Abstract
Autism spectrum disorder (ASD) now affects one in 68 births in the United States and is the fastest growing neurodevelopmental disability worldwide. Alarmingly, for the majority of cases, the causes of ASD are largely unknown, but it is becoming increasingly accepted that ASD is no longer defined simply as a behavioral disorder, but rather as a highly complex and heterogeneous biological disorder. While research has focused on the identification of genetic abnormalities, emerging studies increasingly suggest immune dysfunction is a viable risk factor contributing to the neurodevelopmental deficits observed in ASD. This review summarizes the investigations implicating autoimmunity and autoantibodies in ASD.
Keywords: Autism, autoimmunity, maternal autoantibodies, immune, neurodevelopmental, pregnancy
Introduction
Autism spectrum disorder (ASD) is a highly heterogeneous neurodevelopmental disorder characterized by behavioral, social, and cognitive deficits(1). Since its first description nearly seventy years ago, our understanding of the disorder has changed considerably(2). While the diagnosis always relies on behavioral domains and symptomology, there are likely multiple, biologically defined subgroups within the ASD spectrum(3–7). Specifically, there is growing evidence that supports maternal immune dysfunction may underlie the behavioral abnormalities observed in a subset of children affected with the disorder(8). Several immunologic risk factors have been described including: genetic associations with immune-related genes(9–16), family history of autoimmune disease(15, 17–21), maternal inflammation and infection during pregnancy(22–27), and altered immune responses in the children, and are associated with increased impairments in core and associated features of ASD(28). More specific to this review, maternal anti-brain autoantibodies, which are thought to access the fetal compartment during gestation, have been identified as one risk factor for developing ASD and are proposed to contribute to early neurodevelopmental perturbations in the developing fetus(29–31).
The Relationship between Autoimmunity and ASD
ASD shares many of the features typically recognized in autoimmune disorders; there is a strong familial predisposition and association with immune abnormalities, the genetic predisposition is complex and believed to be polygenic, environmental factors can increase or modulate risk, and there are substantial gender disparities(17, 32). Many of the genes implicated in autoimmunity are also clustered within families with ASD. However, while several candidate genes have been implicated in the disorder, replication of the majority of these findings has been elusive, and hints that in addition to genetic factors, environmental influences, like those observed in autoimmune diseases, may be contributing to the disorder in some cases (33). Further, ASD is four times more prevalent in males, and recent studies suggest the prevalence in boys is closer to 1 in 42(34). The epidemiological links between autoimmunity and ASD are compelling, and the similarities have spurred several investigators to connect biologically rooted autoimmune disorders with behaviorally defined ASD.
The first investigations supporting the idea that autoimmunity could be etiologically relevant to ASD were described in a 1971 case report that presented on a child with ASD that had a strong family history of autoimmune disorders (35). Since that time, numerous other reports of autoimmune or immune mediated disorders associated with an increased ASD risk have emerged (15, 17–19, 36, 37). In one of the largest studies to date that included close to 700,000 children, an increased risk, as expressed by an increased risk ratio (IRR), of ASD was observed in mothers with rheumatoid arthritis (IRR: 1.70) and celiac disease (IRR: 2.97); a familial history of type I diabetes (IRR: 1.78) was also found to increase the risk of having child with ASD(18). Similar results were seen in a recent systematic review and meta analysis performed by Wu et al. (2015), which suggested a family history of autoimmune disease was associated with a higher risk of having an affected child, and a statistically significant association was observed in families with hypothyroidism, type I diabetes, rheumatoid arthritis and psoriasis(20). A similar analysis by Chen et al. (2016) found children born to mothers with autoimmune disease were 34% more likely to develop ASD(21). A genetic predisposition in autoimmune diseases is often attributed to specific major histocompatibility complex (MHC) haplotypes and polymorphisms in genes involved in establishing self-tolerance and immune regulation(38). Similar polymorphisms and associations have been found in ASD. Most notably, studies have correlated the null allele of the C4B gene(9, 14), the extended haplotype B44-C30-DR4, the third hypervariable region (HVR-3) of certain DRP 1 alleles (10), and HLA-A2 alleles with an increased susceptibility for ASD (11, 39). Interestingly, these alleles and haplotypes are also associated with the same aforementioned autoimmune diseases that are linked with an increased risk of having a child with ASD. Another important functional polymorphism that often clusters in families with ASD is a genetic variant that disrupts transcription of the gene encoding the MET receptor tyrosine kinase(12), which has important roles in both immune regulation and neurodevelopment. The similarities between the findings in ASD and autoimmunity suggest an immune-related subtype of ASD (Table 1).
Table 1.
Studies linking Autoimmunity and ASD
| Type of Study | Research Group | Year | Findings |
|---|---|---|---|
| Case Study | Money et al. (35) | 1971 | Child with ASD had a family history of autoimmune disease |
| Research Study | Warren et al. (9) Mostafa et al. (14) |
1991 2010 |
The null allele of the C4B gene and the extended haplotypeB44-C30-DR4 is associated with autism. |
| Research Study | Warren et al. (10) | 1996 | The third hypervariable region (HVR-3) of certain DRP 1 alleles have very strong association with ASD. |
| Research Study | Torres et al. (11) | 2006 | The frequency of HLA-A2 alleles was significantly increased in autistic subjects compared with normal allelic frequencies |
| Research Study | Campbell et al. (12) |
2008 | The MET promoter variant rs1858830 C allele was associated with ASD |
| Population Study | Atladottir et al. (18) |
2009 | Increased risk of ASD in mothers with Rheumatoid Arthritis and Celiac Disease and in families with Type I Diabetes |
|
Systemic Review and Meta Analysis |
Wu et al. (20) | 2015 | A statistically significant association with ASD was observed in families with hypothyroidism, type I diabetes, rheumatoid arthritis and psoriasis |
|
Systemic Review and Meta Analysis |
Chen et al. (21) | 2016 | Children born to mothers with autoimmune disease were 34% more likely to develop ASD |
The notion that immune system dysfunction could be a plausible factor in the etiology of ASD is derived from the now recognized importance of the immune system in healthy neurodevelopment, and the ability of immune dysregulation to influence patterns of behavior, especially during gestation (28, 39–41). Thus, there is growing recognition that the maternal immune response during critical windows of development has a long lasting impact on neurodevelopmental outcomes.
