Abstract
Autism spectrum disorder (ASD) represents a heterogeneous group of disorders characterized by alterations in three behavioral symptom domains: social interactions, verbal and nonverbal communication, and repetitive behaviors. Increasing prevalence of ASD in recent years suggests that exposure to environmental toxicants may be critical in modulating etiology of this disease. As clinical diagnosis of autism still relies on behavioral evaluation, it is important to be able to assess similar behavioral traits in animal models, to provide biological plausibility of associations between environmental exposures and ASD. Rodents naturally exhibit a large number of behaviors that can be linked to similar behaviors in human. In this unit, behavioral tests are described that are relevant to the domains affected in ASD. For the repetitive domain, the T-maze spontaneous alternation test and marble burying test are described. For the communication domain, neonatal ultrasonic vocalization and olfactory habituation test toward social and non-social odor are described. Finally, for the sociability domain, the three chambered social preference test and the reciprocal interaction test are presented.
Keywords: Behavior, Mouse, Autism spectrum disorders
INTRODUCTION
Autism spectrum disorder (ASD) represents a heterogeneous group of disorders characterized by difficulties in social interaction, verbal and nonverbal communication and repetitive behaviors. Etiology of ASD is believed to start early in development, while both architectural organization and circuitry connectivity in brain have been identified as major contributors toward progression of symptoms (Crippa et al. 2016). A 2012 survey from CDC showed 1 in 68 American children are affected by autism spectrum disorder, indicating a ten-fold increase in prevalence over the last forty years (Christensen et al. 2016). An epidemiological study in California estimated that 26.4% of the increased autism prevalence can be attributed to change in diagnostic practices between 1992 and 2005 (King and Bearman, 2009). Since genetic factors alone have been shown to only account for a small percentage of ASD patients (Torre-Ubieta et al. 2016), there has been increasing interest in identifying environmental factors that may be involved in the etiology of ASD. Exposure to environmental toxicants such as air pollution, pesticides, heavy metals, industrial compounds, as well as contracting infectious diseases during pregnancy, have been associated with increased ASD risk in both epidemiological and animal studies (Allen et al. 2016; Kalkbrenner et al. 2014; Lo Pumo et al. 2006; Ornoy et al. 2015; De Felice et al. 2015).
The diagnosis of ASD is primarily based on behavioral evaluation (Hennel et al. 2016), as potential reliable biomarkers are still in an investigative phase (Varcin and Nelson, 2016). In recent years, morphological changes in the brain (e.g. ventriculomegaly, disorganization of cortical layering, and a decreased number of cerebellar Purkinje cells) have been found to be occur in ASD (Bennett and Lagopoulos, 2015; Blackmon et al. 2016; Blatt, 2012; Hampson and Blatt, 2015; Stoner et al. 2014; Turner et al. 2016). In order to investigate potential therapeutic interventions for ASD, as well as the contribution of exposure to environmental factors to this disorder, the ability of reproducing the equivalent behavioral traits in animals is of the utmost importance. This Unit describes six different behavioral tests in the three main areas affected in ASD (repetitive behavior, communication, social interactions). These tests have been modified and adjusted from similar tests that have been used to characterize genetic animal models of this disease (e.g. the BTBR T=tf/J or the reelin deficient mouse) and in other studies investigating potential environmental etiological factors of ASD such as prenatal infection (maternal immune activation, MIA; Patterson, 2011). We have utilized such tests to profile the developmental effects of traffic-related air pollution in mice. They would have application in studies aimed at defining the potential involvement of other environmental factors, including pollutants, in the etiology of ASD, in studies investigating gene-environment interactions using known or novel genetic animal models, and in defining potential therapeutic strategies.
All protocols must be reviewed and approved by an Institutional Animal Care and Use Committee (IACUC) and must conform to governmental regulations regarding the care and use of laboratory animals before experiments can proceed.
REPETITIVE DOMAIN
BASIC PROTOCOL 1: MARBLE BURYING
The marble burying task has been widely applied to assess repetitive/persistent behavior relevant to obsessive-compulsive disorder (OCD) and ASD in rodent models (Amodeo et al. 2012; Andersen et al. 2010; Deacon et al. 2006; Moy et al. 2014; Scattoni et al. 2008; Scwartzer et al. 2013; Silverman et al. 2015; Takeuchi et al. 2002). In this test, the numbers of marbles buried serves as a proxy measurement for the extent of repetitive digging behavior exhibited by mice. Increased repetitive digging has been observed in the marble burying test in animal models of ASD, such as the BTBR T+ tf/J mouse, eIF4E-transgenic mice (4E Tg), as well as in the maternal immune activation mouse model (Amodeo et al. 2012; Santini et al. 2013; Choi et al. 2016). The marble burying test protocol described below is modified from Deacon et. al. (2006) and Angoa-Pérez et. al. (2013)
Materials
Mice (at least 6 weeks of age)
Clean standard polycarbonate mouse cage (29.75″ × 61.5″ × 74″ high)
Shaved aspen bedding
24 Marbles (5/8 inch/1.58 cm in diameter)
Clear acrylic ceiling: a sturdy clear acrylic sheet with ventilation holes helps keep mice in the cage during the recording session
Paper towel and 70% ethanol for cleaning
Camera (Microsoft LifeCam HD-6000 720p HD Webcam) and mounting tripod
Ethovision® XT11 software for videotracking and data collection
Light meter (used in measuring lighting intensity of the testing room)
Preparation of the experimental setup
(This setup allows testing of two mice at the same time)
-
1
In a quiet room, set lighting intensity to 100 lux.
-
2
Fill two clean standard mouse housing cages with finely shaved aspen bedding to 5 cm in depth.
Run through aspen bedding with gloved hands a few times to break up clumps developed during shipment. Densely packed bedding material requires more effort from mice to perform marble burying task. For consistent results it is important to make sure the aspen bedding is evenly distributed and without clumps before continuing to the next step.
-
3
Place the cages next to each other, joined by the long sides to allow recording of both cages at the same time.
Line the gap between two cages with clean paper towel as a visual barrier.
-
4
Place 12 clean marbles on top of the aspen bedding in each cage. Make sure the marbles are evenly spaced throughout the cage in a 3 by 4 grid. After each use, wash marbles with mild detergent and rinse thoroughly with tap water, then spray with 70% ethanol to remove any residual odor. Allow the marbles to air-dry before returning them back to a clean container.
-
5
Clean the acrylic ceiling with 70% ethanol, and dry thoroughly with a paper towel to avoid exposing mice to residual ethanol during testing.
-
6
Position camera to ensure unrestricted view of both cages. Set up Ethovision® software to collect video recording for 30 minutes.
Testing procedure
-
7
Before the start of testing, record identity (group, sex, age, etc.) of each mouse, as well as trial number, and label the testing cage.
-
8
Using a clean housing cage, transfer two mice from the housing room to the testing room. Gently pick up both mice by the tail and place them into separate testing cages at the same time.
-
9
Quickly and gently place the cleaned acrylic ceiling with ventilation holes on top of testing cages.
-
10
Start recording for 30 minutes. To allow uncompromised data collection investigator should leave the room during the 30 minutes recording session.
It is crucial for investigator(s) to leave the testing room during the recording session since mice tend to avoid the part of the cage closer to the investigator(s), resulting in unburied marbles in parts of the cage. As long as the clear plastic lid is maintained on top of the testing cages to prevent escape, it is ok to leave mice unsupervised in the testing room.
-
11
At the end of the 30-minute recording session, return mice back to their home cage.
-
12
Before starting next trial, repeat setup steps 2 to 5.
Data Analysis
-
13
To assess extent of repetitive digging behavior, number of marbles buried is counted at 0, 5, 10, 15, 20, 25, and 30 minutes during each recording trial. A marble is considered buried when more than 2/3 of its volume is covered by shaved aspen bedding (see Fig. 1).
-
14
Data can be presented as a line graph with time on X axis and number of marbles buried on Y axis. For statistical analysis, repeated-measures ANOVA would be an appropriate way to assess repetitive digging response over multiple time-points.
Figure 1. Marble burying test.

Screen shots taken at beginning (left), middle (center), and the end (right) of 30 minute trial period (video recorded).
