SUMMARY
Legumain (AEP) is a lysosomal cysteine protease that is a lysosomal cysteine protease that was first characterized in leguminous seeds and later discovered in higher eukaryotes. AEP up-regulation is linked to a number of diseases including inflammation, arteriosclerosis and tumorigenesis. Thus legumain is an excellent molecular target for the development of new chemical markers. We deployed a hybrid combinatorial substrate library (HyCoSuL) approach to obtain P1-Asp fluorogenic substrates and biotin-labeled inhibitors that targeted legumain. Since this approach led to probes that were also recognized by caspases, we introduced a Counter Selection Substrate Library (CoSeSuL) approach that biases the peptidic scaffold against caspases, thus delivering highly selective legumain probes. The selectivity of these tools was validated using M38L and HEK293 cells. We also propose that the CoSeSuL methodology can be considered as a general principle in the design of selective probes for other protease families where selectivity is difficult to achieve by conventional sequence-based profiling.
INTRODUCTION
Cysteine proteases constitute one of the largest group of proteolytic enzymes, consisting of several separate clans (Rawlings et al., 2014). One of these clans is clan CD which includes legumain, clostripains, caspases, paracaspases, metacaspases, gingipains, and separases/separins (Chen et al., 1998). Clan CD members are distinct from other groups of proteases in their stringent preference for a single amino acid in the P1 position (McLuskey and Mottram, 2015). Together, the human members of clan CD participate in a number of critical cellular pathways including cell survival and cell death signaling, inflammation, antigen presentation, and cell cycle regulation.
Legumain (asparaginyl endopeptidase - AEP, EC 3.4.22.34) displays high selectivity and cleaves substrates after Asn (P1 position) (Chen et al., 1997; Chen et al., 2000; Dall and Brandstetter, 2016). However, at acid pH this enzyme can also hydrolyze peptide bonds after Asp, which overlaps with caspases substrate specificity (Dall and Brandstetter, 2012). Moreover, it was also reported that this enzyme is inhibited by commonly used synthetic caspase inhibitors containing Asp residue at P1 (Rozman-Pungercar et al., 2003). Legumain was first identified and characterized in 1993 as a cysteine protease in leguminous seed (Kembhavi et al., 1993), and its paralog was first identified in kidney (Chen et al., 1997). Today it is known that legumain is expressed in diverse cell types, and it is mainly located in late endosomes and lysosomes, since acidic pH is a prerequisite for prolegumain activation (Dall and Brandstetter, 2012; Hashimoto et al., 1999; Lecaille et al., 2004). Unexpectedly, legumain can also be active extracellularly when stabilized by integrins on the cell surface (Dall and Brandstetter, 2013). Legumain intra- and extracellular activity is controlled by endogenous cysteine protease inhibitors called type 2 cystatins (Alvarez-Fernandez et al., 1999; Briggs et al., 2010; Smith et al., 2012). Although displaying a caspase-like fold, legumain is not inhibited by natural caspase inhibitors (Dall and Brandstetter, 2016; Snipas et al., 2001). Legumain is considered to play an important role in living cells, since it is the only enzyme known in humans able to recognize Asn in P1 position of peptide substrates indicating regulatory functions (Rawlings et al., 2014). It has been demonstrated that this enzyme can participate in a number of biological events including extracellular matrix remodeling (Morita et al., 2007), inhibition of osteoclast formation (Choi et al., 1999), MHC class II-mediated antigen presentation (Manoury et al., 2003), conversion of pro-matrix metalloproteinase-2 (MMP2) into active-MMP2 (Chen et al., 2001), and processing of lysosomal cysteine cathepsins such as cathepsins B, H, and L (Mattock et al., 2010; Shirahama-Noda et al., 2003). It is also required for normal kidney physiology and homeostasis (Miller et al., 2011). Legumain over-expression has been linked with inflammation (Chan et al., 2009), atherosclerosis (Clerin et al., 2008), and tumorigenesis. Several recent studies have reported that over-expression of legumain can be used as a prognostic factor in various human cancer types, including breast (Gawenda et al., 2007), colorectal (Haugen et al., 2013), gastric (Li et al., 2013), glioma (Qiu et al., 2008), ovarian (Wang et al., 2012), and prostate (Ohno et al., 2013) cancers. Therefore, legumain is a good candidate for molecular targeting in cancer tumor therapy (Bajjuri et al., 2011; Liu et al., 2003; Luo et al., 2006). On the other hand, it has been reported that legumain deficiency is involved in disorders resembling hemophagocytic syndrome (HLH) (Chan et al., 2009). Moreover, parasite legumain plays a significant role in the development of parasitic infections, for example schistosomatosis, which along with malaria is the most widespread parasitic disease (Gotz et al., 2008; James et al., 2003; Ovat et al., 2009).
