Abstract
Background & Aims
Mucosal‐associated invariant T (MAIT) cells are important innate T cells with antimicrobial and immunoregulatory activity, recently found to be depleted in blood of patients with HIV and HCV mono‐infections. In this study, we assessed the impact of HIV, HCV and HCV/HIV co‐infection on circulating and intrahepatic MAIT‐cells and correlations with liver fibrosis.
Methods
In this cross‐sectional study, nine healthy subjects, nine HIV, 20 HCV and 22 HCV/HIV co‐infected patients were included. Blood and liver fine needle aspirate biopsies were studied using flowcytometry for CD3+ CD161+Vα7.2+ MAIT‐cell frequency, phenotype and function in HCV mono‐infected and HCV/HIV co‐infected patients without or with mild fibrosis (Metavir‐score F0‐F1) or severe fibrosis to cirrhosis (Metavir‐score F3‐F4).
Results
Circulating MAIT‐cells were decreased in blood of HCV, HIV and HCV/HIV patients with F0‐F1. In HCV/HIV co‐infected individuals with severe fibrosis to cirrhosis, the frequency of circulating MAIT‐cells was even further depleted, whereas their function was comparable to HCV/HIV co‐infected patients with low or absent fibrosis. In contrast, in HCV mono‐infected patients, MAIT‐cell frequencies were not related to fibrosis severity; however, MAIT‐cell function was impaired in mono‐infected patients with more fibrosis. More advanced liver fibrosis in HCV or HCV/HIV‐infected patients was not reflected by increased accumulation of MAIT‐cells in the affected liver.
Conclusions
Severe liver fibrosis is associated with dysfunctional MAIT‐cells in blood of HCV mono‐infected patients, and lower MAIT frequencies in blood of HCV/HIV co‐infected patients, without evidence for accumulation in the liver.
Keywords: fibrosis, hepatitis C virus, human immunodeficiency virus, mucosal‐associated invariant T cell
Abbreviations
- cART
combined Antiretroviral therapy
- IFN
interferon
- IL
interleukin
- MAIT
mucosal‐associated invariant T cells
- MR1
major histocompatibility complex class I‐related protein 1
- TCR
T‐cell receptor
Key points.
Hypothesis: MAIT‐cells are important innate T cells with antimicrobial and immunoregulatory activity that may play a role in HCV and HIV/HCV‐related liver pathology.
This study: MAIT‐cells are severely depleted in HIV/HCV patients with advanced fibrosis.
This study: MAIT‐cells seem dysfunctional in blood of HCV mono‐infected patients.
This study: There is no evidence for MAIT‐cell accumulation in the liver.
1. INTRODUCTION
Co‐infection with the hepatitis C virus (HCV) affects an estimated number of 2.3 million people living with the human immunodeficiency virus (HIV) worldwide, which translates into a co‐infection prevalence of 6.2% overall, 6.4% in men who have sex with men, and 82.4% in people who inject drugs. Moreover, viral hepatitis is a leading and increasingly important cause of disability and mortality worldwide, with a doubling of the quality adjusted life years lost because of HCV infection between 1990 and 2013.1, 2 In HIV‐infected patients not receiving combined antiretroviral therapy (cART), HCV co‐infection accelerates the development of liver fibrosis compared to HCV mono‐infection,3 and increases the risk for liver‐related and non‐liver‐related causes of death.4 This progressive liver fibrosis in HCV/HIV infection may be rescued by cART, especially when initiated early after HIV infection before the development of significant immunodeficiency.5
Mucosal‐associated invariant T (MAIT) cells are a group of innate‐like T cells that play an important role in the antimicrobial response to a range of bacteria and yeast species.6, 7 Recently, it has been shown that viral infections, including HCV infection, also activate MAIT‐cells in a TCR‐independent fashion dependent on cytokines.8 MAIT‐cells are identified in blood as CD3+CD161+Vα7.2+ cells, and comprise about 5% of the total T cells in blood of healthy individuals.9, 10 Similar to NK‐cells, MAIT‐cells can be activated by interleukin (IL)‐12 and IL‐1811 to produce high levels of IFN‐gamma and the cytotoxic enzyme granzyme‐B, but also by interaction of the semi‐invariant T‐cell receptor (TCR) Vα7.2 with the non‐polymorphic, major histocompatibility complex class I‐related protein 1 (MR1) on antigen presenting cells.6, 12, 13 MR1 presents riboflavin metabolites synthesized by microbes to the TCR of the MAIT‐cell.14 Viruses do not metabolize riboflavin and were shown to activate MAIT‐cells in a TCR‐independent fashion dependent on IL‐18 in synergy with IL‐12, IL‐15 and/or type I interferon.8, 11
Recently, many studies demonstrated that MAIT‐cells are depleted from blood of HIV‐infected individuals, and express exhaustion markers. Interestingly, their numbers are not fully restored despite effective cART. Recently, we and others demonstrated similar findings for HCV‐infected patients.8, 15, 16 In these patients, MAIT‐cells were depleted from peripheral blood, and successful interferon‐free therapy was unable to restore their numbers and function,15, 16 while restoration was observed for impaired CD8+ T cells and NK‐cells.16, 17, 18 The mechanisms underlying MAIT‐cell depletion from blood during HIV and HCV infection remain unclear. Activation‐induced cell death and accumulation at the site of infection and inflammation, such as the liver in HCV infection, may explain this phenomenon.8, 15, 16, 19 However, at least for HIV infection this hypothesis seems unlikely, as MAIT‐cells were systemically depleted in simian immunodeficiency virus‐infected rhesus macaques, a model often used to investigate HIV.20
Enhanced fibrosis development in HCV/HIV co‐infection has been associated with enhanced translocation of gut microbial products in HIV, which drives continuous immune activation.3, 4 Since MAIT‐cells are abundantly present in human liver perfusates,21, 22 depleted during HIV and HCV infection, and are important in antimicrobial immunity, we hypothesized that MAIT‐cells play a role in HCV‐related liver pathology and especially in HCV/HIV co‐infection. To examine this hypothesis, we compared peripheral blood vs liver residing MAIT‐cell frequencies, immune phenotype and function in individuals with HCV mono‐infections and HCV/HIV co‐infections with different fibrosis scores.