The Detection of Autoantibodies in ASD
The recognition that maternal antibodies may lead to developmental defects in the fetal nervous system is not novel. Experiments performed in the late 1950’s demonstrated when female mice were immunized with a brain emulsion and later mated, subsequent offspring had gross brain abnormalities, which the author attributed to maternal antibodies directed against the brain and nervous system of the embryos(42). This idea gained favor again in the early 1970’s, when researchers, concerned that maternal environmental exposures could have detrimental effects on fetal nervous system development, discovered maternal IgG was present in fetal cerebral spinal fluid (CSF), and was able to gain access to brain during gestation and early life due the permissive nature of the blood brain barrier during that period(43–45). Succeeding experiments by several groups showed that antibrain antibodies could induce behavioral changes in the exposed offspring(46, 47). However, it was not until the 1990’s that the first studies implicating maternal antibodies in the etiology of ASD emerged.
The earliest study performed by Warren et al. (1990) confirmed their hypothesis that maternal autoantibodies reactive to proteins displayed on paternal lymphocytes, which are often found in women with a history of miscarriage, are disproportionately observed in mothers with children with ASD (48). While the sample sizes of the study were small, it prompted other investigators to conduct similar etiological studies into the role of maternal antibodies and aberrant neurodevelopment associated with ASD. Subsequently, a single sample from the serum of a mother with a child with ASD was used to demonstrate that antibodies purified and developmental delay reacted to mouse neurons and when injected into a dam during gestation resulted in deficits in the exploratory behaviors in the resulting offspring (49).
Acknowledging the importance of these pivotal pilot studies, researchers began to expand these endeavors to include larger clinical populations (Table 1). One of the first expanded, case-controlled studies published was performed by Braunschweig et al. (2008) and included equal numbers of children with autism (n=61) and mothers of typically developing children (n=62), along with a subset of mothers that had children with developmental delay (n=40). This study found a significant correlation between the paired maternal antibody reactivity to fetal brain proteins located within the 37 and 73kDa molecular weight bands on a human fetal brain immunoblot and a diagnosis of ASD in the child. Further, this reactivity was only in plasma samples obtained from families affected with ASD, as reactivity was not seen in families that had typically developing children or children diagnosed with developmental delay (50). In another small study by Zimmerman et al. (2007), it was shown via immunoblotting that only antibodies from mothers of autistic children recognized fetal rat brain proteins, as no antibody reactivity was detected in the control maternal group, and they were specific to fetal antigens, as they did not bind to proteins derived from postnatal and adult rat brains(51). These reports were closely followed by a study that examined the serum reactivity of 100 mothers of children with ASD and 100 age-matched control mothers, and determined antibody reactivity to fetal brain proteins observed in mothers of children with ASD significantly differed from the control groups(52).
A similar study utilizing banked mid-pregnancy (prospective) blood samples also observed maternal autoantibody binding to antigens near 37 and 73 kDa was only found in women whose children later received a diagnosis of ASD(53). Surprisingly, all of the aforementioned reports were unable to find a correlation between a family history of autoimmunity and the presence of maternal anti-brain antibodies. However, a later study conducted by Heuer et al. reported 95% of mothers with autoantibodies to both the 37 and 73 kDa fetal brain bands (found only in ASD) possessed the MET ‘C’ allele,, which provided the first link between a functional immune-related outcome and an ASD susceptibility gene(54). In this study population, among the 346 mothers of both ASD and TD children that were negative for the 37/73-kDa bands, 101 (29%) were C/C, 154 (45%) were C/G and 91 (26%) were G/G. Thus, the MET ‘C’ allele appears to confer susceptibility rather than cause for the production of these autoantibodies. Furthermore, as this allelic variant was shown to lead to decreased levels of IL-10, a crucial anti-inflammatory cytokine, mothers with this allele are hypothesized to be at increased risk of autoimmunity and autoantibody generation, hinting at a potential mechanism behind the loss of self-tolerance in these mothers. Studies conducted by Brimberg et al. support this hypothesis, as their studies not only found that mothers of children with ASD preferentially carried autoantibodies to fetal brain tissue, but these mothers were also significantly more likely to have anti-nuclear autoantibodies than mothers of typically developing children and mothers of children with ASD that are anti-brain autoantibody negative. Interestingly, it was also discovered mothers with anti-brain autoantibodies had increased incidence of rheumatoid arthritis (RA) and systemic lupus erythematosus (SLE), and, conversely, anti-brain autoantibodies were detected in women with RA, providing additional evidence that brain-reactive maternal autoantibodies are related to autoimmunity(55). While the methods for the Brimberg study, in which serum samples were incubated with mouse tissue sections and autoantibodies were detected using immunofluorescence, differed from previous studies utilizing denatured proteins and detecting autoantibodies via immunoblotting, similar results were achieved in that maternal autoantibodies were elevated in the mothers of children with ASD. Although the specific maternal anti-fetal brain antibodies were disproportionately detected in mothers of children with ASD in the earlier studies, and were associated with immune dysfunction and autoimmunity, there was still little evidence supporting the notion that maternal autoantibodies could impact behavior at that time.
In order to address the association between maternal autoantibodies and behavior, another large study was conducted by Braunschweig et al. (2012) that provided further suggestion of a potential role for maternal autoantibodies in ASD behaviors, and reported an association between a the presence of anti-fetal brain antibodies in the mother and ASD-related deficits in the child. The Braunschweig study discovered that paired brain (i.e. 37 and 73kDa bands) reactivity correlated with lower expressive language scores in the child(56). Additionally, it was noted in a subsequent study that children born to mothers with this antibody-binding pattern also exhibited abnormal brain enlargement when compared to both children with ASD born to mothers that did not harbor anti-brain antibodies as well as typically developing control children(57). Further, reactivity to a band near 39kDa was later discovered, and paired reactivity to proteins at 39 kDa and 73 kDa correlated with a broader diagnosis of ASD (which was distinct from full autism at that time) as well as increased irritability on the Aberrant Behavioral Checklist (ABC) scale(56).
