Abstract
The orphan nuclear receptor steroidogenic factor 1 (SF-1, also called Ad4BP, encoded by the NR5A1 gene) is an essential regulator of endocrine development and function. Initially identified as a tissue-specific transcriptional regulator of cytochrome P450 steroid hydroxylases, studies of both global and tissue-specific knockout mice have demonstrated that SF-1 is required for the development of the adrenal glands, gonads, and ventromedial hypothalamus and for the proper functioning of pituitary gonadotropes. Many genes are transcriptionally regulated by SF-1, and many proteins, in turn, interact with SF-1 and modulate its activity. Whereas mice with heterozygous mutations that disrupt SF-1 function have only subtle abnormalities, humans with heterozygous SF-1 mutations can present with XY sex reversal (i.e. testicular failure), ovarian failure, and occasionally adrenal insufficiency; dysregulation of SF-1 has been linked to diseases such as endometriosis and adrenocortical carcinoma. The current state of knowledge of this important transcription factor will be reviewed with a particular emphasis on the pioneering work on SF-1 by the late Keith Parker.
The orphan nuclear receptor steroidogenic factor 1 (SF-1, encoded by the NR5A1 gene) is an essential regulator of endocrine development and function.
The orphan nuclear receptor, steroidogenic factor 1 (SF-1), plays a key role in the development and function of steroidogenic tissues. It was discovered by Keith Parker, who died tragically at age 54 in 2008. Parker’s work established an extremely active area of investigation that has resulted in more than 1000 publications listed in PubMed. Our contributions to SF-1 biology (and connections to Parker) began in 1984 when we met at a symposium. White had recently cloned the cytochrome P450 (CYP)21 gene encoding steroid 21-hydroxylase, which is mutated in most cases of congenital adrenal hyperplasia. Schimmer suggested a collaboration to explore the regulation of this gene, and steroidogenesis in general, using mouse Y1 adrenocortical cells, which lack Cyp21 expression. White referred him to another collaborator, Jon Seidman at Harvard, whose laboratory had isolated mouse cosmids carrying the Cyp21 gene. In turn, Seidman assigned the project to Keith Parker, a postdoctoral fellow in his laboratory at the time.
These studies demonstrated that Y1 cells stably transfected with the mouse Cyp21 cosmid recovered hormonally regulated expression of 21-hydroxylase ( 1). Sequences essential for expression were localized to the proximal 330 bp of 5′-flanking DNA ( 2, 3). When genes encoding other steroid hydroxylases were isolated and their 5′-flanking regions compared, shared AGGTCA promoter elements were identified in several of these genes that interacted with the same DNA-binding protein in steroidogenic cell lines. This protein was designated steroidogenic factor 1 (SF-1) or adrenal 4-binding protein (Ad4BP) (see Ref. 4 for a review of these studies). The selective expression of SF-1 in steroidogenic cells and its regulation of genes encoding several distinct steroid hydroxylases provided the first clues that it was a key determinant of cell-selective expression of steroidogenic enzymes.
Parker and colleagues ( 5) reasoned that the AGGTCA DNA recognition motif represented a binding site for an atypical member of the nuclear hormone receptor family and cloned mouse SF-1 cDNA from a Y1 cell cDNA library using a hybridization probe comprising the DNA-binding region of retinoid X receptor. Independently, Morohashi and colleagues ( 6) used an oligonucleotide affinity column to purify the protein from bovine adrenal glands, ultimately allowing them to obtain amino acid sequence and clone a bovine cDNA with an oligonucleotide probe. Subsequent studies established that the mouse and bovine cDNAs encoded orthologs of a protein that transactivated the steroid hydroxylase promoters in steroidogenic and nonsteroidogenic cells. The sequences of these cDNAs confirmed that SF-1 belonged to the nuclear hormone receptor family, with striking homology to the Drosophila nuclear receptor fushi tarazu factor 1. SF-1 was also similar in sequence to an orphan receptor cloned from mouse liver, designated LRH1, and its human homolog, PHR-1. Although LRH1 clearly derives from a separate gene and has an expression profile that is distinct from that of SF-1 (e.g. in liver and pancreas), the LRH1 and SF-1 sequences are sufficiently similar to group them as members of the same subfamily of nuclear hormone receptors, designated NR5A. The SF-1 gene is now officially termed NR5A1 (in this review, we will continue to use SF-1 to refer to the protein or mRNA) and the LRH1 gene is named NR5A2. Early work on SF-1 structure and function has been reviewed elsewhere ( 4).
Like other nuclear hormone receptors, SF-1 has a modular domain structure comprised of an N-terminal zinc finger DNA-binding domain (DBD), a ligand-binding domain, a C-terminal AF-2 activation domain, and an intervening proline-rich hinge region that has AF-1 like activation activity (Fig. 1). SF-1 also contains a fushi tarazu factor 1 or A box (i.e. a 30-amino acid extension of the DBD) that mediates specific binding to sequences 5′ to the consensus hexamer. As determined by X-ray crystallography of the DBD complexed with a sequence in the proximal promoter region of the INHA (inhibin-α) gene, SF-1 forms a specific complex with DNA by binding to the major and minor grooves through the core DBD and the N-terminal segment of the A box, respectively. Additionally, a helix in the C-terminal segment of the A box interacts with both the core DBD and DNA and serves as an important determinant of stability of the complex. This helix may also interact with coactivators and other DNA-bound factors ( 7).
Fig. 1.
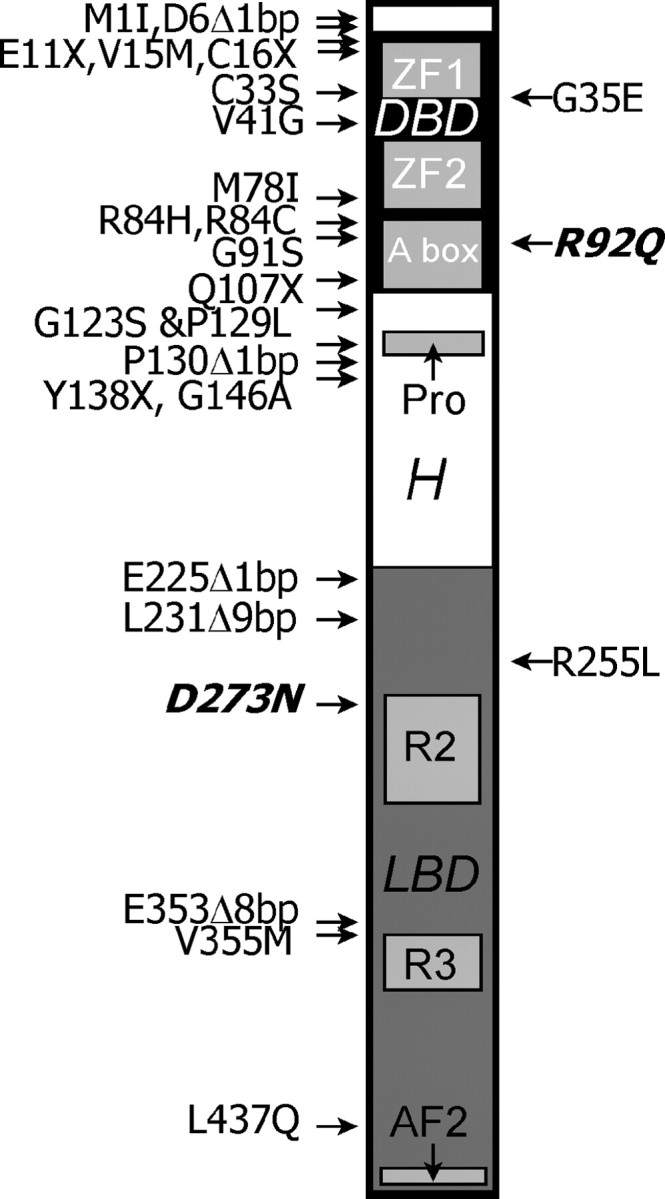
Schematic of SF-1 with mutations causing human disease (only coding sequence mutations are shown). The N terminus is at the top. A DBD (denoted by the black box) is near the N terminus, containing two zinc finger (ZF) domains and an accessory binding domain (A box). A proline-rich (pro) region is followed by a hinge (H) region. A ligand-binding domain (LBD, denoted by the dark gray box), contains two highly conserved regions (R2 and R3) and an activation domain (AF2) near the C terminus. Mutations to the right of the schematic are associated with adrenal insufficiency with or without gonadal failure, whereas those to the left are associated with gonadal failure (XY sex reversal or XX ovarian failure) without adrenal insufficiency. All of these mutations were found in the heterozygous state except for R92Q and D273N (in bold and italic), which cause disease only in homozygous individuals. Nearby mutations separated by commas are independent, but G123S and P129L occur in cis in the same patient. Δ, Deletion.
Regulation of SF-1 Expression and Function
Patterns of expression in adult and embryonic tissues
The localization of SF-1 in adult tissues has been investigated extensively in mice, rats, humans, and other vertebrates. Consistent with its role as a master regulator of steroidogenesis, SF-1 is expressed in the major steroidogenic tissues, i.e. the three zones of the adrenal cortex, testicular Leydig and Sertoli cells and ovarian interstitium, theca cells, granulosa cells, and, to a lesser degree, corpus luteum ( 6, 8, 9, 10, 11). It also is expressed in pituitary gonadotrophs where it regulates the expression of the gonadotropins ( 12, 13) and the GnRH receptor ( 14). Apart from these tissues, SF-1 expression is limited to neurons in the dorsomedial portion of the ventromedial hypothalamus that are distinct from those expressing GnRH, to the endothelial linings of the venous sinuses and pulp veins in the spleen ( 11, 15), and to a subset of neurons in the hippocampus that coexpress steroidogenic acute regulatory protein (StAR) and aromatase ( 16).
In developing embryos, SF-1 first appears in the urogenital ridge and represents the earliest marker of adrenal and gonadal differentiation ( 17, 18, 19, 20). As development proceeds, SF-1 is expressed in the steroid-secreting cortical zones of the adrenal gland, in testicular Leydig and Sertoli cells, and in the human ovary; however, in mice and rats, ovarian expression of SF-1 is turned off as development proceeds and does not resume until just before birth ( 17, 19, 20). SF-1 also appears in the fetal spleen ( 15) and in the prosencephalon—that region of the brain that includes the hypothalamic primordium ( 19).
Regulation of NR5A1 promoter activity
Regulatory elements and transcription factors
The first 110 bp in the 5′-flanking region of NR5A1 do not contain a TATA box but do include a small number of regulatory elements including a SRY (sex determining region Y)-box (SOX) binding site, which may contribute to Sertoli cell expression of SF-1 ( 21), and an E box, a CCAAT box, and an Sp1/Sp3 site that may function more broadly ( 22, 23, 24, 25, 26)(Fig. 2). In vitro, these elements bind combinations of proteins that differ somewhat among cell lines of adrenal, Leydig, Sertoli, and pituitary origin ( 21, 22, 23, 25, 26, 27). Two other Sp1/Sp3 sites situated downstream of the transcription start site between +10 and +30 also contribute to optimal activity of the promoter ( 25). Longer NR5A1 promoter fragments with 589 bp of 5′-flanking sequence contain binding sites for GATA-4 ( 28) and for WT1 and Lhx9 ( 29) that enhance the activity of the promoter in testes-derived cells. Other transcription factors implicated in NR5A1 regulation include SOX15, SOX30, and TEAD-4 ( 21, 30). Finally, the Polycomb ortholog, chromobox homolog 2 (CBX2, M33), may be an important regulator of NR5A1. CBX2 binds the NR5A1 promoter in Y1 cells. Mice carrying CBX2 null mutations have reduced SF-1 expression and have phenotypes reminiscent of those seen with SF-1 mutations (see below) including XY sex reversal, small adrenal glands, and abnormalities in the spleen ( 31). XY sex reversal also is seen in humans with CBX2 mutations ( 32).
Fig. 2.
Transcriptional regulation of NR5A1, which encodes SF-1. This gene is transcribed left to right. A, The genomic region surrounding NR5A1 on chromosome 9q33.3. A 100-kb scale is shown. Arrows denote direction of transcription. Vertical bars in each gene denote exons. NR6A1 encodes germ cell nuclear factor (GCNF); only the 3′-portion of this gene is shown. GPR144 encodes a G protein-coupled receptor, and PSMB7 encodes the β7 proteosome subunit. Almost the entire depicted region is required for full NR5A1 transgene expression in mice. B, The NR5A1 gene. A 10-kb scale is shown; exons are numbered. A graph of sequence conservation is below, and aligned with, the schematic of the gene. Note that exons are relatively highly conserved, but there are highly conserved intronic regions as well. Tissue-specific enhancers have been localized to several of these highly conserved intronic regions and are denoted by horizontal bars. C, The proximal promoter of NR5A1. The scale is marked in base pairs, with the main transcriptional start site at 0, denoted by an arrow. Several conserved elements are shown as shaded boxes, with binding proteins denoted by ovals. Not all elements are used in all cell types, and many more proteins than are depicted here are assembled in transcriptional complexes on these sites. Sx, A binding site for SOX9 (used in Sertoli cells); E, an E box that binds upstream factors 1 and 2 (USF1 and USF2); C, a CCAAT box that binds isoforms of nuclear factor Y (NFY); Sp, a binding site for Sp1 and Sp3 transcription factors.
