This longitudinal observational study determines if the combination of smell identification testing followed by dopamine transporter imaging can accurately and efficiently identify individuals from the general population at risk for conversion to a clinical diagnosis of Parkinson disease.
Key Points
Question
Can a 2-step screening strategy testing for hyposmia and dopamine transporter imaging (DAT) deficit identify a population with no clinical diagnosis of Parkinson disease (PD) for later conversion to PD?
Findings
In this observational trial of 280 hyposmic and normosmic individuals, 14 of 21 patients (67%) with hyposmia and a DAT deficit at baseline converted to PD compared with 2 of 22 (9%) with DAT in an indeterminate range and 3 of 109 (2.8%) with no DAT deficit during a 4-year period.
Meaning
A sequential biomarker strategy of olfactory testing followed by DAT imaging can identify individuals at risk for PD and provides a framework for planning PD prevention trials.
Abstract
Importance
Detecting individuals at risk for Parkinson disease (PD) during the prodromal phase could clarify disease mechanisms and allow for treatment earlier in the disease process to possibly slow or prevent the onset of motor PD.
Objective
To determine if the combination of smell identification testing followed by dopamine transporter (DAT) imaging can accurately and efficiently identify individuals from the general population at risk for conversion to a clinical diagnosis of PD.
Design, Setting, and Participants
Participants were identified from the community by olfactory testing assessed longitudinally with DAT imaging 2 and 4 years after baseline and by annual clinical follow-up to determine whether they had clinical evidence to establish a PD diagnosis. Participants were contacted by mail and completed olfactory testing at home. Longitudinal follow-up of clinical measures and DAT imaging occurred at specialty centers. There were 203 hyposmic and 100 normosmic participants. A total of 185 hyposmic and 95 normosmic individuals had at least 1 follow-up visit, and 152 hyposmic participants (82.2%) were either observed for 4 years or converted to PD during follow-up.
Main Outcomes and Measures
Percentage of individuals with hyposmia and a DAT deficit that converted to PD and the change in PD clinical scale scores (Unified Parkinson’s Disease Rating Scale) and DAT imaging during 4-year follow-up.
Results
Of 280 total participants, 140 (50.0%) were male, and the mean (SD) age of the cohort was 63 (8.7) years. Among 21 participants with hyposmia and a DAT deficit (65% or less of age-expected lowest putamen binding ratio) at baseline, 14 (67%) converted to PD at 4 years compared with 2 of 22 participants (9%) with a DAT in an indeterminate range (greater than 65%-80%) and 3 of 109 participants (2.8%) with no DAT deficit (greater than 80%) at baseline. Individuals with a baseline DAT deficit experienced a 4-year decline in DAT binding of 20.23% (SD, 15.04%) compared with 3.68% (SD, 18.36%) and 5.45% (SD, 13.58%) for participants with an indeterminate and no DAT deficit, respectively (P = .002). The relative risk of conversion to a diagnosis of PD in hyposmic individuals with a DAT deficit was 17.47 (95% CI, 7.02-43.45) compared with individuals with either indeterminate or no DAT deficit.
Conclusions and Relevance
The combination of hyposmia and DAT deficit was highly predictive of conversion to PD within 4 years of clinical follow-up. Individuals with hyposmia and a DAT deficit had a 5% reduction in DAT binding annually, similar to early PD. These results provide a framework for planning disease prevention studies in PD.
Introduction
Emerging evidence from preclinical and biomarker studies indicates that the pathology underlying neurodegenerative diseases begins many years before a clinical diagnosis is apparent. In Parkinson disease (PD), the second most common neurodegenerative disorder with a societal cost of more than $15 billion (2010-adjusted) per year in the United States, there is a prodromal phase characterized by pathologic alterations in dopaminergic integrity and subtle nonmotor features in the absence of cardinal motor signs. Earlier detection of PD would enable testing of potential disease-modifying treatments when pathology is less advanced and when treatments may be more effective. Identifying individuals with prodromal PD requires an assessment strategy that is both widely available and highly specific. We describe a sequential biomarker paradigm, starting with a highly sensitive, widely available tool (olfaction) to identify a risk group followed by a highly specific test (dopamine transporter [DAT] imaging) to confirm a high-risk group.
Olfactory dysfunction is a consistent finding in early PD, present at the time of diagnosis in 80% to 90% of patients. It is easily and accurately tested in the general population using the University of Pennsylvania Smell Identification test, a simple scratch-and-sniff test. In addition, olfactory impairment has been shown to predict increased risk of PD within the next 4 years and is increased in asymptomatic carriers of genes associated with PD, suggesting that the olfactory deficit is present at least several years prior to diagnosis.
Dopaminergic imaging with either positron emission tomography or single-photon emission computed tomography (SPECT) is highly specific for the dopaminergic deficit seen in parkinsonian disorders. Studies in individuals at risk for PD because of genetic mutation or rapid eye movement sleep behavior disorder (RBD) have demonstrated that a dopaminergic imaging deficit precedes a PD diagnosis, likely by several years. Therefore, while this technology is not suitable for widespread screening, dopaminergic imaging can be used to identify a high-risk group in an already prescreened enriched population.
