Abstract
The immune context of tumors has significant prognostic value and is predictive of responsiveness to several forms of therapy, including immunotherapy. We report here that CD8+ T cell frequency and functional orientation within the tumor microenvironment is regulated by β2-adrenergic receptor (β-AR) signaling in host immune cells. We used three strategies - physiologic (manipulation of ambient thermal environment), pharmacologic (β-blockers), and genetic (β2-adrenergic receptor knockout mice) to reduce adrenergic stress signaling in two widely studied preclinical mouse tumor models. Reducing β-AR signaling facilitated conversion of tumors to an immunologically active tumor microenvironment with increased intra-tumoral frequency of CD8+ T cells with an effector phenotype and decreased expression of PD-1, in addition to an elevated effector CD8+ T cell to CD4+ regulatory T cell ratio (IFN-γ+CD8+:Treg). Moreover, this conversion significantly increased the efficacy of anti-PD-1 checkpoint blockade. These data highlight the potential of adrenergic stress and norepinephrine-driven β-adrenergic receptor signaling to regulate the immune status of the tumor microenvironment and supports the strategic use of clinically available β-blockers in patients to improve responses to immunotherapy.
Keywords: Adrenergic signaling, Chronic stress, Checkpoint inhibitors, Breast cancer, Melanoma, Animal models of cancer
INTRODUCTION
Immune checkpoint blockade is becoming first line therapy for patients with melanoma and several other types of cancer (1,2). However, despite remarkable and durable responses, long term control of tumors is achieved in only a fraction of patients. Extensive pre-clinical and clinical studies to identify the differences between responders and nonresponders demonstrate a lack of anti-tumor T cell activity within the tumor microenvironment (TME) of non-responders (3–6). Multiple lines of evidence indicate that checkpoint inhibitors are more effective in combination with treatments that generate a robust CD8+ T cell infiltrate, including radiation (7), chemotherapy (8), and immune activating agents (9). Additionally, clinical studies indicate that the immunoscore, a measurement of the functional orientation, density, and location of specific immune cell subsets in the TME, has significant prognostic value (5,10,11). However, understanding underlying mechanisms that could be targeted to improve T cell infiltration remains a challenge (3,6).
We recently reported that ambient housing temperature plays an unexpected role in regulating the anti-tumor immune response in murine tumor models. We found that the mild, chronic cold stress caused by the required sub-thermoneutral housing temperatures (~22°C) mandated by the Institutional Animal Care and Use Committee (12,13) suppresses the anti-tumor immune response which can be reversed by housing mice at thermoneutral temperatures (~30°C) (14). Housing mice at 30°C increases the frequency of intra-tumoral effector CD8+ T cells, correlating with significantly improved control of tumor growth (14). However, the underlying mechanisms were not identified in this study.
Cold exposure causes activation of the sympathetic nervous system (SNS) and norepinephrine (NE) mediated adaptive thermogenesis to maintain a normal core body temperature (~37°C). Previously, we demonstrated that the mild cold stress experienced by laboratory mice at 22°C is, in fact, sufficient to cause elevated norepinephrine (NE) in comparison to mice housed at 30°C (15,16). In addition to the role of NE in heat production, several investigators have shown that increased signaling of NE through β-adrenergic receptors (β-ARs) on immune cells can significantly suppress immune cell function (17). However, the role of adrenergic signaling in regulating anti-tumor immune suppression remains unclear. Therefore, in this study, we sought to determine if adrenergic signaling was the mechanism mediating suppression of the anti-tumor immune response in mice housed at 22°C compared to 30°C.
Previous studies showing that tumors actually release neurotrophic factors which stimulate outgrowth of fibers from sympathetic ganglia was first observed in vitro in a landmark study by Cohen et al. in 1954 (18). Recently, Magnon et al. (19) demonstrated that sympathetic input to tumors is required for the initiation and growth of primary tumors in a model of prostate cancer, thus demonstrating that neurogenesis of autonomic fibres plays a significant role in tumor growth and progression. Cumulatively, these and many other studies have made it clear that the release of catecholamines, primarily NE, in response to a variety of stresses facilitates tumor initiation, growth and progression (20–22).
In non-tumor settings, adrenergic signaling clearly inhibits CD8+ T cell responses. Grebe et al. (23) have shown that anti-influenza CD8+ T cell responses in vivo are limited by adrenergic signaling, and Estrada et al. (24) clearly demonstrate suppression of effector function by β2-AR signaling in both human and mouse CD8+ T cells. These studies support the idea that adrenegic signaling could suppress anti-tumor immunity, however, the impact of adrenergic stress on the development of anti-tumor immunity, the immune contexture of tumors, or the role that β-AR signaling may have in dictating the sensitivity or resistance of tumors to checkpoint inhibitor therapy has received virtually no attention.
Overall, these inhibitory effects of adrenergic signaling on CD8+ T cell responses, taken together with our previous work on the effects of ambient housing temperature on NE levels, tumor growth, and the anti-tumor immune response, suggest that increased adrenergic signaling is a critical mechanism underlying suppression of the anti-tumor immune response. Here, using the pan-β-AR blocker propranolol, as well as β2-AR receptor knockout mice (Adrb2−/−) in which the immune cells are non-responsive to adrenergic signaling (24), we report for the first time that the mechanism mediating differences in tumor growth and the anti-tumor immune response observed between mice housed at 22°C and 30°C, are dependent upon β-AR signaling. Furthermore, reducing β-AR signaling either by housing mice at 30°C or by treating mice at 22°C with propranolol, we discovered that we could “reset” the baseline immune response in murine tumor models and significantly improve responses to anti-PD-1 checkpoint blockade therapy.
These data highlight factors that directly affect interpretation of data obtained in murine tumor models since nearly all preclinical mouse work is conducted at 22°C. Additionally, they also reveal, for the first time, the critical role that adrenergic stress plays in shaping the phenotype of CD8+ T cells in the TME and its role in constraining the efficacy of checkpoint inhibitors. Taken together, these data provide the first evidence that using FDA approved β-blockers is a viable strategy to improve checkpoint inhibitor efficacy.
METHODS
Mice
Female BALB/cAnNcr (BALB/c) and C57BL/6NCr (C57BL/6) were purchased from Charles River and C.B. Igh-1b Icr Tac Prkdc scid (SCID) mice from the Laboratory Animal Resource at RPCI. BALB/c mice globally deficient in β2-ARs (Adrb2−/−) were provided by David Farrar (University of Texas Southwestern Medical Center) (24). OT-1 (B6.129S7-Rag1tm1Mom Tg[TcraTcrb]1,100Mjb) mice were provided by Dr. Minhyung Kim (RPCI). Mice were 8–12 weeks old and were maintained in specific pathogen-free facilities. All studies followed approved protocols and were performed under the guidelines established by the Institutional Animal Care and Use Committee at RPCI.
Cell culture and tumor models
4T1 tumor cells were purchased from and authenticated by ATCC in 2014. B16-OVA tumor cells were provided by Dr. Protul Shrikant in 2012 and have not been genetically authenticated by our laboratory. Cell lines were confirmed to be Mycoplasma negative using the Mycoplasma Plus PCR Primer Set (Aligent Technologies, 302008). Both cell lines were cultured in RPMI 1640 (Gibco) supplemented with 10% FBS, 1% L-glutamine, and 1% Penicillin/Streptomycin. Once thawed, cells were passed twice prior to use. 1×104 4T1 cells in 100μl PBS were injected into 4th mammary fat-pad. 2×105 B16-OVA cells in 100μl PBS were injected subcutaneously into the lower left abdomen. Tumor growth was monitored throughout experiments; perpendicular diameters (width/length) were measured 2–3x/week and tumor volume calculated = W2 × L/2 mm3.