Studies of transgenic animals give a somewhat different picture of the DNA segments required for normal NR5A1 expression. The very short NR5A1 promoter fragments are insufficient to recapitulate gene expression in sites where SF-1 is normally expressed ( 33); the somewhat larger fragment from −590 to +85 is able to direct gene expression in the indifferent gonads of transgenic mouse embryos in a WT1-dependent manner but is insufficient to drive expression in any other SF-1-expressing tissue ( 29). A much larger reporter gene driven by mouse genomic DNA, extending from exon 2 into the upstream gene NR6A1 gene encoding germ cell nuclear factor, is able to drive gene expression in the adrenal cortex, testicular Leydig cells, ovarian theca cells, the ventromedial hypothalamus (VMH), and the spleen, but not in the pituitary gland or corpus luteum ( 33). So far, only a rat genomic fragment including the NR5A1 gene as well as flanking DNA extending into the NR6A1 and PSMB7 genes on either side duplicates the patterns of endogenous SF-1 expression, both spatially and quantitatively ( 34). Within this very large genomic fragment lie highly conserved regions within intron 6 of NR5A1 that are required to recapitulate VMH-specific ( 35) or gonadotrope-specific expression (36) of SF-1 from the fetal stage to the adult (Fig. 2).
Expression of the NR5A1 gene in the fetal adrenal gland, on the other hand, depends on binding sites for Pbx1-Prep and Pbx1-hox complexes and for SF-1 itself within a highly conserved region of intron 4 ( 37). This region is unable to sustain SF-1 expression in the adult gland and thus is considered to be a fetal adrenal-specific enhancer of NR5A1 expression that, by binding SF-1, functions in an autoregulatory manner. Consistent with this, mice carrying an SF-1 transgene that contains this region (along with 5.8 kb of 5′-flanking sequences) have increased adrenal size and ectopic adrenal tissue in the thorax, suggesting that increased levels of SF-1 may divert uncommitted precursors to the steroidogenic lineage ( 38). Moreover, all cells in the murine adult adrenal cortex descend from cells that express SF-1 under the control of this enhancer before d 14.5 of embryogenesis (Fig. 2; Ref. 39).
Contributions of DNA methylation
Whereas transcription factor-binding sites and their interacting proteins are primary determinants of NR5A1 expression, DNA methylation appears to act as a secondary layer of control. CpG islands in the NR5A1 promoter isolated from endometriotic tissue are hypomethylated when compared with those from normal endometrial stroma and are associated with the aberrant expression of NR5A1 and other genes involved in steroidogenesis ( 40). Treatment of SF-1-negative, normal endometrial stromal cells with the demethylating reagent, 5-aza-deoxycytidine, leads to demethylation of the NR5A1 promoter and the appearance of NR5A1 transcripts, whereas methylation of the promoter leads to a loss of its activity. The correlation between methylation of the NR5A1 promoter and NR5A1 expression also is seen in steroid-secreting cell lines and normal steroid-secreting tissues ( 41). DNA methylation at CpG-rich sequences generally increases with cellular differentiation, providing an efficient mechanism for transcriptional repression and maintenance of cell-specific phenotypes ( 42). Consistent with this general finding, the NR5A1 proximal promoter is unmethylated in the developing testis and ovary, whereas it is hypermethylated in most embryonic tissues in which SF-1 is not expressed. Embryonic mouse and human kidneys are an exception because SF-1 is never expressed but, for unknown reasons, the NR5A1 gene is nevertheless hypomethylated ( 41). On balance, the data suggest that methylation of the proximal promoter region of NR5A1 is established early in differentiation and contributes to the tissue-restricted expression of SF-1 during development.
Chromatin interactions
NR5A1 is located only 13 kb downstream of the last exon of the NR6A1gene (Fig. 2), which has a distinct expression pattern, suggesting the presence of an intervening insulator element (a DNA fragment between two genes that functions as an enhancer blocker and a barrier to modified chromatin). In Y-1 cells, there are three hypersensitive sites between the last exon of NR6A1 and the first exon of NR5A1. The most upstream site contains a binding site for CCCTC-binding factor, a zinc finger protein known to bind insulator elements, whereas the other sites associate with the nuclear matrix (i.e. they constitute a matrix attachment region). As determined by chromatin immunoprecipitation analysis, there is a discontinuous pattern of histone acetylation upstream of these sites, suggesting that the chromatin architecture specified by CCCTC-binding factor and the matrix contribute to the distinct pattern of transcriptional regulation of NR5A1 and NR6A1 ( 43).
Signal transduction pathways affecting SF-1 function
cAMP-dependent signaling
The effects of SF-1 on cell-selective gene expression are intimately associated with cAMP-regulated expression of many of the same genes, e.g. CYP11A1, CYP11B1, CYP11B2, CYP17, CYP21, STAR ( 44), SCARB1 (45, 46), MC2R ( 47, 48), and INHA (49). This interplay between SF-1 and the cAMP/protein kinase A-signaling cascade is complex and involves multiple factors acting in promoter-specific contexts that have not been fully defined. Although SF-1 contains a consensus site for phosphorylation by protein kinase A ( 6), mutating this does not affect the cAMP-dependent regulation of its activity, so it is unlikely that this synergy results from protein kinase A-mediated phosphorylation of SF-1 ( 50, 51). One facet of cAMP-dependent signaling may be the generation of activating ligands (see below) for SF-1 via activation of diacylglycerol kinase ϑ and the resultant synthesis of phosphatidic acid ( 52, 53). The cAMP-signaling cascade also appears to affect the recruitment of SF-1 to active foci of transcription within the nucleus and the dynamic interaction of SF-1 with its regulatory cofactors ( 48, 54, 55). This effect may be secondary to ligand activation of SF-1 ( 52) or to cAMP-mediated induction of p300 ( 54). Finally, cAMP may affect the levels of SF-1 RNA and protein; however, evidence supporting this hypothesis is conflicting ( 56, 57, 58, 59).
MAPK and cyclin-dependent kinase 7 (CDK7)
SF-1 is phosphorylated primarily at S203, within the activation factor helix 1 domain, the hinge region between the DBD and ligand-binding domain of the protein ( 60, 61). The MAPK, Erk2, can phosphorylate SF-1 at S203 in vitro and possibly in vivo ( 60, 62) and has been implicated in the estrogen-dependent activation of SF-1 via the G protein-coupled receptor, GPR30 ( 63). CDK7 also may contribute significantly to the phosphorylation of SF-1 at S203 ( 64). Both the ligand-binding domain of SF-1 and the extent of posttranslational modification via small ubiquitin-related modifier (SUMO)-ylation (see below) modify the extent of SF-1 phosphorylation at S203. Phosphorylation of SF-1 at S203 does not affect its half-life, its subcellular localization, or its binding to DNA ( 60); however, it does enhance its ability to interact with cofactors such as glucocorticoid receptor-interacting protein 1 (GRIP1) and silencing mediator of retinoid and thyroid hormone receptor (SMRT) ( 60), to sustain SF-1-dependent activity at some promoters but not others ( 51, 64), and to adopt an active conformation within its ligand-binding domain ( 61).
Protein kinase C-δ
SF-1 expression may be under the control of a regulatory module comprised of erb-b3, ebp1, and protein kinase C-δ, because the knockdown of these three transcripts using specific small interfering RNAs in a heterologous cell culture system enhances SF-1 promoter activity ( 30); conversely, overexpression of protein kinase C-δ inhibits SF-1 promoter activity. The extent to which the regulatory activities of protein kinase C-δ, erb-b3, and ebp1 at the SF-1 promoter can be extrapolated to SF-1 expression in situ in cells that normally express SF-1 has yet to be determined.
Wnt and hedgehog
The Wnt signaling pathway also impacts on SF-1-regulated gene expression. This pathway is comprised of Wnt ligands, the Frizzled family of G protein-coupled receptors, low-density lipoprotein receptor-related protein 5/6 coreceptors, Dishevelled, ß-catenin, and a ß-catenin inactivation complex that keeps ß-catenin at low levels and localized to the plasma membrane ( 65). The activation of the Wnt pathway leads to phosphorylation of Dishevelled, the consequent inhibition of the ß-catenin inactivation complex, and accumulation of ß-catenin in the nucleus. ß-Catenin binds directly to SF-1 and synergistically regulates the expression of Anti-Müllerian hormone (AMH; Mullerian-inhibiting substance) receptor 2 (AMHR2) α-inhibin, DAX-1 (dosage-sensitive sex reversal-AHC critical region on the X chromosome gene 1), and several genes involved in steroidogenesis, including STAR and CYP19A1 (see Table 1). Targeted inactivation of β-catenin in SF-1-expressing cells using an SF-1/Cre transgene leads to adrenal agenesis, supporting the idea that proper functioning of the Wnt/β-catenin pathway is critical for normal adrenal development ( 66).
Table 1.
Sites of action and target genes for SF-1
| Site of action | Target genes | Comments |
|---|---|---|
| Ventromedial hypothalamus | NMDAR1 | N-methyl-d-aspartate receptor |
| BDNF | Brain-derived neurotrophic factor | |
| A2BP1, AMIGO2, CDH4, NPTX2, SEMA3A, SLIT3, NETRIN3 | Cell adhesion and cell guidance molecules | |
| FEZF1, NKX2-2 | Transcription factors | |
| Gonadotropes | CGA | α-Subunit of glycoprotein hormones |
| LHB | Luteinizing hormone (LH) ß | |
| FSHB | Follicle-stimulating hormone (FSH) ß | |
| GNRHR | Gonadotropin-releasing hormone receptor | |
| Adrenal cortex | CYP11A1, CYP17, CYP21, CYP11B1, CYP11B2 | Cytochrome P450 steroid hydroxylases |
| HSD3B2 | 3ß-Hydroxysteroid dehydrogenase; Hsd3b1 in rodents | |
| STAR | Steroidogenic acute regulatory protein | |
| MC2R | Corticotropin receptor | |
| SCARB1 | Scavenger receptor-B1 | |
| NR0B1 | Dosage-sensitive sex reversal, adrenal hypoplasia congenital, X chromosome (DAX-1) | |
| AKR1B7 | Aldose reductase-like protein | |
| SULT2A1 | Steroid sulfotransferase 2A1 | |
| ADCY4 | Adenylyl cyclase type 4 (repressor activity) | |
| Gonads | ||
| Leydig cells | CYP11A1, CYP17 | Cytochrome P450 steroid hydroxylases |
| STAR | Steroidogenic acute regulatory protein | |
| LHR | LH receptor | |
| INSL3 | Insulin-like 3 | |
| PRLR | Prolactin receptor | |
| AMHR2 | Anti-Mullerian hormone/Mullerian inhibiting substance receptor | |
| Sertoli cells | AMH | Anti-Mullerian hormone/Mullerian inhibiting substance |
| INHA | Inhibin α−subunit | |
| FSHR | FSH receptor | |
| SRY | Sex-determining region Y (SRY) | |
| SOX9 | SOX9 (SRY box 9) | |
| Theca and granulosa cells | CYP11A1, CYP17, CYP19 | Cytochrome P450 steroid hydroxylases |
| STAR | Steroidogenic acute regulatory protein | |
| INHA | Inhibin α subunit | |
| OXT | Oxytocin |
The hedgehog pathway consists of three signaling ligands: sonic hedgehog, desert hedgehog (the main signaling ligand in the male gonad), and Indian hedgehog. Each of these ligands interacts with its membrane receptor Patch to free Smoothen, a member of the G protein-coupled receptor family, for downstream effects that include inhibition of cleavage of the GLI3 transcription factor. GLI3 acts as a transcriptional activator, whereas its cleavage product represses the expression of target genes ( 67, 68). The hedgehog pathway appears to have a role in SF-1 expression because disruption of the pathway decreases the gonadal expression of SF-1 ( 69), whereas activation of the pathway has the opposite effect ( 70).
Regulation of function through protein-protein and protein-RNA interactions
Although SF-1 interacts with its target DNA element as a monomer ( 71), its activity is strongly influenced by its interactions with other transcription factors and coregulators (for brief review see Ref. 72) (Table 2). Some interacting proteins are expressed in a tissue-specific manner whereas others are more widely expressed, and it is unclear how each contributes to the coordinate and cell-selective expression of SF-1-dependent genes. Analyses of transcription complex assembly on the MC2R ( 48) and CYP17 ( 73) promoters indicate that SF-1-containing complex formation is dynamic and cyclical. Thus the nature of the complexes formed is likely to be influenced by the relative abundance of the interacting partners and by their relative affinities for the promoter and for SF-1 at any given spatiotemporal interval.
Table 2.
