Abstract
Objective
Previous studies have estimated future PD prevalence based on population aging. This study revisits that projection by accounting for the potential impact of declining rates of smoking.
Methods
The age- and gender-stratified smoking prevalence in the United States from 2000–2040 were obtained from the US Census Bureau and the US Surgeon General’s Smoking Report. PD prevalence was estimated based on population aging with and without an account of the impact of declining smoking rates. Relative risks of 0.56 and 0.78 were applied for current and former smokers, respectively.
Results
Accounting for aging alone, ~700,000 PD cases are predicted by 2040. After accounting for the declining smoking prevalence, ~770,000 cases, an increase of ~10% over the estimate without smoking, are predicted.
Conclusions
If the epidemiological association of smoking and PD is causal, projecting future cases without considering smoking may underestimate disease burden, underscoring the urgency of adequate resource allocation.
Keywords: Parkinson’s projection, smoking, health care burden, chronic disease, aging
Introduction
Public health initiatives targeting tobacco use have impacted health behaviors and average life expectancy in the US.1,2 The US Surgeon General and the Department of Health and Human Services predict decreases in the prevalence of smoking in coming decades, whereas US Census Bureau projections suggest substantial growth in the US population ≥50 years old.3,4 Both trends reflect improved population health, but may lead to an increase in the prevalence of Parkinson’s disease (PD) whose risk is known to increase with age.5 In addition, smoking is associated robustly with lower PD risk, although a mechanism for causality is not known.5–9
Dorsey et al.10 estimated the prevalence of PD in the year 2005 in 15 countries (including the US) according to age group. The study projected PD prevalence for the year 2030, and has become a landmark for estimates of future economic and social burdens of PD. It projected, however, PD prevalence based only on the aging of the population, not taking into account the trend towards decline of smoking that suggests that decline of smoking will contribute to a higher PD incidence in the years to come. Thus, we have included the predicted smoking trend in re-examining the previous projection of PD prevalence to provide a more accurate estimate of the future prevalence.
Methods
Data on both historical and projected US populations by gender from the years 1990 to 2040 were obtained from the US Census Bureau.3 The data were tabulated based on age-gender-specific cohorts of ages 50–64, 65–74, and 75+ for each calendar year.
Data regarding historical and future estimates of smoking prevalence from 1990 to the year 2040 were obtained from the Surgeon General’s Report on smoking,11 specifically projections by Holford et al.12 Prevalence rates for current, former, and non-smokers were derived for age- and gender-specific cohorts of ages 50–64, 65–74, and 75+ for each calendar year.
In PD, dopamine cell loss may start 5–13 years or more before clinical diagnosis.13,14 Thus, we implemented a 10-year lag of smoking behavior to predict PD prevalence in a given year in order to account for the temporal effect of smoking on PD incidence.
A large meta-analysis including 44 case-control studies and four cohort studies determined a pooled relative risk of PD of 0.59 for former smokers, and a relative risk of 0.39 for current smokers.9 The largest study in the US with detailed smoking data determined odds ratios of 0.56 for current and 0.78 for former smokers.15 As the incidence of PD is so low in the general population, the odds ratio can reasonably be interpreted as relative risk.16 The model incorporates odds ratios for PD of 0.78 for former smokers and 0.56 for current smokers; this represents the more conservative US estimates.15,17
We applied the population data to PD prevalence models, where P is prevalence and Pop is population. First, we utilized the same prevalence estimates from Dorsey et al. 10 for each age cohort (P50–64, P65–74, P75+) to calculate the PD prevalence in each year until the year 2040 (Equation 1). This model does not account for smoking.
| Equation 1 |
Then we projected PD prevalence until the year 2040 by accounting for smoking prevalence. The overall prevalence of PD for a given year resulted from a summation of the output of the following model for each age-gender cohort (Equation 2):
| Equation 2 |
Results
From year 2000 to 2040, the US population ≥50 years of age will increase by 46.2% while the percentage of smokers ≥50 years of age will decrease by 59.8% (Figure 1). The absolute number of former smokers in the US will plateau, although the percentage of the population comprised by this group will decrease. By the year 2040, active smokers are expected to be only 3.5% of the US population ≥50 years of age, whereas former smokers are predicted to be 26.4%.