2. MATERIALS AND METHODS
2.1. Inclusion of patients and healthy subjects
Ethical approval for this study was obtained from the Ethical Review Boards of Erasmus MC, University MC Utrecht, and University MC Hamburg. Participants signed informed consent and patient data were anonymized. At the outpatient clinics, heparinized blood was collected from patients with chronic HCV infection (n = 20), chronic HCV patients with cART‐suppressed HIV (HCV/HIV; n = 22), patients with cART‐suppressed HIV mono‐infection (n = 9). In addition, blood was collected from healthy volunteers working at Erasmus MC after written informed consent (n = 9). The patient characteristics are presented in Table 1. Fine needle aspiration biopsies (FNAB) were collected from patients with chronic HCV infection (n = 10) and cART‐suppressed HCV/HIV infection (n = 8). The characteristics of these patients are presented in Table 2.
Table 1.
Characteristics of healthy controls and patients
| Healthy | HCV F0‐F1 | HCV F3‐F4 | HIV | HCV/HIV F0‐F1 | HCV/HIV F3‐F4 | |
|---|---|---|---|---|---|---|
| N | 9 | 9 | 11 | 9 | 13 | 9 |
| Gender (% male) | 100% | 100% | 100% | 100% | 100% | 100% |
| Age (y) | 40 (28‐50) | 54 (45‐68) | 51 (47‐57) | 47 (31‐58) | 48 (23‐71) | 52 (50‐67) |
| ALT (U/L) | 57 (24‐95) | 100 (38‐211) | 25 (21‐31) | 56 (20‐270) | 67 (32‐132) | |
| HCV RNA (IU/mL) | 4.38 × 106 | 2.90 × 106 | 2.15 × 106 | 1.30 × 106 | ||
| HCV genotype 1/2/3/4 | 8/0/0/1 | 8/1/1/1 | 9/1/0/3 | 7/0/2/0 | ||
| HIV load (geg/mL) | All <20 | All <20 | All <20 |
Table 2.
Characteristics of patients who donated fine needle aspirates of the liver
| HCV F0‐F1 | HCV F3‐F4 | HCV/HIV F0‐F1 | HCV/HIV F3‐F4 | |
|---|---|---|---|---|
| N | 6 | 4 | 5 | 3 |
| Gender (% male) | 100% | 100% | 100% | 100% |
| Age (y) | 58 (53‐62) | 51 (45‐58) | 58 (52‐62) | 51 (45‐58) |
| ALT (U/L) | 57 (24‐95) | 103 (60‐147) | 49 (20‐79) | 61 (45‐81) |
| HCV RNA (IU/mL) | 2.72 × 106 | 5.4 × 106 | 1.28 × 106 | 1.24 × 106 |
| HCV genotype 1/2/3/4 | 6/0/0/0 | 2/0/2/0 | 4/0/0/1 | 3/0/0/0 |
| HIV load (geq/mL) | All <20 | All <20 | ||
| Fibrosis F0/1/2/3/4 | 3/3/0/0/0 | 0/0/0/3/1 | 2/3/0/0/0 | 0/0/0/1/2 |
Liver fibrosis was scored using ultrasound and liver elastography (Fibroscan®). Liver pathology was either categorized as low, meaning absent to mild liver fibrosis (Metavir‐score F0‐F1) or high, meaning severe liver fibrosis to cirrhosis (Metavir‐score F3‐F4). Only males were included, aged between 20 to 71 years old, with undetectable viral HIV RNA levels, compliant use of cART and no further comorbidity like: autoimmune disease, cancer, co‐infection with non‐related viral or sexual transmitted infections.
2.2. Acquisition of peripheral blood mononuclear cells and liver cells
FNAB for intrahepatic cells were performed as described before.23, 24 Peripheral blood mononuclear cells (PBMC) were isolated using ficoll separation (Ficoll‐Paque™ plus, GE Healthcare Bio‐Sciences AB, Uppsala, Sweden). Approximately, 15 million blood lymphocytes were used for analyses and the remainder was frozen at −150°C.
2.3. Phenotyping by flowcytometry
PBMC were thawed, and washed with RPMI 1640 supplemented with 10% fetal calf serum (Lonza, Walkersville, MD, USA). For flowcytometry, 500 000 viable PBMC were stained with anti‐CD8‐FITC(RPA‐T8); anti‐CD3‐Amcyan(SK7); anti‐CD161‐eFluor450(HP‐3G10); Anti‐TCR Vα7.2‐PE(3C10); anti‐CD56‐APC(N901); anti‐CD56‐APC‐eFluor780(CMSSB); anti‐CD38‐PerCp‐eFluor710(HB7); or anti‐HLA‐DR‐PerCP‐Cyanine5.5(LN3) for 20 minutes at 4°C. Marker expression was detected by flowcytometry (MACSQuant Analyser 10 [Miltenyi Biotec, Cologne, Belgium]). MAIT‐cells were defined as CD3+CD161+Vα7.2+ cells within the lymphocyte gate and NK‐cells were defined as CD3−CD56+ cells within the lymphocyte gate.
