Abstract
Aims
The aim of this study was to identify early foci of α-synuclein (α-syn pathology) accumulation, subsequent progression and neurodegeneration in multiple system atrophy of the cerebellar type (MSA-C).
Methods
We analysed 70-µm-thick sections of 10 cases with MSA-C and 24 normal controls.
Results
MSA-C cases with the lowest burden of pathology showed α-syn glial cytoplasmic inclusions (GCIs) in the cerebellum as well as in medullary and pontine cerebellar projections. Cerebellar pathology was highly selective and severely involved subcortical white matter, whereas deep white matter and granular layer were only mildly affected and the molecular layer was spared. Loss of Purkinje cells increased with disease duration and was associated with neuronal and axonal abnormalities. Neocortex, basal ganglia and spinal cord became consecutively involved with the increasing burden of α-syn pathology, followed by hippocampus, amygdala, and, finally, the visual cortex. GCIs were associated with myelinated axons, and the severity of GCIs correlated with demyelination.
Conclusions
Our findings indicate that cerebellar subcortical white matter and cerebellar brainstem projections are likely the earliest foci of α-syn pathology in MSA-C, followed by involvement of more widespread regions of the central nervous system and neurodegeneration with disease progression.
Keywords: α-synuclein, cerebellum, multiple system atrophy
Introduction
Multiple system atrophy (MSA) is a relentlessly progressive adult-onset neurodegenerative disease of unknown aetiology for which no disease-modifying therapy exists [1]. Two major clinico-pathological subtypes can de delineated: MSA-C [olivo-ponto-cerebellar atrophy (OPCA)], mainly characterized by cerebellar ataxia, and MSA-P (striato-nigral degeneration), presenting as a parkinsonian syndrome that is poorly responsive to dopaminergic therapy [2–4]. To a variable extent, the clinical features of both subtypes also include autonomic failure, which can predate motor symptoms, as well as pyramidal signs [5–7]. While MSA is generally believed to be a sporadic disease, emerging evidence indicates that rare genetic variants increase susceptibility to the disease [8–10]. Indeed, some of these mutations cause significant oligodendroglial α-synuclein (α-syn) deposition and cause morphological phenotypes that resemble MSA, as exemplified by the G51D SNCA mutation which shows a remarkable convergence of neuronal and oligodendroglial α-syn inclusions [11].
The neuropathological hallmarks of MSA are α-syn-positive oligodendroglial cytoplasmic inclusions (GCIs) and, to a lesser extent, infrequent α-syn-positive neuronal inclusions (NIs) in the central nervous system (CNS) [2,7,11–13]. α-Syn is a 17-kDa protein encoded by the SNCA gene at location 4q22.1 that is predominantly expressed in neurons, where it localizes to synaptic terminals and plays a role in vesicle transport and turnover [14,15]. When misfolding occurs, the amyloidogenic region of α-syn forms β-sheets, leading to protofibrils and the formation of fibrils that accumulate into intra-cytoplasmic amyloid aggregates as GCIs and rarely as NIs in MSA. Similar α-syn aggregates also characterize the pathology of Parkinson’s disease (PD) and dementia with Lewy bodies (DLB), albeit in a predominantly neuronal localization to form Lewy bodies with no GCIs [16,17]. Nonetheless, both Lewy bodies and GCIs show a similar epitope profile of α-syn, thereby suggesting that similar α-syn protein domains aggregate to form these inclusions which show properties of amyloid deposits [11,18].
Progressive spreading disease protein aggregates in many of non-prion neurodegenerative disorders is increasingly recognized as a unifying pathological principle of these clinically diverse disorders, including synucleinopathies [19–21]. Furthermore, converging lines of evidence from cell culture and animal model experiments as well as from human tissue studies support the idea of cell-to-cell α-syn transmission in PD and DLB [22–28]. However, given the mainly oligodendroglial localization of α-syn aggregates in MSA, it is currently debated whether this concept of protein propagation can be extended to MSA. While there seems to be little or no oligodendroglial expression of α-syn under normal conditions [29], this could be changed in a pathological setting like MSA [18]. Moreover, it remains enigmatic where and how α-syn aggregation begins in MSA, what possibly characterizes the earliest dissemination of α-syn pathology, and what potentially determines oligodendroglial and neuronal vulnerability to α-syn pathology.
In this study, we aimed to identify early foci of α-syn pathology in a cohort of clinically well-defined cases with MSA-C by implementing a methodological approach pioneered by Braak and colleagues that uses 70-µm-thick sections to enable a highly sensitive analysis of protein aggregation pathology and its anatomical localization in neurodegenerative diseases [30].
Methods
Autopsy cohort
Of 47 patients with a clinical and confirmed neuropathological diagnosis of MSA [2,7,31,32] who were followed longitudinally to autopsy in the Center for Neurodegenerative Disease Research (CNDR) at the University of Pennsylvania between 1989 and 2013, we included all cases with a clinical picture of MSA-C and a neuropathological picture of OPCA (n = 10, 1 female, 9 males; mean age at onset ± SD: 58 ± 7.7 years, range 51–74 years, mean disease duration ± SD: 6.1 ± 3.3 years, range 3.5–14 years) as well as 24 controls age-matched to the MSA-C group that did not show clinical or neuropathological signs of neurodegenerative disease (7 female, 17 male, mean age at death 63.9 ± 6.0 years). Informed consent for autopsy was obtained for all patients or from their next of kin. The study was approved by the University of Pennsylvania Institutional Review Board. Detailed clinical characteristics (gender, age at onset, age at death, clinical symptoms of onset, disease duration) were ascertained from an integrated autopsy database, as described previously [33,34] and by retrospective chart review of clinical visits within the University of Pennsylvania Health System (Table 1).
Table 1.
Demographic data of cases with multiple system atrophy of the cerebellar type (MSA-C) and severity of a-syn pathology in different regions of the central nervous system as rated on a semi-quantitative rating scale
| Case | Sex | Age | Dur | NFT | CE | Phase | AMY | HIP DEN | HIP CA3,4 | HIP CA1,2 | HIP SR | ENT | ENT WM | OF | OF WM |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 55 | 45 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 2 | M | 53 | 48 | 1 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 3 | M | 62 | 42 | 1 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 4 | M | 53 | 48 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 5 | M | 51 | 49 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 |
| 6 | M | 74 | 66 | 0 | 0 | 3 | 0 | 1 | 1 | 1 | 1 | ||||
| 7 | M | 69 | 72 | 2 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 2 | |
| 8 | M | 55 | 84 | 0 | 0 | 4 | 1 | 1 | 1 | 1 | 2 | 1 | 2 | 1 | 2 |
| 9 | M | 54 | 108 | 1 | 0 | 4 | 1 | 1 | 1 | 1 | 2 | 1 | 2 | 1 | 2 |
| 10 | F | 53 | 168 | 0 | 2 | 4 | 1 | 1 | 1 | 1 | 2 | 1 | 2 | 1 | 2 |
| Case | MF | MF WM | MOT | Mot WM | CG | CG WM | SMT | SMT WM | ANG | ANG WM | SEN | SEN WM | VIS | VIS WM | PUT |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 4 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| 5 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 2 |
| 6 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | ||
| 7 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 1 |
| 8 | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 3 | 1 | 3 | 1 | 2 | 0 | 1 | 2 |
| 9 | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 | 0 | 1 | 2 |
| 10 | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 | 0 | 1 | 2 |
| Case | PAL | CI | THA | MB CC | MB SN | MB RN | MB ML | PON LC | PON TF | PON CST | PON SCP | PON MCP |
MED CST |
MED ML |
MED IO |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 1 | 2 | 0 | 0 | 0 |
| 2 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 2 | 2 | 0 | 0 | 0 |
| 3 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 2 | 1 | 2 | 2 | 1 | 1 | 0 |
| 4 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 2 | 1 | 2 | 1 | 0 | ||
| 5 | 1 | 2 | 1 | 2 | 0 | 0 | 1 | 2 | 1 | 2 | 3 | 3 | 2 | 2 | |
| 6 | 1 | 2 | 1 | 2 | 0 | 1 | 1 | 1 | 2 | 1 | 3 | 3 | 3 | 2 | 2 |
| 7 | 1 | 1 | 1 | 2 | 1 | 3 | 3 | 2 | 2 | ||||||
| 8 | 2 | 2 | 1 | 2 | 1 | 3 | 1 | 3 | 3 | 3 | 2 | 2 | |||
| 9 | 2 | 2 | 2 | 3 | 1 | 1 | 2 | 1 | 3 | 2 | 3 | 3 | 3 | 3 | 3 |
| 10 | 2 | 3 | 2 | 3 | 2 | 2 | 2 | 2 | 3 | 2 | 3 | 3 | 3 | 3 | 3 |
| Case | MED OCF |
MED ARC |
MED XII |
MED X |
MED ICP |
CB MOL |
CB PCL |
CB GL |
CB SWM |
CB DWM |
CB DEN |
CSC CST |
CSC EPT |
CSC SCT |
CSC STT |
LAM IX |
LAM I–VIII |
CSC SENT |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1 | 0 | 0 | 0 | 2 | 0 | 0 | 1 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 2 | 2 | 0 | 0 | 0 | 2 | 0 | 0 | 1 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 3 | 2 | 0 | 0 | 0 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | ||||||