Ambient temperature manipulation
Mice were housed five/cage in Precision Refrigerated Plant-Growth Incubators (Thermo Fisher Scientific) maintained at either standard temperature (~22°C) or thermoneutral temperature (~30°C) as previously described (14–16). Humidity was controlled using a Top Fin Air Pump AIR 1000 with Top Fin tubing. Mice were acclimated to the assigned temperature for at least 2 weeks.
Propranolol (β-blocker) studies
For studies in which propranolol was used to assess the impact of adrenergic signaling on the endogenous anti-tumor immune response, mice were acclimated to 22°C or 30°C housing temperature for 2 weeks; propranolol treatment in mice at 22°C and 30°C was initiated 4 days prior to tumor cell implantation and given daily until the experimental endpoint. Mice received 200μg propranolol (P0884, Sigma-Aldrich) in 100μl of PBS by i.p. injection; control mice received 100μl PBS for 4 days. Tumor cells were implanted (see above) and treatment continued for the duration of the experiment.
Immunotherapy and propranolol combination studies
Anti-mouse PD-1 antibody (RMP1-14) and rat IgG2a isotype control antibody (2A3) were purchased from BioXCell. The experimental design detailed above was modified for these studies in that treatment was not initiated until tumors were detectable to ensure that tumors in all groups were the same size when treatment was started. This experimental strategy was also designed to mimic clinical implementation of combining β-blockers with checkpoint inhibitors in patients who present to clinic with a tumor. For combination immunotherapy +/− propranolol studies, tumor cells were implanted into mice housed at 22°C prior to starting treatment and mice monitored daily for tumor formation. On a rolling basis beginning the day after tumors became detectable, mice were randomized to receive either PBS (100μl) + isotype antibody (200μg), propranolol (200μg) + isotype antibody (200μg), PBS (100μl) + anti-PD-1 (200μg), or propranolol (200μg) + anti-PD-1 (200μg). Mice received 5–6 injections of antibody spaced 2–3 days apart while propranolol and PBS were given daily.
CD8+ and CD4+ T cell depletion
Mice received weekly i.p. injections (400μg in 100 μl PBS) of CD8+ T cell depleting antibody (53-6.72, BioXCell) or CD4+ T cell depleting antibody (GK1.5, BioXCell) beginning 4 days prior to tumor implantation. Control animals received either rat IgG2a (2A3, BioXCell) or rat IgG2b (LTF-2, BioXCell) isotype controls for CD8+ and CD4+ T cell depletion studies respectively. Depletion was confirmed using flow cytometry.
Flow Cytometry
Single-cell suspensions were created by excising and cutting mouse tumors into 2–3mm pieces. 4T1 tumors were dissociated with collagenase/hyaluronidase (STEMCELL Technologies, 07912) and B16-OVA tumors with a murine tumor dissociation kit (Miltenyi, 130-096-730) following the manufacturers’ protocols prior to passing samples through a 70μm nylon cell strainer (Corning). Spleens were mechanically disrupted and directly passed through a 70μm nylon cell strainer (Corning). Red blood cells were lysed using ACK buffer (Gibco). Cells were then washed with flow running buffer (0.1% BSA in PBS) and incubated with anti-CD16/32 (Fc receptors blocker, 1:200) at 4°C for 15 minutes.
Cells were then stained with the following extracellular antibodies: anti-CD8α BUV395 (53-6.7), anti-CD8β PE (H35-17.2), anti-CD4 BV786 or BUV395 (GK1.5), anti-CD3 BV786, or APC-Cy7 (145-2C11), anti-CD45 FITC or BUV395 (30-F11), anti-PD-1 BV605 (J43), anti-CD11b BUV395 (M1/70), and anti-Gr-1 BV605 (RB6-8C5) all purchased from BD biosciences. CD8+ T cells specific for B16-OVA tumors were identified using the H-2Kb OVA (SIINFEKL) tetramer (APC or BV421) from MBL International Corporation; OT-1 splenocytes were used as a positive control for tetramer staining. Live/dead violet, aqua, or yellow dyes (Thermo Fisher) were used to gate out dead cells.
For intracellular staining, cells were surface-stained as above, then fixed and permeabilized using the FoxP3/Transcription Factor Staining Buffer Set (eBiosciences) as per the manufacturer’s protocol. Cells were then stained with anti-FoxP3 Ax647 (MF23), anti-T-bet Ax647 (4B10), or anti-IFN-γ BV421 (XMG1.2) from BD biosciences, anti-IFN-γ APC (XMG1.2) from eBiosciences, or anti-granzyme B Ax647 (GB11) from Biolegend.
All data was collected on a LSR Fortessa flow cytometer (BD biosciences) and analyzed with FlowJo v10 software (Tree Star, Inc).
ELISA
Noradrenaline Research ELISA kits were purchased from Rocky Mountain Diagnostics (BA E-5200). Blood was collected at the experimental endpoint by retro-orbital bleeding and NE was determined in serum.
Statistical analysis
Students t-test was used to compare data between 2 groups and tumor growth statistics were calculated using two-way ANOVA with Tukey analysis using GraphPad Prism. One-way ANOVA with Tukey posthoc tests was used to compare data between 4 groups using SAS v9.4 (Cary, NC). All data are depicted as mean ± SEM.
RESULTS
Tumor growth in mice housed at ST is promoted by β-adrenergic signaling in non-tumor host cells and can be inhibited by propranolol treatment
We previously showed that several syngeneic tumors and a carcinogen-induced tumor grow more slowly when mice were housed at thermoneutrality (~30°C) than at standard temperatures (~22°C) and this is dependent on the adaptive immune system (14). However, the mechanism(s) mediating immune suppression at 22°C was not identified. We later showed that pancreatic tumor-bearing mice housed at 22°C have significantly higher norepinephrine (NE) levels than those housed at 30°C (16).
In this study, NE levels were also found to be significantly elevated in 4T1 tumor-bearing mice at 22°C compared to 30°C, indicating that this is not a tumor-type dependent phenomenon (Fig. 1A). To test the hypothesis that increased β-AR signaling at 22°C caused accelerated tumor growth when compared to 30°C, we acclimated mice to 22°C or 30°C, began treatment with propranolol (a pan-β-AR antagonist) or PBS 4 days prior to tumor implantation, then implanted tumor cells and monitored tumor formation and growth. A detailed experimental design is depicted in Fig. 1B. Pharmacologic blockade of adrenergic signaling by propranolol significantly slowed both B16-OVA (Fig. 1C) and 4T1 (Fig. 1D) tumor growth in mice at 22°C to a degree comparable to that achieved by housing mice at 30°C. Furthermore, addition of propranolol to mice at 30°C did not improve tumor growth control which is likely because NE levels are already significantly lower at baseline.
Figure 1. Reducing β-adrenergic signaling by physiologic, pharmacologic, and genetic strategies controls tumors at 22°C comparable to 30°C.