Liver aspirates and paired blood were stained with anti‐CD3‐Alexa‐Fluor700(OKT‐3); anti‐CD56‐APC‐eFluor780(CMSSB); anti‐CD45‐PE‐eFluor610(HI30); anti‐CD235a‐FITC(HIR2) or anti‐CD4‐FITC(13B8.2); anti‐CD3‐eVolve605(RPA‐T8); anti‐TCR Vα7.2‐PE(3C10); anti‐CD161‐eFluor450(HP‐3G10); and Live/Dead Aqua (Life Technologies, Carlsbad, CA, USA). Cells were analysed using a FACS ARIA cell sorter (BD‐Biosciences), San Jose, CA, USA and MACSQuant Analyser 10. All antibodies were purchased from eBioscience (San Diego, CA, USA), Beckman (Brae, CA, USA) or Biolegend (London, UK).
2.4. Analysis of intracellular cytokines by flowcytometry
The percentages of cells producing IFN‐γ and granzyme‐B were measured by flowcytometry using various stimuli. For each condition, duplicate wells with 250 000 PBMCs were cultured in a 96 wells plate. Various combinations of the following stimuli were used as indicated; IL‐12 (0.25 ng/mL, Miltenyi), IL‐18 (50 ng/mL, MBL), CD28 monoclonal antibody (2 ug/mL, eBiosciences), R848 (1 μg/mL, Invivogen, San Diego, CA, USA), Escherichia coli ATCC 25922 (fixed for 20 minutes in 2% formaldehyde, 25 bacteria per lymphocyte), and E. coli K12 (fixed for 5 minutes in 1% formaldehyde, 25 bacteria per lymphocyte). For all conditions, cells were incubated for a total of 24 hours at 37°C at 5% CO2. Brefeldin A (10 μg/mL, Sigma) was added after 6 or 21 hours of culture as indicated in the figure legend. Cells were stained with anti‐CD3‐PerCp‐Cy5.5(UCHT1), anti‐CD8‐APC‐H7(SK3), anti‐CD161‐eFluor450(HP‐3G10), anti‐TCR Vα7.2‐PE(3C10), CD56‐APC(N901, Beckman) and Live/dead Aqua, fixed, permeabilized and stained with anti‐IFN‐γ‐PE‐Cy7(4S.B3) and anti‐granzyme‐B‐FITC(GB11). Cytokine‐producing cells were detected by flowcytometry using a MACSQuant Analyser 10. Gating of cells was set on internal controls with low or absent expression on lineage negative cells. Only samples with more than 80 MAIT‐cell events were included for expression of surface markers, IFN‐γ and granzyme‐B.
2.5. Statistics
Flowcytometric data were analysed using flow jo TM (treestar, windows 7 version 10.0.8). Statistical comparison was performed using the Kruskal‐Wallis and Mann‐Whitney test for unpaired non‐parametric analyses. A P value ≤ .05 was considered significant.
3. RESULTS
3.1. MAIT‐cells are severely depleted in blood of HCV, HIV and HCV/HIV patients
It has been reported that MAIT‐cells are depleted in blood of HIV and HCV patients.8, 11, 15, 16, 19, 25, 26, 27, 28, 29, 30 We confirmed these findings by performing flowcytometry on CD3+CD161+TCR Vα7.2+MAIT‐cells in blood of 20 chronic HCV patients, nine HIV patients on cART, and 22 HIV patients on cART co‐infected with HCV, as compared to nine healthy individuals (Table 1, Figure 1A). Only patients without or with only mild liver fibrosis (F0‐F1) were included for comparison. The frequencies of circulating MAIT‐cells, but not CD56+CD3− NK‐cells, were significantly lower in HCV‐, HIV‐ and HCV/HIV‐infected patients as compared to healthy individuals (Figure 1B), whereas MAIT‐cells obtained from these virus‐infected patients were more activated as demonstrated by higher frequencies of CD38 and HLA‐DR‐expressing MAIT‐cells (Figure 1C). An increase in the frequencies of the CD161−TCR Vα7.2+ cell population was observed only in HCV/HIV‐infected patients (Fig. S1).
Figure 1.

Mucosal‐associated invariant T (MAIT)‐cells are severely depleted in blood of HCV, HIV and HCV/HIV patients. (A) Viable MAIT‐cells were identified using flowcytometry as lymphocytes expressing CD3, CD161 and TCR Vα7.2. (B) MAIT‐cell and NK‐cell frequencies and (C) the frequency of CD38+ or HLA‐DR + MAIT‐cells or NK‐cells were determined in blood of healthy individuals, HCV, HIV and HCV/HIV patients, all with no or low levels of fibrosis (F0‐F1)
3.2. Effector functions of blood MAIT‐cells are preserved in HCV, HIV and HCV/HIV patients with low levels of liver disease
MAIT‐cells can be triggered by stimuli, such as the TLR7/8 agonist R848, E. coli and the cytokines IL‐12/IL‐18 to exert their effector functions.9, 11, 22 MAIT‐cells of healthy individuals stimulated with E. coli or R848 alone exhibited low frequencies of cells producing IFN‐γ or the cytolytic enzyme granzyme‐B, whereas IL‐12/IL‐18 stimulation resulted in 18% IFN‐γ+ and 7.5% granzyme‐B+ MAIT‐cells (Figure 2). Additional triggering of IL‐12/IL‐18 with either R848 or E. coli further increased the frequencies of effector‐MAIT‐cells in healthy individuals. Stronger IFN‐γ responses were detected after alteration of the E. coli stimulation in line with an optimized protocol recently published by Dias and colleagues31: E. coli strain K12 instead of ATCC 25922 was used, the E. coli bacteria were fixed for 5 minutes instead of 20 minutes in 1% formaldehyde, and brefeldin A was added to the culture after 6 hours instead of 21 hours of stimulation. This resulted in robust IFN‐γ production by MAIT‐cells (see Figs. S4 and S5). IFN‐γ production by MAIT‐cells could be further enhanced by the addition of either anti‐CD28 or IL‐12/IL‐18 (see Figs. S4 and S5).