REPETITIVE DOMAIN
BASIC PROTOCOL 2: T-maze Spontaneous Alternation
The T-maze spontaneous alternation test has been frequently used to assess both repetitive behavior and spatial working memory. Driven by innate exploratory needs, mice tend to exhibit heightened interest in exploring different parts of their surroundings when placed in a novel environment. In a laboratory scenario where the novel environment is a T-shaped maze, the innate exploratory drive encourages mice to explore different arms of the maze in each subsequent trial; this behavior is referred to as spontaneous alternation. Animals with increased repetitive tendencies (such as autism and OCD mouse models; Kirsten et al. 2012; Naviaux et al. 2014; Xue et al. 2016; Favre et al. 2015), or with deficits in spatial working memory (e.g. mouse models of Alzheimer’s disease; D’Agostino et al. 2012; Peng et al. 2014) usually exhibit a higher number of repetitive entries into the same arm. As an alternative, the Y-maze could be used in a similar protocol, as the Y-shaped maze provides a more natural turning angle (Lainiola et al. 2014). The spontaneous alternation protocol described below is modified from Deacon and Rawlins (2006).
Materials
Mice (at least 6 weeks of age)
T-maze (composed of three 30 × 10 cm arms joined by a 10 × 10 cm center)
Physical Barrier to prevent arm entry (custom-made guillotine doors, or we have used a square-shaped water bottle)
70% ethanol and clean paper towel for cleaning
Timer
Recording camera and computer.
Light meter (for measuring lighting intensity of the testing room)
Preparation of the Experimental Setup
-
1
Reduce lighting of testing room to 10 lux. Adjust position of T-maze apparatus in the testing room to allow even lighting in all three arms.
Mice exhibit a natural affinity toward shadowed and enclosed space; potential preference toward one arm over the others can be better controlled when even lighting is applied to all three arms of the T-maze apparatus. Dimmed lighting in the testing room decreases level of anxiety during testing.
-
2
Clean T-maze apparatus and water bottle with 70% ethanol before starting each trial.
It is important to thoroughly towel dry all apparatus to prevent testing mice in the presence of alcohol
-
3
Position camera to allow full coverage of the T-maze apparatus. Set up the computer screen to allow real time monitoring of mouse position.
Mice tend to flee when approached by researcher. Screening live recording through a computer monitor enables the researcher to track the position of the mouse in the T-maze apparatus without scaring the animal.
-
4
(Optional if live tracking of mice is desired): Set up tracking software (Ethovision® XT11 is used here) to start and end each testing trial manually. For arena setting, designate the center arm as starting point and the other two arms as goal arms.
Sometimes mice will hesitate between the two goal arms for a while before making the choice of which arm to enter. To ensure testing result reflects fully committed choices by tester mice, the end-goal area should start 10 cm away from the center point of the T-maze.
Testing Procedure
-
5
The mouse is placed in the clean T-maze apparatus facing away from end-goal arms and the timer is started.
-
6
In each trial, the mouse is allowed two minutes to make the choice of which end-goal arm to enter.
-
7
Once the mouse enters one of the end-goal arms, a physical barrier is placed to restrict the mouse in the chosen goal arm for 30 seconds before continuing onto the next trial.
If tester mouse fails to make a choice within two minutes, the trial is marked as “fail” and the animal is restricted in the previous chosen arm for 30 seconds before starting the next trial.
-
8
To start the next trial the tester mouse is returned back to the starting point facing away from the end-goal arms.
To maintain exploratory drive, which is important for alternation behavior, cleaning T-maze apparatus with paper towel slightly dampened with 70% ethanol between trials is recommended.
-
9
Procedure is repeated for a total of 16 trials per mouse.
If a tester mouse fails to choose an end-goal arm more than twice in 16 trials, or fails to choose an end goal in two consecutive trials, testing of the mouse should be terminated and data should be excluded from analysis.
-
10
T-maze apparatus and physical barrier are cleaned with 70% ethanol between trials.
Data Collection and Analysis
-
11
The following parameters are collected for analysis:
Identification of tester mice (treatment group, sex, and ID number should be recorded)
Choice made in each trial is denoted as R= right end goal; L= left end goal; O= failed trial. At the end of the experiment, the number of repeated entries and number of failed trials are scored.
-
12
Results can be presented as:
Number of repeated entries.
Alternatively, repeated or alternated entries for each mouse can be presented after normalizing for the number of successful trials. This is useful when working with a mouse strain of low mobility.
COMMUNICATION DOMAIN
BASIC PROTOCOL 1: NEONATAL ULTRASONIC VOCALIZATION
Ultrasonic vocalization is a common method for assessing vocal communication in autism rodent models, as discussed in many review articles (Santini et al. 2013; Kazdoba et al. 2015; Fischer and Hammerscmidt, 2011; Crawley, 2012; Silverman et al. 2010). Mouse pups emit high frequency vocalization calls in both the audible and the ultrasonic range (Warburton et al. 1989; Miller and Engstrom, 2010). When separated from the dam and littermates, mouse pups emit calls more frequently to elicit retrieval behavior in the dam; several protocols take advantage of this adaptive behavior to increase the number of calls emitted by neonates for robust data collection during short recording sessions (Scattoni et al. 2008; Choi et al. 2016; Kas et al. 2014; Branchi et al. 1998; 2006; Wöhr and Scattoni, 2013). In this protocol an ultrasonic range microphone is used for recording of pup vocalization calls. During the five minutes recording session mouse pups are isolated in the sound attenuating chamber away from its dam and littermates.
Materials
Mouse pups (postnatal day 2 or 6 or 8)
Sound attenuating chamber (this allows recording to take place without interference of ambient noise).
For easy transport, a thick walled Styrofoam box could be used to keep out ambient noise. Alternatively, any box with thick walls can be lined with acoustic material for sound proofing effect. When installing the microphone and a heating pad in the box, make sure to fill any gaps with high density foam strips to maximize sound proofing effect.
Electric heating pad (very important when recording neonatal vocalization to help maintain body temperature during the five minutes recording session)
500 ml Glass beaker
Thermometer
Noldus UltraVox™ XT system for recording and data analysis; the system includes a digital microphone that is able to capture the ultrasonic signal up to 125kHZ.
A chlorine dioxide based sterilant (e.g. Clidox®) and paper towel for cleaning
Preparation of the experimental setup
-
1
This experiment should be conducted in a quiet testing room.
-
2
In the sound attenuating chamber fix the microphone 30 cm above where pups will be positioned.
-
3
Line the bottom of the chamber with the electrical heating pad to help neonates maintain body temperature during the recording session.
-
4
Clean 500 ml glass beaker with Clidox® and place the beaker on top of heating pad inside of the chamber. Make sure the beaker is placed directly underneath the microphone.
-
5
Five minutes before the first recording, turn on heating pad to high heat setting until the temperature inside of glass beaker reaches 37°C, then dial down to low heat setting before continuing the experiment.
Depending on the model of heating pad, longer preheating period and different heat setting may be required to hold the recording chamber at 37°C.
-
6
Set up the recording system with the Noldus microphone. Set sample rate to 250,000 Hz (default for Noldus microphones) and microphone gain to 40. The gain function allows researchers to adjust signal amplification of the microphone. The UltraVox™ manual suggests increasing gain setting right before signal starting to saturate the amplitude plot. For neonatal recording we have found a loss of significant portion of calls with gain setting below 40. This could probably be attributed to the fact that neonatal vocalizations are much weaker in amplitude than vocalization of older animals. Although automatic call identification in the UltraVox software works much better with a lower gain setting, we have made the decision to record with gain setting at 40 to avoid missing informative signals with neonatal mice.
-
7
Start audio recording for 5 min once mice are in the chamber. It is important to keep the testing room as quiet as possible.
Testing procedure
-
8
To organize a large number of recording files, a daily recording log containing: a) recording date and time; b) Recording file name; and c) Recording order and identification of pups within each file; pup ID should include information such as age, sex, treatment group, and dam ID.
-
9
Retrieve mouse pup from its home cage, gently place it inside the clean and warm glass beaker in normal upright position (with all four paws resting on the base of the beaker)
-
10
Carefully place the beaker in pre-heated chamber, positioning the beaker directly below the microphone. Confirm that the pup is still in the upright position before closing the chamber door, then start recording.
