SUMMARY
The rat A3 adenosine receptor (AR) is a recently characterized AR subtype cloned from testis and brain cDNA libraries. N6-2-(4-Amino-3-[125I]iodophenyl)ethyladenosine, a high affinity A1AR agonist, has served as the only radioligand available for study of the A3AR. The relatively low affinity of N6-2-(4-amino-3-[125I]iodophenyl)ethyladenosine for the A3AR and its greater A1AR selectivity necessitate the development of more appropriate radioligands for A3AR analysis. This report characterizes 125I-4-aminobenzyl-5′-N-methylcarboxamidoadenosine (125I-AB-MECA), a high affinity radioligand for the A3AR, in two cell lines that express this AR subtype. Membranes from Chinese hamster ovary (CHO) cells expressing the rat A3AR and from the rat mast cell line RBL-2H3 bound 125I-AB-MECA with Kd values of 1.48 ± 0.33 nM and 3.61 ± 0.30 nM, respectively. As determined by 125I-AB-MECA binding, levels of A3AR expresssion in the A3AR-CHO cell line and RBL-2H3 cells were 3.06 ± 0.21 pmol/mg and 1.02 ± 0.13 pmol/mg, respectively. Binding of 125I-AB-MECA was characterized in competition assays. In the A3AR-CHO cell line a potency order of cyclohexyl-5′-N-ethylcarboxamidoadenosine (cyclohexyl-NECA) = benzyl-NECA > (−)-N6-[(R)-phenylisopropyl]adenosine = NECA was observed, and in RBL-2H3 cells (−)-N6-[(R)-phenylisopropyl]adenosine and NECA were equipotent. Xanthine amine congener (XAC) and 8-cyclopentyl-1,3-dipropylxanthine did not significantly inhibit 125I-AB-MECA binding. The parent compound, AB-MECA, dose-dependently inhibited forskolin-stimulated adenylyl cyclase activity in A3AR-CHO cell membranes. 125I-AB-MECA bound to the rat A1AR and canine A2aAR expressed in COS-7 cells with Kd values of 3.42 ± 0.43 nM and 25.1 ± 12.6 nM, respectively. This binding was significantly reduced in the presence of 1 μM XAC. In RBL-2H3 cells, XAC had no effect on 125I-AB-MECA affinity and reduced the level of radioligand binding by ~5%.
Many of the varied physiologic effects of adenosine are the result of its activation of specific cell surface ARs. Classification of ARs into subtypes has been based on the biochemical effects of receptor activation, the pharmacologic properties of the ARs, and, more recently, cDNA structures (1, 2). Presently, based on these criteria, four AR subtypes have been described, the A1AR, A2aAR, A2bAR, and A3AR. The entire cDNA for the A3AR was originally isolated from a rat testis cDNA library (3), and characterization of this cDNA was performed after its isolation from a rat brain cDNA library (4). The classification of the protein encoded by this cDNA as a previously unrecognized AR subtype was based on 1) the fact that in its putative transmembrane domain regions the protein has 58% and 57% amino acid identity to the A1AR and A2aAR, respectively, and 2) the ability of the expressed protein to specifically bind adenosine and several of its agonist analogs, including 125I-APNEA, (R)-PIA, and NECA, with relatively high affinity (4). Several properties of the expressed A3AR are unique to this subtype, compared with the A1AR and A2ARs, including its 15 nM affinity for 125I-APNEA, the potency series of (R)-PIA = NECA > (S)-PIA in displacing 125I-APNEA binding, and its insensitivity to the methylxanthine class of compounds, several of which are high affinity AR antagonists (4).
PCR analysis of reverse transcribed rat mRNA revealed the highest level of A3AR expression to be in the testis, with moderate expression in the kidney, lung, and heart (4). Lower levels of expression were observed in brain regions such as cerebral cortex, striatum, and olfactory bulb (4).
Recently, a cDNA coding for a protein of identical size (317 amino acids) and 72% overall amino acid identity, compared with the rat A3AR, was isolated from a sheep pars tuberalis library (5). Based on distinct pharmacologic differences of the expressed protein and significant variations in tissue expression, relative to the rat A3AR, it is unclear whether this sheep AR represents a species homolog of the A3AR or a distinct receptor subtype.