Figure 1.
US populations age ≥50 years of smokers (S), former smokers (FS) and never smokers (NS) between the years 2000 and 2040.
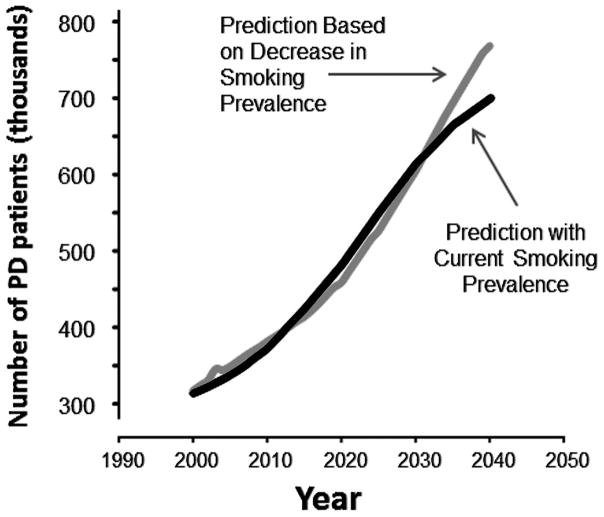
The number of people in the US with PD in the year 2040 is projected (Equation 1) to be approximately 700,000 individuals due to aging alone (Figure 2). This number, however, will increase to approximately 770,000 (10% higher) if smoking truly decreases PD risk and the current trend towards decreased smoking continues (Equation 2). This represents an approximately 56% increase in the PD population between 2005 and 2040. Our model also demonstrates an overall disease prevalence rate of 0.535% of the entire US population in 2040, compared to a prevalence rate of 0.401% in the year 2005. As shown in Figures 1 and 2, the burden of PD in the US will grow substantially as the prevalence of smoking continues to decrease. The divergence between PD prevalence projections (Figure 2) occurs because the prevalence of smoking in the US is expected to continue to decrease.10 Of note, the US Surgeon General’s projected changes in smoking habits within the US population and population growth are non-linear, resulting in the non-monophasic increase in cases of PD and the initial crossover of the models shown in Figure 2. Although the proportion of smokers in the overall population is expected to decrease over time, certain age cohorts are projected to experience increases in the number of smokers at various time points rather than a continual decrease. The divergence of the PD prevalence projections occurs farther in the future when the number of smokers across all cohorts is decreased in contrast to decreases only in certain age cohorts earlier in time 12.
Figure 2.
PD population age ≥50 in the US between the years 2000 and 2040 based upon models that do and do not account for decreasing smoking prevalence over time.
Discussion
Chronic diseases constitute a major health care burden in the US.18 Estimates of annual disease-related medical costs per PD patient range from $10,378 to $14,400 (in 2010 U.S. dollars), not including indirect costs.19–22 Our study suggests that previous projections may have underestimated PD prevalence by 10% in the coming decade by not accounting for the impact of decreasing rates of smoking. Even by our conservative estimates, these 70,000 additional PD cases would add an annual cost of greater than $700 million for the U.S.
Estimates of PD prevalence vary widely, although there is a lack of current large studies for the US.23 A commonly-cited estimate of US prevalence of PD based upon a single door-to-door survey was extrapolated to an estimated PD prevalence of 430,000 in 2010 for the population older than 40.24 More recently, Strickland et al.25 used a PD registry combined with capture-recapture methodology in Nebraska in 2000, and estimated a 2010 PD prevalence estimate for people 40 or older of nearly 920,000 individuals in the U.S., somewhat greater than than Kowal et al.19 whose estimate was 630,000 people. Other studies also have focused on estimated incidence of PD.26–30 The most conservative estimates have generally utilized door to door surveys or autopsy data, whereas studies that focus on claims data yielded higher estimates.30
In the current study, we selected the conservative, well-accepted and widely-cited estimate of PD prevalence from Dorsey et al.10 as our base model. They utilized International Data Base’s population pyramids, in combination with door-to-door studies with age-specific prevalence, to create a model that estimated 373,968 individuals in 2010.10 Our model also utilized conservative modifiers for the effect of former and current smoking on the risk of developing PD. Other studies report risk reductions as profound as 74% in current smokers.31 Therefore, it stands to reason that our estimate of disease prevalence for the year 2040 is also conservative, and in reality the number may be much greater.