Figure 2.
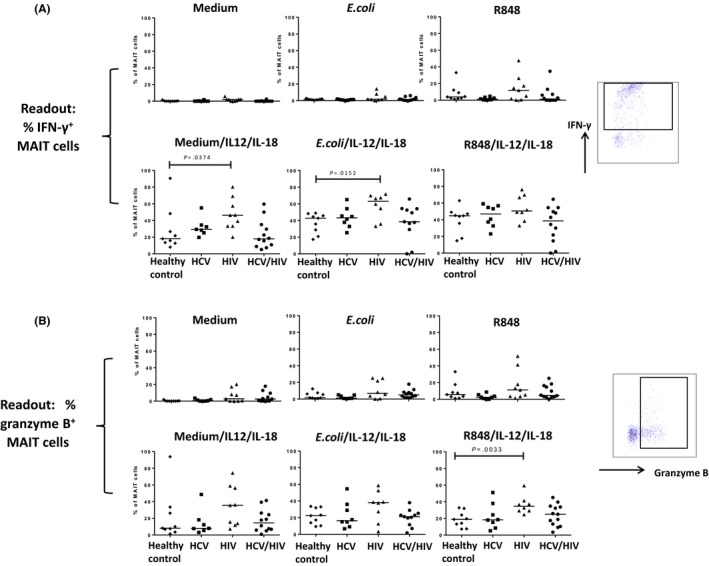
Effector functions of blood mucosal‐associated invariant T (MAIT)‐cells are preserved in HCV, HIV and HCV/HIV patients with no or low levels of liver fibrosis. PBMC from subjects with no or low levels of fibrosis (F0‐F1) were stimulated for 24 hours with medium, Escherichia coli ATCC 25922, and R848, alone or in combination with IL‐12/IL‐18, with brefeldin A being added after 21 hours. Intracellular staining for IFN‐γ (A) and granzyme‐B (B) was assessed for MAIT‐cells
Next, we studied the impact of chronic HCV, HIV and chronic HCV/HIV infection on MAIT effector functions in patients without or with only mild liver fibrosis (F0‐F1). To rule out the impact of liver fibrosis on these effector functions, a subset of F0‐F1 patients from Figure 1 were studied, and patients with severe fibrosis (F3‐F4) were not included in this comparison. Albeit that MAIT‐cells were severely depleted in blood of HCV, HIV and HCV/HIV patients (Figure 1B), we observed no reduction in MAIT‐cell functions as compared to healthy individuals. HIV mono‐infected patients generally showed increased IFN‐γ and granzyme‐B production and upon E. coli in addition to IL‐12/IL‐18 stimulation, we even observed a 65% increase in IFN‐γ‐producing MAIT‐cells (Figure 2A). It is important to note that the frequencies of monocytes, which are triggered by E. coli or R848 to secrete MAIT‐cell‐activating cytokines, were comparable in all experimental groups (mean 13.5% of viable PBMC, range 10.5%‐15.1%).
Moreover, the frequency of IFN‐γ‐producing blood NK‐cells from the same patients was also comparable between the four experimental groups, except from HIV patients who showed reduced frequencies of cytokine‐producing NK‐cells upon certain stimuli. The majority of blood NK‐cells spontaneously expressed granzyme‐B, which was increased to almost all blood NK‐cells upon stimulation with IL‐12/IL‐18, E. coli or R848 (Fig. S2 and data not shown).
Overall, these findings show that despite lower frequencies of MAIT‐cells in blood, the function of MAIT‐cells obtained from HCV or HCV/HIV patients without or with minimal fibrosis (F0‐F1) is maintained as compared to healthy individuals, or even enhanced upon stimulation with E. coli and IL‐12/IL‐18 in HIV patients.
3.3. MAIT‐cell frequencies are more depleted in blood of HCV/HIV co‐infected patients with severe fibrosis and cirrhosis vs no to mild fibrosis
Next, we determined the peripheral blood MAIT‐cell frequencies in individuals with HCV mono‐infections and HCV/HIV co‐infections with different fibrosis scores. To examine this, we compared patients with severe liver fibrosis to cirrhosis (F3‐F4) vs patients without or with only mild fibrosis (F0‐F1) that were also included in Figures 1 and 2. As shown in Figure 3A, we observed that in HCV mono‐infected patients the frequencies of MAIT‐cells in blood were comparable in patients with F0‐F1 vs F3‐F4. However, significant lower MAIT‐cell frequencies were detected in blood of HCV/HIV patients with F3‐F4 as compared to F0‐F1 (P = .024; median 0.350 and 0.880 respectively). Importantly, the frequencies of CD3+CD161−TCR Vα7.2+ cells were not significantly different in patients with F3‐F4 as compared to F0‐F1, and loss of CD161 could not explain the decrease in CD3+CD161+TCR Vα7.2+MAIT‐cells (data not shown). In addition, frequencies of NK‐cells were not modulated as a consequence of differences in fibrosis scores in either patient group (Figure 3A).
Figure 3.

