Abstract
In multiple system atrophy (MSA), progressive neurodegeneration results from the protein α-synuclein misfolding into a self-templating prion conformation that spreads throughout the brain. MSA prions are transmissible to transgenic (Tg) mice expressing mutated human α-synuclein (TgM83+/−), inducing neurological disease following intracranial inoculation with brain homogenate from deceased patient samples. Noting the similarities between α-synuclein prions and PrP scrapie (PrPSc) prions responsible for Creutzfeldt–Jakob disease (CJD), we investigated MSA transmission under conditions known to result in PrPSc transmission. When peripherally exposed to MSA via the peritoneal cavity, hind leg muscle, and tongue, TgM83+/− mice developed neurological signs accompanied by α-synuclein prions in the brain. Iatrogenic CJD, resulting from prion adherence to surgical steel instruments, has been investigated by incubating steel sutures in contaminated brain homogenate before implantation into mouse brain. Mice studied using this model develop disease, whereas wire incubated in control homogenate had no effect on the animals. Notably, formalin fixation does not inactivate α-synuclein prions. Fixed MSA patient samples also transmitted disease to TgM83+/− mice, even after incubating in fixative for 244 months. Finally, at least 10% sarkosyl was found to be the concentration necessary to partially inactivate MSA prions. These results demonstrate the robustness of α-synuclein prions to denaturing conditions. Moreover, they establish the parallel characteristics between PrPSc and α-synuclein prions, arguing that clinicians should exercise caution when working with materials that could contain α-synuclein prions to prevent disease transmission.
Keywords: α-synuclein, neurodegeneration, propagation, proteinopathies, transmission models
INTRODUCTION
Despite the steady accumulation of evidence arguing that most neurodegenerative diseases are caused by prions, most of these diseases have not transmitted neurological disease to rodents, with the exception of the PrP scrapie (PrPSc) diseases (reviewed in [21]). In contrast, brain homogenate from deceased multiple system atrophy (MSA) patients readily transmit neurodegeneration to transgenic mice expressing mutant (A53T) α-synuclein (TgM83+/−) [51]. This finding provided the opportunity to determine if α-synuclein adopts an alternative conformation that undergoes self-propagation, i.e., it becomes a prion. Testing this hypothesis, we found that 19 MSA patient samples from three continents transmitted diseases to the TgM83+/− mice, but six Parkinson’s disease (PD) patient samples from two continents did not [40]. Moreover, using human embryonic kidney (HEK293T) cells expressing mutated α-synuclein fused to yellow fluorescent protein (α-syn140*A53T–YFP), we also found that we could infect the cells with α-synuclein prions isolated from MSA patient samples but not from PD patient samples [53]. The existence of two distinct strains of α-synuclein prions in MSA and PD patients is consistent with work published by others demonstrating that α-synuclein misfolding into distinct conformations produces differing physiological effects [5, 37].
To determine if these putative α-synuclein prions exhibit properties similar to those observed for PrPSc, we investigated the ability of MSA to transmit disease to TgM83+/− mice following peripheral inoculation. Using four MSA patient samples, we found both intraperitoneal (ip) and intramuscular (im) inoculations resulted in disease transmission. In addition, analogous to PrPSc, stainless steel wires were incubated with brain homogenate from MSA patients, and the contaminated wires transmitted disease to TgM83+/− mice following permanent insertion into the brain. We also investigated the resistance of α-synuclein prions to formalin inactivation as well as to the denaturing detergent sarkosyl. MSA patient samples that were fixed in formalin up to 244 months (more than 20 years) transmitted disease to the inoculated mice. Furthermore, measuring prion infectivity in the α-syn140*A53T–YFP cells, up to 10% sarkosyl was needed to partially denature MSA prions.
MSA is a neurodegenerative disease resulting in the accumulation of glial cytoplasmic inclusions (GCIs) in oligodendrocytes [36]. With an incidence rate of 0.6 to 0.7 per 100,000 individuals annually [13, 45], MSA is characterized by rapidly progressing autonomic dysfunction and motor deficits presenting as either cerebellar symptoms or parkinsonism [22]. Despite the identification of GCIs as the neuropathological hallmark of MSA in 1989 [36], α-synuclein was not recognized as the primary protein component until 1998 [44, 49, 50]. Nearly 20 years later, α-synuclein was shown to misfold into a prion conformation capable of self-templating in MSA patient samples, and it is now recognized as the second neuropathogenic prion [40, 51].
Deep brain stimulation (DBS) has widely been used for the last 20 years to ameliorate motor symptoms, including tremors and rigidity, in PD patients. MSA patients, who are typically unresponsive to DBS [34], are most often misdiagnosed with PD [18, 24, 27, 35, 52] and may receive DBS as a treatment. In addition, DBS is increasingly being used to help patients with a number of disorders including major depression [1, 30], obsessive-compulsive disorder [14, 30], Tourette syndrome [10], and schizophrenia [9]. Accidental iatrogenic transmission of CJD previously occurred when DBS depth electrodes were reused without proper decontamination [3, 20]. Given the growing use of DBS as a therapeutic intervention, the findings reported here demonstrate the need for further research into decontamination methods for surgical equipment that may become contaminated with α-synuclein prions. These studies will be critical to minimize the risk of lateral transmission of MSA, particularly in light of the ability of α-synuclein prions to transmit disease to the central nervous system (CNS) following peripheral exposure.
RESULTS
MSA prions transmit intraperitoneally
Previously, we used the TgM83+/− mouse model, which expresses human α-synuclein with the familial A53T mutation under the prion protein (Prnp) promoter [19], to test the ability of MSA patient samples to transmit disease [40, 51, 53]. Following ic inoculation, brain homogenate from 14 MSA patient samples induced neurological disease and α-synuclein neuropathology in this mouse model. Notably, while ic inoculation induced disease in both hemizygous and homozygous TgM83 animals, TgM83+/+ mice develop spontaneous disease, whereas TgM83+/− mice do not [51]. In these hemizygous animals, inoculation with control patient samples had no effect [40]. Importantly, inoculation using α-synuclein aggregates isolated from patient sample MSA14 following nucleic acid digestion with benzonase induced synucleinopathy in the TgM83+/− mice, demonstrating the transmission of neurological disease is a result of α-synuclein prions present in the brains of MSA patients [53].
Given the observed similarities between α-synuclein and PrPSc prions, as well as the ability of PrPSc to transmit disease via ip inoculation [26], we compared the transmissibility of four MSA patient samples (basal ganglia from MSA6 and substantia nigra including the surrounding midbrain from MSA12, MSA13, and MSA18) with one control patient sample (C2) following both ic (30 μL 1% brain homogenate; Online Resource, Fig. S1) and ip (100 μL 1% brain homogenate; Fig. 1a) inoculation. While ic inoculation of the four MSA samples transmitted disease to all inoculated TgM83+/− mice (P < 0.0001), the MSA18 sample showed a reduced titer with a longer incubation period. This observation was consistent with ip inoculation, where all four cases again transmitted disease (P < 0.0001), but MSA18 resulted in the longest incubation period and only transmitted disease to 2 of the 8 inoculated mice by 450 days post inoculation (dpi). Intraperitoneal inoculation using brain homogenate from patient C2 had no effect on the TgM83+/− mice by 450 dpi.
Fig. 1. Intraperitoneal transmission of MSA prions.
TgM83+/− mice received intraperitoneal (ip) injection of 1% brain homogenate from one control and four MSA patient samples. (a) Kaplan–Meier plot shows the onset of neurological signs. Upticks indicate mice that did not die from synucleinopathy. (b) Alpha-synuclein prions were measured from frozen half-brains in the α-syn140*A53T–YFP cell assay (× 103 A.U.). Closed circles indicate mice containing prions (above dotted line), while triangles indicate mice that died from other causes. Open circles indicate mice terminated at 450 days post inoculation (dpi). Mice inoculated with MSA6 (P < 0.01), MSA12 (P < 0.05), and MSA13 (P < 0.01) contained significantly more α-synuclein prions than controls. (c) Quantification of phosphorylated α-synuclein neuropathology in C2-inoculated mice compared to mice positive for synucleinopathy. The percent area containing pathology in the thalamus (Thal), hypothalamus (HTH), midbrain (Mid), and pons was increased in MSA-inoculated mice. Measurable staining was detected in the hippocampus (HC) from MSA6- and MSA-13 inoculated mice. Data shown as mean ± standard deviation. (d, e) Representative images of phosphorylated α-synuclein (EP1536Y; green) and glial fibrillary acidic protein (GFAP; red) staining in the brainstem of C2 (e) and MSA6 (e) inoculated mice. Nuclei in blue. Scale bar = 200 μm. (f) Detergent-insoluble phosphorylated α-synuclein aggregates were visualized by immunoblot. * = P < 0.05, ** = P < 0.01, **** = P < 0.0001.
