Abstract
Rationale: Chronic rhinosinusitis (CRS) without nasal polyps (CRSsNP) and CRS with nasal polyps (CRSwNP) are associated with Th1 and Th2 cytokine polarization, respectively; however, the pathophysiology of CRS remains unclear. The importance of innate lymphoid cells in Th2-mediated inflammatory disease has not been clearly defined.
Objectives: The objective of this study was to investigate the role of the epithelial cell–derived cytokine IL-33 and IL-33–responsive innate lymphoid cells in the pathophysiology of CRS.
Methods: Relative gene expression was evaluated using quantitative real-time polymerase chain reaction. Innate lymphoid cells in inflamed ethmoid sinus mucosa from patients with CRSsNP and CRSwNP were characterized using flow cytometry. Cytokine production from lymphoid cells isolated from inflamed mucosa of patients with CRS was examined using ELISA and intracellular cytokine staining.
Measurements and Main Results: Elevated expression of ST2, the ligand-binding chain of the IL-33 receptor, was observed in inflamed sinonasal mucosa from CRSwNP compared with CRSsNP and healthy control subjects. An increased percentage of innate lymphoid cells was observed in inflamed sinonasal mucosa from CRSwNP compared with CRSsNP. ST2+ innate lymphoid cells are a consistent source of IL-13 in response to IL-33 stimulation. Significant induction of IL-33 was observed in epithelial cells derived from patients with CRSwNP compared with patients with CRSsNP in response to stimulation with Aspergillus fumigatus extract.
Conclusions: These data suggest a role for sinonasal epithelial cell–derived IL-33 and an IL-33–responsive innate lymphoid cell population in the pathophysiology of CRSwNP demonstrating the functional importance of innate lymphoid cells in Th2-mediated inflammatory disease.
Keywords: epithelial cells, Aspergillus fumigatus, Alternaria alternata, IL-33, innate lymphoid cells
At a Glance Commentary
Scientific Knowledge on the Subject
The epithelial cell–derived cytokine IL-33 is known to play a key role in the development and regulation of a Th2 immune response, mediated in part by an IL-33–responsive innate lymphoid cell population. However, there is little direct evidence of an important role for these cells in Th2-mediated inflammatory disease in humans.
What This Study Adds to the Field
Here we show that the percentage of innate lymphoid cells is significantly elevated in diseased mucosa in chronic rhinosinusitis with nasal polyps, a Th2-mediated inflammatory process, and that these cells represent a consistent and potentially important source of IL-13 in response to IL-33 stimulation. We therefore demonstrate for the first time the functional importance of innate lymphoid cells in Th2-mediated inflammatory disease in humans.
Chronic rhinosinusitis (CRS) is a chronic inflammatory process of the nasal and paranasal sinus mucosa affecting more than 30 million people annually, resulting in significant direct healthcare costs (1). CRS without nasal polyps (CRSsNP) and CRS with nasal polyps (CRSwNP) are associated with Th1 and Th2 cytokine polarization, respectively (2, 3), although the cellular and molecular mechanisms driving the Th2-mediated inflammation in CRSwNP are not clearly understood (4, 5). Due largely to a lack of understanding of the pathophysiology of CRS, current treatment options, including high-dose glucocorticosteroids and surgeries, remain noncurative, generic, and largely unchanged over the past decade.
IL-33, a member of the IL-1 family of cytokines, is expressed by epithelial cells, endothelial cells, fibroblasts, smooth muscle cells, macrophages, and dendritic cells (6–8). The IL-33 receptor, a heterodimeric complex composed of ST2 and IL-1 receptor accessory protein (IL1RAP), is expressed on numerous immune cells, including Th2 cells, mast cells, basophils, eosinophils, and macrophages (7–9). IL-33 is a chemoattractant for Th2 cells (10) and promotes Th2 polarization of naive CD4+ T cells, enhancing production of IL-5 and IL-13 independent of IL-4 (11). IL-33 can also induce proinflammatory cytokine and chemokine production by mast cells and enhance degranulation (12), stimulate basophils and eosinophils (13), and enhance IL-13–driven polarization of alternatively activated macrophages (14). More recently, an important role for IL-33 in regulating the development and function of type 2 innate lymphoid cells (ILC) was described (15, 16). Type 2 ILC are members of a heterogeneous family of cells with lymphoid morphology that lack expression of lineage markers, express CD127, the α chain of the IL-7 receptor, and produce IL-13 in response to IL-33 stimulation (15–25). In animal models, these cells play an important role in the clearance of helminth infections (18, 19) and the development of airway hyperreactivity (23, 25). Although Type 2 ILC have recently been described in human tissues (21, 26), their functional importance in Th2-mediated disease in humans is not clearly understood. In addition, the mechanism of induction of IL-33 expression in human airway epithelial cells remains unclear. Here we examine the role of IL-33 and an IL-33–responsive ILC population in the pathophysiology of CRSwNP. Some of these results have been previously reported in an abstract (27).
Methods
Additional details on patient characteristics and methods can be found in the online supplement.
Patients
The research protocol for this study was approved by the Institutional Review Board of The University of Texas Health Science Center, Houston, TX. Ethmoid sinus mucosa removed during the course of medically indicated endoscopic sinus surgery was collected from patients providing informed consent. Patient characteristics are summarized in Table 1.
TABLE 1.
DEMOGRAPHICS OF PATIENTS USED IN VARIOUS EXPERIMENTS
| Healthy Control | CRSsNP | CRSwNP | Total | |
|---|---|---|---|---|
| Total (male) | 8 (4) | 30 (15) | 73 (52) | 111 (64) |
| Mean age (range), yr | 58.5 (31–83) | 48.9 (24–70) | 40 (16–75) | 43.7 (16–83) |
| Allergy | 0 (0%) | 13 (43%) | 36 (49%) | 49 (44%) |
| Asthma | 0 (0%) | 4 (13%) | 17 (23%) | 21 (19%) |
| Preoperative prednisone | 0 (0%) | 0 (0%) | 18 (25%) | 18 (16%) |
| PCR | 8 | 20 | 36 | 64 |
| Flow cytometry | 0 | 4 | 7 | 11 |
| ELISA/ICS | 0/0 | 3/0 | 17/6 | 20/6 |
| Epithelial cell | 0 | 7 | 15 | 22 |
Definition of abbreviations: CRSsNP = chronic rhinosinusitis without nasal polyps; CRSwNP = chronic rhinosinusitis with nasal polyps; ICS = intracellular staining; PCR = polymerase chain reaction.