Because it is the activity of a protease that produces a biological event, small molecule substrates, inhibitors and activity based probes (ABPs) that can interrogate this activity provide excellent tools for studying protease functions in living organisms (Kasperkiewicz et al., 2014; Powers et al., 2002; Sadaghiani et al., 2007). The first step in the design of these tools is to probe protease substrate specificity in detail, allowing the generation of structural motifs that are selective for the protease of interest (Poreba and Drag, 2010). The substrate specificity of legumain was first characterized by Mathieu and others in 2002 (Mathieu et al., 2002) revealing an optimal sequence in the P3-P2-P1 positions (Pro-Thr-Asn). Shortly after the Kalbacher group using a simple peptide library demonstrated that legumain has a broad substrate specificity in the P1′ position, and that peptides with Pro at P1′ were poorly hydrolyzed (Schwarz et al., 2002). While these results shed some light on legumain specificity, none of the sequences identified were used in synthesis of legumain-selective substrates. The first legumain fluorogenic substrate Cbz-Ala-Ala-Asn-AMC was developed by Kembhavi et al. based on the P3-P2-P1 sequence Ala-Ala-Asn (Kembhavi et al., 1993). The presence of Asn in P1 made this substrate specific and very promising in the context of designing selective legumain inhibitors. However, it was later reported that the close proximity of the Asn residue and an electrophile warhead induced intramolecular cyclization making the inhibitor inactive (Loak et al., 2003). To overcome this problem several types of aza-Asn legumain inhibitors have been described, including Michael acceptors (Ekici et al., 2004; Lee and Bogyo, 2012), epoxides (Asgian et al., 2002; Lee and Bogyo, 2012), and halomethylketones (Niestroj et al., 2002). All these compounds inhibited legumain in vitro, but their potency and selectivity in vivo have never been tested. A new group of legumain inhibitors and activity based probes was developed by the Bogyo group (Edgington et al., 2013; Lee and Bogyo, 2010; Sexton et al., 2007). These compounds contain Asp in P1 position, which makes them more stable. One of these probes (LE-28 with the P3-P1 sequence Glu-Pro-Asp) is also legumain-selective in vivo (Edgington et al., 2013). However, this selectivity was not obtained by the Glu-Pro-Asp sequence itself (which is also recognized by caspases), but by introducing bulky groups (Cy5 - fluorophore and QSY21 - fluorescence quencher) directing the probe to the lysosomes through endocytosis/macropinocytosis.
The design of legumain selective peptidic probes with Asp in P1 position is very challenging due to the fact that most of these peptides are recognized by caspases. This problem can be overcome by using our previously reported Hybrid Combinatorial Substrate Library (HyCoSuL) containing more than 100 various unnatural amino acids in each position making it an excellent tool for the determination of broad specificity of proteases (Kasperkiewicz et al., 2014; Poreba et al., 2014a). Using this approach we have been able to identify substrates that can distinguish individual caspases (Poreba et al., 2014a). This library contains Asp in P1, which makes it suitable also for legumain screening. The challenge thus becomes to develop substrates that can distinguish caspases from legumain. To this end we developed a novel counter selection screen where instead of exploiting optimal substrate/enzyme interactions we defined non-productive interactions that would allow for the generation of highly selective substrates. This Counter Selection Substrate Library (CoSeSuL) approach allowed us to identify a peptidic motif that is highly preferred by legumain. The major benefit of this approach is that this peptide ligand can be used to simultaneously obtain legumain-selective substrates, inhibitors and activity based probes (ABPs). In this example the selectivity is generated by the P4-P1 peptide, not by the fluorescent tag or warhead, making this approach universal in context of developing new low molecular weight legumain tools. In this study we describe the step-by-step procedure for development and validation of new legumain P1-Asp fluorogenic substrates and ABPs that are able to enter living cells and selectively label active legumain.
