Summary
Fragile X syndrome (FXS) patients present neuronal alterations that lead to severe intellectual disability, but the underlying neuronal circuit mechanisms are poorly understood. An emerging hypothesis postulates that reduced GABAergic inhibition of excitatory neurons is a key component in the pathophysiology of FXS. Here, we directly test this idea in a FXS Drosophila model. We show that FXS flies exhibit strongly impaired olfactory behaviors. In line with this, olfactory representations are less odor specific due to broader response tuning of excitatory projection neurons. We find that impaired inhibitory interactions underlie reduced specificity in olfactory computations. Finally, we show that defective lateral inhibition across projection neurons is caused by weaker inhibition from GABAergic interneurons. We provide direct evidence that deficient inhibition impairs sensory computations and behavior in an in vivo model of FXS. Together with evidence of impaired inhibition in autism and Rett syndrome, these findings suggest a potentially general mechanism for intellectual disability.
Keywords: fruit fly, fragile X syndrome, olfaction, antennal lobe, lateral inhibition, neural circuits, mental disabilities, autism, Drosophila melanogaster, calcium imaging
Graphical Abstract
Highlights
-
•
Lack of dFMRP leads to reduced olfactory attraction and aversion in fruit flies
-
•
Odor selectivity of antennal lobe projection neurons is impaired in dfmr1− flies
-
•
GABAergic lateral inhibition within the antennal lobe is weaker in dfmr1− flies
-
•
Deficient lateral inhibition impairs sensory computations and animal behavior
Franco et al. show that a Drosophila model of fragile X syndrome exhibits reduced GABAergic transmission, which leads to impaired lateral inhibition across the antennal lobe. These alterations in neuronal connectivity directly affect olfactory computations by reducing the specificity of odor responses and lead to behavioral defects.
Introduction
Fragile X syndrome (FXS) is a common inherited intellectual disability disorder. FXS patients exhibit neurological symptoms that include learning disabilities, social anxiety, attention deficits, hyperarousal, hypersensitivity, autism, and epilepsy [1]. Notwithstanding the complexity of neurophysiological and behavioral alterations, FXS is caused by the silencing, deletion, or loss-of-function mutation of a single gene, FMR1. As a result, FMRP (fragile X mental retardation protein), its protein product, is not expressed in the majority of cases or is non-functional in the rare cases with a point mutation [2, 3, 4]. FMRP is an mRNA-binding protein [5] that regulates several aspects of mRNA metabolism such as nuclear export, transport to synaptic terminals, activity-dependent ribosome stalling and gene expression [6, 7, 8]. Although much of FMRP activity is thought to be related to regulation of synaptic function [9, 10, 11], little is known about the potential defects in neuronal function caused by the absence of FMRP, in particular how these neurophysiological alterations lead to impairment in neuronal computations and behavior in patients with FXS.
Initial studies revealed that dendritic spine number is increased in the cortex of FXS patients [12, 13]. In fact, dendritic abnormalities are the most consistent anatomical correlates of intellectual disability [14]. Studies on animal models of FXS showed that FMRP regulates neuronal branching [15, 16, 17] as well as dendritic spine morphology and density [11, 18]. In addition to defects in synaptic structure and axonal branching, impairments in animal behavior have been observed [11, 16]. However, further studies showed that neuroanatomical and behavioral defects can be genetically uncoupled [17], suggesting that unknown impairments in neuronal circuit function may underlie behavioral deficits.
FMRP regulates translation of mRNAs at synapses, some of which encode proteins involved in synaptic plasticity [19, 20]. Importantly, the absence of FMRP leads to abnormally enhanced group 1 mGluR (metabotropic glutamate receptor) signaling, which results in exaggerated long-term depression [21], with a net loss of AMPA and NMDA receptors [22, 23]. Additionally, enhanced group 1 mGluR signaling contributes to the elongation of dendritic spines in rodent models of FXS [18, 24] and leads to increased intrinsic neuronal excitability through the downregulation of potassium channels controlling resting membrane potential and action potential afterhyperpolarization [25, 26]. Moreover, FMRP directly influences neuronal excitability by regulating expression of potassium channels [27, 28] and by interacting with potassium channels in a translation-independent manner [29]. Nevertheless, the recent failure of FXS clinical trials targeting group 1 mGluR signaling [30] has led the field to re-examine the group 1 mGluR hypothesis.
Loss of FMRP was shown to increase network-level hyperexcitability in the rodent cortex [31, 32], which has been associated with the symptoms observed in FXS patients, such as hypersensitivity, hyperarousal, hyperactivity, anxiety, and epilepsy [33]. Interestingly, absence of FMRP downregulates GABAA receptor subunits in both mice and flies [34, 35]. Furthermore, the enzymes for GABA synthesis and degradation, GABA membrane transporters, a GABA receptor scaffolding protein, and a protein that regulates GABAB receptor signaling are downregulated in the absence of FMRP [36, 37]. These observations suggest a tantalizing, yet poorly understood, link between GABAergic signaling, network hyperexcitability, and behavioral deficits in FXS models and patients.
In contrast to the group 1 mGluR component of FXS, the potential effects of altered synaptic inhibition on neuronal circuit excitability and how these changes might impact sensory computations and animal behavior remain unexplored. In this study, we explore the changes in neuronal circuit function and connectivity underlying FXS by using a combination of behavioral assays, functional brain imaging, optogenetics, and electrophysiology in a fly FXS model. We focused on the Drosophila melanogaster olfactory system, which is a well-understood and genetically tractable neuronal circuit. Specifically, we evaluated olfactory computations in the antennal lobe, a circuit constituted by excitatory projection neurons, which receive synaptic input from their cognate olfactory receptor neurons, as well as inhibitory local interneurons involved in mediating lateral inhibition [38].
We find that the absence of dFMRP, the fly homolog of the human FMRP, results in reduced olfactory attraction and aversion. Calcium imaging data show that antennal lobe projection neurons have broader odor tuning in dfmr1− flies, leading to reduced specificity in odor coding and alterations in olfactory representations. Consistent with these results, we observe that lateral inhibition across olfactory glomeruli, as well as the inhibitory connections between local interneurons and projection neurons, are impaired in dfmr1− flies. Finally, downregulation of GABA receptors in projection neurons is sufficient to produce olfactory behavioral defects. We propose that absence of dFMRP leads to defective lateral inhibition across olfactory glomeruli, which, in turn, results in impaired odor coding and olfactory behaviors.
Results
dfmr1− Flies Exhibit Deficits in Odor-Induced Attraction and Aversion
dfmr1− flies were previously shown to have learning deficits in olfactory behavioral assays [39]. The authors suggested that this was not due to a sensory deficit; however, no detailed analysis of olfactory processing was performed. To evaluate whether the olfactory system of Drosophila melanogaster is affected by the absence of FMRP, we conducted olfactory attraction and aversion assays (Figures 1A and S1A–S1C). Ethyl acetate is known to induce attraction in flies, whereas benzaldehyde induces aversion [40, 41]. We presented these odors to starved flies and quantified attraction and aversion by counting the number of flies in odorized and non-odorized sections of the behavioral arena, before and during odor delivery. We found that dfmr1− flies exhibit significantly weaker olfactory attraction and aversion compared to wild-type (WT) flies (Figures 1B, 1C, 1H, 1I, 1L, and 1M). We observed that dfmr1− flies spend less time exploring the quadrant odorized with the attractive odor ethyl acetate (Figures 1B, 1H, and 1L). Similarly, dfmr1− flies were not repelled as much as WT flies by the aversive odor benzaldehyde (Figures 1C, 1I, and 1M). Furthermore, impaired olfactory performance in dfmr1− flies can be restored by the genomic construct [42] of dFMRP (Figures 1D, 1E, 1H, 1I, 1L, and 1M). To test whether reduced olfactory performance was due to the absence of dFMRP in the antennal lobe circuit, we knocked down dFMRP expression specifically in excitatory antennal lobe projection neurons. Downregulation of dFMRP in the antennal lobe projection neurons led to a significant impairment of olfactory behaviors (Figures 1F, 1G, and 1J–1M), confirming the role of dFMRP for antennal lobe circuit function and olfactory behaviors. Similar results were obtained by knocking down dFMRP expression in inhibitory antennal lobe local interneurons (Figure S1E).
Figure 1.