In earlier studies, we analyzed brain samples from TgM83+/− mice using the α-syn140*A53T–YFP cell assay, which expresses full-length human α-synuclein with the A53T mutation fused to yellow fluorescent protein [53]. Infection in these cells is quantified by measuring the total fluorescence in the aggregates divided by the cell count (× 103 arbitrary units, A.U.). Infected cells emit a fluorescence measurement above 10 × 103 A.U., whereas uninfected cells yield lower values. To confirm transmission of α-synuclein prions from the MSA patient samples to the ip inoculated TgM83+/− mice, we tested the mouse brains in the α-syn140*A53T–YFP cell assay. The 10% brain homogenates were prepared from the frozen half-brains collected when the inoculated TgM83+/− mice developed disease (Fig. 1b). The α-synuclein prions were isolated using sodium phosphotungstic acid (PTA) following digestion of all nucleic acids by benzonase [42, 53], and the resulting protein pellets were incubated with the cells for 4 d prior to analysis. Following inoculation with one of the four MSA patient samples, brains from mice that developed neurological signs contained α-synuclein prions (closed circles), whereas brains from mice that died as a result of other causes (triangles) did not. Most mice terminated at 450 dpi, including the C2-inoculated mice, did not contain α-synuclein prions (open circles); however, one asymptomatic mouse inoculated with MSA12 contained α-synuclein prions. Compared to C2-inoculated animals, brains from mice ip inoculated with MSA6, MSA12, and MSA13 contained significantly more α-synuclein prions (P < 0.01, P < 0.05, and P < 0.01, respectively).
Fixed half-brains collected from mice that tested positive for α-synuclein prions, as well as C2-inoculated control animals, were processed and embedded in paraffin. Sections of 8 μm thickness were cut and stained for phosphorylated α-synuclein, astrogliosis (visualized using the glial fibrillary acidic protein, or GFAP, antibody), p62, and ubiquitin (Fig. 1c–e, S2). Phosphorylated α-synuclein neuropathology was measured as the percent area of each brain region positive for immunostaining. Measurements were made in the hippocampus (HC), thalamus (Thal), hypothalamus (HTH), midbrain (Mid), and pons and were averaged from two adjacent sections from each animal. The Thal, HTH, Mid, and pons all contained phosphorylated α-synuclein pathology following ip inoculation with all four MSA cases (Fig. 1c). MSA6 and MSA13 both induced significant pathological changes in the HC, but the magnitude of change compared to C2-inoculated mice was negligible. Notably, the α-synuclein neuropathology was localized to neurons within the affected areas as a result of the neuronal Prnp promoter used to express the transgene. The accumulation of phosphorylated α-synuclein was accompanied by an increase in astrogliosis, as demonstrated in the brainstem (Fig. 1d, e). The two proteins p62 and ubiquitin are known to play a role in the degradation of aggregated protein in the proteinopathies and are often co-localized with α-synuclein in the GCIs present in MSA patients [29, 32, 33]. Both p62 (Online Resource, Fig. S2a, b) and ubiquitin (Online Resource, Fig. S2c, d) co-localized with phosphorylated α-synuclein in MSA-inoculated animals (merge shown in white in bottom panels of b, d; magnified view of co-localization shown from inset).
Finally, the frozen half-brains from two mice individually inoculated with each homogenate were tested for the presence of detergent-insoluble α-synuclein. Protein aggregates were extracted from the 10% brain homogenate prepared from each mouse using 0.5% Nonidet P-40 (NP-40) and 0.5% sodium deoxycholate (DOC). The resulting protein pellets were resuspended in 1× Dulbecco’s phosphate-buffered saline (DPBS), and phosphorylated α-synuclein was visualized by immunoblot (Fig. 1f). Consistent with the cell assay and neuropathology data, mice inoculated with the MSA patient samples developed phosphorylated α-synuclein aggregates, but mice inoculated with C2 brain homogenate did not.
MSA prions transmit intramuscularly via the hind leg and tongue
PrPSc prions have been shown to transmit neurological disease to animals following a number of inoculation routes, including im inoculation into the hind leg and tongue [2, 47]. To test the ability of α-synuclein prions to transmit neurological disease to mice following im exposure, we injected 5 μL of a 1% brain homogenate from C2 and MSA6, MSA12, MSA13, and MSA18 patient samples into TgM83+/− mice (Fig. 2, S3). Inoculation into the thigh transmitted neurological disease more efficiently than the tongue inoculation (Fig. 2a, b). However, inoculation of MSA patient samples via both routes transmitted disease (thigh, P < 0.0001; tongue, P < 0.01), whereas the C2 sample did not. To confirm the transmission of MSA, frozen half-brains from the inoculated mice were tested in the α-syn140*A53T–YFP cell assay (Fig. 2c, d). Mice inoculated into the thigh with MSA6, MSA12, and MSA13 samples contained significantly more α-synuclein prions in their brains compared to C2-inoculated mice (P < 0.0001, P < 0.01, and P < 0.001, respectively). Although MSA18-inoculated mice were not significantly different from control-inoculated animals, all mice inoculated with MSA samples that showed neurological signs tested positive for α-synuclein prions (closed circles). Healthy mice collected at 450 dpi tested negative for MSA prions (open circles). Only mice inoculated with MSA6 into the tongue contained significantly more α-synuclein prions than the C2-inoculated mice (P < 0.01), but a more thorough assessment of the data again shows a strong association between neurological symptoms and robust levels of α-synuclein prions, as measured by the cell assay. Overall, the observed differences in the cell infectivity of the MSA patient samples after im inoculation into the thigh (Fig. 2a) and the tongue (Fig. 2b) are consistent with the infectivity differences seen between MSA18 and the other three MSA patient samples following ic inoculation (Online Resource, Fig. S1).
Fig. 2. Intramuscular transmission of MSA prions.
TgM83+/− mice received intramuscular injection of 1% brain homogenate from one control and four MSA patient samples. (a, b) Kaplan–Meier plots show the onset of neurological signs following right thigh (a) and tongue (b) inoculation. Upticks indicate mice that did not die from synucleinopathy. (c, d) Frozen half-brains from all mice were tested in the α-syn140*A53T–YFP cell assay (× 103 A.U.). Closed circles indicate mice containing prions (above dotted line), while triangles indicate mice that died from other causes. Open circles indicate mice terminated at 450 dpi. (c) Mice inoculated into the thigh with MSA6 (P < 0.0001), MSA12 (P < 0.01), and MSA13 (P < 0.001) and (d) into the tongue with MSA6 (P < 0.01) contained significantly more α-synuclein prions than C2-inoculated mice. (e, f) Quantification of phosphorylated α-synuclein immunostaining in the HC, Thal, HTH, Mid, and pons of inoculated mice. MSA inoculation into the thigh (e) and tongue (f) induced α-synuclein pathology, but C2 inoculation had no effect. Data shown as mean ± standard deviation. (g, h) Detergent-insoluble phosphorylated α-synuclein was detected in the brains of mice inoculated with MSA in the thigh (g) and the tongue (h) but was not detected in C2-inoculated animals. * = P < 0.05, ** = P < 0.01, *** = P < 0.001, **** = P < 0.0001.