RESULTS
Legumain P1 specificity
To examine the dual nature of legumain’s Asp and Asn specificity we synthesized two 7-amino-4-carbamoylmethyl-coumarin (ACC)-labeled substrates, Z-AAN-ACC and Z-AAD-ACC, and measured kinetics of their hydrolysis by legumain. Z-AAN-ACC was cleaved ~22 time faster (kcat/Km = 36100 s−1M−1) than its P1-Asp analog (kcat/Km = 1600 M−1s−1). Legumain’s ability to recognize Asp in P1 overlaps with caspases, however the mechanisms for Asp recognition are different because these enzymes are active in different pH windows. Cytosolic caspases display the highest catalytic activity at neutral pH range, varying from 6.5 to 8.0 (Asp residue is deprotonated and occupies the positively charged S1 pocket of caspases) (Garcia-Calvo et al., 1999; Stennicke and Salvesen, 1997). On the other hand, legumain prefers the acidic environment of lysosomes and endosomes (below pH 6.0) (Dall and Brandstetter, 2013). Under these conditions legumain can cleave substrates not only after Asn, but also after Asp, since below pH 6.0 the aspartic acid side chain is partially protonated. The maximal legumain activity towards a P1-Asp substrate has been observed at pH 4.0 (Dall and Brandstetter, 2012), which opened the doors for the development of novel P1-Asp probes potentially useful for selective legumain detection.
The influence of peptide length for legumain activity
The ultimate goal of this work was to obtain a legumain-selective peptidic motif that could be used as a scaffold for the synthesis of substrates, inhibitors and activity based probes selectively targeting only this enzyme. Therefore, we first examined the influence of substrate length for legumain activity. To do this, two series of ACC-labeled substrates were synthesized in which the peptide chains were systematically elongated (Ac-TN-ACC, Ac-GTN-ACC, Ac-DGTN-ACC (series 1) and Ac-SN-ACC, Ac-DSN-ACC, Ac-YDSN-ACC (series 2)) (Figure S1). These sequences were selected based on the legumain substrate specificity profile obtained through PS-SCL profiling. Kinetic analysis (kcat/Km measurements) revealed that there is no correlation between the length of the peptide chain and legumain activity. All substrates displayed similar kinetic parameters (Table S1). Taking into account that caspases display almost no activity towards dipeptide substrates and inhibitors, at first glance it would seem rational to use a short scaffold (P2-Asp-warhead/fluorophore) to construct legumain-selective chemical tools. However, this is not appropriate when substrates are converted to ABPs, since simple biotinylated acyloxymethylketone containing only one amino acid (biotin-linker-Asp-AOMK) are able to bind both legumain and caspases (Kato et al., 2005). We therefore postulated that biotin and a linker would decrease the ABP potency toward legumain versus caspases. In an attempt to develop novel legumain-selective ABPs we decided to dissect legumain’s preferences in the P4-P2 positions using a traditional PS-SCL approach as well as the recently developed HyCoSuL strategy to define those amino acids that discriminate legumain from the caspase family.
Legumain specificity for natural amino acids
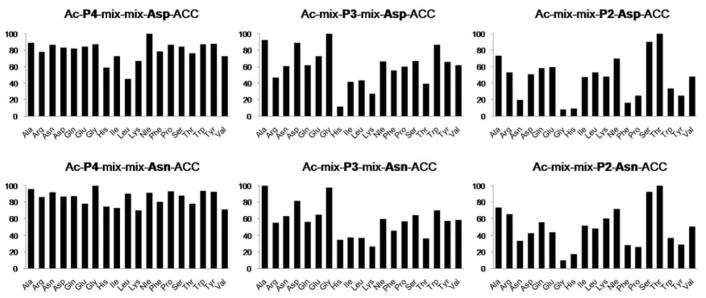
We employed two tetrapeptide combinatorial fluorogenic substrate libraries: Ac-X-X-X-Asp-ACC and Ac-X-X-X-Asn-ACC (Figure S2). Bearing in mind that legumain activity is generated by a pH shift we decided to test legumain specificity at two different pH values, pH 4.5 (Asp specific) and 5.8 (Asn specific) (Figure S3). We found that legumain cleaved P1-Asn substrates approximately 20–30 times more efficient than the P1-Asp counterparts. Kinetic analysis of both libraries revealed that legumain preferred Thr (100%) in the P2 position, however other amino acid residues (Ser, Ala, Val, Nle) were also recognized (30–70% of Thr). The P3 specificity depended on the library used for screening. In the P1-Asp library only a few amino acid residues were recognized at a substantial level: Asp (100%), Ala (50%), Gly (46%), and Glu (40%). The P3 position in the P1-Asn library was less specific and 13 out of 20 amino acids were recognized very well (> 60% compared to the best, Gly). The specificity in the P4 position also differed depending on the library; however, in both cases this enzyme showed almost no specificity, which suggested that legumain does not have a well-defined S4 pocket (Figure 1).