Flow Cytometry Analysis of ILC
Cells dissociated from sinonasal mucosa using Collagenase D and DNase I (Roche Diagnostics, Mannheim, Germany) were stained with fluorescein isothiocyanate (FITC)-labeled anti-human CD3, CD14, CD19, CD34, αβ-TCR, γδ-TCR, allophycocyanin (APC)-labeled anti-human CD117, and phycoerythrin (PE)-labeled anti-human CD127 (Beckman Coulter, Brea, CA), and ILC were identified as FITCneg CD127+ events. In other experiments, cells were stained with FITC-labeled anti-human CD3, CD14, CD19, CD34, αβ-TCR, γδ-TCR, APC-labeled anti-human CD117, and PE-Cy7–labeled anti-human CD127 (BD Biosciences, San Jose, CA) together with PE-labeled anti-CD161 (Beckman Coulter), anti-CRTH2 (Miltenyi Biotec, Auburn, CA), anti-ST2 (R&D Systems, Minneapolis, MN), or relevant isotype controls. Data were collected using a FACS Calibur (Beckton Dickinson, San Jose, CA) and analyzed using FlowJo software (Tree Star, Ashland, OR). All antibodies are from eBioscience (San Diego, CA) unless otherwise noted.
Lymphoid Cell Culture and Stimulation
A CD45-enriched population was obtained from a single cell suspension from inflamed sinonasal mucosa by positive selection using human CD45 microbeads (Miltenyi Biotec). Cells were plated at a density of 106 cells/ml and stimulated with 10 U/ml recombinant human IL-2 (Chiron, Emeryville, CA) or 100 ng/ml recombinant human IL-33 (Peprotech, Placentia, CA) alone or in combination. Supernatant was collected after 96 hours of stimulation, and IL-13 levels were determined by ELISA (eBioscience). For intracellular staining experiments, cells were stimulated with recombinant IL-2 alone or together with recombinant IL-33 for 6 hours, then stained with FITC-labeled anti-human CD3, CD14, CD19, CD34, αβ-TCR, γδ-TCR, APC-labeled anti-human CD117, and PE-Cy7–labeled anti-human CD127, then fixed and permeabilized and stained with PE-labeled anti-human IL-13 (BD Biosciences).
Epithelial Cell Culture and Stimulation
Epithelial cells were cultured for 24 hours in medium alone or containing extracts from Aspergillus fumigatus, Alternaria alternata, or Cladosporium herbarum (Greer, Lenoir, NC) at a concentration of 10 μg/ml; relative expression of IL-33 was then determined by quantitative real-time polymerase chain reaction (qPCR).
Statistics
Data were analyzed with Prism version 6 software (GraphPad, La Jolla, CA). Data are represented as mean ± SEM. Normally distributed data were analyzed by Student t test, paired t test, or one way analysis of variance with Tukey posttest as appropriate. Nonparametric data were analyzed by Mann-Whitney test or Wilcoxon signed rank test as appropriate. A P value less than 0.05 was considered significant.
Results
Elevated Expression of ST2 in Inflamed Ethmoid Sinus Mucosa from Patients with CRSwNP Compared with Patients with CRSsNP and Healthy Control Subjects
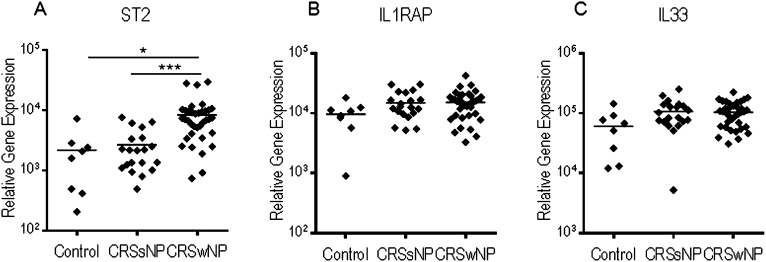
Using qPCR, we examined the expression of IL-33, ST2, and IL1RAP in inflamed ethmoid sinus mucosa from patients with CRSwNP (n = 36), patients with CRSsNP (n = 20), or healthy control subjects (HC) (n = 8) with no history of CRS or allergic disease. Relative expression of ST2 was significantly elevated in inflamed ethmoid sinus mucosa from patients with CRSwNP compared with patients with CRSsNP (P < 0.001) and HC (P < 0.05) (Figure 1A). Expression of IL-33 and IL1RAP was observed in inflamed ethmoid sinus mucosa from patients with CRSwNP, patients with CRSsNP, and HC. Although no significant difference in the relative expression of IL-33 was observed in the inflamed ethmoid sinus mucosa from patients with CRSwNP compared with CRSsNP and HC (Figures 1B and 1C), a high level of expression of IL-33 was evident in tissue from all groups, raising the possibility that IL-33 could interact with an ST2+ cell population specifically present within the inflamed mucosa of patients with CRSwNP.
Figure 1.
Elevated expression of ST2 in inflamed sinonasal mucosa from chronic rhinosinusitis (CRS) with nasal polyps (CRSwNP) as compared with CRS without nasal polyps (CRSsNP) and healthy control subjects (HC). Expression of (A) ST2, (B) IL1RAP, and (C) IL-33 relative to β-actin was determined in inflamed sinonasal mucosa from CRSsNP (n = 20), CRSwNP (n = 36), and HC (n = 8) by quantitative real-time polymerase chain reaction. Each point represents the average of duplicate results for an individual patient sample, and the horizontal line represents the mean. Results were analyzed by one-way analysis of variance with Tukey posttest, *P < 0.05 and ***P < 0.001.