Phosphorylated α-synuclein neuropathology was quantified in the brains of mice containing α-synuclein prions following im inoculation, as well as C2-inoculated animals (Fig. 2e, f). Assessing the effect of im inoculation on α-synuclein aggregation, we found C2-inoculated mice contained no immunostaining, while the four MSA patient samples induced phosphorylated α-synuclein aggregation in the Thal, HTH, Mid, and pons, regardless of whether mice were inoculated in the thigh (Fig. 2e) or the tongue (Fig. 2f). MSA13 and MSA6 induced significantly more phosphorylated α-synuclein accumulation in the HC than the control mice following thigh or tongue inoculation, respectively, but the difference in magnitude was negligible. These increases in α-synuclein deposition following im inoculation were accompanied by an increase in astrogliosis, which was not seen in C2-inoculated animals (Online Resource, Fig. S3a, b). Additionally, α-synuclein co-localized with p62 and ubiquitin following im inoculation into both the thigh and the tongue (Online Resource, Fig. S3).
Frozen half-brains from these same animals were analyzed by immunoblot for the presence of detergent-insoluble α-synuclein (Fig. 2g, h). Both routes of inoculation with MSA homogenate induced accumulation of insoluble phosphorylated α-synuclein in the brains of TgM83+/− mice, but mice inoculated with the control sample did not contain α-synuclein aggregates (Fig. 2g, h).
MSA prions adhere to stainless-steel wires
Multiple studies have assessed the ability of PrPSc prions to adhere to surgical stainless steel. After incubating steel suture in brain homogenate containing PrPSc prions, both permanent and transient insertion of contaminated wire into the brain of an animal resulted in disease transmission [15, 38, 54, 55]. Due to cases of iatrogenic Creutzfeldt–Jakob disease (CJD) that resulted from improperly sterilized neurosurgical instruments after use on an asymptomatic CJD patient [21, 39, 46], this assay has been particularly important for studying the conditions required for the denaturation of PrPSc. To explore additional parallels in disease transmission between PrPSc and α-synuclein prions, we tested the ability of α-synuclein prions in MSA patient samples to adhere to stainless-steel suture. 3-0 steel suture was cut into 4 mm pieces, which were incubated in 10% brain homogenate from C2 and MSA6, MSA12, MSA13, and MSA18 patient samples for 16 h at room temperature. The wires were washed in DPBS five times for 10 min each and were allowed to air-dry in a biosafety cabinet, as described [38]. One wire was then permanently implanted into the right hemisphere of each TgM83+/− mouse.
Wires incubated in C2 brain homogenate did not transmit disease to the mice, and the animals did not show any other deficits. In contrast, wires incubated in homogenate from MSA6, MSA12, and MSA13 samples transmitted neurological symptoms to TgM83+/− mice (P < 0.001; Fig. 3a) with a substantially longer incubation period than observed after ic inoculation with the same samples (Online Resource, Fig. S1). Consistent with the lower titer observed for patient sample MSA18 in previous experiments, only one mouse implanted with wires incubated in MSA18 developed neurological signs. Testing the brains of all mice in the α-syn140*A53T–YFP cell assay showed that the mice implanted with wire incubated with MSA samples were infected with α-synuclein prions (Fig. 3b). The number of α-synuclein prions in the brains of mice implanted with MSA6- and MSA13-incubated wires was significantly greater compared to control animals (P < 0.01 and P < 0.05, respectively). Brains from mice that tested positive for α-synuclein prions also showed significant phosphorylated α-synuclein accumulation in the Thal, HTH, Mid, and pons (Fig. 3c) accompanied by an increase in astrogliosis in comparison to control animals (Fig. 3d, e). The MSA-induced α-synuclein aggregates co-localized with both p62 and ubiquitin (Online Resource, Fig. S4). These brain samples were also assessed for the presence of detergent-insoluble α-synuclein (Fig. 3f). Mice implanted with MSA-coated wire developed phosphorylated α-synuclein aggregates, but C2-incubated wires did not induce α-synuclein accumulation.
Fig. 3. MSA prions adhere to stainless-steel surgical wires.
Stainless-steel wires (4 mm) were incubated in 10% brain homogenate from one control and four MSA samples for 16 h at room temperature. The wires were washed in DPBS (5 × 10 min), dried, and implanted into the right hemisphere of TgM83+/− mice. (a) Kaplan–Meier plot shows the onset of neurological signs following wire implantation. Upticks indicate mice that did not die from synucleinopathy. (b) Frozen half-brains from all mice were tested in the α-syn140*A53T–YFP cell assay (× 103 A.U.). Closed circles indicate mice containing prions (above dotted line), while triangles indicate mice that died from other causes. Open circles show mice terminated at 450 dpi. Wires incubated with MSA6 and MSA13 transmitted significantly more α-synuclein prions than the control (P < 0.01 and P < 0.05, respectively). (c) Quantification of phosphorylated α-synuclein immunostaining. Wire incubation in C2 homogenate did not induce α-synuclein aggregation, but MSA incubation induced accumulation in the Thal, HTH, Mid, and pons. Data shown as mean ± standard deviation. (d, e) Representative images of phosphorylated α-synuclein (green) and GFAP (red) immunostaining in the brainstem of mice implanted with C2 (d) and MSA6 (e) incubated wires. Nuclei in blue. Scale bar = 200 μm. (f) Detergent-insoluble phosphorylated α-synuclein aggregates were visualized by immunoblot. * = P < 0.05, ** = P < 0.01, *** = P < 0.001.
Formalin fixation does not inactivate MSA prions
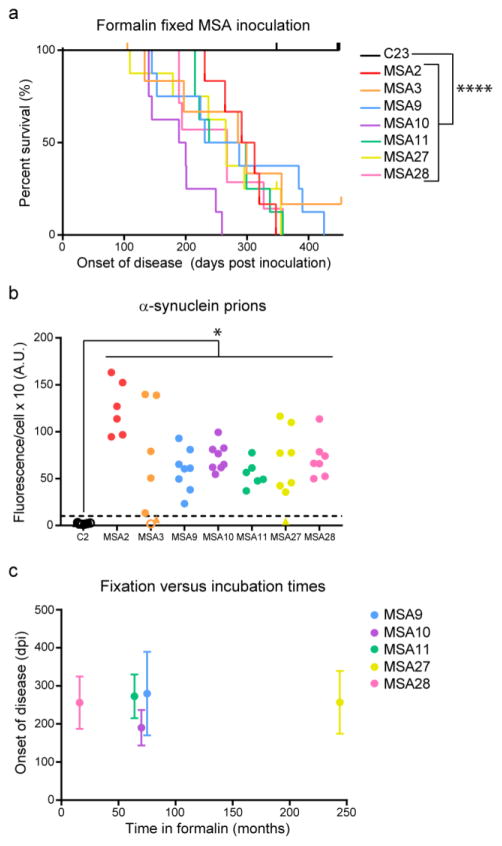
It is well established that formalin fixation does not inactivate PrPSc prions. The earliest report examined sheep inoculated with a vaccine for the louping ill RNA virus but subsequently developed scrapie two years after vaccination [23]. The vaccine used 0.35% formalin to inactivate the RNA virus in brain homogenate from sheep that had contracted louping ill. However, one batch of the virus was made from sheep that had also developed scrapie; as the formalin did not inactivate PrPSc prions in the homogenate, the sheep receiving the vaccine developed disease. To assess the ability of formalin to inactivate α-synuclein prions in MSA patient samples, we homogenized formalin-fixed tissue from one control patient sample (C23; substantia nigra) and seven MSA patient samples (substantia nigra from MSA2 and MSA3; pons from MSA9, MSA10, MSA11, MSA27, and MSA28) and ic inoculated 30 μL of a 1% homogenate into TgM83+/− mice. While mice inoculated with C23 did not develop neurological signs, mice inoculated with MSA patient samples developed disease (P < 0.0001; Fig. 4a).
Fig. 4. Formalin fixation does not inactivate MSA prions.
Formalin-fixed tissue from one control and seven MSA patient samples was homogenized in DPBS, and 30 μL of a 1% homogenate was inoculated intracranially into TgM83+/− mice. (a) Kaplan–Meier plot shows onset of neurological signs in TgM83+/− mice following inoculation. Upticks indicate mice that died for reasons other than synucleinopathy. (b) Frozen half-brains from TgM83+/− mice were tested in the α-syn140*A53T–YFP cell assay (× 103 A.U.). Brains from the mice inoculated with all seven formalin-fixed MSA samples contained α-synuclein prions (closed circles above dotted line), whereas control mice terminated at 450 dpi (open circles) and animals that died from other causes (triangles) did not. Transmission of α-synuclein prions from the formalin-fixed MSA samples was significant compared to C23-inoculated mice (MSA2, P < 0.0001; MSA3, MSA9, and MSA27, P < 0.01; MSA10 and MSA28, P < 0.001; MSA11, P < 0.05). (c) Plot comparing the length of time that five of the MSA samples spent in formalin versus the incubation time in TgM83+/− mice. Fixation times ranged from 16 months (MSA28) to 244 months (MSA27), but the infectivity remained constant. Incubation times plotted as mean ± standard deviation. * = P < 0.05, **** = P < 0.0001.
