studies have noted that the inflammatory signals, not the specific pathogen, contribute to the changes in fetal brain development (Boksa, 2010; Brown, 2012). Most preclinical investigations of maternal immune activation have relied exclusively on the use of viral and bacterial agents, most notably poly(I:C) and LPS, to identify behavioral consequences of systemic prenatal infection (Meyer, 2013). While these findings demonstrate a link between maternal immune activation and behavioral deficits, they are limited in identifying whether other immunogenic agents, particularly those stimuli that are currently on the rise such as allergy and asthma, equally impact neurodevelopmental and neuropsychiatric disease prevalence. Importantly, allergy/asthma responses represent a distinct immune response driven by TH2 immune cascades, raising the question of whether TH2-driven immune activation imparts similar neurodevelopmental alterations.
Using a recently developed animal model of maternal allergic asthma (Schwartzer et al., 2015), pregnant dams of two immunologically distinct backgrounds (i.e. C57 and FVB) underwent repeated OVA-inductions and their sera analyzed for differences in immunogenic phenotype between the two strains. Mice in our MAA model show elevated IL-4, IL-5, and IFNγ, but no treatment effects for IL-13. Although IL-4, IL-5, and IL-13 are canonical TH2 cytokines associated with allergy/asthma responses, and IFNγ is traditionally associated with TH1 responses, IFNγ signaling is important for maintaining chronic allergic inflammation (Ngoc et al., 2005). Clinical reports identified elevations in IL-4, IL-5, and IFNγ, but not IL-13, in mothers who later had a child diagnosed with ASD (Goines et al., 2011), suggesting chronic allergic/asthma inflammation as a mediating factor in neurodevelopmental disorders. The increased levels if IL-4, IL-5, and IFNγ observed in MAA dams closely parallels the clinical findings (Goines et al., 2011) and corroborate our model as a valid paradigm for testing the effects of MAA during mid to late gestation. In fact, mouse serum was collected at 17 days gestation, following the third induction. This period of development is comparable to the mid- to late-second trimester in humans (Clancy et al., 2001), the time period identified to correlate with ASD risk (Goines et al., 2011). Interestingly, while TH2-associated cytokines were observed to be elevated in OVA-treated dams of both strains, elevations in innate and TH17-associated signals following OVA induction were more apparent in the C57 strain. In particular, IL-1β, TNFα, IL-6, and IL-17 were significantly elevated in C57 OVA-dams but not in FVB/Ant OVA-dams. These differences in cytokine responses between FVB/Ant and C57 strains suggest that FVB/Ant mice may possess a more skewed TH2 phenotype in response to allergic/asthma inductions compared to C57 mice that show both TH1/inflammatory and TH2-driven responses. Importantly, increased levels of both IL-6 and IL-17 have been implicated in eliciting ASD-like behaviors in mouse models of viral/bacterial immune activation (Choi et al., 2016; Smith et al., 2007) and IL-6 is an important effector signal in the development of TH17 T-cell responses (Bettelli et al., 2006). However, both FVB/Ant and C57 offspring of MAA dams elicited alterations in social and repetitive behaviors, even though FVB/Ant dams did not express elevations in IL-6 and IL-17 following OVA treatment. These data suggest that other signaling factors that may mediate TH2-responses, as well as differences in signaling factors observed in maternal innate immune activation models, can contribute to changes in offspring behavior through hitherto unexplored pathways. Moreover, differences in immune system responses between strains underscores the importance of considering strain selection when utilizing the OVA-induction model to measure the impact of TH2 over-activation in offspring development.