The results indicate that if tobacco non-use is indeed causally related to an increased risk of PD, past projections may underestimate the prevalence of PD in 2040 by at least 10%. The PD prevalence that our model projects would vary based on the source data for current PD prevalence, but we emphasize that accounting for declining rates of smoking, regardless of baseline disease prevalence, is necessary for a more accurate depiction of future PD burden. This also holds true for the degree of risk modification due to smoking that is utilized in the model, which also varies in the literature.9,15
A recent study by Savica et al.32 demonstrated that the incidence of PD might have increased over the 30-year time period from 1976 to 2005. Although intriguing, this finding needs confirmation, as one may speculate that it may be due, at least to some extent, to the decreasing trend of smoking in the past 50 years in the US.32 Therefore, our model incorporating the diminishing trend in smoking may offer a more accurate projection of future PD prevalence if smoking is indeed causally linked to PD risk. Smoking prevalence is projected to continue to decline with ongoing nationwide smoking cessation health initiatives.12 The Centers for Disease Control has set a goal of decreasing smoking prevalence to 12% of the population by the year of 2020.33 Until smoking prevalence reaches its nadir, it is reasonable to expect continual increases in yearly PD incidence with a plateau beginning in 2030, and with subsequent effects on disease prevalence.
Our model relies on the hypothesis that smoking is neuroprotective for PD, thus PD prevalence will decrease as smoking decreases in the US. It has been proposed, however, that this effect is the result of decreased responsiveness to nicotine in the PD prodromal period, thereby leading to decreased rates of smoking in individuals later diagnosed with PD.34 Although this latter idea is not widely accepted, if true it would nullify the current calculations. This limitation is important, as causality has not been proven. We also utilized a 10-year lag in estimating the influence of smoking on PD risk, but we recognize this is somewhat arbitrary and also limits the accuracy of our projections. Finally, projection accuracy is predicated upon the estimates of future numbers of smokers, former smokers, and non-smokers; this is dependent upon the continued success of public health measures related to smoking cessation.
Above and beyond medical costs, as a chronic condition, PD imposes a significant burden on families and caregivers that is not easily quantified. This will continue to grow as the size of the elderly population in the US continues to increase. Our study suggests additional resources are likely to be needed above those currently estimated.
Acknowledgments
Funding Sources: Tobacco CURE funds grant from the Pennsylvania Department of Health; NIH (NS060722 and NS082151 to XH); the Hershey Medical Center GCRC (National Institute of Health M01RR10732); the GCRC Construction Grant (C06RR016499) The Center for Applied Studies in Health Economics (CASHE).
Footnotes
Financial Disclosures: The authors have nothing to disclose as it relates to this research.
Documentation of Author Roles
Alexander Rossi: Research project conception, organization and execution, statistical analysis execution, manuscript writing of the first draft, review, and critique.
Kristin Berger: Manuscript writing of the first draft, review and critique.
Dr. Honglei Chen: Statistical analysis review and critique, manuscript review and critique.
Dr. Douglas Leslie: Research project conception, organization, and execution, statistical analysis design, review, and critique, manuscript review and critique.
Dr. Richard Mailman: Review and critique of analysis, manuscript review and critique.
Dr. Xuemei Huang: Research project conception, organization, and execution; manuscript organization, review, and critique.
Financial Disclosures for last three years:
Xuemei Huang: Has received consultant fees from the National Institute of Environmental Health Sciences unrelated to this study.
Honglei Chen: Nothing to disclose
Richard Mailman Has received consultant fees from Pfizer Inc. and several law firms unrelated to this study.