To confirm these neurological signs were a result of α-synuclein prion transmission, we tested the brains from the MSA-inoculated mice in the α-syn140*A53T–YFP cell assay and found they contained significantly more α-synuclein prions than mice inoculated with control patient tissue (MSA2: P < 0.0001; MSA3, MSA9, MSA27: P < 0.01; MSA10, MSA28: P < 0.05; MSA11: P < 0.05; Fig. 4b). We assessed neuropathological accumulation of phosphorylated α-synuclein in the brains of mice containing MSA prions (Online Resource, Fig. S5). Compared to C23-inoculated animals, MSA inoculation induced robust α-synuclein immunostaining in the Thal, HTH, Mid, and pons (Online Resource, Fig. S5a). Six of the seven MSA patient samples also induced significant accumulation in the HC; however, the magnitude of change from the control was biologically insignificant. This neuropathology was accompanied by an increase in astrogliosis (Online Resource, Fig. S5b) and was co-localized with p62 and ubiquitin immunostaining as well (Online Resource, Fig. S5b, c). Detergent-insoluble, high molecular weight phosphorylated α-synuclein aggregates were also detected in the brains of MSA-inoculated animals but were not detected in C23-inoculated mice, as shown by immunoblot (Online Resource, Fig. S5d).
Finally, we compared the length of time the MSA patient samples spent in formalin with the disease incubation times in TgM83+/− mice to assess if the amount of time a sample spent in fixative affected its infectivity, or titer (Fig. 4c). Comparing patient sample MSA28, which spent 16 months in formalin, with patient sample MSA27, which spent 244 months (or more than 20 years) in formalin, we observed no differences in incubation times, indicating that formalin does not inactivate α-synuclein prions in MSA patients, regardless of the fixation time.
Extraction in 10% sarkosyl partially denatures MSA prions
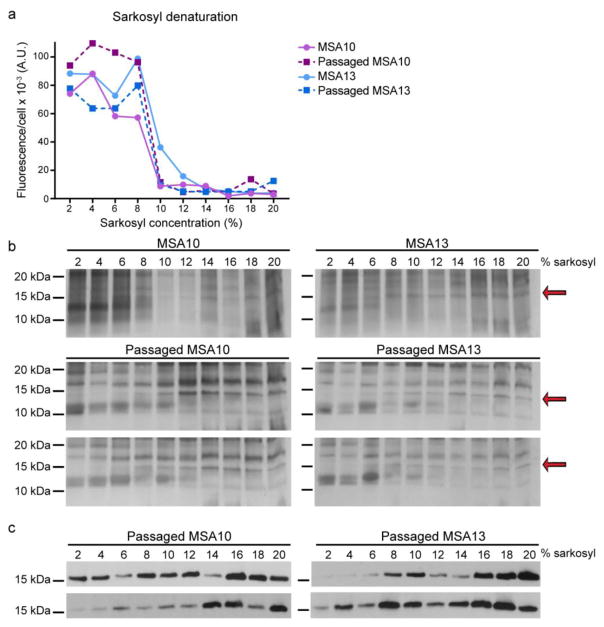
Following the discovery that formalin fixation does not inactivate α-synuclein prions in MSA patient samples, we tested their resistance to denaturation by extraction with increasing concentrations of sarkosyl. It is known that extracting brain homogenate in 2% sarkosyl during PTA precipitation increases the density of PrPSc prions isolated from a sample [31]. Moreover, the addition of 2% sarkosyl to MSA patient samples also enables isolation of α-synuclein prions using PTA [53]. However, the stability of α-synuclein prions to higher concentrations of sarkosyl has been previously unexplored. Using two MSA patient samples (MSA10 and MSA13) and two mice individually inoculated with these samples, we altered the PTA precipitation protocol to extract α-synuclein prions with increasing concentrations of sarkosyl, ranging from 2–20%. PTA was used to precipitate the remaining protein aggregates after extraction, and the isolated protein pellets were tested in the α-syn140*A53T–YFP cell assay (Fig. 5a). While all six samples showed at least partial resistance to denaturation in up to 8% sarkosyl, most of the infectivity measured in the cell assay was eliminated by extraction in 10% sarkosyl. Hypothesizing that increasing the detergent concentration resulted in the degradation of α-synuclein prions into monomeric protein, we examined the total protein present in each sample by silver stain (Fig. 5b). In the lower dilution samples, a 15 kDa band coinciding with α-synuclein monomer was absent, but 15 kDa bands became increasingly abundant for the two human and four mouse samples as the sarkosyl concentration increased (red arrows). To confirm the 15 kDa band was monomeric α-synuclein, we analyzed the mouse samples via immunoblot using the MJFR1 total α-synuclein antibody (Fig. 5c). A weak monomeric band at 15 kDa became increasingly more abundant as the sarkosyl concentration increased, starting at 10% sarkosyl, corresponding with the cell infection and silver stain data. These blots confirmed that the denaturation of α-synuclein prions in the samples resulted in an increase in monomeric protein.
Fig. 5. Stability of MSA prions to denaturation with sarkosyl.
Ten percent brain homogenates from two MSA patient samples (MSA10 and MSA13) and two mice inoculated with each sample were extracted using increasing concentrations of sarkosyl (2–20%). Residual protein aggregates were collected using PTA precipitation. (a) Protein pellets were diluted in DPBS and tested in the α-syn140*A53T–YFP cell assay. The titration curve shows an inflection point between 8% and 10% sarkosyl, with infectivity significantly reduced following extraction in 10% sarkosyl. (b) Silver stains of protein pellets from all six samples tested reveal a 15 kDa band, which corresponds to the molecular weight of α-synuclein. This band is not present in the lower sarkosyl concentrations but becomes more abundant as the sarkosyl concentration increases (red arrows). (c) Immunoblot using the total α-synuclein antibody MJFR1 from the four mouse samples shows a faint monomeric band in the lower sarkosyl concentrations, but it becomes increasingly more abundant starting at 10% sarkosyl.
DISCUSSION
Increasing evidence indicates that in patients with MSA, α-synuclein misfolds into a prion conformation and spreads throughout the brain causing progressive neurodegeneration. The biochemical parallels between α-synuclein and PrPSc led us to compare the transmissibility of the two prions by assessing the infectiousness of α-synuclein prions in MSA patient samples using transmission paradigms previously reported for PrPSc. While iatrogenic transmission of MSA has not been identified, the demonstrated ability of α-synuclein prions to adhere to stainless-steel wires and resist formalin inactivation warrants investigation into the possibility of lateral disease transmission, particularly given the ability of α-synuclein prions to affect the CNS after peripheral exposure.
Several studies have used wild-type and Tg mouse models to demonstrate peripheral transmission of proteinopathies giving rise to CNS disease [2, 26, 47] and protein accumulation [8, 12]. Along these lines, previous work using fibrillized recombinant α-synuclein demonstrated that ip and intraglossal inoculation [6] and im inoculation into the hind leg [41] induced neurological signs and α-synuclein pathology in TgM83 mice. Testing MSA brain homogenate for the ability to transmit disease following systemic exposure, we compared ic inoculation (30 μL) with ip (100 μL) and im (5 μL) inoculations using 1% homogenate prepared from four MSA patient samples. While three of the patient samples produced similar incubation times following ic inoculation, patient sample MSA18 yielded a longer incubation time, likely due to reduced titer. This difference in infectivity between MSA18 and the other MSA patient samples was seen across the three peripheral transmission paradigms tested. Both ip and im inoculation into the hind leg produced extended incubation times compared to ic inoculation, but the relatively longer im incubation times are likely attributable to the small volume used. Intraglossal inoculation was the least efficient transmission route, despite proximity to the CNS. This was consistent with data reported by Breid et al. who found that only one of five mice inoculated with recombinant α-synuclein fibrils into the tongue developed disease [6].