Mouse pup vocalization frequency tends to increase when they are placed in a belly-up orientation. In order to limit confounding effects due to handling differences by researchers or the pup’s ability to resume upright position, it is important to make sure that the pups are oriented in upright position before recording starts. If possible, designate one researcher to handle pups for all recording experiments for more consistent results.
-
11
After the end of the 5-minute recording session, return pup back to the home cage and clean the glass beaker before proceeding to record the next pup.
As Clidox® is toxic, be sure to thoroughly dry and ventilate the beaker with clean paper towel before starting the next recording session.
Data collection and analysis
This section is based on practical experience working with the UltraVox™ system (Noldus Information Technologies), but the working concept should be easily applicable to other systems as well. The UltraVox™ system enables recording of ultrasonic vocalization up to 125 kHz, and the signals are depicted in spectrograph after Fourier transformation, with frequency (kHz) on the y-axis and time (milliseconds) on the x-axis.
-
12
Recorded files can be filtered to remove background noise to improve sensitivity, and specificity in the settings for automatic call detection.
-
13
The following parameters are automatically collected for analysis: number of vocalization calls, mean call duration, standard deviation of call duration, minimum and maximum call duration, cumulative call duration, and maximum frequency (Hz).
-
14
Spectrographic analysis enables researchers to characterize vocalization patterns based on previously published categorizations (Scattoni et al. 2008; Branchi et al. 1998; Brudzynski et al. 1999). Ultrasonic vocalization calls are categorized into patterns as follows:
Short: Single syllable punctuated calls shorter than 5 ms.
Chevron: Single syllable calls with pattern resembling a caret sign; starting with an upward slope followed by downward slope in a symmetrical manner.
Flat: Single syllable calls with flat line pattern, with no change in frequency from beginning to end of the call.
Upward: Single syllable calls exhibiting constant increase in frequency over time
Downward: Single syllable calls exhibiting constant decrease in frequency over time
Complex: Single syllable calls with more than two directional changes in frequency.
Two-syllable: Consists of two components, a long linear component at lower frequency ending with a punctuated downward component at higher frequency.
Frequency Steps: Consists of two or more syllable components at various frequency ranges emitted seamlessly. Spectrograph representation appears as discontinuous vertical steps.
Composite: Two parallel syllables simultaneously emitted
Harmonic: Consists of one principle call paralleled by harmonic processes that are decreased in intensity.
Unstructured: Broken/deformed calls of isolated frequency range unrecognizable as other patterns listed above.
-
15
Call pattern data can be represented qualitatively by showing the percentage of calls of each category on the same pie chart (see Fig. 2). Alternatively, quantitative assessment of calls can be done by plotting out the total number of calls for each call category. In situations where the variability in total number of calls is significant enough to confound categorical analysis, the number of calls in each category can be normalized to the total number of calls before proceeding with quantitative analysis.
Figure 2. Ultrasonic vocalization.

Proportions of each call category expressed as USV profile in PND6 male and female C57BL/6J mice.
COMMUNICATION DOMAIN
BASIC PROTOCOL 2: OLFACTORY HABITUATION TEST
Olfactory cues have been found to play a critical role in social communication in many animal species. Compounds excreted in urine or by scent glands were shown to modulate social and sexual behaviors (Beynon and Hurst, 2004; Arakawa et al. 2008; Vosshall, 2005). Olfactory habituation responses toward social odor and non-social odors have been used extensively to assess olfactory communication in various autism mouse models (Crawley, 2012; Silverman et al. 2010; Kas et al. 2014; Wöhr and Scattoni, 2013; Yang and Crawley, 2009). The protocol described in this Unit is modified from several existing protocols (Silverman et al. 2010; Yang and Crawley, 2009; Zou et al. 2015), in which mice are subjected to three repeated presentations of five odor samples: water, almond, banana, urine from same sex mouse, and urine from opposite sex mouse. This protocol thus includes presentation of both non-social odors (almond and banana) and social odors (same sex urine and opposite sex urine).
Materials
Mice (at least 6 weeks of age). If the mice are presented with opposite sex urine as one of the olfactory cues, 9-week old mice should be used, to avoid variability in timing of sexual maturation.
Clean housing cage setup, including standard polycarbonate mouse cage (29.75″ × 61.5″ × 74″ high) lined with corn cob bedding, wire top, and water bottle.
Camera (Microsoft LifeCam HD-6000 720p HD Webcam) and mounting tripod
Ethovision® XT11 software (Noldus Information Technologies) for data collection
Small plastic weigh boat (4.1 × 4.1 × 0.8 cm, VWR # 89106-764)
Unscented double-sided tape (SCOR-TAPE, 1/8″ wide from Scor-Pal). Scotch tape products should be avoided as most of their tapes come with honey-like scent.
Clean filter paper
-
Olfactory scent samples:
-
○
Almond extract (McCormick).
-
○
Banana extract (McCormick).
-
○
Pooled urine from age- and gender-match female and male mice (for urine collection protocol please refer to support protocol 1)
-
○
Various sized pipettes (20, 200, and 1000 μl), and tips
Distilled water
Preparation of the experimental setup
-
1
Prepare olfactory scent presentation apparatus. First, cut filter paper into 1 × 2 cm squares to better fit into the small weigh boats. Second, tape a piece of filter paper inside the base of each weigh boat with double sided tape. Researcher should prepare 16 weigh boats per test subject, plus some extra just in case.
This scent presentation method is a no-contact method; it is better suited for scents likely to elicit stronger response by tester mouse such as social scents like mouse urine. Alternatively, scents can be presented with a cotton-tipped swab hanging from the top of the cage, as described by Zou et al. (2015). Having weigh boats prepared in advance of the experiment will help the experiment proceed more smoothly. The extra weigh boats will come in handy in situations where weigh boats are accidentally dropped on the floor or contaminated in other ways.
-
2
Change the lighting in testing room to 100 lux before the beginning of the experiment.
-
3
Transfer each mouse in a clean caging setup complete with metal wiretop and water bottle. Place a clean olfactory presentation setup, as described in step one, on the flat side of the wiretop opposite the water nozzle. Place the cages in testing room to acclimate for at least an hour before testing.
Acclimating with clean olfactory presentation setup desensitizes tester mice from the visual stimuli of a novel object (i.e. the weigh boat), allowing the investigator to capture responses purely based on olfactory stimuli.
-
4
Set up the camera and EthoVision® software for video recording from the side of the cage facing water bottle nozzle. For easier file organization, five recording files are created per testing subject to record responses toward water, almond, banana, same sex urine, and opposite sex urine. Each recording file will contain three trials of repeated presentation of the same scent.
-
5
Prepare samples at 4% concentration (by volume) with deionized water. For almond and banana scents, 40 μl of flavor extract is combined with 960 μl of water in a 1.75 ml Eppendorf tube and mixed by vortexing. For same sex and opposite sex urine scents, 40 μl aliquots of pooled urine samples collected from age-matched mice is combined with 960 μl of deionized water. For methods on collection and storage of urine samples please refer to support protocol 1.
The 4% dilution samples should be made freshly right before testing, especially for urine samples, to avoid degradation of pheromones which could negatively impact testing results.
Testing Procedure
-
6
Olfaction samples are presented to the mouse for two minutes per trial in the following order: water, water, water, almond, almond, almond, banana, banana, banana, same sex urine, same sex urine, same sex urine, opposite sex urine, opposite sex urine, opposite sex urine.
-
7
First, pipette 10 μl of water onto the filter paper in the weigh boat, and place it on the wire top of caging setup in the same location. Allow video recording for 2 min while the researcher stays more than 5 ft. away in the room. Repeat for a total of three presentations with the same scent before starting with the next scent.
A new presentation should be prepared for each repeated trial. In each trial, the mouse is allowed two minutes to react to olfactory cues, with a 30-second inter-trial period.
-
8
After each round of testing (from water to opposite sex urine) the testing room should be aired out by opening the door a few times to promote air circulation before starting the next round of testing. This will help eliminating residual odor in the testing room that may confound with testing results in future trials.
Data collection and analysis
-
9
Number of sniffs and duration of sniffs are often used to quantify olfactory response. When a tester mouse places its nose within 2 cm of the weighting boat carrying the olfactory cue, the behavior is considered a sniff.