Proteins that interact with SF-1 and modulate its activity
| Protein | Gene(s) affected | Effect | Reference |
|---|---|---|---|
| Transcription factors | |||
| AR | LHB | − | 139 |
| c-JUN | CYP11A1 | + | 140 |
| DAX1 | AMH | − | 76 78 141 |
| EGR1 | LHB | + | 42 |
| FOXL2 | CYP19A1 | + | 143 |
| GATA4 | AMH | + | 144 |
| GR | DAX1 | + | 79 |
| NFKB | AMH | − | 145 |
| NFYA | FSHB | + | 117 |
| PTX1 | LHB | + | 146 |
| SOX8 | AMH | + | 147 |
| SOX9 | AMH | + | 148 |
| Sp1 | CYP11A1 | + | 149 150 |
| WT1 | AMH | + | 141 |
| Coactivators | |||
| CBP/p300 | CYP11A1 | + | 151 |
| PCAF | CYP17 | + | 85 |
| GRIP1 | CYP21 | + | 60 |
| MBF1 | CYP11B1 | + | 152 |
| p/CIP | CYP17 | + | 153 |
| PNRC | CYP19 | + | 154 |
| SNURF | LHB | + | 155 |
| SRC1 | SF-1 binding site | + | 156 157 |
| TIF2 | CYP17 | + | 153 |
| TReP-132 | CYP11A1 | + | 158 |
| Corepressors | |||
| CtBP1 | CYP17 | − | 73 159 |
| DP103 | CYP11A1 | − | 160 |
| EID1 | CYP21 | − | 161 |
| GIOT1 | CYP21 | − | 162 |
| RIP140 | STAR, CYP17 | − | 163 164 |
| SMRT | CYP21 | − | 60 |
| Zip67 | CYP11A1, CYP17 | − | 165 |
| Other proteins | |||
| ß-Catenin | AMHR2, INHA, DAX1, STAR, CYP19A1 | + | 166 167 168 169 170 |
| P54nrb/NonO | CYP17 | − | 171 |
| PSF | CYP17 | − | 171 |
| PIAS1, PIAS3 | SF-1 binding site | 172 |
Interactions between SF-1 and DAX-1 deserve particular comment (early work is reviewed in Ref. 74). Mutations in DAX-1 (NR0B1) cause X-linked adrenal hypoplasia congenita (AHC) and hypogonadotrophic hypogonadism, phenotypes similar to those seen with SF-1 mutations (see below). In early stages of mouse embryonic development, SF-1 expression in the urogenital ridge and brain either precedes or coincides with DAX-1 expression. As embryonic development proceeds, the two transcription factors exhibit similar expression patterns in the adrenal cortex, testis, ovary, hypothalamus, and anterior pituitary. The slightly earlier onset of SF-1 expression and its ability to bind specifically to a conserved sequence in the NR0B1 5′-flanking region suggest that SF-1 may activate DAX-1 expression, and indeed SF-1 stimulates expression of the NR0B1 promoter in transient expression assays in SF-1-deficient cells ( 75, 76).
Coexpression of DAX-1 and SF-1 inhibits SF-1-mediated transactivation of many target genes. DAX-1 interacts directly with SF-1 in vitro via the carboxy-terminal regions of each protein ( 77). Additionally, DAX-1 interferes with interactions between SF-1 and WT1 (the Wilm’s tumor suppressor protein) or GATA4, each of which normally associates and synergizes with SF-1 to promote AMH expression. Finally, DAX-1 acts as an adaptor that recruits other factors, such as the nuclear receptor corepressor, to SF-1 ( 78).
Expression of DAX-1 in adrenal cells is decreased by ACTH and increased by glucocorticoid treatment, the latter occurring through a glucocorticoid receptor-SF-1 complex on the NR0B1 promoter. ACTH disrupts the formation of this complex by shifting SF-1 binding away from the NR0B1 promoter and toward the MC2R and STAR promoters ( 79, 80).
Whereas all of these findings suggest that DAX-1 acts mainly to negatively regulate SF-1 actions (and thus steroidogenesis), the inhibitory domain in DAX-1 is deleted or mutated in most AHC mutants, suggesting that loss of the inhibitory property in DAX-1 is associated with the development of AHC. Given that inactivating mutations in DAX-1 and SF-1 have similar phenotypes, this seems paradoxical and possibly reflects DAX-1 functions in development that are distinct from its regulatory roles in postnatal life. Indeed, DAX-1 can function as an SF-1 coactivator in some contexts. Both SF-1 and DAX-1 bind to steroid receptor RNA activator (SRA), a noncoding RNA that functions as a coactivator. In cotransfection experiments, an SRA expression plasmid increases the ability of SF-1 (or DAX-1) plasmids to transactivate reporter constructs, whereas knockdown of SRA expression abolishes coactivation by Dax-1. Moreover, SRA is physically associated with both SF-1 and DAX-1 in Y1 cells and is expressed in both the adrenal glands and gonads. The coactivator TIF2 also associates with DAX-1 and synergistically coactivates SF-1 target gene transcription. Corepressor vs. coactivator functions of DAX-1 may be dose dependent; although transfection of DAX-1 expression constructs usually inhibits expression of steroidogenic proteins, knockdown of endogenous DAX-1 actually down-regulates the expression of CYP11A1 and STAR in both H295R adrenal and MA-10 Leydig cells ( 81).
Regulation through posttranslational modification
In addition to phosphorylation at S203 as describe above, SF-1 is subject to SUMO conjugation and acetylation at ε-amino groups of target lysine residues. SUMO-ylation represses SF-1 function ( 82, 83, 84), whereas acetylation enhances its activity ( 54, 85).
For a brief review of protein SUMO-ylation and its consequences on gene expression, see Ref. 86 . SF-1 is SUMO-ylated within its DBD at K119 and within its hinge region at K194 ( 82, 83, 84); K194 appears to be the primary site of SUMO-ylation and suppression of SF-1 activity whereas K119 makes a minor contribution ( 82, 83, 84). Disruption of these SUMO-ylation sites increases the promoter-regulatory activity of SF-1 at CYP19, HSD 3B2, STAR, and at a number of isolated SF-1 response elements ( 82, 84); preventing the SUMO-ylation of SF-1 by overexpressing the SUMO-isopeptidase, SENP1, also enhances SF-1 activity ( 84). SUMO-ylation does not affect the stability, cellular localization, or dynamic association of SF-1 with chromatin or its ability to bind to canonical SF-1 response elements but does inhibit its phosphorylation by CDK7 at S203 ( 87, 88) and may (84) or may not (82) affect its ability to interact with repressors such as DP10; SUMO-ylation at the minor K119 site interferes with the ability of SF-1 to bind to and function at noncanonical response elements such as those found in the rat INHA promoter ( 87).
Two histone acetyltransferase proteins, PCAF ( 85) and p300, ( 54) stimulate the incorporation of labeled acetate into SF-1 and increase its transcriptional activity. Disruption of putative acetylation sites prevents acetylation of SF-1 and decreases its transcriptional activity ( 54, 85), whereas inhibition of the deacetylation of SF-1 using trichostatin A increases both its half-life and activity ( 85, 89). The acetylation of SF-1 by p300, but not by PCAF, appears to increase its localization within transcriptionally active nuclear foci ( 54). Other coactivators with histone acetyltransferase activity interact with, and modulate, the activity of SF-1 as well (Table 2).
The dynamics of SUMO-ylation and acetylation are incompletely understood. Less than 10% of SF-1 appears to be SUMO-ylated in vivo ( 82, 83, 84, 88), and it is not clear how this small fraction can have a dominant effect on SF-1-dependent transcription. The same can be said for acetylated SF-1, based on the presumption that it too represents only a small fraction of the total. Perhaps the posttranslational modifications of SF-1 are influenced by the cyclical and dynamic recruitment of SF-1 into active transcription complexes because, for example, DNA-bound SF-1 is refractory to SUMO-ylation at K113 ( 87).
Phospholipid-dependent regulation
The finding that SF-1 is a member of the nuclear receptor family of transcription factors engendered a search for ligands that regulate its function. Oxysterols enhance SF-1 function in Chinese hamster CV-1 lung cells ( 90, 91) but not in other cell types ( 61, 91, 92). X-ray crystallography and mass spectrophotometry of bacterially expressed SF-1 indicate that its ligand-binding pocket is very large, very hydrophobic, and occupied by phosphatidylethanolamine or phosphatidylglycerol ( 93, 94, 95, 96). These bacterial phospholipids can be exchanged for other potential regulatory ligands such as phosphatidic acid, phosphatidylcholine, and phosphatidylinositol di- and triphosphates ( 93, 94, 95) with little consequence on the conformation of the AF2-activating domain of SF-1, but with some effect on conformation at the entrance to the ligand-binding pocket of the protein ( 95). SF-1 purified from H295R adrenal tumor cells is associated with sphingosine, which acts as an inhibitor of SF-1 function in reporter gene assays ( 53). Treatment of cells with cAMP increases sphingosine catabolism, decreases the amount of sphingosine bound to SF-1, and enhances SF-1-dependent gene expression, whereas inhibitors of sphingosine synthesis enhance SF-1 activity. Sphingosine can be displaced from SF-1 to varying degrees by the phospholipid ligands discussed above, suggesting that SF-1 function is controlled by changes in the lipid environment secondary to regulated changes in lipid metabolism. This may have a teleological explanation given the key role of SF-1 in regulating steroidogenesis, a process requiring high concentrations of cholesterol.
As regards pharmacological ligands, low-molecular weight compounds with a rigid cis-bicyclo[3.3.p]oct-2-ene core structure selectively increase SF-1 activity ( 97) whereas 4-alkyloxy-phenol derivatives act as inverse agonists, suppressing its constitutive activity ( 98). Given the importance of SF-1 in steroidogenesis and in appetite control, these compounds may lead to the development of drugs useful in the treatment of steroid hormone excess, steroid hormone-dependent tumors, obesity, and related metabolic disorders.
The Role of SF-1 as a Regulator of Gene Expression—Lessons from In Vitro Studies
Gene targets of SF-1 in adrenal glands and gonads
Many SF-1 target genes (Table 1) have been identified with use of transient transfection assays using relatively limited stretches of promoter/regulatory DNA.
In adrenocortical cells, SF-1 increases expression of the corticotropin receptor (MCR2), STAR, and all of the enzymes required for cortisol or corticosterone biosynthesis. It also increases levels of scavenger receptor B1, which is required for cellular importation of high-density lipoprotein cholesterol, the main source of cholesterol for steroidogenesis, and AKR1B7 (aldose reductase-like protein), which metabolizes the isocaproaldehyde generated by cleavage of the cholesterol side chain ( 99). Although SF-1 is generally regarded as an activator of gene expression, it is a strong negative regulator of the type 4 adenylyl cyclase, thereby regulating adenylyl cyclase isoform composition ( 100) and of CYP11B2 (aldosterone synthase), which catalyzes the final step in aldosterone biosynthesis ( 101), thereby contributing to the restriction of aldosterone biosynthesis to the adrenal zona glomerulosa. In the ACTH-responsive, Y1 mouse adrenocortical tumor cell line, cells with a mutation affecting SF-1 function exhibit decreased expression of the MC2R CYP11B1 (11ß-hydroxylase) CYP11A1 (cholesterol side-chain cleavage enzyme), and STAR ( 102). In this cell line, the SF-1 defect affects the expression of the MC2R and CYP11B1 to a much greater degree than CYP11A1 or STAR, suggesting heterogeneity among these target genes in their regulation by SF-1.
SF-1 increases the expression of many testicular genes required to develop and maintain the male phenotype. Analogous to its effects in adrenocortical cells, SF-1 stimulates expression in Leydig cells of the LH receptor, STAR, and the CYP11A1 and CYP17 enzymes required for testosterone biosynthesis. Additionally, it increases expression of insulin-like polypeptide 3, which regulates testicular descent and acts in adults as a male germ cell survival factor ( 103, 104), and the AMHR2 receptor for AMH, which is required for normal male reproductive tract development and serves as a negative regulator of Leydig cell steroidogenesis ( 105).
In Sertoli cells, SF-1 is required for the expression of the testes-determining gene products, sex-determining region Y, and SOX9 AMH, the receptor for FSH, and INHA. Thus SF-1 is necessary for male sexual differentiation, a conclusion underscored by the XY sex reversal seen in either humans or mice carrying SF-1 mutations (see below).
Whereas SF-1 is required for ovarian development (as determined from studies of knockout mice, described later), interpreting the role of SF-1 in the adult ovary is complicated by the cyclic nature of follicular maturation, steroidogenesis, and corpus luteum formation. SF-1 is expressed most highly in theca and interstitium, at lower levels in granulosa cells and at even lower levels in the corpus luteum. In contrast, LRH-1 (NR5A2) expression is abundant in cells involved in estrogen biosynthesis, granulosa cells during the estrous cycle (in rodents), and in corpora luteum in both rodents ( 106) and humans (107). LRH-1 potently transactivates both CYP19 ( 106) and hydroxysteroid dehydrogenase ( 107). Thus, LHR-1 may have a more prominent role than SF-1 in regulating luteal steroidogenesis, e.g. during pregnancy.