(A) Norepinephrin e levels in the serum of 4T1 tumor-bearing mice housed at 22°C and 30°C. (B) Experimental design for β blocker studies with propranolol at 22°C and 30°C. Mice were acclimated to 22°C or 30°C and were then treated with or without daily propranolol 4 days before tumor challenge until the experimental endpoint. (C) B16-OVA and (D) 4T1 tumor growth in wildtype mice housed at 22°C or 30°C treated with or without propranolol. (E) 4T1 tumor growth in wildtype BALB/c and Adrb2−/− mice housed at 22°C or 30°C or (F) housed at 22°C treated with or without propranolol. Data are presented as mean ± SEM. Comparison of norepinephrine levels by Student’s t-test. N = 4–5 per group. Tumor growth statistics analyzed using two-way ANOVA with Tukey analysis. * P < 0.05, ** P < 0.01, *** P < 0.001, **** P < 0.0001. N = 4–8 per group.
We next utilized a β2-AR global receptor knockout BALB/c mouse (Adrb2−/−) to assess the role of β2-AR in host cells by comparing tumor growth between Adrb2−/− and wildtype (WT) BALB/c mice (8). First, we physiologically manipulated adrenergic stress by housing mice at 22°C or 30°C. Then we implanted 4T1 tumor cells, which we and others have shown to lack functional β-ARs (25) (data not shown), and monitored tumor growth. Housing temperature had no impact on tumor growth in Adrb2−/− mice and grew at a rate similar to that observed in WT mice housed at 30°C. However, tumors in WT mice at 22°C grew significantly faster than the other three groups (Fig. 1E) indicating that accelerated tumor growth at 22°C is dependent on functional β2-ARs on host cells.
Lastly, we housed mice at 22°C and reduced adrenergic signaling pharmacologically with propranolol. As shown in Fig. 1F, propranolol treatment significantly slowed tumor growth in WT mice but had minimal effect in Adrb2−/− mice, further supporting the conclusion that signaling through β2-ARs in host cells drives immune suppression at 22°C. Unlike housing at 30°C however, there was a the small, but significant, reduction in tumor growth in Adrb2−/− mice treated with a pan-β-AR antagonist suggesting a minor role for other β-AR isoforms not found on immune cells (i.e., β1-AR and β3-AR). These data suggest that increased signaling through β2-ARs on host cells causes tumors to grow faster in mice housed at 22°C compared to those housed at 30°C.
Reduced tumor growth following β-AR blockade results from enhanced CD8+ T cell-mediated anti-tumor immune response
NE signaling through β2-ARs on lymphocytes has recently been shown to suppress immune cell function in non-oncological settings (17,23,24), therefore we asked whether this signaling pathway mediates immune suppression in mice housed at 22°C. We repeated the experiments shown above (Fig. 1C, 1D) in SCID mice and found that neither physiologic (30°C housing) nor pharmacologic (propranolol) reductions in adrenergic signaling had any effect on tumor growth (Fig. 2A, 2B), indicating that the inhibition of tumor growth observed in Fig. 1C, 1D is dependent on the adaptive immune system.
Figure 2. Reduced tumor growth in propranolol treated mice depends on the adaptive immune system and is CD8+ T cell dependent.
(A) B16-OVA and (B) 4T1 tumor growth in SCID mice housed at 22°C or 30°C treated and treated with PBS or. (C) B16-OVA and (D) 4T1 tumor growth in wildtype C57BL/6 mice and wildtype BALB/c mice respectively depleted of CD8+ T cells and treated with or without propranolol. (E) 4T1 tumor growth in wildtype BALB/c and Adrb2−/− mice depleted of CD8+ T cells. Data are presented as mean ± SEM. Tumor growth statistics analyzed using two-way ANOVA with Tukey analysis. * P < 0.05, *** P < 0.001, **** P < 0.0001. N = 5–8 per group.
In order to determine which immune cell(s) were responsible for the improved tumor growth control following β-blockade, we selectively depleted either CD8+ or CD4+ T cells from WT mice. Depletion of CD8+ T cells abrogated the effect of propranolol on both B16-OVA and 4T1 tumor growth (Fig. 2C, 2D), indicating dependence on CD8+ T cells. Furthermore, depletion of CD8+ T cells in Adrb2−/− mice also accelerated tumor growth rates similar to that seen in WT mice following CD8+ T cell depletion (Fig. 2E). In contrast to CD8+ T depletion, the benefit of β-blockade was not lost following CD4+ T cell depletion (Supplementary Fig. S1) indicating that propranolol was slowing tumor growth in mice housed at 22°C in a CD8+ T cell-dependent manner.
Since T cell killing is dependent upon contact with tumor cells in the tumor microenvironment (TME), we examined the functional orientation of tumor infiltrating lymphocytes (TILs) (5,10,11). We evaluated the frequency and relative number (# cells/mg tumor) of CD8+ T cells in tumors using flow cytometry. Gating on all CD8+ TILs (independent of effector markers) revealed no difference in the frequency of CD8+ TILs from propranolol treated mice bearing 4T1 or B16-OVA tumors compared with controls (Supplementary Fig. S2A, S2B). Moreover, using an OVA-tetramer to evaluate antigen-specific CD8+ TILs in the B16-OVA tumor model further showed no difference in the frequency or relative number of antigen-specific CD8+ TILs (Supplementary Fig. S2C–S2E).
We next assessed the frequency and relative number of CD8+ TILs expressing the transcription factor T-bet which drives expression of the effector molecules IFN-γ and granzyme B (GzmB) in CD8+ T cells (26). As shown in Fig. 3A–3C for B16-OVA (4T1 data shown in Supplementary Fig. S3A–S3C), the frequency and relative number of CD8+ TILs expressing T-bet was significantly elevated in propranolol treated mice compared with controls. Similar observations were also made regarding the frequency and relative number of CD8+ TILs expressing IFN-γ (Fig. 3D–3F; 4T1 data in Supplementary Fig. S3D–S3F). We did not observe differences in the frequency or relative number of CD8+ TILs that expressed GzmB (Fig. 3G–3I). However, further analysis of the GzmB+ fraction of CD8+ TILs revealed that the GzmB MFI was significantly increased in the CD8+ T cell population of propranolol treated mice (Fig. 3J, 3K), indicating that CD8+ TILs with detectable GzmB were producing more GzmB in propranolol treated mice than PBS controls.
Figure 3. Frequencies of intra-tumoral effector T cells are increased in β-blocker treated mice.
Single cell suspensions were made from B16-OVA tumors from mice housed at 22°C treated with PBS or propranolol and analyzed by flow cytometry. (A) Representative flowplots of T-bet expression in CD8+ TILs. (B) Frequency and (C) number of T-bet+ CD8+ T cells per mg of tumor. (D) Representative flowplots of IFN-γ expression in CD8+ TILs. (E) Frequency and (F) number of IFN-γ+ CD8+ T cells per mg of tumor. (G) Representative flowplots of GzmB expression in CD8+ TILs. (H) Frequency and (I) number of GzmB+ CD8+ T cells per mg of tumor. (J) Representative histogram of GzmB expression in CD8+ TILs. (K) Quantification of the GzmB MFI in the GzmB+ CD8+ TIL populations. All data are presented as mean ± SEM. Statistics analyzed by Student’s t-test: * P < 0.05, ** P < 0.01, *** P < 005. N = 5–7 per group.
Thus far, these data indicate that β-AR blockade reduces tumor growth in a CD8+ T cell-dependent manner and increases the frequency of effector CD8+ TILs in both the 4T1 and B16-OVA tumor models.