With the recent recognition of the existence of the A3AR, this receptor has been implicated as the AR involved in various physiologic responses to adenosine. Ramkumar et al. (6) demonstrated that the ability of adenosine to enhance antigen-induced secretory responses in the rat mast cell line RBL-2H3 is the result of A3AR activation. Findings also suggest that the A3AR mediates the hypotensive response produced by APNEA, (R)-PIA, and NECA administration in the pithed rat (7).
Currently, 125I-APNEA is the preferred ligand for the study of the rat A3AR (4, 6). However, the affinity of 125I-APNEA (Kd of 15 nM and 34 nM for CHO cells expressing the A3AR and RBL-2H3 cells, respectively) for the A3AR requires dilution of the specific activity of the radioligand with unlabeled iodinated APNEA to obtain a valid determination of A3AR binding parameters in saturation analyses. Furthermore, the use of 125I-APNEA to study the A3AR in native tissues containing multiple AR subtypes may be hampered by the approximately 10–20-fold greater affinity of this radioligand for the A1AR, compared with the A3AR. This paper describes the characterization of 125I-AB-MECA as an agonist radioligand for the A3AR, demonstrating approximately 10-fold greater affinity for this receptor subtype, compared with 125I-APNEA.
Experimental Procedures
Materials
All cell culture supplies and G418 (Geneticin) were from GIBCO-BRL (Gaithersburg, MD). (R)-PIA and papaverine were from Sigma Chemical Co. (St. Louis, MO). XAC and CPX were purchased from Research Biochemicals International (Natick, MA). NECA, adenosine deaminase, and all restriction enzymes were from Boehringer-Mannheim (Indianapolis, IN). Forskolin was obtained from Calbiochem (La Jolla, CA). Na125I and [α-32P]ATP were from DuPont-New England Nuclear (Boston, MA). The synthesis of cyclohexyl-NECA (8) and benzyl-NECA (9) was as described 125I-APNEA was prepared as described previously (10).
Cell culture and DNA transfection
CHO cells were maintained in Ham’s F12 nutrient mixture containing 10% fetal bovine serum. COS-7 cells and RBL-2H3 cells (supplied by Dr. L. A. Kindman, Duke University) were both grown in Dulbecco’s modified Eagle’s medium containing 10% fetal bovine serum. All media were supplemented with penicillin (100 units/ml) and streptomycin (100 μg/ml). A CHO cell line stably expressing the rat A3AR cDNA (4) subcloned into the HindIII/SmaI sites of the expression vector pCMV5 (Dr. D. Russell, University of Texas Southwestern) was created by co-transfection of the expression vector-cDNA construct with the neomycin resistance vector pSVneo (Pharmacia, Piscataway, NJ) via a calcium phosphate precipitation and glycerol shock technique (11), as described previously for the bovine A1AR (12). Cell colonies resistant to G418 (400 μg/ml) were selected, expanded, and screened for A3AR expression by 125I-APNEA binding. The rat A1AR cDNA and sheep A3AR (both kindly provided by Dr. S. Reppert, Harvard Medical School) subcloned into pCMV5 (rat, EcoRI/XbaI sites; sheep, HindIII/XbaI sites) and canine A2aAR cDNA subcloned into pBC12BI (provided by Dr. G. Vassart, Université Libre de Bruxelles, Brussels, Belgium) were transiently transfected into COS-7 cells by a DEAE-dextran procedure (13). COS-7 cells were used for binding assays approximately 72 hr after transfection.