Elevated Percentage of ST2+ ILC in Inflamed Sinonasal Mucosa from Patients with CRSwNP Compared with Patients with CRSsNP
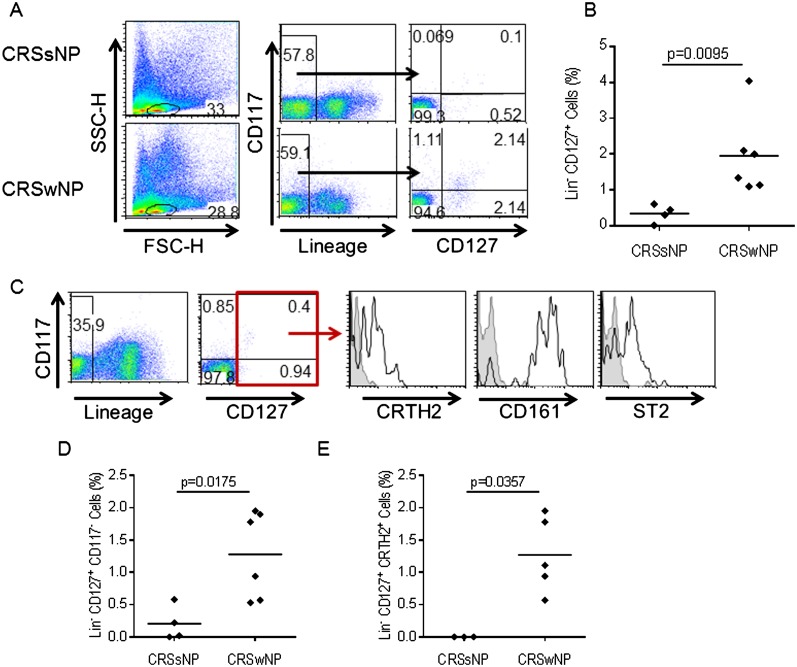
Because we previously identified elevated expression of ST2 in inflamed ethmoid sinus mucosa from patients with CRSwNP as compared with patients with CRSsNP and HC, we next sought to identify the cells present within inflamed sinonasal mucosa of patients with CRSwNP expressing ST2. We examined the percentage of ILC, an important cell type expressing ST2, in inflamed sinonasal mucosa of patients with CRS using flow cytometry. We stained cells from inflamed ethmoid sinus mucosa from patients with CRSsNP (n = 4) and patients with CRSwNP (n = 6) with a cocktail of FITC-conjugated antibodies recognizing several lineage markers, including CD3, αβ-TCR, and γδ-TCR, which are expressed on T cells; CD14, which is expressed on monocytes, macrophages, and some dendritic cell subsets; CD19, which is expressed on B cells; and CD34, which is expressed by hematopoietic progenitor cells. The cells were also stained with a PE-conjugated antibody recognizing CD127, the α chain of the interleukin 7 receptor, and an APC-conjugated antibody recognizing CD117, also known as c-kit. We gated on cells lacking lineage markers (lineageneg) and examined expression of CD117 and CD127 within this population. Results are expressed as the percentage of CD127+ cells within the total lineageneg population. We considered all lineageneg CD127+ cells to be ILC, and observed donor-dependent variable expression of CD117. Representative data from one patient with CRSsNP and one patient with CRSwNP are shown in Figure 2A, and in Figure 2B data from patients with CRSsNP (n = 4) and CRSwNP (n = 6) are compiled. The percentage of lineageneg CD127+ ILC was significantly higher in inflamed ethmoid sinus mucosa from patients with CRSwNP (mean = 1.953 ± 1.11%) compared with patients with CRSsNP (mean = 0.35 ± 0.25%) (P = 0.0095). We then analyzed the surface phenotype of these ILC in greater detail. We stained cells from inflamed mucosa of patients with CRSwNP (n = 4) with the cocktail of FITC-conjugated lineage antibodies described previously, APC-conjugated anti-human CD117, PE-Cy7–conjugated anti-human CD127, and PE-conjugated antibodies recognizing CD161 and CRTH2, markers previously shown to be expressed by ILC (21), and ST2. As shown in Figure 2C, lineageneg CD127+ ILC present in inflamed ethmoid sinus mucosa of patients with CRSwNP expressed ST2 as well as CRTH2 and CD161 in agreement with previously published data (21). Lineageneg CD127+ CD117+ cells within the lineageneg CD127+ population could include mast cells, which also express CD117. However, the percentage of lineageneg CD127+ CD117neg cells was also significantly elevated in inflamed ethmoid sinus mucosa from patients with CRSwNP compared with CRSsNP (Figure 2D). The percentage of lineageneg CD127+ CRTH2+ cells was significantly higher in inflamed ethmoid sinus mucosa from CRSwNP as compared with CRSsNP (Figure 2E). We also observed expression of ST2 on both CD4+ T cells and mast cells within inflamed ethmoid sinus mucosa of patients with CRSwNP (data not shown). We previously demonstrated increased frequency of both CD4+ T cells and mast cells in inflamed sinonasal mucosa from patients with CRSwNP compared with patients with CRSsNP independent of atopy (28), and these cells likely contribute to the elevated expression of ST2 observed in inflamed ethmoid sinus mucosa from patients with CRSwNP.
Figure 2.
Elevated percentage of innate lymphoid cells in chronic rhinosinusitis (CRS) with nasal polyps (CRSwNP). (A) Identification of innate lymphoid cells in inflamed sinonasal mucosa. Representative data from one patient with CRS without nasal polyps (CRSsNP) and one patient with CRSwNP are shown. (B) Percentage of innate lymphoid cells (ILC) in inflamed sinonasal mucosa from CRSsNP (n = 4) and CRSwNP (n = 6). (C) The surface phenotype of ILC from patients with CRSwNP was analyzed by flow cytometry. Data shown are representative of four independent patients. Shaded histogram represents isotype control, and open histogram represents staining with antigen-specific antibody. (D) Percentage of cells lacking lineage markers (lineageneg), CD117neg CD127+ ILC in inflamed sinonasal mucosa from CRSsNP (n = 4) and CRSwNP (n = 6). (E) Percentage of lineageneg CD127+ CRTH2+ ILC in inflamed sinonasal mucosa from CRSsNP (n = 3) and CRSwNP (n = 4). Each point (B, D, E) represents an individual patient sample, and the horizontal bar represents the mean. Results were analyzed by Mann-Whitney test. P < 0.05 was considered significant.