Behavioral measures from MAA offspring corroborate previous mouse model findings linking maternal allergic asthma to altered patterns of restructured/repetitive behaviors (Schwartzer et al., 2015) and extend this model by identifying altered patterns of social interaction present at a juvenile age. Animal models of autism and other neurodevelopmental disorders, including models of maternal immune activation, have relied on the social approach tasks to identify changes in social behavior deficits (Crawley, 2007; Meyer, 2013). Although this assay has the benefit of high-throughput and unbiased computer tracking, it is limited in identifying specific changes in the quality of social interaction as conspecifics are restricted by a tether or cage that may interfere with the naturalistic dynamic of social interactions (Nadler et al., 2004). The reciprocal social interaction task promotes more complex and intricate social behaviors, providing new insights into underlying features of social interaction. Offspring of maternal allergic/asthma dams exhibited altered reciprocal social interaction at the juvenile age. At this prepubertal period, social interactions are not directly driven by reproductive, territorial, or parenting processes and rough-and-tumble play is uncommon (Pellis and Pasztor, 1999; Wolff, 1981). Therefore, studying social interactions at this young age affords the benefit of identifying disturbances in basic social processes that are not directly related to resource acquisition.
Maternal allergic asthma offspring of both FVB/Ant and C57 strains showed alterations in juvenile social interactions, but these differences occurred in opposing directions. Specifically, C57 MAA offspring engaged in reduced social interaction with a conspecific, suggesting the presence of social behavior deficits at an early age. Conversely, FVB/Ant offspring of MAA dams engaged in significantly more social interactions compared to strain matched control mice. These strain-dependent differences in social interaction may reflect differences in stress responding following weaning. While the two background strains were selected given their previously reported similarities in sociability and anxiety-like behaviors (Moy et al., 2007), the specific genetic lines used to generate the FVB/Ant mice included outcrossing with a 129-background (Errijgers et al., 2007), a strain previously reported to show differences in stress responses (Moloney et al., 2015). Therefore, it remains unknown whether strain-specific differences in social behavior observed in our MAA model may be dependent on differences in responses to stress following the weaning process.
The increased social engagement observed in FVB/Ant offspring of OVA-treated dams may reflect deficits in processing social cues from stimulus mice. That is, increases in social interaction time in FVB/Ant mice of MAA dams may be a result of persistent/excessive social engagement when other mice may retreat in response to dominance or hierarchical social cues from a conspecific. Importantly excessive sociability is observed in some neurodevelopmental disorders, such as William's syndrome (Gosch and Pankau, 1994), and increased sociability has been reported in mouse models of neurodevelopmental disorders (Sakurai et al., 2011; Young et al., 2008) highlighting both excessive and reduced social interactions as atypical social behavior profiles (Barak and Feng, 2016). While it is tempting to conclude that the social behavior deficits in MAA offspring are strain-specific, the use of C57 mice as stimulus partners for all social dyads limits this notion.. Bolivar et al (Bolivar et al., 2007) demonstrated that social interaction time is dependent on the strain of the partner. That is, the time a mouse spends engaged in social sniffing is dependent on whether the stimulus mouse is a highly social or low social strain. In our MAA paradigm, mice from all groups were paired with an untreated C57 stimulus mouse. This experimental approach has the benefit of controlling for variability in social stimuli by providing all mice with a similar conspecific. As a result, differences in social behavior between treatment conditions are unlikely a result of differences in the sociability of the stimulus mouse. However, the high sociability of C57 stimulus mice may have masked some of the treatment and strain effects that would have been more easily detected if mice were paired with a novel stimulus of the same treatment and strain. Follow-up studies should consider pairing offspring of MAA exposed dams to other novel strain-matched MAA offspring from a different dam to further elucidate differences in social engagement.
Aside from changes in social behavior, analysis of restricted/repetitive behaviors revealed consistent treatment effects across both strains, with offspring of OVA-treated dams burying significantly more marbles compared to PBS controls. Conversely, both FVB/Ant and C57 offspring of OVA-dams engaged in significantly less stereotypical grooming behavior compared to control mice of the same strain. These findings are consistent with previous work characterizing the behavioral effects of maternal allergic asthma (Schwartzer et al., 2015). The discrepancies in these repetitive grooming and marble burying tasks have been previously observed (Muehlmann et al., 2012; Sungur et al., 2014) and underscore the differences in neuronal mechanisms that drive these behaviors. For example, changes in serotonin signaling are associated with altered repetitive marble burying behavior (Ichimaru et al., 1995), and increases in marble burying, but not grooming, have been correlated with elevated level of the serotonin transporter protein in the brains of mice born to maternal allergic asthma primed dams (Schwartzer et al., 2015). Importantly, these behavioral changes were observed in the absence of changes in anxiety-like measures, indicating that social and repetitive behavioral changes are unlikely confounded by increased arousal. Overall, the similar patterns of repetitive behavior changes across both FVB/Ant and C57 strains suggest that the mechanisms at play contributing to these behavioral changes are independent of genetics or that the genetic factors contributing to MAA-induced alterations in grooming and marble burying are conserved across these two strains.