Synthesis and radioiodination of AB-MECA
A detailed synthesis of AB-MECA is described elsewhere (9) and followed the general method of Olsson et al. (8). The compound was characterized by NMR spectroscopy, mass spectroscopy, and elemental analysis. The melting point was determined to be 135° (decomposition). A genuine sample of 3-[127I]iodo-AB-MECA (m.p. 132°, decomposition) was prepared by an unambiguous synthetic method, starting from 4-amino-3-iodobenzylamine (9), as a reference standard for identification of the iodinated product and for structural assignment of the product as shown in Fig. 1. Radioactive (see below) and nonradioactive iodinated AB-MECA coeluted upon HPLC separation, using two solvent systems. The solvents used were isocratic 60% (v/v) methanol/40% (v/v) 20 mM ammonium formate, pH 8.0, and 50 mM NH4H2PO4, pH 5.2/9% (v/v) methanol/1% (v/v) acetonitrile, to give retention times of 9.2 min and 3.9 min, respectively (Waters μBondapak C18 column; 25 × 0.46 cm; flow rate, 0.75 ml/min).
Fig. 1.
Structure of 125I-AB-MECA. The radioiodination of the compound is described in the text.
Radioiodination of AB-MECA and separation of the labeled ligand by HPLC was essentially as described previously for 125I-APNEA (10). Briefly, 0.2 mg of AB-MECA was dissolved in 1 ml of methanol, and 10 μl of this solution were taken to dryness under vacuum. The dried material was resuspended in 40 μl of 0.3 M NaH2PO4, pH 7.55. To this 1.5 mCi of Na125I was added, followed by 10 μl of a 1 mg/ml chloramine T solution. After 4 min at room temperature, the reaction was stopped by addition of 15 μl of a 0.5 mg/ml sodium metabisulfite solution. The entire solution was applied to a Waters 501 HPLC system. Products were separated using an isocratic protocol and a Waters μBondapak C18 column equilibrated with a mobile phase of 60% (v/v) methanol/40% (v/v) 20 mM ammonium formate, pH 8.0. The 125I-AB-MECA peak was defined by both UV absorbance and incorporation of 125I.
Radioligand binding assays
Radioligand binding assays for the rat A3AR and A1AR, using both transfected CHO and COS-7 cells, were performed as described previously (12). Briefly, cells were washed twice with 10 ml of 10 mM Tris, 5 mM EDTA, pH 7.4 at 5°, and then scraped into 5 ml of the same buffer. The cells were disrupted by 20 strokes by hand in a glass homogenizer on ice. The suspension was centrifuged at 43,000 × g for 10 min and the resulting pellet was resuspended in 50 mM Tris, 10 mM MgCl2, 1 mM EDTA, pH 8.26 at 5° (50/10/1 buffer), to yield a protein concentration of 0.02 mg/ml. Adenosine deaminase was added to give a final concentration of 2 units/ml. Assays were conducted at 37° for 60 min and terminated by filtration using a Brandel cell harvester and rapid washing with 50/10/1 buffer containing 0.01% CHAPS, over glass fiber filters that had been pretreated with 0.3% polyethylenimine. Radioligand binding assays with membranes prepared from COS-7 cells transfected with the A2aAR cDNA were performed in a similar manner except that the binding buffer consisted of 50 mM HEPES, 10 mM MgCl2, pH 6.8 (14). Saturation binding assays were performed using 10 μM (R)-PIA to define nonspecific binding and typically used concentrations of 125I-AB-MECA ranging from 0.25 nM to 3.0–6.0 nM. Additionally, a set of experiments were performed with RBL-2H3 cell membranes in which nonradioactive 127I-AB-MECA was used to dilute the specific activity of the radioligand, to enable concentrations of ligand of 100–150 nM to be used. Competition binding assays were performed with a concentration of 125I-AB-MECA approximately equal to the Kd value for the radioligand in the particular cell type. For 125I-AB-MECA binding experiments conducted in the presence of XAC, the membrane suspension was treated with the antagonist and then immediately added to assay tubes and the incubation was started. Data were analyzed by a previously described computer modeling system (15). For all competing ligands, Hill coefficients were not significantly different from unity. IC50 values obtained from computer analysis of competition curves were converted to Ki values by using the Cheng-Prusoff equation (16). Protein concentrations were determined by the method of Bradford (17).