The primary goal of the Parkinson Associated Risk Study (PARS) is to determine if the combination of smell identification testing followed by DAT imaging can accurately and efficiently identify individuals from the general population at risk for conversion to a clinical diagnosis of PD. The PARS cohort was evaluated longitudinally, with annual clinical examinations and biannual DAT imaging with iodine 123–labeled (123I) β-carboxymethyoxy-3-β-(4-iodophenyl) tropane (CIT) SPECT during a 4-year interval. We report the rate of conversion to a clinical diagnosis of PD in those individuals at risk based on olfactory testing and DAT imaging results and compare the clinical features and changes in (123I)β-CIT SPECT imaging in the at-risk and nonrisk groups during the prodromal stage.
Methods
Study Design
PARS is a longitudinal observational study aimed at testing a 2-tiered biomarker strategy to identify individuals at risk for PD. Details of the study design have been reported previously. The study is coordinated at the Institute for Neurodegenerative Disorders in New Haven, Connecticut, and includes 15 clinical centers in the United States. The protocol received approval by the Western Institutional Review Board, the Human Research Protection Office at the US Army Medical Research Material and Command, and the local institutional review boards at participating centers. Written informed consent was obtained prior to study participation. PARS is funded by the US Department of Defense, which had no role in study design, data collection, analysis, interpretation, writing, or the decision to submit the report.
Study Population
Individuals were initially contacted by mail and completed olfactory testing and written informed consent at home. Details of the cohort contacted by mail have been described previously. Respondents with and without hyposmia were selected at a ratio of 2:1 to participate in longitudinal clinical and DAT imaging follow-up. Participants were examined by a movement disorder neurologist and were excluded from the study at baseline if they had clinical signs sufficient for a diagnosis of PD. All participants who completed a baseline visit were contacted by their local enrolling site at year 1 and 2 for a clinic visit and at year 2 by the Institute for Neurodegenerative Disorders to return for (123I)β-CIT SPECT imaging. All hyposmic individuals and 26 normosmic individuals (including all normosmic individuals with a DAT deficit) were contacted to return for clinic visits locally at years 3 and 4 and a (123I)β-CIT SPECT imaging visit at the Institute for Neurodegenerative Disorders at year 4. The remainder of the normosmic individuals was discontinued from the study to conserve resources.
Clinical Evaluations
Clinical evaluations were conducted annually and included a screening neurological examination and a diagnostic questionnaire. Individuals were also assessed for constipation, subtle parkinsonism signs using the PD Symptom Rating Scale, and RBD. Hyposmia was defined as a University of Pennsylvania Smell Identification test score that fell below the 15th percentile for age and sex based on cohort-specific norms. All clinical assessments were conducted by investigators blinded to imaging data.
Assessment of conversion to a clinical diagnosis of PD was made on an annual basis. Conversion was defined by clinical site investigators’ assignment of the best current diagnosis of PD or a parkinsonian syndrome. Parkinsonian syndromes expected to have a DAT loss (ie, progressive supranuclear palsy, multiple systems atrophy, striatonigral degeneration, corticobasal degeneration, and diffuse Lewy body disease) were included in the definition of conversion, as they are often clinically indistinguishable from PD at an early stage.
In addition to the neurological evaluation, individuals completed a neuropsychological battery, heart rate variability testing, and blood collection (for genomics, RNA profiling, and other biomic analyses), and a subset of individuals had cerebrospinal fluid collection (for α-synuclein, phosphorylated synuclein, tau, phosphorylated tau, and β-amyloid 1-42). The results of these assessments will be described in separate reports.
(123I)β-CIT SPECT Imaging
Dopamine transporter imaging using (123I)β-CIT SPECT was completed at baseline and at year 2 and 4, and analysis of the specific binding ratio (SBR) was performed as previously described. The percentage of age-expected lowest putamen (123I)β-CIT binding, based on comparison with a previously acquired database of 99 healthy individuals, was used to categorize individuals as having a DAT deficit (65% or less of age-expected lowest putamen SBR), DAT in an indeterminate range (greater than 65% to 80% of age-expected lowest putamen SBR), or no DAT deficit (greater than 80% age-expected lowest putamen SBR). All SPECT imaging analyses were conducted blind to clinical assessments and diagnosis and olfactory status.
Data Analysis
χ2 Tests, Fisher exact tests, Cochran-Armitage tests, and Kruskal-Wallis tests were used to compare baseline characteristics for individuals with and without follow-up visits, for hyposmic individuals and normosmic individuals in the follow-up cohort, and for converters vs nonconverters. Kaplan-Meier analyses for time to conversion were stratified by baseline DAT status. Relative risk of conversion (with 95% CIs) was calculated for dichotomized baseline variables, with significance levels based on Fisher exact test. All P values are 2-sided, and significance was set at P < .05.