Absence of dFMRP Results in Deficits in Olfactory Attraction and Aversion
(A) Olfactory behavior arena with a four-input port and a central vacuum generates a four-quadrant air profile. In olfactory attraction assays, ethyl acetate was delivered from a single port (arrowhead), and clean air (white quadrants) was delivered through the remaining ports. In olfactory aversion assays, benzaldehyde was delivered from three ports (arrowheads), while clean air (white quadrant) was delivered through the remaining port. Fifty 20-hr-starved flies were recorded during 10 min (odorless air, 2 min; odor, 3 min; odorless air, 5 min).
(B–G) Heatmaps showing the density of flies across all experiments during the last minute of odor exposure. Note that WT, dfmr1 rescue, and GH146 flies cluster in the ethyl acetate quadrant (dotted quadrant) in attraction assays and in the clean-air quadrant (dotted quadrant) in aversion assays using benzaldehyde. dfmr1− and GH146>dfmr1-RNAi flies, by contrast, are distributed across all quadrants and exhibited a poorer performance in olfactory attraction or aversion assays.
(H–K) Temporal course showing the change in number of flies in the odorized quadrant for olfactory attraction assays and in the clean-air quadrant for olfactory aversion assays, depicted by a dotted line in (B)–(G). Note that WT and dfmr1 rescue flies performed better than dfmr1− flies. Also note that GH146>dfmr1-RNAi flies performed significantly worse than GH146 flies. Shades represent SEM (n = 200 flies per genotype in four experimental sessions and three repetitions of each odor).
(L and M) Preference index defined by the fraction of flies in the odorized (for ethyl acetate) or clean-air (for benzaldehyde) quadrant during the last minute of odor exposure. dfmr1− and GH146>dfmr1-RNAi flies exhibited a lower performance in ethyl acetate attraction (n = 12 trials, Wilcoxon rank-sum test; WT versus dfmr1−, p = 3.0 × 10−5; dfmr1 rescue versus dfmr1−, p = 6.2 × 10−5; GH146 versus GH146>dfmr1-RNAi, p = 1.8 × 10−2) as well as in benzaldehyde aversion (n = 12 trials, Wilcoxon rank-sum test; WT versus dfmr1−, p = 1.8 × 10−5; dfmr1 rescue versus dfmr1−, p = 3.7 × 10−4; GH146 versus GH146>dfmr1-RNAi, p = 3.4 × 10−2).
In this and all other figures, ∗p < 0.05; ∗∗p < 0.03; ∗∗∗p < 0.01. See also Figure S1.
Broader Odor Response Tuning in Projection Neurons Leads to Less Selective Olfactory Representations in dfmr1− Flies
Reduced performance of dfmr1- flies in olfactory behaviors suggests that odor coding is compromised in these animals. To evaluate whether olfactory computations are affected by the absence of dFMRP, we measured the odor responses of antennal lobe projection neurons using calcium imaging (Figures 2A, 2B, and S2) in WT and dfmr1− flies (Figure 2D). We extracted the location of individual glomeruli using independent component analysis [43], which is effective in identifying even the sister glomeruli across antennal lobes with very similar locations and response profiles (Figures 2C and S3).
Figure 2.
Odor Representations in the Antennal Lobe of WT and dfmr1− Flies
(A) Experimental setup depicting a transgenic fly expressing GCaMP6 in projection neurons and the tube for odor delivery (for details, see Figure S2).
(B) Odor maps in the antennal lobes of WT and dfmr1− flies, calculated by the percent change of fluorescence intensity (ΔF/F) during 1 s after response onset. Warmer colors signify strong responses. Note that glomerular responses are more dispersed and localized in WT than in dfmr1− flies. Scale bar, 20 μm.
(C) Examples for the location and the odor responses of antennal lobe glomeruli in WT and in dfmr1− flies. Note that potential sister glomeruli identified via our detection algorithm (for details, see Figure S3) exhibit similar response profiles. Scale bars, 10 μm.
(D) Immunostainings on fly brains used in calcium imaging experiments confirmed expression of dFMRP in WT flies (left) and lack of dFMRP in dfmr1− flies (right). Scale bars, 50 μm.
See also Figures S2 and S3.
Next, we investigated the glomerular activation patterns of projection neurons and compared the representations of 24 odors in WT and dfmr1− flies. We observed that overall responsiveness of olfactory glomeruli is significantly altered, with more excitatory and fewer inhibitory odor responses in dfmr1− flies (Figures S4A–S4D). Specifically, WT flies exhibited more inhibitory responses, more silent glomeruli, and more strong excitatory responses, whereas dfmr1− flies presented an increased number of weak excitatory responses (Figures S4A–S4D), reflecting that not only are dfmr1− projection neurons hyperexcitable, but excitation of strongly responding neurons is also impaired. This indicates a deficit in contrast enhancement of olfactory representations, which might be a consequence of reduced lateral inhibition [44].
To further evaluate olfactory coding, we carried out a pairwise comparison of odor-evoked glomerular activation patterns using two commonly used and complementary measures of similarity, cosine distance and Euclidean distance. Cosine distance compares odor responses regardless of amplitude, while Euclidean distance takes the strength of odor responses into account. High cosine and Euclidean distances indicate increased difference among odor representations and, hence, greater specificity in odor encoding. Our results show significantly lower cosine and Euclidean distances between pairs of odors in dfmr1− flies (Figures 3A–3D). This indicates that loss of dFMRP causes odor-evoked glomerular activation patterns to become less distinct from each other and, therefore, harder to discriminate. Reduced odor specificity of glomerular activation patterns could explain why dfmr1− flies are impaired in both attractive and aversive olfactory behavioral tasks.
Figure 3.
Broader Glomerular Odor Tuning Leads to Less Specific Odor Representations in dfmr1− Flies
(A and B) Cosine (A) and Euclidean (B) distance matrices representing pairwise similarities among glomerular responses to 24 odors in WT and dfmr1− flies (WT, n = 10 flies, 492 glomeruli; dfmr1−, n = 12, 560 glomeruli). Reduced cosines (A) and Euclidean (B) distances (cooler colors) indicate more similar odor representations in dfmr1− flies.
(C and D) Cumulative distribution of cosine (C) and Euclidean (D) distances in WT and in dfmr1− flies. Significantly lower cosine (C) and Euclidean (D) distances indicate more similar odor representations in dfmr1− flies (WT, n = 10 flies, 492 glomeruli; dfmr1−, n = 12 flies, 560 glomeruli; Kolmogorov-Smirnov test; cosine, p = 7.4 × 10−16; Euclidean, p = 4.1 × 10−7).
(E) Normalized odor responses of all individual WT and dfmr1− glomeruli (WT, n = 10 flies, 492 glomeruli; dfmr1−, n = 12 flies, 560 glomeruli). Glomeruli are sorted based on their odor selectivity, from the least selective (top) to the most selective (bottom). Responses are sorted based on their strength, after normalizing to the strongest response of individual glomeruli (warmest colors) on the left. A dashed line is added to help with comparison.
(F) Cumulative distribution of lifetime sparseness for all recorded glomeruli in WT and dfmr1− flies. Reduced lifetime sparseness indicates that dfmr1− glomeruli exhibited broader odor tuning, reflecting reduced specificity (WT, n = 10 flies, 492 glomeruli; dfmr1−, n = 12 flies, 560 glomeruli; Kolmogorov-Smirnov test, p = 9.8 × 10−14).
(G) Cumulative distribution of population sparseness for all measured odors in WT and dfmr1− flies. Lower population sparseness indicates that odors activate more glomeruli in dfmr1− flies (WT, n = 10 flies, 492 glomeruli; dfmr1−, n = 12 flies, 560 glomeruli; Kolmogorov-Smirnov test, p = 8.4 × 10−22).
See also Figures S4 and S5.