Adenylyl cyclase assays
Assays of adenylyl cyclase activity of membranes prepared from CHO cells stably expressing the A3AR were performed by the method of Salomon et al. (18). Briefly, cell membranes were prepared and pelleted as described for radioligand binding assays. Membranes were resuspended in 75 mM Tris, 200 mM NaCl, 1.25 mM MgCl2, pH 8.12 at 4° (TNM buffer), to give a final concentration of 0.1 mg/ml, and 2 units/ml adenosine deaminase was added. Adenylyl cyclase assays consisted of 40 μl of membrane suspension, 40 μl of cyclase mixture (TNM buffer supplemented with 140 μM dATP, 5 μM GTP, 30 units/ml creatine kinase, 5 mM creatine phosphate, 2.2 mM dithiothreitol, 100 μM papaverine, and 1.5 μCi of [α-32P]ATP), and 20 μl of test compounds. Assays were conducted at 30° for 15 min and terminated by addition of a stop solution containing 20,000 cpm/ml [3H]cAMP. Labeled cAMP was isolated by sequential chromatography over Dowez and alumina columns, and quantities were determined by liquid scintillation counting. For all assays, adenylyl cyclase was activated with 1 μM forskolin, which typically induced a 6–8-fold stimulation of the enzyme.
Results and Discussion
An analysis of ligand-receptor structure-activity relationships for the rat A3AR has revealed that, of the compounds examined, N6,5′-disubstituted adenosine analogs demonstrate the highest affinity for this AR subtype.1 One such compound, AB-MECA, was radioiodinated (Fig. 1) and its binding to membranes prepared from CHO cells stably expressing the rat A3AR was examined. Initial experiments using approximately 1 nM 125I-AB-MECA demonstrated specific binding of the radioligand by the A3AR. Specific binding of 125I-AB-MECA to membranes obtained from nontransfected CHO cells was negligible. When conducted at 37°, 125I-AB-MECA binding to stably transfected CHO cells reached equilibrium at approximately 25 min of incubation and remained relatively stable over 90 min (data not shown). Saturation binding analysis demonstrated that binding parameters (Kd and Bmax values) did not vary if the incubation was conducted for 30 min or 60 min at 37°. An incubation of 60 min was used for all subsequent assays. As shown in Fig. 2A, 125I-AB-MECA binding was saturable, specific, and of high affinity. In four experiments, Kd and Bmax values of 1.48 ± 0.33 nM and 3.06 ± 0.21 pmol/mg, respectively, were obtained. In contrast, 125I-APNEA bound with a Kd of approximately 15 nM (data not shown). To further analyze the binding properties of 125I-AB-MECA, this radioligand was used in competition binding assays with a series of adenosine analogs (Fig. 2B; Table 1). The rank order of potencies for displacement of 125I-AB-MECA from CHO cell membranes was cyclohexyl-NECA = benzyl-NECA > (R)-PIA = NECA. The relatively high affinities of the N6,5′-disubstituted analogs and the equal potencies of (R)-PIA and NECA are in agreement with previous studies of the A3AR using 125I-APNEA as radioligand1 It was found that, among N6 substituents, the benzyl group was best suited for A3AR selectivity, and the group was compatible with the A3AR-enhancing effect of the 5′-uronamides (9). Additionally, 125I-AB-MECA binding was insensitive to xanthine antagonist compounds, inasmuch as the potent A1AR antagonist CPX, at a concentration of 10 μM, inhibited binding by 8.5 ± 2.7% (three experiments).
Fig. 2.
Binding of 125t-AB-MECA to CHO cells stably expressing the rat A3AR. Membrane preparation and radioligand binding procedures are described in the text. A, Binding isotherm of 1251-AB-MECA. ●, Total binding; ■, nonspecific binding, which was defined with 10 μM (R)-PIA. Points represent the mean of duplicate samples. Computer analysis of this experiment yielded Kd and Bmax values of 1.29 nm and 2.57 pmol/mg, respectively. Inset, Scatchard transformation of the same data, with symbols representing specific binding. This experiment was repeated three times with similar results. B, Agonist competition for 125I-AB-MECA binding. Concentration of agonist is shown on the x-axis, and 125I-AB-MECA was present at a concentration of 1.1 nM. The amount of 125l-AB-MECA binding was normalized and expressed as percentage. The best fit lines for (R)-PIA and NECA were superimposable. In this experiment, Ki values for cydohexyl-NECA (CH-NECA), benzyl-NECA, (R)-PIA, and NECA were 21.8 nM, 50.9 nM, 123.3 nM, and 130.6 nM, respectively. Similar experiments were performed five to eight times.
