Abstract
Serum amyloid A (SAA) is expressed locally in chronic inflammatory conditions such as chronic obstructive pulmonary disease (COPD), where macrophages that do not accord with the classic M1/M2 paradigm also accumulate. In this study, the role of SAA in regulating macrophage differentiation was investigated in vitro using human blood monocytes from healthy subjects and patients with COPD and in vivo using an airway SAA challenge model in BALB/c mice. Differentiation of human monocytes with SAA stimulated the proinflammatory monokines IL-6 and IL-1β concurrently with the M2 markers CD163 and IL-10. Furthermore, SAA-differentiated macrophages stimulated with lipopolysaccharide (LPS) expressed markedly higher levels of IL-6 and IL-1β. The ALX/FPR2 antagonist WRW4 reduced IL-6 and IL-1β expression but did not significantly inhibit phagocytic and efferocytic activity. In vivo, SAA administration induced the development of a CD11chighCD11bhigh macrophage population that generated higher levels of IL-6, IL-1β, and G-CSF following ex vivo LPS challenge. Blocking CSF-1R signaling effectively reduced the number of CD11chighCD11bhigh macrophages by 71% and also markedly inhibited neutrophilic inflammation by 80%. In conclusion, our findings suggest that SAA can promote a distinct CD11chighCD11bhigh macrophage phenotype, and targeting this population may provide a novel approach to treating chronic inflammatory conditions associated with persistent SAA expression.—Anthony, D., McQualter, J. L., Bishara, M., Lim, E. X., Yatmaz, S., Seow, H. J., Hansen, M., Thompson, M., Hamilton, J. A., Irving, L. B., Levy, B. D., Vlahos, R., Anderson, G. P., Bozinovski, S. SAA drives proinflammatory heterotypic macrophage differentiation in the lung via CSF-1R-dependent signaling.
Keywords: macrophage biology, COPD, lung inflammation, ALX/FPR2 signaling
In the steady state, tissue-resident lung macrophages display a very slow turnover and are capable of self-renewal through macrophage colony-stimulating factor 1 (CSF-1) and granulocyte macrophage-colony-stimulating factor (GM-CSF)-dependent mechanisms (1). Alveolar macrophages (AMs) also display a very slow turnover rate in the steady state, with a reported 40% replacement rate over 1 yr (2), which are primarily derived from blood monocytes and a self-renewing population of progenitor cells within the lung parenchyma (3). Like tissue-resident lung macrophages, the AM phenotype appears to be predominately controlled by high levels of GM-CSF in the lung environment (4), which drive this unique immunosuppressive phenotype that resembles dendritic cells. During acute lung injury and infection, circulating monocytes rapidly migrate to the peripheral tissue and become actively involved in the inflammatory process by regulating the later phases of neutrophilic inflammation (5). Peripheral blood monocytes also replenish depleted lung macrophage populations during acute inflammation (2) by differentiating into mature macrophages that display specialized functional properties under the influence of the local cytokine milieu. Acute lung immune responses to lipopolysaccharide (LPS) and influenza promote macrophage phenotypic heterogeneity that overlaps between the classic M1/M2 paradigm (6). Indeed, it is now accepted that distinct macrophage subsets can share characteristics among phenotypic classifications, and that a spectrum of populations adapt physiological functions in response to environmental signals (7).
Altered macrophage behavior is central to the pathogenesis of chronic obstructive pulmonary disease (COPD), which is predicted to become the third leading cause of death by 2030 (8). Chronic airway inflammation is a prominent feature of COPD where neutrophilic inflammation is abundant and refractory to currently available anti-inflammatory medications (9). Macrophages accumulate within the lung tissue and alveolar space and are positively associated with the severity of COPD (10). This in part reflects increased migration of blood monocytes toward CXC chemokines, which are increased in COPD airways (11). In an experimental model of COPD, the depletion of lung macrophages conferred protection against the development of emphysema-like changes (12), thereby demonstrating a pathological role for this cell population. Airway and systemic inflammation is further worsened during acute exacerbations of COPD that are typically triggered by a respiratory infection (13). Hence, there is a diverse milieu of environmental and endogenous mediators that can influence monocyte activation and maturation in COPD airways. These mediators may also influence the function of the CSF-1 receptor (CSF-1R), which is highly expressed on mononuclear phagocytes. CSF1-R signaling is central to the regulation of monocytes/macrophage proliferation, differentiation, adaptation, and survival, and targeting this receptor may offer therapeutic opportunities in autoimmunity, cancer, and inflammatory conditions (14).
Diversity in polarizing mediators promotes unique macrophage transcription signatures that control physiological processes including modulation of genes involved in metabolic activities, cell cycling, lipid metabolism, and chemokine profile (15). This is also true for COPD, where there is a distinct transcriptional profile for AMs isolated from patients with COPD. This profile was associated with the progressive induction of M2-related programs that contribute to global AM phenotypes associated with smoking and COPD (16). Since there is a poor understanding of the endogenous mediators that contribute to this altered macrophage profile, the focus of this study was to establish the importance of serum amyloid A (SAA), which has recently been implicated in the pathogenesis of COPD (17–19). SAA is classically viewed as an acute-phase protein, and circulating SAA levels have been shown to markedly increase during acute exacerbations of COPD (18). In addition, its transcript levels were significantly elevated in the bronchus and parenchyma of COPD compared to control tissue (20) and in experimental models, its lung transcript levels increase in response to cigarette smoke, LPS, and influenza exposure (19). Furthermore, SAA immunoreactivity was prominent in COPD lung resection tissue (19), and this intense staining was positively associated with the accumulation of tissue neutrophils (17). This study focuses on how SAA modulates the differentiation of macrophage populations in vitro using human blood monocytes from control subjects and patients with COPD. We show that SAA is a potent inducer of TH17 polarizing inflammatory genes IL-6 and IL-1β and also concurrently induced the M2-related genes CD163 and IL-10. Furthermore, in vivo characterization of macrophage populations in response to acute and chronic SAA administration was performed, and also demonstrates the emergence of a proinflammatory macrophage population that is dependent on CSF-1R signaling.
MATERIALS AND METHODS
Reagents
Cell culture medium (RPMI 1640), FBS, trypsin EDTA, and gentamicin were purchased from Life Technologies (Carlsbad, CA, USA). Acradine Orange/Ethidium Bromide (Life Technologies), Hemacolor for microscopy (Merck Millipore, Billerica, MA, USA), TaqMan Fast Universal PCR Master Mix, TaqMan preoptimized target primers, high-capacity RNA-to-cDNA kit (Life Technologies), RNeasy kit (Qiagen, Hilden, Germany), and human recombinant SAA (rSAA; PeproTech, Rocky Hill, NJ, USA) were dissolved in sterile 0.1% BSA in dH2O to a stock concentration of 1 mg/ml and stored at −80°C prior to use. WRW4 was sourced from Tocris Biosciences (Ellisville, MO, USA). Human GM-CSF and CSF-1 (PeproTech Rocky Hill, NJ, USA) were dissolved in 0.1% BSA in dH2O at a stock concentration of 10 μg/ml. The Vybrant Phagocytosis Kit was purchased from Life Technologies. All antibodies were purchased from eBioscience (San Diego, CA, USA) unless otherwise stated. Anti-human antibodies; phycoerythrin (PE)-conjugated CD163 (clone eBioGH/61), and mouse IgG1K isotype control were used to determine cell surface expression of receptors. Viability was determined by annexin V (BD Biosciences, Franklin Lakes, NJ, USA) or DAPI (Life Technologies). The PKH26PCL kit was purchased from Sigma-Aldrich (St. Louis, MO, USA). All other reagents were purchased from Sigma-Aldrich unless otherwise stated.