What underlies the increased similarity among odor representations in dfmr1− flies? To answer this question, we visualized odor selectivity by plotting the responses of each glomerulus normalized to its maximum odor response. We observed that dfmr1− glomeruli have broader response profiles and, thus, reduced odor selectivity, represented by warmer colors (Figure 3E). To quantify this, we calculated the lifetime sparseness, a measure of response selectivity [45], of all glomeruli. A glomerulus with high sparseness value responds to only one or very few odors. Conversely, a glomerulus with low sparseness value responds to many odors equally. We found that dfmr1− glomeruli have significantly lower lifetime sparseness values (Figure 3F), suggesting they are less odor selective. Complementary to this, we computed the population sparseness, which is a measure of the number of glomeruli activated by a single odor. A high population sparseness value signifies that few glomeruli were activated by a given odor, whereas a low population sparseness value signifies that many glomeruli were similarly activated. We found that the antennal lobe of dfmr1− flies has significantly lower population sparseness (Figure 3G), which is consistent with our complementary analysis showing reduced response to background (signal-to-noise) ratios (Figure S4D) and increased correlations across antennal lobe glomeruli in dfmr1− flies (Figure S4E).
Our results reveal an impairment in olfactory coding and odor selectivity in dfmr1− flies due to broader tuning and reduced odor selectivity of antennal lobe projection neurons. This reduced odor selectivity can, in principle, arise from less selective glomerular innervation patterns of individual projection neurons in dfmr1− flies. However, we did not observe changes in glomerular morphology or size in any of the genetically identified projection neurons of dfmr1− flies (Figure S5).
Impaired Lateral Interactions Alter Olfactory Information Processing in dfmr1− Flies
The lack of any obvious morphological alterations in projection neurons, combined with our observations of increased excitatory and reduced inhibitory odor responses, suggests that defective lateral interactions among antennal lobe neurons might be responsible for the reduced specificity of olfactory representations in dfmr1− flies. In the fly antennal lobe, lateral interactions across olfactory glomeruli were shown to mediate the spread of both excitation [46], through gap junctions [47], and inhibition, through local interneurons [44].
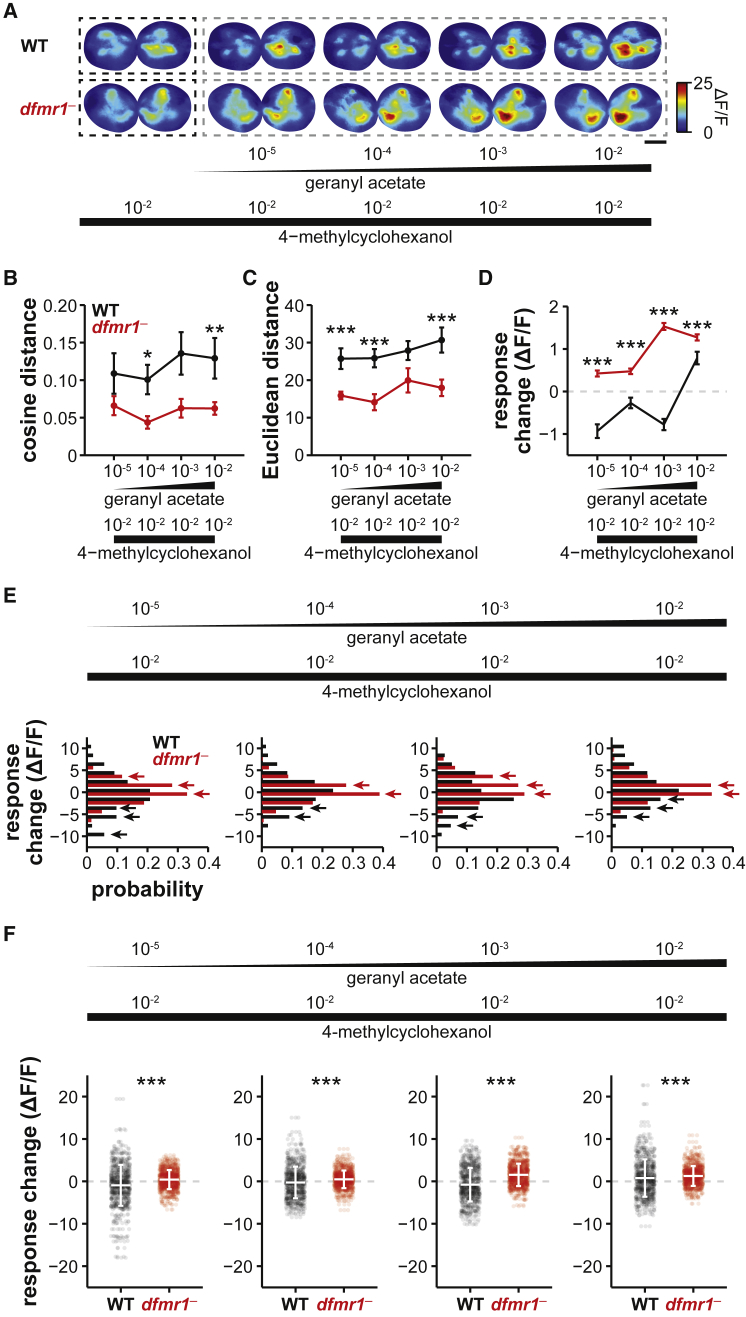
It has been shown that, when odors are mixed, lateral interactions across antennal lobe glomeruli can alter odor representations, both through lateral excitation and lateral inhibition [48]. To compare the level of lateral interactions in WT and dfmr1− flies, we applied mixtures of odorants, in which the concentration of one of the components is kept constant while the concentration of the other mixture component is gradually increased (Figures 4A and S6A). Different odors recruit different subsets of projection neurons and different local interneurons. This, in turn, will change the odor-evoked activity patterns, creating new odor representations depending on the degree of lateral interactions among all recruited neurons [46].
Figure 4.
Lateral Interactions across Olfactory Glomeruli Are Reduced in dfmr1− Flies
(A) Activity maps elicited by odor mixtures of 4-methylcyclohexanol and a progressively increasing concentration of geranyl acetate in WT and dfmr1− flies. Single odors and mixtures are circumscribed by dashed black and gray boxes, respectively. Scale bar, 20 μm.
(B and C) Cosine (B) and Euclidean (C) distances representing pairwise similarities among representations of individual odors and mixtures. Note that the 4-methylcyclohexanol representation becomes progressively different as the concentration of geranyl acetate increases. These changes are reduced in dfmr1− flies (mean ± SEM; WT, n = 6 flies, 298 glomeruli; dfmr1−, n = 7 flies, 345 glomeruli; Wilcoxon rank-sum test; cosine: [10−5], p = 6.9 × 10−2; [10−4], p = 3.7 × 10−2; [10−3], p = 5.1 × 10−2; [10−2], p = 1.1 × 10−2; Euclidean: [10−5], p = 5.8 × 10−4; [10−4], p = 2.3 × 10−3; [10−3], p = 6.9 × 10−2; [10−2], p = 4.1 × 10−3).
(D) Average change in 4-methylcyclohexanol responses of all glomeruli when mixed with different geranyl acetate concentrations. On average, responses to 4-methylcyclohexanol were decreased in WT flies. By contrast, dfmr1− responses increase their amplitude when geranyl acetate was mixed (mean ± SEM; WT, n = 6 flies, 298 glomeruli; dfmr1−, n = 7 flies, 345 glomeruli; Wilcoxon rank-sum test; [10−5], p = 7.5 × 10−16; [10−4], p = 6.4 × 10−11; [10−3], p = 7.9 × 10−46; [10−2], p = 3.7 × 10−7).
(E) Histograms representing the response changes for every recorded glomeruli in WT (n = 6 flies, 298 glomeruli) and dfmr1− flies (n = 7 flies, 345 glomeruli). Note that WT responses exhibited a prominent decrease (black arrows). By contrast, dfmr1− responses showed increase or no change (red arrows).
(F) Scatterplots depicting the response changes in every glomeruli when 4-methylcyclohexanol is mixed with geranyl acetate in WT and dfmr1− flies. Larger variability of changes is observed in WT glomeruli (mean ± SD; WT, n = 6; dfmr1−, n = 7; F test; [10−5], p = 1.6 × 10−7; [10−4], p = 1.4 × 10−8; [10−3], p = 1.5 × 10−10; [10−2], p = 2.6 × 10−15).
See also Figure S6.