| 4 | 3 | 0 | 0 | 0 | 2 | 0 | 0 | 1 | 2 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 |
| 5 | 3 | 1 | 0 | 0 | 3 | 0 | 0 | 1 | 2 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 |
| 6 | 3 | 1 | 0 | 0 | 2 | 0 | 0 | 1 | 3 | 1 | 0 | 2 | 2 | 2 | 2 | 1 | 1 | 0 |
| 7 | 3 | 1 | 0 | 0 | 2 | 0 | 0 | 2 | 3 | 1 | 0 | 2 | 2 | 2 | 2 | 1 | 1 | 0 |
| 8 | 3 | 1 | 1 | 1 | 3 | 0 | 0 | 2 | 3 | 2 | 1 | 2 | 2 | 2 | 2 | 2 | 2 | 1 |
| 9 | 3 | 1 | 1 | 1 | 3 | 0 | 0 | 2 | 3 | 2 | 1 | 2 | 2 | 2 | 2 | 2 | 2 | 1 |
| 10 | 3 | 1 | 1 | 1 | 3 | 0 | 0 | 3 | 3 | 2 | 1 | 2 | 2 | 2 | 2 | 2 | 2 | 1 |
Age, age in years; AMY, amygdala; ANG, angular gyrus; ANG WM, angular gyrus white matter; CB DEN, cerebellum, dentate nucleus; CB DWM, cerebellum, deep white matter; CB GL, cerebellum, granular layer; CB MOL, cerebellum, molecular layer; CB PCL, cerebellum, Purkinje cell layer; CB SWM, cerebellum, subcortical white matter; CE, CERAD score; CG, anterior cingulate gyrus; CG WM, anterior cingulate gyrus white matter; CI, internal capsule; CSC CST, cervical spinal cord, corticospinal tract; CST EPT, CSC, extrapyramidal tracts (rubro-, reticulo-, olivospinal tracts); CSC SCT, CSC, spinocerebellar tract; CSC ST, CSC, spinobulbar tracts; CSC STT, CSC, spinothalamic tract; Dur, duration of disease (months); ENT, entorhinal cortex; ENT WM, entorhinal cortex white matter, HIP CA1,2, hippocampus, cornuammonis regions 1 and 2; HIP CA3,4, hippocampus, Ammon’s horn regions 3 and 4; HIP DEN, dentate gyrus of hippocampus; HIP SR, hippocampus stratum radiatum, LAM I–XIII -laminae 1 to 8 of spinal cord gray matter; LAM IX, lamina 9 of spinal cord gray matter; MBCC, crus cerebri; MB ML, midbrain, medial lemniscus; MB RN, midbrain, red nucleus; MB SN, midbrain substantia nigra; MED IO, medulla, inferior olive; MED ARC, medulla, arcuate nucleus; MED CST, medulla, corticospinal tract; MED ICP, medulla, inferior cerebellar peduncle; MED ML, medulla, medial lemniscus; MED OCF, medulla, olivo-cerebellar fibres; MED X, medulla, dorsal motor nucleus of the vagal nerve; MED XII, medulla, hypoglossal nucleus; MF, middle frontal gyrus; MF WM, middle frontal gyrus white matter, MOT, motor cortex (Brodmann areas 4 and 6); MOT WM, motor cortex white matter, NFT, Braak stage of neurofibrillary tangles; OF, orbital gyrus and gyrus rectus; OF WM, orbital gyrus and gyrus rectus white matter; PAL, pallidum; PON CST, pons, corticospinal tract; PON LC, pons, locus coeruleus; PON MCP, pons, middle cerebellar peduncle; PON SCP, pons, superior cerebellar peduncle; PON TF, pontocerebellar fibres; PUT, putamen; SEN, somatosensory cortex; SEN WM, somatosensory cortex white matter; SMT, superior and middle temporal gyrus, THA, thalamus, VIS, visual cortex (Brodmann areas 17 and 18); VIS WM, visual cortex white matter. Colours indicate severity of pathology from green colour = no pathology to red colour = severe pathology.
Tissue preparation, staining and immunohistochemistry
Pathology was examined in 20 regions of the CNS: the middle frontal gyrus, gyrus rectus and orbital gyri, the agranular motor cortex (Brodmann areas 4 and 6), somatosensory cortex, superior or middle temporal gyrus, angular gyrus, precuneus (Brodmann area 7), visual cortex (Brodmann areas 17 and 18), anterior cingulate gyrus, hypothalamus, amygdala, hippocampal formation, striatum and pallidum (the striatum block included anterior portions of the caudate nucleus and putamen plus the accumbens nucleus, where the caudate nucleus and putamen merge), the thalamus, midbrain [including the substantia nigra (SN) and red nucleus (RN)], upper pons at the level of the locus coeruleus, lower pons (including the motor nucleus of cranial nerve VII), the medulla oblongata at the level of the hypoglossal nucleus (XII) and inferior olive (IO), the cerebellum (including both a portion of the cerebellar cortex and dentate nucleus), and the cervical spinal cord. After overnight fixation of thin 0.5–1-cm-thick tissue blocks to ensure uniform penetration of 10% neutral buffered formalin, all tissue samples were embedded in paraffin using standardized cassettes, sectioned at 6–7 µm, and stained with haematoxylin and eosin (HE) and for immunohistochemistry (IHC) as previously described [17,35]. Briefly, IHC was performed with antibodies to α-syn (monoclonal antibody (mab) Syn303; 1:4000, generated in CNDR) [36], hyperphosphorylated tau (mab PHF1; 1:1000, gift from Dr. Peter Davies), pTDP-43 (rat antibody p409/410, 1:1000, gift from Dr. Manuela Neumann) [37] and amyloid-β (mab NAB228; 1:15 000; generated in CNDR) [38], whereas histochemical staining with HE as well as thioflavin S were performed as reviewed recently [39]. Neurofibrillary tangle stages and CERAD neuritic plaque scores are shown in Table 1.
To study each of the CNS regions in greater neuroanatomical detail, additional sets of 70 µm sections were prepared as described previously [17,30] from the same paraffin blocks as those used above. This thick section technique is performed on free-floating sections and permits recognition of specific cell types and cytoarchitectonic units owing to the visualization of large numbers of biological structures in a more three-dimensional manner than is possible with conventional 6-µm-thick sections [17,30]. The sections were stained for analysis of α-syn pathology and to provide for a more topographical overview and to assess neuronal loss (NL), we combined α-syn IHC with a pigment-Nissl stain (PN) for lipofuscin pigment (aldehyde fuchsin) and basophilic Nissl material (Darrow red) as described [30]. In addition, Campbell-Switzer silver staining (CS) was performed as previously described [40]. Severity of α-syn pathology and NL were assessed according to a 4-point semiquantitative rating scale (0, absent or not detectable; 1, ≤2 aggregates per region + mild; 2, moderate ++; 3, severe/numerous +++) [28,41] and was adjusted for NL. Double-labelling IHC was performed on selected 70 µm sections using the α-syn antibody described above together with the Vector Labs, Burlingame, CA, USA, SG (SK-4700; Vector) blue chromogen and the SMI-311 mab (1:1000; Covance, Princeton, NJ, USA), the rabbit polyclonal antibody (pab) for oligodendrocyte-specific protein (1:400; Abcam, Cambridge, UK) and the anti-oligo2 antibody (1:400; Millipore, Billerica, MA, USA).
For double-labelling immunofluorescence (IF), 15 µm frozen sections were microwaved in 10 mM citrate buffer, pH 6.0, for 15 min; blocked in 2% FBS with 0.1 M Tris and incubated with the mouse mab syn-506 (specific for α-synuclein, amino acids 1–89; 1:10 000; CNDR [42]) and rabbit pab Olig2 (specific for oligodendrocytes; 1:250; Millipore), rat mab TA51 (specific for phosphorylated heavy and medium molecular weight neurofilament; 1:10; CNDR [43]), rabbit pab NFL1/2 (specific for low molecular weight neurofilament; 1:1000; CNDR [44]) or rabbit pab 17028 (a microtubule associated protein 2 specific antibody; 1:500; CNDR, unpubl. data), followed by a goat anti-mouse Alexa Fluor® 546-labelled IgG (H+L) antibody, a goat anti-rabbit Alexa Fluor® 588-labelled IgG (H+L) antibody and a goat anti-rat Alexa Fluor® 588-labeld IgG (H+L) antibody (1:500; Invitrogen, Carlsbad, CA, USA) in 2%FBS in 0.1 M Tris. Slides were cover-slipped using Vectashield mounting media with DAPI (Vector) and digitalized images were obtained using a Leica TCS SPE-II scanning laser confocal microscope.
Targeted next-generation sequencing
Genomic DNA was extracted from frozen brain tissue using commercial reagents (DNA mini kit; Qiagen, Valencia, CA, US). To exclude mutations in COQ2 which have been associated with MSA, targeted sequencing was performed using a neurodegenerative disease-focused panel that targets the coding exons of 45 genes, including COQ2. The library of the target regions was prepared with Haloplex enrichment kit (Agilent Technologies, Santa Clara, CA, USA) according to the manufacturer’s protocol. Sequencing was performed on an Illumina, San Diego, CA, USA, Mi-Seq using 150 base paired-end reads. Following quality control procedures, alignment of sequence reads and variant calling was assessed using SureCall software (Agilent).
Statistical analysis
Data analysis was performed using SPSS (version 17.0; SPSS Inc., Chicago, IL, USA). The average (and range) of data on patient characteristics were estimated by calculating the median (and 25th–75th percentiles). Differences between two clinical subgroups were compared using Wilcoxon Mann–Whitney test. To compare raw data of multiple subgroups, Kruskal–Wallis analysis of variance on ranks was applied and in case of significance, by Dunn’s method. Trend analysis was conducted using the Mantel–Haenszel chi-square test. All correlations were studied using Spearman’s rank order correlation coefficient. Bonferroni-correction for multiple testing was applied when contrasts were not driven by a specific hypothesis. For all other tests, P-values < 0.05 were considered significant. All statistical tests were two-sided.