Iatrogenic transmission of CJD has occurred from the incomplete sterilization of PrPSc prions adhered to stainless-steel surgical instruments after use on an asymptomatic CJD patient [39, 46]. To model this transmission in animals, stainless-steel suture incubated in brain homogenate containing PrPSc prions was permanently implanted into the brain [55]. After determining permanent insertion of the wires transmitted disease to the animals, transient insertion models were designed to better replicate surgical conditions. These studies found that PrPSc transmission occurred even when wires were inserted into the brain for only 15 min [15]. Likewise, wires incubated in brain homogenate from aged TgAPP23 mice, a model for β-amyloid (Aβ) accumulation in Alzheimer’s disease (AD), and implanted in young TgAPP23 mice induced plaque formation [11]. These results, along with ip transmission of Aβ plaques to TgAPP23 mice [12], led the authors to suggest iatrogenic human transmissions may be concealed by longer incubation times than are seen with CJD patients [11]. To test the possibility of lateral α-synuclein prion transmission via contaminated neurosurgical instruments, stainless-steel wires were incubated with brain homogenate from four MSA patient samples and one control sample. Mice implanted with MSA-incubated wires developed neurological signs, while control wires implanted in TgM83+/− mice had no effect. Notably, these studies were performed using permanently implanted wires; studies testing the transmissibility of MSA prions following transient insertion have not been performed.
After using peripheral inoculations and wire implants to transmit disease, we tested brain samples from the TgM83+/− mice in the α-syn140*A53T–YFP cell assay [53] to confirm the apparent neurological signs were caused by α-synuclein prions. Brain samples from control-inoculated mice terminated 450 dpi did not infect the cells. However, brain homogenate from MSA-inoculated animals induced robust α-synuclein aggregation, demonstrating that both peripheral and wire transmissions resulted in α-synuclein prion propagation in TgM83+/− mice. Brains from sick animals also contained detergent-insoluble α-synuclein aggregates and phosphorylated α-synuclein neuropathology that co-localized with p62 and ubiquitin. Notably, regardless of exposure method, inoculation with MSA samples induced neuropathology in the same brain regions (Thal, HTH, Mid, and pons). These findings could be explained either by the specific α-synuclein prion strain in MSA patients inducing consistent pathology in the mice or by the neuron-specific promoter driving transgene expression in the model, neither of which was investigated here.
The unusual resistance of PrPSc prions to formalin fixation [23], a method used to inactivate viruses for vaccine production, has prompted other groups to examine the effect of formalin on additional misfolded proteins, including Aβ prions in AD patient samples [16]. Brain homogenate, prepared from two AD patient samples after fixation in formalin for 2 years, inoculated into the hippocampus of TgAPP23 mice induced Aβ plaque deposition within 4 months. Resistance to formalin inactivation was also demonstrated for α-synuclein prions using Tg mouse samples. Formalin-fixed brains from spontaneously symptomatic mice expressing human α-synuclein with the familial A30P mutation were shown to transmit disease to younger Tg mice [43]. Here, we found that seven formalin-fixed MSA patient samples also transmitted synucleinopathy to TgM83+/− mice, but inoculation with a fixed control sample had no effect. We compared the incubation times in the animal bioassay with the length of time five of the samples spent in formalin and found no correlation; remarkably, disease transmission time using a sample that spent 16 months in formalin was almost identical to disease transmission using a sample that spent 244 months in formalin. These data indicate that more than 20 years in fixative does not reduce the titer of α-synuclein prions in an MSA sample any more than approximately one year in fixative.
Given the robust stability of α-synuclein prions, it is critical to identify methods for denaturing and inactivating the protein to prevent human-to-human transmission. Work by two groups has measured residual protein following exposure to denaturing conditions using two different methods. In the first method, Thomzig et al. used a Western blot to show recoverable protein levels from a stainless-steel grid coated with human α-synuclein after exposure to several harsh alkaline treatments [48]. In the second, Bousset et al. labelled synthetic α-synuclein fibrils with a fluorescent tag prior to spotting the fibrils onto a number of laboratory surfaces, including glass and steel. After washing with various solutions, the residual protein was measured by fluorescence [4]. In both reports, the authors aimed to reduce detectable α-synuclein but did not differentiate between the infective and non-infective species. Previous work testing conditions for PrPSc inactivation has demonstrated variability in results dependent upon prion strain and assay method(s) used (reviewed in [21]). Given the convergence between PrPSc and α-synuclein prions, it is critical that future studies investigate these disinfection methods in animal bioassays that directly measure transmissibility of MSA prions following decontamination. Work by our group and others highlighting the stability of α-synuclein prions argue that validated protocols are not only needed in cleaning and decontaminating surgical instruments but also in decontaminating research equipment to prevent contamination across studies. For the inoculations reported here, single use syringes were discarded after each injection to prevent contamination across inoculum.
The diagnostic accuracy of MSA among movement disorder patients has ranged from 29% to 86% [18, 24, 27, 35, 52], and the majority of misdiagnosed pathologically confirmed MSA cases are clinically diagnosed as PD. While PD patients often see amelioration of symptoms following DBS treatment, the effects of DBS are not sustained in MSA patients, and it is therefore not a recommended treatment [34]. Concern about decontaminating neurosurgical instruments used for DBS in PD patients, or misdiagnosed MSA patients, has increased in light of recent data suggesting lateral transmission of Aβ pathology. Reanalysis of tissue from iatrogenic CJD patients who developed disease after receiving contaminated human growth hormone or dura mater grafts showed some patients also developed Aβ plaques earlier than age-matched controls [17, 25, 28]. Our finding that 10% sarkosyl is needed to at least partially denature α-synuclein prions in MSA patient samples highlights the difficulty of disinfecting contaminated materials and suggests, analogous to PrPSc prions, most commonly used methods for disinfection will be ineffective. On account of the stability of α-synuclein prions and their ability to transmit disease through peripheral exposure routes, additional care should be utilized when decontaminating instruments used during DBS procedures in PD patients until a diagnostic test for MSA is available.
The convergence of the neuropathogenic prions found in MSA and CJD patients, including their resistance to formalin and detergents and their ability to transmit disease through peripheral routes of exposure, emphasizes the need for further research to prevent any possible iatrogenic transmission of MSA. Future studies to establish successful methods for handling and sterilizing contaminated neurosurgical instruments and laboratory equipment should measure the remaining activity of α-synuclein prions in sensitive animal and cellular bioassays. These studies should be carried out using preparations from MSA patient samples, rather than synthetic fibrils, to ensure the methods developed are effective against the α-synuclein prion strain responsible for disease. In addition, improved diagnostic testing for MSA patients is needed to help prevent iatrogenic transmission. Importantly, currently available data indicate health workers and researchers should use caution when decontaminating equipment and working with materials potentially contaminated with MSA prions.
MATERIALS AND METHODS
Experimental design
The overall objective of this study was to assess the transmissibility and stability of α-synuclein prions in MSA patient samples using animal and cellular bioassays to measure protein activity. A subsequent objective was to develop models of MSA transmission to be used for measuring α-synuclein prion inactivation. Based on several decades of prion transmission experiments, we have determined that 8 animals per inoculation group provide a sufficient sample size to achieve at least 80% power with an alpha of 0.05. This sample size accounts for some early animal loss following inoculation, which can occasionally occur if a mouse recovers poorly from the procedure. In all studies reported here, 8 mice were inoculated with each inoculum. At least four individual MSA patient samples were tested for each transmission route investigated. Two human and four mouse samples were used in the sarkosyl denaturation experiments. While ic inoculation with MSA transmits disease in ~120 days [40], experiments were terminated 450 dpi to account for extended incubation periods resulting from the indirect routes of exposure tested. Transmission of MSA prions was determined by testing the mouse brains in the α-syn140*A53T–YFP cell assay. After assessing the dynamic range of the assay, we defined infection with a measurement above 10 × 103 A.U. Mice that tested positive for α-synuclein prions were then assessed neuropathologically and biochemically for phosphorylated α-synuclein aggregates, as were control-inoculated mice. No outliers were excluded.