-
10
Comparing habituation response following repeated presentations for social odor (urine samples) vs. non-social odor (almond and banana) may help researchers identify abnormal patterns in olfactory communication. Starting with plotting out number of sniffs and duration of sniffs in every trial chronologically is helpful for observing the habituation trend. To analyze habituation response statistically, differences in sniffing response between the first and the third presentation of the same odor can be plotted (see Fig. 3).
Figure 3. Olfactory habituation test.
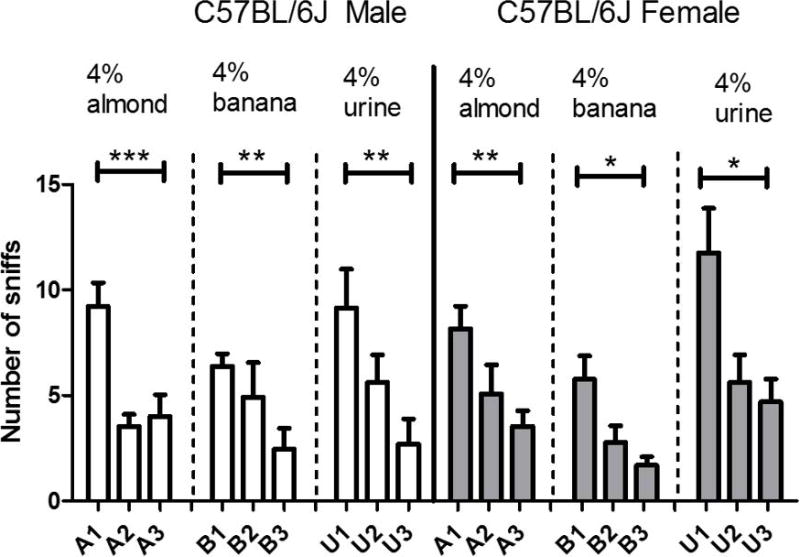
The habituation response in the olfactory test is defined as decreasing level of interest in subsequent presentation (three in this study) of the same odor. Results are expressed as mean +/− SE. Statistical significance determined by one-way ANOVA followed by Dunnett’s test (*p<0.05; **p<0.01).
SUPPORT PROTOCOL 1
Collection and storage of urine samples
Mouse urine is often used as a social olfactory cue in many olfactory habituation protocols. Some protocols test response between different sexes or strains. A method for urine collection is described below, together with some additional information for preventing contamination and for storage of urine samples.
Materials
10 male and 10 female mice. Strain and age should match those of mice to be used for the olfactory habituation test.
1.75 ml Eppendorf tubes
Non-toxic marker or ear tag
Pipettes
Preparing Animals
-
1
Mice used for urine collection should be strain- and age-matched with the tester mice used for the olfactory habituation test. They should be housed in groups of five for at least a week before urine collection.
-
2
Since urine samples will typically be collected over a period of multiple days, it is important to set up a mouse identification system either by ear mark or tail marking with non-toxic markers. Keep in mind that tail marking may need to be re-applied daily. Using both methods is recommended, in case the ear tag falls off or tail marking fades before re-application.
Collection procedure and storage methods
-
3
Urine can be collected twice a day. Depending on the amount of urine needed, collection period could range from four days up to three weeks. Usually, 200 μl of urine can be collected from a 6–7 week old mouse every day.
A minimum of four days of urine collection is recommended to account for hormone modulation during different stages of estrous cycle, since a complete estrous cycle in mice takes four days.
-
4
Before starting urine collection label one Eppendorf tube for each urine donor.
-
5
To collect urine, restrain a mouse by grabbing the scruff of its neck and position the urethra right above a marked and opened Eppendorf tube. To stimulate urine release, gently press on the abdominal side of the mouse by hand.
Some female mice urinate quickly when restrained, which could present a challenge for urine collection. Placing mouse quickly onto a collection Eppendorf tube will help maximize volume of collected urine.
Be careful to avoid contamination with feces. If possible try to position the edge of the Eppendorf tube in between urethra and anus.
Urine collected should be kept on ice and stored in −20°C. A new set of tubes should be prepared for every collection.
-
6
After the last urine collection, thaw all urine samples on ice and combine urine samples collected from the same mouse into one tube. Vortex to mix.
-
7
Combine equal amounts of urine sample from each mouse into one tube and vortex to mix. Keep urine samples on ice.
-
8
Aliquot 40 μl of pooled urines into 1.75ml tubes and store at −20°C.
-
9
Before the olfactory habituation test, prepare 4% urine by adding 960 μl of water to 40 μl urine, and mix by vortexing.
SOCIABILITY DOMAIN
BASIC PROTOCOL 1: THREE-CHAMBERED SOCIAL PREFERENCE TEST
The three chambered social preference test was developed by Jacqueline Crawley’s group specifically for assessing social impairment phenotypes in autism rodent models (Kazdoba et al. 2015). This test is one of the most frequently used tests in studies involving rodent models of ASD (Amodeo et al. 2012; Schwartzer et al. 2013; Crawley, 2012; Silverman et al. 2010; Wöhr and Scattoni, 2013). This test assesses sociability, the tendency to spend time with another mouse, and preference for social novelty, including the ability to discriminate and choose between familiar and new mice. The testing procedure consists of three chronological phases: habituation, sociability, and social novelty. In the habituation phase the testing mouse is placed into the middle chamber and allowed to explore all three chambers freely for 10 minutes. In the sociability phase an age- and gender-matched novel C57BL/6J mouse (raised in a different facility) is placed into one of the holding cups. In the social novelty phase, a second age- and gender-matched novel mouse is added in the second holding cup that was empty during the sociability phase. The tester mouse’s preference in social novelty is quantified by measuring the time spent in the “interaction zone” near the second novel mouse vs. the time spent in the “interaction zone” near the now-familiar first novel mouse.
Materials
Mice at 7 weeks of age
Two age- and sex-matched mice of the same strain (novel mice)
Novel mice should be kept in a different housing facility than tester mice to ensure all aspects of social approach (tactical, olfactory, vocalization) stay novel.
Three-chambered Plexiglas box
Two metal restraining cups. The metal cups are 11 cm high, and consist of a solid 10.5 cm diameter bottom and stainless steel bars spaced at 1 cm intervals.
The cups are designed to allow non-aggressive tactical interaction and olfactory communication.
70% Ethanol for cleaning
Ethovision® XT videotracking system
Preparation of experimental setup
-
1
Change testing room lighting to 100 lux.
-
2
Clean the testing apparatus (three chambered box and two metal cups) with 70% ethanol. Be sure to thoroughly dry the apparatus to avoid exposure of mice to alcohol.
-
3
Place metal cups in each of the side chambers. Position the metal cups on the same side in both side chambers at a 5 cm distance from three adjacent walls.
-
4
Set up the digital recorder for a 10 min. recording session per trial, and Ethovision® XT for live tracking of tester mice.
Testing Procedure
-
5
The testing for each mouse is comprised of three trials: habituation, sociability, social novelty. In the habituation trial, the mouse is placed in the middle chamber and allowed to freely explore all three chambers for 10 min. During the habituation phase, the holding cups in the two side-chambers are empty. At the end of the trial, the mouse is temporally moved to a clean housing cage while the investigator sets up for the sociability trial.
-
6
In the sociability trial, an age- and sex-matched novel mouse is placed into one of the holding cups. The test subject (first mouse) is then re-introduced in the middle chamber of the testing apparatus, and allowed to freely explore all three chambers for 10 min. At the end of the trial, the tester mouse is temporally moved to a clean housing cage while the investigator sets up for social novelty trial (see Fig. 4).
Figure 4. Three chamber test.

A. Apparatus set-up for the sociability phase of social preference test. B. Apparatus set-up for the social novelty phase of social preference test.
The placement of the first novel mouse should be randomized and equally distributed between the two holding cups. This is to control for chamber preference by the tester mice, which may represent a confounding factor.
-
7
In the social novelty trial, a second age- and gender-matched novel mouse is added into the holding cup that was empty during the previous sociability trial. The test mouse is placed back into the center chamber and allowed to freely explore all three chambers for 10 min. (see Fig. 4).