Differentiation of blood monocyte-derived macrophages (MDMs) and THP-1 cells
Healthy volunteers were recruited and all subjects gave written informed consent as approved by the Royal Melbourne Hospital Ethics committee. For experiments involving the comparison of healthy control subjects and patients with COPD, subject demographics are summarized in the Supplemental Table S1. Venous blood was collected in BD vacutainers containing sodium heparin (Becton Dickinson, Franklin Lakes, NJ, USA). Peripheral blood mononuclear cells (PBMCs) were isolated using Ficoll Paque Plus (GE Healthcare, Little Chalfont, UK, USA), washed, and resuspended in MAC buffer (2 mM EDTA, 0.5% BSA in PBS). Blood monocytes were purified from the PBMCs using the Monocyte Isolation kit (Miltenyi Biotec, Auburn, CA, USA) according to manufacturer's instructions.
Differentiation was promoted by culturing cells in complete medium (10% FBS in RPMI 1640 supplemented with 25 mM HEPES, 2 mM l-glutamine, 50 μM β-mercaptomethanol, 1.5 g/L sodium bicarbonate, 1 mM sodium pyruvate, and 5 μg/mL gentamicin) containing 10 ng/ml CSF-1 and 10 ng/ml GM-CSF in the absence or presence of rSAA at 0.1 μg/ml (8 nM) at 37°C in humidified atmosphere with 5% CO2. Cells were maintained for 7 d with replacement of differentiation medium on d 3. On d 7, cells were harvested with trypsin-EDTA, and a viability cell count was performed. In a separate set of experiments, MDMs were also differentiated in the presence of the ALX/FPR2-selective antagonist WRW4. Cells were pretreated with WRW4 (5 μM) for 15 min prior to the addition of rSAA. On generation of MDMs, 3 × 105 cells were retained for quantitative PCR (qPCR). In addition, differentiated MDMs were harvested and replated in 24-well format (2.5×105 cells/well) and stimulated with LPS (1 μg/ml) for 2 h prior to analysis by qPCR. The human THP-1 cell line was also maintained in the same complete medium and differentiated under identical conditions in a subset of experiments.
RNA purification and qPCR
RNA was extracted using the RNeasy Plus Mini kit according to the manufacturer's instructions (Qiagen) and as described previously (21). RNA concentrations were quantified using NanoDrop 1000 (Thermo Fisher Scientific, Waltham, MA, USA). Total RNA was used as a template to generate cDNA with the High Capacity RNA-to-cDNA Kit, and qPCR was performed using predeveloped TaqMan primers and the ABI Prism 7900HT sequence detection system (Life Technologies). Threshold cycle values were normalized to 18S rRNA, and the ΔΔCt relative expression method was used to generate fold increase above the control group as described previously (22). Each assay was performed in triplicates.
Phagocytosis and efferocytosis assays
Phagocytosis was assessed using fluorescein isothiocyanate (FITC)-labeled Escherichia coli (K12 strain) bioparticles using a modified protocol derived from the Vybrant Phagocytosis Assay Kit (Life Technologies). Briefly, 20 μl of reconstituted FITC-E. coli was incubated with 1 × 105 MDMs in 100 μl complete medium for 45 min at 37°C. MDMs were retrieved by centrifugation (400 g, 5 min, 4°C), and nonphagocytized E. coli was quenched with trypan blue incubation for 1 min. Fluorescence quantitation of FITC was performed by flow cytometry using an LSR Fortessa cell analyzer (BD Biosciences), and data were collected for 5 × 106 viable cell events. Macrophages were resolved from E. coli alone on the basis of forward-scatter size, and forward- and side-scatter gates were set to exclude aggregates. Propidium iodide (PI) staining was also used to exclude dead cells. Positive selection gates for phagocytosis were set as FITC fluorescence greater than that of single E. coli bioparticles alone, so that positive events represented phagocytosis of multiple E. coli (Supplemental Fig. S1).
For efferocytosis, cryopreserved blood lymphocytes isolated from the same donor used to generate MDMs were induced to undergo apoptosis using a UV transilluminator (100% UV for 15 min) followed by incubation at 37°C for 2 h. Apoptosis was confirmed using annexin V-FITC/PI staining. Apoptotic lymphocytes were labeled using the PKH26-PCL Red Fluorescent Cell Linker Kit (Sigma-Aldrich) as per the manufacturer's instructions. Next 4 × 105 apoptotic lymphocytes were incubated with 1 × 105 MDM for a final ratio of 4:1. Fluorescence quantitation of PKH26 was performed by flow cytometry using an LSR Fortessa cell analyzer (BD Biosciences), and data were collected for 5 × 106 viable cell events. For quantitation of efferocytic macrophages, MDMs were resolved from lymphocytes alone on the basis of forward-scatter size, and PI staining was used to exclude dead cells and forward and side scatter gates were set to exclude aggregates. Positive selection gates for efferocytosis were set as PKH26 fluorescence equal to or greater than that of single lymphocytes alone so that positive events represented efferocytosis of apoptotic lymphocytes (Supplemental Fig. S1). Data were analyzed using FlowJo X software (TreeStar, Ashland, OR, USA).
In vivo SAA challenge of mice and administration of anti-CSF-1R
Specific pathogen-free (SPF) male BALB/c mice were obtained from the Animal Resource Centre (Perth, Australia) and maintained in SPF conditions at the University of Melbourne. All experimental protocols were approved and conducted in compliance with the National Health and Medical Research Council of Australia. Mice (6–8 wk old) were lightly anesthetized by inhalation of Penthrane vapor, and each treatment was administered into the lungs of mice using the intranasal method, as described previously, in a volume ≤ 50 μl (19). Recombinant SAA (2 μg) and/or 100 μg/mouse of rat anti-mouse IgG2a mAb (isotype control, in PBS) or anti-CSF-1R mAb (AFS298 in PBS) was administered intranasally, as described previously (23). Anti-CSF-1R was instilled into the lungs 2 h prior to rSAA treatment. At the specified times, brochoalveolar lavage (BAL) was performed via tracheotomy, and lung homogenates were obtained as described previously (17). To avoid nonspecific binding of Abs to FcRγ, FACS buffer containing anti-mouse CD16/32 mAb was added to all primary stains.