We observed that the odor representation of the component with fixed concentration became progressively different with increasing concentrations of the second mixture component. These mixing-related changes in odor representations were more pronounced in WT flies than in dfmr1− flies (Figures 4A–4C and S6A–S6C). Next, we quantified the changes in response amplitudes of individual glomeruli. Our results showed that, on average, WT flies exhibited significantly more mixture-related suppression, whereas dfmr1− flies exhibited significantly more mixture-related excitation (Figures 4D, 4E, S6D, and S6E). This suggests that, while lateral inhibition is impaired in dfmr1− flies, lateral excitatory interactions might be spared (Figure S7). These lateral inhibitory and excitatory effects were variable across populations of projection neurons. In line with this, our results suggest that populations of individual glomeruli in WT flies have a significantly larger variety of both inhibitory and excitatory effects at all mixture concentrations, when compared to dfmr1− flies (Figures 4F and S6F). Altogether, these results support the idea that lateral inhibitory interactions are impaired in the antennal lobe of dfmr1− flies, which eventually results in reduced contrast across odor representations and, therefore, poorer performance in olfactory behaviors.
Lateral Inhibition Is Impaired in the Antennal Lobe of dfmr1− Flies
Our findings using odor mixtures point to reduced lateral inhibition among olfactory glomeruli in dfmr1− flies. In line with this, several components of the GABAergic transmission machinery are reported to be downregulated in mouse and fruit fly models of FXS [34, 35]. Moreover, GABAergic signaling appears to be disrupted in the brains of autistic patients [49], a recurrent phenotype in FXS. All this evidence led to the hypothesis that reduced inhibition may be a major mechanism underlying neuronal deficits in FXS [50]. However, direct in vivo physiological evidence that inhibitory connections between neurons are impaired in any in vivo model of FXS is lacking.
In the fruit fly antennal lobe, lateral inhibition across olfactory glomeruli is mediated by GABAergic local interneurons that can act on both olfactory receptor neuron terminals and on projection neurons [44, 51, 52]. To directly test the action of local interneurons on the activity of projection neurons, we performed intracellular recordings of projection neurons while optogenetically stimulating GABAergic local interneurons expressing channelrhodopsin-2 (Figure 5A) [53, 54]. Optogenetic activation of local interneurons consistently hyperpolarized the membrane potential of WT projection neurons (Figures 5B–5D). In contrast, dfmr1− projection neurons exhibited significantly smaller or no hyperpolarization in their membrane potential (Figures 5B–5D). Importantly, we observed that dfmr1− projection neurons exhibit a prominent excitation upon optogenetic local interneuron stimulation (Figure 5C), which is mediated by the gap junctions between local interneurons and projection neurons [47].
Figure 5.
Lateral Inhibition of Projection Neurons Is Impaired in dfmr1− Flies
(A) Experimental setup depicting a fly expressing the light-inducible channel ChR2 in GABAergic [53, 54] local interneurons (LNs). Patch-clamp recordings were conducted in projection neurons (PNs), while stimulating LNs with blue light. ORN, olfactory receptor neuron; LN, local interneuron; PN, projection neuron.
(B) Representative voltage traces of PNs. In response to optogenetic activation of LNs, WT PNs typically show a hyperpolarization and inhibition of action potentials. By contrast, dfmr1− PNs exhibit little hyperpolarization and mild suppression of action potentials. Blue shade depicts the 500-ms blue-light stimulation.
(C) Average membrane potential of every individual PN (transparent traces) in response to optogenetic LN activation in WT and dfmr1− flies (WT, n = 16 cells; dfmr1−, n = 16 cells). On average (full-color traces), optogenetic activation of LNs drives an initial depolarization followed by a more pronounced hyperpolarization in WT flies. By contrast, activation of LNs drives a more pronounced initial depolarization followed by little or no hyperpolarization in dfmr1− flies.
(D) Scatterplot depicting the light-evoked changes in the membrane potential of WT and dfmr1− PNs calculated during a 1-s window after the onset of the light stimulus. Inhibitory responses in WT PNs were significantly larger than in dfmr1− PNs (mean ± SEM; WT, n = 16 cells; dfmr1−, n = 16 cells; Wilcoxon rank-sum test, p = 7.6 × 10−5).
(E) Firing rates (top) and raster plots (bottom) of representative PNs from a WT fly and a dfmr1− fly, respectively. Note that optogenetic activation of LNs suppresses action potential firing in the WT PN. This effect is reduced in this dfmr1− PN.
(F) Firing rates (transparent traces) of all recorded WT and dfmr1− PNs (WT, n = 16 cells; dfmr1−, n = 16 cells). On average (full-color traces), activation of LNs consistently decreases the spontaneous firing of WT PNs. By contrast, firing of dfmr1− PNs is slightly increased, albeit some delayed suppression.
(G) Scatterplot illustrating the spontaneous firing of all recorded WT and dfmr1− PNs, quantified during a 500-ms window before the onset of the light stimulus. No differences were found between the spontaneous firing of WT and that of dfmr1− PNs (mean ± SEM; WT, n = 16 cells; dfmr1−, n = 16 cells; Wilcoxon rank-sum test, p = 8.0 × 10−2). n.s., not significant.
(H) Scatterplot showing the suppression in the spontaneous firing of all recorded WT and dfmr1− PNs by optogenetic activation of LNs, calculated as the difference between the spontaneous firing in (G) and the firing during the 500-ms blue-light stimulation period. The firing rates of WT PNs are significantly more suppressed when compared to those of dfmr1− PNs (mean ± SEM; WT, n = 16 cells; dfmr1−, n = 16 cells; Wilcoxon rank-sum test, p = 4.3 × 10−4).
n.s., not significant. See also Figure S7.
During these recordings, we kept the antennae dry and the olfactory nerve intact, which ensures that the olfactory receptor neurons are undamaged and sustain a healthy level of background activity. As previously shown [55], this remaining olfactory receptor neuron background firing results in prominent subthreshold synaptic activity and spontaneous action potential firing in our recorded projection neurons (Figures 5B, 5E, and 5G). The optogenetic activation of local interneurons reduced the firing rate of WT projection neurons significantly more than that of dfmr1− projection neurons (Figures 5E–5H). In line with the remaining gap-junction-mediated lateral excitation, we observed a slight increase in projection neuron firing rates of dfmr1− flies (Figures 5E and 5F).
The observed impaired inhibition in the projection neurons of dfmr1− flies could, in principle, be the consequence of a less effective optogenetic activation of local interneurons. To rule out this possibility, we recorded the responses to optogenetic activation in local interneurons expressing channelrhodopsin-2, both in WT and dfmr1− flies (Figures 6A and 6B). Our results showed that optogenetic stimulation elicited significantly larger depolarization and higher firing rates in dfmr1− local interneurons, when compared to WT local interneurons (Figures 6C–6H). Furthermore, optogenetic stimulation of local interneurons consistently inhibit the local interneurons that do not express channelrhodopsin-2 in WT flies (Figures 6I–6K). By contrast, little or no inhibition was observed in dfmr1− local interneurons not expressing channelrhodopsin-2 (Figures 6I–6K). These results indicate that the reduced inhibition observed in dfmr1− projection neurons (Figure 5) cannot be due to less effective optogenetic activation of dfmr1− local interneurons. In fact, our results suggest that optogenetic stimulation is more effective in activating dfmr1− local interneurons, especially at the later phase of the stimulation, presumably, due to less effective GABAergic inhibition across dfmr1− local interneurons. In summary, these experiments revealed that deficient inhibition of dfmr1− projection neurons (Figure 5) is due to less effective GABAergic inhibition from local interneurons onto the whole antennal lobe circuit, at the level of both projection neurons and local interneurons.
Figure 6.
Lateral Inhibition of Local Interneurons Is Impaired in dfmr1− Flies
(A) Experimental setup depicting a fly expressing the light-inducible channel ChR2 in GABAergic [53, 54] local interneurons (LNs). Patch-clamp recordings were conducted in LNs expressing Ch2 while stimulating them with blue light. ORN, olfactory receptor neuron; LN, local interneuron; PN, projection neuron.
(B) Representative voltage traces of LNs. In response to optogenetic stimulation, WT LNs show a transient membrane depolarization and action potential firing, followed by hyperpolarization. By contrast, dfmr1− LNs exhibit a sustained depolarization and action potential firing. Blue shade depicts the 500-ms blue-light stimulation.
(C) Average membrane potential of every individual LN (transparent traces) in response to optogenetic LN activation in WT and dfmr1− flies (WT, n = 8 cells; dfmr1−, n = 16 cells). On average (full color traces), optogenetic activation of LNs drives an initial depolarization followed by hyperpolarization in WT flies. By contrast, in dfmr1− flies, activation of LNs drives a sustained depolarization with little or no inhibition.