Data collection and analysis
-
8
Analysis for the three-chambered sociability test usually consists of tracking location of the test mouse over the duration of the test and measuring the time spent in each of the three chambers during all three trials. The presence of a novel mouse is going to elicit stronger exploratory interest, resulting in more time spent by the test subject near the novel mouse compared to the side with the empty holding cup. Furthermore, the novel mouse will elicit more interest than the familiar mouse. To assess physical interactions between the test mouse and the novel mouse, an interaction zone (defined as 10 cm. radius from the base of the metal cup) can be added as a sub-region in the experimental arena design.
-
9
Additionally, grooming and rearing responses can also be scored to assess parameters that are known to influence social behavior such as anxiety and exploratory drive.
BASIC PROTOCOL 2: RECIPROCAL INTERACTION
The test for reciprocal interaction provides an environment allowing detailed assessment of intrinsic mouse interactions, and is commonly used to assess social interactions in rodent models of ASD (Kazdoba et al. 2015; Crawley, 2012; Siverman et al. 2010; Kas et al. 2014; Wöhr and Scattoni, 2013; Terranova and Laviola, 2005). Each test subject is introduced to an age- and gender-matched socially naïve mouse for 10 min of unrestricted interaction. Total social contact including sniffing (nose–nose, anogenital, body), push–play behavior, rearing and digging behaviors are scored.
Materials
Mice at 7 weeks of age
Age- and sex-matched mice (novel mice)
Novel mice should be kept in a different housing facility than test mice to ensure that all aspects of social approach (tactical, olfactory, vocalization) are novel.
Non-toxic marker (such as acrylic marker) for animal identification
Clean housing cage setup including standard polycarbonate mouse cage (29.75″ × 61.5″ × 74″ high) lined with corn cob bedding, wire top, and water bottle.
Clear acrylic ceiling: a sturdy clear acrylic sheet with ventilation holes helps keep mice in cages during recording session
Camera (Microsoft LifeCam HD-6000 720p HD Webcam) and mounting tripod
Ethovision® XT11 software for data collection.
Preparation of animals for testing
-
1
Change testing room lighting to 100 lux. Habituate singly-housed test mice and novel mice in the testing room for at least an hour before testing.
-
2
Fifteen minutes before testing, apply a non- toxic marker on the back fur of the test and the novel mice. The procedure may be repeated just before testing.
If the color marking drastically increases self-grooming to the point that prevents social behaviors from taking place try marking the tail instead.
Preparation of the experimental setup
-
3
To record two test subjects in separate arenas at the same time, place two clean cages filled with corn cob bedding side by side with the long walls touching. Insert a piece of clean paper towel in between the two cages as visual barrier.
-
4
Clean acrylic ceiling with 70% ethanol. Make sure to dry thoroughly to avoid ethanol exposure of experimental mice.
-
5
Setup Ethovision® XT and digital camera to record and track mice for 10 min.
Testing procedure
-
6
Place one test mouse and one age- and sex-matched novel mouse in each clean testing cage. Place cleaned acrylic ceiling on top of cages and start recording.
It is important not to reuse cages used for habituation as it may be considered as established territory, and any additional mouse could be considered an intruder.
-
7
At the end of the 10 min recording session return test mouse and novel mouse back to the home cage and clean the acrylic ceiling with 70% ethanol. Start the next recording session with new clean cages.
Data collection and analysis
-
8
Frequency and duration of nose-anogenital sniffing, nose-nose sniffing, side sniffing, self-grooming, rearing, and aggressive behaviors (pushing and biting) are scored manually with assistance from EthoVision® XT software.
COMMENTARY
Background Information
Autism spectrum disorder (ASD) represents a heterogeneous group of disorders characterized by difficulties in social interaction, verbal and nonverbal communication and repetitive behaviors. As rodents do naturally exhibit a large number of behaviors that can be associated with such behaviors in humans, animal models (either genetic of treatment-related) can be utilized to assess whether they display alterations in the three characteristic domains of ASD. In this unit behavioral tests relevant to three characteristic domains of autism have been described in operational level of detail.
To assess behaviors in the repetitive domain, this Unit describes methods for the marble burying test and the T-maze spontaneous alternation test. Historically, both of these tests have been used to assess repetitive/persistent behaviors with in OCD models (Andersen et al. 2010; Deacon, 2006; Moy et al. 2014; Angoa-Perez et al. 2013). Alternative tests are represented by measurement of repetitive grooming behavior and by the Morris water maze test with reversal learning task (Silverman et al. 2010). In comparison to other tests, the marble burying test and T-maze spontaneous alternation test are very reliable and easy to score, with a low false positive rate.
Olfactory and vocal communications are integral components of rodent communication, and there are many behavioral tests addressing both types of communication in a wide range of age groups and under different experimental conditions (Scattoni et al. 2008; Branchi et al. 1998; 2006; Brudzynski et al. 1999; Yang and Crawley, 2009; Zou et al. 2015). Neonatal ultrasonic vocalization and olfactory habituation toward non-social and social odor are described in this unit to assess both vocal and olfactory communication. Mice emit vocalization calls with complex patterning in the ultrasonic frequency range (Warburton et al. 1989; Branchi et al. 1998; 2006). Though mouse vocal communication in various social situations still needs to be fully understood, in many autism mouse models a shift in vocalization patterning and an increased production of unviable (unstructured) vocalization calls have been observed (Scattoni et al. 2008; Choi et al. 2016; Ey et al. 2013; Romano et al. 2013; Ju et al. 2014; Yang et al. 2012). In the wild, urinary steroidal pheromones are used as territory markers. Mice innately exhibit intense interest in investigating urinary scent markers from other mice. The olfactory habituation test allows assessment of initial response and habituation response toward repeated presentation of social vs. non-social olfactory cues. The olfactory habituation protocol described in this unit has been modified from several previously published protocols (Zou et al. 2015; Terranova and Laviola, 2005; Silverman et al. 2010). Important modifications are the non-contact odor presentation method, and automatic scoring of sniffing response by Ethovision XT® (Noldus Information Technologies) which eliminates odor contamination during testing and minimizes potential human error and bias during data analysis.
Mice are very social animals and intrinsically exhibit a high level of reciprocal social interactions, communal nesting, parenting behaviors, territorial scent marking, as well as aggressive behaviors (Terranova and Laviola, 2005). In the sociability domain, methods for the three chambered social preference test and reciprocal interaction test are described in this unit. The three chambered social preference test, developed by Jacqueline Crawley’s group (Silverman et al. 2010) utilizes innate social preference reported in various rodent species to investigate social tendencies such as pair-bonding, dominance hierarchies and social memory (Nadler et al. 2004). Observations of reciprocal social interactions have also been utilized for assessment of spontaneous social behaviors that are representative of natural behavioral repertoire in various rodent species (Silverman et al. 2010; Wöhr and Scattoni, 2013; Terranova and Laviola, 2005). As alternative methods, the social transmission of food preference test (Nadler et al. 2004) or different variations of social preference test such as the partition test and the social approach test can also be used to assess sociability (Silverman et al. 2010).
Critical Parameters/Troubleshooting
Marble burying test
The setup for the marble burying test consists of a standard mouse housing cage filled with a thick layer (5 cm) of shaved aspen bedding. If a transparent ceiling with breathing holes is placed on top of testing cage, this will prevent the test subject from leaving the cage while allowing unrestricted video recording. The latter is important, as during each trial the investigator is allowed to leave the room thus avoiding the possibility that the test mouse would preferentially bury marbles located further from her/him. We have also found that the density of bedding material critically affects test mouse ability to bury marbles. With higher density bedding material (such as 1/8′ corn cob), instead of burying marbles underneath bedding material the mouse only pushes marbles around the cage. Fine aspen shaving bedding provides more airiness, allowing the mouse to tunnel underneath the marbles and cover them with bedding material. A marble is considered buried when 2/3 of the marble is covered by bedding material. If the mouse blocks view of a marble in the still frame of any of the time points, video footage from 30 sec before and after that time point can be examined to determine exact burying status of that marble.