Figure 1. P4-P2 legumain substrate specificity profile determined with the use of two fluorogenic substrate libraries (P1-Asp and P1-Asn).
The y-axis represents amino acid activity (optimal residue set to 100%) and the x-axis presents amino acids.
Legumain substrate specificity using the HyCoSuL approach
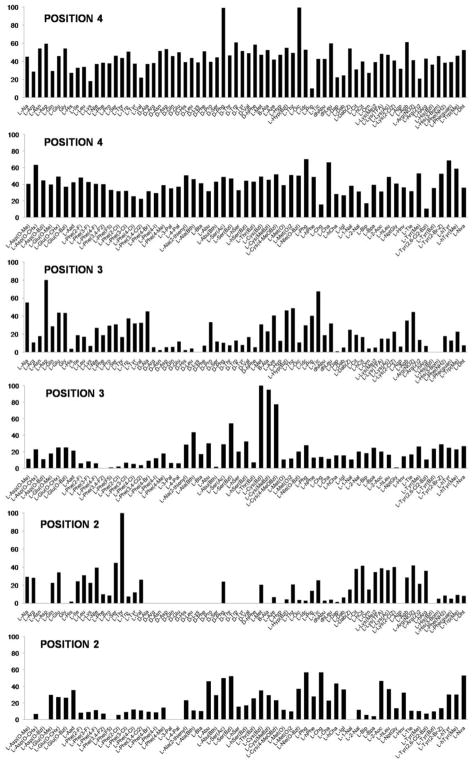
HyCoSuL is a fluorophore-labeled tetrapeptide library that in the P4-P2 positions contains, in addition to natural amino acids, more than 100 unnatural amino acids of different structures and physicochemical properties, thus providing an excellent tool for studying proteolytic enzymes displaying overlapping substrate specificity profiles (i.e. legumain and caspases). The tetrapeptide architecture of HyCoSuL is based on the assumption that a protease would have a well-defined S4 pocket – but this is not the case for legumain (Figure S3) (Dall and Brandstetter, 2013). The best amino acid recognized by legumain in P2 position was still the natural Thr (Figure 2), however, this enzyme also tolerated small/medium-size hydrophobic (Phg, Chg, Abu, Ser(Ac)) or neutral (Cit, hCit and Lys(tfa)) amino acids (for unnatural amino acids abbreviation list, see Table S3). Legumain poorly recognized large hydrophobic amino acids like Bip, Bpa, 1-Nal, substituted Phe and did not recognize D-amino acids. Legumain specificity was more liberal in the P3 position recognizing most amino acids, however, the best were Cys(Bzl), Cys(MeBzl), Cys(Me-O-Bzl) and secondary Tic. The analysis of the P4 position confirmed the observation from the natural amino acids library analysis that legumain had no preferences in the S4 pocket. Indeed, almost all amino acids (even those with D-stereochemistry) were recognized at the same level. The detailed analysis of legumain preferences and the comparison of its specificity matrix with that previously obtained with caspases allowed us to extract some differences and design short peptide sequences recognized only by legumain.
Figure 2. Substrate specificity profile of legumain obtained via HyCoSuL.
The figure presents legumain preferences in P4, P3 and P2 positions. The y-axis represents amino acid activity (optimal residue set to 100%) and the x-axis presents amino acids (natural and unnatural) used for screening.
The influence of pH on legumain activity
Legumain activity is pH-dependent. To examine this phenomenon we synthesized substrates with Asp or Asn in the P1 position (Ac-D-Tyr-Tic-Thr-Asn-ACC and Ac-D-Tyr-Tic-Thr-Asp-ACC), revealing that the optimal pH for legumain activity towards the P1-Asn substrate was 5.8, whereas the pH optimum for the P1-Asp substrate was shifted to 4.5 (Table S2), which is likely due to protonation of the Asp residue at lower pH (Dall and Brandstetter, 2012, 2013, 2016).