IL-33 Induces IL-13 Production from a Lineageneg ILC Population within Sinonasal Mucosa of Patients with CRSwNP
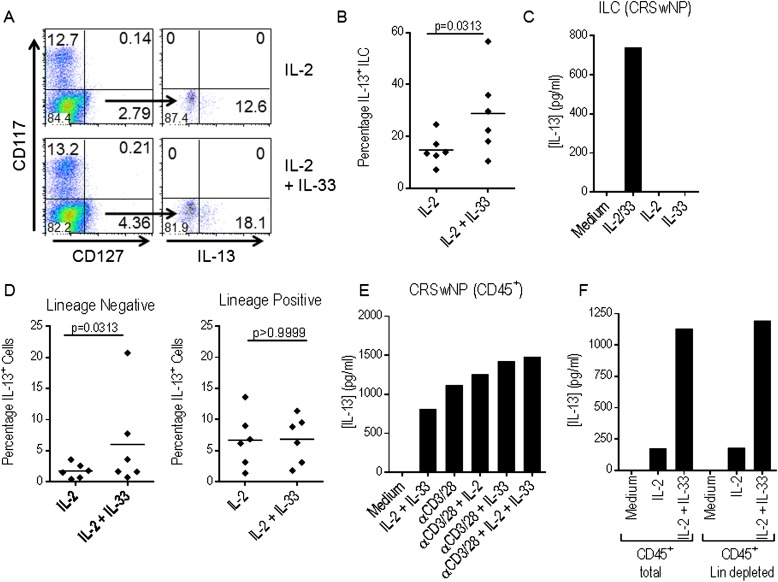
Having identified an elevated percentage of ST2+ ILC within inflamed ethmoid sinus mucosa from patients with CRSwNP compared with patients with CRSsNP, we next sought to determine whether ST2+ ILC present within inflamed sinonasal mucosa of patients with CRSwNP could respond to IL-33 stimulation. In initial experiments, we examined the production of IL-13 from total lymphoid cells isolated from inflamed mucosa of patients with CRSwNP (n = 10) in response to stimulation with recombinant IL-33 alone or together with recombinant IL-2, a cytokine necessary for lymphoid cell activation. We isolated cells expressing CD45, a marker present on all lymphoid cells, from dissociated sinonasal mucosa from patients with CRSwNP and cultured them for 4 days in medium alone or with recombinant IL-2, IL-33, or both. After stimulation, supernatant was collected and IL-13 production was assessed by ELISA. As shown in Figure 3A, we did not observe a significant increase in IL-13 production from CD45+ cells from patients with CRSwNP in response to IL-2 (mean = 173.0 ± 48 pg/ml) or IL-33 (mean = 34.5 ± 12.6 pg/ml) compared with medium control (mean = 2.9 ± 2.1 pg/ml). However, we observed a significantly elevated level of IL-13 production in response to stimulation with IL-2 and IL-33 together (609.0 ± 170.6 pg/ml) compared with stimulation with IL-2 (P < 0.01) or IL-33 (P < 0.001) alone or medium control (P < 0.001). In similar experiments with CD45+ cells isolated from inflamed sinonasal mucosa from patients with CRSsNP (n = 3), no significant increase in IL-13 production was observed in response to stimulation with IL-2 and IL-33 together compared with stimulation with IL-2 alone (Figure 3B). Also, the amount of IL-13 produced by CD45+ cells from patients with CRSwNP stimulated with IL-2 and IL-33 (range = 80.0–1,620.0 pg/ml) was significantly higher than the level of IL-13 produced by CD45+ cells from patients with CRSsNP (range = 32.7–127 pg/ml) (Figure 3C). We did not observe IL-13 production from CD45neg cells from patients with CRSwNP or CRSsNP in response to stimulation with recombinant IL-2, IL-33, or both (data not shown). This indicates that a CD45+ cell population within the inflamed sinonasal mucosa of patients with CRSwNP can produce IL-13 in response to IL-33 stimulation in the presence of IL-2.
Figure 3.
Increased IL-13 production from cells lacking lineage markers (lineageneg) CD127+ innate lymphoid cells in chronic rhinosinusitis (CRS) with nasal polyps (CRSwNP) in response to IL-33 stimulation. IL-13 production from CD45+ cells isolated from inflamed sinonasal mucosa of (A) patients with CRSwNP (n = 10), or (B) patients with CRS without nasal polyps (CRSsNP) (n = 3) stimulated with recombinant IL-2, IL-33, or both was determined by ELISA. (C) IL-13 production from CD45+ cells from CRSwNP (n = 10) and CRSsNP (n = 3) stimulated with recombinant IL-2 and IL-33 was determined by ELISA. Each point represents the results for an individual patient sample, and the horizontal line represents the mean. Results were analyzed by one-way analysis of variance with Tukey posttest (A, B) or Mann-Whitney test (C). P < 0.05 was considered significant. **P < 0.01 and ***P < 0.001.
To identify the cells producing IL-13 in response to stimulation with IL-2 and IL-33, CD45+ cells from inflamed sinonasal mucosa from patients with CRSwNP were cultured for 6 hours with IL-2 alone or together with IL-33, and IL-13+ ILC were subsequently detected by intracellular staining. Briefly, cells were stained, as described previously, with a cocktail of FITC-conjugated lineage antibodies, APC-conjugated anti-human CD117, PE-Cy7–conjugated anti-human CD127, then fixed and permeabilized and stained with PE-conjugated anti-human IL-13. Representative data from one patient are shown in Figure 4A, and data from six independent experiments, each with one patient, are compiled in Figure 4B. Lineageneg CD127+ CD117+ cells were not observed after in vitro stimulation; therefore, the percentage of IL-13+ cells within the lineageneg CD127+ CD117neg population is shown. After stimulation with IL-2 together with IL-33, the percentage of lineageneg CD127+ ILC that are IL-13+ (mean = 28.9 ± 6.6%) is significantly higher than after stimulation with IL-2 alone (mean = 14.8 ± 2.4%) (P = 0.0313). As shown in Figure 4C, sorted ILC produce IL-13 in response to stimulation with recombinant IL-2 and IL-33. These data indicate that ILC present within diseased mucosa in CRSwNP produce IL-13 in response to IL-33 stimulation.
Figure 4.