Human tissue samples
Frozen brain tissue samples from neuropathologically confirmed cases of MSA were provided by the Massachusetts Alzheimer’s Disease Research Center, the Parkinson’s UK Tissue Bank at Imperial College, and the Sydney Brain Bank. Control patient tissue was provided by Dr. Martin Ingelsson (Uppsala University). Demographic information about samples used are included in Online Resource, Table S1.
Mice
Animals were maintained in an AAALAC-accredited facility in compliance with the Guide for the Care and Use of Laboratory Animals. All procedures used in this study were approved by the University of California, San Francisco, Institutional Animal Care and Use Committee. All animals were maintained under standard environmental conditions with a 12:12-h light:dark cycle and free access to food and water. To generate the hemizygous TgM83+/− mice used in this study, homozygous TgM83+/+ mice [19], which express human α-synuclein with the A53T mutation on a B6;C3 background, were purchased from the Jackson Laboratory (USA) and were bred with B6C3F1 mice.
Inoculations
Fresh frozen human tissue was used to create a 10% (wt/vol) homogenate using calcium- and magnesium-free 1× DPBS using the Omni Tissue Homogenizer (Omni International). For inoculation studies, the homogenate was diluted to 1% using 5% (wt/vol) bovine serum albumin in 1× DPBS. Fixed frozen tissue was homogenized to use as inoculum under the same conditions. Prior to homogenization, the fixed tissue was incubated in sterile 1× DPBS for 2 h at room temperature with shaking to remove residual formaldehyde.
Ten-week-old mice were anesthetized with isoflurane prior to inoculation. Inoculations were performed using 1% brain homogenate inoculated into the right parietal lobe (30 μL), peritoneal cavity (100 μL), right hind thigh (5 μL), or tongue (5 μL). Transmission studies using stainless-steel wires contaminated with MSA prions were carried out as previously reported [38]. Following inoculation, all mice were assessed twice a week for the onset of neurological signs based on standard diagnostic criteria for prion disease [7] and were euthanized within 2 d of demonstrated CNS dysfunction. Following euthanasia, the brain was removed and bisected down the midline. The left hemisphere was frozen for biochemical analysis, and the right hemisphere was fixed in formalin for neuropathological assessment.
Alpha-synuclein prion precipitation
A 10% (wt/vol) brain homogenate was prepared using frozen human tissue in calcium- and magnesium-free 1× DPBS using the Omni Tissue Homogenizer with disposable soft tissue tips (Omni International). Aggregated protein was isolated from the patient samples using sodium PTA (Sigma) as described [42, 53]. Isolated protein pellets were diluted 1:10 in 1× DPBS before testing in the cell aggregation assay.
Cell aggregation assay
HEK293T cells expressing α-syn140*A53T–YFP were previously reported, and culture and assay conditions were used as described [53]. Briefly, α-syn140*A53T–YFP cells were cultured and plated in 1× Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% (vol/vol) fetal bovine serum (Thermo Fisher), 50 units/mL penicillin, and 50 μg/mL streptomycin (Thermo Fisher). Cells were maintained in a humidified atmosphere of 5% CO2 at 37 °C.
The α-syn140*A53T–YFP cells were plated at a density of 2,500 cells/well in a 384-well plate with black polystyrene walls (Greiner) with 0.012 μg/well of Hoechst 33342. Plates were returned to the incubator for 2–4 h. Lipofectamine 2000 (1.5% final volume; Thermo Fisher) was incubated with each sample for 1.5 h at room temperature prior to adding OptiMEM (78.5% final volume; Thermo Fisher). Samples were then added to the α-syn140*A53T–YFP cells in six replicate wells. After the plates were incubated for 4 d at 37 °C in a humidified atmosphere with 5% CO2, individual plates were imaged using the IN Cell Analyzer 6000 (GE Healthcare). DAPI and FITC channels were used to collect two images from five different regions in each well; these images were analyzed with the IN Cell Developer software using an algorithm designed to detect intracellular aggregates in living cells, quantified as total fluorescence per cell (× 103, arbitrary units, A.U.).
Statistical analysis
Data are presented as mean ± standard deviation. Statistical analysis comparing the onset of neurological symptoms in mice was compared using a log-rank (Mantel–Cox) test to assess the distribution of survival curves. Statistical analysis of data collected from the α-syn140*A53T–YFP cell assay was analyzed using a one-way ANOVA with a Dunnett multiple comparison post hoc test. Mouse neuropathology was analyzed using a two-tailed Student’s t-test with unequal variance to compare MSA-inoculated mice with control-inoculated mice by brain region. Significance was determined with a P value < 0.05.
Supplementary Material
Acknowledgments
We thank the Hunters Point animal facility staff for breeding and caring for the mice used in this study and Martin Ingelsson (Uppsala University) for providing control tissue. This work was supported by grants from the National Institutes of Health (AG002132 and AG031220), as well as by gifts from the Glenn Foundation and Daiichi Sankyo. The Massachusetts Alzheimer’s Disease Research Center is supported by the National Institutes of Health (AG005134); the Parkinson’s UK Brain Bank at Imperial College London is funded by Parkinson’s UK, a charity registered in England and Wales (948776) and in Scotland (SC037554); and the Sydney Brain Bank is supported by Neuroscience Research Australia and the University of New South Wales. Glenda M. Halliday is a National Health and Medical Research Council of Australia Senior Principal Research Fellow (1079679).
FUNDING
This work was supported by grants from the National Institutes of Health (AG002132 and AG031220), as well as by gifts from the Glenn Foundation and Daiichi Sankyo. The Massachusetts Alzheimer’s Disease Research Center is supported by the National Institutes of Health (AG005134); the Parkinson’s UK Brain Bank at Imperial College London is funded by Parkinson’s UK, a charity registered in England and Wales (948776) and in Scotland (SC037554); and the Sydney Brain Bank is supported by Neuroscience Research Australia and the University of New South Wales. Glenda M. Halliday is a National Health and Medical Research Council of Australia Senior Principal Research Fellow (1079679).
Footnotes
AUTHOR CONTRIBUTIONS
A.L.W., S.H.O., and S.B.P. designed research; A.L.W., S.A.K., S.P., Y.F., and A.O. performed research; A.A., D.A.M., G.M.H., L.T.M., and S.M.G. contributed new reagents/analytic tools; A.L.W., S.A.K., S.P., S.H.O., and S.B.P. analyzed data; and A.L.W., S.H.O., and S.B.P. wrote the paper.
COMPLIANCE WITH ETHICAL STANDARDS
Conflict of interest
The Institute for Neurodegenerative Diseases has a research collaboration with Daiichi Sankyo (Tokyo, Japan). S.B.P. is the chair of the Scientific Advisory Board of Alzheon, Inc., which has not contributed financial or any other support to these studies.
Ethical approval
Animals were maintained in an AAALAC-accredited facility in compliance with the Guide for the Care and Use of Laboratory Animals. All procedures used in this study were approved by the University of California, San Francisco, Institutional Animal Care and Use Committee.