Strong evidence for a developmental role of SF-1 comes from studies of forced SF-1 expression in several different SF-1-negative cell types in primary culture. In the first of these studies, embryonic stem cells were induced to express the cholesterol side-chain cleavage enzyme after the forced expression of SF-1 ( 108). Subsequently, mesenchymal cells from mouse and human bone marrow cells were transformed into cells that expressed most of the enzymes required for steroid biosynthesis after the forced expression of SF-1. These cells synthesized steroid hormones de novo, including progesterone, corticosterone, cortisol, dehydroepiandrosterone, testosterone, and estradiol in response to ACTH. In the human cells, SF-1 dramatically induced the expression of the receptors for both ACTH and LH ( 109, 110). Similar results were obtained using adipose tissue-derived mesenchymal cells, but in these cells, adrenal steroid biosynthesis (e.g. corticosterone) predominated, whereas gonadal steroids (e.g. testosterone) were more apparent in bone marrow-derived cells ( 111). These results suggest the potential utility of adipose tissue-derived mesenchymal cells for autologous cell regeneration therapy for patients with adrenal insufficiency.
Although it was anticipated that SF-1 knockout mice might provide in vivo evidence for the importance of SF-1 in gene expression, particularly for those genes involved in steroidogenesis, the failure of the knockout mice to develop the steroidogenic organs and the ventromedial hypothalamus (see below) precluded such analyses in these tissues. However, supporting in vivo roles of SF-1 have been provided for several genes listed in Table 1 including AMH ( 112, 113) and LHB (114).
Gene targets of SF-1 in pituitary and hypothalamus
The finding of SF-1 expression in pituitary gonadotropes and in the VMH (see above) prompted a search for SF-1-regulated genes in these tissues. A series of in vitro DNA-binding assays and reporter gene assays of promoter activity suggested that the expression of the α-subunit of glycoprotein hormones [α-GSU ( 12)], LH ß (115), the GnRH receptor ( 116), FSH ß (117) in pituitary gonadotropes were all regulated by SF-1 As discussed below, SF-1 clearly plays an important role in the expression of these genes in vivo as well.
In silico analyses of putative SF-1-binding sites among VMH-enriched transcripts, followed by promoter activity assays in vitro, suggested roles for SF-1 in the expression of the N-methyl-d-aspartate receptor, the cell adhesion molecules Amigo2, Cdh4, Sema3a, Slit3, and Netrin3, and other genes within the VMH, including Fezf1, Nptx2, Nkx2-2, and A2bp1 ( 118). It remains to be determined whether the SF-1-dependent expression of these transcripts can be confirmed in the VMH-specific SF-1 knockout mice discussed below.
SF-1 as a Regulator of Differentiation and Development—Lessons from Knockout Mouse Models
Total disruption of SF-1
Consistent with the hypothesis that SF-1 is required for adrenal and gonadal steroidogenesis, SF-1 knockout mice die shortly after birth from adrenocortical insufficiency and exhibit male-to-female sex reversal of the external genitalia. However, these abnormalities are not due to poor expression of steroidogenic enzymes, but instead to complete absence of the adrenal glands and gonads ( 119, 120). The initial stages of adrenal and gonadal development occur in the absence of SF-1 but subsequently regress. Because their gonads regress before male sexual differentiation normally occurs, the internal and external urogenital tracts of SF-1 knockout mice are female, irrespective of genetic sex. Heterozygous SF-1 knockout mice have decreased adrenal volume associated with impaired corticosterone production in response to stress ( 121).
The gonadotropes of SF-1 knockout mice also have impaired expression of a number of gene products that regulate reproduction, including LH-ß, FSH-ß, αGSU, and the receptor for GnRH ( 13, 122). Moreover, knockout mice lack the VMH, a hypothalamic region linked to feeding and appetite regulation and female reproductive behavior ( 122, 123). Finally, although the functional consequences remain to be defined, the SF-1 knockout mice have defects in their splenic parenchyma ( 15).
Tissue-specific knockout of SF-1
Anterior pituitary gland
Because mice with SF-1 completely disrupted are globally deficient in SF-1, they die in the immediate postnatal period without corticosteroid replacement and cannot be used to delineate the roles of SF-1 at specific sites of expression. Therefore the Cre/loxP system has been used to produce tissue-specific knockouts of SF-1. With use of a transgenic mouse line in which Cre recombinase expression is directed to the anterior pituitary gland by the 5′-flanking sequences of the α-subunit of glycoprotein hormones, mice with pituitary-specific disruption of SF-1 have been generated. These αGSU-Cre/loxP mice selectively lack SF-1 immunoreactivity in the anterior pituitary ( 124) but have normal levels at other sites, including the adrenal cortex and VMH. These mice have markedly diminished levels of pituitary gonadotropins and exhibit severe hypoplasia of the gonads and external genitalia. In males, the testes exhibit some signs of differentiation and development; however, germ cell numbers are considerably lower than normal and fail to progress to mature spermatids. Leydig cells are markedly reduced in number and are devoid of the histological features of steroidogenesis. In females, the ovaries develop through the antral stage but do not produce large preovulatory follicles or corpora lutea. These results confirm earlier promoter-driven reporter gene studies indicating that SF-1 was required for the expression of the gonadotropins and the GnRH receptor (reviewed in Ref. 4) and demonstrate that the local production of SF-1 in mice is essential for normal pituitary gonadotrope function.
Gonads
An anti-Müllerian hormone type 2 receptor-driven Cre recombinase transgene was used to generate mice with SF-1 specifically disrupted in testicular Leydig cells and ovarian granulosa cells ( 125). In the SF1-disrupted males, the testes failed to descend and were structurally abnormal and hypoplastic. Two hallmarks of Leydig cell function, CYP11A and STAR expression, were impaired, indicating a defect in androgen biosynthesis. In females, ovaries were hypoplastic; adults were sterile and the ovaries had reduced numbers of oocytes and lacked corpora lutea ( 126). These observations thus provide compelling evidence for the essential role of SF-1 in normal reproductive function.
Central nervous system (CNS)
To assess functions of SF-1 within the VMH, SF-1 was ablated within the CNS (the VMH being the sole site of SF-1 expression within the CNS) using a nestin-Cre transgene ( 127). Adrenals, gonads, and pituitaries all developed and functioned normally in these mice, but the VMH nuclei were disrupted.
The VMH may be part of a medial hypothalamic defensive system mediating responses to predators ( 128). Consistent with this idea, these VMH-disrupted mice have increased anxiety-like behavior using many different tests, and their mediobasal hypothalami have decreased expression or altered distribution of genes implicated in anxiety-like behavior including those encoding brain-derived neurotrophic factor, the type 2 CRH receptor (CRHR2), and urocortin 3. The BDNF ( 129) and CRHR2 genes both contain known SF-1-binding sites, suggesting that both are direct transcriptional targets of SF-1.
The VMH is also important for CNS regulation of appetite and energy homeostasis (see Ref. 130 for review). VMH neurons are implicated in satiety sensing, increasing excitatory input to proopiomelanocortin neurons in the arcuate nucleus and thereby activating anorexigenic neural circuits. In particular, SF-1-expressing neurons in the VMH make essential contributions to energy homeostasis as regulated by leptin and glucose signals. The cannabinoid receptor 1 (CB1R), which is associated with hypothalamic regulation of energy homeostasis, is highly expressed and functional in SF-1 neurons of the VMH and requires SF-1 for this expression. CB1R agonists administered systemically normally increase nocturnal food consumption in a partial satiety state but have blunted activities in CNS-specific SF-1 knockout mice. Conversely, the anorexic effect of a CB1R antagonist is significantly diminished in these mice, arguing that regulatory roles of SF-1 on CB1R expression in the VMH play an important role in cannabinoid-induced effects on energy intake.
SF-1 and Human Disease
Mutations of SF-1
In contrast to homozygous SF-1 null mice, which can be kept alive with glucocorticoid replacement, no human has been identified who is homozygous for null mutations in the NR5A1 gene encoding SF-1. The first two SF-1-defective patients identified were heterozygous for mutations that abolished the ability of SF-1 to bind DNA, thus rendering the protein transcriptionally inactive without creating a dominant-negative effect. Both affected patients had adrenal insufficiency; one was a phenotypic female XY infant, and the other was an apparently normal XX female who was not diagnosed with adrenal insufficiency until 14 months of age ( 131, 132). A third infant, who had adrenal failure and complete 46,XY sex reversal, was homozygous for a mutation that altered a highly conserved residue in a secondary DBD that causes partial loss of DNA binding and transcriptional activity ( 133). Heterozygous carriers of this mutation were normal; thus, heterozygosity for apparently null mutations or homozygosity for a mutation that only partially reduces activity yield similar phenotypes.
These data suggested that, in humans, male gonad development and adrenal development in both sexes required SF-1 expression in a dosage-sensitive manner. However, heterozygous SF-1 mutations subsequently were identified mainly among undervirilized 46,XY individuals (i.e. XY disorders of sexual development) who do not have adrenal insufficiency ( 134). Thus, the SF-1 genotype alone seems insufficient to explain why some patients have adrenal insufficiency, whereas others do not.
Additionally, SF-1 mutations impairing transcriptional activity have been identified among 46,XX women with premature ovarian failure in four kindreds with histories of both 46,XY disorders of sex development and 46,XX primary ovarian insufficiency and in two of 25 subjects with sporadic ovarian insufficiency. None of these subjects had clinically apparent adrenal insufficiency. A range of ovarian anomalies was found including 46,XX gonadal dysgenesis and primary ovarian insufficiency ( 135).
The adverse effects of heterozygous SF-1 mutations on reproductive function in both males and females could explain the failure to identify humans who are homozygous for null mutations, although it remains possible that such a condition is embryonically lethal in humans, perhaps (for example) owing to adverse effects on CNS development.
Dysregulation of SF-1
Endometriosis
SF-1 may play a significant role in the pathogenesis of endometriosis; it is undetectable in normal endometrial stromal cells but is expressed in endometriotic cells. This seems to be due, in part, to hypomethylation of a CpG-rich region in the proximal promoter region of the gene (see above). Expression of SF-1 leads to aberrant expression of the STAR and CYP19 genes, which, in turn, increase the endogenous synthesis of estrogen within endometriotic tissue, a key causative factor in the disease. Additionally, the E-box sequence (CACGTG) at −82/−77 in the SF-1 promoter is required for its activity in endometriotic stromal cells as in most other cell types. This element interacts with upstream stimulatory factors (USF) 1 and 2; USF2 mRNA and immunoreactive USF2 levels are increased in endometriotic tissues compared with normal endometrium, and knockdown of USF2 abolishes the overexpression of SF-1 (for review see Ref. 136).
Adrenocortical carcinoma
Because deficiency of SF-1 causes adrenal agenesis, it would not be surprising if SF-1 overexpression was associated, conversely, with adrenocortical overgrowth. In a cohort of children with adrenocortical carcinoma from Brazil (where this condition occurs relatively frequently), the 9q33.3 chromosomal region carrying the NR5A1 gene is amplified in almost all cases, and NR5A1 gene copy number is increased ( 137). SF-1 protein is overexpressed as well, but levels of expression do not correlate with gene copy number or with clinical grade ( 138). The latter fact implies that SF-1 overexpression is permissive for tumorigenesis but not related to malignant progression.
Conclusions
Since its discovery in 1992, much has been learned about the roles of SF-1 in gene expression, differentiation and development, and disease. Nevertheless, many of the questions that were raised 5 yr after its discovery ( 4) remain unresolved today. Some of these are touched on below along with new questions raised as the result of recent progress.
What mechanisms regulate the expression of SF-1?
Whereas specific genetic regions have been identified as necessary for SF-1 expression in the gonads, fetal adrenal gland, pituitary gonadotropes, and VMH (see above), the regions required for SF-1 expression in the adult adrenal cortex are as yet unknown, as are many of the transacting factors that presumably regulate the ordered expression of SF-1 in the various tissues.
What mechanisms regulate SF-1 function?
SF-1 clearly is a master regulator of transcription that directs cell-selective gene expression within the endocrine system; however, its activity is regulated by phospholipids, by a large array of transcription factors and coregulators, and by posttranscriptional modification. Whether phospholipids ligands serve as SF-1 activators or merely as stabilizers of SF-1 function is uncertain and although a regulatory role for sphingosine as an inhibitory ligand is somewhat more compelling, it remains to be determined whether its actions can be extended beyond a simple reporter gene assay in a specific cell line. Furthermore, the fact that SF-1 interacts with such a large number of proteins that modify its activity in vitro (Table 2) suggests a promiscuity that seems inconsistent with the tissue-specific functions of SF-1. Ultimately, these interactions must be placed in a context that explains the temporal as well as the tissue-specific and gene-specific effects of SF-1.
What contributions does SF-1 make to appetite control and anxiety?
The VMH-specific knockout of SF-1 in mice leads to appetite and anxiety disorders; however, it is not clear whether SF-1 function in this context is merely permissive and related to the development of the VMH, or whether SF-1 regulates the expression of genes in the VMH that directly contribute to the aberrant phenotypes.
How do SF-1 mutations in humans impact on phenotype?
The apparent discordance in individual phenotypes produced by SF-1 mutations that disrupt SF-1 function requires further investigation. Although the SF-1 mutations described to date have been found in patients with adrenal and/or gonadal deficiency, one wonders if new mutations will be found with phenotypes resulting from effects at other sites, e.g. the VMH or spleen.
Acknowledgments
We respectfully dedicate this paper to our late friend and colleague, Keith Parker.
NURSA Molecule Pages:
Nuclear Receptors: SF-1.
Footnotes
This work was supported by Grant MOP-64325 from the Canadian Institutes of Health Research (to B.P.S.) P.C.W. is the Audry Newman Rapoport Distinguished Chair in Pediatric Endocrinology.