Surface marker analysis by flow cytometry
For CD163 surface expression on MDMs, cells were dislodged using accutase, washed twice in FACS buffer (2% FCS in PBS), and incubated with FACS buffer containing mAb or isotype control for 30 min at 4°C in the dark. After antibody incubation, cells were washed twice in FACS buffer and retrieved by centrifugation (400 g, 3 min, 4°C). Analysis was performed using the LSR Fortessa cell analyzer (BD Biosciences), and data were collected for up to 2 × 105 viable cell events.
For in vivo staining of mice at the specified times, BAL was performed via tracheotomy, and lung homogenates were obtained as described previously (17). To avoid nonspecific binding of Abs to FcRγ, FACS buffer containing anti-mouse CD16/32 mAb (Mouse BD Fc Block; 2.4G2; BD Biosciences) was added to all primary stains. All antibodies were purchased from BD Biosciences unless otherwise stated. Allophycocyanin-conjugated CD11b and PE-Cy7-conjugated CD11c was purchased from eBioscience. A strict gating strategy was used to determine different macrophage cell populations as follows: single-cell gate (exclusion of aggregates; FSC-H vs. FSC-A), viability (PI exclusion), granularity/size cell gate (FSC-A vs. SSC-A), and specific surface marker gates. Macrophages and neutrophils were initially gated as single, live, large cells. Macrophages were deemed to be autofluoresent cells that were F/480+ and CD11c+. The level of CD11b expression was used to further distinguish macrophage subpopulations. Neutrophils were classified as F4/80−, CD11C−, and Ly6Ghigh. Cells were sorted on the Aria II Cell Sorter (BD Biosciences Australia). FlowJo software (TreeStar) was used to analyze data.
Statistical analysis
Statistical analyses were conducted using GraphPad 5.0 (GraphPad, San Diego, CA, USA), and normally distributed data were expressed as means ± sem. Significance between treatment groups was tested using an unpaired t test or 1-way or 2-way ANOVA, and values of P < 0.05 were considered statistically significant. For the phagocytosis and efferocytosis assay, a paired t test was used.
RESULTS
SAA promotes a mixed M1/M2 profile during monocyte to macrophage differentiation
Differentiation of blood monocytes in the presence of SAA (at 8 nM) resulted in a morphologically distinct macrophage population characterized by a heavily vacuolated cytoplasm when compared to MDMs differentiated in the absence of SAA (Fig. 1A–C). qPCR analysis revealed that the levels of IL-6 and IL-1β transcript were 6- and 4-fold higher, respectively, in the MDMs generated in the presence of SAA relative to control (Fig. 1D, E). Detailed kinetic analysis of THP-1 monocytes demonstrates a rapid induction of IL-6 and IL-1β within 6 h, peaking at 24 h and remaining elevated at d 7 (100- and 10-fold above vehicle-treated cells, respectively; Supplemental Fig. S2A, B). In addition, the M2 marker CD163 was significantly increased in MDMs by SAA (∼6-fold, Fig. 1F) with kinetic analysis revealing a gradual induction of CD163 over 6 d (Supplemental Fig. S2C). Concentration response curve analysis in THP-1 monocytes differentiated under identical conditions generated an EC50 value of 8.1 nM for SAA-induced CD163 mRNA expression (Supplemental Fig. S2D). Increasing concentrations of SAA (8 and 80 nM) also significantly increased cell surface expression of CD163, as determined by flow cytometry (Supplemental Fig. S2E).
Figure 1.
SAA initiates expression of inflammatory genes and CD163 in primary blood monocytes. A–C) Representative images of monocytes at d 0 (A) and MDMs following differentiation over 7 d (D7) in the (B) absence (−SAA; B) or presence of SAA (+SAA; C). D–F) At d 7, gene expression of IL-6 (D), IL-1β (E), and CD163 (F) was determined in the MDM populations. Data represent n = 4 individual blood samples. *P < 0.05.
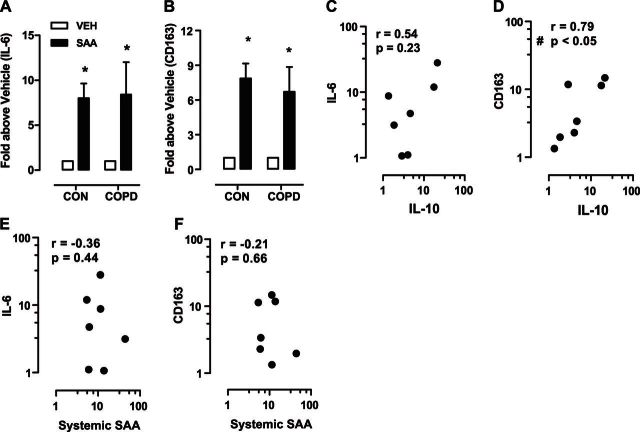
SAA also stimulated expression of IL-6 and CD163 in MDMs differentiated from COPD blood monocytes, which was a very similar response when compared to healthy controls (IL-6, 8.0±1.6 vs. 8.4±3.6, and CD163, 7.9±1.3 vs. 6.7±2.2 -fold increase, control vs. COPD-derived MDMs; Fig. 2A, B). In addition, IL-10 expression was increased by SAA in MDMs isolated from subjects with COPD (7.7±3.1-fold increase above vehicle-derived MDMs; Supplemental Fig. S2F). Since IL-10 is a key antiinflammatory gene that can promote expression of M2 markers, its association with CD163 and IL-6 was investigated. IL-10 and CD163 levels were positively associated (P<0.05, r=0.79; Fig. 2D), whereas no significant correlation was observed between IL-10 and IL-6 expression (P=0.23, r=0.54; Fig. 2C). Levels of systemic SAA in matching serum samples collected at time of monocyte isolation were not associated with the in vitro response to SAA, as assessed by directly comparing serum SAA levels with IL-6 (Fig. 2E) and CD163 (Fig. 2F) expression.
Figure 2.
IL-10 regulates CD163, but not IL-6, in COPD MDMs generated in the presence of SAA. A, B) Gene expression of IL-6 (A) and CD163 (B) was compared between MDMs generated from healthy controls and subjects with COPD. C, D) Spearman correlation between IL-10 and IL-6 (C) and IL-10 and CD163 (D) in SAA-induced MDMs from patients with COPD. E, F) No relationship was observed between systemic serum SAA levels and expression of IL-6 (E) or CD163 (F). Data represent n = 6 for control and n = 7 for COPD individual blood samples. *P < 0.05 vs. vehicle-treated MDM expression; #P < 0.05; Spearman test.
The functional characteristics of SAA-generated MDMs were assessed by measuring the phagocytic (uptake of E. coli) and efferocytic (uptake of apoptotic lymphocytes) capacity of the cells. SAA significantly increased the percentage of macrophages that phagocytosed FITC-labeled E. coli compared to vehicle control (SAA: 78.88±5.11% vs. veh: 45.12±5.68%; Fig. 3A) by an average of 2-fold (Fig. 3C). This indicates that SAA is a potent promoter of phagocytic function. When assessing efferocytosis (using matching MDMs and PKH26-labeled apoptotic lymphocytes) SAA-induced MDMs significantly increased (∼2.4-fold increase, P=0.006) their ability to engulf dying lymphocytes (SAA: 40.79±8.51% vs. veh: 18.51±4.78%; Fig. 3B, D).
