Much of our understanding of the immune system has come from studying immune cells drawn from blood. Such samples are minimally invasive and relatively easy to collect, but they provide an incomplete and often misleading picture. Put simply, the biology of the immune system in blood doesn’t match the biology in the tissues where most infections play out, explains David Masopust, a Howard Hughes Medical Institute microbiologist at the University of Minnesota. “By basing concepts solely on blood and lymphoid populations, the field was missing out on a major part of the immune surveillance story,” says Masopust.
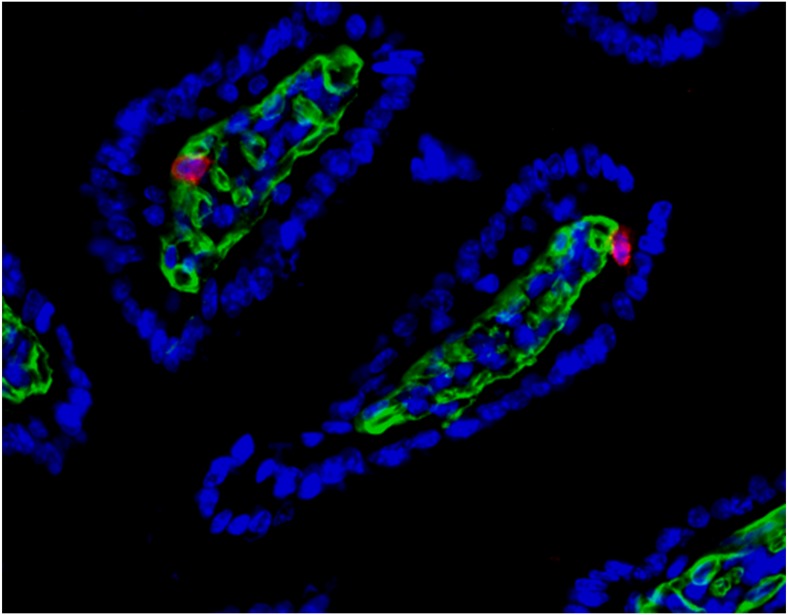
Fig. 1.
Virus-specific tissue-resident memory cells (red) patrol a mouse’s small intestine. Image courtesy of Sathi Wijeyesinghe (University of Minnesota, Minneapolis).
In 2001, as a graduate student under the direction of the late Leo Lefrancois, chair of immunology at University of Connecticut, Masopust identified a group of immune cells that remain in the tissue after their encounter with a pathogen (1). He later dubbed them tissue resident memory (TRM) T cells. TRM cells, it seems, are qualitatively and functionally different from other immune cells.
The notion that memory cells aren’t just circulating in the blood has changed the way we view the immune system, according to Masopust and other immunologists. The existence of TRMs—their location, physical and functional differences, and a still-emerging sense of their generation and maintenance—could help develop better vaccines and cancer immunotherapies. “TRMs are going to become a key component of the cancer immunotherapy conversation.” says Masopust.
Updating the Paradigm
The classic paradigm of immunology entails two forms of immune defense. Innate immunity, a mix of antimicrobial chemicals and immune cells residing in barrier and other tissue, provides immediate but generic protection from pathogens. The innate immune response simultaneously releases chemical signals that trigger migration of T cells, part of the second line of defense, the adaptive immune component.
Continuously circulating T cells are adapted to a specific pathogen and provide a more potent secondary response than innate immune cells. As naïve T cells in a lymph node, they are exposed to an antigen from a pathogen; they then proliferate, migrate through capillaries, and follow signals to the innate components already engaged with the pathogen to finish off the invader. The T cells that don’t die in the encounter then migrate back into the blood as veteran memory cells, trained for the next exposure to that microbe.
That theoretical construct of the immune system, in which the T cell component only migrated and did not reside in tissue, began to change with Masopust’s 2001 article. He found that TRMs remained in the tissue after their encounter with a pathogen rather than returning to the blood. Initially, it wasn’t clear whether the residents were permanent or were a rotating deployment of circulating T cells. But a consensus has emerged, based on research from multiple labs, that TRMs are permanent, slowly replacing themselves.
Importantly, Masopust’s article also demonstrated a functional difference between memory T cells residing in tissue and memory T cells in the blood and lymph nodes. The distinction harkens back to the differences between innate and adaptive immunity. T cells mount a far more specific and potent immune defense against microbes than do their innate counterparts. And by localizing close to where a pathogen has previously entered, TRMs are poised to react to a later incursion within seconds rather than the hours or days it would take circulating T cells to bring their antimicrobial weapons to bear. This quicker reaction from TRMs also means the bolus of the invading pathogens they encounter is smaller, less established, and hence easier to neutralize.
Publications on TRMs began to take off in 2009 with a mouse study of herpes simplex virus infection, conducted by Francis Carbone’s lab at the University of Melbourne (2). Using a green florescent protein marker and skin transplantation, the group found that one component of the adaptive immune system, CD8 T cells, not only remained in the transplanted tissue, but when exposed again to herpes, the cells dramatically expanded their numbers and released chemicals (cytokines) to kill the virus. Clearly TRMs had to be considered when evaluating total immune response.