Results
Participant Tracking for All Visits
The baseline cohort included 203 hyposmic individuals and 100 normosmic individuals. One individual was excluded at baseline because of PD diagnosis. A total of 185 hyposmic individuals and 95 normosmic individuals had at least 1 follow-up visit and were considered eligible (eTable in the Supplement). Of the 280 participants in the follow-up cohort, the 185 hyposmic individuals were significantly older than the 95 normosmic individuals (mean age, 64.5 years vs 62.3 years; P = .01) (Table 1).
Table 1. Baseline Demographic and Imaging Characteristics for Normosmic and Hyposmic Individuals Completing Baseline Visits and at Least 1 Follow-up Clinic or Imaging Visit.
| Characteristic | No. (%) | P Value | |
|---|---|---|---|
| Normosmic (n = 95) | Hyposmic (n = 185) | ||
| Sex | .06 | ||
| Male | 40 (42) | 100 (54.1) | |
| Female | 55 (58) | 85 (45.9) | |
| ≥1 Family member with PD | 51 (54) | 84 (45.4) | .19 |
| Age-expected lowest putamen binding ratio | <.001 | ||
| ≤65% | 1 (1) | 21 (11.4) | |
| >65%-80% | 6 (6) | 30 (16.2) | |
| >80% | 88 (93) | 134 (72.4) | |
| Age, mean (SD), y | 62.3 (9.9) | 64.5 (7.9) | .009 |
| Total UPDRS score, mean (SD) | 2.5 (3.7) | 3.3 (4.2) | .16 |
| UPDRS motor score, mean (SD) | 1.3 (2.0) | 1.8 (2.7) | .32 |
Abbreviations: PD, Parkinson disease; UPDRS, Unified Parkinson’s Disease Rating Scale.
Of 185 individuals with hyposmia, 152 (82.2%) were either observed for 4 years or converted to PD during follow-up. Of the 95 individuals with normosmia, 26 (27%) were observed for 4 years (none converted to PD). Fifty-six individuals with normosmia (59%) were discontinued from the study before the year 4 follow-up clinic visit (55 observed for 2 years), allowing more efficient use of study resources. Details of progress through the study are shown in Figure 1.
Figure 1. Progression of Participants Through the Study.
Flow diagram showing the number of hyposmic individuals and normosmic individuals observed through the study. No normosmic individuals converted to Parkinson disease (PD) during the first 2 years of follow-up, and 56 were discontinued from the study at that point to conserve resources. Subsequently, no normosmic individual who was observed for 4 years converted to a clinical diagnosis. PARS indicates Parkinson Associated Risk Study.
aFive hyposmic individuals who converted to a diagnosis of PD (or dementia with Lewy bodies) before year 4 and did not have further follow-up are considered to have completed the study (endpointed), even if they did not have a year 4 visit.
Conversion to PD Among Hyposmic Individuals
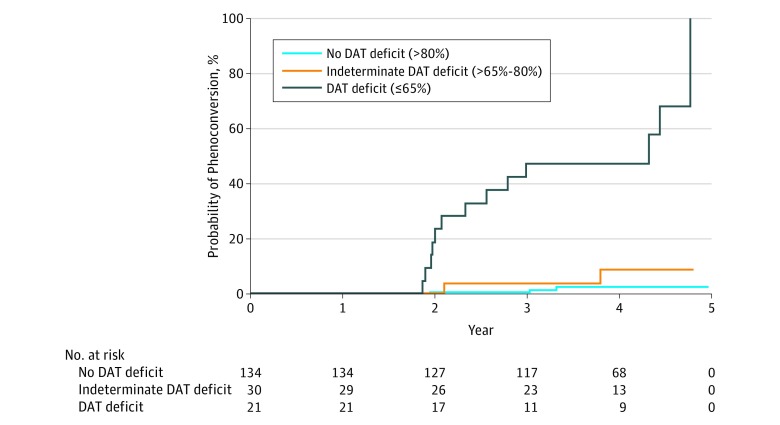
During the 4-year follow-up period, 19 individuals (all of whom were hyposmic at baseline) were clinically diagnosed as having PD. Hyposmic individuals with a baseline DAT deficit had the highest rate of conversion to clinical PD by 4 years of follow-up (67% [14 of 21 individuals]) compared with hyposmic individuals with an indeterminate DAT deficit (9% [2 of 22]) and hyposmic individuals with no baseline DAT deficit (3% [3 of 109]). The relative risk of conversion to a diagnosis of PD in hyposmic individuals with a DAT deficit was 17.47 (95% CI, 7.02-43.45) compared with individuals with either indeterminate or no DAT deficit. Using a less stringent definition of 80% or less of expected DAT at baseline, the relative risk for conversion was 13.52-fold higher compared with individuals without a DAT deficit (95% CI, 4.15-44.06; P < .001). Kaplan-Meier analysis of time-to-conversion by baseline DAT status (n = 185) showed a significantly higher conversion rate for hyposmic individuals who had a DAT deficit of 65% or less at baseline (log-rank test, 71.3; degrees of freedom, 2; P < .001) (Figure 2). While some instability of diagnosis at yearly follow-up was noted, among individuals with hyposmia and a DAT deficit, 5 of 7 (72%) who had at least 1 follow-up visit after conversion were consistent at year 4, and 7 individuals with hyposomia and a DAT deficit converted at year 4 or did not yet have follow-up visits.