β-AR blockade significantly increases the ratio of effector CD8+ T cells to Tregs in B16-OVA tumors and decreases the accumulation of MDSCs in the spleens of 4T1 tumor-bearing mice
Tumor infiltration by effector CD8+ T cells is a favorable prognostic indicator in many tumor types (5,10,11). However, the presence of CD4+ regulatory T cells (Tregs; CD4+ FoxP3+), as well as a low CD8+ T cell to Treg ratio, is often a negative prognostic indicator (5,27,28). We therefore asked whether propranolol treatment decreased numbers of Tregs and, if so, did this favorably alter the CD8:Treg ratio. At endpoint, we found that the frequency CD4+ T cells in B16-OVA tumors with a Treg phenotype was significantly reduced in propranolol treated mice (Fig. 4A, 4B). We next evaluated the ratio of total CD8+ T cells to Tregs, but did not observe a difference between groups (Fig. 4C). However, we did observe a significant increase in the ratio of effector (IFN-γ+) CD8+ T cells to Tregs in propranolol treated mice compared to controls (Fig. 4D).
Figure 4. Treating B16-OVA tumor-bearing mice at 22°C with β-blockade significantly increases the ratio of CD8 T cells to suppressive Tregs.
Single cell suspensions were made from B16-OVA tumors from mice housed at 22°C treated with or without propranolol and analyzed by flow cytometry. (A) Representative flowplots of FoxP3 expression in CD4+ TILs. (B) Percentage CD4+ TILs positive for FoxP3 staining. The ratio between (C) total CD8+ T cells and (D) effector IFN-γ+ CD8+ T cells to Tregs in B16-OVA tumors. All data are presented as mean ± SEM. Statistics analyzed by Student’s t-test: * P < 0.05, *** P < 005. N = 5–7 per group.
In 4T1, we assessed the frequency of another potent suppressor cell type, myeloid derived suppressor cells (MDSC; CD11b+ Gr-1+), since they accumulate in the spleens of mice bearing 4T1 and other mammary tumors (29). Using flow cytometry, we observed that the mass of the spleens in propranolol treated mice was significantly less than that of untreated mice (Supplementary Fig. S4A). We also observed a significant reduction in the frequency and absolute number of MDSCs in the spleens of 4T1 tumor-bearing mice housed at 22°C treated with propranolol compared with PBS controls (Supplementary Fig. S4B, S4C).
Since the MDSC frequency correlates with tumor burden (29), we next asked whether adrenergic signaling played a role in the accumulation of MDSCs in addition to tumor burden. Here, we evaluated spleens of both non-tumor-bearing and 4T1 tumor-bearing mice treated with propranolol or PBS following the experimental outline detailed in Supplementary Fig. S5A. In the absence of a tumor, propranolol treatment did not alter MDSC frequency. However, analysis of spleens at three different time-points post 4T1 tumor implantation (days 7, 12, and 20) revealed that the frequency and absolute number of MDSCs were decreased in the propranolol treated cohorts despite the fact that tumor volumes were starting to separate but were not yet significantly different (Supplementary Fig. S5B–S5F).
Overall, these data indicate that β-blockade reduces the frequency of Tregs and increases the ratio of IFN-γ+ CD8 TILs to Tregs while also decreasing the accumulation of MDSCs in the spleens of 4T1 tumor-bearing mice.
Fewer effector CD8+ TILs express PD-1 in β-blocker treated mice compared to controls
Because we found that β-blockade reversed immunosuppression at 22°C, we next examined whether this would also change the expression of immune checkpoint molecules which naturally suppress the anti-tumor immune response, specifically PD-1 (programmed death receptor-1). Recent work indicates that tumors, as well as suppressive cells in the TME, can express the ligand PD-L1 which can bind to PD-1 and suppress anti-tumor immune responses. Moreover, one recent study concluded that the percentage of PD-1 expressing CD8+ T cells in murine tumors is one factor that dictates the responsiveness of tumors to anti-PD-1 (30). Specifically, responsive tumors had a low frequency of PD-1+ CD8+ TILs while non-responding tumors had a high frequency of PD-1+ CD8+ TILs.
Therefore, prior to testing anti-PD-1 checkpoint blockade in our stress reducing murine tumor models, we first investigated whether β-AR signaling had any impact on PD-1 expression. Following treatment of mice housed at 22°C with propranolol, we observed that the percentage of CD8+ TILs expressing PD-1 was decreased in both 4T1 (Fig. 5A, 5B) and B16-OVA tumors (Fig. 5C, 5D). Further analysis of the PD-1 MFI on the PD-1+ CD8+ TIL population from 4T1 tumors revealed that the expression of PD-1 was significantly decreased when mice were treated with propranolol compared with controls (Fig. 5E, 5F). In contrast, the PD-1 MFI was increased on PD-1+ CD8+ TILs isolated B16-OVA tumors in mice treated with propranolol compared with control animals (Fig. 5G, 5H).
Figure 5. Expression of PD-1 by effector CD8+ TILs is reduced by propranolol treatment.
Single cell suspensions were made from B16-OVA tumors from mice housed at 22°C treated with PBS or propranolol and analyzed by flow cytometry. Representative flowplots of PD-1 expression on CD8+ TILs isolated from (A) 4T1 and (C) B16-OVA tumors. Quantification of CD8+ TILs expressing PD-1 isolated from (B) 4T1 and (D) B16-OVA tumors. Representative histograms of PD-1 expression on CD8+ TILs isolated from (E) 4T1 and (G) B16-OVA tumors. Quantification of PD-1 MFI on CD8+ TILs isolated from (F) 4T1 and (H) B16-OVA tumors. The percentage of effector CD8+ TILs isolated from 4T1 tumors that co-express both PD-1 and (I) T-bet or (J) IFN-γ. All data are presented as mean ± SEM. Statistics analyzed by Student’s t-test: *** P < 005. B16-OVA studies N = 5 per group. 4T1 studies N = 8–10 per group.
To determine if the cells that expressed PD-1 had an effector phenotype, as evidenced by expression of T-bet and IFN-γ, we analyzed CD8+ TILs from 4T1 tumors that co-expressed effector molecules and PD-1. Here, we observed that the frequency of CD8+ TILs co-expressing PD-1 and either T-bet+ (Fig. 5I) or IFN-γ+ (Fig. 5J) was decreased in propranolol treated mice compared with PBS controls.
Taken together, these data indicate that the frequency of CD8+ TILs isolated from 4T1 and B16-OVA tumors which express PD-1 is decreased in mice housed at 22°C and treated with propranolol. In addition, fewer effector CD8+ TILs from 4T1 tumors express PD-1 in β-blocker treated mice, suggesting that not only does adrenergic signaling suppress the function of CD8+ TILs, but these effector cells are also more susceptible to suppression via PD-1/PD-L1 signaling.
β-adrenergic stress signaling impairs the efficacy of anti-PD-1 checkpoint blockade in murine tumor models
After determining that β-AR blockade facilitates development of endogenous anti-tumor immunity in murine tumor models, which was associated with a decreased percentage of PD-1 expressing CD8+ TILs, we next evaluated whether anti-PD-1 checkpoint blockade would have greater efficacy when adrenergic signaling was reduced. Since all previously reported murine studies assessing checkpoint inhibitor efficacy have been conducted at 22°C, we first used a physiologic approach to test whether housing temperature induced adrenergic signaling influenced anti-PD-1 efficacy in mice bearing B16-OVA and 4T1 tumors. Here, we acclimated mice to 22°C or 30°C, implanted tumor cells prior to initiation of treatment to ensure that tumors in all groups were established and of comparable size, and subsequently began treatment with anti-PD-1 or isotype antibodies as tumors became detectable. The treatment protocol is depicted in Supplementary Fig. S6A.