Taking together the differences in offspring behavioral profiles along with cytokine responses to MAA induction between strains, our data suggest the presence of gene by environment interactions that influence the effects of maternal immune activation on offspring behavioral development. In particular, we highlight altered social phenotypes, beginning at the juvenile period, between C57 and FVB/Ant offspring of OVA-treated dams. These synergistic effects did not exacerbate restricted repetitive behaviors, raising the question of whether different cytokine signals in the mother are responsible for distinct ASD-like behaviors in offspring. Importantly, experimental procedures were conducted in parallel across two institutions, with all four experimental groups evenly distributed across both institutions. Differences in behavioral outcomes in mouse studies have been well noted to occur between institutions, even when procedures and environmental conditions are well controlled (Crabbe et al., 1999). Our data overcome this potential confound by demonstrating significant behavioral alterations in MAA-exposed offspring after controlling for random differences between institutions statistically. In fact, Wahlsten et al. (2003)) note the robust nature of strain-differences in behavioral outcomes across laboratories. These limitations notwithstanding, our data underscore the importance of strain selection in the development of a mouse model of MAA and support the ongoing hypothesis linking ASD to a combination of genetic predispositions and environmental insults.
Highlights.
Compared to C57 dams, FVB/Ant mice exhibit a TH2 response to allergic asthma induction
Altered social interactions are strain dependent in maternal allergic asthma offspring
Maternal OVA-induction decreases grooming and increases marble burying behavior in offspring
Acknowledgments
The authors would like to acknowledge Katherine Suen, Samantha Bilton, Felicity Emerson, Megan Johnson, and Maddy Berkowitz-Cerasano for their technical expertise. The authors further acknowledge support from NIH R15HD082638, U54HD079125, NARSAD Foundation, Jane Botsford Johnson Foundation, Peter Emch Foundation, and Autism Speaks Foundation (#7567).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflicts of interest.
REFERENCES
- Abdallah MW, Larsen N, Grove J, Norgaard-Pedersen B, Thorsen P, Mortensen EL, Hougaard DM. Amniotic fluid inflammatory cytokines: potential markers of immunologic dysfunction in autism spectrum disorders. The world journal of biological psychiatry : the official journal of the World Federation of Societies of Biological Psychiatry. 2013;14:528–538. doi: 10.3109/15622975.2011.639803. [DOI] [PubMed] [Google Scholar]
- Association, A.P., editor. Diagnostic and statistical manual of mental disorders. 5th 2013. [Google Scholar]
- Atladottir HO, Pedersen MG, Thorsen P, Mortensen PB, Deleuran B, Eaton WW, Parner ET. Association of family history of autoimmune diseases and autism spectrum disorders. Pediatrics. 2009;124:687–694. doi: 10.1542/peds.2008-2445. [DOI] [PubMed] [Google Scholar]
- Barak B, Feng G. Neurobiology of social behavior abnormalities in autism and Williams syndrome. Nat Neurosci. 2016;19:647–655. doi: 10.1038/nn.4276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- Boksa P. Effects of prenatal infection on brain development and behavior: a review of findings from animal models. Brain, behavior, and immunity. 2010;24:881–897. doi: 10.1016/j.bbi.2010.03.005. [DOI] [PubMed] [Google Scholar]