Figure 3.
SAA induces increased phagocytosis and efferocytosis in primary human MDMs. A, B) Representative histograms show phagocytosis of E. coli (A) and apoptotic lymphocytes (B) by primary MDMs in the absence (dotted line) or presence of SAA (solid line). C, D) Quantification of phagocytic (C) and efferocytic (D) activity of MDMs from individual donors in the absence (vehicle) or presence of SAA. Data from n = 5–6 samples. *P < 0.05.
Maximal activation of SAA-derived MDMs is dependent on ALX/FPR2, and stimulation with LPS demonstrates enhanced production of IL-6 and IL-1β monokines
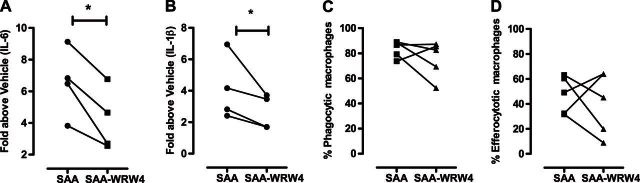
Since SAA can potentially interact with multiple receptors, including ALX/FPR2, TLR2, and CD36, the peptide antagonist WRW4 was used to selectively target ALX/FPR2. As shown in Fig. 4A, B, SAA-induced expression of IL-6 and IL-1β was significantly reduced by 38 ± 11 and 37 ± 10%, respectively, in MDMs treated with WRW4. The effect of ALX/FPR2 antagonism on the phagocytic and efferocytic function of MDMs was also determined. In contrast to gene expression, no significant reduction was observed in phagocytosis (SAA: 83.4±2.9% vs. SAA-WRW4: 75.4±6.6%, P=0.31; Fig. 4C) or efferocytosis (SAA: 47.3±6.7% vs. SAA-WRW4: 40.3±11.3%, P=0.62; Fig. 4D). MDMs were also stimulated with LPS to further characterize their activation status. LPS markedly increased IL-6 expression by 223-fold in control MDMs, and this was further increased to 436-fold in MDMs generated with SAA, representing a 96% increase in IL-6 expression (Fig. 5A). IL-1β expression was also increased by 22-fold in control MDMs by LPS, and this was further enhanced to 31-fold in MDMs generated with SAA, representing a 41% increase in IL-1β expression (Fig. 5B).
Figure 4.
Targeting ALX/FPR2 inhibits inflammatory gene expression without compromising macrophage function. Expression of IL-6 and IL-1β was determined in SAA-induced MDMs in the absence or presence of WRW4. WRW4 reduced (A) IL-6 and (B) IL-1β by 37% and 38%, respectively. On analysis of (C) phagocytosis and (D) efferocytosis in primary human MDMs generated with SAA, no significant inhibition of function was observed. Data represent n = 4 individual blood samples, *P < 0.05.
Figure 5.
SAA-generated MDMs express higher IL-1β and IL-6 in response to LPS. SAA-induced MDMs were stimulated with or without LPS (1 μg/ml) for 2 h, and expression of IL-6 (A) and IL-1β (B) was determined by qPCR. Gene expression is expressed relative to the housekeeping gene 18S and presented by fold change above vehicle treatment (VEH). Data represent n = 4 individual blood samples.
In vivo appearance of an SAA-induced proinflammatory macrophage subpopulation that is dependent on CSF-1R signaling
Lung macrophage subpopulations were investigated in BALB/c mice in response to in vivo stimulation with SAA, which initiates neutrophilic inflammation via an ALX/FPR2 dependent mechanism (19). In control mice, the majority (>95%) of resident alveolar macrophages were CD11chighCD11blow (Fig. 6A), and, as previously shown were CD115+ and Ly6c− (6). After challenge with SAA the number of resident CD11chighCD11blow cells did not significantly change in the lung tissue (Fig. 6C) or BAL compartment (Fig. 6D). In contrast, a CD11chighCD11bhigh population appeared following SAA challenge (Fig. 6B) and was significantly elevated in the lung tissue (Fig. 6E) and BAL (Fig. 6F) at the 24–72 h time points, disappearing by d 7 postchallenge. To investigate the consequences of chronic SAA exposure on macrophage lung subpopulations, BALB/c were challenged 1×/wk over 5 wk with either vehicle or SAA, with the final challenge given 7 d prior to analysis of the BAL compartment. A significant increase in BAL CD11chighCD11bhigh macrophages was observed in SAA-treated mice, where numbers increased 3.3-fold above vehicle-treated mice (Fig. 7A), demonstrating the persistence of the population due to chronic SAA exposure. A cell-sorting strategy was applied to isolate CD11chighCD11blow and CD11chighCD11bhigh macrophages from mice treated with vehicle or SAA in the chronic challenge model. On ex vivo stimulation with LPS, the resident CD11chighCD11blow population from vehicle- or SAA-treated lungs expressed similar levels of IL-6, IL-1β, and G-CSF (Fig. 7B–D). In contrast, CD11chighCD11bhigh macrophages isolated from SAA-challenged mice generated significantly higher levels of IL-6 (245- vs. 15-fold increase; Fig. 7B), IL-1β (22- vs. 10-fold increase; Fig. 7C) and G-CSF (27- vs. 9-fold increase; Fig. 7D) in comparison to CD11chighCD11blow control macrophages stimulated with LPS.
Figure 6.
Acute rSAA treatment into murine lung results in the emergence of CD11chighCD11bhigh macrophage population. CD11c and CD11b expression was used to determine macrophage subsets. A, B) Representative dot plots of macrophage subpopulations in BAL fluid 48 h after vehicle (A) or SAA treatment (B). C, D) Number of CD11chighCD11blow cells per milligram of lung tissue (C) and BAL (D). E, F) Number of CD11chighCD11bhigh cells per milligram of lung tissue (E) and BAL (F). Pooled data from 2 experiments n = 4–8 samples. *P ≤ 0.05.
Figure 7.
Chronic administration of rSAA results in the sustained presence of the CD11chighCD11bhigh population that expresses high levels of IL-6, IL-1β, and G-CSF. A) Percentage of CD11chighCD11bhigh macrophages in the BAL compartment. B–D) Macrophage populations were isolated from lung tissue using a cell sorting strategy and stimulated ex vivo with saline (control) or LPS. Expression of IL-6 (B), IL-1β (C), and G-CSF (D) were determined by quantitative PCR and expressed as a fold increase above resident CD11chighCD11blow macrophages. Pooled data from 2 experiments; n = 4–8 samples. *P ≤ 0.05.