TABLE 1. Agonist competition for 125l-AB-MECA binding.
Binding was performed using two different cell types that contain the A3AR. A3CHO represents CHO cells stably transferred with the rat A3AR cDNA. Ki values represent the mean ± standard error. The number of times each experiment was performed is given in parentheses.
| Ligand |
Ki
|
|
|---|---|---|
| A3CHO | RBL-2H3 | |
| nM | ||
| Cyctobexyt-NECA | 33.1 ± 10.1 (5) | NDa |
| Benzyl-NECA | 43.7 ± 3.6 (5) | ND |
| (R)-PIA | 217.9 ±42.7 (8) | 166.0 ±40.0 (3) |
| NECA | 260.5 ± 36.1 (8) | 223.3 ± 37.0 (3) |
ND, not determined.
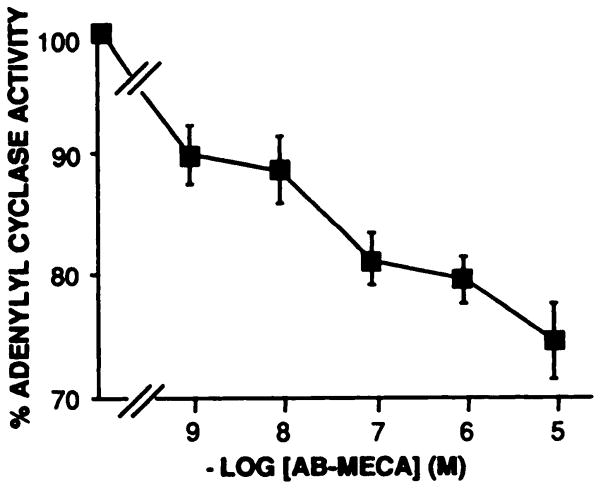
The A3AR has previously been shown to be coupled to the inhibition of adenylyl cyclase via a pertussis toxin-sensitive G protein (4). To demonstrate the agonist activity of AB-MECA, the ability of the noniodinated compound to inhibit forskolin-stimulated adenylyl cyclase activity in membranes prepared from stably transfected CHO cells was examined. As shown in Fig. 3, AB-MECA inhibited forskolin-stimulated adenylyl cyclase activity in a dose-dependent fashion, with inhibition of approximately 25% at 10 μM. The agonist nature of 125I-AB-MECA was also indicated by the effect of guanine nucleotides on binding of the radioligand to membranes prepared from the stably transfected CHO cells. In three experiments, the inclusion of 10 μM Gpp(NH)p in the binding assay did not affect the affinity of the binding interaction (Kd values of 1.25 ± 0.16 nM and 1.70 ± 0.18 nM in control and Gpp(NH)p-treated membranes, respectively). However, the population of receptors in this high affinity state was reduced by 49.7 ± 4.3% by Gpp(NH)p treatment.
Fig. 3.
Inhibition of forskolin-stimulated adenytyl cyclase activity by AB-MECA. The preparation of membranes from CHO cells stably expressing the rat A3AR and conditions of the assay are described In the text. Forskolin was present at a concentration of 1 μM, which typically produced a 6–8-fold stimulation of adenytyl cyclase activity (100% response). Each point represents the mean ± standard error of three experiments, each performed in duplicate.