Further insights came from the study of a very different disease. Doctors have known for years that the autoimmune disease vitiligo, a condition that results in patches of lost skin pigmentation, confers protection against the deadly skin cancer melanoma. This spring, microbiologist Mary Jo Turk at Dartmouth and colleagues demonstrated in mice that TRMs are the mechanism providing that protection. Furthermore, a specific molecule on the surface of those CD8 T cells, CD103, was required to generate and maintain those cells in skin (3). When they knocked out CD103 in genetically altered mice, the mice couldn’t generate those cells and had no protection against melanoma.
The vitiligo-associated TRMs were more plentiful in white patches where the mouse skin had lost pigment but were also found in smaller numbers in areas of skin that looked normal. However, they were not found in other types of tissue, such as the lymph nodes, spleen, or blood, Turk explains. “So when we rechallenged these mice, put the melanoma back in the skin, regardless of what skin site we challenged them on, they were protected,” she says.
Thus far, TRMs have been found in every tissue where researchers have looked, in both animal models and in humans, according to Masopust and others.
Challenge of Heterogeneity
Studying TRMs presents big challenges. Researchers have a tough time identifying resident memory cells and distinguishing them from their circulating counterparts. Tissue sites differ by pH, oxygen level, nutrient availability, and myriad other factors. “T cells have had to make accommodations to adapt to those environments,” Masopust explains. The result: a variety of different protein markers on their surface. Heterogeneity is the rule, which makes phenotyping incredibly challenging, Masopust notes.
“It is like everything with immunology, once you start breaking it up into subsets you get sub, sub, sub populations and you can really go crazy,” says Donna Farber, an immunologist at Columbia University who has looked extensively at TRMs in human tissue. She expects that specific cell markers and levels of adhesions and integrins—molecules that are important to anchoring the cell in a tissue location rather than migrating out—will differ by tissue compartment.
The biology of where these immune cells reside differs by cell type, says Yale University immunologist Akiko Iwasaki. “CD8 T cells don’t need any antigen to reside in a tissue,” she explains. But CD4 T cells, in at least some tissues, appear dependent on the presence of antigens from the pathogen, secreted from macrophages, to keep the CD4 T cells from returning to the blood.
Studies have reached differing conclusions on the location and coverage of TRMs generated by a single infection. Such details could provide clues as to whether TRMs can protect against re-exposure to a given pathogen. Some research suggests that the coverage is narrow and focused, while other work suggests it’s quite broad. This variability is likely due to the interaction among the pathogen, tissue compartment, and breadth of the infection before being resolved, say researchers.
The density of TRM populations and its role in protecting against later exposure to the pathogen also remains an open question. “I bet it is going to differ between organs and pathogens,” says Iwasaki. “Some pathogens are very acute and robust, others are very quiescent. So I bet a lot of times you are not going to get a lot of density.”
Clinical Applications
Attempts at moving TRM insights from bench to bedside have already begun. Iwasaki has proposed what she calls a “prime and pull” vaccine strategy that manipulates TRMs for the prevention or treatment of disease. The vaccine component creates numerous circulating memory cells; researchers then pull these cells into the tissue using chemokines to establish their residence as TRMs, Iwasaki explains. “The advantage is that you can selectively recruit the T cells to the tissue of your choice,” she adds, noting that the strategy focuses TRMs where they are desired and minimizes off-target effects.
Physician colleagues at Yale, Alessandro Santin and Sangini Sheth, are putting that strategy to the test with a therapeutic clinical trial to treat HPV-related cervical lesions. Researchers are combining two agents already on the market, a 9-valent HPV vaccine to prevent infection and imiquimod, a potent immune stimulator. The goal is to generate a stronger immune response and pull more TRMs into cervical tissue. The hypothesis: an influx of TRMs will clear the precancerous lesions and leave a lasting memory response that will protect against future exposure to the cancer-causing HPV.
Treatment for cancer or chronic infections “is not just one and done,” Turk explains. Often a few cells will escape treatment and seed future disease. “If we are looking at therapies that are going to provide a durable cure,” she adds, “I think it is going to be crucial to generate a TRM response.”
Masopust has his own translational medicine possibilities in mind; his lab is, for example, repurposing pathogen-specific TRMs in mice in an attempt to develop a tumor immunotherapy. “I think their full significance is not yet known,” he says of TRMs. “But I predict that with more research, we’ll learn they are playing a key role in a broad array of diseases.”
References
- 1.Masopust D, Vezys V, Marzo AL, Lefrançois L. Preferential localization of effector memory cells in nonlymphoid tissue. Science. 2001;291:2413–2417. doi: 10.1126/science.1058867. [DOI] [PubMed] [Google Scholar]
- 2.Gebhardt T, et al. Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nat Immunol. 2009;10:524–530. doi: 10.1038/ni.1718. [DOI] [PubMed] [Google Scholar]
- 3.Malik M, et al. Resident memory T cells in the skin mediate durable immunity to melanoma. Sci Immunol. 2017;2:eaam6346. doi: 10.1126/sciimmunol.aam6346. [DOI] [PMC free article] [PubMed] [Google Scholar]