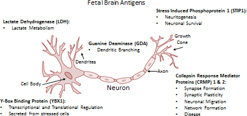
In order to understand how these anti-brain antibodies could potentially lead to aberrant developmental trajectories, the identity of the proteins corresponding to the 37, 39, and 73 kDa bands needed to be elucidated. Studies by our laboratory, which utilized 2-D gel electrophoresis followed by tandem mass spectrometry peptide sequencing determined that the maternal autoantibodies recognize seven developmentally regulated proteins in the fetal brain that include lactate dehydrogenase A and B (LDH-A, LDH-B), stress-induced phosphoprotein 1 (STIP1), Guanine Deaminase (GDA), collapsin response mediator proteins 1 and 2 (CRMP1, CRMP2), and Y-box binding protein 1(YBX1) (Figure 1). The antigens recognized by maternal autoantigens are significant as a number of them are critical for normal brain development, specifically processes essential for neuronal migration and neural network formation; GDA has an integral role in dendritic branching of hippocampal neurons, while STIP1, in combination the cellular prion protein, is responsible for neuritogenesis and increases neuronal survival(58, 59),. Further, the collapsin response mediator proteins (CRMPs 1–5) are necessary for proper growth cone collapse and are required for proper cell migration and axon-dendrite specification(60, 61). The effects of YBX1 and LDH in neuronal development are more widespread; YBX1 is involved in almost all DNA and mRNA-dependent processes and LDH is essential for energy metabolism(62, 63). Subsequent experiments using western blots containing the purified protein targets confirmed these findings leading to a finding of 23% incidence of the highly specific antigen reactivity patterns associated with ASD. Further, we characterized behavioral outcomes in the children of autoantibody positive mothers that associated with the presence of the most common autoantibody pattern, which included combined reactivity to LDH, STIP1, and CRMP 1. Combined maternal antibody reactivity to this autoantigen pattern was found in 7% of mothers with children with ASD and correlated with an increase in stereotypic behaviors in the child(64) (Table 2). Maternal autoantibodies are increasingly implicated in the behavioral and cognitive deficits that characterize ASD, but as these studies have been conducted in the human subjects, it is impossible to determine the mechanism by which these antibodies lead to the symptoms observed in ASD. In order to understand the potential pathogenic role of maternal antibodies, it was necessary to move to experimental animal studies. Moreover, studies are currently underway to elucidate the peptide epitopes on each fetal antigen recognized by the maternal autoantibody related (MAR) ASD antibodies, as determining the precise binding site(s) of the autoantibodies may offer insight into the etiology and mechanism by which these antibodies mediate changes in behavior.
Figure 1. Fetal Brain Antigens.
The maternal autoantibodies detected in mothers of children affected with ASD bind to fetal brain antigens that are responsible for critical process in the developing brain.
Table 2.
Studies linking Autoimmunity and ASD
| Antibody Target | MW | Number in Study |
Autoantibody Prevalence in ASD Population |
Research Group |
Year |
|---|---|---|---|---|---|
| Fetal Rat Brain | Low MW bands and 250kDa |
AU=11 TD=10 |
45%-Low MW bands 55%–250kDa |
Zimmerman et al. (51) |
2007 |
|
Fetal Human Brain |
37 and 73 kDa | AU=61 DD=40 TD=62 |
12% | Braunschweig et al. (50) |
2008 |
|
Fetal Human Brain |
39 kDa | AU=84 DD=49 TD=160 |
7% | Croen et al. (53) | 2008 |
|
Fetal Human Brain |
36kDa | AU=100 TD=100 |
10% | Singer et al. (52) | 2008 |
|
Fetal Monkey Brain |
37, 39, and 73 kDa | AU=201 ASD=71 DD=102 TD=185 |
7% (37 & 73 kDa) 4% (39 & 73 kDa) |
Braunschweig et al. (69) |
2012 |
|
LDH, STIP1, CRMP1, GDA, CRMP2, YBX1 |
37, 39, 48, 62 and 68 kDa |
ASD=246 TD=149 |
7% | Braunschweig et al. (64) |
2013 |
| Mouse Brain | Undetermined | ASD=2431 TD=653 |
10.7% | Brimberg et al. (55) |
2013 |
Abbreviations: Autism (AU), Typically Developing (TD), Developmental Delay (DD), and Autism Spectrum Disorders (ASD)
Animal Models of ASD
It is possible that the maternal antibodies represent purely a biological marker of damage that occurred during gestation, and may not necessarily underlie the neurodevelopmental dysfunction detected in ASD. In order to determine if maternal antibodies are clinically significant, and not just an immune epiphenomenon, studies were initiated by several research labs utilizing animal models. Typically, in autoimmune diseases where antibodies are suspected to have a deleterious role, passive transfer studies, where antibodies are transferred from a diseased animal to a healthy animal, are often used to establish a pathogenic capacity(65, 66).
Non-Human Primate Models
The first animal studies, which utilized rhesus macaques due to their increased social repertoire and established battery of social tests, demonstrated that the group of monkeys that were prenatally exposed through passive transfer to IgG purified from mothers of children with ASD had significantly more stereotypic behaviors and higher levels of motor activity when placed in a novel social setting than monkeys treated with IgG from mothers with typically developing children (67). Further, these findings were recently replicated in a larger study utilizing non-human primates (NHPs) administered with targeted IgG from mothers of ASD children that was specific for the dominant 37/73 kDa band pattern (now known to correspond to LDH, CRMP1 and STIP1). In the second NHP model, it was observed that offspring treated with IgG from mothers of children with ASD had abnormal social behaviors and enlarged brain volume compared to control IgG-treated animals. They also found that the dams receiving the ASD-associated IgG displayed a heightened maternal protectiveness towards their progeny(68). While these studies were not without limitations, they provide insight into the potential pathologic significance of maternal autoantibodies in ASD, and how their interaction with fetal brain antigens may alter the course of brain and behavioral development.
Murine Models
Concurrent to the studies conducted in rhesus macaques, researchers revealed similar findings utilizing a murine passive transfer model, in which pregnant mice exposed to a single, intravenous dose of human maternal plasma predetermined by immunoblotting to be reactive to the 37/37kDa-banding pattern had offspring with increased anxiety and response to stress, along with impaired motor and sensory development(69). This outcome was also seen in a previous study utilizing pooled IgG from mothers of children with ASD but not based on any 37 or 73kDa band patterns. The authors determined that the offspring born to the dams injected with IgG from mothers with autistic children, and not from mothers with typically developing children, mimicked some of the symptoms seen in ASD, including alterations in sociability and increased activity and anxiety(70). The most recent studies, aiming to further illuminate the mechanism by which maternal autoantibodies alter fetal brain development, transferred human, antigen-specific maternal IgG into the cerebral ventricles of embryonic mice. The first of these studies focused on the behavioral outcomes of the exposed offspring. Those offspring injected with antibodies reactive to the 37/37kDa-banding pattern had increased stereotypic behaviors and altered social phenotypes reminiscent of the children born to mothers with this brain pattern reactivity(71). In parallel studies, the physiological effects of maternal IgG revealed increased cellular proliferation in the sub-ventricular zone, increased size of adult cortical neurons, and increased adult brain size and weight compared to animals exposed to autoantibody-negative control IgG(72). Although these studies were highly experimental and not representative of a natural exposure, they offer further evidence supporting the role of maternal anti-brain antibodies in the incidence of ASD, as well as, insight as to the cellular target: radial glial stem cells. Further, as these studies are limited by the passive transfer of IgG during pregnancy at a single time point, our laboratory has recently created an antigen-driven mouse model by breaking tolerance to the specific antigenic determinants of LDHA, LDHB, CRMP1 and STIP1 to create the most clinically relevant animal model in which to study MAR autism. Behavioral studies are currently underway in these mice.