To characterize 125I-AB-MECA binding in a native tissue expressing the A3AR, the rat mast cell line RBL-2H3 was selected. The presence of the A3AR in these cells was recently demonstrated pharmacologically in ligand binding assays, functionally in antigen-induced secretion assays, and by Northern blotting and PCR analysis of reverse-transcribed mRNA obtained from the cells (6). Furthermore, PCR analysis indicated that these cells do not express A1ARs or A2ARs (6). As shown in Fig. 4A, 125I-AB-MECA binding to membranes prepared from RBL-2H3 cells was similar to that observed for the stable CHO cell line. In five experiments, the calculated Kd and Bmax values were 3.61 ± 0.30 nM and 1.02 ± 0.13 pmol/mg, respectively. Dilutional saturation curves using maximum concentrations of ligand of 100–150 nM yielded Kd and Bmax values of 3.04 ± 0.33 nM and 0.87 ± 0.08 pmol/mg, respectively (four experiments). Interestingly, the approximately 2.5-fold lower affinity of the RBL-2H3 cell A3AR, compared with the receptor in the stable A3AR-CHO cell line, is similar to the difference observed for 125I-APNEA affinities in the two cell types (34 nM versus 15 nM). Again, to demonstrate the pharmacologic characteristics of 125I-AB-MECA and A3AR binding, competition binding assays were performed (Fig. 4B; Table 1). As described previously (4, 6), (R)-PIA and NECA were equipotent at the A3AR and 10 μM CPX displaced only 4.65 ± 0.3% of the 125I-AB-MECA binding (two experiments).
Fig. 4.
Binding of 125I-AB-MECA to membranes prepared from RBL-2H3 cells. Membrane preparation and radioligand binding procedures are described in the text. A, Binding isotherm of 125I-AB-MECA. ●, Total binding; ■, nonspecific binding, which was defined with 10 μM (R)-PIA. Points represent the mean of duplicate samples. Computer analysis of this experiment yielded Kd and Bmax values of 2.99 nM and 0.99 pmol/mg, respectively. Inset, Scatchard transformation of the same data, with the symbols representing specific binding. The experiment was repeated four times with similar results. B, Agonist competition for 125I-AB-MECA binding. Concentration of agonist is shown on the x-axis, and 125I-AB-MECA was present at a concentration of 1.1 nM. The amount of 125I-AB-MECA binding was normalized and expressed as percentage. In this experiment, Ki values for (R)-PIA and NECA were 140.0 nM and 240.0 nM, respectively. This experiment was performed three times.
To ascertain the utility of 125I-AB-MECA in the study of A3AR8 in tissues containing a mixed population of AR subtypes, the binding of the radioligand to membranes prepared from COS-7 cells transfected with either the rat A1AR or canine A2aAR cDNAs was examined. 125I-AB-MECA bound to the rat AiAR with a Kd of 3.42 ± 0.43 nM (eight experiments), compared with a Kd of 1.32 ± 0.35 nM (four experiments) for 125I-APNEA. Binding of 125I-AB-MECA to the A2aR was of lower affinity (Kd = 25.1 ± 12.6 nM, six experiments) and a much lower level of total binding was obtained, thus resulting in a substantially lower percentage of specific binding, compared with the rat A3AR and A1AR. The lack of binding of xanthine antagonists by the A3AR (4) enables the use of these compounds to mask 125I-AB-MECA binding by other AR subtypes, while A3AR binding of the radioligand is unaffected. Ki values for XAC of 4.6 nM and 50 nM for the purified rat brain A1AR (19) and rat striatal A2aAR (20), respectively, have been reported. Therefore, 125I-AB-MECA saturation curves were constructed in the presence of 1 μM XAC using membranes prepared from COS-7 cells expressing either the A1AR or the A2aAR and from RBL-2H3 cells. In three experiments, binding of 125I-AB-MECA to the A1AR was virtually abolished by coincubation with 1 μ XAC. In general, coincubation of the A2aAR with XAC resulted in complete inhibition of 125I-AB-MECA binding at lower concentrations of the radioligand, whereas binding was reduced ~50–75% at the maximum concentration (~4 nM) used. Conversely, in the presence of 1 μM XAC the affinity of 125I-AB-MEC A for the RBL-2H3 cell A3AR was unchanged and the Bmax value was decreased by only 5.7 ± 0.9% (three experiments).