As shown in Supplementary Fig. S6B, although anti-PD-1 was modestly effective in slowing B16-OVA tumor growth in mice housed at 22°C, its efficacy was significantly improved in mice which had been acclimated to 30°C housing prior to tumor implantation. In the 4T1 model, anti-PD-1 had no impact on tumor growth in mice housed at 22°C (Supplementary Fig. S6C), whereas significantly improved efficacy of anti-PD-1 was observed in mice housed at 30°C.
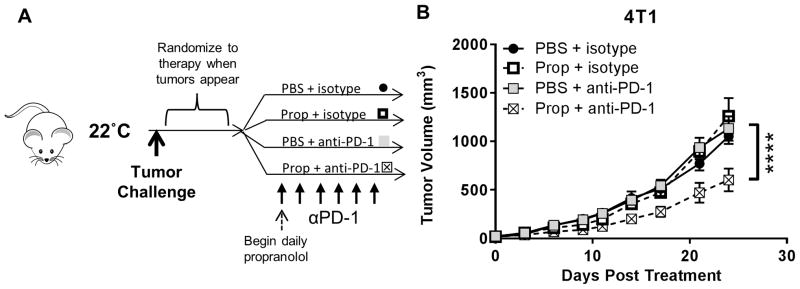
We next tested the hypothesis that treating mice housed at 22°C with propranolol should recapitulate the beneficial effects on anti-PD-1 efficacy observed when housing mice at 30°C. To ensure that the tumors in each group were of comparable size, tumors were again implanted prior to treatment. In this clinically relevant therapeutic model, we began propranolol treatment after tumors became detectable. Mice treated with propranolol or PBS were then randomized to receive anti-PD-1 or isotype antibodies as detailed in Fig. 6A and Supplementary Fig. S7A.
Figure 6. β-blockade increases the efficacy of anti-PD-1 immunotherapy in the 4T1 murine tumor model.
(A) Experimental design for combination propranolol and anti-PD-1 studies at 22°C. Mice at 22°C were challenged with 4T1 tumors. Mice were randomized to receive anti-PD-1 or isotype antibody (200μg) with or without daily propranolol treatments (200μg) beginning the day after tumors became detectable. Mice received 5 doses of anti-PD-1 or isotype antibody on days 0, 3, 6, 9, and 12. (B) 4T1 tumor growth. Data are presented as mean ± SEM. Tumor growth statistics analyzed using two-way ANOVA with Tukey analysis. **** P < 0.0001. N = 6–7 per group.
In the B16-OVA tumor model, we again observed that anti-PD-1 alone had limited efficacy (Supplementary Fig. S7B) at 22°C. However, the addition of propranolol significantly improved responses to anti-PD-1 (Supplementary Fig. S7B). In 4T1 tumor-bearing mice, treatment with anti-PD-1 alone had no impact on tumor growth (Fig. 6B). In contrast, combining anti-PD-1 with propranolol significantly slowed 4T1 tumor growth (Fig 6B). Note that propranolol treatment alone had little effect on the ability of the immune response to control these established tumors in contrast to the beneficial effect seen when propranolol was given prior to tumor implantation.
Next, we sought to determine whether the improved efficacy of anti-PD-1 and propranolol in 4T1 tumor-bearing mice at 22°C was associated with changes in the immune contexture of the TME. We analyzed the effector phenotype of CD8+ TILs in tumor tissue taken from the experiment in Fig. 6B. We observed that propranolol, anti-PD-1, or a combination of the two had no influence on the frequency of total CD8+ T cells (Supplementary Fig. S8). However, the frequency and relative number of effector CD8+ TILs (IFN-γ+) in tumors from mice receiving combination therapy was significantly higher than all other groups (Fig. 7A–7C).
Figure 7. Anti-PD-1 and β-blocker therapy synergize to increase the frequency of IFN-γ expressing intra-tumoral effector CD8+ T cells in 4T1 tumors.
Single cell suspensions were made from 4T1 tumors harvested from mice housed at 22°C treated with anti-PD-1 or isotype antibody with or without propranolol. IFN-γ expression was determined in intra-tumoral CD8+ T cells using flow cytometry. (A) Representative flowplots of IFN-γ expression in CD8+ TILs. (B) Quantification of IFN-γ expressing CD8+ TILs in all treatment groups. (C) The number of IFN-γ+ CD8+ T cells per mg of tumor. All data are presented as mean ± SEM. Log-transformed data analyzed using one-way ANOVA with Tukey adjusted post-hoc tests. In figures 7B and 7C, there was a significant overall association with treatment group (P = 0.002 and P = 0.001, respectively). * P < 0.05, ** P < 0.01. N = 6–7 per group.
Altogether, these data show that combining propranolol and anti-PD-1 in mice housed at 22°C significantly changes the immune contexture of tumors to a more inflamed TME as evidenced by an overall increase in the frequency and relative number of effector CD8+ TILs. Importantly, these changes correlate with a significant improvement in the efficacy of anti-PD-1 therapy.
DISCUSSION
In this study, we exploited a physiologic model of mild cold stress to investigate the role of chronic adrenergic signaling in the development of anti-tumor immunity, the immune contexture of the TME, and the efficacy of anti-PD-1 checkpoint blockade. We report here, for the first time, that increased adrenergic signaling at 22°C, by suppressing CD8+ T-cell mediated anti-tumor immunity, is the underlying mechanism by which cool housing temperatures suppress the anti-tumor immune response. Notably, this suppression can be reversed by treating mice with the pan-β-AR blocker propranolol, and the improved immune contexture of treated tumors correlated with increased anti-PD-1 efficacy.
While many studies have shown that β-blockade slows tumor growth, these previous studies have either focused on how tumor cell intrinsic adrenergic signaling affects growth and metastasis or other pro-tumorigenic processes such as increased angiogenesis (16,19–21,31). In contrast, our study investigated the effects of propranolol on the anti-tumor immune response. Importantly, we demonstrated that the benefit of propranolol is lost in the absence of CD8+ T cells in these models. Moreover, propranolol treatment improved the functional orientation of CD8+ TILs as evidenced by increased expression of markers of effector function (T-bet, IFN-γ, and GzmB). We also found a significantly increased ratio of IFN-γ+ CD8+:Treg cells which is indicative of an inflammatory TME (28). Furthermore, the tumor promoting effects of cool housing were lost in mice lacking β2-ARs (Adrb2−/−) as was the immune enhancing role of propranolol, pointing to a specific role for the β2-AR.
We also found that effector CD8+ TILs isolated from the tumors of mice treated with propranolol expressed lower levels of PD-1 and thus are likely less susceptible to PD-L1 mediated suppression. Furthermore, we showed that reducing adrenergic signaling by either housing mice at 30°C or treating mice housed at 22°C with propranolol, significantly improved responses to anti-PD-1 in both the B16-OVA and 4T1 tumor models. It should be noted that in these clinically relevant studies, we started treatments only after tumors were established (detectable) because if we pretreated the mice with propranolol before implanting tumors, tumors in the propranolol groups would already be significantly smaller when the anti-PD-1 was begun. Consistent with previously published work by Ngiow et al. (30), we observed that a decreased frequency of PD-1+ CD8+ TILs corresponded with an increased efficacy of anti-PD-1 therapy. However, to date, definitive data regarding PD-1 expression on CD8+ TILs and how this impacts responses to anti-PD-1 is debatable (6). Moreover, our data regarding PD-1 expression on CD8+ TILs is observational and warrants further investigation to more definitively evaluate if low PD-1 expression on CD8+ TILs in our model is directly linked to increased anti-PD-1 efficacy.