Autoantibodies in other forms of psychopathology
A number of research groups have established an association with autoimmunity and the presence of anti-brain autoantibodies and the incidence of numerous behavioral disorders(73). Antibodies reactive to neuronal tissues have also been detected Schizophrenia(74–77), Tourette’s syndrome(78–84), and Obsessive Compulsive Disorder (OCD)(85–87), seemingly linking these related and often comorbid disorders. Further, anti-brain autoantibodies have also been observed in children with ASD(88–94). However, unlike the anti-brain autoantibodies observed in mothers of children with autism, the anti-brain antibodies observed in children, and in other disorders, have been found to preferentially bind to adult, rather than fetal brain substrates, suggesting they are reactive to a different repertoire of protein targets. The variance in diseases associated with these anti-brain autoantibodies may be due to their antigen-specificity and/or the window of exposure by which they interact with their brain-derived protein targets. There is more support for the etiologic relevance of fetal brain reactive maternal antibodies, as maternal antibody exposure overlaps major processes in neurodevelopment, and maternal antibodies have greater access to the fetal brain due to increased permissiveness of the blood brain barrier during gestation(95). In other forms of psychopathology that arise later in life, autoantibodies may still mediate deleterious effects on the brain leading to aberrations in behavior. Nevertheless, the presence of anti-brain autoantibodies postnatally does not necessarily lead to disorders of brain; it is hypothesized there must be another event that increases barrier permeability allowing antibodies to traverse blood-brain barrier and gain access to brain tissue to inhibit and alter neuronal processes(96, 97). However, it may be this necessary preliminary event that accounts for the episodic nature of many psychiatric disorders. While anti-brain autoantibodies may represent a common etiological agent leading to related psychopathological disorders such as ASD and schizophrenia, there are likely many other aspects, including genetic predisposition, environmental factors, and timing of exposure during development, that contribute to the differing symptoms and manifestations of non-ASD autoantibody related neuropsychiatric disorders.
Conclusion
MAR ASD has been noted by numerous researchers describing the presence of maternal autoantibodies reactive to fetal brain proteins in a subset of mothers of children with ASD. Further, there is now an abundance of evidence supporting their deleterious role in neurodevelopment. For the most part, these studies have described similar experimental outcomes and, given the clinical and biological heterogeneity of ASD, there likely exists a complex relationship between the presence of maternal anti-fetal brain antibodies and developmental trajectory of exposed offspring. It is still unclear how and when these maternal autoantibodies arise, but studies currently underway may provide increased insight into their ontogeny. Further, the generation of more clinically relevant animal models will enable the illumination of the mechanism by which maternal antibodies impair neurodevelopment, resulting in the social and behavioral deficits observed in ASD. Moreover, by determining their mechanism of action, appropriate therapeutic interventions could be implemented, thus raising the optimistic prospect that some future cases of ASD or related neurodevelopmental and psychiatric disorders may be prevented.
Acknowledgments
We thank the staff of the CHARGE study, and families involved in this research.
This study was funded by the NIEHS Center for Children’s Environmental Health and Environmental Protection Agency (EPA) grants (2P01ES011269-11, 83543201 respectively), the NIEHS-funded CHARGE study (R01ES015359), and the NICHD funded IDDRC 054 (U54HD079125). There are 3 patents associated with the work at UCD noted in this review: A provisional ‘Maternal Diagnostic Marker for Autism Risk and Therapeutic Intervention’ UC Case No. 2014-467-1. There are 2 issued patents: The US patent Methods of Diagnosing and Treating Autism, U.S. Patent No. 8,383,360 B2 or Pat. 8,383,360 was filed August 12, 2010 and issued February 26, 2013. The client reference number for this patent is 2009-737-2US. This patent is currently in the process of National filing. The first issued patent is Diagnostic Methods for Detection of Autism Spectrum Disorder, U.S. Patent No. US 7,452,681 B2. This patent was filed May 5, 2006, and was issued November 18, 2008. This patent was filed in the US only. Dr. Van de Water received consulting fees from Pediatric Bioscience from Jan 1, 2015 until October 2015.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: Drs. Edmiston and Ashwood report no biomedical financial interests or potential conflicts of interest.