Signaling through the CSF-1R can contribute to the differentiation of myeloid progenitors into heterogeneous populations of macrophages. To determine the importance of CSF-1R receptor signaling on the appearance of CD11chighCD11bhigh cells, a neutralizing antibody to CSF-1R was administered intranasally 2 h prior to challenge with SAA. Blocking CSF-1R did not significantly alter the resident CD11chighCD11blow population (Fig. 8A); however, it markedly reduced the emergence of the SAA-induced CD11chighCD11bhigh population (71% reduction, SAA-treated isotype control vs. anti-CSF-1R; Fig. 8B) and BAL neutrophils (80% reduction, SAA-treated isotype control vs. anti-CSF-1R; Fig. 8C).
Figure 8.
The emergence of SAA-induced CD11ChiCD11bhi macrophage subpopulation is dependent on CSF-1R signaling. BALB/c mice were intranasally pretreated with anti-CSF-1R or control followed by rSAA. BAL cells were analyzed by flow cytometry 24 h later for macrophage subset populations. Neutrophils were identified as CD11c−Ly6G+ cells. Graphs show total number of CD11chighCD11blow macrophages (A), CD11chighCD11bhigh macrophages (B), and neutrophils (C); n = 4–6 individual samples/group. *P ≤ 0.05.
DISCUSSION
SAA is abundant in COPD, where its immunoreactivity colocalizes with the accumulation of macrophages (19). SAA is also prominent in the inflammatory milieu of rheumatoid arthritis, where its expression is related to the accumulation of disease-associated macrophage populations (24). Although there is evidence for SAA acting as a potent chemoattractant that initiates monocyte migration and calcium mobilization with comparative activity to chemokine family members, including RANTES, MCP-1, and MIP-1α (25, 26), its influence on macrophage polarization remains poorly characterized. In this study, we now reveal a prominent role for SAA in modulating the differentiation of human monocytes into macrophages that express increased levels of the inflammatory genes IL-6 and IL-1β in conjunction with the M2-related scavenger receptor CD163. Systemic levels of SAA did not influence in vitro responses to SAA. It is known that in the circulation SAA interacts with high density lipoproteins and in this lipid-bound state displays less biological activity, which may represent a protective mechanism to prevent excessive systemic inflammation. This is in contrast to lipid-poor SAA aggregates that are produced within an inflamed tissue microenvironment, which can potently stimulate pathogen recognition receptors such as ALX/FPR2. In addition, our findings of a mixed M1/M2 macrophage profile are consistent with the disease setting, where considerable heterogeneity within the inflamed COPD lung environment exists. It is already established that in a microenvironment where inflammatory markers such IL-1β and IL-6 and proteinases such as MMP-9 are abundant, COPD airway macrophages express an M2-skewed gene profile (16). The findings within this study identify SAA as a major mediator that can contribute to this mixed M1/M2 disease macrophage profile.
The induction of CD163 is commonly recognized as a marker for M2-alternatively activated macrophages involved in wound healing and angiogenesis (27, 28) and to the best of our knowledge, this study is the first to identify SAA as a prominent inducer of CD163 expression. Using COPD monocytes, IL-10 was shown to be up-regulated by SAA and positively associated with CD163 expression, which is consistent with CD163 being the most strongly induced gene in monocytes stimulated with IL-10 (29). The data presented here support the concept that SAA-induced IL-10 can contribute to increased expression of CD163 in MDMs, but do not exclude additional SAA-induced mediators such as IL-6, which can also induce CD163 expression (30). Since CD163+ macrophages are highly prominent in the BAL compartment of current and ex-smokers with COPD (31), our findings identify SAA as a disease-relevant mediator underlying accumulation of a distinct macrophage population. CD163 may also constitute a major defense mechanism to protect the lung because it functions as a scavenger receptor for hemoglobin–haptoglobin (HbHp) complexes, which on internalization leads to degradation of HbHp complexes and signaling that induces expression of Heme-oxygenase 1 (HO-1) (28). HO-1 subsequently degrades heme to biliverdin, carbon monoxide, and iron, each with candidate roles in cytoprotection (32). The persistence of HO-1 in COPD airways (33) is consistent with an environment where there is excessive oxidative stress and a deficiency in the resolution of inflammation.
It is also well established that IL-10 can exert anti-inflammatory actions through autoinhibitory mechanisms on macrophages; however, in this study increased expression of IL-10 was not inversely associated with IL-6 expression in MDMs derived from patients with COPD. Furthermore, increased IL-10 expression in SAA-induced MDMs did not appear to exert an immunosuppressive response in this macrophage population, as IL-6 and IL-1β were markedly increased in response to LPS stimulation above control MDMs. Utilizing an ALX/FPR2 receptor selective antagonist (WRW4), the maximal release of IL-6 and IL-1β in response to SAA was shown to be dependent on ALX/FPR2 signaling in primary human monocytes. This response was not fully inhibited by the ALX/FPR2 antagonist, hence alternative receptors reported to interact with SAA including TLR2 (34), CD36 (35), and scavenger receptor class B type I (36) may also contribute to maximal release. The ALX/FPR2 receptor is a member of the GPCR superfamily that is highly expressed in myeloid cells (37), where the transcription factors OCT1 and SP1 coordinate the transcription of the ALX/FPR2 gene on monocytes (38). Consistent with the findings presented here, SAA-induced NFκB activity, MMP-9, CCL2, and TNF-α expression were shown to be dependent on ALX/FPR2 signaling in human monocytes (39–41).
One of the paradoxes of COPD is that, despite there being an accumulation of M2-skewed macrophages, there is a deficiency in clearance of bacteria and apoptotic cells, which is thought to be mediated by excessive oxidative stress (42). Our data support the concept that in nonpathological conditions, SAA enhances host immunity through promotion of phagocytic activity and recruitment of inflammatory monocytes and neutrophils that further promote clearance of pathogen and/or injured tissue. However, in chronic conditions such as COPD, the persistence of SAA may maintain inflammatory cell recruitment, thereby establishing a damaging cycle of inflammation and tissue injury. This can also directly contribute to excessive oxidative stress, which can directly modify and inhibit the phagocytic machinery, such as carbonylation of the actin cytoskeleton (22). Hence, there is a need to reduce inflammation and concurrently improve clearance mechanisms in COPD. An alternative approach to achieving this outcome may be to target the ALX/FPR2 receptor as reviewed previously (9). It is known that ALX/FPR2 displays diverse ligand affinities (43), where lipoxins can directly oppose the inflammatory actions of SAA (19, 44, 45). It is now becoming increasingly apparent that the ALX/FPR2 receptor can display ligand-biased signaling, where alternative receptor conformations can be induced in a ligand-specific fashion (46, 47). Lipoxin A4 has been shown to enhance nonphlogistic phagocytosis by monocyte-derived macrophages (48, 49) and inhibit lung neutrophilic inflammation (17, 19). Hence, the use of alternative ALX/FPR2 agonists such as lipoxins may help to restore clearance pathways and concurrently antagonize inflammatory pathways activated by SAA. In addition, ALX/FPR2 can modulate macrophage activity implicated in tumorigenesis, where SAA promoted tumor invasiveness (50). SAA-stimulated CSF-1 and MCP-1 expression promoted an alternative macrophage phenotype associated with tumor invasiveness, in contrast to lipoxin, which displayed antitumorigenic activity. Intriguingly, CSF-1R blockage has been shown to reduce tumor burden through the modulation of tumor associated macrophages (51). Since COPD confers a substantial risk to developing lung cancer, and as SAA is upregulated in lung cancer (52), the modulation of the SAA-ALX/FPR2-CSF1R tumor macrophage axis represents an unexplored therapeutic locus for lung cancer.