To extend the characterization of 125I-AB-MECA as a high affinity A3AR ligand and to further compare the rat A3AR with the recently cloned sheep A3AR (5), the binding of the radioligand to membranes from COS-7 cells transiently expressing the sheep A3AR was examined. 125I-AB-MECA bound with Kd and Bmax values of 4.36 ± 0.48 nM and 1.35 ± 0.10 pmol/mg, respectively (six experiments). Thus, 125I-AB-MECA has an affinity for the sheep A3AR similar to that of N6-(4-amino-3-[125I]iodobenzyl)adenosine (Kd = 6 nM), which was the radioligand used to initially characterize this receptor (5). The high affinity binding of 125I-AB-MECA does not appear to be limited to the rat A3AR, and this compound may be a radioligand of choice for receptors of this subtype. Although it possesses slightly higher affinity for the rat versus sheep A3AR, 125I-AB-MECA would not appear useful for the further definition of these two receptors as unique AR subtypes or as species homologs. The lack of discrimination demonstrated by 125I-AB-MECA is certainly not apparent for other classical AR ligands such as cyclopentyladenosine and several members of the xanthine antagonist series. Thus, these compounds would be preferred for the characterization of ARs constituting the A3AR subtype.
In summary, this report characterizes 125I-AB-MECA as an agonist radioligand for the rat A3AR. 125I-AB-MECA has much greater affinity for the A3AR than does 125I-APNEA, which had previously been the most useful radioligand for the study of this recently described AR subtype. The availability of 125I-AB-MECA should permit better analysis of the tissue distribution of the A3AR via radioligand binding assays and autoradiography. The high affinity of 126I-AB-MECA for the A3AR will be useful in future studies of receptor regulation and in the study of the structural requirements of AR-ligand interactions.
Acknowledgments
G.L.S. was supported by National Heart, Lung, and Blood Institute Specialized Center of Organized Research Grant P50HL17670 in Ischemic Disease, in part by National Heart, Lung, and Blood Institute Grant RO1HL35134, and by Grant-in-Aid 91008200 from the American Heart Association.
ABBREVIATIONS
- AR
adenosine receptor
- APNEA
N6-2-(4-aminophenyl)ethyladenosine
- (R)-PIA
(−)-N6-[(R)-phenylisopropyl]adenosine
- NECA
5′-N-ethylcarboxamidoadenosine
- (S)-PIA
(+)-N6-[(S)-phenylisopropyl]adenosine
- AB-MECA
4-aminobenzyl-5′-N-methylcarboxamidoadenosine
- CPX
8-cyclopentyl-1,3-dipropylxanthine
- CHO
Chinese hamster ovary
- XAC
xanthine amine congener
- benzyl-NECA
N6-benzyl-5′-N-ethylcarboxamidoadenosine
- cyclohexyl-NECA
N6-cyclohexyl-5′-N-ethylcarboxamidoadenosine
- PCR
polymerase chain reaction
- HPLC
high performance liquid chromatography
- HEPES
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid
- CHAPS
3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate
- Gpp(NH)p
guanosine-5′-(β,γ-imido)triphosphate
Footnotes
P. J. M. van Galen, A. H. Van Bergen, C. Gallo-Rodriguez, N. Melman, M. E. Olah, A. P. IJzerman, G. L. Stiles, and K. A. Jacobson. A binding site model and structure-activity relationships for the rat A3 adenosine receptor. Manuscript in preparation.