- Bolivar VJ, Walters SR, Phoenix JL. Assessing autism-like behavior in mice: variations in social interactions among inbred strains. Behav Brain Res. 2007;176:21–26. doi: 10.1016/j.bbr.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AS. Epidemiologic studies of exposure to prenatal infection and risk of schizophrenia and autism. Developmental neurobiology. 2012 doi: 10.1002/dneu.22024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi GB, Yim YS, Wong H, Kim S, Kim H, Kim SV, Hoeffer CA, Littman DR, Huh JR. The maternal interleukin-17a pathway in mice promotes autism-like phenotypes in offspring. Science. 2016;351:933–939. doi: 10.1126/science.aad0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clancy B, Darlington RB, Finlay BL. Translating developmental time across mammalian species. Neuroscience. 2001;105:7–17. doi: 10.1016/s0306-4522(01)00171-3. [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Wahlsten D, Dudek BC. Genetics of mouse behavior: interactions with laboratory environment. Science. 1999;284:1670–1672. doi: 10.1126/science.284.5420.1670. [DOI] [PubMed] [Google Scholar]
- Crawley JN. Mouse behavioral assays relevant to the symptoms of autism. Brain Pathol. 2007;17:448–459. doi: 10.1111/j.1750-3639.2007.00096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croen LA, Grether JK, Yoshida CK, Odouli R, Van de Water J. Maternal autoimmune diseases, asthma and allergies, and childhood autism spectrum disorders: a case-control study. Arch Pediatr Adolesc Med. 2005;159:151–157. doi: 10.1001/archpedi.159.2.151. [DOI] [PubMed] [Google Scholar]
- Deacon RM. Digging and marble burying in mice: simple methods for in vivo identification of biological impacts. Nat Protoc. 2006;1:122–124. doi: 10.1038/nprot.2006.20. [DOI] [PubMed] [Google Scholar]
- Errijgers V, Van Dam D, Gantois I, Van Ginneken CJ, Grossman AW, D'Hooge R, De Deyn PP, Kooy RF. FVB.129P2-Pde6b(+) Tyr(c-ch)/Ant, a sighted variant of the FVB/N mouse strain suitable for behavioral analysis. Genes Brain Behav. 2007;6:552–557. doi: 10.1111/j.1601-183X.2006.00282.x. [DOI] [PubMed] [Google Scholar]
- Goines PE, Croen LA, Braunschweig D, Yoshida CK, Grether J, Hansen R, Kharrazi M, Ashwood P, Van de Water J. Increased midgestational IFN-gamma, IL-4 and IL-5 in women bearing a child with autism: A case-control study. Molecular autism. 2011;2:13. doi: 10.1186/2040-2392-2-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosch A, Pankau R. Social-emotional and behavioral adjustment in children with Williams-Beuren syndrome. Am J Med Genet. 1994;53:335–339. doi: 10.1002/ajmg.1320530406. [DOI] [PubMed] [Google Scholar]
- Hallmayer J, Cleveland S, Torres A, Phillips J, Cohen B, Torigoe T, Miller J, Fedele A, Collins J, Smith K, Lotspeich L, Croen LA, Ozonoff S, Lajonchere C, Grether JK, Risch N. Genetic heritability and shared environmental factors among twin pairs with autism. Arch Gen Psychiatry. 2011;68:1095–1102. doi: 10.1001/archgenpsychiatry.2011.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichimaru Y, Egawa T, Sawa A. 5-HT1A-receptor subtype mediates the effect of fluvoxamine, a selective serotonin reuptake inhibitor, on marble-burying behavior in mice. Jpn J Pharmacol. 1995;68:65–70. doi: 10.1254/jjp.68.65. [DOI] [PubMed] [Google Scholar]
- Jiang HY, Xu LL, Shao L, Xia RM, Yu ZH, Ling ZX, Yang F, Deng M, Ruan B. Maternal infection during pregnancy and risk of autism spectrum disorders: A systematic review and meta-analysis. Brain, behavior, and immunity. 2016 doi: 10.1016/j.bbi.2016.06.005. [DOI] [PubMed] [Google Scholar]