References
- 1.Anderson RJ, Frye MA, Abulseoud OA, Lee KH, McGillivray JA, Berk M, Tye SJ. Deep brain stimulation for treatment-resistant depression: Efficacy, safety and mechanisms of action. Neurosci Biobehav Rev. 2012;36:1920–1933. doi: 10.1016/j.neubiorev.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 2.Bartz JC, Kincaid AE, Bessen RA. Rapid prion neuroinvasion following tongue infection. J Virol. 2003;77:583–591. doi: 10.1128/JVI.77.1.583-591.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bernoulli C, Siegfried J, Baumgartner G, Regli F, Rabinowicz T, Gajdusek DC, Gibbs CJ., Jr Danger of accidental person-to-person transmission of Creutzfeldt-Jakob disease by surgery. Lancet. 1977;309:478–479. doi: 10.1016/s0140-6736(77)91958-4. [DOI] [PubMed] [Google Scholar]
- 4.Bousset L, Brundin P, Böckmann A, Meier B, Melki R. An efficient procedure for removal and inactivation of alpha-synuclein assemblies from laboratory materials. J Parkinsons Dis. 2016;6:143–151. doi: 10.3233/JPD-150691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bousset L, Pieri L, Ruiz-Arlandis G, Gath J, Jensen PH, Habenstein B, Madiona K, Olieric V, Böckmann A, Meier BH, et al. Structural and functional characterization of two alpha-synuclein strains. Nat Commun. 2013;4:2575. doi: 10.1038/ncomms3575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Breid S, Bernis ME, Babila JT, Garza MC, Wille H, Tamgüney G. Neuroinvasion of α-synuclein prionoids after intraperitoneal and intraglossal inoculation. J Virol. 2016;90:9182–9193. doi: 10.1128/JVI.01399-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carlson GA, Kingsbury DT, Goodman PA, Coleman S, Marshall ST, DeArmond S, Westaway D, Prusiner SB. Linkage of prion protein and scrapie incubation time genes. Cell. 1986;46:503–511. doi: 10.1016/0092-8674(86)90875-5. [DOI] [PubMed] [Google Scholar]
- 8.Clavaguera F, Hench J, Lavenir I, Schweighauser G, Frank S, Goedert M, Tolnay M. Peripheral administration of tau aggregates triggers intracerebral tauopathy in transgenic mice. Acta Neuropathol. 2014;127:299–301. doi: 10.1007/s00401-013-1231-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Corripio I, Sarró S, McKenna PJ, Molet J, Álvarez E, Pomarol-Clotet E, Portella MJ. Clinical improvement in a treatment-resistant patient with schizophrenia treated with deep brain stimulation. Biol Psychiatry. 2016;80:e69–e70. doi: 10.1016/j.biopsych.2016.03.1049. [DOI] [PubMed] [Google Scholar]
- 10.Dowd RS, Pourfar M, Mogilner AY. Deep brain stimulation for Tourette syndrome: A single-center series. J Neurosurg. 2017:1–9. doi: 10.3171/2016.10.JNS161573. [DOI] [PubMed] [Google Scholar]
- 11.Eisele YS, Bolmont T, Heikenwalder M, Langer F, Jacobson LH, Yan ZX, Roth K, Aguzzi A, Staufenbiel M, Walker LC, et al. Induction of cerebral β-amyloidosis: Intracerebral versus systemic Aβ inoculation. Proc Natl Acad Sci USA. 2009;106:12926–12931. doi: 10.1073/pnas.0903200106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eisele YS, Obermuller U, Heilbronner G, Baumann F, Kaeser SA, Wolburg H, Walker LC, Staufenbiel M, Heikenwalder M, Jucker M. Peripherally applied A beta-containing inoculates induce cerebral beta-amyloidosis. Science. 2010;330:980–982. doi: 10.1126/science.1194516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fanciulli A, Wenning GK. Multiple-system atrophy. N Engl J Med. 2015;372:249–263. doi: 10.1056/NEJMra1311488. [DOI] [PubMed] [Google Scholar]
- 14.Fayad SM, Guzick AG, Reid AM, Mason DM, Bertone A, Foote KD, Okun MS, Goodman WK, Ward HE. Six-nine year follow-up of deep brain stimulation for obsessive-compulsive disorder. PLoS One. 2016;11:e0167875. doi: 10.1371/journal.pone.0167875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flechsig E, Hegyi I, Enari M, Schwarz P, Collinge J, Weissmann C. Transmission of scrapie by steel-surface-bound prions. Mol Med. 2001;7:679–684. [PMC free article] [PubMed] [Google Scholar]
- 16.Fritschi SK, Cintron A, Ye L, Mahler J, Bühler A, Baumann F, Neumann M, Nilsson KPR, Hammarström P, Walker LC, et al. Aβ seeds resist inactivation by formaldehyde. Acta Neuropathol. 2014;128:477–484. doi: 10.1007/s00401-014-1339-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frontzek K, Lutz MI, Aguzzi A, Kovacs GG, Budka H. Amyloid-β pathology and cerebral amyloid angiopathy are frequent in iatrogenic Creutzfeldt-Jakob disease after dural grafting. Swiss Med Wkly. 2016;146:w14287-14281–w14287-14285. doi: 10.4414/smw.2016.14287. [DOI] [PubMed] [Google Scholar]
- 18.Geser F, Wenning GK, Seppi K, Stampfer-Kountchev M, Scherfler C, Sawires M, Frick C, Ndayisaba J-P, Ulmer H, Pellecchia MT, et al. Progression of multiple system atrophy (MSA): A prospective natural history study by the European MSA Study Group (EMSA SG) Mov Disord. 2006;21:179–186. doi: 10.1002/mds.20678. [DOI] [PubMed] [Google Scholar]
- 19.Giasson BI, Duda JE, Quinn SM, Zhang B, Trojanowski JQ, Lee VM. Neuronal α-synucleinopathy with severe movement disorder in mice expressing A53T human α-synuclein. Neuron. 2002;34:521–533. doi: 10.1016/s0896-6273(02)00682-7. [DOI] [PubMed] [Google Scholar]
- 20.Gibbs CJ, Jr, Asher DM, Kobrine A, Amyx HL, Sulima MP, Gajdusek DC. Transmission of Creutzfeldt-Jakob disease to a chimpanzee by electrodes contaminated during neurosurgery. J Neurol Neurosurg Psychiatry. 1994;57:757–758. doi: 10.1136/jnnp.57.6.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Giles K, Woerman AL, Berry DB, Prusiner SB. Bioassays and inactivation of prions. In: Prusiner SB, editor. Prion Biology. Cold Spring Harbor Laboratory Press; City: 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gilman S, Wenning GK, Low PA, Brooks DJ, Mathias CJ, Trojanowski JQ, Wood NW, Colosimo C, Dürr A, Fowler CJ, et al. Second consensus statement on the diagnosis of multiple system atrophy. Neurology. 2008;71:670–676. doi: 10.1212/01.wnl.0000324625.00404.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gordon WS. Advances in veterinary research. Vet Rec. 1946;58:516–520. [PubMed] [Google Scholar]
- 24.Hughes AJ, Daniel SE, Ben-Shlomo Y, Lees AJ. The accuracy of diagnosis of parkinsonian syndromes in a specialist movement disorder service. Brain. 2002;125:861–870. doi: 10.1093/brain/awf080. [DOI] [PubMed] [Google Scholar]
- 25.Jaunmuktane Z, Mead S, Ellis M, Wadsworth JDF, Nicoll AJ, Kenny J, Launchbury F, Linehan J, Richard-Loendt A, Walker AS, et al. Evidence for human transmission of amyloid-β pathology and cerebral amyloid angiopathy. Nature. 2015;525:247–250. doi: 10.1038/nature15369. [DOI] [PubMed] [Google Scholar]
- 26.Kimberlin RH, Walker CA. Pathogenesis of scrapie (strain 263K) in hamsters infected intracerebrally, intraperitoneally or intraocularly. J Gen Virol. 1986;67:255–263. doi: 10.1099/0022-1317-67-2-255. [DOI] [PubMed] [Google Scholar]
- 27.Koga S, Aoki N, Uitti RJ, van Gerpen JA, Cheshire WP, Josephs KA, Wszolek ZK, Langston JW, Dickson DW. When DLB, PD, and PSP masquerade as MSA: An autopsy study of 134 patients. Neurology. 2015;85:404–412. doi: 10.1212/WNL.0000000000001807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kovacs GG, Lutz MI, Ricken G, Ströbel T, Höftberger R, Preusser M, Regelsberger G, Hönigschnabl S, Reiner A, Fischer P, et al. Dura mater is a potential source of Aβ seeds. Acta Neuropathol. 2016;131:911–923. doi: 10.1007/s00401-016-1565-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kuusisto E, Salminen A, Alafuzoff I. Ubiquitin-binding protein p62 is present in neuronal and glial inclusions in human tauopathies and synucleinopathies. Neuroreport. 2001;12:2085–2090. doi: 10.1097/00001756-200107200-00009. [DOI] [PubMed] [Google Scholar]