Mucosal‐associated invariant T (MAIT)‐cell frequencies are more depleted in blood of HCV/HIV co‐infected patients with severe fibrosis vs low fibrosis. (A). The frequencies of MAIT‐cells and NK‐cells, and (B) the frequencies of CD38+ or HLA‐DR + MAIT‐cells or NK‐cells were determined in blood of HCV mono‐infected and HCV/HIV co‐infected patients with F0‐F1 or F3‐F4 fibrosis score
The frequency of CD38+ and HLA‐DR+ MAIT‐cells in HCV and HCV/HIV co‐infected patients was significantly increased compared to those of healthy individuals (Figure 1C), but when stratifying for liver fibrosis we observed no significant modulation of the frequency of CD38+ or HLA‐DR+ MAIT‐cells in patients with F0‐F1 vs F3‐F4 (Figure 3B). Also for NK‐cells, no significant differences were observed when comparing the patients with different fibrosis scores.
3.4. Reduced function of MAIT‐cells in blood of HCV mono‐infected patients with severe fibrosis and cirrhosis vs no to mild fibrosis
To determine whether fibrosis scores affected the functionality of MAIT‐cells in these patients, PBMC were stimulated as before. As shown in Figure 4, we observed in HCV mono‐infected patients a significant reduction of the frequencies of IFN‐γ+ MAIT‐cells in blood of patients with severe liver fibrosis to cirrhosis (F3‐F4) vs patients without or with only mild fibrosis (F0‐F1) upon stimulation with IL‐12/IL‐18 alone, or in combination with E. coli or R848. Using the optimized protocol for E. coli stimulation of MAIT‐cells,31 we confirmed these findings. Further, we observed the same trend for E. coli stimulation alone and E. coli in combination with anti‐CD28, albeit that this effect did not reach significance (Fig. S5). Moreover, upon stimulation with IL‐12/IL‐18 alone, a significant reduction of the frequencies of granzyme‐B+ MAIT‐cells was observed in the HCV mono‐infected patients with F3‐F4 vs F0‐F1 (P = .0229). In contrast, patients with HCV/HIV co‐infections exhibited comparable frequencies of IFN‐γ+ MAIT‐cells and granzyme‐B+ MAIT‐cells upon stimulation. These alterations were observed for blood MAIT‐cells, but not for NK‐cells from the same patients (Fig. S3).
Figure 4.

Mucosal‐associated invariant T (MAIT)‐cell function is reduced in blood of HCV mono‐infected patients with severe fibrosis vs low fibrosis. PBMC of HCV mono‐infected and HCV/HIV co‐infected patients with F0‐F1 or F3‐F4 fibrosis score were stimulated with medium, Escherichia coli ATCC 25922, and R848, alone or in combination with IL‐12/IL‐18, with brefeldin A being added after 21 hours. Intracellular staining for IFN‐γ (A) and granzyme‐B (B) was assessed for MAIT‐cells
In summary, our findings suggest that MAIT‐cell effector functions are reduced in HCV mono‐infected patients with severe liver fibrosis and cirrhosis as reflected by reduced frequencies of granzyme‐B+ MAIT‐cells upon stimulation with IL‐12/IL‐18.
3.5. Ex vivo intrahepatic MAIT‐cells do not differ in frequency in HCV mono‐infected vs HCV/HIV patients
A possible explanation for the reduced numbers of circulating MAIT‐cells in HCV mono‐ c evaluation of the aspirates demonstrated that the frequencies of MAIT‐cells in the liver of HCV and HCV/HIV patients without or with only mild fibrosis (F0‐F1) were relatively low (HCV liver: median 1.11%; HCV/HIV liver: median 1.08% of the total intrahepatic T cells), but higher than in blood. Importantly, the percentage of intrahepatic CD161−TCR Vα7.2+ cells was comparable in all patient groups (data not shown). Also, as shown in Figure 5B, no increased MAIT‐cell frequencies were observed in the liver of the patients with severe fibrosis or cirrhosis as compared to patients without fibrosis. These findings suggest that the more pronounced depletion of MAIT‐cells in blood of HCV/HIV co‐infected patients with high fibrosis scores, as presented in Figure 3A, is not reflected by higher frequencies in the liver of this population in the same patient.
Figure 5.

The frequencies of mucosal‐associated invariant T (MAIT)‐cells are higher in liver than blood in HCV mono‐infected and HCV/HIV co‐infected individuals. (A) Viable MAIT‐cells were identified in liver aspirates using flowcytometry as viable CD45+ cells, lacking CD235a and expressing CD3, CD161 and TCR Vα7.2. (B) The frequencies of MAIT‐cells within the CD3+ T‐cell compartment is higher in liver than blood, but overall not different between individuals with F0‐F1 vs F3‐F4 scores in HCV and HCV/HIV patients
4. DISCUSSION
In line with an increasing number of studies, we confirmed that the frequency of MAIT‐cells in blood of HCV‐ and HIV mono‐infected patients is reduced, while these cells are more activated as evidenced by higher expression of CD38 and HLA‐DR8, 15, 16, 19, 25, 26, 27, 28, 29, 30 We now show, for the first time, that MAIT‐cells are also depleted and more activated in blood of patients chronically co‐infected with HCV and HIV as compared to healthy individuals, without evidence for accumulation in the liver as shown using aspirate biopsies. In line with literature, we observed that MAIT‐cells retain their cytokine‐producing capacity in patients infected with HIV29, 30 as well as HCV16 as compared to healthy individuals. Further supported by the findings that in all patient groups enhanced CD38 and HLA‐DR expression was observed, it is clear that blood MAIT‐cells are fully functional in these patients. Interestingly, in HIV mono‐infected patients, but not in HCV mono‐infected or co‐infected patients, we even observed increased frequencies of IFN‐γ‐ and granzyme‐B‐producing MAIT‐cells with certain stimuli, which further supports that their functionality is not impaired.