Results
Morphology of α-syn pathology in MSA
Main types of α-syn inclusions in MSA-C
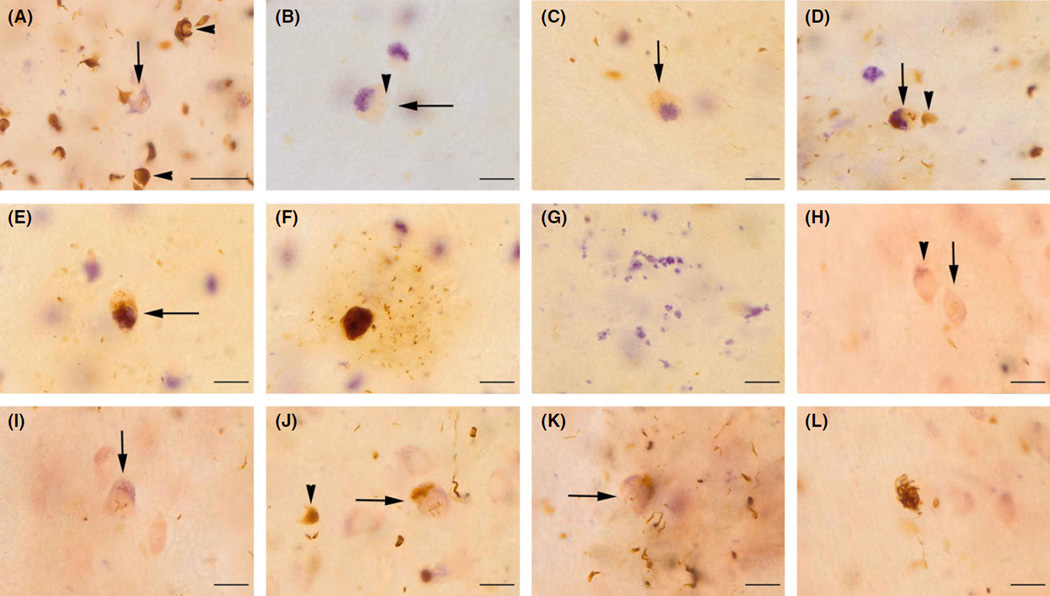
We first analysed the morphology of α-syn pathology in MSA-C in 70-µm-thick sections which allows for a more three-dimensional view of the histology than conventional 6-µm-thick sections wherein objects have a more two-dimensional appearance [17,30]. We observed three main types of α-syn inclusions in MSA-C and none of these pathologies were observed in any of the age-matched controls: The most abundant pathology consisted of GCIs in white and (to a lesser extent) grey matter of the CNS. GCIs and neuritic α-syn inclusions (NRI) in axons constituted the earliest changes in regions with a low overall burden of α-syn pathology (Figure 1A). GCIs frequently were associated with considerable swelling of the oligodendroglial nucleus and cytoplasm. NIs were rare and not as widespread as GCIs but appeared to gradually develop with the increasing burden of α-syn pathology.
Figure 1.
Morphology and timeline of glial and neuronal α-syn aggregation in multiple system atrophy of the cerebellar type (MSA-C). Here and in the subsequent two figures we show images of 70-µm-thick sections because they were used to enable to see the histology and pathology in a more three-dimensional manner than conventional 6-µm-thick sections wherein objects have a more two-dimensional appearance. Hence, this figure shows a richer morphology of α-syn aggregation in the inferior olive (IO) (A–G) and the pontine nuclei (H–L) as depicted by Darrow red aldehyde fuchsin staining combined with α-syn immunohistochemistry. (A) The most frequent pathology in MSA-C consisted of α-syn-positive glial cytoplasmic inclusions (GCIs; arrowheads), whereas α-syn-positive Nis (arrow) were rare. GCIs frequently were seen in oligodendroglial cells with a swollen nucleus and expanded cytoplasm. (B) Nis were often seen in association with neurons that showed cytoplasmic and nuclear swelling as well as a loosening of cytoplasmic lipofuscin pigment and the arrow identifies swollen cell of the IO, arrowhead shows enlarged nucleus. (C) This may represent changes that are followed by the development of α-syn inclusions (arrow), and, consecutively, by the occurrence of increasingly bulky cytoplasmic neuronal inclusion (NI) (D) and the arrow depicts a neuron with both nuclear and cytoplasmic NIs, arrowhead shows GCI). (E, F) Cytoplasmic NIs (as shown by arrow in E) increased to fill most of the neuronal perikarya. In parallel, neurons became surrounded by an increasing numbers of neuritic α-syn inclusions (NRIs), presumablly in dendrites. (G) Finally, neuronal loss became apparent by free lipofuscin pigment remnants in the extracellular space. (H) Arrowhead depicts neuronal abnormalities as evidenced by cytoplasmic and nuclear swelling of a pontine neuron and the arrow shows an incipient nuclear NI. (I) Arrow depicts pontine neuron with nuclear NI. (J) Arrow shows pontine neuron including both nuclear and cytoplasmic NI, arrowhead shows GCI. (K) Affected neuron (arrow) is increasingly surrounded by GCI. (L) After neuronal degeneration, large cytoplasmic NI is observed free in the extracellular space. All scale bars in this figure represent 20 µm.
Sequence of neuronal α-syn aggregates in MSA-C
Initially, and prior to the development of NI, neurons showed abnormalities which included cytoplasmic and nuclear swelling, cytoplasmic loosening of lipofuscin pigment or changes in dendritic architecture (Figure 1B, H). With disease progression, these changes were accompanied by the development of nuclear α-syn skeins, and, then, by the formation of granular cytoplasmic aggregates (Figure 1C–E and I–L). With increasing accumulation of cytoplasmic α-syn aggregates, neurons became increasingly surrounded by dendritic NRI (Figure 1F).
In addition, accumulation of α-syn inclusions was accompanied by progressive NL, which was accompanied by the extracellular retention of α-syn aggregates and extracellular lipofuscin pigment remnants in the neuropil (Figure 1G). Remarkably, NL was frequently observed in groups of neurons that did not (or only to a very mild extent) show NI or NRI. As an example, severe loss of cerebellar Purkinje cells (PC) was detected in cases with a short disease duration, and these cells did not show any NI or NRI. Similarly, neurons of the SN and the locus coeruleus showed severe NL but only mild NI.
Four phases of α-syn pathology in MSA-C
On the basis of the different types of α-syn inclusions described above, we proceeded to analyse phases of α-syn pathology in MSA-C according to an increasing overall burden of pathology (Table 1).
Phase 1: cerebellum, and cerebellar brainstem connectivities
Generally, MSA-C cases with the lowest overall burden of pathology showed involvement of cerebellar subcortical white matter, olivo-cerebellar fibres in the medulla, the inferior cerebellar peduncle of the medulla, pontocerebellar fibres and the middle and (to a lesser extent) the superior cerebellar peduncles (Table 1).
We observed a highly selective pattern of cerebellar involvement, which persisted with an increasing burden of α-syn pathology (Figure 2). Cerebellar pathology was prominent in the corpus cerebelli, whereas the nodulus (vestibulo-cerebellum) was hardly affected. In addition, pathology more severely affected the vermis cerebelli than the hemispheres. GCIs severely involved the cerebellar subcortical white matter, whereas deep white matter and the granular layer were only mildly involved (Figure 2A–C). Double-labelling IF demonstrated that α-syn inclusions in the granular layer were GCIs and not NIs (Figure S1F). The dentate nucleus developed NIs only in cases with a high burden of GCI pathology, whereas the molecular layer was entirely spared of NIs (Table 1, Figure 2A–C). Using combined CS + myelin staining and CS + PN staining, we observed GCI in areas with severe pathology to be mostly argyrophilic, whereas GCIs in areas with a lower burden of this α-syn pathology were less positively stained by the CS method (Figure 2E). Importantly, we found a close spatial relationship between GCIs and myelinated axons, which suggests that these GCIs were in myelinating oligodendroglial cells (Figure 2F–H). In addition, we observed argyrophilic aggregates in oligodendroglial processes that ensheathed myelinated axons (Figure 2H), which further suggests that these GCIs are in myelinating oligodendroglia rather than in satellite cells. Moreover, the regional severity of GCI pathology correlated with the severity of myelin pallor, with the most severe myelin loss observed in subcortical white matter, whereas myelin in the deep white matter was much better preserved (Figure 2D). In addition, cerebellar involvement was characterized by loss of PCs that increased with disease duration (Figure 2I, J). Remarkably, the PCs themselves did not show any α-syn inclusions but showed other evidence of neuronal abnormalities, including distortion of dendrites as well as axonal swellings mainly in the proximal parts of PC axons (Figure 2K, L). In contrast to PCs, NL of dentate nucleus neurons was only observed in cases with a later phase of α-syn pathology and long disease duration.
Figure 2.