Innate lymphoid cells (ILC) from chronic rhinosinusitis (CRS) with nasal polyps (CRSwNP) produce IL-13 in response to IL-33. CD45+ cells from inflamed mucosa of patients with CRSwNP (n = 6) were stimulated with recombinant IL-2 alone or together with recombinant IL-33. The percentage of IL-13+ ILC was determined by intracellular staining. Data from one representative patient (A) and cumulative data from six independent patients (B) are shown. (C) IL-13 production from lineageneg CD127+ CRTH2+ ILC sorted from inflamed ethmoid sinus mucosa from CRSwNP and stimulated with recombinant IL-2 alone or together with recombinant IL-33 was determined by ELISA. (D) The total percentage of IL-13+ cells within the lineage+ and lineageneg population was determined. (E) IL-13 production from CD45+ cells from inflamed mucosa of patients with CRSwNP stimulated with recombinant IL-2 and IL-33 or plate-bound anti-CD3 and soluble anti-CD28 alone or together with recombinant IL-2, IL-33, or both was determined by ELISA. (F) IL-13 production from total or lineage-depleted CD45+ cells sorted from inflamed ethmoid sinus mucosa of CRSwNP, plated at a density of 106/ml (total) or 9.3 × 104/ml (lineage-depleted) to represent the frequency of lineage+ cells within the total CD45+ population, and stimulated for 4 days with IL-2 alone or together with IL-33 was determined by ELISA. Each point represents the results for an individual patient sample, and the horizontal line represents the mean. Data from one experiment representative of two (C, F) or three (E) independent experiments each with one patient are shown. Results were analyzed by one-way analysis of variance with Tukey posttest (A) or Wilcoxon signed rank test (B, D). P < 0.05 was considered significant.
However, other CD45+ cells present within diseased mucosa, including T cells and mast cells, can produce IL-13 in response to IL-33 stimulation. We reexamined the data from the intracellular staining experiments described previously and determined the total percentage of IL-13+ cells within the lineageneg or lineage+ cell populations after stimulation with IL-2 alone or together with IL-33. There is a significant increase in the percentage of total IL-13+ cells within the lineageneg population in response to stimulation with IL-2 and IL-33 (mean = 6.0 ± 3.1%) compared with IL-2 alone (mean = 1.75 ± 0.5%) (P = 0.0313). However, no significant difference is observed within the lineage+ population (Figure 4D). Mast cells are present within the lineageneg population; however, published data indicate that they respond to IL-33 stimulation without a requirement for concurrent IL-2 (12), and no significant increase in IL-13 production from CD45+ cells from patients with CRSwNP was observed in response to stimulation with IL-33 alone (Figure 3A). To confirm that T cells present within diseased mucosa are not a significant source of IL-13 in response to IL-33 stimulation, we cultured CD45+ cells from CRSwNP (n = 3) with IL-2 and IL-33 alone or in combination or with plate-bound anti-human CD3 and soluble anti-human CD28, to specifically activate T cells, alone or together with IL-2, IL-33, or both. After 4 days of culture, supernatant was collected and IL-13 levels were determined by ELISA. As shown in Figure 4E, there is little difference in IL-13 production from CD45+ cells from patients with CRSwNP in response to stimulation with plate-bound anti-CD3 and soluble anti-CD28 alone or together with IL-2, IL-33, or IL-2 and IL-33, suggesting that T cells present within diseased mucosa are not a significant source of IL-13 in response to IL-33 stimulation. Finally, we stained dissociated cells from CRSwNP mucosa with PE-labeled anti-human CD45, the cocktail of FITC-labeled lineage antibodies described previously, and FITC-labeled anti-human FcεR1α, which is expressed on mast cells but not ILC. We sorted total or lineage-depleted CD45+ cells, cultured them for 4 days with recombinant IL-2 alone or with recombinant IL-33, then measured IL-13 in the supernatant by ELISA. The amount of IL-13 produced by total and lineage-depleted CD45+ cells is similar (Figure 4F), suggesting that ILC rather than mast cells or T cells are the primary source of IL-13 in response to IL-33 stimulation.
Induction of IL-33 Expression in Primary Sinonasal Epithelial Cells from Patients with CRSwNP in Response to Stimulation with an Extract of Aspergillus fumigatus
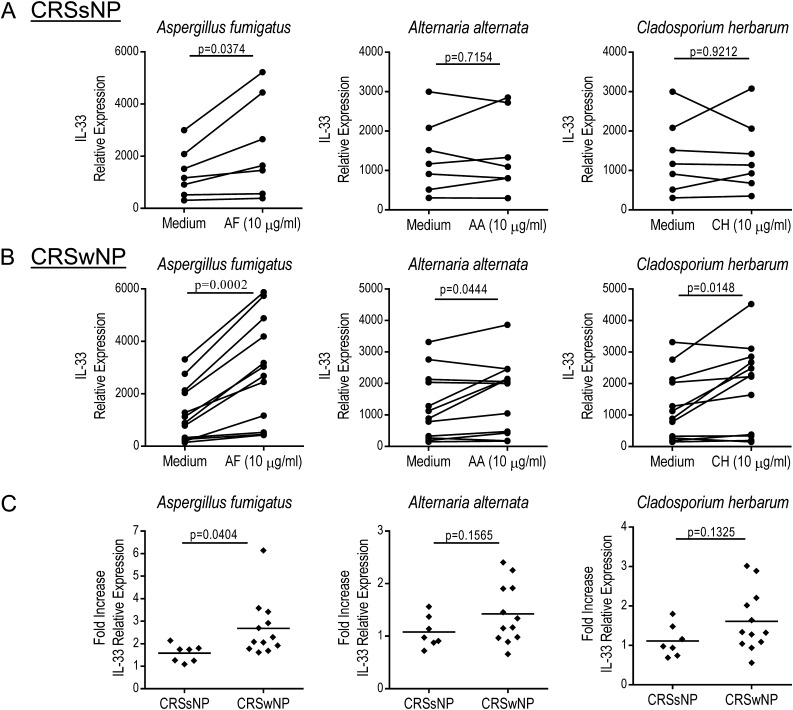
We have demonstrated an elevated percentage of ST2+ ILC in inflamed ethmoid sinus mucosa from patients with CRSwNP compared with patients with CRSsNP and determined that these cells produce IL-13 in response to stimulation with recombinant IL-2 and IL-33. This raises the question of which cells are producing IL-33 and how its expression is regulated. Numerous cell types, including epithelial cells, express IL-33, and its expression can be up-regulated in response to stimulation with microbial antigens. Numerous environmental fungi are associated with CRS (5), so we investigated their ability to induce IL-33 expression in sinonasal epithelial cells from patients with CRS. We cultured primary sinonasal epithelial cells from patients with CRSsNP (n = 7) and patients with CRSwNP (n = 12) and stimulated them for 24 hours with extracts of several fungi commonly associated with CRS, including Aspergillus fumigatus, Alternaria alternata, and Cladosporium herbarum (5), then determined the relative expression of IL-33 in the cultured epithelial cells using qPCR. We observed significant induction of IL-33 expression in epithelial cells from patients with CRSsNP in response to stimulation with an extract of A. fumigatus (P = 0.0374) but not A. alternata (P = 0.7154) or C. herbarum (P = 0.9212) (Figure 5A). We