Since MAIT‐cells are abundantly present in human liver perfusates21, 22 and important in antimicrobial immunity, we examined the possible association between the frequency and function of MAIT‐cells and the degree of HCV‐related liver pathology. Interestingly, we observed that HCV/HIV co‐infected patients with severe liver fibrosis or cirrhosis (F3‐F4) had lower frequencies of MAIT‐cells in blood as compared to patients without fibrosis (F0‐F1). This was observed for co‐infected patient, but not for HCV mono‐infected patients. The lack of correlation of MAIT‐cell frequencies and fibrosis scores in HCV mono‐infected patients as opposed to co‐infected patients confirms our earlier study in an independent cohort.16 Moreover, MAIT‐cells from HCV/HIV co‐infected patients with severe fibrosis are not more activated, and do not produce more IFN‐γ than their counterpart from co‐infected patients without fibrosis. Interestingly, in contrast to HCV/HIV co‐infected patients, MAIT‐cells obtained from HCV mono‐infected patients with severe fibrosis are not more depleted, but show lower frequencies of IFN‐γ‐producing cells as well as a clear trend towards reduced frequencies of granzyme‐B+ cells as compared to their counterparts with low fibrosis scores. Both in F3‐F4 patients with HCV mono‐infections as well as in patients with HCV/HIV co‐infections, MAIT‐cells seem impaired. However, in HCV/HIV co‐infections only MAIT‐cell numbers are decreased, whereas in HCV mono‐infections only MAIT‐cell functionality is hampered, reflected by reduced IFN‐γ production and granzyme‐B expression.
Likely, similar processes may be responsible for these divergent findings. MAIT‐cell depletion in HCV/HIV co‐infected patients may be the consequence of the interplay between inflammation caused by enhanced microbial translocation from the gut via the portal vein to the liver and sustained immune activation as a consequence of HIV infection. HIV infection possibly further reduces the number of MAIT‐cells in advanced fibrosis patients. On the other hand, we show in Figure 2 that HIV induces higher IFN‐γ production and granzyme‐B expression in MAIT‐cells which may explain why HCV/HIV co‐infected patients with advanced fibrosis show similar MAIT‐cell functions as in patients without or with only mild fibrosis. This balance is likely different in HCV mono‐infected patients, and possibly leakage of microbial products from the intestine only occurs in these patients after the liver has become severely fibrotic.
To rule out that our experimental set‐up was responsible for our findings, we repeated our MAIT IFN‐γ production experiments on identical samples using an optimized protocol recently published by Dias and colleagues.31 Based on availability of samples, we repeated the experiments on samples of 46 subjects including six healthy controls, all nine HIV mono‐infected and all 20 HCV mono‐infected patients. Because of limited availability of archived PBMC samples of some patients, only seven HCV/HIV co‐infected patients without significant fibrosis (F0‐F1) and four with severe fibrosis to cirrhosis (F3‐F4) could be included. Using a different E. coli strain, fixation method and kinetics, we confirmed our previous findings that the frequencies of IFN‐γ+ MAIT‐cells in blood of HCV mono‐infected patients with severe liver fibrosis to cirrhosis (F3‐F4) vs patients without or with only mild fibrosis (F0‐F1) were significantly reduced after E. coli/IL‐12/IL‐18 or IL‐12/IL‐18 stimulation. Further, we observed the same trend for E. coli stimulation alone and E. coli in combination with anti‐CD28, albeit that this effect did not reach significance (Fig. S5).
A suggested theory for the observed MAIT‐cell depletion from blood is that the MAIT‐cells of HCV/HIV co‐infected patients migrate to the liver in response to the chronic infection.10, 19, 26, 29, 32 However, we observed no indications for enhanced migration of MAIT‐cells to the liver in co‐infected patients with F3‐F4 as compared to patients with F0‐F1. In fact, although the number of liver aspirate biopsies that could be examined is small, it even appears that in HCV/HIV co‐infected patients with severe fibrosis the frequency is lower, making it unlikely that enhanced MAIT‐cell migration to the liver is occurring in these patients. Importantly, the percentage of MAIT‐cells as a proportion of T cells does not necessarily reflect absolute MAIT frequencies. However, the number of lymphocytes we purified from F0‐F1 livers was similar or slightly higher than the number purified from F3‐F4 livers. Therefore, it is unlikely that in HCV/HIV co‐infected patients with advanced fibrosis, enhanced intrahepatic cellular infiltration accompanied by a concurrent migration of MAIT‐cells to these livers explains our finding that MAIT‐cells are depleted more profoundly in blood of patients with high fibrosis scores. However, it should be noted that the frequencies of MAIT‐cells in the livers of patients with HCV and HCV/HIV obtained by fine needle aspiration are much lower than the frequencies reported by other studies performed using liver perfusates of healthy individuals.21, 22 It is important to examine in future studies whether patient cohorts and/or methodological factors account for these differences in frequencies. More studies are also needed to investigate whether MAIT‐cells migrate to the hepatic lymph nodes of HCV mono‐ and HCV/HIV co‐infected patients. Also, information on the distribution of MAIT‐cells in healthy individuals and HIV mono‐infected patients is needed. Notwithstanding the profound relevance, acquisition of these data is challenging because of difficult inclusion of patients and healthy volunteers, and partly because of ethical constraints.