α-syn pathology in the cerebellum of multiple system atrophy of the cerebellar type. (A) Darrow red aldehyde fuchsin staining combined with α-syn immunohistochemistry (IHC) [pigment-Nissl (PN) + α-syn] depicts the selective pattern of cerebellar involvement by α-syn pathology and neuronal loss: glial cytoplasmic inclusions (GCIs) most severely affect subcortical white matter (SWM), whereas the deep white matter (DWM) surrounding the dentate nucleus (indicated by arrowheads) is much less affected. Mild GCI pathology can be detected in the granular layer (GL), whereas the molecular layer (MOL) remains free of α-syn inclusions. (B, C) PN + α-syn IHC shows more detailed view of GCI (examples shown by arrowheads) in SWM and GL, whereas the ML is unaffected. (D) Myelin stain shows severe demyelination of SWM, whereas DWM surrounding dentate nucleus (arrowheads) is well preserved. (E) Combined Campbell-Switzer silver staining (CS) and α-syn IHC shows GCI that are argyrophilic (arrow) and not argyrophilic (arrowhead) in SWM of cerebellum. (F, G) Combined CS and myelin staining show spatial relation of GCI (arrowhead) to myelinated axons (arrow), and the asterisk in G indicates a Purkinje cell (PC). (H) Combined CS and myelin staining shows a GCI (arrowhead) and myelinated axon (arrow) that is ensheathed by argyrophilic processes, most likely oligodendroglial processes. (I) Combined CS + PN staining shows loss of PCs in of cerebellar cortex (area indicated by arrowhead). (J) Provides more detailed view of i) showing PCs (example depicted by arrow), area with PC loss (arrowhead) and swelling of PC axon (‘torpedo’, indicated by asterisk). (K) Combined CS + myelin staining shows multiple torpedoes (asterisks) of proximal PC (arrow) axons; asterisk indicates GCI in subcortical white matter. (L) Combined CS + myelin staining shows PC (arrow) with distortion of dendrites (arrowheads) and axonal swelling (asterisk). Scale Bar is equivalent to 1 mm in A and D, 200 µm in B, F, G, and K, 500 µm in I, 100 µm in C, E, and M, and 50 µm in H.
Similar to our observations on the cerebellum, the earliest pathology in the brainstem prominently involved white matter tracts (Figure 3). In the medulla oblongata, α-syn pathology was observed to a lesser extent in the IO (Figure 3A, B) than elsewhere in the medulla (Figure 3C), and abundant GCIs were seen in olivo-cerebellar fibres. In addition, α-syn-positive GCIs were observed in the inferior cerebellar peduncle. While no NIs were detectable in the IO, IO neurons were often surrounded by a dust-like cloud of α-syn inclusions, presumably in dendrites, and there was mild loss of IO neurons (Figure 3A, B). In the pons, GCIs and NRIs were observed in pontocerebellar fibres (Table 1, Figure 3D, E), especially in lower pontine areas. In addition, we observed α-syn-positive GCIs in the middle and superior cerebellar peduncles. In contrast to cerebellum and brainstem, the basal ganglia, substantia nigra, neocortical fields, limbic system centres, and the spinal cord were uninvolved in phase 1 cases. None of the control cases showed any α-syn pathology.
Figure 3.
α-syn pathology beyond the cerebellum in multiple system atrophy of the cerebellar type. All images are Darrow red aldehyde fuchsin staining (pigment-Nissl) combined with α-syn immunohistochemistry (IHC). (A, B) Images depict severe involvement of inferior olive (IO) neurons (example shown by arrow in B) surrounded by a dust-like cloud of α-syn inclusions presumably in dendrites; arrowhead indicates a glial cytoplasmic inclusion (GCI) and the asterisk in B identifies region in the IO with neuronal loss (NL) and free extracellular lipofuscin. (C) While the medulla oblongata shows severe and widespread GCI pathology, the hypoglossal nucleus (XII) and the dorsal motor nucleus of the vagus (X) are relatively spared. (D) Image shows severe neuritic α-syn inclusion (NRI) and GCI pathology in the transverse pontine white matter fibres (arrow), whereas the bundles of corticospinal tract fibres (arrowheads) passing through the pons are less affected. (E) Image shows involvement of pontine transverse fibres in more detail. (F) The SN pars compacta shows dopaminergic neurons (arrows), GCIs (arrowheads) and NL (asterisks). (G) NRIs (arrowheads) and a GCI (arrow) in the internal capsule (ic) whereas pu indicates putamen. (H) Neuronal inclusion (NI) and GCI pathology in the motor cortex with α-syn pathology mainly affecting lower cortical layers (arrow). (I) Image shows example of severe dentate gyrus involvement by NIs and GCIs in the hippocampus. (J, K) Severe NRI α-syn pathology in the Ammon’s horn. (L) In cases with the highest burden of α-syn pathology (8–9 in Table 1), GCIs were seen in the hypoglossal nucleus. Scale bar in A, C and D indicates 500 µm, scale bars in B and in E–N indicate 100 µm.
Phase 2: pyramidal and extrapyramidal white matter
With an increasing overall burden of α-syn-positive GCI pathology, in addition to the findings described above, α-syn inclusions became detectable in cerebellar deep white matter, whereas the dentate nucleus still remained free of NI (Table 1). Moreover, there was increasing loss of PC in the cerebellar cortex (Figure 2I, J).
In the medulla, GCIs became detectable in the medial lemniscus and also in fibres of the corticospinal tract within the medullary pyramids. The pontine pyramidal bundles also displayed α-syn-positive GCIs, though to a lesser extent than the pontocerebellar fibres (Figure 2D). In the midbrain, GCIs and NRIs prominently involved fibres within the crura cerebri (Table 1). In the basal ganglia, cases with the lowest overall burden of α-syn pathology, GCIs and NRIs were present in fibre tracts of the internal capsule (Table 1, Figure 2G). Double-labelling IHC and IF revealed that these long NRIs in the internal capsule were axonal aggregates of α-syn (Figure S1C–E).
GCIs and NRIs were seen to involve different fibre tracts in the spinal cord, including the anterior as well as the lateral portion of the corticospinal tracts, the spino-thalamic, spino-cerebellar and spino-olivary tracts, whereas sensory white matter pathways were not involved until later disease phases (Table 1). In addition to white matter fibre tracts, GCIs were also observed in the spinal cord gray matter, whereas NIs were only rarely detected there. Moreover, loss of α-motoneurons was rare and limited to cases with the highest overall burden of α-syn-positive GCI pathology (Table 1). Sections of the thoracic, lumbar and sacral spinal cord were available for only n = 3 cases, but the spinal cord α-syn GCI pathology there extended all the way down to sacral spinal cord sections and also involved the intermediolateral nucleus.
Phase 3: neocortex and basal ganglia gray matter
Cortical α-syn inclusions were first detectable in frontal and temporal cortical areas, whereas the parietal cortex (including the sensory and angular gyrus) was only involved in later phases of MSA-C (Table 1). Within the cortex, GCIs and NRIs first became detectable in lower cortical layers (layers V, VI), subcortical white matter and deep white matter (Figure 3H), whereas NIs and NL were not observed in cases with a low burden of cortical pathology. With an increasing burden of α-syn-positive GCI pathology, NIs also were present, especially in pyramidal cells of lower cortical layers (V, VI), when white matter GCI pathology was very severe.
In the lower medulla, phase 3 cases showed NIs in the IO, although the lesions were infrequent and mild compared to white matter GCIs. With the increasing overall burden of α-syn pathology, NIs in the IO became more common and frequently were surrounded by a dust-like cloud of α-syn inclusions that appeared to be intra-dendritic inclusions (Figure 2A, B). Similarly, NL was present in the dorsal motor nucleus of the vagal nerve, whereas NIs were sparse there (Figure 2C). In addition, NL as opposed to only infrequent NIs was detected in the locus coeruleus (Table 1), and in the midbrain, α-syn inclusions were increasingly observed in the RN (Table 1). Moderate α-syn inclusions (GCIs, NIs) and NL were detectable within the hypothalamus of seven 7 MSA-C cases, which were not among those with the lowest overall burden of pathology. Finally, the thalamus became gradually involved with increasing burden of pathology.
Phase 4: amygdala and hippocampus
Cases with further increases in the burden of α-syn inclusions were characterized by involvement of the hippocampus and amygdala (Table 1). In the hippocampus, GCIs and NRIs were observed in the external plexiform layer as well as in the stratum radiatum and lacunosum, whereas NIs were rarer and were first detected in the outer sectors of the Ammon’s horn (CA1, CA2) as well as in the subiculum. In contrast, the inner sectors of the Ammon’s horn (CA3, CA4) and the dentate gyrus were less severely involved and only became involved in the MSA-C cases with the most severe burden of α-syn pathology and NL (Table 1, Figure 3I–L). Occasionally, extensive NRI could be detected within the Ammon’s horn (Figure 3J, K). Hippocampal involvement was associated with the presence of α-syn pathology in the entorhinal cortex and in the basolateral subnucleus of the amygdala (Table 1).
In addition to these findings, MSA-C cases with a burden of α-syn inclusions corresponding to phase 4 showed NIs in the cerebellar dentate nucleus (Table 1) and showed a very mild burden of NIs in the dorsal motor nucleus of the vagal nerve and hypoglossal nucleus (Figure 3L).
Clinicopathological correlations and genetic results
We next analysed clinicopathological correlations for the entire cohort of MSA-C cases. The patterns of α-syn pathology described above correlated significantly with duration of disease (ρ = 0.83, P < 0.001) but were not related to age at onset (ρ = −0.13, P = 0.71) or age at death (ρ = 0.3, P = 0.38). Furthermore, severity of α-syn pathology increased significantly with duration of disease across many of the regions analysed here (Table S1). In contrast, we observed no significant correlation between the regional severity of α-syn inclusions and tau or Aβ pathology (P > 0.05 and ρ < 0.04 each). Myelin loss in pons and cerebellum correlated significantly with severity of GCI pathology, and increased with disease duration (ρ > 0.4, P < 0.05 each).
Although mutations in COQ2 have been associated with an increased risk of MSA, the overwhelming majority of MSA cases present as sporadic disease [45]. We screened the MSA-C cases in this study for genetic variations in COQ2. No pathogenic mutations were identified.