Disclosure Summary: P.C.W. has nothing to disclose. B.P.S. receives royalties from McGraw-Hill Inc. for his contributions as an author of Goodman and Gilman’s The Pharmacological Basis of Therapeutics (11th ed. New York; McGraw-Hill, Inc.).
First Published Online March 4, 2010
Abbreviations: AHC, Adrenal hypoplasia congenital; AMH, anti-Müllerian hormone (Müllerian-inhibiting substance); CB1R, cannabinoid receptor 1; CDK, cyclin-dependent kinase; CNS, central nervous system; CYP, cytochrome P450; DAX-1, dosage-sensitive sex reversal-AHC critical region on the X chromosome gene 1; DBD, DNA-binding domain; α-GSU, glycoprotein hormone α-subunit; MC2R, melanocortin receptor 2 (corticotropin receptor); SF-1, steroidogenic factor 1; SOX, SRY (sex determining region Y)-box; SRA, steroid receptor RNA activator; StAR, steroidogenic acute regulatory protein; SUMO, small ubiquitin-related modifier; USF, upstream stimulatory factor; VMH, ventromedial hypothalamus.
References
- 1.Parker KL, Chaplin DD, Wong M, Seidman JG, Smith JA, Schimmer BP1985. Expression of murine 21-hydroxylase in mouse adrenal glands and in transfected Y1 adrenocortical tumor cells. Proc Natl Acad Sci USA 82:7860–7864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Handler JD, Schimmer BP, Flynn TR, Szyf M, Seidman JG, Parker KL1988. An enhancer element and a functional cyclic AMP-dependent protein kinase are required for expression of adrenocortical 21-hydroxylase. J Biol Chem 263:13068–13073 [PubMed] [Google Scholar]
- 3.Parker KL, Schimmer BP, Chaplin DD, Seidman JG1986. Characterization of a regulatory region of the steroid 21-hydroxylase gene. J Biol Chem 261:15353–15355 [PubMed] [Google Scholar]
- 4.Parker KL, Schimmer BP1997. Steroidogenic factor 1: a key determinant of endocrine development and function. Endocr Rev 18:361–377 [DOI] [PubMed] [Google Scholar]
- 5.Lala DS, Rice DA, Parker KL1992. Steroidogenic factor 1, a key regulator of steroidogenic enzyme expression, is the mouse homolog of Fushi Tarazu-Factor 1. Mol Endocrinol 6:1249–1258 [DOI] [PubMed] [Google Scholar]
- 6.Honda S, Morohashi K, Nomura M, Takeya H, Kitajima M, Omura T1993. Ad4BP regulating steroidogenic P-450 gene is a member of steroid hormone recepter superfamily. J Biol Chem 268:7494–7502 [PubMed] [Google Scholar]
- 7.Little TH, Zhang Y, Matulis CK, Weck J, Zhang Z, Ramachandran A, Mayo KE, Radhakrishnan I2006. Sequence-specific deoxyribonucleic acid (DNA) recognition by steroidogenic factor 1: a helix at the carboxy terminus of the DNA binding domain is necessary for complex stability. Mol Endocrinol 20:831–843 [DOI] [PubMed] [Google Scholar]
- 8.Ikeda Y, Lala DS, Luo X, Kim E, Moisan MP, Parker KL1993. Characterization of the mouse FTZ-F1 gene, which encodes a key regulator of steroid hydroxylase gene expression. Mol Endocrinol 7:852–860 [DOI] [PubMed] [Google Scholar]
- 9.Morohashi K, Iida H, Nomura M, Hatano O, Honda S, Tsukiyama T, Niwa O, Hara T, Takakusu A, Shibata Y, Omura T1994. Functional difference between Ad4BP and ELP, and their distributions in steroidogenic tissues. Mol Endocrinol 8:643–653 [DOI] [PubMed] [Google Scholar]
- 10.Morohashi Ki1999. Gonadal and extragonadal functions of Ad4BP/SF-1: developmental aspects. Trends Endocrinol Metab 10:169–173 [DOI] [PubMed] [Google Scholar]
- 11.Ramayya MS, Zhou J, Kino T, Segars JH, Bondy CA, Chrousos GP1997. Steroidogenic factor 1 messenger ribonucleic acid expression in steroidogenic and nonsteroidogenic human tissues: Northern blot and in situ hybridization studies. J Clin Endocrinol Metab 82:1799–1806 [DOI] [PubMed] [Google Scholar]
- 12.Barnhart KM, Mellon PL1994. The orphan nuclear receptor, steroidogenic factor-1, regulates the glycoprotein hormone α-subunit gene in pituitary gonadotropes. Mol Endocrinol 8:878–885 [DOI] [PubMed] [Google Scholar]
- 13.Ingraham HA, Lala DS, Ikeda Y, Luo X, Shen WH, Nachtigal MW, Abbud R, Nilson JH, Parker KL1994. The nuclear receptor steroidogenic factor 1 acts at multiple levels of the reproductive axis. Genes Dev 8:2302–2312 [DOI] [PubMed] [Google Scholar]
- 14.Ngan ES, Cheng PK, Leung PC, Chow BK1999. Steroidogenic factor-1 interacts with a gonadotrope-specific element within the first exon of the human gonadotropin-releasing hormone receptor gene to mediate gonadotrope-specific expression. Endocrinology 140:2452–2462 [DOI] [PubMed] [Google Scholar]
- 15.Morohashi K, Tsuboi-Asai H, Matsushita S, Suda M, Nakashima M, Sasano H, Hataba Y, Li CL, Fukata J, Irie J, Watanabe T, Nagura H, Li E1999. Structural and functional abnormalities in the spleen of an mFTZ–F1 gene-disrupted mouse. Blood 93:1586–1594 [PubMed] [Google Scholar]
- 16.Wehrenberg U, Prange-Kiel J, Rune GM2001. Steroidogenic factor-1 expression in marmoset and rat hippocampus: co-localization with StAR and aromatase. J Neurochem 76:1879–1886 [DOI] [PubMed] [Google Scholar]
- 17.Hanley NA, Ball SG, Clement-Jones M, Hagan DM, Strachan T, Lindsay S, Robson S, Ostrer H, Parker KL, Wilson DI1999. Expression of steroidogenic factor 1 and Wilms’ tumour 1 during early human gonadal development and sex determination. Mech Dev 87:175–180 [DOI] [PubMed] [Google Scholar]
- 18.Hanley NA, Rainey WE, Wilson DI, Ball SG, Parker KL2001. Expression profiles of SF-1, DAX1, and CYP17 in the human fetal adrenal gland: potential interactions in gene regulation. Mol Endocrinol 15:57–68 [DOI] [PubMed] [Google Scholar]
- 19.Ikeda Y, Shen WH, Ingraham HA, Parker KL1994. Developmental expression of mouse steroidogenic factor-1, an essential regulator of the steroid hydroxylases. Mol Endocrinol 8:654–662 [DOI] [PubMed] [Google Scholar]
- 20.Morohashi K, Hatano O, Nomura M, Takayama K, Hara M, Yoshii H, Takakusu A, Omura T1995. Function and distribution of a steroidogenic cell-specific transcription factor, Ad4BP. J Steroid Biochem Mol Biol 53:81–88 [DOI] [PubMed] [Google Scholar]
- 21.Shen JH, Ingraham HA2002. Regulation of the orphan nuclear receptor steroidogenic factor 1 by Sox proteins. Mol Endocrinol 16:529–540 [DOI] [PubMed] [Google Scholar]
- 22.Daggett MA, Rice DA, Heckert LL2000. Expression of steroidogenic factor 1 in the testis requires an E box and CCAAT box in its promoter proximal region. Biol Reprod 62:670–679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harris AN, Mellon PL1998. The basic helix-loop-helix, leucine zipper transcription factor, USF (upstream stimulatory factor), is a key regulator of SF-1 (steroidogenic factor-1) gene expression in pituitary gonadotrope and steroidogenic cells. Mol Endocrinol 12:714–726 [DOI] [PubMed] [Google Scholar]
- 24.Nomura M, Bärtsch S, Nawata H, Omura T, Morohashi K1995. An E box element is required for the expression of the ad4bp gene, a mammalian homologue of ftz-f1 gene, which is essential for adrenal and gonadal development. J Biol Chem 270:7453–7461 [DOI] [PubMed] [Google Scholar]
- 25.Scherrer SP, Rice DA, Heckert LL2002. Expression of steroidogenic factor 1 in the testis requires an interactive array of elements within its proximal promoter. Biol Reprod 67:1509–1521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Woodson KG, Crawford PA, Sadovsky Y, Milbrandt J1997. Characterization of the promoter of SF-1, an orphan nuclear receptor required for adrenal and gonadal development. Mol Endocrinol 11:117–126 [DOI] [PubMed] [Google Scholar]
- 27.Tamura M, Kanno Y, Chuma S, Saito T, Nakatsuji N2001. Pod-1/Capsulin shows a sex- and stage-dependent expression pattern in the mouse gonad development and represses expression of Ad4BP/SF-1. Mech Dev 102:135–144 [DOI] [PubMed] [Google Scholar]
- 28.Tremblay JJ, Viger RS2001. GATA factors differentially activate multiple gonadal promoters through conserved GATA regulatory elements. Endocrinology 142:977–986 [DOI] [PubMed] [Google Scholar]
- 29.Wilhelm D, Englert C2002. The Wilms tumor suppressor WT1 regulates early gonad development by activation of Sf1. Genes Dev 16:1839–1851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sakai N, Terami H, Suzuki S, Haga M, Nomoto K, Tsuchida N, Morohashi K, Saito N, Asada M, Hashimoto M, Harada D, Asahara H, Ishikawa T, Shimada F, Sakurada K2008. Identification of NR5A1 (SF-1/AD4BP) gene expression modulators by large-scale gain and loss of function studies. J Endocrinol 198:489–497 [DOI] [PubMed] [Google Scholar]
- 31.Katoh-Fukui Y, Owaki A, Toyama Y, Kusaka M, Shinohara Y, Maekawa M, Toshimori K, Morohashi K2005. Mouse Polycomb M33 is required for splenic vascular and adrenal gland formation through regulating Ad4BP/SF1 expression. Blood 106:1612–1620 [DOI] [PubMed] [Google Scholar]
- 32.Biason-Lauber A, Konrad D, Meyer M, DeBeaufort C, Schoenle EJ2009. Ovaries and female phenotype in a girl with 46,XY karyotype and mutations in the CBX2 gene. Am J Hum Genet 84:658–663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stallings NR, Hanley NA, Majdic G, Zhao L, Bakke M, Parker KL2002. Development of a transgenic green fluorescent protein lineage marker for steroidogenic factor 1. Mol Endocrinol 16:2360–2370 [DOI] [PubMed] [Google Scholar]
- 34.Karpova T, Presley J, Manimaran RR, Scherrer SP, Tejada L, Peterson KR, Heckert LL2005. A FTZ-F1-containing yeast artificial chromosome recapitulates expression of steroidogenic factor 1 in vivo Mol Endocrinol 19:2549–2563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shima Y, Zubair M, Ishihara S, Shinohara Y, Oka S, Kimura S, Okamoto S, Minokoshi Y, Suita S, Morohashi K2005. Ventromedial hypothalamic nucleus-specific enhancer of Ad4BP/SF-1 gene. Mol Endocrinol 19:2812–2823 [DOI] [PubMed] [Google Scholar]
- 36.Shima Y, Zubair M, Komatsu T, Oka S, Yokoyama C, Tachibana T, Hjalt TA, Drouin J, Morohashi K2008. Pituitary homeobox 2 regulates adrenal4 binding protein/steroidogenic factor-1 gene transcription in the pituitary gonadotrope through interaction with the intronic enhancer. Mol Endocrinol 22:1633–1646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zubair M, Ishihara S, Oka S, Okumura K, Morohashi K2006. Two-step regulation of Ad4BP/SF-1 gene transcription during fetal adrenal development: initiation by a Hox-Pbx1-Prep1 complex and maintenance via autoregulation by Ad4BP/SF-1. Mol Cell Biol 26:4111–4121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zubair M, Oka S, Parker KL, Morohashi K2009. Transgenic expression of Ad4BP/SF-1 in fetal adrenal progenitor cells leads to ectopic adrenal formation. Mol Endocrinol 23:1657–1667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zubair M, Parker KL, Morohashi K2008. Developmental links between the fetal and adult zones of the adrenal cortex revealed by lineage tracing. Mol Cell Biol 28:7030–7040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xue Q, Lin Z, Yin P, Milad MP, Cheng YH, Confino E, Reierstad S, Bulun SE2007. Transcriptional activation of steroidogenic factor-1 by hypomethylation of the 5′ CpG island in endometriosis. J Clin Endocrinol Metab 92:3261–3267 [DOI] [PubMed] [Google Scholar]
- 41.Hoivik EA, Aumo L, Aesoy R, Lillefosse H, Lewis AE, Perrett RM, Stallings NR, Hanley NA, Bakke M2008. Deoxyribonucleic acid methylation controls cell type-specific expression of steroidogenic factor 1. Endocrinology 149:5599–5609 [DOI] [PubMed] [Google Scholar]