Figure 2. Phenoconversion Rate Depending on Degree of Baseline Dopamine Transporter (DAT) Deficit.
Kaplan-Meier curves showing rates of survival to conversion to a diagnosis of Parkinson disease among 185 hyposmic individuals. Individuals are grouped into no DAT deficit (>80% of age-expected lowest putamen binding ratio), indeterminate DAT deficit (>65%-80% expected), and DAT deficit (≤65% expected). The relative hazard for conversion to Parkinson disease was significantly higher in the DAT deficit group (log-rank test, 71.3; degrees of freedom, 2; P < .001).
Baseline Clinical Characteristics of Converters
At baseline, the mean (SD) age of individuals who subsequently converted was 67.1 (6.8) years compared with 63.7 (7.8) years for nonconverters (P = .04) (Table 2). Mean Unified Parkinson’s Disease Rating Scale (UPDRS) total score and motor scores were significantly higher for subsequent converters than nonconverters (total: 7.1 [range, 1-28] vs 2.8 [range, 0-24]; P < .001; motor: 3.9 [range, 0-19] vs 1.4 [range, 0-13]; P < .001), and the mean number of nonmotor symptoms experienced by converters was significantly greater than nonconverters (1.2 [range, 0-5] vs 0.6 [range, 0-6]; P = .02). However, there was substantial overlap between converters and nonconverters on these clinical scales, which would not allow identification of converters based solely on clinical features.
Table 2. Change in Demographic, Imaging, and Clinical Features From Baseline to Follow-up Among Hyposmic Converters and Nonconvertersa.
| Characteristic | No. (%) | |||
|---|---|---|---|---|
| Converters (n = 19) | Nonconverters at Year 4 (n = 133) | |||
| Baseline | Conversion Visit | Baseline | Year 4 Visit | |
| Demographic information | ||||
| Male sex | 14 (74) | NA | 71 (53.4) | NA |
| Age, mean (SD), y | 67.1 (6.8) | NA | 63.7 (7.8) | NA |
| ≥1 Family member with PD | 12 (63) | NA | 60 (45.1) | NA |
| Imaging characteristicsb | ||||
| Age-expected lowest putamen binding ratio | ||||
| ≤65% | 14 (74) | NA | 7 (5.3) | NA |
| >65%-80% | 2 (11) | NA | 20 (15.0) | NA |
| >80% | 3 (16) | NA | 106 (79.7) | NA |
| Age-expected lowest putamen binding ratio, mean (SD), % | 61.6 (21.9) | 52.1 (25.0) | 96.2 (20.3) | 89.9 (23.2) |
| Striatum, mean (SD) | 4.2 (1.2) | 3.5 (1.3) | 5.7 (1.2) | 5.4 (1.3) |
| Motor signs | ||||
| UPDRS total, mean (SD) | 7.1 (6.2) | 26.0 (17.1) | 2.8 (3.7) | 4.4 (4.7) |
| UPDRS motor score, mean (SD) | 3.9 (4.4) | 17.5 (11.6) | 1.4 (2.3) | 2.2 (3.2) |
| Resting tremor | 0 | 5 (26) | 1 (0.8) | 3 (2.3) |
| Action tremor | 4 (21) | 6 (32) | 7 (5.3) | 8 (6.0) |
| Rigidity | 2 (11) | 14 (74) | 3 (2.3) | 9 (6.8) |
| Bradykinesia | 5 (26) | 17 (89) | 18 (13.5) | 22 (16.5) |
| Gait disturbances | 2 (11) | 7 (37) | 3 (2.3) | 5 (3.8) |
| Nonmotor symptoms | ||||
| Sleep disorder | 5 (26) | 7 (37) | 12 (9.0) | 12 (9.0) |
| Depression | 4 (21) | 2 (11) | 11 (8.3) | 12 (9.0) |
| Cognitive loss | 1 (5) | 4 (21) | 1 (0.8) | 9 (6.8) |
| Anxiety | 3 (16) | 3 (16) | 9 (6.8) | 7 (5.3) |
| Excessive sweating | 1 (5) | 0 | 9 (6.8) | 5 (3.8) |
| Lightheadedness | 2 (11) | 1 (5) | 4 (3.0) | 4 (3.0) |
| Constipation | 3 (16) | 4 (21) | 16 (12.0) | 29 (21.8) |
| Urinary urgency/frequency | 4 (21) | 7 (37) | 15 (11.3) | 17 (12.8) |
Abbreviations: PD, Parkinson disease; NA, not applicable; UPDRS, Unified Parkinson’s Disease Rating Scale.