References
- 1.Association AP. Diagnositc and Statistical Manual of Mental Disorders. 5th. Washington, DC: 2013. [Google Scholar]
- 2.Kanner L. Irrelevant and metaphorical language in early infanitle autism. American Journal of Psychiatry. 1946;103:242–246. doi: 10.1176/ajp.103.2.242. [DOI] [PubMed] [Google Scholar]
- 3.Lord C, Risi S, Lambrecht L, Cook EH, Leventhal BL, DiLavore PC, et al. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. Journal of autism and developmental disorders. 2000;30:205–223. [PubMed] [Google Scholar]
- 4.Newschaffer CJ, Croen La, Daniels J, Giarelli E, Grether JK, Levy SE, et al. The epidemiology of autism spectrum disorders. Annual review of public health. 2007;28:235–258. doi: 10.1146/annurev.publhealth.28.021406.144007. [DOI] [PubMed] [Google Scholar]
- 5.Snow AV, Lecavalier L, Houts C. The structure of the Autism Diagnostic Interview-Revised: diagnostic and phenotypic implications. Journal of child psychology and psychiatry, and allied disciplines. 2009;50:734–742. doi: 10.1111/j.1469-7610.2008.02018.x. [DOI] [PubMed] [Google Scholar]
- 6.Ousley O, Cermak T. Autism spectrum disorder: Defining dimensions and subgroups. Current Developmental Disorders Reports. 2013;1:20–28. doi: 10.1007/s40474-013-0003-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McDougle CJ, Landino SM, Vahabzadeh A, O’Rourke J, Zurcher NR, Finger BC, et al. Toward an immune-mediated subtype of autism spectrum disorder. Brain Research. 2015;1617:72–92. doi: 10.1016/j.brainres.2014.09.048. [DOI] [PubMed] [Google Scholar]
- 8.Onore C, Careaga M, Ashwood P. The role of immune dysfunction in the pathophysiology of autism. Brain, Behavior, and Immunity. 2012;26:383–392. doi: 10.1016/j.bbi.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Warren RP, Singh VK, Cole P, Odell JD, Pingree CB, Warren WL, et al. Increased frequency of the null allele at the complement C4b locus in autism. Clinical & Experimental Immunology. 1991;83:438–440. doi: 10.1111/j.1365-2249.1991.tb05657.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Warren RP, Odell JD, Warren WL, Burger RA, Maciulis A, Daniels WW, et al. Strong association of the third hypervariable region of HLA-DRβ1 with autism. Journal of Neuroimmunology. 1996;67:97–102. doi: 10.1016/0165-5728(96)00052-5. [DOI] [PubMed] [Google Scholar]
- 11.Torres AR, Sweeten TL, Cutler A, Bedke BJ, Fillmore M, Stubbs EG, et al. The association and linkage of the HLA-A2 class I allele with autism. Human immunology. 2006;67:346–351. doi: 10.1016/j.humimm.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 12.Campbell DB, Li C, Sutcliffe JS, Persico AM, Levitt P. Genetic evidence implicating multiple genes in the MET receptor tyrosine kinase pathway in autism spectrum disorder. Autism Research. 2008;1:159–168. doi: 10.1002/aur.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thanseem I, Nakamura K, Miyachi T, Toyota T, Yamada S, Tsujii M, et al. Further evidence for the role of MET in autism susceptibility. Neuroscience Research. 2010;68:137–141. doi: 10.1016/j.neures.2010.06.014. [DOI] [PubMed] [Google Scholar]
- 14.Mostafa GA, Shehab AA. The link of C4B null allele to autism and to a family history of autoimmunity in Egyptian autistic children. Journal of Neuroimmunology. 2010;223:115–119. doi: 10.1016/j.jneuroim.2010.03.025. [DOI] [PubMed] [Google Scholar]
- 15.Jung JY, Kohane IS, Wall DP. Identification of autoimmune gene signatures in autism. Translational psychiatry. 2011;1:e63–e63. doi: 10.1038/tp.2011.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Torres AR, Westover JB, Gibbons C, Johnson RC, Ward DC. Activating killer-cell immunoglobulin-like receptors (KIR) and their cognate HLA ligands are significantly increased in autism. Brain, behavior, and immunity. 2012;26:1122–1127. doi: 10.1016/j.bbi.2012.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Comi AM, Zimmerman AW, Frye VH, Law PA, Peeden JN. Familial Clustering of Autoimmune Disorders and Evaluation of Medical Risk Factors in Autism. Journal of Child Neurology. 1999;14:388–394. doi: 10.1177/088307389901400608. [DOI] [PubMed] [Google Scholar]
- 18.Atladóttir HO, Pedersen MG, Thorsen P, Mortensen PB, Deleuran B, Eaton WW, et al. Association of family history of autoimmune diseases and autism spectrum disorders. Pediatrics. 2009;124:687–694. doi: 10.1542/peds.2008-2445. [DOI] [PubMed] [Google Scholar]
- 19.Vinet É, Pineau CA, Clarke AE, Scott S, Fombonne É, Joseph L, et al. Increased Risk of Autism Spectrum Disorders in Children Born to Women With Systemic Lupus Erythematosus: Results From a Large Population-Based Cohort. Arthritis & rheumatology (Hoboken, NJ) 2015;67:3201–3208. doi: 10.1002/art.39320. [DOI] [PubMed] [Google Scholar]
- 20.Wu S, Ding Y, Wu F, Li R, Xie G, Hou J, et al. Family history of autoimmune diseases is associated with an increased risk of autism in children: A systematic review and meta-analysis. Neuroscience and biobehavioral reviews. 2015;55:322–332. doi: 10.1016/j.neubiorev.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 21.Chen S-W, Zhong X-S, Jiang L-N, Zheng X-Y, Xiong Y-Q, Ma S-J, et al. Maternal autoimmune diseases and the risk of autism spectrum disorders in offspring: A systematic review and meta-analysis. Behavioural brain research. 2016;296:61–69. doi: 10.1016/j.bbr.2015.08.035. [DOI] [PubMed] [Google Scholar]
- 22.Chess S. Autism in children with congenital rubella. Journal of autism and childhood schizophrenia. 1971;1:33–47. doi: 10.1007/BF01537741. [DOI] [PubMed] [Google Scholar]
- 23.Meyer U, Nyffeler M, Engler A, Urwyler A, Schedlowski M, Knuesel I, et al. The time of prenatal immune challenge determines the specificity of inflammation-mediated brain and behavioral pathology. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26:4752–4762. doi: 10.1523/JNEUROSCI.0099-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith SEP, Li J, Garbett K, Mirnics K, Patterson PH. Maternal Immune Activation Alters Fetal Brain Development through Interleukin-6. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:10695–10702. doi: 10.1523/JNEUROSCI.2178-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Atladóttir HÓ, Thorsen P, Østergaard L, Schendel DE, Lemcke S, Abdallah M, et al. Maternal Infection Requiring Hospitalization During Pregnancy and Autism Spectrum Disorders. Journal of Autism and Developmental Disorders. 2010;40:1423–1430. doi: 10.1007/s10803-010-1006-y. [DOI] [PubMed] [Google Scholar]