Another suggested explanation for the depletion of CD3+CD161+TCR Vα7.2+MAIT‐cells is downregulation of CD161.28, 33 Indeed, we observed an increase in CD3+CD161−TCR Vα7.2+ cells in HCV/HIV‐infected patients (Fig. S1). However, this did not explain the lower proportion of MAIT‐cells found in our HCV/HIV co‐infected patients with advanced fibrosis (F3‐F4) as opposed to patients without or with only mild fibrosis (F0‐F1), and the percentage of intrahepatic CD161−TCR Vα7.2+ cells was comparable in all patient groups. Moreover, there is continuous debate whether these CD3+TCR Vα7.2+ cells lacking CD161 expression are truly MAIT‐cells. Important in this is that MAIT‐cells activated via their T‐cell receptor by vitamin B metabolites bound by MR1, are almost absent among CD3+CD161−TCR Vα7.2+ cells in healthy individuals,34 and in HIV‐infected patients, MR1 tetramers did not bind to CD3+CD161−TCR Vα7.2+ cells.29
At present, it is unknown if the dysfunctional blood MAIT‐cells in HCV mono‐infected patients and reduced MAIT‐cell numbers in blood of HCV/HIV co‐infected individuals are the cause or the consequence of the more severe liver fibrosis. Low MAIT‐cell frequencies as compared to healthy individuals might predispose for fibrosis in HCV mono‐infected and this situation could be accelerated in HCV/HIV co‐infected individuals with high fibrosis scores. A dysfunctional MAIT‐cell compartment likely impacts the antimicrobial activity, which affects clearance of microbial products released to the liver via enhanced microbial translocation.11 Moreover, IFN‐γ has been described to possess antifibrotic activity, and consequently a reduction of IFN‐γ levels in the liver because of decreased levels of IFN‐γ production or lower numbers of IFN‐γ ‐producing MAIT‐cells might also promote fibrosis development. Unfortunately, because of the small numbers of intrahepatic leucocytes collected by aspiration of the liver, sufficient numbers of cells were available for flowcytometry, but not for additional functional testing of intrahepatic MAIT‐cells.
In conclusion, our findings demonstrate that the degree of impairment of the MAIT‐cell compartment in blood of HCV and HCV/HIV patients is more pronounced in individuals with severe liver fibrosis or cirrhosis as compared to those without or with only mild fibrosis. Moreover, evaluation of aspirate biopsies of the liver provided no evidence for enhanced MAIT‐cell migration to the liver during chronic viral infections or advanced fibrosis.
CONFLICT OF INTEREST
None of the authors declare a conflict of interest in connection with the submitted manuscript.
Supporting information
ACKNOWLEDGEMENTS
The authors thank the research nurses Heleen van Santen en Melek Polat for their excellent and dedicated support.
Beudeker BJB, van Oord GW, Arends JE, et al. Mucosal‐associated invariant T‐cell frequency and function in blood and liver of HCV mono‐ and HCV/HIV co‐infected patients with advanced fibrosis. Liver Int. 2018;38:458–468. https://doi.org/10.1111/liv.13544
Funding information
SzW is supported by the German Center for Infection (DZIF) and the German research agency (DFG SFB 841 project A6). The Virgo consortium (funded by the Dutch government, project FES0908) supported AB.
Handling Editor: Mario Mondelli
REFERENCES
- 1. Stanaway JD, Flaxman AD, Naghavi M, et al. The global burden of viral hepatitis from 1990 to 2013: findings from the Global Burden of Disease Study 2013. The Lancet. 2016;388:1081‐1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Platt L, Easterbrook P, Gower E, et al. Prevalence and burden of HCV co‐infection in people living with HIV: a global systematic review and meta‐analysis. Lancet Infect Dis. 2016;16:797‐808. [DOI] [PubMed] [Google Scholar]
- 3. Graham CS, Baden LR, Yu E, et al. Influence of human immunodeficiency virus infection on the course of hepatitis C virus infection: a meta‐analysis. Clin Infect Dis. 2001;33:562‐569. [DOI] [PubMed] [Google Scholar]
- 4. Hernando V, Perez‐Cachafeiro S, Lewden C, et al. All‐cause and liver‐related mortality in HIV positive subjects compared to the general population: differences by HCV co‐infection. J Hepatol. 2012;57:743‐751. [DOI] [PubMed] [Google Scholar]
- 5. Arends JE, Lieveld FI, Boeijen LL, et al. Natural history and treatment of HCV/HIV coinfection: is it time to change paradigms? J Hepatol. 2015;63:1254‐1262. [DOI] [PubMed] [Google Scholar]
- 6. Le Bourhis L, Martin E, Peguillet I, et al. Antimicrobial activity of mucosal‐associated invariant T cells. Nat Immunol. 2010;11:701‐708. [DOI] [PubMed] [Google Scholar]
- 7. Gold MC, Cerri S, Smyk‐Pearson S, et al. Human mucosal associated invariant T cells detect bacterially infected cells. PLoS Biol. 2010;8:e1000407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. van Wilgenburg B, Scherwitzl I, Hutchinson EC, et al. MAIT cells are activated during human viral infections. Nat Commun. 2016;7:11653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Le Bourhis L, Dusseaux M, Bohineust A, et al. MAIT cells detect and efficiently lyse bacterially‐infected epithelial cells. PLoS Pathog. 2013;9:e1003681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dusseaux M, Martin E, Serriari N, et al. Human MAIT cells are xenobiotic‐resistant, tissue‐targeted, CD161hi IL‐17‐secreting T cells. Blood. 2011;117:1250‐1259. [DOI] [PubMed] [Google Scholar]