(D) Scatterplot depicting the light-evoked membrane potential changes in WT and dfmr1− LNs calculated during a 500-ms window after the onset of the light stimulus. dfmr1− LNs exhibited a larger depolarization as compared to WT LNs (mean ± SEM; WT, n = 8 cells; dfmr1−, n = 16 cells; Wilcoxon rank-sum test, p = 6.5 × 10−4).
(E) Firing rates (top) and raster plots (bottom) of representative LNs from a WT fly and a dfmr1− fly, respectively. Note that the initial action potential firing is effectively suppressed after 250 ms in the WT LN. This is less pronounced in the dfmr1− LN.
(F) Firing rates (transparent traces) of all recorded WT and dfmr1− LNs (WT, n = 8 cells; dfmr1−, n = 16 cells). On average (full color traces), the initial light-induced firing is suppressed after 250 ms in WT LNs. By contrast, the initial firing is only slightly decreased in dfmr1− LNs.
(G) Scatterplot illustrating the spontaneous firing of all recorded WT and dfmr1− LNs, quantified during a 500-ms window before the onset of the light stimulus. No differences were found between the spontaneous firing of WT and dfmr1− LNs (mean ± SEM; WT, n = 8 cells; dfmr1−, n = 16 cells; Wilcoxon rank-sum test, p = 1.6 × 10−1). n.s., not significant.
(H) Scatterplot showing the changes in the firing of optogenetically activated WT and dfmr1− LNs, calculated as the difference between the spontaneous firing in (G) and the evoked firing during the 500-ms window of the light stimulation. The firing of action potentials evoked by light stimulation is significantly higher in dfmr1− LNs (mean ± SEM; WT, n = 8 cells; dfmr1−, n = 16 cells; Wilcoxon rank-sum test, p = 3.4 × 10−4).
(I) Patch-clamp recordings were conducted in LNs not expressing Ch2 while stimulating the subpopulation of LNs expressing ChR2 with blue light.
(J) Representative voltage traces of LNs not expressing ChR2. In response to optogenetic stimulation, WT LNs typically show a hyperpolarization, whereas dfmr1− LNs exhibit little or no hyperpolarization. Blue shade depicts the 500-ms blue-light stimulation.
(K) Average membrane potential of every individual LN not expressing ChR2 (transparent traces) in response to optogenetic LN activation in WT and dfmr1− flies (WT, n = 7 cells; dfmr1−, n = 8 cells). On average (full color traces), optogenetic activation of LNs expressing ChR2 hyperpolarizes WT LNs not expressing ChR2. By contrast, activation of LNs expressing ChR2 drives little or no inhibition in LNs not expressing ChR2 in dfmr1− flies.
(L) Scatterplot depicting the light-evoked changes in the membrane potential of WT and dfmr1− LNs not expressing ChR2 calculated during a 1-s window after the onset of the light stimulus. WT LNs exhibited a larger hyperpolarization (mean ± SEM; WT, n = 8 cells; dfmr1−, n = 8 cells; Wilcoxon rank-sum test, p = 9.3 × 10−4).
We observed that dfmr1− flies present less strongly activated glomeruli (Figures S4A–S4D), which could be caused by reduced lateral excitation. We, therefore, recorded lateral excitatory responses in projection neurons of flies, in which the antennae were removed and, hence, did not present spontaneous activity (Figure S7A). This experimental arrangement minimizes the effects produced by presynaptic lateral inhibition [44]. Optogenetic activation of local interneurons produced an excitatory response in both WT and dfmr1− projection neurons (Figures S7B and S7C). However, lateral excitatory responses decay faster in dfmr1− projection neurons (Figures S7B and S7C) and were smaller in amplitude (Figure S7D). This observation could, in part, explain the lower incidence of strongly activated glomeruli upon odor stimulation in dfmr1− flies (Figures S4A–S4D).
Downregulation of GABAergic Rdl Receptors in the Antennal Lobe Impairs Olfactory Behavior in Flies
Our observations, indicating that inhibition is reduced in both the projection neurons (Figure 5) and the local interneurons (Figure 6) of the antennal lobe, suggest that lack of inhibition is the neurophysiological cause of the behavioral abnormalities observed in the absence of dFMRP (Figure 1). We directly tested this idea by knocking down the expression of the GABAergic Rdl receptor. Downregulation of Rdl receptors selectively in projection neurons (Figure 7A) or in local interneurons (Figure 7B) resulted in lower olfactory behavioral performance in fruit flies. Taken together with the previously reported decreased expression of GABAA receptors in the absence of FMRP [34], the electrophysiological and behavioral evidence presented in this study strongly suggests that reduced inhibition of neuronal circuits contributes to the pathophysiology of FXS.
Figure 7.
Downregulation of Rdl Receptors in the Fly Antennal Lobe Impairs Olfactory Attraction
(A and B) Preference index showing that downregulation of GABAergic Rdl receptors in both projection neurons (A) and local interneurons (B) reduces olfactory attraction in flies (n = 12 trials, Wilcoxon rank-sum test; GH146 versus GH146>Rdl-RNAi, p = 8.3 × 10−4; Rdl-RNAi versus GH146>Rdl-RNAi, p = 2.3 × 10−2; NP2426 versus NP2426>Rdl-RNAi, p = 2.2 × 10−2; Rdl-RNAi versus NP2426>Rdl-RNAi, p = 1.3 × 10−1). n.s., not significant.
Discussion
Since the discovery of reduced GABAA receptor subunit expression in the absence of FMRP [34], accumulated evidence has pointed toward alterations in GABAergic transmission as a key component in the neurophysiology of FXS [50, 56]. In fact, intracellular recordings on acute brain slices suggested that reduced inhibitory input from interneurons onto pyramidal neurons could result in an excitation/inhibition imbalance [35, 57]. Whether this is true in vivo and how it might impact neuronal circuit function and behavior remained unclear.
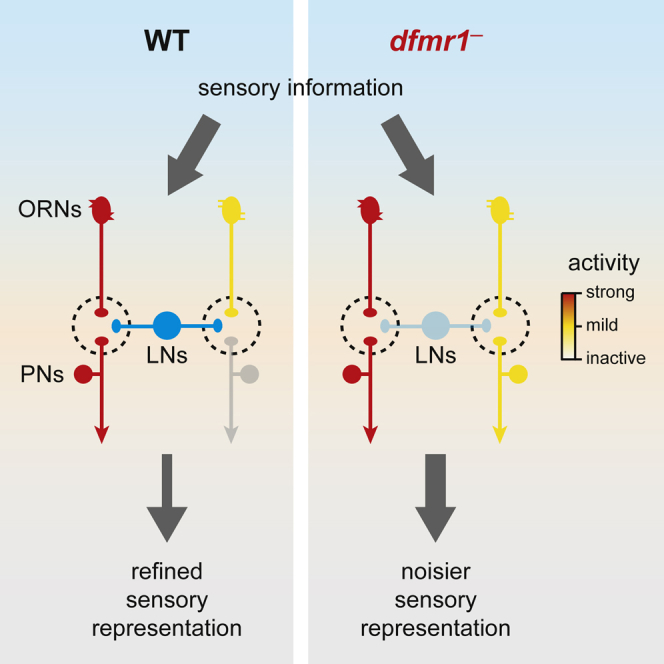
We tested this using the fruit fly antennal lobe circuit and demonstrate that GABAergic connections established by local interneurons, which mediate lateral inhibition [44, 51, 58], are impaired in a Drosophila melanogaster model of FXS. Moreover, we show that deficits in GABAergic lateral inhibition leads to increased circuit excitability, which results in reduced stimulus selectivity in projection neurons. With lower selectivity comes impaired olfactory computations leading to strong odor discrimination deficits. We postulate that similar deficits in lateral inhibition impair neuronal computations in other sensory modalities. In consonance with this, it has been reported that circuit hyperexcitability leads to behavioral alterations in tactile, auditory, and olfactory tasks in mouse models of FXS [32, 59, 60].