Design of legumain selective substrates - CoSeSuL
To design new legumain selective P1-Asp fluorogenic substrates we analyzed legumain and caspase specificity profiles and selected some amino acid candidates for use as substrate building blocks. The best amino acid for legumain in the P2 position is Thr, however, it is also recognized by caspases so we selected the less active, but more specific Ser (Poreba et al., 2014a). Another reason for using Ser at P2 instead of Thr (the best amino acid from library screening) is because inhibitors with Thr at P2 have decreased potency toward legumain for unknown reasons (Sexton et al., 2007).
In the P3 position we selected the preferred Cys(Bzl), Hyp(Bzl) and Tic as the most promising candidates. Finally, we looked towards preferences in the S4 pocket, and exploited the differential requirement for occupancy of this pocket between caspases and legumain. Legumain had no preferences in this position and also tolerated some D-amino acids (D-Arg, D-Tyr and D-Phg), whereas caspases did not (although caspase 8 displayed a very minimal tolerance for these D-amino acids) (Poreba et al., 2014a). After selection of the amino acid building blocks we synthesized several substrates and measured kinetic parameters of their hydrolysis by legumain and caspases in their optimal buffer and pH conditions (Figure 3, Table S3).
Figure 3. Two novel legumain selective ACC-labeled fluorogenic substrates containing Asp in P1 position.
The optimal pH for legumain cleavage at Asp was 4.5, and pH for caspases was 7.4. Standard errors calculated from three measurements were in the range of 5–15%.
Design and binding of legumain-selective activity-based probes
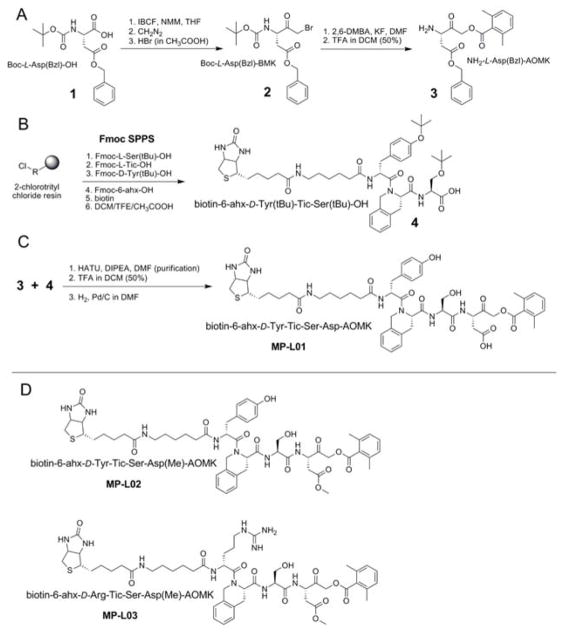
One of the main aims of this work was to find irreversible activity-based probes that could selectively label the active form of legumain in a cellular system where other enzymes (i.e. caspases) are present. Through profiling we obtained several P1-Asp legumain selective substrates, which were in the next step used to design legumain-selective probes. From six substrate candidates two champions were selected: Ac-D-Tyr-Tic-Ser-Asp-ACC and Ac-D-Arg-Tic-Ser-Asp-ACC (Figure 3, and Table S3). These two substrates were highly sensitive to legumain and most selective for this enzyme compared to apoptotic caspases (Table S3). Based on the sequences mentioned above we synthesized three biotin-labeled activity-based probes. The first probe biotin-6-ahx-D-Tyr-Tic-Ser-Asp-AOMK (MP-L01) had a biotin tag on the N-terminus and acyloxymethylketone (AOMK) as a warhead (Figure 4). The aminohexanoic linker (6-ahx) was used, as it was shown previously that this spacer increases the potency of inhibitors toward endogenous legumain (Sexton et al., 2007). The kinetic analysis with recombinant enzymes revealed that this probe was very potent towards legumain (pH 5.8), and showed slight off-target activity against caspases when used at pH 7.4 and elevated concentrations (Figure 4).
Figure 4. Synthesis and structures of the legumain selective activity based probes.
The synthesis of MP-L01 probe (panels A-C) is presented in details, and probes with methylated P1-Asp (MP-L02 and MP-L03; panel D) were synthesized in a similar manner using NH2-Asp(Me)-AOMK instead of NH2-Asp(Bzl)-AOMK. MP-L01 has a free aspartic acid, whereas MP-L02 and MP-L03 have an aspartic acid methyl ester at P1. The only difference between two methylated probes is P4 position (D-Tyr in MP-L02 and D-Arg in MP-L03).