Statistical analysis:
Honglei Chen, Douglas Leslie
References
- 1.Holford TR, Meza R, Warner, et al. Tobacco Control and the Reduction in Smoking-Related Premature Deaths in the United States, 1964–2012. JAMA. 2017;311(2):164–171. doi: 10.1001/jama.2013.285112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Taylor DH, Jr, Hasselblad V, Henley SJ, Thun MJ, Sloan FA. Benefits of smoking cessation for longevity. Am J Public Health. 2002;92(6):990–996. doi: 10.2105/ajph.92.6.990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anonymous. Population Estimates Program. Washington DC: United States Census Bureau; 2017. [Google Scholar]
- 4.Colby SL, Ortman JM. Commerce, editor. Projections of the size and composition of the U.S. population: 2014–2060. Washington DC: United States Census Bureau; 2015. p. 13. [Google Scholar]
- 5.Kalia LV, Lang AE. Parkinson’s disease. Lancet. 2015;386(9996):896–912. doi: 10.1016/S0140-6736(14)61393-3. [DOI] [PubMed] [Google Scholar]
- 6.Grandinetti A, Morens DM, Reed D, MacEachern D. Prospective study of cigarette smoking and the risk of developing idiopathic Parkinson’s disease. Am J Epidemiol. 1994;139(12):1129–1138. doi: 10.1093/oxfordjournals.aje.a116960. [DOI] [PubMed] [Google Scholar]
- 7.Paganini-Hill A. Risk factors for parkinson’s disease: the leisure world cohort study. Neuroepidemiology. 2001;20(2):118–124. doi: 10.1159/000054770. [DOI] [PubMed] [Google Scholar]
- 8.Hernan MA, Zhang SM, Rueda-deCastro AM, Colditz GA, Speizer FE, Ascherio A. Cigarette smoking and the incidence of Parkinson’s disease in two prospective studies. Ann Neurol. 2001;50(6):780–786. doi: 10.1002/ana.10028. [DOI] [PubMed] [Google Scholar]
- 9.Hernan MA, Takkouche B, Caamano-Isorna F, Gestal-Otero JJ. A meta-analysis of coffee drinking, cigarette smoking, and the risk of Parkinson’s disease. Ann Neurol. 2002;52(3):276–284. doi: 10.1002/ana.10277. [DOI] [PubMed] [Google Scholar]
- 10.Dorsey ER, Constantinescu R, Thompson JP, et al. Projected number of people with Parkinson disease in the most populous nations, 2005 through 2030. Neurology. 2007;68(5):384–386. doi: 10.1212/01.wnl.0000247740.47667.03. [DOI] [PubMed] [Google Scholar]
- 11.Warren GW, Alberg AJ, Kraft AS, Cummings KM. The 2014 Surgeon General’s report: “The health consequences of smoking--50 years of progress”: a paradigm shift in cancer care. Cancer. 2014;120(13):1914–1916. doi: 10.1002/cncr.28695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holford TR, Levy DT, McKay LA, et al. Patterns of birth cohort-specific smoking histories, 1965–2009. American journal of preventive medicine. 2014;46(2):e31–37. doi: 10.1016/j.amepre.2013.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marek K, Jennings D, Seibyl J. Dopamine agonists and Parkinson’s disease progression: what can we learn from neuroimaging studies. Ann Neurol. 2003;53(Suppl 3):S160–S166. doi: 10.1002/ana.10486. [DOI] [PubMed] [Google Scholar]
- 14.Marek K, Jennings D. Can we image premotor Parkinson disease? Neurology. 2009;72(7 Suppl):S21–S26. doi: 10.1212/WNL.0b013e318198df97. [DOI] [PubMed] [Google Scholar]
- 15.Chen H, Huang X, Guo X, et al. Smoking duration, intensity, and risk of Parkinson disease. Neurology. 2010;74(11):878–884. doi: 10.1212/WNL.0b013e3181d55f38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davies HT, Crombie IK, Tavakoli M. When can odds ratios mislead? Bmj. 1998;316(7136):989–991. doi: 10.1136/bmj.316.7136.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thacker EL, O’Reilly EJ, Weisskopf MG, et al. Temporal relationship between cigarette smoking and risk of Parkinson disease. Neurology. 2007;68(10):764–768. doi: 10.1212/01.wnl.0000256374.50227.4b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bodenheimer T, Chen E, Bennett HD. Confronting the growing burden of chronic disease: can the U.S. health care workforce do the job? Health affairs (Project Hope) 2009;28(1):64–74. doi: 10.1377/hlthaff.28.1.64. [DOI] [PubMed] [Google Scholar]