- 26.Patterson PH. Maternal infection and immune involvement in autism. Trends in Molecular Medicine. 2011;17:389–394. doi: 10.1016/j.molmed.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garay PA, Hsiao EY, Patterson PH, McAllister AK. Brain, Behavior, and Immunity Maternal immune activation causes age- and region-specific changes in brain cytokines in offspring throughout development. 2012 doi: 10.1016/j.bbi.2012.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ashwood P, Van de Water J. Is autism an autoimmune disease? Autoimmunity reviews. 2004;3:557–562. doi: 10.1016/j.autrev.2004.07.036. [DOI] [PubMed] [Google Scholar]
- 29.Braunschweig D, Van de Water J. Maternal autoantibodies in autism. Archives of neurology. 2012;69:693–699. doi: 10.1001/archneurol.2011.2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fox E, Amaral D, Van de Water J. Maternal and fetal antibrain antibodies in development and disease. Developmental Neurobiology. 2012;72:1327–1334. doi: 10.1002/dneu.22052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fox-Edmiston E, Van De Water J. Maternal Anti-Fetal Brain IgG Autoantibodies and Autism Spectrum Disorder: Current Knowledge and its Implications for Potential Therapeutics. CNS Drugs. 2015;29:715–724. doi: 10.1007/s40263-015-0279-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mallampalli MP, Davies E, Wood D, Robertson H, Polato F, Carter CL. Role of environment and sex differences in the development of autoimmune diseases: a roundtable meeting report. Journal of women's health (2002) 2013;22:578–586. doi: 10.1089/jwh.2013.4462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hertz-Picciotto I, Croen LA, Hansen R, Jones CR, van de Water J, Pessah IN. The CHARGE study: an epidemiologic investigation of genetic and environmental factors contributing to autism. Environ Health Perspect. 2006;114:1119–1125. doi: 10.1289/ehp.8483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Christensen Dl, Braun KvBJ, et al. Prevalence of autism spectrum disorders - autism and developmental disabilities monitoring network, 11 sites, United States, 2012. MMWR Surveill Summ. 2016;65:1–23. doi: 10.15585/mmwr.ss6503a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Money J, Bobrow NA, Clarke FC. Autism and autoimmune disease: A family study. Journal of autism and childhood schizophrenia. 1971;1:146–160. doi: 10.1007/BF01537954. [DOI] [PubMed] [Google Scholar]
- 36.Croen LA, Grether JK, Yoshida CK, Odouli R, Van de Water J. Maternal autoimmune diseases, asthma and allergies, and childhood autism spectrum disorders: A case-control study. Archives of Pediatrics & Adolescent Medicine. 2005;159:151–157. doi: 10.1001/archpedi.159.2.151. [DOI] [PubMed] [Google Scholar]
- 37.Keil A, Daniels JL, Forssen U, Hultman C, Cnattingius S, Söderberg KC, et al. Parental Autoimmune Diseases Associated With Autism Spectrum Disorders in Offspring. Epidemiology (Cambridge, Mass) 2010;21:805–808. doi: 10.1097/EDE.0b013e3181f26e3f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Christen U, von Herrath MG. Initiation of autoimmunity. Current opinion in immunology. 2004;16:759–767. doi: 10.1016/j.coi.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 39.Ashwood P, Van de Water J. A Review of Autism and the Immune Response. Clinical and Developmental Immunology. 2004;11:165–174. doi: 10.1080/10446670410001722096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Garay Pa, McAllister aK. Novel roles for immune molecules in neural development: implications for neurodevelopmental disorders. Frontiers in synaptic neuroscience. 2010;2:136–136. doi: 10.3389/fnsyn.2010.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Filiano AJ, Gadani SP, Kipnis J. Interactions of innate and adaptive immunity in brain development and function. Brain Research. 2015;1617:18–27. doi: 10.1016/j.brainres.2014.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gluecksohn-Waelsch S. The Effect of Maternal Immunization against Organ Tissues on Embryonic Differentiation in the Mouse. Development. 1957;5:83–92. [Google Scholar]
- 43.Adinolfi M, Beck SE, Haddad SA, Seller MJ. Permeability of the blood-cerebrospinal fluid barrier to plasma proteins during foetal and perinatal life. Nature. 1976;259:140–141. doi: 10.1038/259140a0. [DOI] [PubMed] [Google Scholar]
- 44.Plum F. The concept of a blood-brain barrier. Annals of Neurology. 1981;9:622–622. [Google Scholar]
- 45.Adinolfi M. The development of the human blood-CSF brain barrier. Developmental Medicine & Child Neurology. 1985;27:532–537. doi: 10.1111/j.1469-8749.1985.tb04581.x. [DOI] [PubMed] [Google Scholar]
- 46.Karpiak SE, Jr, Rapport MM. Behavioral changes in 2-month-old rats following prenatal exposure to antibodies against synaptic membranes. Brain Research. 1975;92:405–413. doi: 10.1016/0006-8993(75)90325-x. [DOI] [PubMed] [Google Scholar]
- 47.Rick JT, Gregson AN, Leibowitz S, Adinolfi M. Behavioural Changes in Adult Rats Following Administration of Antibodies again Brain Gangliosides. Developmental Medicine & Child Neurology. 1980;22:719–724. doi: 10.1111/j.1469-8749.1980.tb03738.x. [DOI] [PubMed] [Google Scholar]
- 48.Warren RP, Cole P, Odell JD, Pingree CB, Warren WL, White E, et al. Detection of maternal antibodies in infantile autism. J Am Acad Child Adolesc Psychiatry. 1990;29:873–877. doi: 10.1097/00004583-199011000-00005. [DOI] [PubMed] [Google Scholar]
- 49.Dalton P, Deacon R, Blamire A, Pike M, McKinlay I, Stein J, et al. Maternal neuronal antibodies associated with autism and a language disorder. Ann Neurol. 2003;53:533–537. doi: 10.1002/ana.10557. [DOI] [PubMed] [Google Scholar]