In summary, this study characterizes a novel mechanism by which chronic airway inflammation can be maintained via SAA-dependent activation and differentiation of monocyte derived macrophages. SAA was the first described chemotactic ligand for ALX/FPR2, where binding initiated calcium mobilization and monocyte migration (26). More recently, the activation of monocytes has been shown to regulate second phase neutrophilic infiltration in inflammatory lung injury models (5). This study demonstrates that SAA not only interacts with ALX/FPR2 on monocytes to promote migration to the site of tissue injury, but also initiates expression of the cytokines IL-6 and IL-1β that are strongly implicated in neutrophilic inflammation. These findings were replicated in vivo, where chronic administration of SAA promoted the persistence of a CD11chighCD11bhigh macrophage population that generated significantly higher expression of IL-6, IL-1β and G-CSF, which stimulates the bone marrow to produce and mobilize granulocytes. IL-6 and IL-1β are also potent TH17 polarizing mediators that promote the emergence of IL-17 producing T cell lineages. Most recently, we have identified an integral role for IL-17A in SAA-mediated lung inflammation, as we have shown that inhibition of TH17 and γδ T cell derived IL-17A markedly inhibited neutrophilic infiltration (17). Since CSF-1R signaling can govern macrophage lineage development (as reviewed in ref. 14, 53), this study also demonstrates that the targeting of CSF-1R through a blocking antibody can inhibit the emergence of CD11chighCD11bhigh BAL macrophages and the recruitment of BAL neutrophils in response to SAA.
In conclusion, we identify SAA as a novel modulator of macrophage polarity that coordinates the generation of a distinct phenotype, which is both proinflammatory and displays some characteristic features of M2 macrophages. The persistence of this population in chronic diseases may contribute to the deleterious remodeling of the injured tissue and persistence of TH17-mediated inflammatory mechanisms. Two potential therapeutic applications were explored, where ALX/FPR2 antagonism reduced the initial phase of inflammatory gene expression in response to SAA in vitro and inhibition of CSF-1R signaling markedly suppresses the emergence of this macrophage population in vivo.
Supplementary Material
Acknowledgments
This work was supported by the National Health and Medical Research Council (NHMRC) of Australia.
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
- AM
- alveolar macrophage
- BAL
- brochoalveolar lavage
- COPD
- chronic obstructive pulmonary disease
- CSF-1
- colony-stimulating factor 1
- CSF-1R
- colony stimulating factor 1 receptor
- FITC
- fluorescein isothiocyanate
- GM-CSF
- granulocyte macrophage-colony-stimulating factor
- HO-1
- heme-oxygenase 1
- LPS
- lipopolysaccharide
- MDM
- monocyte-derived macrophage
- PBMC
- peripheral blood mononuclear cell
- PE
- phycoerythrin
- PI
- propidium iodide
- qPCR
- quantitative PCR
- rSAA
- recombinant serum amyloid A
- SAA
- serum amyloid A
REFERENCES
- 1. Hashimoto D., Chow A., Noizat C., Teo P., Beasley M. B., Leboeuf M., Becker C. D., See P., Price J., Lucas D., Greter M., Mortha A., Boyer S. W., Forsberg E. C., Tanaka M., van Rooijen N., Garcia-Sastre A., Stanley E. R., Ginhoux F., Frenette P. S., Merad M. (2013) Tissue-resident macrophages self-maintain locally throughout adult life with minimal contribution from circulating monocytes. Immunity 38, 792–804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Maus U. A., Janzen S., Wall G., Srivastava M., Blackwell T. S., Christman J. W., Seeger W., Welte T., Lohmeyer J. (2006) Resident alveolar macrophages are replaced by recruited monocytes in response to endotoxin-induced lung inflammation. Am. J. Respir. Cell Mol. Biol. 35, 227–235 [DOI] [PubMed] [Google Scholar]
- 3. Guth A. M., Janssen W. J., Bosio C. M., Crouch E. C., Henson P. M., Dow S. W. (2009) Lung environment determines unique phenotype of alveolar macrophages. Am. J. Physiol. Lung Cell. Mol. Physiol. 296, L936–L946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shibata Y., Berclaz P. Y., Chroneos Z. C., Yoshida M., Whitsett J. A., Trapnell B. C. (2001) GM-CSF regulates alveolar macrophage differentiation and innate immunity in the lung through PU. 1. Immunity 15, 557–567 [DOI] [PubMed] [Google Scholar]
- 5. Dhaliwal K., Scholefield E., Ferenbach D., Gibbons M., Duffin R., Dorward D. A., Morris A. C., Humphries D., MacKinnon A., Wilkinson T. S., Wallace W. A., van Rooijen N., Mack M., Rossi A. G., Davidson D. J., Hirani N., Hughes J., Haslett C., Simpson A. J. (2012) Monocytes control second-phase neutrophil emigration in established lipopolysaccharide-induced murine lung injury. Am. J. Respir. Crit. Care Med. 186, 514–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Duan M., Li W. C., Vlahos R., Maxwell M. J., Anderson G. P., Hibbs M. L. (2012) Distinct macrophage subpopulations characterize acute infection and chronic inflammatory lung disease. J. Immunol. 189, 946–955 [DOI] [PubMed] [Google Scholar]
- 7. Mosser D. M., Edwards J. P. (2008) Exploring the full spectrum of macrophage activation. Nature Rev. Immunol. 8, 958–969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jemal A., Ward E., Hao Y., Thun M. (2005) Trends in the leading causes of death in the United States, 1970–2002. JAMA 294, 1255–1259 [DOI] [PubMed] [Google Scholar]
- 9. Bozinovski S., Anthony D., Anderson G. P., Irving L. B., Levy B. D., Vlahos R. (2013) Treating neutrophilic inflammation in COPD by targeting ALX/FPR2 resolution pathways. Pharmacol. Ther. 140, 280–289 [DOI] [PubMed] [Google Scholar]
- 10. Hogg J. C., Chu F., Utokaparch S., Woods R., Elliott W. M., Buzatu L., Cherniack R. M., Rogers R. M., Sciurba F. C., Coxson H. O., Pare P. D. (2004) The nature of small-airway obstruction in chronic obstructive pulmonary disease. N. Engl. J. Med. 350, 2645–2653 [DOI] [PubMed] [Google Scholar]
- 11. Traves S. L., Smith S. J., Barnes P. J., Donnelly L. E. (2004) Specific CXC but not CC chemokines cause elevated monocyte migration in COPD: a role for CXCR2. J. Leukoc. Biol. 76, 441–450 [DOI] [PubMed] [Google Scholar]