- 42.Mohn F, Schübeler D2009. Genetics and epigenetics: stability and plasticity during cellular differentiation. Trends Genet 25:129–136 [DOI] [PubMed] [Google Scholar]
- 43.Ishihara SL, Morohashi K2005. A boundary for histone acetylation allows distinct expression patterns of the Ad4BP/SF-1 and GCNF loci in adrenal cortex cells. Biochem Biophys Res Commun 329:554–562 [DOI] [PubMed] [Google Scholar]
- 44.Sewer MB, Dammer EB, Jagarlapudi S2007. Transcriptional regulation of adrenocortical steroidogenic gene expression. Drug Metab Rev 39:371–388 [DOI] [PubMed] [Google Scholar]
- 45.Cao G, Zhao L, Stangl H, Hasegawa T, Richardson JA, Parker KL, Hobbs HH1999. Developmental and hormonal regulation of murine scavenger receptor, class B, type 1. Mol Endocrinol 13:1460–1473 [DOI] [PubMed] [Google Scholar]
- 46.Lopez D, Sandhoff TW, McLean MP1999. Steroidogenic factor-1 mediates cyclic 3′,5′-adenosine monophosphate regulation of the high density lipoprotein receptor. Endocrinology 140:3034–3044 [DOI] [PubMed] [Google Scholar]
- 47.Naville D, Barjhoux L, Jaillard C, Lebrethon MC, Saez JM, Bègeot M1994. Characterization of the transcription start site of the ACTH receptor gene: presence of an intronic sequence in the 5′-flanking region. Mol Cell Endocrinol 106:131–135 [DOI] [PubMed] [Google Scholar]
- 48.Winnay JN, Hammer GD2006. Adrenocorticotropic-mediated signaling cascades coordinate a cyclic pattern of steroidogenic factor-1-dependent transcriptional activation. Mol Endocrinol 20:147–166 [DOI] [PubMed] [Google Scholar]
- 49.Ito M, Park Y, Weck J, Mayo KE, Jameson JL2000. Synergistic activation of the inhibin α-promoter by steroidogenic factor-1 and cyclic adenosine 3′,5′-monophosphate. Mol Endocrinol 14:66–81 [DOI] [PubMed] [Google Scholar]
- 50.Aesøy R, Mellgren G, Morohashi K, Lund J2002. Activation of cAMP-dependent protein kinase increases the protein level of steroidogenic factor-1. Endocrinology 143:295–303 [DOI] [PubMed] [Google Scholar]
- 51.Lopez D, Nackley AC, Shea-Eaton W, Xue J, Schimmer BP, McLean MP2001. Effects of mutating different steroidogenic factor-1 protein regions on gene regulation. Endocrine 14:353–362 [DOI] [PubMed] [Google Scholar]
- 52.Li D, Urs AN, Allegood J, Leon A, Merrill Jr AH, Sewer MB2007. Cyclic AMP-stimulated interaction between steroidogenic factor 1 and diacylglycerol kinase θ facilitates induction of CYP17. Mol Cell Biol 27:6669–6685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Urs AN, Dammer E, Sewer MB2006. Sphingosine regulates the transcription of CYP17 by binding to steroidogenic factor-1. Endocrinology 147:5249–5258 [DOI] [PubMed] [Google Scholar]
- 54.Chen WY, Juan LJ, Chung BC2005. SF-1 (nuclear receptor 5A1) activity is activated by cyclic AMP via p300-mediated recruitment to active foci, acetylation, and increased DNA binding. Mol Cell Biol 25:10442–10453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fan W, Yanase T, Wu Y, Kawate H, Saitoh M, Oba K, Nomura M, Okabe T, Goto K, Yanagisawa J, Kato S, Takayanagi R, Nawata H2004. Protein kinase A potentiates adrenal 4 binding protein/steroidogenic factor 1 transactivation by reintegrating the subcellular dynamic interactions of the nuclear receptor with its cofactors, general control nonderepressed-5/transformation/transcription domain-associated protein, and suppressor, dosage-sensitive sex reversal-1: a laser confocal imaging study in living KGN cells. Mol Endocrinol 18:127–141 [DOI] [PubMed] [Google Scholar]
- 56.Chau YM, Crawford PA, Woodson KG, Polish JA, Olson LM, Sadovsky Y1997. Role of steroidogenic-factor 1 in basal and 3′,5′-cyclic adenosine monophosphate-mediated regulation of cytochrome P450 side-chain cleavage enzyme in the mouse. Biol Reprod 57:765–771 [DOI] [PubMed] [Google Scholar]
- 57.Nomura M, Kawabe K, Matsushita S, Oka S, Hatano O, Harada N, Nawata H, Morohashi K1998. Adrenocortical and gonadal expression of the mammalian Ftz-F1 gene encoding Ad4BP/SF-1 is independent of pituitary control. J Biochem 124:217–224 [DOI] [PubMed] [Google Scholar]
- 58.Mamluk R, Greber Y, Meidan R1999. Hormonal regulation of messenger ribonucleic acid expression for steroidogenic factor-1, steroidogenic acute regulatory protein, and cytochrome P450 side-chain cleavage in bovine luteal cells. Biol Reprod 60:628–634 [DOI] [PubMed] [Google Scholar]
- 59.Zhang P, Mellon SH1996. The orphan nuclear receptor steroidogenic factor-1 regulates the cyclic adenosine 3′,5′-monophosphate-mediated transcriptional activation of rat cytochrome P450c17 (17a-hydroxylase/c17-20 lyase). Mol Endocrinol 10:147–158 [DOI] [PubMed] [Google Scholar]
- 60.Hammer GD, Krylova I, Zhang Y, Darimont BD, Simpson K, Weigel NL, Ingraham HA1999. Phosphorylation of the nuclear receptor SF-1 modulates cofactor recruitment: integration of hormone signaling in reproduction and stress. Mol Cell 3:521–526 [DOI] [PubMed] [Google Scholar]
- 61.Desclozeaux M, Krylova IN, Horn F, Fletterick RJ, Ingraham HA2002. Phosphorylation and intramolecular stabilization of the ligand binding domain in the nuclear receptor steroidogenic factor 1. Mol Cell Biol 22:7193–7203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fowkes RC, Desclozeaux M, Patel MV, Aylwin SJ, King P, Ingraham HA, Burrin JM2003. Steroidogenic factor-1 and the gonadotrope-specific element enhance basal and pituitary adenylate cyclase-activating polypeptide-stimulated transcription of the human glycoprotein hormone α-subunit gene in gonadotropes. Mol Endocrinol 17:2177–2188 [DOI] [PubMed] [Google Scholar]
- 63.Lin BC, Suzawa M, Blind RD, Tobias SC, Bulun SE, Scanlan TS, Ingraham HA2009. Stimulating the GPR30 estrogen receptor with a novel tamoxifen analogue activates SF-1 and promotes endometrial cell proliferation. Cancer Res 69:5415–5423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lewis AE, Rusten M, Hoivik EA, Vikse EL, Hansson ML, Wallberg AE, Bakke M2008. Phosphorylation of steroidogenic factor 1 is mediated by cyclin-dependent kinase 7. Mol Endocrinol 22:91–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Angers S, Moon RT2009. Proximal events in Wnt signal transduction. Nat Rev Mol Cell Biol 10:468–477 [DOI] [PubMed] [Google Scholar]
- 66.Kim AC, Reuter AL, Zubair M, Else T, Serecky K, Bingham NC, Lavery GG, Parker KL, Hammer GD2008. Targeted disruption of β-catenin in Sf1-expressing cells impairs development and maintenance of the adrenal cortex. Development 135:2593–2602 [DOI] [PubMed] [Google Scholar]
- 67.Cohen Jr MM2003. The hedgehog signaling network. Am J Med Genet A 123A:5–28 [DOI] [PubMed]
- 68.Philipp M, Caron MG2009. Hedgehog signaling: is Smo a G protein-coupled receptor? Curr Biol 19:R125–R127 [DOI] [PubMed]
- 69.Yao HH, Whoriskey W, Capel B2002. Desert Hedgehog/Patched 1 signaling specifies fetal Leydig cell fate in testis organogenesis. Genes Dev 16:1433–1440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Barsoum IB, Bingham NC, Parker KL, Jorgensen JS, Yao HH2009. Activation of the Hedgehog pathway in the mouse fetal ovary leads to ectopic appearance of fetal Leydig cells and female pseudohermaphroditism. Dev Biol 329:96–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wilson TE, Fahrner TJ, Milbrandt J1993. The orphan receptors NGFI-B and steroidogenic factor 1 establish monomer binding as a third paradigm of nuclear receptor-DNA interaction. Mol Cell Biol 13:5794–5804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hammer GD, Parker KL, Schimmer BP2005. Transcriptional regulation of adrenocortical development. Endocrinology 146:1018–1024 [DOI] [PubMed] [Google Scholar]
- 73.Dammer EB, Leon A, Sewer MB2007. Coregulator exchange and sphingosine-sensitive cooperativity of steroidogenic factor-1, general control nonderepressed 5, p54, and p160 coactivators regulate cyclic adenosine 3′,5′-monophosphate-dependent cytochrome P450c17 transcription rate. Mol Endocrinol 21:415–438 [DOI] [PubMed] [Google Scholar]
- 74.Parker KL, Schimmer BP2002. Genes essential for early events in gonadal development. Ann Med 34:171–178 [PubMed] [Google Scholar]
- 75.Ikeda Y, Swain A, Weber TJ, Hentges KE, Zanaria E, Lalli E, Tamai KT, Sassone-Corsi P, Lovell-Badge R, Camerino G, Parker KL1996. Steroidogenic factor 1 and Dax-1 colocalize in multiple cell lineages: potential links in endocrine development. Mol Endocrinol 10:1261–1272 [DOI] [PubMed] [Google Scholar]
- 76.Ito M, Yu R, Jameson JL1997. DAX-1 inhibits SF-1-mediated transactivation via a carboxy-terminal domain that is deleted in adrenal hypoplasia congenita. Mol Cell Biol 17:1476–1483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Suzuki T, Kasahara M, Yoshioka H, Morohashi K, Umesono K2003. LXXLL-related motifs in Dax-1 have target specificity for the orphan nuclear receptors Ad4BP/SF-1 and LRH-1. Mol Cell Biol 23:238–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Crawford PA, Dorn C, Sadovsky Y, Milbrandt J1998. Nuclear receptor DAX-1 recruits nuclear receptor corepressor N-CoR to steroidogenic factor 1. Mol Cell Biol 18:2949–2956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gummow BM, Scheys JO, Cancelli VR, Hammer GD2006. Reciprocal regulation of a glucocorticoid receptor-steroidogenic factor-1 transcription complex on the Dax-1 promoter by glucocorticoids and adrenocorticotropic hormone in the adrenal cortex. Mol Endocrinol 20:2711–2723 [DOI] [PubMed] [Google Scholar]
- 80.Ragazzon B, Lefrançcois-Martinez AM, Val P, Sahut-Barnola I, Tournaire C, Chambon C, Gachancard-Bouya JL, Begue RJ, Veyssière G, Martinez A2006. Adrenocorticotropin-dependent changes in SF-1/DAX-1 ratio influence steroidogenic genes expression in a novel model of glucocorticoid-producing adrenocortical cell lines derived from targeted tumorigenesis. Endocrinology 147:1805–1818 [DOI] [PubMed] [Google Scholar]
- 81.Xu B, Yang WH, Gerin I, Hu CD, Hammer GD, Koenig RJ2009. Dax-1 and steroid receptor RNA activator (SRA) function as transcriptional coactivators for steroidogenic factor 1 in steroidogenesis. Mol Cell Biol 29:1719–1734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Komatsu T, Mizusaki H, Mukai T, Ogawa H, Baba D, Shirakawa M, Hatakeyama S, Nakayama KI, Yamamoto H, Kikuchi A, Morohashi K2004. Small ubiquitin-like modifier 1 (SUMO-1) modification of the synergy control motif of Ad4 binding protein/steroidogenic factor 1 (Ad4BP/SF-1) regulates synergistic transcription between Ad4BP/SF-1 and Sox9. Mol Endocrinol 18:2451–2462 [DOI] [PubMed] [Google Scholar]
- 83.Chen WY, Lee WC, Hsu NC, Huang F, Chung BC2004. SUMO modification of repression domains modulates function of nuclear receptor 5A1 (steroidogenic factor-1). J Biol Chem 279:38730–38735 [DOI] [PubMed] [Google Scholar]
- 84.Lee MB, Lebedeva LA, Suzawa M, Wadekar SA, Desclozeaux M, Ingraham HA2005. The DEAD-box protein DP103 (Ddx20 or Gemin-3) represses orphan nuclear receptor activity via SUMO modification. Mol Cell Biol 25:1879–1890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jacob AL, Lund J, Martinez P, Hedin L2001. Acetylation of steroidogenic factor 1 protein regulates its transcriptional activity and recruits the coactivator GCN5. J Biol Chem 276:37659–37664 [DOI] [PubMed] [Google Scholar]
- 86.Garcia-Dominguez M, Reyes JC2009. SUMO association with repressor complexes, emerging routes for transcriptional control. Biochim Biophys Acta 1789:451–459 [DOI] [PubMed] [Google Scholar]