Converters showed significantly greater decline in dopamine transporter and motor performance (measured by UPDRS) as well as a greater number of nonmotor symptoms compared with nonconverters.
Includes data from most recent scan.
Other demographic and putative prodromal features of PD had a minor effect on risk of conversion. Only constipation (relative risk, 2.70; 95% CI, 1.19-6.16; P = .03), defined as having less than 1 bowel movement per day, and older age (older than 65 years; relative risk, 2.49; 95% CI, 1.04-5.96; P = .046) were significantly associated with conversion. Rapid eye movement sleep behavior disorder (relative risk, 2.84; 95% CI, 1.06-7.58; P = .052), defined in our cohort as having RBD symptoms at least once per month, and depression (relative risk, 2.43; 95% CI, 0.93-6.40; P = .10) were not found to be significant.
Clinical Characteristics and Conversion to PD
Individuals who were classified as converters had a phenotype at clinic visit largely consistent with idiopathic PD. All individuals were diagnosed as having PD, with the exception of 1 individual diagnosed as having dementia with Lewy bodies. All individuals had at least 1 cardinal feature of PD, and most had rigidity and bradykinesia (Table 2 and Table 3). Rest tremor was noted in 5 converts (26%), gait difficulty was present in 7 (37%), and cognitive impairment was reported in 4 (21%). Frequency of autonomic features, including excessive sweating, lightheadedness, constipation, and urinary urgency/frequency, were similar for converters compared with nonconverters. Notably, 3 converters did not have a DAT deficit at the time of conversion. All 3 converters without DAT deficit had action tremor and bradykinesia.
Table 3. Imaging and Clinical Characteristics at the Time of Conversion for 19 Hyposmic Individuals Diagnosed as Having PD During Follow-upa.
| Characteristic | Patient Number | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | |
| Age-expected SBR, %b | 29.5 | 32.7 | 35.9 | 37.2 | 37.5 | 39.4 | 39.5 | 40.5 | 41.2 | 42.6 | 44.2 | 45.2 | 45.9 | 50.9 | 51.9 | 61.3 | 85.4 | 89.2 | 112.5 |
| Motor UPDRS score | 19 | 30 | 36 | 5 | 38 | 6 | 12 | 20 | 12 | 14 | 6 | 12 | 42 | 16 | 5 | 26 | 13 | 7 | 14 |
| Rigidity | X | X | X | X | X | X | X | X | X | X | X | X | X | X | |||||
| Bradykinesia | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | ||
| Rest tremor | X | X | X | X | X | ||||||||||||||
| Action tremor | X | X | X | X | X | X | |||||||||||||
| Gait disturbance | X | X | X | X | X | X | X | ||||||||||||
| Cognitive impairment | X | X | X | X | |||||||||||||||
| Depression | X | X | |||||||||||||||||
| Sleep disorder | X | X | X | X | X | X | X | ||||||||||||
Abbreviations: PD, Parkinson disease; SBR, specific binding ratio; UPDRS, Unified Parkinson’s Disease Rating Scale; X, characteristic present.
Almost all individuals had bradykinesia and rigidity. Three individuals who were clinically diagnosed did not have a dopamine deficit on imaging (ie, had scans without evidence of dopaminergic deficit). Action tremor was more common in these individuals than in converters with a dopamine transporter deficit.
Percentage of age-expected uptake in most affected putamen.
Longitudinal Change in Disease Severity of Hyposmic Individuals
Regardless of conversion, hyposmic individuals who also had a baseline DAT deficit showed significantly greater decline in biomarker and clinical measures than comparable individuals without a baseline DAT deficit. Among 131 individuals with complete imaging data (ie, scans at baseline, year 2, and year 4), there was a 20% decline at 4 years in mean SBR for those with a baseline DAT deficit compared with declines of 4% and 5% for those with indeterminate or normal DAT binding, respectively (χ2 = 12.1; degrees of freedom, 2; P = .002) (Figure 3). Three individuals with hyposmia exposed to modafinil (known to reduce DAT) at baseline were excluded. Similarly, individuals with a DAT deficit also showed the greatest change in clinical measures of parkinsonism. The total UPDRS score increased by 9.8 in this group compared with an increase of 1.8 in the other 2 groups (χ2 = 9.4; degrees of freedom, 1; P = .002), and the motor subscore increased by 6.2 compared with 1.2 (χ2 = 5.2; degrees of freedom, 1; P = .02). The 6 individuals with DAT deficit who did not convert to clinical PD showed a decline in SBR of 9.8% (SD, 16.8%), and their UPDRS motor score increased by 5.2 (SD, 4.2) points, midway between the DAT deficit hyposmic individuals and the non–DAT deficit hyposmic individuals.
Figure 3. Mean Change in Whole Striatum Specific Binding Ratio (SBR).