- 12. Beckett E. L., Stevens R. L., Jarnicki A. G., Kim R. Y., Hanish I., Hansbro N. G., Deane A., Keely S., Horvat J. C., Yang M., Oliver B. G., van Rooijen N., Inman M. D., Adachi R., Soberman R. J., Hamadi S., Wark P. A., Foster P. S., Hansbro P. M. (2013) A new short-term mouse model of chronic obstructive pulmonary disease identifies a role for mast cell tryptase in pathogenesis. J. Allergy Clin. Immunol. 131, 752–762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hurst J. R., Perera W. R., Wilkinson T. M., Donaldson G. C., Wedzicha J. A. (2006) Systemic and upper and lower airway inflammation at exacerbation of chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 173, 71–78 [DOI] [PubMed] [Google Scholar]
- 14. Hamilton J. A. (2008) Colony-stimulating factors in inflammation and autoimmunity. Nat. Rev. Immunol. 8, 533–544 [DOI] [PubMed] [Google Scholar]
- 15. Martinez F. O., Gordon S., Locati M., Mantovani A. (2006) Transcriptional profiling of the human monocyte-to-macrophage differentiation and polarization: new molecules and patterns of gene expression. J. Immunol. 177, 7303–7311 [DOI] [PubMed] [Google Scholar]
- 16. Shaykhiev R., Krause A., Salit J., Strulovici-Barel Y., Harvey B. G., O'Connor T. P., Crystal R. G. (2009) Smoking-dependent reprogramming of alveolar macrophage polarization: implication for pathogenesis of chronic obstructive pulmonary disease. J. Immunol. 183, 2867–2883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Anthony D., Seow H. J., Uddin M., Thompson M., Dousha L., Vlahos R., Irving L. B., Levy B. D., Anderson G. P., Bozinovski S. (2013) Serum amyloid A promotes lung neutrophilia by increasing IL-17A levels in the mucosa and gammadelta T cells. Am. J. Respir. Crit. Care Med. 188, 179–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bozinovski S., Hutchinson A., Thompson M., Macgregor L., Black J., Giannakis E., Karlsson A. S., Silvestrini R., Smallwood D., Vlahos R., Irving L. B., Anderson G. P. (2008) Serum amyloid A is a biomarker of acute exacerbations of chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 177, 269–278 [DOI] [PubMed] [Google Scholar]
- 19. Bozinovski S., Uddin M., Vlahos R., Thompson M., McQualter J. L., Merritt A. S., Wark P. A., Hutchinson A., Irving L. B., Levy B. D., Anderson G. P. (2012) Serum amyloid A opposes lipoxin A(4) to mediate glucocorticoid refractory lung inflammation in chronic obstructive pulmonary disease. Proc. Natl. Acad. Sci. U. S. A. 109, 935–940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lopez-Campos J. L., Calero C., Rojano B., Lopez-Porras M., Saenz-Coronilla J., Blanco A. I., Sanchez-Lopez V., Tobar D., Montes-Worboys A., Arellano E. (2013) C-reactive protein and serum amyloid a overexpression in lung tissues of chronic obstructive pulmonary disease patients: a case-control study. Int. J. Med. Sci. 10, 938–947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Liu K., Anderson G. P., Bozinovski S. (2008) DNA vector augments inflammation in epithelial cells via EGFR-dependent regulation of TLR4 and TLR2. Am. J. Respir. Cell Mol. Biol. 39, 305–311 [DOI] [PubMed] [Google Scholar]
- 22. Bozinovski S., Vlahos R., Zhang Y., Lah L. C., Seow H. J., Mansell A., Anderson G. P. (2011) Carbonylation caused by cigarette smoke extract is associated with defective macrophage immunity. Am. J. Respir. Cell Mol. Biol. 45, 229–236 [DOI] [PubMed] [Google Scholar]
- 23. Lenzo J. C., Turner A. L., Cook A. D., Vlahos R., Anderson G. P., Reynolds E. C., Hamilton J. A. (2012) Control of macrophage lineage populations by CSF-1 receptor and GM-CSF in homeostasis and inflammation. Immunol. Cell Biol. 90, 429–440 [DOI] [PubMed] [Google Scholar]
- 24. O'Hara R., Murphy E. P., Whitehead A. S., FitzGerald O., Bresnihan B. (2000) Acute-phase serum amyloid A production by rheumatoid arthritis synovial tissue. Arthritis Res. 2, 142–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Badolato R., Wang J. M., Murphy W. J., Lloyd A. R., Michiel D. F., Bausserman L. L., Kelvin D. J., Oppenheim J. J. (1994) Serum amyloid A is a chemoattractant: induction of migration, adhesion, and tissue infiltration of monocytes and polymorphonuclear leukocytes. J. Exp. Med. 180, 203–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Su S. B., Gong W., Gao J. L., Shen W., Murphy P. M., Oppenheim J. J., Wang J. M. (1999) A seven-transmembrane, G protein-coupled receptor, FPRL1, mediates the chemotactic activity of serum amyloid A for human phagocytic cells. J. Exp. Med. 189, 395–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mantovani A., Sica A., Sozzani S., Allavena P., Vecchi A., Locati M. (2004) The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 25, 677–686 [DOI] [PubMed] [Google Scholar]
- 28. Van Gorp H., Delputte P. L., Nauwynck H. J. (2010) Scavenger receptor CD163, a Jack-of-all-trades and potential target for cell-directed therapy. Mol. Immunol. 47, 1650–1660 [DOI] [PubMed] [Google Scholar]
- 29. Williams L., Jarai G., Smith A., Finan P. (2002) IL-10 expression profiling in human monocytes. J. Leukoc. Biol. 72, 800–809 [PubMed] [Google Scholar]
- 30. Buechler C., Ritter M., Orso E., Langmann T., Klucken J., Schmitz G. (2000) Regulation of scavenger receptor CD163 expression in human monocytes and macrophages by pro- and antiinflammatory stimuli. J. Leukoc. Biol. 67, 97–103 [PubMed] [Google Scholar]
- 31. Kunz L. I., Lapperre T. S., Snoeck-Stroband J. B., Budulac S. E., Timens W., van Wijngaarden S., Schrumpf J. A., Rabe K. F., Postma D. S., Sterk P. J., Hiemstra P. S., and Groningen Leiden Universities Corticosteroids in Obstructive Lung Disease Study. (2011) Smoking status and anti-inflammatory macrophages in bronchoalveolar lavage and induced sputum in COPD. Respir. Res. 12, 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ryter S. W., Kim H. P., Nakahira K., Zuckerbraun B. S., Morse D., Choi A. M. (2007) Protective functions of heme oxygenase-1 and carbon monoxide in the respiratory system. Antioxid. Redox Signal. 9, 2157–2173 [DOI] [PubMed] [Google Scholar]