Our results indicate that, in the absence of dFMRP, neuronal computations are impaired in the antennal lobe of Drosophila melanogaster. Consequently, flies exhibit deficits in olfactory behaviors. This is in apparent contradiction with a previous study showing long-term memory defects in dfmr1− flies and no sensory deficits [39]. As we report, responses to many odors are still elicited in projection neurons of dfmr1− flies. They are just less selective due to reduced lateral inhibition. We suggest that this difference may be due to a more extensive and quantitative analysis of behavior and physiology in our study that revealed defects that may have not been previously detected. Alternatively, the penetrance and severity of phenotypes in FMR1 mutant animals, both mice and flies, can be sensitive to genetic background. It is possible that the previous study did not account for this. At any rate, both null alleles and RNAi flies analyzed using behavioral, imaging, and electrophysiological approaches revealed that dfmr1 mutants exhibit reduced odor specificity and, thus, deficient olfactory processing.
Lateral inhibition across Drosophila olfactory glomeruli has been proposed to be important for increasing contrast among odor representations and, therefore, for discriminating odors [44, 48]. Interestingly, such a mechanism has been suggested to be relevant for other sensory modalities [61]. In this winner-take-all model, glomeruli with most prominent odor responses would strongly activate surrounding interneurons, spreading inhibition to nearby weakly activated glomeruli. The spread of lateral inhibition, in turn, would inhibit the odor responses of weakly activated glomeruli, while strongly activated glomeruli remain as the unique encoder of the particular odor. This model also suggests that the lack of many weakly activated glomeruli, in addition to few strongly responding but very odor-specific glomeruli, enhances the separation of odor response patterns from one another. In line with this model, we observed that lack of lateral inhibition in the antennal lobe of dfmr1− flies, indeed, leads to an increase in the number of weakly activated and less odor-specific glomeruli. By contrast, WT flies present more inhibitory and less weak excitatory responses, sparing strongly responding olfactory glomeruli that are more odor specific. This is probably a consequence of reduced lateral inhibition, which is important for contrast enhancement of odor representations [44]. Additionally, the slight decrease in lateral excitation observed in dfmr1− flies could result in less strongly represented glomeruli. Importantly, defects in olfactory processing have been observed in other animal models of FXS [59], as well as in human patients, which display hypersensitivity to smells [62] and to other sensory modalities involving lateral inhibitory mechanisms such as tactility and audition [62].
Beyond the olfactory system, several studies have shown that CNS neurons are hyperexcitable in the absence of FMRP [31, 32, 57]. Since activation of the group 1 mGluR signaling pathway results in increased neuronal excitability [25, 26], circuit hyperexcitability has been attributed to the constitutively enhanced group 1 mGluR signaling observed in mouse FXS models. Here, we provide the first direct in vivo evidence showing that defects in lateral GABAergic inhibition significantly contribute to circuit hyperexcitability. This is consistent with downregulation of proteins involved in GABAergic transmission both in fruit flies and in rodents [34, 36]. Thus, reduced inhibition could be a consequence of decreased GABA release from local interneurons, reduced expression of postsynaptic GABA receptors, or both. Further studies of protein expression profiles for the specific neuron types are needed to elucidate this. It is possible that this mechanism might explain phenotypes observed in FXS patients such as hypersensitivity, hyperarousal, hyperactivity, and epilepsy, all of which reflect hyperexcitable brain states.
In summary, we demonstrate that lateral inhibition within the antennal lobe is strongly affected in dfmr1− flies due to impaired inhibitory connections from local interneurons onto projection neurons and other local interneurons. The lack of this lateral inhibition on projection neurons is probably the major cause for their increased excitability and reduced odor specificity. We propose that this compromised olfactory coding consequently leads to impaired olfactory behaviors in dfmr1− flies. More generally, we provide the missing in vivo evidence that the lack of dFMRP has a direct impact on sensory processing and animal behavior through a weakening of lateral inhibitory connections, which broadens response tuning of principal neurons. This mechanism might be ubiquitously present in the brain of FXS patients. For instance, reduced GABAergic inhibition could produce hyperexcitable neuronal circuits in FXS patients, which not only explains symptoms such as hypersensitivity, hyperarousal, or epilepsy but also potentially contributes to the misprocessing of information across the brain, which would have severe effects on human behavior. Finally, given the overlap between the phenotypes of FXS and those of other neurological diseases, such as autism, Rett syndrome, or Dravet syndrome, and their corresponding perturbations in GABAergic transmission [33, 49, 63], it is possible that similar mechanisms involving reduced lateral inhibition are also present in these neurological syndromes, which are yet to be discovered.
Author Contributions
L.M.F., B.A.H., and E.Y. conceived the study, designed the experiments, and wrote the manuscript. L.M.F. conducted behavioral experiments, calcium imaging, electrophysiology, and all data analyses. Z.O. performed immunohistochemistry, generated transgenic animals, and wrote the manuscript. G.A.L. performed behavioral experiments.
Acknowledgments
We thank David Akalal and Ron Davis for early work leading to this project; Patrick Callaerts, Sebastian Haesler, and Yaksi and Hassan lab members for advice; Ilona Kadow (who shared UAS-GCaMP6m flies); Rachel Wilson (who shared UAS-ChR2, GH146-GAL4, NP5221-GAL4, and NP3062-GAL4 flies); the DGRC (who shared NP2426-GAL4 flies); the VDRC (who shared UAS-dfmr1-RNAi flies); the BDSC (who shared UAS-Rdl-RNAi flies); and Hiromu Tanimoto for initial ideas to design our behavioral arena. This work was funded by VIB (B.A.H. and E.Y.), NERF (E.Y.), the VIB International PhD Program (L.M.F.), ERC Starting Grant 335561 (E.Y.), the BELSPO WiBrain Interuniversity Attraction Pole P7/20, FWO grants G.0871.12N (E.Y.) and G.0503.12 (B.A.H.), EMBO grant ALTF 1591-2014, and European Commission Marie Skłodowska-Curie grant 656824 (G.A.L.). The E.Y. lab is currently funded by the Kavli Institute for Systems Neuroscience at NTNU. The B.A.H. lab is currently funded by the Institut Hospitalier Universitaire (IHU), the Institut du Cerveau et de la Moelle Epinière (ICM), the Paul G. Allen Frontiers Group, and the Einstein Foundation. E.Y. acknowledges support from the FENS-Kavli Network of Excellence.
Published: March 30, 2017
Footnotes
Supplemental information includes seven figures and Supplemental Experimental Procedures and can be found with this article online at http://dx.doi.org/10.1016/j.cub.2017.02.065.
Contributor Information
Bassem A. Hassan, Email: bassem.hassan@icm-institute.org.
Emre Yaksi, Email: emre.yaksi@ntnu.no.