Discussion
The main aim of this study was to determine early foci of α-syn pathology in MSA-C. The major findings can be summarized as follows: (i) cerebellar subcortical white matter and cerebellar brainstem projections represent early foci of α-syn pathology in MSA-C; (ii) cerebellar pathology in MSA-C shows a highly specific pattern of involvement, namely, severe involvement of subcortical white matter, only mild involvement of deep white matter and granular layer, and sparing of the molecular layer. (iii) This pattern appears to be determined by the early involvement of PCs; and; (iv) the pattern of α-syn pathology in MSA-C is indicative of a progressive spread and, therefore, is reminiscent of what has been observed in several other neurodegenerative disorders [19–21].
Morphology of α-syn inclusions in thick sections of MSA-C
We first analysed the morphology of α-syn aggregates in 70-µm-thick sections. As noted above and reported elsewhere [17,30,40], we emphasize illustrations of 70-µm-thick sections because they allowed us to view the histology and pathology in a more three-dimensional manner than what is possible by examining conventional 6-µm-thick sections. We also examined 6-µm-thick sections for this study, but these provide a shorted depth of viewing that result in objects having a more two-dimensional appearance. In line with previous evidence [2,7,12,13,39,46–53], we found the main α-syn pathology to consist of white matter GCIs. GCIs were reported to be partially argyrophilic [54], especially in cases with longer duration of disease (Figure 2E). Moreover, similar to Lewy bodies in PD and DLB, GCIs also show properties of amyloids including positivity for thioflavin S and other amyloid binding dyes [18]. Consequently, argyrophilia and positivity for amyloid binding dyes are likely to be a characteristic of more mature α-syn inclusions in MSA-C. NIs and NRIs were less frequent than GCIs, which is also in accordance with previous studies of MSA [55–57].
Importantly, our study indicates that neurons in MSA-C manifest other abnormalities that variably include cytoplasmic and nuclear swelling, intraneuronal loosening of lipofuscin pigment (Figure 1B, H), and dendritic morphological changes prior to the development of NI (Figure 2K–L), which we speculate may reflect a form of cellular stress or incipient neurodegeneration. In addition, our study indicates that nuclear α-syn aggregates in neurons are more frequent than previously reported [49]. Although α-syn is mainly a presynaptic protein, it was also detected at the inner nuclear membrane [58]. Such nuclear α-syn aggregates could have potentially severe functional consequences in cells that do not show the prominent cytoplasmic aggregates observed in other synucleinopathies [16,28].
Cerebellum and its projections as early foci of pathology in MSA-C
On the basis of our analysis of α-syn inclusion morphology, we proceeded to look for evidence of a possible spreading of α-syn pathologyin MSA-C. We focused on MSA-C as we expected the spread of α-syn in MSA-P to be different and to require a separate study. Our intention was not to propose a new grading system of MSA-C [7,12,46] but, rather, to determine where α-syn pathology might begin and how it progressively spreads in autopsy-confirmed MSA-C cases.
Cases with the lowest overall burden of pathology (phase 1, Table 1) showed prominent GCIs in the cerebellar white matter and in medullar and pontine white matter cerebellar projections, which is in agreement with previous neuropathology and neuroimaging studies [2,7,12,32,46,59–61]. GCIs and NRIs were associated with myelin loss in the pontocerebellar fibres (Figure 2D, E) [62,63], which has been linked with the characteristic ‘cross-bun sign’ detectable in magnetic resonance imaging of living MSA-C patients [64,65].
Cerebellar involvement was highly selective: Subcortical white matter showed severe GCIs already in cases with a low burden of pathology/short disease duration (Table 1). In contrast, deep white matter and the granular layer were affected to a much lesser degree, and the molecular layer remained free of any α-syn inclusions (Table 1, Figure 2A–C). The dentate nucleus showed NIs only in cases with a long disease duration (Table 1), which coincides with previous observations [7,46,66]. Importantly, GCIs showed a close spatial relation to myelinating axons (Figure 2F–H), thereby indicating that GCIs are α-syn lesions that occur in myelinating oligodendroglia (and not in satellite cells). In line with this and previous findings [46,54], we found severe myelin loss in regions characterized by early and severe GCI formation, such as cerebellar subcortical white matter or pontocerebellar fibres (Figure 2D). We hypothesize that this highly selective pattern of cerebellar involvement in MSA-C could be partially explained by the association of GCI with myelinated fibres. Thus, we found that the cerebellar molecular layer, which contains the unmyelinated parallel fibres of granular layer neurons, remained free of GCI pathology even in cases with the longest disease duration [67]. Similarly, the distal parts of PC axons, which penetrate the cerebellar deep white matter surrounding the dentate nucleus, have been reported to be unmyelinated [68,69], which could explain the relative sparing of the cerebellar deep white matter by GCI pathology (Figure 2A).
With an increasing burden of α-syn pathology (phase 3, Table 1), GCIs, Nis, and NRIs became detectable in the neocortex (Figure 3H). In accordance with previous studies, neocortical NIs developed only gradually and initially affected chiefly lower cortical layers [70,71]. Although cortical involvement in MSA is reportedly rare [61,70], some studies have reported NL in the motor and supplementary motor cortex [72] and have suggested a relationship with striato-nigral involvement [73]. In the midbrain, GCIs and NRIs prominently involved the crura cerebri, which contains striatonigral ‘comb’ fibres [74], the involvement of which might be linked to NL in the SN [12,46,75]. Similarly, the presence of severe GCIs and NRIs in the internal capsule (Figure 3G) could contribute to NL in the putamen and SN. In line with previous studies, we observed loss of hypothalamic neurons in cases of MSA-C (Table 1) [46, 76, 77]. The hypothalamus is of primary importance for thermoregulation, glucoregulation, osmoregulation, stress responses and immunomodulation [78]. Thus, its involvement could contribute to autonomic dysfunction in MSA-C [76]. Finally, MSA-C cases with a further increasing burden of pathology (phase 4, Table 1), were characterized by involvement of the entorhinal cortex, hippocampus (Figure 3I–L) and the basolateral subnucleus of the amygdala.
Mechanisms of NL in MSA-C
Remarkably, NL in MSA-C frequently affected groups of neurons that showed only mild α-syn aggregates (including neurons of the SN and the locus coeruleus, whereas the pathology is characterized by massive involvement of oligodendroglia [7,46,66,75]. Furthermore, previous studies on cases of minimal change MSA with NL restricted to the substantia nigra and locus coeruleus, but more widespread GCI pathology, suggested that oligodendroglial pathology could lead to neuronal dysfunction and clinical symptoms before overt NL in MSA [79,80]. We hypothesize that neuronal dysfunction and NL could be caused by GCI along myelinated axons at a considerable anatomical distance from the neuronal cell body. The mechanisms by which α-syn accumulation in GCIs could possibly lead to axonal dysfunction and neuronal death are still unknown, but both were seen in an earlier transgenic mouse model of MSA induced by α-syn overexpression [81]. There is increasing evidence that oligodendrocytes provide essential metabolic support to neurons by transferring glycolytic intermediates [82,83]. Furthermore, oligodendrocytes are responsible for maintaining brain lipid homeostasis, and myelin instability was recently suggested to precede α-syn pathology in MSA [84,85]. In addition, altered myelin protein composition including changes in the cellular interactions between MBP and p25α have been suggested to contribute to MSA pathology [20].
Finally, it is unclear if the concept of ‘prion-like’ propagation attributed to other synucleinopathies can be extended to MSA [22,23,25,27,86–88]. Our findings point to a stereotypical spreading pattern of α-syn-containing lesions similar to what has been described for PD and DLB [28,41]. A limitation of our study is the comparatively low number of cases with MSA-C available. It should therefore be regarded as a pilot-study that should entail a multi-centre follow-up effort to validate our findings and establish stages of α-syn pathology in MSA-C.
Supplementary Material
Figure S1. Double-labelling immunofluorescence (IF) analysed by confocal microscopy.
Table S1. Correlation of α-synuclein pathology in multiple system atrophy of the cerebellar type (MSA-C) with duration of disease, age at onset and age at death.
Acknowledgments
We thank the patients who contributed to this study. We are grateful to Terry Schuck, Sigrid Baumann, Gabriele Ehmke, Simone Feldengut, Julia Straub and Thu Brettschneider for technical support. This study was supported by a grant from the MSA Coalition/Cure PSP to JB. VM-YL is the John H. Ware, 3rd, Professor of Alzheimer’s Disease Research. JQT is the William Maul Measey-Truman G. Schnabel, Jr. Professor of Geriatric Medicine and Gerontology. These studies were supported in part by P50 NS053488 (JQT).
Footnotes
Author contributions
JB contributed to the acquisition, analysis of data and drafting of the manuscript. DI and JR participated in analysis of data and drafting of the manuscript. SB, ERS, VVD, JBT, MG, HH, RD, SP, VML and JQT were involved in analysis of data and reviewing of the manuscript. MB and LF were involved in acquisition, analysis of data and reviewing of the manuscript. EBL contributed to the drafting and reviewing of the manuscript.