Percentage of change in mean SBR for 131 hyposmic individuals with complete dataa divided into 3 groups based on baseline dopamine transporter (DAT) status; 96 had no DAT deficit (>80% of age-expected lowest putamen binding ratio), 20 had an indeterminate DAT deficit (>65%-80% expected), and 15 had a DAT deficit (≤65% expected). The DAT deficit group experienced significantly greater loss of striatal DAT than the other 2 groups (χ2 = 12.1; degrees of freedom, 2; P = .002). Error bars indicate standard error.
aAt baseline, 4 hyposmic individuals and 1 normosmic individual were receiving modafinil, a drug known to bind to the DAT. Three hyposmic individuals (1 with a DAT deficit and 2 with no DAT deficit) had baseline year, year 2, and year 4 scans but were excluded. None of the 5 modafinil-exposed individuals converted to PD or had abnormal DAT imaging at year 4, when modafinil was either discontinued or withheld at the time of imaging. All individuals exposed to modafinil were excluded from this analysis.
Discussion
The results of this longitudinal study show that approximately two-thirds of hyposmic individuals identified as being at high risk based on abnormal DAT imaging converted to a clinical diagnosis of PD within 4 years of observation. Hyposmic individuals with indeterminate DAT deficits also converted to PD at a rate that was approximately 3-fold higher than hyposmic individuals without a DAT deficit. Notably, none of the 95 individuals with normosmia were diagnosed with PD during a mean follow-up of 2.9 years.
We recognize that diagnosis of PD at disease threshold is challenging and that several research diagnostic criteria for PD have been proposed. In this study, we chose to mimic clinical practice and instructed study investigators, all movement disorder experts, to determine diagnosis at each visit according to their best clinical judgement. Importantly, the expert neurologists were blind to the participants’ DAT imaging results at all clinical assessments.
These data further demonstrate that a sequential biomarker strategy of olfactory testing followed by DAT imaging can systematically identify individuals at high risk for PD. Our screening approach was chosen to exploit the ease of widespread testing of olfaction outside of the clinic setting. The results of this study show that University of Pennsylvania Smell Identification testing may be completed at home and returned by mail to be centrally scored.
We chose our screening strategy to enhance generalizability for idiopathic PD, as both olfactory loss and DAT deficit are present in the overwhelming majority of individuals with PD. While it is difficult to distinguish between PD and other parkinsonian syndromes at disease onset and DAT imaging does not distinguish between PD and other parkinsonian syndromes, almost every participant diagnosed in this study had a clinical presentation consistent with typical, uncomplicated PD. At the time of PD diagnosis, the UPDRS score for converters was consistent with patients with early PD. While rest tremor was only noted in one-third of converters, most converters had typical PD features, including asymmetric rigidity and bradykinesia. Other than 1 individual diagnosed with dementia with Lewy bodies, no participants had dementia at the time of diagnosis, and cognitive decline was noted in 21% of our cohort, a rate similar to that noted in incident PD cohorts. Other nonmotor features, including sleep disorders, depression, and constipation, were similarly present at a frequency consistent with early PD. These results indicate that the combination of hyposmia and DAT deficit likely identifies a group at high risk for typical PD.
In 3 individuals (2 with hyposmia and 1 with normosmia) treated at baseline with modafinil, there was a DAT deficit at baseline that subsequently normalized when modafinil was stopped (2 individuals; 1 individual only at baseline scan). None of these participants converted to PD. The individual with normosmia treated with modafinil was the only normosmic individual with a DAT deficit. Excluding the 2 hyposmic individuals treated with modafinil who had a DAT deficit would further enhance the risk of conversion of hyposmic individuals with a DAT deficit for conversion.
The 4-year longitudinal follow-up data of all hyposmic individuals demonstrated that among the group with hyposmia and a DAT deficit, UPDRS scores changed at an annualized rate that is consistent with clinical trials in de novo patients with PD. Similarly, annualized change in mean SBR was consistent with prior longitudinal studies assessing β-CIT changes in patients with newly diagnosed PD. These UPDRS and DAT imaging changes were observed both in individuals with a DAT deficit who were diagnosed as having clinical PD and in those not diagnosed at 4-year follow-up, suggesting that additional follow-up of this cohort may yield additional individuals who will convert after 4 years.
The results of this study provide a framework for planning PD prevention studies. First, the size of our study and the number of conversions to PD that occurred allow for a relatively stable estimate of the expected rate of conversion to PD among individuals with a DAT deficit. Prior studies suggested a similar conversion rate but were not large enough to estimate this rate with confidence. Second, our study suggests that the screening strategy of olfactory testing followed by DAT imaging identifies individuals with typical PD. The fact that the converters in our cohort had relatively uncomplicated disease and homogeneous characteristics means that a standardized diagnostic approach focusing on conversion to the most frequent phenotype of PD could be used as an endpoint in a PD prevention trial. While definition is difficult, conversion is an inherently meaningful clinical outcome. Third, our finding that both DAT and UPDRS score change in individuals with a DAT deficit, regardless of whether they received a PD diagnosis, suggests that change in these metrics during the prodromal period (as continuous outcome variables) could be used to assess the efficacy of therapies in high-risk populations.