Supplemental Information
References
- 1.Penagarikano O., Mulle J.G., Warren S.T. The pathophysiology of fragile X syndrome. Annu. Rev. Genomics Hum. Genet. 2007;8:109–129. doi: 10.1146/annurev.genom.8.080706.092249. [DOI] [PubMed] [Google Scholar]
- 2.Okray Z., de Esch C.E., Van Esch H., Devriendt K., Claeys A., Yan J., Verbeeck J., Froyen G., Willemsen R., de Vrij F.M., Hassan B.A. A novel fragile X syndrome mutation reveals a conserved role for the carboxy-terminus in FMRP localization and function. EMBO Mol. Med. 2015;7:423–437. doi: 10.15252/emmm.201404576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sutcliffe J.S., Nelson D.L., Zhang F., Pieretti M., Caskey C.T., Saxe D., Warren S.T. DNA methylation represses FMR-1 transcription in fragile X syndrome. Hum. Mol. Genet. 1992;1:397–400. doi: 10.1093/hmg/1.6.397. [DOI] [PubMed] [Google Scholar]
- 4.Verkerk A.J., Pieretti M., Sutcliffe J.S., Fu Y.H., Kuhl D.P., Pizzuti A., Reiner O., Richards S., Victoria M.F., Zhang F.P. Identification of a gene (FMR-1) containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome. Cell. 1991;65:905–914. doi: 10.1016/0092-8674(91)90397-h. [DOI] [PubMed] [Google Scholar]
- 5.Ashley C.T., Jr., Wilkinson K.D., Reines D., Warren S.T. FMR1 protein: conserved RNP family domains and selective RNA binding. Science. 1993;262:563–566. doi: 10.1126/science.7692601. [DOI] [PubMed] [Google Scholar]
- 6.Bagni C., Greenough W.T. From mRNP trafficking to spine dysmorphogenesis: the roots of fragile X syndrome. Nat. Rev. Neurosci. 2005;6:376–387. doi: 10.1038/nrn1667. [DOI] [PubMed] [Google Scholar]
- 7.Santoro M.R., Bray S.M., Warren S.T. Molecular mechanisms of fragile X syndrome: a twenty-year perspective. Annu. Rev. Pathol. 2012;7:219–245. doi: 10.1146/annurev-pathol-011811-132457. [DOI] [PubMed] [Google Scholar]
- 8.Bassell G.J., Warren S.T. Fragile X syndrome: loss of local mRNA regulation alters synaptic development and function. Neuron. 2008;60:201–214. doi: 10.1016/j.neuron.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Darnell J.C., Van Driesche S.J., Zhang C., Hung K.Y., Mele A., Fraser C.E., Stone E.F., Chen C., Fak J.J., Chi S.W. FMRP stalls ribosomal translocation on mRNAs linked to synaptic function and autism. Cell. 2011;146:247–261. doi: 10.1016/j.cell.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Edbauer D., Neilson J.R., Foster K.A., Wang C.F., Seeburg D.P., Batterton M.N., Tada T., Dolan B.M., Sharp P.A., Sheng M. Regulation of synaptic structure and function by FMRP-associated microRNAs miR-125b and miR-132. Neuron. 2010;65:373–384. doi: 10.1016/j.neuron.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang Y.Q., Bailey A.M., Matthies H.J., Renden R.B., Smith M.A., Speese S.D., Rubin G.M., Broadie K. Drosophila fragile X-related gene regulates the MAP1B homolog Futsch to control synaptic structure and function. Cell. 2001;107:591–603. doi: 10.1016/s0092-8674(01)00589-x. [DOI] [PubMed] [Google Scholar]
- 12.Hinton V.J., Brown W.T., Wisniewski K., Rudelli R.D. Analysis of neocortex in three males with the fragile X syndrome. Am. J. Med. Genet. 1991;41:289–294. doi: 10.1002/ajmg.1320410306. [DOI] [PubMed] [Google Scholar]
- 13.Irwin S.A., Patel B., Idupulapati M., Harris J.B., Crisostomo R.A., Larsen B.P., Kooy F., Willems P.J., Cras P., Kozlowski P.B. Abnormal dendritic spine characteristics in the temporal and visual cortices of patients with fragile-X syndrome: a quantitative examination. Am. J. Med. Genet. 2001;98:161–167. doi: 10.1002/1096-8628(20010115)98:2<161::aid-ajmg1025>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 14.Kaufmann W.E., Moser H.W. Dendritic anomalies in disorders associated with mental retardation. Cereb. Cortex. 2000;10:981–991. doi: 10.1093/cercor/10.10.981. [DOI] [PubMed] [Google Scholar]
- 15.Galvez R., Gopal A.R., Greenough W.T. Somatosensory cortical barrel dendritic abnormalities in a mouse model of the fragile X mental retardation syndrome. Brain Res. 2003;971:83–89. doi: 10.1016/s0006-8993(03)02363-1. [DOI] [PubMed] [Google Scholar]
- 16.Morales J., Hiesinger P.R., Schroeder A.J., Kume K., Verstreken P., Jackson F.R., Nelson D.L., Hassan B.A. Drosophila fragile X protein, DFXR, regulates neuronal morphology and function in the brain. Neuron. 2002;34:961–972. doi: 10.1016/s0896-6273(02)00731-6. [DOI] [PubMed] [Google Scholar]
- 17.Reeve S.P., Bassetto L., Genova G.K., Kleyner Y., Leyssen M., Jackson F.R., Hassan B.A. The Drosophila fragile X mental retardation protein controls actin dynamics by directly regulating profilin in the brain. Curr. Biol. 2005;15:1156–1163. doi: 10.1016/j.cub.2005.05.050. [DOI] [PubMed] [Google Scholar]
- 18.Nimchinsky E.A., Oberlander A.M., Svoboda K. Abnormal development of dendritic spines in FMR1 knock-out mice. J. Neurosci. 2001;21:5139–5146. doi: 10.1523/JNEUROSCI.21-14-05139.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zalfa F., Giorgi M., Primerano B., Moro A., Di Penta A., Reis S., Oostra B., Bagni C. The fragile X syndrome protein FMRP associates with BC1 RNA and regulates the translation of specific mRNAs at synapses. Cell. 2003;112:317–327. doi: 10.1016/s0092-8674(03)00079-5. [DOI] [PubMed] [Google Scholar]
- 20.Brown V., Jin P., Ceman S., Darnell J.C., O’Donnell W.T., Tenenbaum S.A., Jin X., Feng Y., Wilkinson K.D., Keene J.D. Microarray identification of FMRP-associated brain mRNAs and altered mRNA translational profiles in fragile X syndrome. Cell. 2001;107:477–487. doi: 10.1016/s0092-8674(01)00568-2. [DOI] [PubMed] [Google Scholar]
- 21.Huber K.M., Gallagher S.M., Warren S.T., Bear M.F. Altered synaptic plasticity in a mouse model of fragile X mental retardation. Proc. Natl. Acad. Sci. USA. 2002;99:7746–7750. doi: 10.1073/pnas.122205699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huber K.M., Kayser M.S., Bear M.F. Role for rapid dendritic protein synthesis in hippocampal mGluR-dependent long-term depression. Science. 2000;288:1254–1257. doi: 10.1126/science.288.5469.1254. [DOI] [PubMed] [Google Scholar]
- 23.Snyder E.M., Philpot B.D., Huber K.M., Dong X., Fallon J.R., Bear M.F. Internalization of ionotropic glutamate receptors in response to mGluR activation. Nat. Neurosci. 2001;4:1079–1085. doi: 10.1038/nn746. [DOI] [PubMed] [Google Scholar]
- 24.Vanderklish P.W., Edelman G.M. Dendritic spines elongate after stimulation of group 1 metabotropic glutamate receptors in cultured hippocampal neurons. Proc. Natl. Acad. Sci. USA. 2002;99:1639–1644. doi: 10.1073/pnas.032681099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brager D.H., Johnston D. Plasticity of intrinsic excitability during long-term depression is mediated through mGluR-dependent changes in I(h) in hippocampal CA1 pyramidal neurons. J. Neurosci. 2007;27:13926–13937. doi: 10.1523/JNEUROSCI.3520-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sourdet V., Russier M., Daoudal G., Ankri N., Debanne D. Long-term enhancement of neuronal excitability and temporal fidelity mediated by metabotropic glutamate receptor subtype 5. J. Neurosci. 2003;23:10238–10248. doi: 10.1523/JNEUROSCI.23-32-10238.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee H.Y., Ge W.P., Huang W., He Y., Wang G.X., Rowson-Baldwin A., Smith S.J., Jan Y.N., Jan L.Y. Bidirectional regulation of dendritic voltage-gated potassium channels by the fragile X mental retardation protein. Neuron. 2011;72:630–642. doi: 10.1016/j.neuron.2011.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Strumbos J.G., Brown M.R., Kronengold J., Polley D.B., Kaczmarek L.K. Fragile X mental retardation protein is required for rapid experience-dependent regulation of the potassium channel Kv3.1b. J. Neurosci. 2010;30:10263–10271. doi: 10.1523/JNEUROSCI.1125-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brown M.R., Kronengold J., Gazula V.R., Chen Y., Strumbos J.G., Sigworth F.J., Navaratnam D., Kaczmarek L.K. Fragile X mental retardation protein controls gating of the sodium-activated potassium channel Slack. Nat. Neurosci. 2010;13:819–821. doi: 10.1038/nn.2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mullard A. Fragile X disappointments upset autism ambitions. Nat. Rev. Drug Discov. 2015;14:151–153. doi: 10.1038/nrd4555. [DOI] [PubMed] [Google Scholar]