Supporting information
Additional Supporting Information may be found in the online version of this article at the publisher’s web-site:
References
- 1.Ahmed Z, Asi YT, Sailer A, Lees AJ, Houlden H, Revesz T, Holton JL. The neuropathology, pathophysiology and genetics of multiple system atrophy. Neuropathol Appl Neurobiol. 2012;38:4–24. doi: 10.1111/j.1365-2990.2011.01234.x. [DOI] [PubMed] [Google Scholar]
- 2.Trojanowski JQ, Revesz T. Proposed neuropathological criteria for the post mortem diagnosis of multiple system atrophy. Neuropathol Appl Neurobiol. 2007;33:615–620. doi: 10.1111/j.1365-2990.2007.00907.x. [DOI] [PubMed] [Google Scholar]
- 3.Tison F, Yekhlef F, Chrysostome V, Balestre E, Quinn NP, Poewe W, Wenning GK. Parkinsonism in multiple system atrophy: natural history, severity (UPDRS-III), and disability assessment compared with Parkinson’s disease. Mov Disord. 2002;17:701–709. doi: 10.1002/mds.10171. [DOI] [PubMed] [Google Scholar]
- 4.Lin DJ, Hermann KL, Schmahmann JD. Multiple system atrophy of the cerebellar type: clinical state of the art. Mov Disord. 2014;29:294–304. doi: 10.1002/mds.25847. [DOI] [PubMed] [Google Scholar]
- 5.Figueroa JJ, Singer W, Parsaik A, Benarroch EE, Ahlskog JE, Fealey RD, Parisi JE, Sandroni P, Mandrekar J, Iodice V, Low PA, Bower JH. Multiple system atrophy: prognostic indicators of survival. Mov Disord. 2014;29:1151–1157. doi: 10.1002/mds.25927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wenning GK, Geser F, Krismer F, Seppi K, Duerr S, Boesch S, Kollensperger M, Goebel G, Pfeiffer KP, Barone P, Pellecchia MT, Quinn NP, Koukouni V, Fowler CJ, Schrag A, Mathias CJ, Giladi N, Gurevich T, Dupont E, Ostergaard K, Nilsson CF, Widner H, Oertel W, Eggert KM, Albanese A, del Sorbo F, Tolosa E, Cardozo A, Deuschl G, Hellriegel H, Klockgether T, Dodel R, Sampaio C, Coelho M, Djaldetti R, Melamed E, Gasser T, Kamm C, Meco G, Colosimo C, Rascol O, Meissner WG, Tison F, Poewe W. The natural history of multiple system atrophy: a prospective European cohort study. Lancet Neurol. 2013;12:264–274. doi: 10.1016/S1474-4422(12)70327-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cykowski MD, Coon EA, Powell SZ, Jenkins SM, Benarroch EE, Low PA, Schmeichel AM, Parisi JE. Expanding the spectrum of neuronal pathology in multiple system atrophy. Brain. 2015;138:2293–2309. doi: 10.1093/brain/awv114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Collaboration M-SAR. Mutations in COQ2 in familial and sporadic multiple-system atrophy. N Engl J Med. 2013;369:233–244. doi: 10.1056/NEJMoa1212115. [DOI] [PubMed] [Google Scholar]
- 9.Hara K, Momose Y, Tokiguchi S, Shimohata M, Terajima K, Onodera O, Kakita A, Yamada M, Takahashi H, Hirasawa M, Mizuno Y, Ogata K, Goto J, Kanazawa I, Nishizawa M, Tsuji S. Multiplex families with multiple system atrophy. Arch Neurol. 2007;64:545–551. doi: 10.1001/archneur.64.4.545. [DOI] [PubMed] [Google Scholar]
- 10.Soma H, Yabe I, Takei A, Fujiki N, Yanagihara T, Sasaki H. Heredity in multiple system atrophy. J Neurol Sci. 2006;240:107–110. doi: 10.1016/j.jns.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 11.Kiely AP, Ling H, Asi YT, Kara E, Proukakis C, Schapira AH, Morris HR, Roberts HC, Lubbe S, Limousin P, Lewis PA, Lees AJ, Quinn N, Hardy J, Love S, Revesz T, Houlden H, Holton JL. Distinct clinical and neuropathological features of G51D SNCA mutation cases compared with SNCA duplication and H50Q mutation. Mol Neurodegener. 2015;10:41. doi: 10.1186/s13024-015-0038-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wenning GK, Seppi K, Tison F, Jellinger K. A novel grading scale for striatonigral degeneration (multiple system atrophy) J Neural Transm. 2002;109:307–320. doi: 10.1007/s007020200025. [DOI] [PubMed] [Google Scholar]
- 13.Tu PH, Galvin JE, Baba M, Giasson B, Tomita T, Leight S, Nakajo S, Iwatsubo T, Trojanowski JQ, Lee VM. Glial cytoplasmic inclusions in white matter oligodendrocytes of multiple system atrophy brains contain insoluble alpha-synuclein. Ann Neurol. 1998;44:415–422. doi: 10.1002/ana.410440324. [DOI] [PubMed] [Google Scholar]
- 14.Abeliovich A, Schmitz Y, Farinas I, Choi-Lundberg D, Ho WH, Castillo PE, Shinsky N, Verdugo JM, Armanini M, Ryan A, Hynes M, Phillips H, Sulzer D, Rosenthal A. Mice lacking alpha-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron. 2000;25:239–252. doi: 10.1016/s0896-6273(00)80886-7. [DOI] [PubMed] [Google Scholar]
- 15.Jo E, McLaurin J, Yip CM, St George-Hyslop P, Fraser PE. alpha-Synuclein membrane interactions and lipid specificity. J Biol Chem. 2000;275:34328–34334. doi: 10.1074/jbc.M004345200. [DOI] [PubMed] [Google Scholar]
- 16.Spillantini MG, Crowther RA, Jakes R, Hasegawa M, Goedert M. alpha-Synuclein in filamentous inclusions of Lewy bodies from Parkinson’s disease and dementia with Lewy bodies. Proc Natl Acad Sci U S A. 1998;95:6469–6473. doi: 10.1073/pnas.95.11.6469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brettschneider J, Del Tredici K, Toledo JB, Robinson JL, Irwin DJ, Grossman M, Suh E, Van Deerlin VM, Wood EM, Baek Y, Kwong L, Lee EB, Elman L, McCluskey L, Fang L, Feldengut S, Ludolph AC, Lee VM, Braak H, Trojanowski JQ. Stages of pTDP-43 pathology in amyotrophic lateral sclerosis. Ann Neurol. 2013;74:20–38. doi: 10.1002/ana.23937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Asi YT, Simpson JE, Heath PR, Wharton SB, Lees AJ, Revesz T, Houlden H, Holton JL. Alpha-synuclein mRNA expression in oligodendrocytes in MSA. Glia. 2014;62:964–970. doi: 10.1002/glia.22653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jucker M, Walker LC. Self-propagation of pathogenic protein aggregates in neurodegenerative diseases. Nature. 2013;501:45–51. doi: 10.1038/nature12481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Song YJ, Lundvig DM, Huang Y, Gai WP, Blumbergs PC, Hojrup P, Otzen D, Halliday GM, Jensen PH. p25alpha relocalizes in oligodendroglia from myelin to cytoplasmic inclusions in multiple system atrophy. Am J Pathol. 2007;171:1291–1303. doi: 10.2353/ajpath.2007.070201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guo JL, Lee VM. Cell-to-cell transmission of pathogenic proteins in neurodegenerative diseases. Nat Med. 2014;20:130–138. doi: 10.1038/nm.3457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luk KC, Kehm V, Carroll J, Zhang B, O’Brien P, Trojanowski JQ, Lee VM. Pathological alpha-synuclein transmission initiates Parkinson-like neurodegeneration in nontransgenic mice. Science. 2012;338:949–953. doi: 10.1126/science.1227157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luk KC, Kehm VM, Zhang B, O’Brien P, Trojanowski JQ, Lee VM. Intracerebral inoculation of pathological alpha-synuclein initiates a rapidly progressive neurodegenerative alpha-synucleinopathy in mice. J Exp Med. 2012;209:975–986. doi: 10.1084/jem.20112457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kordower JH, Chu Y, Hauser RA, Freeman TB, Olanow CW. Lewy body-like pathology in long-term embryonic nigral transplants in Parkinson’s disease. Nat Med. 2008;14:504–506. doi: 10.1038/nm1747. [DOI] [PubMed] [Google Scholar]
- 25.Volpicelli-Daley LA, Luk KC, Patel TP, Tanik SA, Riddle DM, Stieber A, Meaney DF, Trojanowski JQ, Lee VM. Exogenous alpha-synuclein fibrils induce Lewy body pathology leading to synaptic dysfunction and neuron death. Neuron. 2011;72:57–71. doi: 10.1016/j.neuron.2011.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Desplats P, Lee HJ, Bae EJ, Patrick C, Rockenstein E, Crews L, Spencer B, Masliah E, Lee SJ. Inclusion formation and neuronal cell death through neuron-to-neuron transmission of alpha-synuclein. Proc Natl Acad Sci U S A. 2009;106:13010–13015. doi: 10.1073/pnas.0903691106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mougenot AL, Nicot S, Bencsik A, Morignat E, Verchere J, Lakhdar L, Legastelois S, Baron T. Prion-like acceleration of a synucleinopathy in a transgenic mouse model. Neurobiol Aging. 2012;33:2225–2228. doi: 10.1016/j.neurobiolaging.2011.06.022. [DOI] [PubMed] [Google Scholar]