- 11. Ussher JE, Klenerman P, Willberg CB. Mucosal‐associated invariant T‐cells: new players in anti‐bacterial immunity. Front Immunol. 2014;5:450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Patel O, Kjer‐Nielsen L, Le Nours J, et al. Recognition of vitamin B metabolites by mucosal‐associated invariant T cells. Nat Commun. 2013;4:2142. [DOI] [PubMed] [Google Scholar]
- 13. Treiner E, Duban L, Bahram S, et al. Selection of evolutionarily conserved mucosal‐associated invariant T cells by MR1. Nature. 2003;422:164‐169. [DOI] [PubMed] [Google Scholar]
- 14. Kjer‐Nielsen L, Patel O, Corbett AJ, et al. MR1 presents microbial vitamin B metabolites to MAIT cells. Nature. 2012;491:717‐723. [DOI] [PubMed] [Google Scholar]
- 15. Hengst J, Strunz B, Deterding K, et al. Nonreversible MAIT cell‐dysfunction in chronic hepatitis C virus infection despite successful interferon‐free therapy. Eur J Immunol. 2016;46:2204‐2210. [DOI] [PubMed] [Google Scholar]
- 16. Spaan M, Hullegie SJ, Beudeker BJ, et al. Frequencies of circulating MAIT cells are diminished in chronic HCV, HIV and HCV/HIV co‐infection and do not recover during therapy. PLoS ONE. 2016;11:e0159243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Martin B, Hennecke N, Lohmann V, et al. Restoration of HCV‐specific CD8+ T cell function by interferon‐free therapy. J Hepatol. 2014;61:538‐543. [DOI] [PubMed] [Google Scholar]
- 18. Serti E, Chepa‐Lotrea X, Kim YJ, et al. Successful interferon‐free therapy of chronic hepatitis C virus infection normalizes natural killer cell function. Gastroenterology. 2015;149:190‐200 e192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cosgrove C, Ussher JE, Rauch A, et al. Early and nonreversible decrease of CD161++/MAIT cells in HIV infection. Blood. 2013;121:951‐961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Vinton C, Wu F, Rossjohn J, et al. Mucosa‐associated invariant T cells are systemically depleted in simian immunodeficiency virus‐infected rhesus macaques. J Virol. 2016;90:4520‐4529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tang XZ, Jo J, Tan AT, et al. IL‐7 licenses activation of human liver intrasinusoidal mucosal‐associated invariant T cells. J Immunol. 2013;190:3142‐3152. [DOI] [PubMed] [Google Scholar]
- 22. Jo J, Tan AT, Ussher JE, et al. Toll‐like receptor 8 agonist and bacteria trigger potent activation of innate immune cells in human liver. PLoS Pathog. 2014;10:e1004210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Claassen MA, de Knegt RJ, Janssen HL, Boonstra A. Retention of CD4+ CD25+ FoxP3+ regulatory T cells in the liver after therapy‐induced hepatitis C virus eradication in humans. J Virol. 2011;85:5323‐5330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Spaan M, Claassen MA, Hou J, Janssen HL, de Knegt RJ, Boonstra A. The intrahepatic T cell compartment does not normalize years after therapy‐induced hepatitis C virus eradication. J Infect Dis. 2015;212:386‐390. [DOI] [PubMed] [Google Scholar]
- 25. Eberhard JM, Hartjen P, Kummer S, et al. CD161+ MAIT cells are severely reduced in peripheral blood and lymph nodes of HIV‐infected individuals independently of disease progression. PLoS ONE. 2014;9:e111323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ussher JE, Phalora P, Cosgrove C, et al. Molecular analyses define Valpha7.2‐Jalpha33+ MAIT cell depletion in HIV infection: a case‐control study. Medicine (Baltimore). 2015;94:e1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wong EB, Akilimali NA, Govender P, et al. Low levels of peripheral CD161++CD8+ mucosal associated invariant T (MAIT) cells are found in HIV and HIV/TB co‐infection. PLoS ONE. 2013;8:e83474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Leeansyah E, Ganesh A, Quigley MF, et al. Activation, exhaustion, and persistent decline of the antimicrobial MR1‐restricted MAIT‐cell population in chronic HIV‐1 infection. Blood. 2013;121:1124‐1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fernandez CS, Amarasena T, Kelleher AD, et al. MAIT cells are depleted early but retain functional cytokine expression in HIV infection. Immunol Cell Biol. 2015;93:177‐188. [DOI] [PubMed] [Google Scholar]
- 30. Leeansyah E, Svard J, Dias J, et al. Arming of MAIT cell cytolytic antimicrobial activity is induced by IL‐7 and defective in HIV‐1 infection. PLoS Pathog. 2015;11:e1005072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dias J, Sobkowiak MJ, Sandberg JK, Leeansyah E. Human MAIT‐cell responses to Escherichia coli: activation, cytokine production, proliferation, and cytotoxicity. J Leukoc Biol. 2016;100:233‐240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Saeidi A, Tien Tien VL, Al‐Batran R, et al. Attrition of TCR Valpha7.2+ CD161++ MAIT cells in HIV‐tuberculosis co‐infection is associated with elevated levels of PD‐1 expression. PLoS ONE. 2015;10:e0124659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ussher JE, Bilton M, Attwod E, et al. CD161++ CD8+ T cells, including the MAIT cell subset, are specifically activated by IL‐12+IL‐18 in a TCR‐independent manner. Eur J Immunol. 2014;44:195‐203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Reantragoon R, Corbett AJ, Sakala IG, et al. Antigen‐loaded MR1 tetramers define T cell receptor heterogeneity in mucosal‐associated invariant T cells. J Exp Med. 2013;210:2305‐2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