- 31.Gonçalves J.T., Anstey J.E., Golshani P., Portera-Cailliau C. Circuit level defects in the developing neocortex of fragile X mice. Nat. Neurosci. 2013;16:903–909. doi: 10.1038/nn.3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang Y., Bonnan A., Bony G., Ferezou I., Pietropaolo S., Ginger M., Sans N., Rossier J., Oostra B., LeMasson G., Frick A. Dendritic channelopathies contribute to neocortical and sensory hyperexcitability in Fmr1(-/y) mice. Nat. Neurosci. 2014;17:1701–1709. doi: 10.1038/nn.3864. [DOI] [PubMed] [Google Scholar]
- 33.Braat S., Kooy R.F. The GABAA receptor as a therapeutic target for neurodevelopmental disorders. Neuron. 2015;86:1119–1130. doi: 10.1016/j.neuron.2015.03.042. [DOI] [PubMed] [Google Scholar]
- 34.D’Hulst C., De Geest N., Reeve S.P., Van Dam D., De Deyn P.P., Hassan B.A., Kooy R.F. Decreased expression of the GABAA receptor in fragile X syndrome. Brain Res. 2006;1121:238–245. doi: 10.1016/j.brainres.2006.08.115. [DOI] [PubMed] [Google Scholar]
- 35.Curia G., Papouin T., Séguéla P., Avoli M. Downregulation of tonic GABAergic inhibition in a mouse model of fragile X syndrome. Cereb. Cortex. 2009;19:1515–1520. doi: 10.1093/cercor/bhn159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.D’Hulst C., Heulens I., Brouwer J.R., Willemsen R., De Geest N., Reeve S.P., De Deyn P.P., Hassan B.A., Kooy R.F. Expression of the GABAergic system in animal models for fragile X syndrome and fragile X associated tremor/ataxia syndrome (FXTAS) Brain Res. 2009;1253:176–183. doi: 10.1016/j.brainres.2008.11.075. [DOI] [PubMed] [Google Scholar]
- 37.Gatto C.L., Pereira D., Broadie K. GABAergic circuit dysfunction in the Drosophila fragile X syndrome model. Neurobiol. Dis. 2014;65:142–159. doi: 10.1016/j.nbd.2014.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wilson R.I. Early olfactory processing in Drosophila: mechanisms and principles. Annu. Rev. Neurosci. 2013;36:217–241. doi: 10.1146/annurev-neuro-062111-150533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bolduc F.V., Bell K., Cox H., Broadie K.S., Tully T. Excess protein synthesis in Drosophila fragile X mutants impairs long-term memory. Nat. Neurosci. 2008;11:1143–1145. doi: 10.1038/nn.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Farhan A., Gulati J., Groβe-Wilde E., Vogel H., Hansson B.S., Knaden M. The CCHamide 1 receptor modulates sensory perception and olfactory behavior in starved Drosophila. Sci. Rep. 2013;3:2765. doi: 10.1038/srep02765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Christiaens J.F., Franco L.M., Cools T.L., De Meester L., Michiels J., Wenseleers T., Hassan B.A., Yaksi E., Verstrepen K.J. The fungal aroma gene ATF1 promotes dispersal of yeast cells through insect vectors. Cell Rep. 2014;9:425–432. doi: 10.1016/j.celrep.2014.09.009. [DOI] [PubMed] [Google Scholar]
- 42.Inoue S., Shimoda M., Nishinokubi I., Siomi M.C., Okamura M., Nakamura A., Kobayashi S., Ishida N., Siomi H. A role for the Drosophila fragile X-related gene in circadian output. Curr. Biol. 2002;12:1331–1335. doi: 10.1016/s0960-9822(02)01036-9. [DOI] [PubMed] [Google Scholar]
- 43.Mukamel E.A., Nimmerjahn A., Schnitzer M.J. Automated analysis of cellular signals from large-scale calcium imaging data. Neuron. 2009;63:747–760. doi: 10.1016/j.neuron.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Olsen S.R., Wilson R.I. Lateral presynaptic inhibition mediates gain control in an olfactory circuit. Nature. 2008;452:956–960. doi: 10.1038/nature06864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yaksi E., Judkewitz B., Friedrich R.W. Topological reorganization of odor representations in the olfactory bulb. PLoS Biol. 2007;5:e178. doi: 10.1371/journal.pbio.0050178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Olsen S.R., Bhandawat V., Wilson R.I. Excitatory interactions between olfactory processing channels in the Drosophila antennal lobe. Neuron. 2007;54:89–103. doi: 10.1016/j.neuron.2007.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yaksi E., Wilson R.I. Electrical coupling between olfactory glomeruli. Neuron. 2010;67:1034–1047. doi: 10.1016/j.neuron.2010.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Olsen S.R., Bhandawat V., Wilson R.I. Divisive normalization in olfactory population codes. Neuron. 2010;66:287–299. doi: 10.1016/j.neuron.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Robertson C.E., Ratai E.M., Kanwisher N. Reduced GABAergic action in the autistic brain. Curr. Biol. 2016;26:80–85. doi: 10.1016/j.cub.2015.11.019. [DOI] [PubMed] [Google Scholar]
- 50.Braat S., Kooy R.F. Insights into GABAAergic system deficits in fragile X syndrome lead to clinical trials. Neuropharmacology. 2015;88:48–54. doi: 10.1016/j.neuropharm.2014.06.028. [DOI] [PubMed] [Google Scholar]
- 51.Chou Y.H., Spletter M.L., Yaksi E., Leong J.C., Wilson R.I., Luo L. Diversity and wiring variability of olfactory local interneurons in the Drosophila antennal lobe. Nat. Neurosci. 2010;13:439–449. doi: 10.1038/nn.2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wilson R.I., Laurent G. Role of GABAergic inhibition in shaping odor-evoked spatiotemporal patterns in the Drosophila antennal lobe. J. Neurosci. 2005;25:9069–9079. doi: 10.1523/JNEUROSCI.2070-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Okada R., Awasaki T., Ito K. Gamma-aminobutyric acid (GABA)-mediated neural connections in the Drosophila antennal lobe. J. Comp. Neurol. 2009;514:74–91. doi: 10.1002/cne.21971. [DOI] [PubMed] [Google Scholar]
- 54.Das A., Sen S., Lichtneckert R., Okada R., Ito K., Rodrigues V., Reichert H. Drosophila olfactory local interneurons and projection neurons derive from a common neuroblast lineage specified by the empty spiracles gene. Neural Dev. 2008;3:33. doi: 10.1186/1749-8104-3-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kazama H., Wilson R.I. Origins of correlated activity in an olfactory circuit. Nat. Neurosci. 2009;12:1136–1144. doi: 10.1038/nn.2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Contractor A., Klyachko V.A., Portera-Cailliau C. Altered neuronal and circuit excitability in fragile X syndrome. Neuron. 2015;87:699–715. doi: 10.1016/j.neuron.2015.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gibson J.R., Bartley A.F., Hays S.A., Huber K.M. Imbalance of neocortical excitation and inhibition and altered UP states reflect network hyperexcitability in the mouse model of fragile X syndrome. J. Neurophysiol. 2008;100:2615–2626. doi: 10.1152/jn.90752.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hong E.J., Wilson R.I. Simultaneous encoding of odors by channels with diverse sensitivity to inhibition. Neuron. 2015;85:573–589. doi: 10.1016/j.neuron.2014.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schilit Nitenson A., Stackpole E.E., Truszkowski T.L., Midroit M., Fallon J.R., Bath K.G. Fragile X mental retardation protein regulates olfactory sensitivity but not odorant discrimination. Chem. Senses. 2015;40:345–350. doi: 10.1093/chemse/bjv019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rotschafer S., Razak K. Altered auditory processing in a mouse model of fragile X syndrome. Brain Res. 2013;1506:12–24. doi: 10.1016/j.brainres.2013.02.038. [DOI] [PubMed] [Google Scholar]
- 61.Sachdev R.N., Krause M.R., Mazer J.A. Surround suppression and sparse coding in visual and barrel cortices. Front. Neural Circuits. 2012;6:43. doi: 10.3389/fncir.2012.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rogers S.J., Hepburn S., Wehner E. Parent reports of sensory symptoms in toddlers with autism and those with other developmental disorders. J. Autism Dev. Disord. 2003;33:631–642. doi: 10.1023/b:jadd.0000006000.38991.a7. [DOI] [PubMed] [Google Scholar]
- 63.Banerjee A., Rikhye R.V., Breton-Provencher V., Tang X., Li C., Li K., Runyan C.A., Fu Z., Jaenisch R., Sur M. Jointly reduced inhibition and excitation underlies circuit-wide changes in cortical processing in Rett syndrome. Proc. Natl. Acad. Sci. USA. 2016;113:E7287–E7296. doi: 10.1073/pnas.1615330113. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.