- Knuesel I, Chicha L, Britschgi M, Schobel SA, Bodmer M, Hellings JA, Toovey S, Prinssen EP. Maternal immune activation and abnormal brain development across CNS disorders. Nat Rev Neurol. 2014;10:643–660. doi: 10.1038/nrneurol.2014.187. [DOI] [PubMed] [Google Scholar]
- Krakowiak P, Goines PE, Tancredi DJ, Ashwood P, Hansen RL, Hertz-Picciotto I, Van de Water J. Neonatal Cytokine Profiles Associated with Autism Spectrum Disorder. Biological psychiatry. 2015 doi: 10.1016/j.biopsych.2015.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyall K, Ashwood P, Van de Water J, Hertz-Picciotto I. Maternal immune-mediated conditions, autism spectrum disorders, and developmental delay. J Autism Dev Disord. 2014;44:1546–1555. doi: 10.1007/s10803-013-2017-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarlane HG, Kusek GK, Yang M, Phoenix JL, Bolivar VJ, Crawley JN. Autism-like behavioral phenotypes in BTBR T+tf/J mice. Genes Brain Behav. 2008;7:152–163. doi: 10.1111/j.1601-183X.2007.00330.x. [DOI] [PubMed] [Google Scholar]
- Meyer U. Prenatal Poly(I:C) Exposure and Other Developmental Immune Activation Models in Rodent Systems. Biol Psychiatry. 2013 doi: 10.1016/j.biopsych.2013.07.011. [DOI] [PubMed] [Google Scholar]
- Moloney RD, Dinan TG, Cryan JF. Strain-dependent variations in visceral sensitivity: relationship to stress, anxiety and spinal glutamate transporter expression. Genes Brain Behav. 2015;14:319–329. doi: 10.1111/gbb.12216. [DOI] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Young NB, Perez A, Holloway LP, Barbaro RP, Barbaro JR, Wilson LM, Threadgill DW, Lauder JM, Magnuson TR, Crawley JN. Mouse behavioral tasks relevant to autism: phenotypes of 10 inbred strains. Behav Brain Res. 2007;176:4–20. doi: 10.1016/j.bbr.2006.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muehlmann AM, Edington G, Mihalik AC, Buchwald Z, Koppuzha D, Korah M, Lewis MH. Further characterization of repetitive behavior in C58 mice: developmental trajectory and effects of environmental enrichment. Behav Brain Res. 2012;235:143–149. doi: 10.1016/j.bbr.2012.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadler JJ, Moy SS, Dold G, Trang D, Simmons N, Perez A, Young NB, Barbaro RP, Piven J, Magnuson TR, Crawley JN. Automated apparatus for quantitation of social approach behaviors in mice. Genes Brain Behav. 2004;3:303–314. doi: 10.1111/j.1601-183X.2004.00071.x. [DOI] [PubMed] [Google Scholar]
- Ngoc PL, Gold DR, Tzianabos AO, Weiss ST, Celedon JC. Cytokines, allergy, and asthma. Curr Opin Allergy Clin Immunol. 2005;5:161–166. doi: 10.1097/01.all.0000162309.97480.45. [DOI] [PubMed] [Google Scholar]
- Pellis SM, Pasztor TJ. The developmental onset of a rudimentary form of play fighting in C57 mice. Dev Psychobiol. 1999;34:175–182. [PubMed] [Google Scholar]
- Raz R, Roberts AL, Lyall K, Hart JE, Just AC, Laden F, Weisskopf MG. Autism spectrum disorder and particulate matter air pollution before, during, and after pregnancy: a nested case-control analysis within the Nurses' Health Study II Cohort. Environmental health perspectives. 2015;123:264–270. doi: 10.1289/ehp.1408133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai T, Dorr NP, Takahashi N, McInnes LA, Elder GA, Buxbaum JD. Haploinsufficiency of Gtf2i, a gene deleted in Williams Syndrome, leads to increases in social interactions. Autism research : official journal of the International Society for Autism Research. 2011;4:28–39. doi: 10.1002/aur.169. [DOI] [PubMed] [Google Scholar]