also observed significant induction of IL-33 expression in epithelial cells from patients with CRSwNP in response to all fungal antigens tested including A. fumigatus (P = 0.0002), A. alternata (P = 0.0444), and C. herbarum (P = 0.0148) (Figure 5B). We subsequently compared the fold induction of IL-33 expression in response to fungal antigen stimulation in epithelial cells from CRSsNP and patients with CRSwNP. In response to stimulation with A. fumigatus extract, induction of IL-33 expression was significantly higher in epithelial cells from CRSwNP as compared with CRSsNP (P = 0.0404) (Figure 5C). However, there was no significant difference in the induction of IL-33 expression in epithelial cells derived from patients with CRSsNP and patients with CRSwNP in response to A. alternata (P = 0.1565) or C. herbarum (P = 0.1325) extracts. We observed a trend toward increased IL-33 protein production in epithelial cells from CRSwNP in response to fungal antigen stimulation, but this was not statistically significant and was variable between patients (see Figure E1 in the online supplement). Collectively, these data indicate that epithelial cells from patients with CRSwNP react to stimulation with A. fumigatus extract with increased induction of IL-33 expression compared with epithelial cells from patients with CRSsNP.
Figure 5.
Induction of IL-33 expression in sinonasal epithelial cells from patients with chronic rhinosinusitis (CRS) with nasal polyps (CRSwNP) in response to stimulation with Aspergillus fumigatus extract. Relative expression of IL-33 in sinonasal epithelial cells from patients with CRS without nasal polyps (CRSsNP) (n = 7) (A) or patients with CRSwNP (n = 12) (B) cultured for 24 hours in medium alone or with an extract of A. fumigatus (AF), Alternaria alternata (AA), or Cladosporium herbarum (CH). (C) Fold increase in IL-33 expression in sinonasal epithelial cells from CRSsNP (n = 7) and CRSwNP (n = 12) in response to stimulation with fungal extracts. Each point represents the average of duplicate results from an individual patient and the horizontal line represents the mean. Results were analyzed by paired t test (A, B) or unpaired t test (C). P < 0.05 was considered significant.
Discussion
In this study, we examined the role of the epithelial cell–derived cytokine IL-33 in the pathophysiology of CRS. We demonstrated elevated expression of ST2 in inflamed ethmoid sinus mucosa from patients with CRSwNP compared with CRSsNP and HC, which complements previously published microarray data indicating that ST2 expression is up-regulated in diseased mucosa from patients with asthma with CRSwNP compared with HC (29). We showed that ST2 is expressed on a lineageneg CD127+ ILC population and that the percentage of these cells is significantly elevated in inflamed ethmoid sinus mucosa from patients with CRSwNP compared with patients with CRSsNP. We subsequently determined that within diseased mucosa in CRSwNP, ILC produce IL-13 in response to stimulation with recombinant IL-2 and IL-33. Furthermore, we demonstrated increased induction of IL-33 expression in epithelial cells derived from patients with CRSwNP compared with patients with CRSsNP in response to stimulation with an extract of A. fumigatus. Collectively, these data suggest a role for IL-33 and an IL-33–responsive ILC population in the pathophysiology of CRSwNP and indicate that ILC, rather than T cells or mast cells, are a consistent and potentially important source of IL-13 in response to IL-33 stimulation, demonstrating the functional importance of these cells in Th2-mediated inflammatory disease in humans.
Here we showed that ST2+ ILC, present within inflamed mucosa of patients with CRSwNP, produce IL-13 in response to stimulation with recombinant IL-2 and IL-33. The increased production of IL-13 from CD45+ cells from CRSwNP required concurrent IL-2 stimulation, suggesting that mast cells are not the source of the IL-13, as published data indicate they respond directly to IL-33 stimulation (12). We also observed that IL-33 did not enhance the production of IL-13 from CD45+ cells isolated from CRSwNP mucosa and stimulated with plate-bound anti-CD3 and soluble CD28. Although other cells present within diseased mucosa are capable of producing IL-13, we observed that increased IL-13 production in response to IL-2 and IL-33 stimulation is restricted to cells lacking conventional lineage markers. Collectively these data suggest that ILC are a key source of IL-13 in diseased mucosa in CRSwNP, rather than mast cells or T cells.
IL-13 produced by ILC may promote eosinophilia and mucus production commonly observed in CRSwNP, through the regulation of mucin production and the expression of chemokines that mediate eosinophil recruitment (30). Studies conducted using animal models have demonstrated an important role for IL-33 and ILC in the development of airway inflammation and clearance of intestinal parasites through their production of IL-13 (18, 19, 23, 25). However, microarray analysis of ILC from mouse lung showed expression of several molecules important for wound healing, tissue homeostasis, and maintenance of epithelial cell barrier integrity (24). Therefore, ILC may play an important role in nasal polyp formation through their excessive production of these factors. Most studies of ILC thus far have focused on their role as effector cells, mediating inflammation, helminth expulsion, or wound healing through their production of soluble factors. However, the role of ILC in modulating the function of other immune cells present within inflamed mucosa of patients with CRS, including T cells, mast cells, and dendritic cells, either through the production of soluble mediators or through physical interactions, has not been explored.