Analysis of 4T1 tumors from mice treated with both propranolol and anti-PD-1 showed a significant increase in the frequency of IFN-γ producing CD8+ T cells compared to those receiving anti-PD-1 or propranolol alone. These findings have broad implications related to the baseline immunosuppression of tumor-bearing mice housed at 22°C (15,16,32) and could help to explain why the remarkable responses seen in some patients receiving a single checkpoint inhibitor in a therapeutic setting are generally not observed in mice (33,34).
To date, most of the investigations regarding β-AR signaling and T cell behavior have been conducted in non-oncological models and have focused on CD4+ T cells. For example, Bellinger and Lorton have recently reviewed mechanisms by which the SNS regulates the immune response, focusing on both sympathetic innervation to lymphoid organs and β-AR expression on immune cells (17). As they point out, NE signaling through β-ARs on CD4+ lymphocytes induces production of the TH2 cytokines IL-6 and IL-10 suppressing production of the TH1 cytokines IL-12, TNF-α and INF-γ thereby reducing the generation of cytotoxic CD8+ T cells. Recently, Estrada et al, investigated adrenergic signaling specifically in CD8+ T cells and found that β2-AR activation decreased IFN-γ, TNF-α, and IL-2 production and suppressed the ability of CD8+ T cells to kill virally infected cells (24).
Several questions and future studies emerge from this work. One is the need to identify the mechanisms by which adrenergic signaling ultimately shapes the immune phenotype of tumors. One possibility is that β-AR signaling undermines production of inflammatory cytokines and chemokines within the TME that may regulate the recruitment of both anti-tumor and suppressive immune cells to the tumor. Recent studies assessing lymph node trafficking and T cell activation indicate that adrenergic signaling is an important mediator of egress from lymph nodes which can significantly impact responses to vaccination and the severity of autoimmunity (35,36). Additionally, other work has shown that circadian oscillations in NE regulate chemokines and adhesion molecules on endothelial cells that are essential for T cell trafficking (37).
Another important goal will be to improve our understanding of the degree to which adrenergic signaling shapes anti-tumor immune responses in more naturally developing tumors. We have previously reported that the incidence of a carcinogen-induced tumor model (3-methylcholanthrene; MCA) is reduced in mice housed at 22°C compared to 30°C (14), suggesting that adrenergic signaling likely plays a role in immune surveillance and immune editing. Using a transgenic rat model of melanoma, Wrobel et al. (38) found that propranolol also decreased tumor incidence, reduced the number of myeloid cells, and increased the presence of GzmB+ CD8+ T cells in tumors at endpoint.
Future work must also address the mechanisms by which adrenergic signaling may impact the suppressive functions of myeloid cells. Relatively little is known about the importance of β-AR signaling in MDSCs, but recent work by Jin et al. (39) showed that psychological stress increases MDSC frequency in non-tumor-bearing mice (29). In our study, β-blockade reduced MDSC accumulation in spleens at both early and late time-points post 4T1 tumor implantation. Future work should assess whether adrenergic signaling alters the suppressive functionality of MDSCs.
In addition to MDSCs, M2 macrophages should also be evaluated since they suppress anti-tumor immunity, drive metastasis, and can produce NE (40). Previously, it was shown that cold stress skews macrophages towards an M2 phenotype in brown fat which produce NE for heat generation (41). However, a recent conflicting study by Fischer et al. concluded that M2 macrophages do not participate in adaptive thermogenesis in brown fat (42). While we did not observe differences in the frequency of phenotypic M2 macrophages in our models (data not shown), future work should determine whether M2 macrophages are making NE locally in the TME.
Future studies should also assess whether housing at 30°C or β-blockade at 22°C affects the efficacy of other immunotherapies, such as anti-CTLA-4, or combinations of immunotherapies. The potential role of combining β-AR blockade with anti-CTLA-4 is of particular interest because β2-AR activation in Tregs increases suppressive functions by upregulating expression of CTLA-4 (43). Additionally, it will be important to investigate if reducing adrenergic signaling increases the incidence and severity of autoimmunity often observed in patients receiving but was not predicted by mouse models. Although we did not observe any obvious increases in toxicity in our models, our ongoing studies will assess the impact of combination therapies. Since all pre-clinical studies which tested checkpoint inhibitors in mice were conducted at 22°C, it is possible that the general immune suppression we have demonstrated under these housing conditions also suppresses the function of autoreactive T cells, an observation we previously reported in models of GVHD (15).
Although the work presented here focused specifically on ambient housing temperature, other concurrent forms of stress also likely contributed to stress in mice and may influence data interpretation. These include but are not limited to the presence of an “aggressor” mouse in the cage as well as the frequency of handling by investigators (44). Also, since baseline levels of catecholamines vary between male and female mice (45), these studies should be repeated in male mice.
From a clinical perspective, our findings complement several retrospective studies suggesting that patients taking non-specific β-blockers for indications other than cancer have better outcomes than both those administered specific β1-blockers and non-users. (46–48). Since β-blockers are generally prescribed to treat a variety of cardiovascular conditions, as well as performance anxiety, it would be interesting to see if patients taking β-blockers have improved responses to immunotherapies and/or an increased incidence or severity of autoimmunity, both of which would be predicted by our study.
In addition to assessing retrospective β-blocker use in the context of immunotherapies, analysis of patients taking psychotropic drugs which modulate adrenergic signaling like anti-depressants and anti-psychotics should also be examined. Based on our data, we would predict that patients taking anti-depressants which increase NE levels, such as TCAs, SNRIs, and DNRIs would have poorer responses to checkpoint inhibitors than those taking SSRIs which specifically modulate serotonin. This data could generate prescribing physicians as to which anti-depressants to prescribe or avoid in cancer patients who will receive immunotherapy.
Moreover, it would be of value to determine the percentage of patients who have not responded to checkpoint inhibitors among those who have faced extended periods of stress throughout their lifetime. One study by Mundy-Bosse et al. (49) determined that patients who reported high levels of chronic, but not acute, stress had a significantly increased frequency of MDSCs in circulation. These findings are likely important to checkpoint inhibitor responses since recent work by Waight et al. showed that a high frequency of MDSCs decreases responses to checkpoint inhibitors while also serving as a negative prognostic indicator in breast cancer patients (50).
In summary, our data reveal a fundamental role for adrenergic signaling in regulating the anti-tumor immune response. Our findings support the idea that the effects of catecholamines and β-AR signaling are a significant underlying mechanism that causes suppression of anti-tumor immunity. Additionally, the work presented here suggests that in some patients, strategic combinations with β-blockers should enhance the efficacy of checkpoint blockade.
Disrupting the NE/β-AR axis provides an attractive target for clinicians since there is already an extensive list of FDA-approved β-blockers which have been well studied and used in patients with a long-standing history of safety and efficacy over many decades. The availability of these β-blockers and the potential to rapidly repurpose this class of drugs for use with cancer treatments make the possibility of new combination trials especially feasible.