- 30.Lakhan SE, Callaway E. Deep brain stimulation for obsessive-compulsive disorder and treatment-resistant depression: Systematic review. BMC Res Notes. 2010;3:60. doi: 10.1186/1756-0500-3-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Levine DJ, Stöhr J, Falese LE, Ollesch J, Wille H, Prusiner SB, Long JR. Mechanism of scrapie prion precipitation with phosphotungstate anions. ACS Chem Biol. 2015;10:1269–1277. doi: 10.1021/cb5006239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lowe J, Blanchard A, Morrell K, Lennox G, Reynolds L, Billett M, Landon M, Mayer RJ. Ubiquitin is a common factor in intermediate filament inclusion bodies of diverse type in man, including those of Parkinson’s disease, Pick’s disease, and Alzheimer’s disease, as well as Rosenthal fibres in cerebellar astrocytomas, cytoplasmic bodies in muscle, and mallory bodies in alcoholic liver disease. J Pathol. 1988;155:9–15. doi: 10.1002/path.1711550105. [DOI] [PubMed] [Google Scholar]
- 33.Masuda-Suzukake M, Nonaka T, Hosokawa M, Oikawa T, Arai T, Akiyama H, Mann DM, Hasegawa M. Prion-like spreading of pathological alpha-synuclein in brain. Brain. 2013;136:1128–1138. doi: 10.1093/brain/awt037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meissner WG, Laurencin C, Tranchant C, Witjas T, Viallet F, Guehl D, Damier P, Houeto J-L, Tison F, Eusebio A, et al. Outcome of deep brain stimulation in slowly progressive multiple system atrophy: A clinico-pathological series and review of the literature. Parkinsonism Relat Disord. 2016;24:69–75. doi: 10.1016/j.parkreldis.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 35.Osaki Y, Ben-Shlomo Y, Wenning GK, Daniel SE, Hughes A, Lees AJ, Mathias CJ, Quinn N. Do published criteria improve clinical diagnostic accuracy in multiple system atrophy? Neurology. 2002;59:1486–1491. doi: 10.1212/01.wnl.0000028690.15001.00. [DOI] [PubMed] [Google Scholar]
- 36.Papp MI, Kahn JE, Lantos PL. Glial cytoplasmic inclusions in the CNS of patients with multiple system atrophy (striatonigral degeneration, olivopontocerebellar atrophy and Shy-Drager syndrome) J Neurol Sci. 1989;94:79–100. doi: 10.1016/0022-510x(89)90219-0. [DOI] [PubMed] [Google Scholar]
- 37.Peelaerts W, Bousset L, Van der Perren A, Moskalyuk A, Pulizzi R, Giugliano M, Van den Haute C, Melki R, Baekelandt V. α-Synuclein strains cause distinct synucleinopathies after local and systemic administration. Nature. 2015;522:340–344. doi: 10.1038/nature14547. [DOI] [PubMed] [Google Scholar]
- 38.Peretz D, Supattapone S, Giles K, Vergara J, Freyman Y, Lessard P, Safar JG, Glidden DV, McCulloch C, Nguyen H-OB, et al. Inactivation of prions by acidic sodium dodecyl sulfate. J Virol. 2006;80:322–331. doi: 10.1128/JVI.80.1.322-331.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Poisson M, Magdelenat H, Foncin JF, Bleibel JM, Philippon J, Pertuiset B, Buge A. Récepteurs d’cestrogénes et de progestérone dans les méningiomes. Rev Neurol (Paris) 1980;136:193–203. [PubMed] [Google Scholar]
- 40.Prusiner SB, Woerman AL, Rampersaud R, Watts JC, Berry DB, Patel S, Oehler A, Lowe JK, Kravitz SN, Geschwind DH, et al. Evidence for α-synuclein prions causing multiple system atrophy in humans with signs of Parkinson’s disease. Proc Natl Acad Sci USA. 2015;112:E5308–E5317. doi: 10.1073/pnas.1514475112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sacino AN, Brooks M, Thomas MA, McKinney AB, Lee S, Regenhardt RW, McGarvey NH, Ayers JI, Notterpek L, Borchelt DR, et al. Intramuscular injection of α-synuclein induces CNS α-synuclein pathology and a rapid-onset motor phenotype in transgenic mice. Proc Natl Acad Sci USA. 2014;111:10732–10737. doi: 10.1073/pnas.1321785111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Safar J, Wille H, Itri V, Groth D, Serban H, Torchia M, Cohen FE, Prusiner SB. Eight prion strains have PrPSc molecules with different conformations. Nat Med. 1998;4:1157–1165. doi: 10.1038/2654. [DOI] [PubMed] [Google Scholar]
- 43.Schweighauser M, Bacioglu M, Fritschi SK, Shimshek DR, Kahle PJ, Eisele YS, Jucker M. Formaldehyde-fixed brain tissue from spontaneously ill α-synuclein transgenic mice induces fatal α-synucleinopathy in transgenic hosts. Acta Neuropathol. 2015;129:157–159. doi: 10.1007/s00401-014-1360-5. [DOI] [PubMed] [Google Scholar]
- 44.Spillantini MG, Crowther RA, Jakes R, Cairns NJ, Lantos PL, Goedert M. Filamentous α-synuclein inclusions link multiple system atrophy with Parkinson’s disease and dementia with Lewy bodies. Neurosci Lett. 1998;251:205–208. doi: 10.1016/s0304-3940(98)00504-7. [DOI] [PubMed] [Google Scholar]
- 45.Stefanova N, Bücke P, Duerr S, Wenning GK. Multiple system atrophy: An update. Lancet Neurol. 2009;8:1172–1178. doi: 10.1016/S1474-4422(09)70288-1. [DOI] [PubMed] [Google Scholar]
- 46.Taylor DM. Inactivation of prions by physical and chemical means. J Hosp Infect. 1999;43:S69–S76. doi: 10.1016/s0195-6701(99)90067-1. [DOI] [PubMed] [Google Scholar]
- 47.Thomzig A, Kratzel C, Lenz G, Kruger D, Beekes M. Widespread PrPSc accumulation in muscles of hamsters orally infected with scrapie. EMBO Rep. 2003;4:530–533. doi: 10.1038/sj.embor.embor827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thomzig A, Wagenführ K, Daus ML, Joncic M, Schulz-Schaeffer WJ, Thanheiser M, Mielke M, Beekes M. Decontamination of medical devices from pathological amyloid-β-, tau- and α-synuclein aggregates. Acta Neuropathol Commun. 2014;2:151. doi: 10.1186/s40478-014-0151-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tu PH, Galvin JE, Baba M, Giasson B, Tomita T, Leight S, Nakajo S, Iwatsubo T, Trojanowski JQ, Lee VM. Glial cytoplasmic inclusions in white matter oligodendrocytes of multiple system atrophy brains contain insoluble α-synuclein. Ann Neurol. 1998;44:415–422. doi: 10.1002/ana.410440324. [DOI] [PubMed] [Google Scholar]
- 50.Wakabayashi K, Yoshimoto M, Tsuji S, Takahashi H. α-Synuclein immunoreactivity in glial cytoplasmic inclusions in multiple system atrophy. Neurosci Lett. 1998;249:180–182. doi: 10.1016/s0304-3940(98)00407-8. [DOI] [PubMed] [Google Scholar]
- 51.Watts JC, Giles K, Oehler A, Middleton L, Dexter DT, Gentleman SM, DeArmond SJ, Prusiner SB. Transmission of multiple system atrophy prions to transgenic mice. Proc Natl Acad Sci USA. 2013;110:19555–19560. doi: 10.1073/pnas.1318268110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wenning GK, Tison F, Ben Shlomo Y, Daniel SE, Quinn NP. Multiple system atrophy: A review of 203 pathologically proven cases. Mov Disord. 1997;12:133–147. doi: 10.1002/mds.870120203. [DOI] [PubMed] [Google Scholar]
- 53.Woerman AL, Stöhr J, Aoyagi A, Rampersaud R, Krejciova Z, Watts JC, Ohyama T, Patel S, Widjaja K, Oehler A, et al. Propagation of prions causing synucleinopathies in cultured cells. Proc Natl Acad Sci USA. 2015;112:E4949–E4958. doi: 10.1073/pnas.1513426112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yan ZX, Stitz L, Heeg P, Pfaff E, Roth K. Infectivity of prion protein bound to stainless steel wires: a model for testing decontamination procedures for transmissible spongiform encephalopathies. Infect Control Hosp Epidemiol. 2004;25:280–283. doi: 10.1086/502392. [DOI] [PubMed] [Google Scholar]
- 55.Zobeley E, Flechsig E, Cozzio A, Enari M, Weissmann C. Infectivity of scrapie prions bound to a stainless steel surface. Mol Med. 1999;5:240–243. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.