T-Maze spontaneous alternation test
When restricting the mouse in the chosen goal arm for 30 sec before continuing onto the next trial, it is important to wait until the mouse moves 10 cm into the goal arm. If the physical barrier is dropped too close, the mouse may startle. This stressful experience may prevent the mouse from entering the same goal arm in subsequent trials. In each trial the mouse is allowed 2 min to make a choice between entering two goal arms. If the mouse fails to make a choice, the trial is marked as fail, and the mouse is restricted in the previous chosen arm for 30 sec before starting the next trial. If a mouse fails two consecutive trials or more than two trials out of the 16 planned trials, the mouse is excluded from data analysis. It is essential to maintain a high exploratory drive, which is responsible for the spontaneous alternation behavior. Any olfactory marking left by test mice in the T-maze apparatus should be removed between trials throughout all 16 planned trials per testing subject. A clean paper napkin lightly sprayed with 70% ethanol, can be used to remove any feces or urine left by test mouse.
Neonatal ultrasonic vocalization
Pup vocalization frequency varies by age, room temperature, circadian rhythm, and position of pup (Branchi et al. 1998; 2006). Room temperature can also modulate how frequently vocalization calls are emitted, thus maintaining constant temperature in the recording chamber at 37 ͦC helps minimize variability. Vocalization frequency can also be confounded by the circadian rhythm; this issue can be easily solved by recording at the same time of the day. Furthermore, mouse pups emit more vocalization calls when they are positioned belly side up. Before the start of each recording session, be sure to check that pups are placed in upright position. Conducting neonatal vocalization recordings in a quiet room and sound-attenuating chamber minimizes background noise, making automated call detection easier.
Olfactory habituation test
Experiments performed by us with C57BL/6J mice indicated that social odors such as urine samples from novel mice could be very attractive to test subjects. In our hands, using a hanging cotton swab for odor presentation, as described in Zou et. al. (2015), caused C57BL/6J mice to grasp the cotton swab tip during presentation of urine of novel mice. The non-contact odor presentation method described in this unit is modified from Yang and Crawley (2009). It is important to make sure that the concentrations of odor samples are strong enough to elicit enough initial sniffing response, and the dishabituation odor should be distinctly different from the previous odor presented. As non-social odors, neutral scents are preferred, as highly attractive odorants could elicit behavior that may confound the analysis of the sniffing response. Neutral non-social odors such as almond, banana, and vanilla can only elicit modest level of sniffing response. In contrast, social odors such as urine from novel mice are far more attractive and can elicit stronger initial sniffing response (Kazdoba et al. 2015; Silverman et al. 2010; Yang and Crawley, 2009).
Social Preference test
Possible confounding factors in the social preference test include lighting intensity in the testing room, age, sex and strain of the novel mice, and olfactory interference of residual scent left from previous experiments. Light intensity in the testing room should be set to 100 lux for all trials. Light bulb may dim over time, so it is always good to double check with a photometer before starting the first trial of the day. Age- and sex-matched novel mice from the same strain as the test mouse should be used. To avoid olfactory interference, 70% ethanol can be applied to neutralize the smell of urine and feces. Be sure to dry with clean paper towel thoroughly to avoid exposure of mice to alcohol. If testing more than one mouse in a grouped housing cage, it is important to preserve novelty of the age- and sex-matched novel mice. Do not return the test mouse back to home cage to avoid exposing scent of novel mice to cage mates that have not been tested yet. Cage mates can be housed together only after testing of all animals has ended. Test mice climbing on top of the restraining cup containing novel mice has been reported by several groups (Silverman et al. 2015; Choi et al. 2016; Han et al. 2012). Though there is no interaction between the test mouse and the novel mouse when the test mouse is on top of the restraining cup, this could create false-positive results in interaction time, since the test mouse is located in the interaction zone. Placing a cup or flask filled with water on top of the restraining cups can eliminate the possibility of generating false-positive results.
Reciprocal interaction test
Non-toxic markers of different colors should be used to mark the back fur of test and novel mice, thereby allowing a clear differentiation during the analysis of reciprocal interactions. Because of the marking on the back, increased grooming activity should be expected. It is important to make sure that the amount and the formulation of markers used are not too irritating to the point that overt self-grooming activity prevents other types of behaviors. The marker used in this protocol is a non-toxic acrylic paint produced for black surfaces, which works well on the black fur of C57BL/6J mice. We have also tried livestock markers and non-toxic hair chalk, but both caused skin irritation and were difficult to apply.
Anticipated Results
Marble burying test
In this test the number of marbles buried is used as a proxy measurement for the degree of repetitive digging expressed by the test subjects. Animals exhibiting OCD tendency or ASD-associated repetitive behavior bury more marbles at a faster rate. By the end of a 30 min testing time even naïve C57BL/6J mice should have buried the majority of the marbles. Enhanced marble burying was observed in multiple mouse models of autism including the MIA model (Schwartzer et al. 2013; Choi et al. 2016), and in a number of transgenic mice such as the BTBR T+ tf/J18 mice, the C58J21 mice, and the Fmr1 knock-out mice (Crawley, 2012).
T-maze spontaneous alternation test
For the T-maze spontaneous alternation test the number of repetitive entries into the same goal arm in consecutive trials is used to assess repetitiveness in spontaneous exploratory behavior. Mice with ASD-related repetitive behavior would exhibit higher level of repetitive entries into the same goal arm. For naïve mice, an 80% alternation rate is typically expected. The percent alternation varied from 60% to 10% in animals expressing autism related repetitive tendency, depending on the severity of phenotypic behavior in different models. Higher percent of repetitive entries into the same goal arm was observed in multiple mouse models of autism, including eIF4E-transgenic mice (Santini et al. 2013), the MIA model (Schwartzer et al. 2013; Choi et al. 2016) and BTBR T+ tf/J18 mice.
Neonatal ultrasonic vocalization
The neonatal ultrasonic vocalization allows the assessment of intrinsic vocal communication in rodent pups. Vocalization results are typically quantified by total number of calls and/or call duration emitted in each recording session. Vocalization results among autism mouse models are inconsistent, possibly due to variation in behavioral phenotypes. In both the MIA model of ASD and in BTBR T+ tf/J mice, an increase in the number and duration of vocalization calls compared to controls has been observed (Scattoni et al. 2008; Schwartzer et al. 2013). In contrast, results of ultrasonic vocalization measurements in reelin heterozygous deficient (RLN+/−) transgenic mice are inconsistent (Romano et al. 2013; Michetti et al. 2014), while the neuroligin-4 null mutant mouse (another ASD model) presents decreased vocalizations (Ju et al. 2014). In recent years, vocalization patterns reflecting a shift of call distribution in different call categories within recorded sonograms are being analyzed, providing better insights on the changes observed in ASD mouse models (Scattoni et al. 2008; Silverman et al. 2010; Romano et al. 2013; Michetti et al. 2014). Interestingly, an increase in unstructured calls was observed in BTBR T+ tf/J and in ProSAP1/Shank2 mice (Crawley, 2012; Ey et al. 2013; Scattoni et al. 2011).
Olfactory habituation
Animals with normal olfaction should show gradual reduction in sniffing response (both the number of sniffs and sniffing duration) when the same odor is presented for the 2nd and 3rd time (habituation), and an increase of sniffing when a novel odor is presented for the first time. Both BTBR T+tf/J and Shank1 mutant mice express normal habituation and reinstatement toward non-social odors while exhibiting milder reinstatement and habituation effects toward social odors (Yang et al. 2012; Silverman et al. 2011).
Social preference test
The amount of time spent near a novel animal is used to evaluate social affiliation, social recognition and social memory in this test. Mice expressing normal sociability would spend more time in a chamber or in physical contact with a novel mouse than with an empty cup. Mice expressing normal ability to recognize social novelty would spend more time in a chamber or in physical contact with a novel mouse than with a familiar mouse. Deficits in sociability and ability to recognize social novelty have been observed in BTBR T+ tf/J, ProSAP1/Shank2, reelin deficient (Rln+/−) and in Scn1a+/− mice, as well as in the MIA model af ASD (Amodeo et al. 2012; Schwartzer et al. 2013; Choi et al. 2016; Ey et al. 2013; Romano et al. 2013; Yang et al. 2012; Han et al. 2012; Michetti et al. 2014).
Reciprocal interaction test
Animal models of ASD should show fewer interactions with a socially naïve stimulus partner, which may be accompanied by increased repetitive self-grooming activity. Utilizing the reciprocal interaction protocol, decreased interactions have been observed in Scn1a+/−, BTBR T+tf/J, and C58J mouse models of ASD (Silverman et al. 2015; Yang et al. 2012; Han et al. 2012).