Supplementary Material
Acknowledgments
Grant Support: This research was supported by The Peter T. Rowley Breast Cancer Research Grant C028252 (E.A. Repasky); The Harry J. Lloyd Charitable Trust (E.A. Repasky); The Roswell Park Alliance Foundation (E.A. Repasky); The National Institute of Health Grant 5-T32CA085183 (M.J. Bucsek) and 1-R01CA140622 (S.I. Abrams), and used Shared Resources supported by the Roswell Park Cancer Institute’s Comprehensive Cancer Center Support Grant CA016056.
We thank Drs. Ira Blader, Sarah Holstein, Dana Bovbjerg, Anurag Singh, and Kelly Madden as well as Rosemarie Pitoniak, Daniel Greenberg, Jeremey Kuettel, Tim Winslow, and Brenden Zengari for their helpful discussions or technical assistance. We thank Drs. David Farrar for providing our laboratory with Adrb2−/− mice, Minhyung Kim for providing us with OT-1 RAG deficient mice, and Protul Shrikant for providing us with the B16-OVA tumor cell line. We also thank Dr. Kitty De Jong and the Flow and Image Cytometry Core facility for helpful discussions and assistance in flow cytometry gating as well as Renae Holtz from the Genetics Core Facility for her assistance in testing our cells for mycoplasma. We would also like to thank Jeanne Prendergast for her laboratory and editorial assistance.
Footnotes
Conflict of Interest Statement: The authors declare no potential conflicts of interest
References
- 1.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gettinger S, Rizvi NA, Chow LQ, Borghaei H, Brahmer J, Ready N, et al. Nivolumab Monotherapy for First-Line Treatment of Advanced Non–Small-Cell Lung Cancer. Journal of Clinical Oncology. 2016;34:2980–2987. doi: 10.1200/JCO.2016.66.9929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sharma P, Allison JP. The future of immune checkpoint therapy. Science. 2015;348:56–61. doi: 10.1126/science.aaa8172. [DOI] [PubMed] [Google Scholar]
- 4.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fridman WH, Pagès F, Sautès-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer. 2012;12:298–306. doi: 10.1038/nrc3245. [DOI] [PubMed] [Google Scholar]
- 6.Shen M, Ren X. Highlights on immune checkpoint inhibitors in non–small cell lung cancer. Tumor Biology. 2017;39:1010428317695013. doi: 10.1177/1010428317695013. [DOI] [PubMed] [Google Scholar]
- 7.Sharabi AB, Lim M, DeWeese TL, Drake CG. Radiation and checkpoint blockade immunotherapy: radiosensitisation and potential mechanisms of synergy. The Lancet Oncology. 16:e498–e509. doi: 10.1016/S1470-2045(15)00007-8. [DOI] [PubMed] [Google Scholar]
- 8.Pfirschke C, Engblom C, Rickelt S, Cortez-Retamozo V, Garris C, Pucci F, et al. Immunogenic Chemotherapy Sensitizes Tumors to Checkpoint Blockade Therapy. Immunity. 44:343–354. doi: 10.1016/j.immuni.2015.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Byrne KT, Vonderheide RH. CD40 therapy and surgery: a potential immunological partnership. Journal of immunotherapy (Hagerstown, Md.: 1997) 2013;36:359–361. doi: 10.1097/CJI.0b013e31829fb871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pages F, Galon J, Dieu-Nosjean MC, Tartour E, Sautes-Fridman C, Fridman WH. Immune infiltration in human tumors: a prognostic factor that should not be ignored. Oncogene. 2009;29:1093–1102. doi: 10.1038/onc.2009.416. [DOI] [PubMed] [Google Scholar]
- 11.Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pagès C, et al. Type, Density, and Location of Immune Cells Within Human Colorectal Tumors Predict Clinical Outcome. Science. 2006;313:1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 12.National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals. Guide for the Care and Use of Laboratory Animals. National Academies Press (US); Washington (DC): 2008. [Google Scholar]
- 13.Gordon CJ. Thermal physiology of laboratory mice: Defining thermoneutrality. Journal of Thermal Biology. 2012;37:654–685. [Google Scholar]
- 14.Kokolus KM, Capitano ML, Lee CT, Eng JW, Waight JD, Hylander BL, et al. Baseline tumor growth and immune control in laboratory mice are significantly influenced by subthermoneutral housing temperature. Proc Natl Acad Sci U S A. 2013;110:20176–20181. doi: 10.1073/pnas.1304291110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leigh ND, Kokolus KM, O’Neill RE, Du W, Eng JW, Qiu J, et al. Housing Temperature-Induced Stress Is Suppressing Murine Graft-versus-Host Disease through beta2-Adrenergic Receptor Signaling. J Immunol. 2015;195:5045–5054. doi: 10.4049/jimmunol.1500700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eng JW, Reed CB, Kokolus KM, Pitoniak R, Utley A, Bucsek MJ, et al. Housing temperature-induced stress drives therapeutic resistance in murine tumour models through beta2-adrenergic receptor activation. Nat Commun. 2015;6:6426. doi: 10.1038/ncomms7426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bellinger DL, Lorton D. Autonomic regulation of cellular immune function. Auton Neurosci. 2014;182:15–41. doi: 10.1016/j.autneu.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 18.Cohen S, Levi-Montalcini R, Hamburger V. A Nerve Growth-stimulating Factor Isolated From Sarcomas 37 and 180. Proceedings of the National Academy of Sciences of the United States of America. 1954;40:1014–1018. doi: 10.1073/pnas.40.10.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Magnon C, Hall SJ, Lin J, Xue X, Gerber L, Freedland SJ, et al. Autonomic nerve development contributes to prostate cancer progression. Science. 2013;341:1236361. doi: 10.1126/science.1236361. [DOI] [PubMed] [Google Scholar]
- 20.Cole SW, Sood AK. Molecular pathways: beta-adrenergic signaling in cancer. Clinical cancer research: an official journal of the American Association for Cancer Research. 2012;18:1201–1206. doi: 10.1158/1078-0432.CCR-11-0641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cole SW, Nagaraja AS, Lutgendorf SK, Green PA, Sood AK. Sympathetic nervous system regulation of the tumour microenvironment. Nat Rev Cancer. 2015;15:563–572. doi: 10.1038/nrc3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eng JWL, Kokolus KM, Reed CB, Hylander BL, Ma WW, Repasky EA. A Nervous Tumor Microenvironment: The Impact of Adrenergic Stress on Cancer Cells, Immunosuppression, and Immunotherapeutic Response. Cancer immunology, immunotherapy: CII. 2014;63:1115–1128. doi: 10.1007/s00262-014-1617-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grebe KM, Hickman HD, Irvine KR, Takeda K, Bennink JR, Yewdell JW. Sympathetic nervous system control of anti-influenza CD8+ T cell responses. Proceedings of the National Academy of Sciences. 2009;106:5300–5305. doi: 10.1073/pnas.0808851106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Estrada LD, Agac D, Farrar JD. Sympathetic neural signaling via the beta2-adrenergic receptor suppresses T-cell receptor-mediated human and mouse CD8(+) T-cell effector function. Eur J Immunol. 