Two other legumain-selective probes were synthesized with methylation in the P1 position (biotin-6-ahx-D-Tyr-Tic-Ser-Asp(Me)-AOMK (MP-L02) and biotin-6-ahx-D-Arg-Tic-Ser-Asp(Me)-AOMK (MP-L03)), since methylation of carboxylic acid residues is a common strategy to improve cell permeability (Figure 4). Once methylated probes enter the cell, endogenous esterases might remove the methyl group and make the probe ready for enzyme targeting. The use of D-Arg in the P4 position of MP-L03 was designed to enhance probe solubility, allowing it to be used at a higher concentration. Addition of MP-L01, -L02 and -L03 (0–10 μM) to equal amounts of lysates (3.5 μg total protein) from the stable monoclonal legumain over-expressing M38L cell line (Smith et al., 2012) revealed that all three probes bound and inhibited active legumain (intermediate 46 kDa and mature 36 kDa forms) in a concentration-dependent manner (Figure 5; Figure S4). The potency of MP-L01 was approximately 100-fold higher compared to either methylated probes (MP-L02 or -L03), which showed similar legumain binding and inhibition. These observations were reflected in both legumain activity measurements (Figure 5A) and visualized by co-migration of legumain and each probe on immunoblots (Figure 5B; Figure S4). Interestingly, although equal amount of lysate was used in the incubations, addition of increasing concentrations of probes indicated an accumulation of mature legumain (36 kDa), as well as the intermediate 46/47 kDa forms (Figure S4, lower panels). None of the probes bound the proform of legumain in the cell lysate. Previously, expression and increased processing of cathepsin B and L from single chain to double chain active forms have been found in the legumain over-expressing M38L cells (Smith et al., 2012). However, no immunobands corresponding to cathepsins were detected by the probes, suggesting no probe cross-reactivity with cathepsins (Figure 5B; Figure S4).
Figure 5. Direct probe binding and inhibition of legumain in cell lysates.
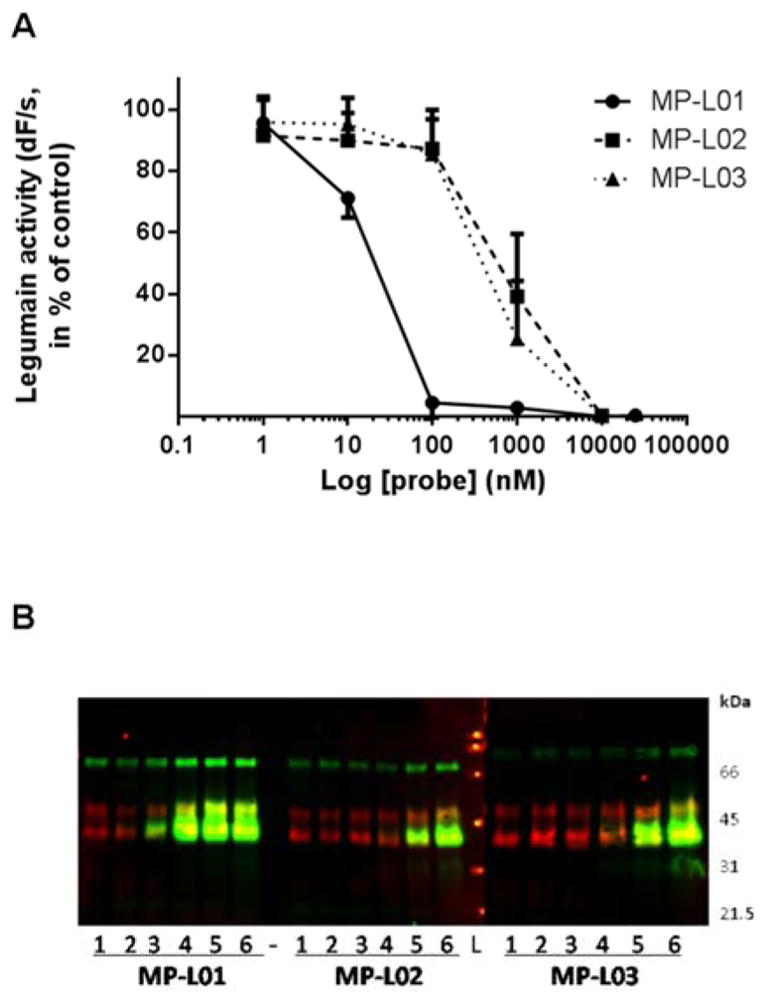
MP-L01, -L02 or -L03 (0–10 μM) was added to equal volumes of M38L cell lysate (3.5 μg total protein) and incubated in legumain assay buffer pH 5.8 (total volume 50 μl) for 30 min at 30°C. A. Residual legumain activity measurements as % of untreated controls. Note the x-axis is logarithmic. B. Detection of legumain (red), probes (green) and overlay (yellow) by immunoblotting (upper panels). β-tubulin (50 kDa) was used as loading control (lower panels). Lane 1, control; lane 2, 1 nM probe; lane 3, 10 nM probe; lane 4, 100 nM probe; lane 5, 1 μM probe; lane 6, 10 μM probe; -, empty lane; L, Broad Range SDS-PAGE Standard Ladder (BioRad). Representative overlay blots are shown, whereas individual blots are shown in Figure S5. n=3–7.