- 87.Campbell LA, Faivre EJ, Show MD, Ingraham JG, Flinders J, Gross JD, Ingraham HA2008. Decreased recognition of SUMO-sensitive target genes following modification of SF-1 (NR5A1). Mol Cell Biol 28:7476–7486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yang WH, Heaton JH, Brevig H, Mukherjee S, Iñiguez-Lluhí JA, Hammer GD2009. SUMOylation inhibits SF-1 activity by reducing CDK7-mediated serine 203 phosphorylation. Mol Cell Biol 29:613–625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tremblay JJ, Robert NM, Laguë E2009. Nuclear receptors, testosterone, and posttranslational modifications in human INSL3 promoter activity in testicular Leydig cells. Ann NY Acad Sci 1160:205–212 [DOI] [PubMed] [Google Scholar]
- 90.Lala DS, Syka PM, Lazarchik SB, Mangelsdorf DJ, Parker KL, Heyman RA1997. Activation of the orphan nuclear receptor steroidogenic factor 1 by oxysterols. Proc Natl Acad Sci USA 94:4895–4900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Christenson LK, McAllister JM, Martin KO, Javitt NB, Osborne TF, Strauss III JF1998. Oxysterol regulation of steroidogenic acute regulatory protein gene expression. J Biol Chem 273:30729–30735 [DOI] [PubMed] [Google Scholar]
- 92.Mellon SH, Bair SR1998. 25-Hydroxycholesterol is not a ligand for the orphan nuclear receptor steroidogenic factor-1 (SF-1). Endocrinology 139:3026–3029 [DOI] [PubMed] [Google Scholar]
- 93.Krylova IN, Sablin EP, Moore J, Xu RX, Waitt GM, MacKay JA, Juzumiene D, Bynum JM, Madauss K, Montana V, Lebedeva L, Suzawa M, Williams JD, Williams SP, Guy RK, Thornton JW, Fletterick RJ, Willson TM, Ingraham HA2005. Structural analyses reveal phosphatidyl inositols as ligands for the NR5 orphan receptors SF-1 and LRH-1. Cell 120:343–355 [DOI] [PubMed] [Google Scholar]
- 94.Li Y, Choi M, Cavey G, Daugherty J, Suino K, Kovach A, Bingham NC, Kliewer SA, Xu HE2005. Crystallographic identification and functional characterization of phospholipids as ligands for the orphan nuclear receptor steroidogenic factor-1. Mol Cell 17:491–502 [DOI] [PubMed] [Google Scholar]
- 95.Sablin EP, Blind RD, Krylova IN, Ingraham JG, Cai F, Williams JD, Fletterick RJ, Ingraham HA2009. Structure of SF-1 bound by different phospholipids: evidence for regulatory ligands. Mol Endocrinol 23:25–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wang W, Zhang C, Marimuthu A, Krupka HI, Tabrizizad M, Shelloe R, Mehra U, Eng K, Nguyen H, Settachatgul C, Powell B, Milburn MV, West BL2005. The crystal structures of human steroidogenic factor-1 and liver receptor homologue-1. Proc Natl Acad Sci USA 102:7505–7510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Whitby RJ, Dixon S, Maloney PR, Delerive P, Goodwin BJ, Parks DJ, Willson TM2006. Identification of small molecule agonists of the orphan nuclear receptors liver receptor homolog-1 and steroidogenic factor-1. J Med Chem 49:6652–6655 [DOI] [PubMed] [Google Scholar]
- 98.Del Tredici AL, Andersen CB, Currier EA, Ohrmund SR, Fairbain LC, Lund BW, Nash N, Olsson R, Piu F2008. Identification of the first synthetic steroidogenic factor 1 inverse agonists: pharmacological modulation of steroidogenic enzymes. Mol Pharmacol 73:900–908 [DOI] [PubMed] [Google Scholar]
- 99.Aigueperse C, Val P, Pacot C, Darne C, Lalli E, Sassone-Corsi P, Veyssiere G, Jean C, Martinez A2001. SF-1 (steroidogenic factor-1), C/EBPβ (CCAAT/enhancer binding protein), and ubiquitous transcription factors NF1 (nuclear factor 1) and Sp1 (selective promoter factor 1) are required for regulation of the mouse aldose reductase-like gene (AKR1B7) expression in adrenocortical cells. Mol Endocrinol 15:93–111 [DOI] [PubMed] [Google Scholar]
- 100.Rui X, Tsao J, Scheys JO, Hammer GD, Schimmer BP2008. Contributions of specificity protein-1 and steroidogenic factor 1 to Adcy4 expression in Y1 mouse adrenal cells. Endocrinology 149:3668–3678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bassett MH, Zhang Y, Clyne C, White PC, Rainey WE2002. Differential regulation of aldosterone synthase and 11β-hydroxylase transcription by steroidogenic factor-1. J Mol Endocrinol 28:125–135 [DOI] [PubMed] [Google Scholar]
- 102.Frigeri C, Tsao J, Czerwinski W, Schimmer BP2000. Impaired steroidogenic factor 1 (NR5A1) activity in mutant Y1 mouse adrenocortical tumor cells. Mol Endocrinol 14:535–544 [DOI] [PubMed] [Google Scholar]
- 103.Tremblay JJ, Robert NM2005. Role of nuclear receptors in INSL3 gene transcription in Leydig cells. Ann NY Acad Sci 1061:183–189 [DOI] [PubMed] [Google Scholar]
- 104.Zimmermann S, Schwärzler A, Buth S, Engel W, Adham IM1998. Transcription of the Leydig insulin-like gene is mediated by steroidogenic factor-1. Mol Endocrinol 12:706–713 [DOI] [PubMed] [Google Scholar]
- 105.Fynn-Thompson E, Cheng H, Teixeira J2003. Inhibition of steroidogenesis in Leydig cells by Mullerian-inhibiting substance. Mol Cell Endocrinol 211:99–104 [DOI] [PubMed] [Google Scholar]
- 106.Hinshelwood MM, Repa JJ, Shelton JM, Richardson JA, Mangelsdorf DJ, Mendelson CR2003. Expression of LRH-1 and SF-1 in the mouse ovary: localization in different cell types correlates with differing function. Mol Cell Endocrinol 207:39–45 [DOI] [PubMed] [Google Scholar]
- 107.Peng N, Kim JW, Rainey WE, Carr BR, Attia GR2003. The role of the orphan nuclear receptor, liver receptor homologue-1, in the regulation of human corpus luteum 3β-hydroxysteroid dehydrogenase type II. J Clin Endocrinol Metab 88:6020–6028 [DOI] [PubMed] [Google Scholar]
- 108.Crawford PA, Sadovsky Y, Milbrandt J1997. Nuclear receptor steroidogenic factor 1 directs embryonic stem cells toward the steroidogenic lineage. Mol Cell Biol 17:3997–4006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gondo S, Yanase T, Okabe T, Tanaka T, Morinaga H, Nomura M, Goto K, Nawata H2004. SF-1/Ad4BP transforms primary long-term cultured bone marrow cells into ACTH-responsive steroidogenic cells. Genes Cells 9:1239–1247 [DOI] [PubMed] [Google Scholar]
- 110.Tanaka T, Gondo S, Okabe T, Ohe K, Shirohzu H, Morinaga H, Nomura M, Tani K, Takayanagi R, Nawata H, Yanase T2007. Steroidogenic factor 1/adrenal 4 binding protein transforms human bone marrow mesenchymal cells into steroidogenic cells. J Mol Endocrinol 39:343–350 [DOI] [PubMed] [Google Scholar]
- 111.Gondo S, Okabe T, Tanaka T, Morinaga H, Nomura M, Takayanagi R, Nawata H, Yanase T2008. Adipose tissue-derived and bone marrow-derived mesenchymal cells develop into different lineage of steroidogenic cells by forced expression of steroidogenic factor 1. Endocrinology 149:4717–4725 [DOI] [PubMed] [Google Scholar]
- 112.Giuili G, Shen WH, Ingraham HA1997. The nuclear receptor SF-1 mediates sexually dimorphic expression of Mullerian inhibiting substance, in vivo. Development 124:1799–1807 [DOI] [PubMed] [Google Scholar]
- 113.Arango NA, Lovell-Badge R, Behringer RR1999. Targeted mutagenesis of the endogenous mouse Mis gene promoter: in vivo definition of genetic pathways of vertebrate sexual development. Cell 99:409–419 [DOI] [PubMed] [Google Scholar]
- 114.Keri RA, Nilson JH1996. A steroidogenic factor-1 binding site is required for activity of the luteinizing hormone β subunit promoter in gonadotropes of transgenic mice. J Biol Chem 271:10782–10785 [DOI] [PubMed] [Google Scholar]
- 115.Halvorson LM, Kaiser UB, Chin WW1996. Stimulation of luteinizing hormone β gene promoter activity by the orphan nuclear receptor, steroidogenic factor-1. J Biol Chem 271:6645–6650 [DOI] [PubMed] [Google Scholar]
- 116.Duval DL, Nelson SE, Clay CM1997. A binding site for steroidogenic factor-1 is part of a complex enhancer that mediates expression of the murine gonadotropin-releasing hormone receptor gene. Biol Reprod 56:160–168 [DOI] [PubMed] [Google Scholar]
- 117.Jacobs SB, Coss D, McGillivray SM, Mellon PL2003. Nuclear factor Y and steroidogenic factor 1 physically and functionally interact to contribute to cell-specific expression of the mouse follicle-stimulating hormone-β gene. Mol Endocrinol 17:1470–1483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kurrasch DM, Cheung CC, Lee FY, Tran PV, Hata K, Ingraham HA2007. The neonatal ventromedial hypothalamus transcriptome reveals novel markers with spatially distinct patterning. J Neurosci 27:13624–13634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Luo X, Ikeda Y, Parker KL1994. A cell-specific nuclear receptor is essential for adrenal and gonadal development and sexual differentiation. Cell 77:481–490 [DOI] [PubMed] [Google Scholar]
- 120.Sadovsky Y, Crawford PA, Woodson KG, Polish JA, Clements MA, Tourtellotte LM, Simburger K, Milbrandt J1995. Mice deficient in the orphan receptor steroidogenic factor 1 lack adrenal glands and gonads but express P450 side-chain-cleavage enzyme in the placenta and have normal embryonic serum levels of corticosteroids. Proc Natl Acad Sci USA 92:10939–10943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bland ML, Jamieson CA, Akana SF, Bornstein SR, Eisenhofer G, Dallman MF, Ingraham HA2000. Haploinsufficiency of steroidogenic factor-1 in mice disrupts adrenal development leading to an impaired stress response. Proc Natl Acad Sci USA 97:14488–14493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Shinoda K, Lei H, Yoshii H, Nomura M, Nagano M, Shiba H, Sasaki H, Osawa Y, Ninomiya Y, Niwa O, Morohashi KI, Li E1995. Developmental defects of the ventromedial hypothalamic nucleus and pituitary gonadotroph in the Ftz-F1 disrupted mice. Dev Dyn 204:22–29 [DOI] [PubMed] [Google Scholar]
- 123.Ikeda Y, Luo X, Abbud R, Nilson JH, Parker KL1995. The nuclear receptor steroidogenic factor 1 is essential for the formation of the ventromedial hypothalamic nucleus. Mol Endocrinol 9:478–486 [DOI] [PubMed] [Google Scholar]
- 124.Zhao L, Bakke M, Krimkevich Y, Cushman LJ, Parlow AF, Camper SA, Parker KL2001. Steroidogenic factor 1 (SF1) is essential for pituitary gonadotrope function. Development 128:147–154 [DOI] [PubMed] [Google Scholar]
- 125.Jeyasuria P, Ikeda Y, Jamin SP, Zhao L, De Rooij DG, Themmen AP, Behringer RR, Parker KL2004. Cell-specific knockout of steroidogenic factor 1 reveals its essential roles in gonadal function. Mol Endocrinol 18:1610–1619 [DOI] [PubMed] [Google Scholar]
- 126.Pelusi C, Ikeda Y, Zubair M, Parker KL2008. Impaired follicle development and infertility in female mice lacking steroidogenic factor 1 in ovarian granulosa cells. Biol Reprod 79:1074–1083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zhao L, Kim KW, Ikeda Y, Anderson KK, Beck L, Chase S, Tobet SA, Parker KL2008. Central nervous system-specific knockout of steroidogenic factor 1 results in increased anxiety-like behavior. Mol Endocrinol 22:1403–1415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Canteras NS2002. The medial hypothalamic defensive system: hodological organization and functional implications. Pharmacol Biochem Behav 71:481–491 [DOI] [PubMed] [Google Scholar]
- 129.Tran PV, Akana SF, Malkovska I, Dallman MF, Parada LF, Ingraham HA2006. Diminished hypothalamic bdnf expression and impaired VMH function are associated with reduced SF-1 gene dosage. J Comp Neurol 498:637–648 [DOI] [PubMed] [Google Scholar]
- 130.Kim KW, Zhao L, Parker KL2009. Central nervous system-specific knockout of steroidogenic factor 1. Mol Cell Endocrinol 300:132–136 [DOI] [PubMed] [Google Scholar]