- 50.Braunschweig D, Ashwood P, Krakowiak P, Hertz-Picciotto I, Hansen R, Croen LA, et al. Autism: Maternally derived antibodies specific for fetal brain proteins. NeuroToxicology. 2008;29:226–231. doi: 10.1016/j.neuro.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zimmerman AW, Connors SL, Matteson KJ, Lee L-CC, Singer HS, Castaneda Ja, et al. Maternal antibrain antibodies in autism. Brain Behav Immun. 2007;21:351–357. doi: 10.1016/j.bbi.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 52.Singer HS, Morris CM, Gause CD, Gillin PK, Crawford S, Zimmerman AW. Antibodies against fetal brain in sera of mothers with autistic children. J Neuroimmunol. 2008;194:165–172. doi: 10.1016/j.jneuroim.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 53.Croen LA, Braunschweig D. Maternal mid-pregnancy autoantibodies to fetal brain protein: the early markers for autism study. Biological Psychiatry. 2008;64:583–588. doi: 10.1016/j.biopsych.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Heuer L, Braunschweig D, Ashwood P, Van de Water J, Campbell DB. Association of a MET genetic variant with autism-associated maternal autoantibodies to fetal brain proteins and cytokine expression. Translational psychiatry. 2011;1:e48–e48. doi: 10.1038/tp.2011.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brimberg L, Sadiq A, Gregersen PK, Diamond B. Brain-reactive IgG correlates with autoimmunity in mothers of a child with an autism spectrum disorder. Mol Psychiatry. 2013;18:1171–1177. doi: 10.1038/mp.2013.101. [DOI] [PubMed] [Google Scholar]
- 56.Braunschweig D, Duncanson P, Boyce R, Hansen R, Ashwood P, Pessah IN, et al. Behavioral correlates of maternal antibody status among children with autism. Journal of autism and developmental disorders. 2012;42:1435–1445. doi: 10.1007/s10803-011-1378-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nordahl CW, Braunschweig D, Iosif A-M, Lee A, Rogers S, Ashwood P, et al. Maternal autoantibodies are associated with abnormal brain enlargement in a subgroup of children with autism spectrum disorder. Brain, Behavior, and Immunity. 2013;30:61–65. doi: 10.1016/j.bbi.2013.01.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Akum BF, Chen M, Gunderson SI, Riefler GM, Scerri-Hansen MM, Firestein BL. Cypin regulates dendrite patterning in hippocampal neurons by promoting microtubule assembly. Nat Neurosci. 2004;7:145–152. doi: 10.1038/nn1179. [DOI] [PubMed] [Google Scholar]
- 59.Lopes MH, Hajj GN, Muras AG, Mancini GL, Castro RM, Ribeiro KC, et al. Interaction of cellular prion and stress-inducible protein 1 promotes neuritogenesis and neuroprotection by distinct signaling pathways. J Neurosci. 2005;25:11330–11339. doi: 10.1523/JNEUROSCI.2313-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Charrier E, Reibel S, Rogemond V, Aguera M, Thomasset N, Honnorat J. Collapsin response mediator proteins (CRMPs) Molecular Neurobiology. 2003;28:51–63. doi: 10.1385/MN:28:1:51. [DOI] [PubMed] [Google Scholar]
- 61.Quach TT, Duchemin A-M, Rogemond V, Aguera M, Honnorat J, Belin M-F, et al. Involvement of collapsin response mediator proteins in the neurite extension induced by neurotrophins in dorsal root ganglion neurons. Molecular and Cellular Neuroscience. 2004;25:433–443. doi: 10.1016/j.mcn.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 62.Eliseeva IA, Kim ER, Guryanov SG, Ovchinnikov LP, Lyabin DN. Y-box-binding protein 1 (YB-1) and its functions. Biochemistry (Moscow) 2011;76:1402–1433. doi: 10.1134/S0006297911130049. [DOI] [PubMed] [Google Scholar]
- 63.Hashimoto T, Hussien R, Cho H-S, Kaufer D, Brooks GA. Evidence for the Mitochondrial Lactate Oxidation Complex in Rat Neurons: Demonstration of an Essential Component of Brain Lactate Shuttles. PLoS ONE. 2008;3:e2915. doi: 10.1371/journal.pone.0002915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Braunschweig D, Krakowiak P, Duncanson P, Boyce R, Hansen RL, Ashwood P, et al. Autism-specific maternal autoantibodies recognize critical proteins in developing brain. Translational psychiatry. 2013;3:e277–e277. doi: 10.1038/tp.2013.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Diamond B, Huerta PT. Losing your nerves? Maybe it's antibodies. Nature Reviews …. 2009;9:449–456. doi: 10.1038/nri2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Diamond B, Honig G, Mader S, Brimberg L, Volpe BT. Brain-reactive antibodies and disease. Annual review of immunology. 2013;31:345–385. doi: 10.1146/annurev-immunol-020711-075041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Martin LA, Ashwood P, Braunschweig D, Cabanlit M, Van de Water J, Amaral DG. Stereotypies and hyperactivity in rhesus monkeys exposed to IgG from mothers of children with autism. Brain, Behavior, and Immunity. 2008;22:806–816. doi: 10.1016/j.bbi.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bauman MD, Iosif a-MM, Ashwood P, Braunschweig D, Lee a, Schumann CM, et al. Maternal antibodies from mothers of children with autism alter brain growth and social behavior development in the rhesus monkey. Translational Psychiatry. 2013;3:e278–e278. doi: 10.1038/tp.2013.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Braunschweig D, Golub MS, Koenig CM, Qi L, Pessah IN, Van de Water J, et al. Maternal autism-associated IgG antibodies delay development and produce anxiety in a mouse gestational transfer model. Journal of neuroimmunology. 2012;252:56–65. doi: 10.1016/j.jneuroim.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Singer HS, Morris C, Gause C, Pollard M, Zimmerman AW, Pletnikov M. Prenatal exposure to antibodies from mothers of children with autism produces neurobehavioral alterations: A pregnant dam mouse model. Journal of neuroimmunology. 2009;211:39–48. doi: 10.1016/j.jneuroim.2009.03.011. [DOI] [PubMed] [Google Scholar]
- 71.Camacho J, Jones K, Miller E, Ariza J, Noctor S, Van de Water J, et al. Embryonic intraventricular exposure to autism-specific maternal autoantibodies produces alterations in autistic-like stereotypical behaviors in offspring mice. Behavioural Brain Research. 2014;266:46–51. doi: 10.1016/j.bbr.2014.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Martínez-Cerdeño V, Camacho J, Fox E, Miller E, Ariza J, Kienzle D, et al. Prenatal Exposure to Autism-Specific Maternal Autoantibodies Alters Proliferation of Cortical Neural Precursor Cells, Enlarges Brain, and Increases Neuronal Size in Adult Animals. Cerebral cortex (New York, NY : 1991) 2014 doi: 10.1093/cercor/bhu291. bhu291--bhu291- [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Benros ME, Eaton WW, Mortensen PB. The Epidemiologic Evidence Linking Autoimmune Diseases and Psychosis. Biological psychiatry. 2013:1–7. doi: 10.1016/j.biopsych.2013.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Heath RG, Krupp IM. Schizophrenia as an immunologic disorder: I. demonstration of antibrain globulins by fluorescent antibody techniques. Archives of General Psychiatry. 1967;16:1–9. doi: 10.1001/archpsyc.1967.01730190003001. [DOI] [PubMed] [Google Scholar]