Time considerations
To control for litter effect, a common confounder for behavioral experiment, only one male and one female from the same litter should be used for each behavioral test. To avoid interference associated with multiple testing, a week of buffer period between tests is highly recommended. For the marble burying test, two subjects can be tested in the same trial, by utilizing physical and visual isolation. Each trial takes five min to setup and 30 min of recording time. For the T-maze spontaneous alternation test only one subject can be tested at a time and each trial takes from 25 to 45 minutes to complete. For the neonatal ultrasonic vocalization recording only one pup can be recorded at a time, and each trial takes two minutes to setup and five minutes to record. For the olfactory habituation test one hour habituation before testing is required. Two subjects (of the same sex) can be tested in the same trial, and each trial takes 45 min to complete. For the social preference test only one subject can be tested at a time, and 45 min are typically required to complete all three phases of this test. For the reciprocal interaction test, one hour habituation before testing is required, and each trial takes about 15 min including setup and recording.
Acknowledgments
Research by the authors has been supported by grants from the National Institutes of Health (R01ES22949, P30ES07033, P42ES04696, T32ES07032, U54HD083091).
LITERATURE CITED
- Allen JL, et al. Developmental neurotoxicity of inhaled ambient ultrafine particle air pollution: Parallels with neuropathological and behavioral features of autism and other neurodevelopmental disorders. Neurotoxicology. 2016 doi: 10.1016/j.neuro.2015.12.014. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amodeo DA, Jones JH, Sweeney JA, Ragozzino ME. Differences in BTBR T+ tf/J and C57BL/6J mice on probabilistic reversal learning and stereotyped behaviors. Behav Brain Res. 2012;227:64–72. doi: 10.1016/j.bbr.2011.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen SL, Greene-Colozzi EA, Sonntag KC. A novel, multiple symptom model of obsessive-compulsive-like behaviors in animals. Biol Psychiatry. 2010;68:741–747. doi: 10.1016/j.biopsych.2010.05.011. [DOI] [PubMed] [Google Scholar]
- Angoa-Pérez M, Kane MJ, Briggs DI, Francescutti DM, Kuhn DM. Marble burying and nestlet shredding as tests of repetitive, compulsive-like behaviors in mice. J Vis Exp. 2013;82:50978. doi: 10.3791/50978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arakawa H, Blanchard DC, Arakawa K, Dunlap C, Blanchard RJ. Scent marking behavior as an odorant communication in mice. Neurosci Biobehav Rev. 2008;32:1236–1248. doi: 10.1016/j.neubiorev.2008.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett MR, Lagopoulos J. Neurodevelopmental sequelae associated with gray and white matter changes and their cellular basis: A comparison between Autism Spectrum Disorder, ADHD and dyslexia. Int J Dev Neurosci. 2015;46:132–143. doi: 10.1016/j.ijdevneu.2015.02.007. [DOI] [PubMed] [Google Scholar]
- Beynon RJ, Hurst JL. Urinary proteins and the modulation of chemical scents in mice and rats. Peptides. 2004;25:1553–1563. doi: 10.1016/j.peptides.2003.12.025. [DOI] [PubMed] [Google Scholar]
- Blackmon K, et al. Periventricular white matter abnormalities and restricted repetitive behavior in autism spectrum disorder. Neuroimage Clin. 2016;10:36–45. doi: 10.1016/j.nicl.2015.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatt GJ. The Neuropathology of Autism. Scientifica (Cairo) 2012;2012:703675. doi: 10.6064/2012/703675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branchi I, Santucci D, Alleva E. Analysis of Ultrasonic Vocalizations Emitted by Infant Rodents. Curr Protoc Toxicol. 2006 doi: 10.1002/0471140856.tx1312s30. Chapter 13, Unit 13. [DOI] [PubMed] [Google Scholar]
- Branchi I, Santucci D, Vitale A, Alleva E. Ultrasonic Vocalizations by Infant Laboratory Mice : A Preliminary Spectrographic Characterization under Different Conditions. Dev Psychobiol. 1998;33:249–256. doi: 10.1002/(sici)1098-2302(199811)33:3<249::aid-dev5>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Brudzynski SM, Kehoe P, Callahan M. Sonographic Structure of Isolation-Induced Ultrasonic Calls of Rat Pups. Dev Psychobiol. 1999;34:195–204. doi: 10.1002/(sici)1098-2302(199904)34:3<195::aid-dev4>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Choi GB, et al. The maternal interleukin-17a pathway in mice promotes autismlike phenotypes in offspring. Science. 2016;351:933–939. doi: 10.1126/science.aad0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen DL, et al. Prevalence and Characteristics of Autism Spectrum Disorder Among Children Aged 8 Years - Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2012. Morb Mortal Wkly report Surveill Summ. 2016;65:1–23. doi: 10.15585/mmwr.ss6503a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawley JN. Translational animal models of autism and neurodevelopmental disorders. Dialogues Clin Neurosci. 2012;14:293–305. doi: 10.31887/DCNS.2012.14.3/jcrawley. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crippa A, et al. Cortico-Cerebellar Connectivity in Autism Spectrum Disorder: What Do We Know So Far? Front Psychiatry. 2016;7:1–7. doi: 10.3389/fpsyt.2016.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Agostino G, et al. Palmitoylethanolamide protects against the amyloid-β25-35-induced learning and memory impairment in mice, an experimental model of Alzheimer disease. Neuropsychopharmacology. 2012;37:1784–1792. doi: 10.1038/npp.2012.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deacon RM, Rawlins JN. T-maze alternation in the rodent. Nat Protoc. 2006;1:7–12. doi: 10.1038/nprot.2006.2. [DOI] [PubMed] [Google Scholar]
- Deacon RM. Digging and marble burying in mice : simple methods for in vivo identification of biological impacts. Nat Protoc. 2006;1:122–124. doi: 10.1038/nprot.2006.20. [DOI] [PubMed] [Google Scholar]
- De Felice A, Scattoni ML, Ricceri L, Calamandrei G. Prenatal exposure to a common organophosphate insecticide delays motor development in a mouse model of idiopathic autism. PLoS One. 2015;10:e0121663. doi: 10.1371/journal.pone.0121663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ey E, et al. The Autism ProSAP1/Shank2 mouse model displays quantitative and structural abnormalities in ultrasonic vocalisations. Behav Brain Res. 2013;256:677–689. doi: 10.1016/j.bbr.2013.08.031. [DOI] [PubMed] [Google Scholar]
- Favre MR, et al. Predictable enriched environment prevents development of hyperemotionality in the VPA rat model of autism. Front Neurosci. 2015;9:1–14. doi: 10.3389/fnins.2015.00127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer J, Hammerschmidt K. Ultrasonic vocalizations in mouse models for speech and socio-cognitive disorders: Insights into the evolution of vocal communication. Genes, Brain Behav. 2011;10:17–27. doi: 10.1111/j.1601-183X.2010.00610.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson DR, Blatt GJ. Autism spectrum disorders and neuropathology of the cerebellum. Front Neurosci. 2015;9:1–16. doi: 10.3389/fnins.2015.00420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S, et al. Autistic behavior in Scn1a+/− mice and rescue by enhanced GABAergic transmission. Nature. 2012;489:385–390. doi: 10.1038/nature11356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennel S, et al. Diagnosing autism: Contemporaneous surveys of parent needs and paediatric practice. J Paediatr Child Health. 2016;52:5016–511. doi: 10.1111/jpc.13157. [DOI] [PubMed] [Google Scholar]
- Ju A, et al. Juvenile manifestation of ultrasound communication deficits in the neuroligin-4 null mutant mouse model of autism. Behav Brain Res. 2014;270:159–164. doi: 10.1016/j.bbr.2014.05.019. [DOI] [PubMed] [Google Scholar]