- Schwartzer JJ, Careaga M, Chang C, Onore CE, Ashwood P. Allergic fetal priming leads to developmental, behavioral and neurobiological changes in mice. Translational psychiatry. 2015;5:e543. doi: 10.1038/tp.2015.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman JL, Yang M, Lord C, Crawley JN. Behavioural phenotyping assays for mouse models of autism. Nat Rev Neurosci. 2010;11:490–502. doi: 10.1038/nrn2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SE, Li J, Garbett K, Mirnics K, Patterson PH. Maternal immune activation alters fetal brain development through interleukin-6. J Neurosci. 2007;27:10695–10702. doi: 10.1523/JNEUROSCI.2178-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sungur AO, Vorckel KJ, Schwarting RK, Wohr M. Repetitive behaviors in the Shank1 knockout mouse model for autism spectrum disorder: Developmental aspects and effects of social context. J Neurosci Methods. 2014 doi: 10.1016/j.jneumeth.2014.05.003. [DOI] [PubMed] [Google Scholar]
- van Wyk M, Schneider S, Kleinlogel S. Variable phenotypic expressivity in inbred retinal degeneration mouse lines: A comparative study of C3H/HeOu and FVB/N rd1 mice. Mol Vis. 2015;21:811–827. [PMC free article] [PubMed] [Google Scholar]
- Vijayakumar NT, Judy MV. Autism spectrum disorders: Integration of the genome, transcriptome and the environment. J Neurol Sci. 2016;364:167–176. doi: 10.1016/j.jns.2016.03.026. [DOI] [PubMed] [Google Scholar]
- Volk HE, Hertz-Picciotto I, Delwiche L, Lurmann F, McConnell R. Residential proximity to freeways and autism in the CHARGE study. Environmental health perspectives. 2011;119:873–877. doi: 10.1289/ehp.1002835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlsten D, Metten P, Phillips TJ, Boehm SL, 2nd, Burkhart-Kasch S, Dorow J, Doerksen S, Downing C, Fogarty J, Rodd-Henricks K, Hen R, McKinnon CS, Merrill CM, Nolte C, Schalomon M, Schlumbohm JP, Sibert JR, Wenger CD, Dudek BC, Crabbe JC. Different data from different labs: lessons from studies of gene-environment interaction. J Neurobiol. 2003;54:283–311. doi: 10.1002/neu.10173. [DOI] [PubMed] [Google Scholar]
- Walf AA, Frye CA. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat Protoc. 2007;2:322–328. doi: 10.1038/nprot.2007.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehead GS, Walker JK, Berman KG, Foster WM, Schwartz DA. Allergen-induced airway disease is mouse strain dependent. American journal of physiology. Lung cellular and molecular physiology. 2003;285:L32–L42. doi: 10.1152/ajplung.00390.2002. [DOI] [PubMed] [Google Scholar]
- Wolff RJ. Solitary and Social Play in Wild Mus-Musculus (Mammalia) J Zool. 1981;195:405–412. [Google Scholar]
- Yang M, Loureiro D, Kalikhman D, Crawley JN. Male mice emit distinct ultrasonic vocalizations when the female leaves the social interaction arena. Frontiers in behavioral neuroscience. 2013;7:159. doi: 10.3389/fnbeh.2013.00159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young EJ, Lipina T, Tam E, Mandel A, Clapcote SJ, Bechard AR, Chambers J, Mount HT, Fletcher PJ, Roder JC, Osborne LR. Reduced fear and aggression and altered serotonin metabolism in Gtf2ird1-targeted mice. Genes Brain Behav. 2008;7:224–234. doi: 10.1111/j.1601-183X.2007.00343.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu W, Gilmour MI. Comparison of allergic lung disease in three mouse strains after systemic or mucosal sensitization with ovalbumin antigen. Immunogenetics. 2009;61:199–207. doi: 10.1007/s00251-008-0353-8. [DOI] [PubMed] [Google Scholar]