- 28.Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 29.Richter-Landsberg C, Gorath M, Trojanowski JQ, Lee VM. alpha-synuclein is developmentally expressed in cultured rat brain oligodendrocytes. J Neurosci Res. 2000;62:9–14. doi: 10.1002/1097-4547(20001001)62:1<9::AID-JNR2>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 30.Feldengut S, Del Tredici K, Braak H. Paraffin sections of 70–100 µm: a novel technique and its benefits for studying the nervous system. J Neurosci Methods. 2013;215:241–244. doi: 10.1016/j.jneumeth.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 31.Gilman S, Low PA, Quinn N, Albanese A, Ben-Shlomo Y, Fowler CJ, Kaufmann H, Klockgether T, Lang AE, Lantos PL, Litvan I, Mathias CJ, Oliver E, Robertson D, Schatz I, Wenning GK. Consensus statement on the diagnosis of multiple system atrophy. J Neurol Sci. 1999;163:94–98. doi: 10.1016/s0022-510x(98)00304-9. [DOI] [PubMed] [Google Scholar]
- 32.Gilman S, Wenning GK, Low PA, Brooks DJ, Mathias CJ, Trojanowski JQ, Wood NW, Colosimo C, Durr A, Fowler CJ, Kaufmann H, Klockgether T, Lees A, Poewe W, Quinn N, Revesz T, Robertson D, Sandroni P, Seppi K, Vidailhet M. Second consensus statement on the diagnosis of multiple system atrophy. Neurology. 2008;71:670–676. doi: 10.1212/01.wnl.0000324625.00404.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Toledo JB, Van Deerlin VM, Lee EB, Suh E, Baek Y, Robinson JL, Xie SX, McBride J, Wood EM, Schuck T, Irwin DJ, Gross RG, Hurtig H, McCluskey L, Elman L, Karlawish J, Schellenberg G, Chen-Plotkin A, Wolk D, Grossman M, Arnold SE, Shaw LM, Lee VM, Trojanowski JQ. A platform for discovery: the University of Pennsylvania Integrated Neurodegenerative Disease Biobank. Alzheimers Dement. 2013;10:477–484. doi: 10.1016/j.jalz.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xie SX, Baek Y, Grossman M, Arnold SE, Karlawish J, Siderowf A, Hurtig H, Elman L, McCluskey L, Van Deerlin V, Lee VM, Trojanowski JQ. Building an integrated neurodegenerative disease database at an academic health center. Alzheimers Dement. 2011;7:e84–e93. doi: 10.1016/j.jalz.2010.08.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Geser F, Brandmeir NJ, Kwong LK, Martinez-Lage M, Elman L, McCluskey L, Xie SX, Lee VM, Trojanowski JQ. Evidence of multisystem disorder in whole-brain map of pathological TDP-43 in amyotrophic lateral sclerosis. Arch Neurol. 2008;65:636–641. doi: 10.1001/archneur.65.5.636. [DOI] [PubMed] [Google Scholar]
- 36.Duda JE, Giasson BI, Mabon ME, Lee VM, Trojanowski JQ. Novel antibodies to synuclein show abundant striatal pathology in Lewy body diseases. Ann Neurol. 2002;52:205–210. doi: 10.1002/ana.10279. [DOI] [PubMed] [Google Scholar]
- 37.Neumann M, Kwong LK, Lee EB, Kremmer E, Flatley A, Xu Y, Forman MS, Troost D, Kretzschmar HA, Trojanowski JQ, Lee VM. Phosphorylation of S409/410 of TDP-43 is a consistent feature in all sporadic and familial forms of TDP-43 proteinopathies. Acta Neuropathol. 2009;117:137–149. doi: 10.1007/s00401-008-0477-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee EB, Leng LZ, Zhang B, Kwong L, Trojanowski JQ, Abel T, Lee VM. Targeting amyloid-beta peptide (Abeta) oligomers by passive immunization with a conformation-selective monoclonal antibody improves learning and memory in Abeta precursor protein (APP) transgenic mice. J Biol Chem. 2006;281:4292–4299. doi: 10.1074/jbc.M511018200. [DOI] [PubMed] [Google Scholar]
- 39.Arnold SE, Toledo JB, Appleby DH, Xie SX, Wang LS, Baek Y, Wolk DA, Lee EB, Miller BL, Lee VM, Trojanowski JQ. Comparative survey of the topographical distribution of signature molecular lesions in major neurodegenerative diseases. J Comp Neurol. 2013;521:4339–4355. doi: 10.1002/cne.23430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Braak H, Rub U, Del Tredici K. Involvement of precerebellar nuclei in multiple system atrophy. Neuropathol Appl Neurobiol. 2003;29:60–76. doi: 10.1046/j.1365-2990.2003.00432.x. [DOI] [PubMed] [Google Scholar]
- 41.Del Tredici K, Braak H. Spinal cord lesions in sporadic Parkinson’s disease. Acta Neuropathol. 2012;124:643–664. doi: 10.1007/s00401-012-1028-y. [DOI] [PubMed] [Google Scholar]
- 42.Waxman EA, Duda JE, Giasson BI. Characterization of antibodies that selectively detect alpha-synuclein in pathological inclusions. Acta Neuropathol. 2008;116:37–46. doi: 10.1007/s00401-008-0375-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sobue G, Hashizume Y, Yasuda T, Mukai E, Kumagai T, Mitsuma T, Trojanowski JQ. Phosphorylated high molecular weight neurofilament protein in lower motor neurons in amyotrophic lateral sclerosis and other neurodegenerative diseases involving ventral horn cells. Acta Neuropathol. 1990;79:402–408. doi: 10.1007/BF00308716. [DOI] [PubMed] [Google Scholar]
- 44.Trojanowski JQ, Kelsten ML, Lee VM. Phosphate-dependent and independent neurofilament protein epitopes are expressed throughout the cell cycle in human medulloblastoma (D283 MED) cells. Am J Pathol. 1989;135:747–758. [PMC free article] [PubMed] [Google Scholar]
- 45.Federoff M, Price TR, Sailer A, Scholz S, Hernandez D, Nicolas A, Singleton AB, Nalls M, Houlden H. Genome-wide estimate of the heritability of multiple system atrophy. Parkinsonism Relat Disord. 2016;22:35–41. doi: 10.1016/j.parkreldis.2015.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jellinger KA, Seppi K, Wenning GK. Grading of neuropathology in multiple system atrophy: proposal for a novel scale. Mov Disord. 2005;20(Suppl. 12):S29–S36. doi: 10.1002/mds.20537. [DOI] [PubMed] [Google Scholar]
- 47.Mochizuki Y, Mizutani T, Warabi Y, Shimizu T, Isozaki E. The somatosensory cortex in multiple system atrophy. J Neurol Sci. 2008;271:174–179. doi: 10.1016/j.jns.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 48.Sakamoto M, Uchihara T, Nakamura A, Mizutani T, Mizusawa H. Progressive accumulation of ubiquitin and disappearance of alpha-synuclein epitope in multiple system atrophy-associated glial cytoplasmic inclusions: triple fluorescence study combined with Gallyas-Braak method. Acta Neuropathol. 2005;110:417–425. doi: 10.1007/s00401-005-1066-9. [DOI] [PubMed] [Google Scholar]
- 49.Armstrong RA, Lantos PL, Cairns NJ. Spatial patterns of alpha-synuclein positive glial cytoplasmic inclusions in multiple system atrophy. Mov Disord. 2004;19:109–112. doi: 10.1002/mds.10637. [DOI] [PubMed] [Google Scholar]
- 50.Nishie M, Mori F, Fujiwara H, Hasegawa M, Yoshimoto M, Iwatsubo T, Takahashi H, Wakabayashi K. Accumulation of phosphorylated alpha-synuclein in the brain and peripheral ganglia of patients with multiple system atrophy. Acta Neuropathol. 2004;107:292–298. doi: 10.1007/s00401-003-0811-1. [DOI] [PubMed] [Google Scholar]
- 51.Gai WP, Pountney DL, Power JH, Li QX, Culvenor JG, McLean CA, Jensen PH, Blumbergs PC. alpha-Synuclein fibrils constitute the central core of oligodendroglial inclusion filaments in multiple system atrophy. Exp Neurol. 2003;181:68–78. doi: 10.1016/s0014-4886(03)00004-9. [DOI] [PubMed] [Google Scholar]
- 52.Dickson DW, Liu W, Hardy J, Farrer M, Mehta N, Uitti R, Mark M, Zimmerman T, Golbe L, Sage J, Sima A, D’Amato C, Albin R, Gilman S, Yen SH. Widespread alterations of alpha-synuclein in multiple system atrophy. Am J Pathol. 1999;155:1241–1251. doi: 10.1016/s0002-9440(10)65226-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Spillantini MG, Crowther RA, Jakes R, Cairns NJ, Lantos PL, Goedert M. Filamentous alpha-synuclein inclusions link multiple system atrophy with Parkinson’s disease and dementia with Lewy bodies. Neurosci Lett. 1998;251:205–208. doi: 10.1016/s0304-3940(98)00504-7. [DOI] [PubMed] [Google Scholar]
- 54.Papp MI, Kahn JE, Lantos PL. Glial cytoplasmic inclusions in the CNS of patients with multiple system atrophy (striatonigral degeneration, olivopontocerebellar atrophy and Shy-Drager syndrome) J Neurol Sci. 1989;94:79–100. doi: 10.1016/0022-510x(89)90219-0. [DOI] [PubMed] [Google Scholar]
- 55.Kato S, Nakamura H. Cytoplasmic argyrophilic inclusions in neurons of pontine nuclei in patients with olivopontocerebellar atrophy: immunohistochemical and ultrastructural studies. Acta Neuropathol. 1990;79:584–594. doi: 10.1007/BF00294235. [DOI] [PubMed] [Google Scholar]
- 56.Arima K, Murayama S, Mukoyama M, Inose T. Immunocytochemical and ultrastructural studies of neuronal and oligodendroglial cytoplasmic inclusions in multiple system atrophy. 1. Neuronal cytoplasmic inclusions. Acta Neuropathol. 1992;83:453–460. doi: 10.1007/BF00310020. [DOI] [PubMed] [Google Scholar]