- 131.Achermann JC, Ito M, Ito M, Hindmarsh PC, Jameson JL1999. A mutation in the gene encoding steroidogenic factor-1 causes XY sex reversal and adrenal failure in humans. Nat Genet 22:125–126 [DOI] [PubMed] [Google Scholar]
- 132.Biason-Lauber A, Schoenle EJ2000. Apparently normal ovarian differentiation in a prepubertal girl with transcriptionally inactive steroidogenic factor 1 (NR5A1/SF-1) and adrenocortical insufficiency. Am J Hum Genet 67:1563–1568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Achermann JC, Ozisik G, Ito M, Orun UA, Harmanci K, Gurakan B, Jameson JL2002. Gonadal determination and adrenal development are regulated by the orphan nuclear receptor steroidogenic factor-1, in a dose-dependent manner. J Clin Endocrinol Metab 87:1829–1833 [DOI] [PubMed] [Google Scholar]
- 134.Köhler B, Lin L, Ferraz-de-Souza B, Wieacker P, Heidemann P, Schröder V, Biebermann H, Schnabel D, Grüters A, Achermann JC2008. Five novel mutations in steroidogenic factor 1 (SF1, NR5A1) in 46,XY patients with severe underandrogenization but without adrenal insufficiency. Hum Mutat 29:59–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lourençco D, Brauner R, Lin L, De Perdigo A, Weryha G, Muresan M, Boudjenah R, Guerra-Junior G, Maciel-Guerra AT, Achermann JC, McElreavey K, Bashamboo A2009. Mutations in NR5A1 associated with ovarian insufficiency. N Engl J Med 360:1200–1210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Bulun SE, Utsunomiya H, Lin Z, Yin P, Cheng YH, Pavone ME, Tokunaga H, Trukhacheva E, Attar E, Gurates B, Milad MP, Confino E, Su E, Reierstad S, Xue Q2009. Steroidogenic factor-1 and endometriosis. Mol Cell Endocrinol 300:104–108 [DOI] [PubMed] [Google Scholar]
- 137.Figueiredo BC, Cavalli LR, Pianovski MA, Lalli E, Sandrini R, Ribeiro RC, Zambetti G, DeLacerda L, Rodrigues GA, Haddad BR2005. Amplification of the steroidogenic factor 1 gene in childhood adrenocortical tumors. J Clin Endocrinol Metab 90:615–619 [DOI] [PubMed] [Google Scholar]
- 138.Pianovski MA, Cavalli LR, Figueiredo BC, Santos SC, Doghman M, Ribeiro RC, Oliveira AG, Michalkiewicz E, Rodrigues GA, Zambetti G, Haddad BR, Lalli E2006. SF-1 overexpression in childhood adrenocortical tumours. Eur J Cancer 42:1040–1043 [DOI] [PubMed] [Google Scholar]
- 139.Jorgensen JS, Nilson JH2001. AR suppresses transcription of the LHβ subunit by interacting with steroidogenic factor-1. Mol Endocrinol 15:1505–1516 [DOI] [PubMed] [Google Scholar]
- 140.Li LA, Chiang EF, Chen JC, Hsu NC, Chen YJ, Chung BC1999. Function of steroidogenic factor 1 domains in nuclear localization, transactivation, and interaction with transcription factor TFIIB and c-Jun. Mol Endocrinol 13:1588–1598 [DOI] [PubMed] [Google Scholar]
- 141.Nachtigal MW, Hirokawa Y, Enyeart-VanHouten DL, Flanagan JN, Hammer GD, Ingraham HA1998. Wilms’ tumor 1 and Dax-1 modulate the orphan nuclear receptor SF-1 in sex-specific gene expression. Cell 93:445–454 [DOI] [PubMed] [Google Scholar]
- 142.Tremblay JJ, Drouin J1999. Egr-1 is a downstream effector of GnRH and synergizes by direct interaction with Ptx1 and SF-1 to enhance luteinizing hormone β gene transcription. Mol Cell Biol 19:2567–2576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wang DS, Kobayashi T, Zhou LY, Paul-Prasanth B, Ijiri S, Sakai F, Okubo K, Morohashi K, Nagahama Y2007. Foxl2 up-regulates aromatase gene transcription in a female-specific manner by binding to the promoter as well as interacting with ad4 binding protein/steroidogenic factor 1. Mol Endocrinol 21:712–725 [DOI] [PubMed] [Google Scholar]
- 144.Tremblay JJ, Viger RS1999. Transcription factor GATA-4 enhances Mullerian inhibiting substance gene transcription through a direct interaction with the nuclear receptor SF-1. Mol Endocrinol 13:1388–1401 [DOI] [PubMed] [Google Scholar]
- 145.Hong CY, Park JH, Seo KH, Kim JM, Im SY, Lee JW, Choi HS, Lee K2003. Expression of MIS in the testis is downregulated by tumor necrosis factor α through the negative regulation of SF-1 transactivation by NF-κB. Mol Cell Biol 23:6000–6012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Tremblay JJ, Marcil A, Gauthier Y, Drouin J1999. Ptx1 regulates SF-1 activity by an interaction that mimics the role of the ligand-binding domain. EMBO J 18:3431–3441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Schepers G, Wilson M, Wilhelm D, Koopman P2003. SOX8 is expressed during testis differentiation in mice and synergizes with SF1 to activate the Amh promoter in vitro. J Biol Chem 278:28101–28108 [DOI] [PubMed] [Google Scholar]
- 148.De Santa Barbara P, Bonneaud N, Boizet B, Desclozeaux M, Moniot B, Sudbeck P, Scherer G, Poulat F, Berta P1998. Direct interaction of SRY-related protein SOX9 and steroidogenic factor 1 regulates transcription of the human anti-Müllerian hormone gene. Mol Cell Biol 18:6653–6665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Liu Z, Simpson ER1997. Steroidogenic factor 1 (SF-1) and SP1 are required for regulation of bovine CYP11A gene expression in bovine luteal cells and adrenal Y1 cells. Mol Endocrinol 11:127–137 [DOI] [PubMed] [Google Scholar]
- 150.Liu Z, Simpson ER1999. Molecular mechanism for cooperation between Sp1 and steroidogenic factor-1 (SF-1) to regulate bovine CYP11A gene expression. Mol Cell Endocrinol 153:183–196 [DOI] [PubMed] [Google Scholar]
- 151.Monté D, DeWitte F, Hum DW1998. Regulation of the human P450scc gene by steroidogenic factor 1 is mediated by CBP/p300. J Biol Chem 273:4585–4591 [DOI] [PubMed] [Google Scholar]
- 152.Kabe Y, Goto M, Shima D, Imai T, Wada T, Morohashi K, Shirakawa M, Hirose S, Handa H1999. The role of human MBF1 as a transcriptional coactivator. J Biol Chem 274:34196–34202 [DOI] [PubMed] [Google Scholar]
- 153.Børud B, Hoang T, Bakke M, Jacob AL, Lund J, Mellgren G2002. The nuclear receptor coactivators p300/CBP/cointegrator-associated protein (p/CIP) and transcription intermediary factor 2 (TIF2) differentially regulate PKA-stimulated transcriptional activity of steroidogenic factor 1. Mol Endocrinol 16:757–773 [DOI] [PubMed] [Google Scholar]
- 154.Zhou D, Quach KM, Yang C, Lee SY, Pohajdak B, Chen S2000. PNRC: a proline-rich nuclear receptor coregulatory protein that modulates transcriptional activation of multiple nuclear receptors including orphan receptors SF1 (steroidogenic factor 1) and ERRα1 (estrogen related receptor α-1). Mol Endocrinol 14:986–998 [DOI] [PubMed] [Google Scholar]
- 155.Curtin D, Ferris HA, Häkli M, Gibson M, Jänne OA, Palvimo JJ, Shupnik MA2004. Small nuclear RING finger protein stimulates the rat luteinizing hormone-beta promoter by interacting with Sp1 and steroidogenic factor-1 and protects from androgen suppression. Mol Endocrinol 18:1263–1276 [DOI] [PubMed] [Google Scholar]
- 156.Crawford PA, Polish JA, Ganpule G, Sadovsky Y1997. The activation function-2 hexamer of steroidogenic factor-1 is required, but not sufficient for potentiation by SRC-1. Mol Endocrinol 11:1626–1635 [DOI] [PubMed] [Google Scholar]
- 157.Ito M, Yu RN, Jameson JL1998. Steroidogenic factor-1 contains a carboxy-terminal transcriptional activation domain that interacts with steroid receptor coactivator-1. Mol Endocrinol 12:290–301 [DOI] [PubMed] [Google Scholar]
- 158.Gizard F, Lavallee B, DeWitte F, Teissier E, Staels B, Hum DW2002. The transcriptional regulating protein of 132 kDa (TReP-132) enhances P450scc gene transcription through interaction with steroidogenic factor-1 in human adrenal cells. J Biol Chem 277:39144–39155 [DOI] [PubMed] [Google Scholar]
- 159.Dammer EB, Sewer MB2008. Phosphorylation of CtBP1 by cAMP-dependent protein kinase modulates induction of CYP17 by stimulating partnering of CtBP1 and 2. J Biol Chem 283:6925–6934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Ou Q, Mouillet JF, Yan X, Dorn C, Crawford PA, Sadovsky Y2001. The DEAD box protein DP103 is a regulator of steroidogenic factor-1. Mol Endocrinol 15:69–79 [DOI] [PubMed] [Google Scholar]
- 161.Park YY, Park KC, Shong M, Lee SJ, Lee YH, Choi HS2007. EID-1 interacts with orphan nuclear receptor SF-1 and represses its transactivation. Mol Cells 24:372–377 [PubMed] [Google Scholar]
- 162.Song KH, Park YY, Kee HJ, Hong CY, Lee YS, Ahn SW, Kim HJ, Lee K, Kook H, Lee IK, Choi HS2006. Orphan nuclear receptor Nur77 induces zinc finger protein GIOT-1 gene expression, and GIOT-1 acts as a novel corepressor of orphan nuclear receptor SF-1 via recruitment of HDAC2. J Biol Chem 281:15605–15614 [DOI] [PubMed] [Google Scholar]
- 163.Mellgren G, Børud B, Hoang T, Yri OE, Fladeby C, Lien EA, Lund J2003. Characterization of receptor-interacting protein RIP140 in the regulation of SF-1 responsive target genes. Mol Cell Endocrinol 203:91–103 [DOI] [PubMed] [Google Scholar]
- 164.Sugawara T, Abe S, Sakuragi N, Fujimoto Y, Nomura E, Fujieda K, Saito M, Fujimoto S2001. RIP 140 modulates transcription of the steroidogenic acute regulatory protein gene through interactions with both SF-1 and DAX-1. Endocrinology 142:3570–3577 [DOI] [PubMed] [Google Scholar]
- 165.Borud B, Mellgren G, Lund J, Bakke M2003. Cloning and characterization of a novel zinc finger protein that modulates the transcriptional activity of nuclear receptors. Mol Endocrinol 17:2303–2319 [DOI] [PubMed] [Google Scholar]
- 166.Hossain A, Saunders GF2003. Synergistic cooperation between the β-catenin signaling pathway and steroidogenic factor 1 in the activation of the Mullerian inhibiting substance type II receptor. J Biol Chem 278:26511–26516 [DOI] [PubMed] [Google Scholar]
- 167.Gummow BM, Winnay JN, Hammer GD2003. Convergence of Wnt signaling and steroidogenic factor-1 (SF-1) on transcription of the rat inhibin α gene. J Biol Chem 278:26572–26579 [DOI] [PubMed] [Google Scholar]
- 168.Mizusaki H, Kawabe K, Mukai T, Ariyoshi E, Kasahara M, Yoshioka H, Swain A, Morohashi K2003. Dax-1 (dosage-sensitive sex reversal-adrenal hypoplasia congenita critical region on the X chromosome, gene 1) gene transcription is regulated by wnt4 in the female developing gonad. Mol Endocrinol 17:507–519 [DOI] [PubMed] [Google Scholar]
- 169.Parakh TN, Hernandez JA, Grammer JC, Weck J, Hunzicker-Dunn M, Zeleznik AJ, Nilson JH2006. Follicle-stimulating hormone/cAMP regulation of aromatase gene expression requires β-catenin. Proc Natl Acad Sci USA 103:12435–12440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Jordan BK, Shen JH, Olaso R, Ingraham HA, Vilain E2003. Wnt4 overexpression disrupts normal testicular vasculature and inhibits testosterone synthesis by repressing steroidogenic factor 1/β-catenin synergy. Proc Natl Acad Sci USA 100:10866–10871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Sewer MB, Nguyen VQ, Huang CJ, Tucker PW, Kagawa N, Waterman MR2002. Transcriptional activation of human CYP17 in H295R adrenocortical cells depends on complex formation among p54(nrb)/NonO, protein-associated splicing factor, and SF-1, a complex that also participates in repression of transcription. Endocrinology 143:1280–1290 [DOI] [PubMed] [Google Scholar]
- 172.Mukai T, Kusaka M, Kawabe K, Goto K, Nawata H, Fujieda K, Morohashi K2002. Sexually dimorphic expression of Dax-1 in the adrenal cortex. Genes Cells 7:717–729 [DOI] [PubMed] [Google Scholar]