Limitations
Our study had limitations. First, normosmic individuals without a DAT deficit did not receive long-term follow-up. However, it is reasonable to assume that conversion in this group would be the same or lower than in hyposmic individuals with similar DAT results, who showed little progression and a rate of conversion similar to what would be expected in the general population. A second limitation is that our assessments for risk factors other than impaired olfaction may not have been sensitive. Specifically, RBD assessments were only completed in patients with a bed partner, and our assessment was constructed from items in a general sleep questionnaire. A validated RBD screening questionnaire, such as exists today, was not available at the time the study was initiated. Using a more sensitive instrument across the entire study population might have revealed a stronger association between RBD and risk of PD in our cohort. It is likely that using RBD and olfactory testing can identify different sub-groups of at-risk individuals, with higher rates of multiple system atrophy and dementia with Lewy bodies among patients identified via prodromal RBD.
Conclusions
Our study has important implications for understanding the prodromal phase of PD and for planning prevention trials. Most important, it confirms that most hyposmic individuals with DAT deficit will develop clinical PD in a relatively short period of time in a larger cohort than was previously studied. Further, we found that adding additional predictors (such as demographic information or nonmotor symptoms) did not substantially improve the efficiency of identifying at-risk individuals, indicating that a simple paradigm of olfactory testing followed by DAT imaging can identify individuals with typical, uncomplicated PD. Finally, those identified as at risk demonstrated progressive loss of clinical and imaging outcomes at a similar rate to early idiopathic PD, suggesting prodromal progression may be considered as an outcome for PD therapeutic trials. Taken together, our results provide a basis for planning biomarker-based trials to further characterize prodromal PD populations and to test potentially disease-modifying therapies during the prediagnostic phase of PD.
eTable. Baseline demographic and imaging characteristics for patients not completing any follow-up visits and patients completing baseline visit plus at least 1 follow-up clinic or imaging visit.
References
- 1.Berg D, Postuma RB, Adler CH, et al. MDS research criteria for prodromal Parkinson’s disease. Mov Disord. 2015;30(12):1600-1611. [DOI] [PubMed] [Google Scholar]
- 2.Jack CR Jr, Knopman DS, Jagust WJ, et al. Tracking pathophysiological processes in Alzheimer’s disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol. 2013;12(2):207-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kowal SL, Dall TM, Chakrabarti R, Storm MV, Jain A. The current and projected economic burden of Parkinson’s disease in the United States. Mov Disord. 2013;28(3):311-318. [DOI] [PubMed] [Google Scholar]
- 4.Streffer JR, Grachev ID, Fitzer-Attas C, et al. Prerequisites to launch neuroprotective trials in Parkinson’s disease: an industry perspective. Mov Disord. 2012;27(5):651-655. [DOI] [PubMed] [Google Scholar]
- 5.Doty RL, Deems DA, Stellar S. Olfactory dysfunction in parkinsonism: a general deficit unrelated to neurologic signs, disease stage, or disease duration. Neurology. 1988;38(8):1237-1244. [DOI] [PubMed] [Google Scholar]
- 6.Hawkes C. Olfaction in neurodegenerative disorder. Mov Disord. 2003;18(4):364-372. [DOI] [PubMed] [Google Scholar]
- 7.Ross GW, Petrovitch H, Abbott RD, et al. Association of olfactory dysfunction with risk for future Parkinson’s disease. Ann Neurol. 2008;63(2):167-173. [DOI] [PubMed] [Google Scholar]
- 8.Sawle GV, Playford ED, Burn DJ, Cunningham VJ, Brooks DJ. Separating Parkinson’s disease from normality: discriminant function analysis of fluorodopa F 18 positron emission tomography data. Arch Neurol. 1994;51(3):237-243. [DOI] [PubMed] [Google Scholar]
- 9.Marshall VL, Reininger CB, Marquardt M, et al. Parkinson’s disease is overdiagnosed clinically at baseline in diagnostically uncertain cases: a 3-year European multicenter study with repeat [123I]FP-CIT SPECT. Mov Disord. 2009;24(4):500-508. [DOI] [PubMed] [Google Scholar]
- 10.Sierra M, Sánchez-Juan P, Martínez-Rodríguez MI, et al. Olfaction and imaging biomarkers in premotor LRRK2 G2019S-associated Parkinson disease. Neurology. 2013;80(7):621-626. [DOI] [PubMed] [Google Scholar]