- 57.Papp MI, Lantos PL. Accumulation of tubular structures in oligodendroglial and neuronal cells as the basic alteration in multiple system atrophy. J Neurol Sci. 1992;107:172–182. doi: 10.1016/0022-510x(92)90286-t. [DOI] [PubMed] [Google Scholar]
- 58.Maroteaux L, Campanelli JT, Scheller RH. Synuclein: a neuron-specific protein localized to the nucleus and presynaptic nerve terminal. J Neurosci. 1988;8:2804–2815. doi: 10.1523/JNEUROSCI.08-08-02804.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Matsusue E, Fujii S, Kanasaki Y, Kaminou T, Ohama E, Ogawa T. Cerebellar lesions in multiple system atrophy: postmortem MR imaging-pathologic correlations. AJNR Am J Neuroradiol. 2009;30:1725–1730. doi: 10.3174/ajnr.A1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wu YT, Shyu KK, Jao CW, Liao YL, Wang TY, Wu HM, Wang PS, Soong BW. Quantifying cerebellar atrophy in multiple system atrophy of the cerebellar type (MSA-C) using three-dimensional gyrification index analysis. NeuroImage. 2012;61:1–9. doi: 10.1016/j.neuroimage.2012.02.057. [DOI] [PubMed] [Google Scholar]
- 61.Ramirez EP, Vonsattel JP. Neuropathologic changes of multiple system atrophy and diffuse Lewy body disease. Semin Neurol. 2014;34:210–216. doi: 10.1055/s-0034-1381732. [DOI] [PubMed] [Google Scholar]
- 62.Makino T, Ito S, Kuwabara S. Involvement of pontine transverse and longitudinal fibers in multiple system atrophy: a tractography-based study. J Neurol Sci. 2011;303:61–66. doi: 10.1016/j.jns.2011.01.014. [DOI] [PubMed] [Google Scholar]
- 63.Yokoyama T, Hasegawa K, Horiuchi E, Yagishita S. Multiple system atrophy (MSA) with massive macrophage infiltration in the ponto-cerebellar afferent system. Neuropathology: official journal of the Japanese Society of. Neuropathology. 2007;27:375–377. doi: 10.1111/j.1440-1789.2007.00777.x. [DOI] [PubMed] [Google Scholar]
- 64.Abe K, Hikita T, Yokoe M, Mihara M, Sakoda S. The ‘cross’ signs in patients with multiple system atrophy: a quantitative study. J Neuroimaging. 2006;16:73–77. doi: 10.1177/1051228405279988. [DOI] [PubMed] [Google Scholar]
- 65.Yang H, Wang X, Liao W, Zhou G, Li L, Ouyang L. Application of diffusion tensor imaging in multiple system atrophy: the involvement of pontine transverse and longitudinal fibers. Int J Neurosci. 2014;125:18–14. doi: 10.3109/00207454.2014.896914. [DOI] [PubMed] [Google Scholar]
- 66.Wenning GK, Tison F, Ben Shlomo Y, Daniel SE, Quinn NP. Multiple system atrophy: a review of 203 pathologically proven cases. Mov Disord. 1997;12:133–147. doi: 10.1002/mds.870120203. [DOI] [PubMed] [Google Scholar]
- 67.Lemkey Johnston N, Larramendi LM. The separation and identification of fractions of nonmyelinated axons from the cerebellum of the cat. Exp Brain Res. 1968;5:326–340. doi: 10.1007/BF00235907. [DOI] [PubMed] [Google Scholar]
- 68.Hamori J, Szentagothai J. Identification of synapses formed in the cerebellar cortex by Purkinje axon collaterals: an electron microscope study. Exp Brain Res. 1968;5:118–128. doi: 10.1007/BF00238701. [DOI] [PubMed] [Google Scholar]
- 69.Palay SL, Chan-Palay V. Cerebellar Cortex: Cytology and Organization. 1st. Berlin: Springer; 1974. [Google Scholar]
- 70.Armstrong RA, Lantos PL, Cairns NJ. Multiple system atrophy: laminar distribution of the pathological changes in frontal and temporal neocortex–a study in ten patients. Clin Neuropathol. 2005;24:230–235. [PubMed] [Google Scholar]
- 71.Papp MI, Lantos PL. The distribution of oligodendroglial inclusions in multiple system atrophy and its relevance to clinical symptomatology. Brain. 1994;117(Pt 2):235–243. doi: 10.1093/brain/117.2.235. [DOI] [PubMed] [Google Scholar]
- 72.Tsuchiya K, Ozawa E, Haga C, Watabiki S, Ikeda M, Sano M, Ooe K, Taki K, Ikeda K. Constant involvement of the Betz cells and pyramidal tract in multiple system atrophy: a clinicopathological study of seven autopsy cases. Acta Neuropathol. 2000;99:628–636. doi: 10.1007/s004010051173. [DOI] [PubMed] [Google Scholar]
- 73.Su M, Yoshida Y, Hirata Y, Watahiki Y, Nagata K. Primary involvement of the motor area in association with the nigrostriatal pathway in multiple system atrophy: neuropathological and morphometric evaluations. Acta Neuropathol. 2001;101:57–64. doi: 10.1007/s004010000273. [DOI] [PubMed] [Google Scholar]
- 74.Fox CA, Rafols JA. The striatal efferents in the globus pallidus and in the substantia nigra. Res Publ Assoc Res Nerv Ment Dis. 1976;55:37–55. [PubMed] [Google Scholar]
- 75.Wenning GK, Tison F, Elliott L, Quinn NP, Daniel SE. Olivopontocerebellar pathology in multiple system atrophy. Mov Disord. 1996;11:157–162. doi: 10.1002/mds.870110207. [DOI] [PubMed] [Google Scholar]
- 76.Benarroch EE, Schmeichel AM, Sandroni P, Low PA, Parisi JE. Differential involvement of hypothalamic vasopressin neurons in multiple system atrophy. Brain. 2006;129:2688–2696. doi: 10.1093/brain/awl109. [DOI] [PubMed] [Google Scholar]
- 77.Benarroch EE, Schmeichel AM, Sandroni P, Low PA, Parisi JE. Involvement of hypocretin neurons in multiple system atrophy. Acta Neuropathol. 2007;113:75–80. doi: 10.1007/s00401-006-0150-0. [DOI] [PubMed] [Google Scholar]
- 78.Thompson RH, Swanson LW. Structural characterization of a hypothalamic visceromotor pattern generator network. Brain Res Brain Res Rev. 2003;41:153–202. doi: 10.1016/s0165-0173(02)00232-1. [DOI] [PubMed] [Google Scholar]
- 79.Wenning GK, Quinn N, Magalhaes M, Mathias C, Daniel SE. ‘Minimal change’ multiple system atrophy. Mov Disord. 1994;9:161–166. doi: 10.1002/mds.870090206. [DOI] [PubMed] [Google Scholar]
- 80.Ling H, Asi YT, Petrovic IN, Ahmed Z, Prashanth LK, Hazrati LN, Nishizawa M, Ozawa T, Lang A, Lees AJ, Revesz T, Holton JL. Minimal change multiple system atrophy: an aggressive variant? Mov Disord. 2015;30:960–967. doi: 10.1002/mds.26220. [DOI] [PubMed] [Google Scholar]
- 81.Yazawa I, Giasson BI, Sasaki R, Zhang B, Joyce S, Uryu K, Trojanowski JQ, Lee VM. Mouse model of multiple system atrophy alpha-synuclein expression in oligodendrocytes causes glial and neuronal degeneration. Neuron. 2005;45:847–859. doi: 10.1016/j.neuron.2005.01.032. [DOI] [PubMed] [Google Scholar]
- 82.Horner PJ, Thallmair M, Gage FH. Defining the NG2-expressing cell of the adult CNS. J Neurocytol. 2002;31:469–480. doi: 10.1023/a:1025739630398. [DOI] [PubMed] [Google Scholar]
- 83.Lee Y, Morrison BM, Li Y, Lengacher S, Farah MH, Hoffman PN, Liu Y, Tsingalia A, Jin L, Zhang PW, Pellerin L, Magistretti PJ, Rothstein JD. Oligodendroglia metabolically support axons and contribute to neurodegeneration. Nature. 2012;487:443–448. doi: 10.1038/nature11314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bleasel JM, Wong JH, Halliday GM, Kim WS. Lipid dysfunction and pathogenesis of multiple system atrophy. Acta Neuropathol Commun. 2014;2:15. doi: 10.1186/2051-5960-2-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bleasel JM, Hsiao JH, Halliday GM, Kim WS. Increased expression of ABCA8 in multiple system atrophy brain is associated with changes in pathogenic proteins. J Parkinsons Dis. 2013;3:331–339. doi: 10.3233/JPD-130203. [DOI] [PubMed] [Google Scholar]
- 86.Kordower JH, Dodiya HB, Kordower AM, Terpstra B, Paumier K, Madhavan L, Sortwell C, Steece-Collier K, Collier TJ. Transfer of host-derived alpha synuclein to grafted dopaminergic neurons in rat. Neurobiol Dis. 2011;43:552–557. doi: 10.1016/j.nbd.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Masuda-Suzukake M, Nonaka T, Hosokawa M, Oikawa T, Arai T, Akiyama H, Mann DM, Hasegawa M. Prion-like spreading of pathological alpha-synuclein in brain. Brain. 2013;136:1128–1138. doi: 10.1093/brain/awt037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Prusiner SB, Woerman AL, Mordes DA, Watts JC, Rampersaud R, Berry DB, Patel S, Oehler A, Lowe JK, Kravitz SN, Geschwind DH, Glidden DV, Halliday GM, Middleton LT, Gentleman SM, Grinberg LT, Giles K. Evidence for alpha-synuclein prions causing multiple system atrophy in humans with parkinsonism. Proc Natl Acad Sci U S A. 2015;112:E5308–E5317. doi: 10.1073/pnas.1514475112. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Double-labelling immunofluorescence (IF) analysed by confocal microscopy.
Table S1. Correlation of α-synuclein pathology in multiple system atrophy of the cerebellar type (MSA-C) with duration of disease, age at onset and age at death.