The mechanism by which expression of IL-33 in airway epithelial cells is regulated by microbial antigens is unclear. Published data indicate that human airway epithelial cells produce IL-33 in response to stimulation with an extract of the fungus Alternaria or oriental cockroach extract (31). Elevated expression of IL-33 has also been observed in human airway epithelial cells in response to microbial DNA (32) and proinflammatory cytokines (33). Here we demonstrate a significant increase in expression of IL-33 in sinonasal epithelial cells from patients with CRSwNP in response to several fungal antigens commonly associated with CRS, including A. fumigatus, A. alternata, and C. herbarum. However, only A. fumigatus extract promoted increased induction of IL-33 in epithelial cells from CRSwNP compared with CRSsNP, suggesting differences in the response of epithelial cells from patients with CRSsNP and patients with CRSwNP to A. fumigatus. Collectively our data suggest that IL-33 produced by sinonasal epithelial cells in response to common environmental fungi may drive IL-13 production from ILC in CRSwNP, which could in turn promote mucous production and tissue eosinophilia typically observed in these patients. This supports a key role for IL-33 and ILC in the pathophysiology of CRSwNP.
Acknowledgments
Acknowledgment
The authors thank Dr. Faramarz Ashoori for his exceptional support in this study and Drs. Seth Isaacs, Li-Xing Man, and Paul Lee, rhinology fellows who assisted in the collection of surgical tissue.
Footnotes
Funded by internal funds from the Department of Otorhinolaryngology–Head and Neck Surgery at the University of Texas Medical Center at Houston. This work was supported in part by the shared instrumentation grant (RP 110776) from the Cancer Prevention and Research Institute of Texas.
Author Contributions: Conception and design: A.L., J.L.S., Y.-J.L.; analysis and interpretation: A.L., J.L.S., S.F., M.J.C., P.C.P., D.B.C., and F.K.; drafting the manuscript for important intellectual content: A.L. and J.L.S.; providing patient tissue: A.L., S.F., and M.J.C.; reading, reviewing, revising, and approving the final manuscript: all authors.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201212-2227OC on June 27, 2013
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Hamilos DL. Chronic rhinosinusitis: epidemiology and medical management. J Allergy Clin Immunol. 2011;128:693–707. doi: 10.1016/j.jaci.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 2.Tieu DD, Kern RC, Schleimer RP. Alterations in epithelial barrier function and host defense responses in chronic rhinosinusitis. J Allergy Clin Immunol. 2009;124:37–42. doi: 10.1016/j.jaci.2009.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van Zele T, Claeys S, Gevaert P, Van Maele G, Holtappels G, Van Cauwenberge P, Bachert C. Differentiation of chronic sinus diseases by measurement of inflammatory mediators. Allergy. 2006;61:1280–1289. doi: 10.1111/j.1398-9995.2006.01225.x. [DOI] [PubMed] [Google Scholar]
- 4.Van Crombruggen K, Zhang N, Gevaert P, Tomassen P, Bachert C. Pathogenesis of chronic rhinosinusitis: inflammation. J Allergy Clin Immunol. 2011;128:728–732. doi: 10.1016/j.jaci.2011.07.049. [DOI] [PubMed] [Google Scholar]
- 5.Fokkens WJ, Ebbens F, van Drunen CM. Fungus: a role in pathophysiology of chronic rhinosinusitis, disease modifier, a treatment target, or no role at all? Immunol Allergy Clin North Am. 2009;29:677–688. doi: 10.1016/j.iac.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 6.Schmitz J, Owyang A, Oldham E, Song Y, Murphy E, McClanahan TK, Zurawski G, Moshrefi M, Qin J, Li X, et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T Helper type 2-associated cytokines. Immunity. 2005;23:479–490. doi: 10.1016/j.immuni.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 7.Sims JE, Smith DE. The IL-1 family: regulators of immunity. Nat Rev Immunol. 2010;10:89–102. doi: 10.1038/nri2691. [DOI] [PubMed] [Google Scholar]
- 8.Liew F, Pitman NI, McInnes IB. Disease-associated functions of IL-33: the new kid in the IL-1 family. Nat Rev Immunol. 2010;10:103–110. doi: 10.1038/nri2692. [DOI] [PubMed] [Google Scholar]
- 9.Chackerian AA, Oldham ER, Murphy EE, Schmitz J, Pflanz S, Kastelein RA. IL-1 receptor accessory protein and ST2 comprise the IL-33 receptor complex. J Immunol. 2007;179:2551–2555. doi: 10.4049/jimmunol.179.4.2551. [DOI] [PubMed] [Google Scholar]
- 10.Komai-Koma M, Xu D, Li Y, McKenzie ANJ, McInnes IB, Liew FY. IL-33 is a chemoattractant for human Th2 cells. Eur J Immunol. 2007;37:2779–2786. doi: 10.1002/eji.200737547. [DOI] [PubMed] [Google Scholar]
- 11.Kurowska-Stolarska M, Kewin P, Murphy G, Russo RC, Stolarski B, Garcia CC, Komai-Koma M, Pitman N, Li Y, Niedbala W, et al. IL-33 induces antigen-specific IL-5+ T cells and promotes allergic-induced airway inflammation independent of IL-4. J Immunol. 2008;181:4780–4790. doi: 10.4049/jimmunol.181.7.4780. [DOI] [PubMed] [Google Scholar]
- 12.Allakhverdi Z, Smith DE, Comeau MR, Delespesse G. Cutting edge: the ST2 ligand IL-33 potently activates and drives maturation of human mast cells. J Immunol. 2007;179:2051–2054. doi: 10.4049/jimmunol.179.4.2051. [DOI] [PubMed] [Google Scholar]
- 13.Pecaric-Petkovic T, Didichenko SA, Kaempfer S, Spiegl N, Dahinden CA. Human basophils and eosinophils are the direct target leukocytes of the novel IL-1 family member IL-33. Blood. 2009;113:1526–1534. doi: 10.1182/blood-2008-05-157818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kurowska-Stolarska M, Stolarski B, Kewin P, Murphy G, Corrigan CJ, Ying S, Pitman N, Mirchandani A, Rana B, van Rooijen N, et al. IL-33 amplifies the polarization of alternatively activated macrophages that contribute to airway inflammation. J Immunol. 2009;183:6469–6477. doi: 10.4049/jimmunol.0901575. [DOI] [PubMed] [Google Scholar]