- 75.Patterson PH. Immune involvement in schizophrenia and autism: Etiology, pathology and animal models. Behavioural Brain Research. 2009;204:313–321. doi: 10.1016/j.bbr.2008.12.016. [DOI] [PubMed] [Google Scholar]
- 76.Margari F, Petruzzelli MG, Mianulli R, Campa MG, Pastore A, Tampoia M. Circulating anti-brain autoantibodies in schizophrenia and mood disorders. Psychiatry Research. 2015;230:704–708. doi: 10.1016/j.psychres.2015.10.029. [DOI] [PubMed] [Google Scholar]
- 77.Margari F, Petruzzelli MG, Mianulli R, Toto M, Pastore A, Bizzaro N, et al. Anti-brain autoantibodies in the serum of schizophrenic patients: A case-control study. Psychiatry Research. 2013;210:800–805. doi: 10.1016/j.psychres.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 78.Kansy JW, Katsovich L, McIver KS, Pick J, Zabriskie JB, Lombroso PJ, et al. Identification of pyruvate kinase as an antigen associated with Tourette syndrome. Journal of Neuroimmunology. 2006;181:165–176. doi: 10.1016/j.jneuroim.2006.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Martino D, Defazio G, Church AJ, Dale RC, Giovannoni G, Robertson MM, et al. Antineuronal antibody status and phenotype analysis in Tourette's syndrome. Movement disorders : official journal of the Movement Disorder Society. 2007;22:1424–1429. doi: 10.1002/mds.21454. [DOI] [PubMed] [Google Scholar]
- 80.Murphy TK, Kurlan R, Leckman J. The immunobiology of Tourette's disorder, pediatric autoimmune neuropsychiatric disorders associated with Streptococcus, and related disorders: a way forward. Journal of child and adolescent psychopharmacology. 2010;20:317–331. doi: 10.1089/cap.2010.0043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cheng Y-h, Zheng Y, He F, Yang J-h, Li W-b, Wang M-l, et al. Detection of autoantibodies and increased concentrations of interleukins in plasma from patients with Tourette's syndrome. Journal of molecular neuroscience : MN. 2012;48:219–224. doi: 10.1007/s12031-012-9811-8. [DOI] [PubMed] [Google Scholar]
- 82.Elamin I, Edwards MJ, Martino D. Immune dysfunction in Tourette syndrome. Behavioural Neurology. 2013;27:23–32. doi: 10.3233/BEN-120295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hornig M, Lipkin WI. Immune-mediated animal models of Tourette syndrome. Neuroscience and Biobehavioral Reviews. 2013;37:1120–1138. doi: 10.1016/j.neubiorev.2013.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Martino D, Madhusudan N, Zis P, Cavanna AE. An introduction to the clinical phenomenology of tourette syndrome. 1. Elsevier Inc.; 2013. [DOI] [PubMed] [Google Scholar]
- 85.Bhattacharyya S, Khanna S, Chakrabarty K, Mahadevan A, Christopher R, Shankar SK. Anti-brain autoantibodies and altered excitatory neurotransmitters in obsessive-compulsive disorder. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2009;34:2489–2496. doi: 10.1038/npp.2009.77. [DOI] [PubMed] [Google Scholar]
- 86.Gause C, Morris C, Vernekar S, Pardo-Villamizar C, Grados MA, Singer HS. Antineuronal antibodies in OCD: Comparisons in children with OCD-only, OCD+chronic tics and OCD+PANDAS. J Neuroimmunol. 2009 doi: 10.1016/j.jneuroim.2009.06.015. [DOI] [PubMed] [Google Scholar]
- 87.Guiseppe M, Albert U, Bogetto F, Borghese C, Berro AC, Mutani R, et al. Anti-brain antibodies in adult patients with obsessive-compulsive disorder. Journal of Affective Disorders. 2009;116:192–200. doi: 10.1016/j.jad.2008.11.019. [DOI] [PubMed] [Google Scholar]
- 88.Silva SC, Correia C, Fesel C, Barreto M, Coutinho AM, Marques C, et al. Autoantibody repertoires to brain tissue in autism nuclear families. Journal of neuroimmunology. 2004;152:176–182. doi: 10.1016/j.jneuroim.2004.03.015. [DOI] [PubMed] [Google Scholar]
- 89.Singer HS, Morris CM, Williams PN, Yoon DY, Hong JJ, Zimmerman AW. Antibrain antibodies in children with autism and their unaffected siblings. Journal of neuroimmunology. 2006;178:149–155. doi: 10.1016/j.jneuroim.2006.05.025. [DOI] [PubMed] [Google Scholar]
- 90.Wills S, Cabanlit M, Bennett J, Ashwood P, Amaral D, Van de Water J. Autoantibodies in autism spectrum disorders (ASD) Annals of the New York Academy of Sciences. 2007;1107:79–91. doi: 10.1196/annals.1381.009. [DOI] [PubMed] [Google Scholar]
- 91.Goines P, Haapanen L, Boyce R. Autoantibodies to cerebellum in children with autism associate with behavior. Brain, behavior, and …. 2011;25:514–523. doi: 10.1016/j.bbi.2010.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Morris CM, Zimmerman AW, Singer HS. Childhood serum anti-fetal brain antibodies do not predict autism. Pediatric neurology. 2009;41:288–290. doi: 10.1016/j.pediatrneurol.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 93.Cabanlit M, Wills S, Goines P, Ashwood P, Van de Water J. Brain-specific autoantibodies in the plasma of subjects with autistic spectrum disorder. Annals of the New York Academy of Sciences. 2007;1107:92–103. doi: 10.1196/annals.1381.010. [DOI] [PubMed] [Google Scholar]
- 94.Wills S, Rossi CC, Bennett J, Martinez Cerdeño V, Ashwood P, Amaral DG, et al. Further characterization of autoantibodies to GABAergic neurons in the central nervous system produced by a subset of children with autism. Molecular autism. 2011;2:5–5. doi: 10.1186/2040-2392-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kowal C, Athanassiou A, Chen H, Diamond B. Maternal antibodies and developing bloodbrain barrier. Immunologic research. 2015;63:18–25. doi: 10.1007/s12026-015-8714-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Coutinho E, Harrison P, Vincent A. Do Neuronal Autoantibodies Cause Psychosis? A Neuroimmunological Perspective. Biological psychiatry. 2013:1–7. doi: 10.1016/j.biopsych.2013.07.040. [DOI] [PubMed] [Google Scholar]
- 97.Hammer C, Stepniak B, Schneider A, Papiol S, Tantra M, Begemann M, et al. Neuropsychiatric disease relevance of circulating anti-NMDA receptor autoantibodies depends on blood-brain barrier integrity. Mol Psychiatry. 2014;19:1143–1149. doi: 10.1038/mp.2013.110. [DOI] [PubMed] [Google Scholar]