References
- 1.Olah ME, Stiles GL. Adenosine receptors. Annu, Rev Physiol. 1992;54:211–225. doi: 10.1146/annurev.ph.54.030192.001235. [DOI] [PubMed] [Google Scholar]
- 2.Tucker AL, Linden J. Cloned receptors and cardiovascular responses to adenosine. Cardiovasc Res. 1993;27:62–67. doi: 10.1093/cvr/27.1.62. [DOI] [PubMed] [Google Scholar]
- 3.Meyerhof W, Muller-Brechlin R, Richter D. Molecular cloning of a novel putative G-protein coupled receptor expressed during rat spermiogenesis. FEBS Lett. 1991;284:155–160. doi: 10.1016/0014-5793(91)80674-r. [DOI] [PubMed] [Google Scholar]
- 4.Zhou Q-Y, Li C, Olah ME, Johnson RA, Stiles GL, Civelli O. Molecular cloning and characterization of an adenosine receptor: the A3 adenosine receptor. Proc Natl Acad Sci USA. 1992;89:7432–7436. doi: 10.1073/pnas.89.16.7432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Linden J, Taylor HE, Robeva AS, Tucker AL, Stehle JH, Rivkees SA, Fink JS, Reppert SM. Molecular cloning and functional expression of a sheep A3 adenosine receptor with widespread tissue distribution. MoL Pharmacol. 1993;44:524–532. [PubMed] [Google Scholar]
- 6.Ramkumar V, Stiles GL, Beaven MA, Ali H. The A3 adenosine receptor is the unique adenosine receptor which facilitates release of allergic mediators in mast cells. J BioL Chem. 1993;268:16887–16890. [PubMed] [Google Scholar]
- 7.Fozard JR, Carruthers AM. Adenosine A3 receptors mediate hypotension in the angiotensin II-supported circulation of the pithed rat. Br J Pharmacol. 1993;109:3–5. doi: 10.1111/j.1476-5381.1993.tb13522.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Olsson RA, Kusachi S, Thompson RD, Ukena D, Padgett W, Daly JW. N6-substituted N-alkyladenosne-5′-uronamides: bifunctional ligands having recognition groups for A1 and A2 adenosine receptors. J Med Chem. 1986;29:1683–1689. doi: 10.1021/jm00159a020. [DOI] [PubMed] [Google Scholar]
- 9.Gallo-Rodriguez C, Ji X-D, Melman N, Siegman BD, Sanders LH, Orlina J, Pu Q, van Galen PJM, Stiles GL, Jacobson KA. Structure-activity relationships at A3-adenosine receptors. J Med Chem. 1994;37:636–646. doi: 10.1021/jm00031a014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stiles GL, Daly DT, Olsson RA. The A1 adenosine receptor identification of the binding subunit by photoaffinity crosslinking. J BioL Chem. 1985;260:10806–10811. [PubMed] [Google Scholar]
- 11.Maniatis T, Fritsch EF, Sambrook J. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory; Cold Spring Harbor, NY: 1989. [Google Scholar]
- 12.Olah ME, Ren H, Ostrowski J, Jacobson KA, Stiles GL. Cloning, expression and characterization of the unique bovine A1 adenosine receptor: studies on the ligand binding site by site-directed mutagenesis. J BioL Chem. 1992;267:10764–10770. [PMC free article] [PubMed] [Google Scholar]
- 13.Cullen BR. Use of eukaryotic expression technology in the functional analysis of cloned genes. Methods EnzymoL. 1987;152:684–704. doi: 10.1016/0076-6879(87)52074-2. [DOI] [PubMed] [Google Scholar]
- 14.Nanoff C, Jacobson KA, Stiles GL. The A2 adenosine receptor. guanine nucleotide modulation of agonist binding is enhanced by proteolysis. MoL Pharmacol. 1991;39:130–135. [PMC free article] [PubMed] [Google Scholar]
- 15.DeLean A, Hancock AA, Lefkowitz RJ. Validation and statistical analysis of a computer modeling method for quantitative analysis of radioligand binding data for mixtures of pharmacological receptor subtypes. MoL Pharmacol. 1982;21:5–16. [PubMed] [Google Scholar]
- 16.Cheng Y, Prusoff WH. Relationship between the inhibition constant (Ki) and the concentration of an inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol. 1973;22:3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- 17.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 18.Salomon Y, Londos C, Rodbell M. A highly sensitive adenylate cyclase assay. Anal Biochem. 1974;58:541–548. doi: 10.1016/0003-2697(74)90222-x. [DOI] [PubMed] [Google Scholar]
- 19.Nakata H. Purification of A1 adenosine receptor from rat brain membranes. J Biol Chem. 1989;264:16545–16551. [PubMed] [Google Scholar]
- 20.Jarvis MF, Schulz R, Hutchison AJ, Do UH, Sills MA, Williams M. [3H]CGS21680, a selective A2 adenosine receptor agonist, directly labels A2 receptors in rat brain. J Pharmacol Exp Ther. 1989;251:888–893. [PubMed] [Google Scholar]