- 11.Khan NL, Jain S, Lynch JM, et al. Mutations in the gene LRRK2 encoding dardarin (PARK8) cause familial Parkinson’s disease: clinical, pathological, olfactory and functional imaging and genetic data. Brain. 2005;128(pt 12):2786-2796. [DOI] [PubMed] [Google Scholar]
- 12.Iranzo A, Tolosa E, Gelpi E, et al. Neurodegenerative disease status and post-mortem pathology in idiopathic rapid-eye-movement sleep behaviour disorder: an observational cohort study. Lancet Neurol. 2013;12(5):443-453. [DOI] [PubMed] [Google Scholar]
- 13.Jennings D, Siderowf A, Stern M, et al. ; PARS Investigators . Imaging prodromal Parkinson disease: the Parkinson Associated Risk Syndrome Study. Neurology. 2014;83(19):1739-1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Siderowf A, Jennings D, Eberly S, et al. ; PARS Investigators . Impaired olfaction and other prodromal features in the Parkinson At-Risk Syndrome Study. Mov Disord. 2012;27(3):406-412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abbott RD, Petrovitch H, White LR, et al. Frequency of bowel movements and the future risk of Parkinson’s disease. Neurology. 2001;57(3):456-462. [DOI] [PubMed] [Google Scholar]
- 16.Tanner CM, Ellenberg J, Mayeux R, Ottman R, Langston JW. A sensitive and specific screening method for Parkinson’s disease. Neurology. 2004;32:267-268. [Google Scholar]
- 17.Comella CL, Nardine TM, Diederich NJ, Stebbins GT. Sleep-related violence, injury, and REM sleep behavior disorder in Parkinson’s disease. Neurology. 1998;51(2):526-529. [DOI] [PubMed] [Google Scholar]
- 18.Doty RL, Shaman P, Dann M. Development of the University of Pennsylvania Smell Identification test: a standardized microencapsulated test of olfactory function. Physiol Behav. 1984;32(3):489-502. [DOI] [PubMed] [Google Scholar]
- 19.Seibyl JP, Marek K, Sheff K, et al. Iodine-123-beta-CIT and iodine-123-FPCIT SPECT measurement of dopamine transporters in healthy subjects and Parkinson’s patients. J Nucl Med. 1998;39(9):1500-1508. [PubMed] [Google Scholar]
- 20.Jennings DL, Seibyl JP, Oakes D, Eberly S, Murphy J, Marek K. (123I) beta-CIT and single-photon emission computed tomographic imaging vs clinical evaluation in Parkinsonian syndrome: unmasking an early diagnosis. Arch Neurol. 2004;61(8):1224-1229. [DOI] [PubMed] [Google Scholar]
- 21.Postuma RB, Berg D, Stern M, et al. MDS clinical diagnostic criteria for Parkinson’s disease. Mov Disord. 2015;30(12):1591-1601. [DOI] [PubMed] [Google Scholar]
- 22.Olanow CW, Rascol O, Hauser R, et al. ; ADAGIO Study Investigators . A double-blind, delayed-start trial of rasagiline in Parkinson’s disease. N Engl J Med. 2009;361(13):1268-1278. [DOI] [PubMed] [Google Scholar]
- 23.Ravina B, Tanner C, Dieuliis D, et al. ; Parkinson Study Group LABS-PD Investigators . A longitudinal program for biomarker development in Parkinson’s disease: a feasibility study. Mov Disord. 2009;24(14):2081-2090. [DOI] [PubMed] [Google Scholar]
- 24.Aarsland D, Andersen K, Larsen JP, Lolk A, Kragh-Sørensen P. Prevalence and characteristics of dementia in Parkinson disease: an 8-year prospective study. Arch Neurol. 2003;60(3):387-392. [DOI] [PubMed] [Google Scholar]
- 25.Foltynie T, Brayne CE, Robbins TW, Barker RA. The cognitive ability of an incident cohort of Parkinson’s patients in the UK: the CamPaIGN study. Brain. 2004;127(pt 3):550-560. [DOI] [PubMed] [Google Scholar]
- 26.Weintraub D, Moberg PJ, Duda JE, Katz IR, Stern MB. Effect of psychiatric and other nonmotor symptoms on disability in Parkinson’s disease. J Am Geriatr Soc. 2004;52(5):784-788. [DOI] [PubMed] [Google Scholar]
- 27.Shults CW, Oakes D, Kieburtz K, et al. ; Parkinson Study Group . Effects of coenzyme Q10 in early Parkinson disease: evidence of slowing of the functional decline. Arch Neurol. 2002;59(10):1541-1550. [DOI] [PubMed] [Google Scholar]
- 28.Ponsen MM, Stoffers D, Booij J, van Eck-Smit BLF, Wolters ECh, Berendse HW. Idiopathic hyposmia as a preclinical sign of Parkinson’s disease. Ann Neurol. 2004;56(2):173-181. [DOI] [PubMed] [Google Scholar]
- 29.Berendse HW, Booij J, Francot CM, et al. Subclinical dopaminergic dysfunction in asymptomatic Parkinson’s disease patients’ relatives with a decreased sense of smell. Ann Neurol. 2001;50(1):34-41. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable. Baseline demographic and imaging characteristics for patients not completing any follow-up visits and patients completing baseline visit plus at least 1 follow-up clinic or imaging visit.