- 15.Barret NA, Austen KF. Innate cells and T helper 2 cell immunity in airway inflammation. Immunity. 2009;31:425–437. doi: 10.1016/j.immuni.2009.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spits H, Di Santo JP. The expanding family of innate lymphoid cells: regulators and effectors of immunity and tissue remodeling. Nat Immunol. 2011;12:21–27. doi: 10.1038/ni.1962. [DOI] [PubMed] [Google Scholar]
- 17.Moro K, Yamada T, Tanabe M, Takeuchi T, Ikawa T, Kawamoto H, Furusawa J, Ohtani M, Fujii H, Koyasu S. Innate production of Th2 cytokines by adipose tissue-associated c-Kit+ Sca-1+ lymphoid cells. Nature. 2010;463:540–544. doi: 10.1038/nature08636. [DOI] [PubMed] [Google Scholar]
- 18.Neill DR, Wong SH, Bellosi A, Flynn RJ, Daly M, Langford TKA, Bucks C, Kane CM, Fallon PG, Pannell R, et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature. 2010;434:1367–1370. doi: 10.1038/nature08900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saenz SA, Siracusa MC, Perrigoue JG, Spencer SP, Urban JF, Jr, Tocker JE, Budelsky AL, Kleinschek MA, Kastelein RA, Kambayashi T, et al. IL25 elicits a multipotent progenitor cell population that promotes Th2 cytokine responses. Nature. 2010;463:1352–1356. doi: 10.1038/nature08901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Crellin NK, Trifari S, Kaplan CD, Satoh-Takayama N, Di Santo JP, Spits H. Regulation of cytokine secretion in human CD127+ LTi-like innate lymphoid cells by toll-like receptor 2. Immunity. 2010;33:752–764. doi: 10.1016/j.immuni.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 21.Mjosberg JM, Trifari S, Crellin NK, Peters CP, van Drunen CM, Piet B, Fokkens WJ, Cupedo T, Spits H. Human IL-25- and IL-33-responsive type 2 innate lymphoid cells are defined by expression of CRTH2 and CD161. Nat Immunol. 2011;12:1055–1062. doi: 10.1038/ni.2104. [DOI] [PubMed] [Google Scholar]
- 22.Liang HE, Reinhardt RL, Bando JK, Sullivan BM, Ho IC, Locksley R. Divergent expression patterns of IL-4 and IL-13 define unique functions in allergic immunity. Nat Immunol. 2012;13:58–66. doi: 10.1038/ni.2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chang YJ, Kim HY, Albacker LA, Baumgarth N, McKenzie ANJ, Smith DE. Innate lymphoid cells mediate influenza-induced airway hyper-reactivity independently of adaptive immunity. Nat Immunol. 2011;12:631–637. doi: 10.1038/ni.2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Monticelli LA, Sonnenberg GF, Abt MC, Alenghat T, Ziegler CGK, Doering TA, Angelosanto JM, Laidlaw BJ, Yang CY, Sathaliyawala T, et al. Innate lymphoid cells promote lung-tissue homeostasis after infection with influenza virus. Nat Immunol. 2011;12:1045–1054. doi: 10.1031/ni.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Halim TY, Krauss RH, Sun AC, Takei F. Lung natural helper cells are a critical source of Th2 cell-type cytokines in protease allergen-induced airway inflammation. Immunity. 2012;36:451–463. doi: 10.1016/j.immuni.2011.12.020. [DOI] [PubMed] [Google Scholar]
- 26.Mjosberg J, Bernink J, Golebski K, Karrich JJ, Peters CP, Blom B, Te Velde AA, Fokkens WJ, van Drunen CM, Spits H.The transcription factor GATA3 is essential for the function of human type 2 innate lymphoid cell. Immunity 201237649–659. [DOI] [PubMed] [Google Scholar]
- 27.Shaw J, Fakhri S, Citardi M, Corry D, Kheradmand F, Liu YJ, Luong A. Sinonasal epithelial cell-derived IL-33 induces IL-13 in ST2+ innate lymphoid cells in chronic rhinosinusitis [abstract] J Immunol. 2012;188:161.8. [Google Scholar]
- 28.Shaw JL, Ashoori F, Fakhri S, Citardi MJ, Luong A. Increased percentage of mast cells within sinonasal mucosa of chronic rhinosinusitis with nasal polyp patients independent of atopy. Int Forum Allergy Rhinol. 2012;2:233–40. doi: 10.1002/alr.21021. [DOI] [PubMed] [Google Scholar]
- 29.Plager DA, Kahl JC, Asmann YW, Nilson AE, Pallanch JF, Friedman O, Kita H. Gene transcription changes in asthmatic chronic rhinosinusitis with nasal polyps and comparison to those in atopic dermatitis. PLoS ONE. 2010;5:e11450. doi: 10.1371/journal.pone.0011450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Munitz A, Brandt EB, Mingler M, Finkelman FD, Rothenberg ME. Distinct roles for IL-13 and IL-4 via IL-13 receptor a1 and the type II IL-4 receptor in asthma pathogenesis. Proc Natl Acad Sci USA. 2008;105:7240–7245. doi: 10.1073/pnas.0802465105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kouzaki H, Iijima K, Kobayashi T, O’Grady SM, Kita H. The danger signal, extracellular ATP, is a sensor for an airborne allergen and triggers IL-33 release and innate Th2-type responses. J Immunol. 2011;186:4375–4387. doi: 10.4049/jimmunol.1003020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reh DD, Wang Y, Ramanathan M, Jr, Lane AP. Treatment-recalcitrant chronic rhinosinusitis with nasal polyps is associated with altered epithelial cell expression of interleukin-33. Am J Rhinol Allergy. 2010;24:105–109. doi: 10.2500/ajra.2010.24.3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chustz RT, Nagarkar DR, Poposki JA, Fovoreto S, Jr, Avila PC, Schleimer RP, Kato A. Regulation and function of IL-1F9 in human bronchial epithelial cells. Am J Respir Cell Mol Biol. 2010;45:145–153. doi: 10.1165/rcmb.2010-0075OC. [DOI] [PMC free article] [PubMed] [Google Scholar]