2016;46:1948–1958. doi: 10.1002/eji.201646395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Szpunar MJ, Burke KA, Dawes RP, Brown EB, Madden KS. The Antidepressant Desipramine and α(2)-Adrenergic Receptor Activation Promote Breast Tumor Progression in Association with Altered Collagen Structure. Cancer prevention research (Philadelphia, Pa.) 2013;6 doi: 10.1158/1940-6207.CAPR-1113-0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lazarevic V, Glimcher LH, Lord GM. T-bet: a bridge between innate and adaptive immunity. Nat Rev Immunol. 2013;13:777–789. doi: 10.1038/nri3536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sato E, Olson SH, Ahn J, Bundy B, Nishikawa H, Qian F, et al. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:18538–18543. doi: 10.1073/pnas.0509182102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Curran MA, Montalvo W, Yagita H, Allison JP. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proceedings of the National Academy of Sciences. 2010;107:4275–4280. doi: 10.1073/pnas.0915174107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Youn JI, Nagaraj S, Collazo M, Gabrilovich DI. Subsets of Myeloid-Derived Suppressor Cells in Tumor-Bearing Mice. The Journal of Immunology. 2008;181:5791–5802. doi: 10.4049/jimmunol.181.8.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ngiow SF, Young A, Jacquelot N, Yamazaki T, Enot D, Zitvogel L, et al. A Threshold Level of Intratumor CD8+ T-cell PD1 Expression Dictates Therapeutic Response to Anti-PD1. Cancer Research. 2015;75:3800–3811. doi: 10.1158/0008-5472.CAN-15-1082. [DOI] [PubMed] [Google Scholar]
- 31.Thaker PH, Han LY, Kamat AA, Arevalo JM, Takahashi R, Lu C, et al. Chronic stress promotes tumor growth and angiogenesis in a mouse model of ovarian carcinoma. Nature medicine. 2006;12:939–944. doi: 10.1038/nm1447. [DOI] [PubMed] [Google Scholar]
- 32.Hylander BL, Repasky EA. Thermoneutrality, Mice, and Cancer: A Heated Opinion. Trends in Cancer. 2:166–175. doi: 10.1016/j.trecan.2016.03.005. [DOI] [PubMed] [Google Scholar]
- 33.Liu J, Blake SJ, Smyth MJ, Teng MWL. Improved mouse models to assess tumour immunity and irAEs after combination cancer immunotherapies. Clin Trans Immunol. 2014;3:e22. doi: 10.1038/cti.2014.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu J, Blake SJ, Harjunpää H, Fairfax KA, Yong MCR, Allen S, et al. Assessing Immune-Related Adverse Events of Efficacious Combination Immunotherapies in Preclinical Models of Cancer. Cancer Research. 2016;76:5288–5301. doi: 10.1158/0008-5472.CAN-16-0194. [DOI] [PubMed] [Google Scholar]
- 35.Nakai A, Hayano Y, Furuta F, Noda M, Suzuki K. Control of lymphocyte egress from lymph nodes through β2-adrenergic receptors. The Journal of Experimental Medicine. 2014;211:2583–2598. doi: 10.1084/jem.20141132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Suzuki K, Hayano Y, Nakai A, Furuta F, Noda M. Adrenergic control of the adaptive immune response by diurnal lymphocyte recirculation through lymph nodes. The Journal of Experimental Medicine. 2016;213:2567–2574. doi: 10.1084/jem.20160723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scheiermann C, Kunisaki Y, Lucas D, Chow A, Jang JE, Zhang D, et al. Adrenergic Nerves Govern Circadian Leukocyte Recruitment to Tissues. Immunity. 2012;37:290–301. doi: 10.1016/j.immuni.2012.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wrobel LJ, Bod L, Lengagne R, Kato M, Prevost-Blondel A, Le Gal FA. Propranolol induces a favourable shift of anti-tumor immunity in a murine spontaneous model of melanoma. Oncotarget. 2016 doi: 10.18632/oncotarget.12833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jin J, Wang X, Wang Q, Guo X, Cao J, Zhang X, et al. Chronic Psychological Stress Induces the Accumulation of Myeloid-Derived Suppressor Cells in Mice. PLoS ONE. 2013;8:e74497. doi: 10.1371/journal.pone.0074497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sica A, Schioppa T, Mantovani A, Allavena P. Tumour-associated macrophages are a distinct M2 polarised population promoting tumour progression: Potential targets of anti-cancer therapy. European Journal of Cancer. 2006;42:717–727. doi: 10.1016/j.ejca.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 41.Nguyen KD, Qiu Y, Cui X, Goh YPS, Mwangi J, David T, et al. Alternatively activated macrophages produce catecholamines to sustain adaptive thermogenesis. Nature. 2011;480:104–108. doi: 10.1038/nature10653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fischer K, Ruiz HH, Jhun K, Finan B, Oberlin DJ, van der Heide V, et al. Alternatively activated macrophages do not synthesize catecholamines or contribute to adipose tissue adaptive thermogenesis. Nature medicine. 2017;23:623–630. doi: 10.1038/nm.4316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guereschi MG, Araujo LP, Maricato JT, Takenaka MC, Nascimento VM, Vivanco BC, et al. Beta2-adrenergic receptor signaling in CD4+Foxp3+ regulatory T cells enhances their suppressive function in a PKA-dependent manner. European Journal of Immunology. 2013;43:1001–1012. doi: 10.1002/eji.201243005. [DOI] [PubMed] [Google Scholar]
- 44.Demas GE, Carlton ED. Ecoimmunology for Psychoneuroimmunologists: Considering Context in Neuroendocrine-Immune-Behavior Interactions. Brain, behavior, and immunity. 2015;0:9–16. doi: 10.1016/j.bbi.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Luo H, Wu Z, Tremblay J, Thorin E, Peng J, Lavoie JL, et al. Receptor Tyrosine Kinase Ephb6 Regulates Vascular Smooth Muscle Contractility and Modulates Blood Pressure in Concert with Sex Hormones. The Journal of Biological Chemistry. 2012;287:6819–6829. doi: 10.1074/jbc.M111.293365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang HM, Liao ZX, Komaki R, Welsh JW, O’Reilly MS, Chang JY, et al. Improved survival outcomes with the incidental use of beta-blockers among patients with non-small-cell lung cancer treated with definitive radiation therapy. Annals of Oncology. 2013;24:1312–1319. doi: 10.1093/annonc/mds616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Watkins JL, Thaker PH, Nick AM, Ramondetta LM, Kumar S, Urbauer DL, et al. Clinical impact of selective and nonselective beta-blockers on survival in patients with ovarian cancer. Cancer. 2015;121:3444–3451. doi: 10.1002/cncr.29392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Barron TI, Connolly RM, Sharp L, Bennett K, Visvanathan K. Beta Blockers and Breast Cancer Mortality: A Population- Based Study. Journal of Clinical Oncology. 2011;29:2635–2644. doi: 10.1200/JCO.2010.33.5422. [DOI] [PubMed] [Google Scholar]
- 49.Mundy-Bosse BL, Thornton LM, Yang HC, Andersen BL, Carson WE. Psychological Stress is Associated with Altered Levels of Myeloid-Derived Suppressor cells in Breast Cancer Patients. Cellular immunology. 2011;270:80–87. doi: 10.1016/j.cellimm.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Waight JD, Netherby C, Hensen ML, Miller A, Hu Q, Liu S, et al. Myeloid-derived suppressor cell development is regulated by a STAT/IRF-8 axis. The Journal of Clinical Investigation. 2013;123:4464–4478. doi: 10.1172/JCI68189. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.