- 33. Dolinay T., Choi A. M., Ryter S. W. (2012) Heme Oxygenase-1/CO as protective mediators in cigarette smoke- induced lung cell injury and chronic obstructive pulmonary disease. Curr. Pharm. Bio/Tech. 13, 769–776 [DOI] [PubMed] [Google Scholar]
- 34. Cheng N., He R., Tian J., Ye P. P., Ye R. D. (2008) Cutting edge: TLR2 is a functional receptor for acute-phase serum amyloid A. J. Immunol. 181, 22–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Baranova I. N., Bocharov A. V., Vishnyakova T. G., Kurlander R., Chen Z., Fu D., Arias I. M., Csako G., Patterson A. P., Eggerman T. L. (2010) CD36 is a novel serum amyloid A (SAA) receptor mediating SAA binding and SAA-induced signaling in human and rodent cells. J. Biol. Chem. 285, 8492–8506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cai L., de Beer M. C., de Beer F. C., van der Westhuyzen D. R. (2005) Serum amyloid A is a ligand for scavenger receptor class B type I and inhibits high density lipoprotein binding and selective lipid uptake. J. Biol. Chem. 280, 2954–2961 [DOI] [PubMed] [Google Scholar]
- 37. Murphy P. M., Ozcelik T., Kenney R. T., Tiffany H. L., McDermott D., Francke U. (1992) A structural homologue of the N-formyl peptide receptor. Characterization and chromosome mapping of a peptide chemoattractant receptor family. J. Biol. Chem. 267, 7637–7643 [PubMed] [Google Scholar]
- 38. Waechter V., Schmid M., Herova M., Weber A., Gunther V., Marti-Jaun J., Wust S., Rosinger M., Gemperle C., Hersberger M. (2012) Characterization of the promoter and the transcriptional regulation of the lipoxin A4 receptor (FPR2/ALX) gene in human monocytes and macrophages. J. Immunol. 188, 1856–1867 [DOI] [PubMed] [Google Scholar]
- 39. Lee H. Y., Kim M. K., Park K. S., Bae Y. H., Yun J., Park J. I., Kwak J. Y., Bae Y. S. (2005) Serum amyloid A stimulates matrix-metalloproteinase-9 upregulation via formyl peptide receptor like-1-mediated signaling in human monocytic cells. Biochem. Biophys. Res. Commun. 330, 989–998 [DOI] [PubMed] [Google Scholar]
- 40. Lee H. Y., Kim M. K., Park K. S., Shin E. H., Jo S. H., Kim S. D., Jo E. J., Lee Y. N., Lee C., Baek S. H., Bae Y. S. (2006) Serum amyloid A induces contrary immune responses via formyl peptide receptor-like 1 in human monocytes. Mol. Pharmacol. 70, 241–248 [DOI] [PubMed] [Google Scholar]
- 41. Lee H. Y., Kim S. D., Shim J. W., Lee S. Y., Lee H., Cho K. H., Yun J., Bae Y. S. (2008) Serum amyloid A induces CCL2 production via formyl peptide receptor-like 1-mediated signaling in human monocytes. J. Immunol. 181, 4332–4339 [DOI] [PubMed] [Google Scholar]
- 42. Harvey C. J., Thimmulappa R. K., Sethi S., Kong X., Yarmus L., Brown R. H., Feller-Kopman D., Wise R., Biswal S. (2011) Targeting Nrf2 signaling improves bacterial clearance by alveolar macrophages in patients with COPD and in a mouse model. Sci. Trans. Med. 3, 78ra32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ye R. D., Boulay F., Wang J. M., Dahlgren C., Gerard C., Parmentier M., Serhan C. N., Murphy P. M. (2009) International Union of Basic and Clinical Pharmacology. LXXIII. Nomenclature for the formyl peptide receptor (FPR) family. Pharmacol. Rev. 61, 119–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. El Kebir D., Jozsef L., Khreiss T., Pan W., Petasis N. A., Serhan C. N., Filep J. G. (2007) Aspirin-triggered lipoxins override the apoptosis-delaying action of serum amyloid A in human neutrophils: a novel mechanism for resolution of inflammation. J. Immunol. 179, 616–622 [DOI] [PubMed] [Google Scholar]
- 45. He R., Sang H., Ye R. D. (2003) Serum amyloid A induces IL-8 secretion through a G protein-coupled receptor, FPRL1/LXA4R. Blood 101, 1572–1581 [DOI] [PubMed] [Google Scholar]
- 46. Cooray S. N., Gobbetti T., Montero-Melendez T., McArthur S., Thompson D., Clark A. J., Flower R. J., Perretti M. (2013) Ligand-specific conformational change of the G-protein-coupled receptor ALX/FPR2 determines proresolving functional responses. Proc. Natl. Acad. Sci. U. S. A. 110, 18232–18237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Filep J. G. (2013) Biasing the lipoxin A4/formyl peptide receptor 2 pushes inflammatory resolution. Proc. Natl. Acad. Sci. U. S. A. 110, 18033–18034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Godson C., Mitchell S., Harvey K., Petasis N. A., Hogg N., Brady H. R. (2000) Cutting edge: lipoxins rapidly stimulate nonphlogistic phagocytosis of apoptotic neutrophils by monocyte-derived macrophages. J. Immunol. 164, 1663–1667 [DOI] [PubMed] [Google Scholar]
- 49. Maderna P., Cottell D. C., Toivonen T., Dufton N., Dalli J., Perretti M., Godson C. (2010) FPR2/ALX receptor expression and internalization are critical for lipoxin A4 and annexin-derived peptide-stimulated phagocytosis. FASEB J. 24, 4240–4249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Li Y., Cai L., Wang H., Wu P., Gu W., Chen Y., Hao H., Tang K., Yi P., Liu M., Miao S., Ye D. (2011) Pleiotropic regulation of macrophage polarization and tumorigenesis by formyl peptide receptor-2. Oncogene 30, 3887–3899 [DOI] [PubMed] [Google Scholar]
- 51. Pyonteck S. M., Akkari L., Schuhmacher A. J., Bowman R. L., Sevenich L., Quail D. F., Olson O. C., Quick M. L., Huse J. T., Teijeiro V., Setty M., Leslie C. S., Oei Y., Pedraza A., Zhang J., Brennan C. W., Sutton J. C., Holland E. C., Daniel D., Joyce J. A. (2013) CSF-1R inhibition alters macrophage polarization and blocks glioma progression. Nat. Med. 19, 1264–1272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sung H. J., Ahn J. M., Yoon Y. H., Rhim T. Y., Park C. S., Park J. Y., Lee S. Y., Kim J. W., Cho J. Y. (2011) Identification and validation of SAA as a potential lung cancer biomarker and its involvement in metastatic pathogenesis of lung cancer J. Proteome Res. 10, 1383–1395 [DOI] [PubMed] [Google Scholar]
- 53. Hume D. A., MacDonald K. P. (2012) Therapeutic applications of macrophage colony-stimulating factor-1 (CSF-1) and antagonists of CSF-1 receptor (CSF-1R) signaling. Blood 119, 1810–1820 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.