- 19.Kowal SL, Dall TM, Chakrabarti R, Storm MV, Jain A. The current and projected economic burden of Parkinson’s disease in the United States. Mov Disord. 2013;28(3):311–318. doi: 10.1002/mds.25292. [DOI] [PubMed] [Google Scholar]
- 20.Noyes K, Liu H, Li Y, Holloway R, Dick AW. Economic burden associated with Parkinson’s disease on elderly Medicare beneficiaries. Mov Disord. 2006;21(3):362–372. doi: 10.1002/mds.20727. [DOI] [PubMed] [Google Scholar]
- 21.Huse DM, Schulman K, Orsini L, Castelli-Haley J, Kennedy S, Lenhart G. Burden of illness in Parkinson’s disease. Mov Disord. 2005;20(11):1449–1454. doi: 10.1002/mds.20609. [DOI] [PubMed] [Google Scholar]
- 22.O’Brien J, Ward A, Michels S, Tzivelekis S. Economic burden associated with Parkinson Disease. Drug Benefit Trends. 2009;21(6):179. [Google Scholar]
- 23.JB, Alcalay R, Bower J, et al. Improving estimates of Parkinson’s Disease prevalence and incidence in the USA[abstract] Movement Disorders. 2014;29(Suppl 1):1475. [Google Scholar]
- 24.Schoenberg BS, Anderson DW, Haerer AF. Prevalence of Parkinson’s disease in the biracial population of Copiah County, Mississippi. Neurology. 1985;35(6):841–845. doi: 10.1212/wnl.35.6.841. [DOI] [PubMed] [Google Scholar]
- 25.Strickland D, Bertoni JM. Parkinson’s prevalence estimated by a state registry. Mov Disord. 2004;19(3):318–323. doi: 10.1002/mds.10619. [DOI] [PubMed] [Google Scholar]
- 26.Morens DM, Davis JW, Grandinetti A, Ross GW, Popper JS, White LR. Epidemiologic observations on Parkinson’s disease: incidence and mortality in a prospective study of middle-aged men. Neurology. 1996;46(4):1044–1050. doi: 10.1212/wnl.46.4.1044. [DOI] [PubMed] [Google Scholar]
- 27.Bower JH, Maraganore DM, McDonnell SK, Rocca WA. Incidence and distribution of parkinsonism in Olmsted County, Minnesota, 1976–1990. Neurology. 1999;52(6):1214–1220. doi: 10.1212/wnl.52.6.1214. [DOI] [PubMed] [Google Scholar]
- 28.Mayeux R, Marder K, Cote LJ, et al. The frequency of idiopathic Parkinson’s disease by age, ethnic group, and sex in northern Manhattan, 1988–1993. Am J Epidemiol. 1995;142(8):820–827. doi: 10.1093/oxfordjournals.aje.a117721. [DOI] [PubMed] [Google Scholar]
- 29.Van Den Eeden SK, Tanner CM, Bernstein AL, et al. Incidence of Parkinson’s disease: variation by age, gender, and race/ethnicity. Am J Epidemiol. 2003;157(11):1015–1022. doi: 10.1093/aje/kwg068. [DOI] [PubMed] [Google Scholar]
- 30.Safarpour D, Thibault DP, DeSanto CL, et al. Nursing home and end-of-life care in Parkinson disease. Neurology. 2015;85(5):413–419. doi: 10.1212/WNL.0000000000001715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morozova N, O’Reilly EJ, Ascherio A. Variations in gender ratios support the connection between smoking and Parkinson’s disease. Mov Disord. 2008;23(10):1414–1419. doi: 10.1002/mds.22045. [DOI] [PubMed] [Google Scholar]
- 32.Savica R, Grossardt BR, Bower JH, Ahlskog JE, Rocca WA. Time Trends in the Incidence of Parkinson Disease. JAMA Neurol. 2016 doi: 10.1001/jamaneurol.2016.0947. in press/on-line. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Anonymous. DATA2020:HealthyPeople.gov. Washington DC: Office of Disease Prevention and Health Promotion; 2017. [Google Scholar]
- 34.Ritz B, Lee PC, Lassen CF, Arah OA. Parkinson disease and smoking revisited: ease of quitting is an early sign of the disease. Neurology. 2014;83(16):1396–1402. doi: 10.1212/WNL.0000000000000879. [DOI] [PMC free article] [PubMed] [Google Scholar]