- Kalkbrenner AE, Schmidt RJ, Penlesky AC. Environmental chemical exposures and autism spectrum disorders: a review of the epidemiological evidence. Curr Probl Pediatr Adolesc Health Care. 2014;44:277–318. doi: 10.1016/j.cppeds.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kas MJ, et al. Assessing behavioural and cognitive domains of autism spectrum disorders in rodents: current status and future perspectives. Psychopharmacology (Berl) 2014;231:1125–46. doi: 10.1007/s00213-013-3268-5. [DOI] [PubMed] [Google Scholar]
- Kazdoba TM, Leach PT, Crawley JN. Behavioral phenotypes of genetic mouse models of autism. Genes, Brain Behav. 2016;15:7–26. doi: 10.1111/gbb.12256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King M, Bearman P. Diagnostic change and the increased prevalence of autism. Int J Epidemiol. 2009;38:1224–34. doi: 10.1093/ije/dyp261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsten TB, et al. Hypoactivity of the central dopaminergic system and autistic-like behavior induced by a single early prenatal exposure to lipopolysaccharide. J Neurosci Res. 2012;90:1903–1912. doi: 10.1002/jnr.23089. [DOI] [PubMed] [Google Scholar]
- Lainiola M, Procaccini C, Linden AM. MGluR3 knockout mice show a working memory defect and an enhanced response to MK-801 in the T- and Y-maze cognitive tests. Behav Brain Res. 2014;266:94–103. doi: 10.1016/j.bbr.2014.03.008. [DOI] [PubMed] [Google Scholar]
- Lo Pumo R, Bellia M, Nicosia A, Micale V, Drago F. Long-lasting neurotoxicity of prenatal benzene acute exposure in rats. Toxicology. 2006;223:227–234. doi: 10.1016/j.tox.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Michetti C, et al. Mapping pathological phenotypes in reelin mutant mice. Front Pediatr. 2014;2:95. doi: 10.3389/fped.2014.00095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JR, Engstrom MD. Stereotypic vocalizations in harvest mice (Reithrodontomys): Harmonic structure contains prominent and distinctive audible, ultrasonic, and non-linear elements. J Acoust Soc Am. 2010;128:1501–1510. doi: 10.1121/1.3455855. [DOI] [PubMed] [Google Scholar]
- Moy SS, et al. Repetitive behavior profile and supersensitivity to amphetamine in the C58/J mouse model of autism. Behav Brain Res. 2014;259:200–214. doi: 10.1016/j.bbr.2013.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadler JJ, et al. Automated apparatus for quantitation of social approach behaviors in mice. Genes, Brain Behav. 2004;3:303–314. doi: 10.1111/j.1601-183X.2004.00071.x. [DOI] [PubMed] [Google Scholar]
- Naviaux JC, et al. Reversal of autism-like behaviors and metabolism in adult mice with single-dose antipurinergic therapy. Transl Psychiatry. 2014;4:e400. doi: 10.1038/tp.2014.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornoy A, Weinstein-Fudim L, Ergaz Z. Prenatal factors associated with autism spectrum disorder (ASD) Reprod Toxicol. 2015;56:155–169. doi: 10.1016/j.reprotox.2015.05.007. [DOI] [PubMed] [Google Scholar]
- Patterson PH. Maternal infection and immune involvement in autism. Trends Mol Med. 2011;17:389–394. doi: 10.1016/j.molmed.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Y, et al. High-Fat-Diet-Induced Weight Gain Ameliorates Bone Loss without Exacerbating AβPP Processing and Cognition in Female APP/PS1 Mice. Front Cell Neurosci. 2014;8:225. doi: 10.3389/fncel.2014.00225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano E, Michetti C, Caruso A, Laviola G, Scattoni ML. Characterization of neonatal vocal and motor repertoire of reelin mutant mice. PLoS One. 2013;8:e64407. doi: 10.1371/journal.pone.0064407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santini E, et al. Exaggerated translation causes synaptic and behavioural aberrations associated with autism. Nature. 2013;493:411–5. doi: 10.1038/nature11782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scattoni ML, Gandhy SU, Ricceri L, Crawley JN. Unusual repertoire of vocalizations in the BTBR T+tf/J mouse model of autism. PLoS One. 2008;3:e3067. doi: 10.1371/journal.pone.0003067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scattoni ML, Ricceri L, Crawley J. Unusual Repertoire of Vocalizations in Adult BTBR T+tf/J Mice Genes Brain Behav. Genes Brain Behav. 2011;10:44–56. doi: 10.1111/j.1601-183X.2010.00623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartzer JJ, et al. Maternal immune activation and strain specific interactions in the development of autism-like behaviors in mice. Transl Psychiatry. 2013;3:e240. doi: 10.1038/tp.2013.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman JL, et al. GABAB Receptor Agonist R-Baclofen Reverses Social Deficits and Reduces Repetitive Behavior in Two Mouse Models of Autism. Neuropsychopharmacology. 2015;40:2228–2239. doi: 10.1038/npp.2015.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman JL, et al. Sociability and motor functions in Shank1 mutant mice. Brain Res. 2011;1380:120–137. doi: 10.1016/j.brainres.2010.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman JL, Yang M, Lord C, Crawley JN. Behavioural phenotyping assays for mouse models of autism. Nat Rev Neurosci. 2010;11:490–502. doi: 10.1038/nrn2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoner R, et al. Patches of disorganization in the neocortex of children with autism. N Engl J Med. 2014;370:1209–19. doi: 10.1056/NEJMoa1307491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi H, Yatsugi S, Yamaguchi T. Effect of YM992, a novel antidepressant with selective serotonin re-uptake inhibitory and 5-HT 2A receptor antagonistic activity, on a marble-burying behavior test as an obsessive-compulsive disorder model. Jpn J Pharmacol. 2002;90:197–200. doi: 10.1254/jjp.90.197. [DOI] [PubMed] [Google Scholar]
- Terranova ML, Laviola G. Scoring of Social Interactions and Play in Mice During Adolescence. Curr Protoc Toxicol. 2005;13:1–11. doi: 10.1002/0471140856.tx1310s26. [DOI] [PubMed] [Google Scholar]
- Torre-Ubieta L, et al. Advancing the understanding of autism disease mechanisms through genetics. Nat Med. 2016;116:1477–1490. doi: 10.1038/nm.4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner AH, Greenspan KS, van Erp TGM. Pallidum and lateral ventricle volume enlargement in autism spectrum disorder. Psychiatry Res Neuroimaging. 2016;252:40–45. doi: 10.1016/j.pscychresns.2016.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varcin KJ, Nelson CA. A developmental neuroscience approach to the search for biomarkers in autism spectrum disorder. Curr Opin Neurol. 2016;29:123–129. doi: 10.1097/WCO.0000000000000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vosshall LB. Social Signals: The Secret Language of Mice. Curr Biol. 2005;15:255–257. doi: 10.1016/j.cub.2005.03.027. [DOI] [PubMed] [Google Scholar]
- Warburton VL, Sales GD, Milligan SR. The emission and elicitation of mouse ultrasonic vocalizations: The effects of age, sex and gonadal status. Physiol Behav. 1989;45:41–47. doi: 10.1016/0031-9384(89)90164-9. [DOI] [PubMed] [Google Scholar]
- Wöhr M, Scattoni ML. Behavioural methods used in rodent models of autism spectrum disorders: Current standards and new developments. Behav Brain Res. 2013;251:5–17. doi: 10.1016/j.bbr.2013.05.047. [DOI] [PubMed] [Google Scholar]
- Wrenn CC, Harris AP, Saavedra MC, Crawley JN. Social transmission of food preference in mice: Methodology and application to galanin-overexpressing transgenic mice. Behav Neurosci. 2003;117:21–31. [PubMed] [Google Scholar]
- Xue R, et al. Selective inhibition of PTEN preserves ischaemic post-conditioning cardioprotection in STZ-induced Type 1 diabetic rats: role of the PI3K/Akt and JAK2/STAT3 pathways. Clin Sci. 2016;130:377–392. doi: 10.1042/CS20150496. [DOI] [PubMed] [Google Scholar]
- Yang M, CrawleyJ A. Simple Behavioral Assessment of Mouse Olfaction. Curr Protoc Neurosci. 2009:1–14. doi: 10.1002/0471142301.ns0824s48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, et al. Low sociability in BTBR T+tf/J mice is independent of partner strain. Physiol Behav. 2012;107:649–662. doi: 10.1016/j.physbeh.2011.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou J, Wang W, Pan YW, Lu S, Xia Z. Methods to measure olfactory behavior in mice. Curr Protoc Toxicol. 2015;2015:11.18.1–11.18.21. doi: 10.1002/0471140856.tx1118s63. [DOI] [PMC free article] [PubMed] [Google Scholar]