The most potent MP-L01 probe (0–100 μM) was incubated with the M38L lysate, and legumain activity in the lysate showed a dose-dependent decrease with increasing probe concentration (Figure S4A). Binding of probe to active legumain (36 kDa) was confirmed by immunoblotting (Figure S4B). Secreted prolegumain (56 kDa) in serum-free conditioned M38L cell media (CM) was allowed to autoactivate at pH 4.0 to the active intermediate legumain form (46 kDa), which was found to bind MP-L01 in a similar manner to mature active legumain (36 kDa) at pH 5.8 (Figure S4C). Autoactivation of recombinant human prolegumain was also performed at pH 4.0, showing similar MP-L01 binding and inhibition (not shown). Importantly, the probe did not bind either cellular, secreted (Figure S4B, C) or recombinant prolegumain (56 kDa). Using probe detection by streptavidin a high molecular weight biotinylated protein (approximately 80 kDa) was detected in the cell lysate, but not in the conditioned medium, and no proteins of the size of other active cysteine proteases like cathepsins (23–30 kDa) or caspases (18–23 kDa).
Determination of the inhibition rate constants (kobs/I) of MP-L01, MP-L02, and MP-L03 was performed with autoactivated recombinant human legumain (R&D Systems) using Z-Ala-Ala-Asn-AMC as substrate (Table S4). Km for the Z-Ala-Ala-Asn-AMC legumain substrate used in these calculations as well as in the cell experiment analyses was determined to be 25.7 μM. Comparison of the kobs/I values (by the method of (Salvesen and Nagase, 2001) for recombinant legumain revealed that the MP-L01 probe had an inhibition rate constant approximately 100-fold higher than the two methylated probes (MP-L02 and -L03), which correlated with the observed legumain inhibition pattern in M38L cell lysate – where the unmethylated MP-L01 probe inhibited the enzyme activity more efficiently (Figure 5A).
Selectivity of legumain activity based probes in living cells
Intracellular levels of the three probes were studied by treating M38L cells for 24 h with 0–100 μM probe. Parallel wells were harvested in either legumain lysis buffer (pH 5.8) or SDS-PAGE sample buffer. SDS-PAGE sample buffer was used to avoid possible false positives resulting from unspecific binding of probes to cell surfaces or well plastic and subsequent binding to legumain after cell lysis. Lysates in each buffer were analyzed by immunoblotting and showed similar results, confirming cell entrance and probe binding to legumain in the cells before lysis. All three probes were detected by immunoblotting at probe concentrations ≥ 10 μM (Figure 6A, Figure S5). As with direct addition of probes to cell lysate (Figure 5), treatment of living cells resulted in probe binding to and accumulation of mature active legumain (36 kDa). Furthermore, probe binding and inhibition of legumain resulted in an accumulation and decreased autoprocessing of prolegumain (56 kDa; Figure 6A, Figure S5). As revealed by probe incubation directly in cell lysates (Figure 5) or with whole cells (Figure 6, Figure S5), as well as kinetic analyses using pure enzymes (Table 1), none of the probes bound to caspases, indicating legumain specificity. High molecular weight proteins (>80 kDa) detected by streptavidin were most pronounced in the samples harvested in SDS-PAGE sample buffer since direct lysis in this buffer allowed loading of higher total protein concentrations on the gels. Legumain activity measurements of the samples in legumain lysis buffer reflected the immunoblotting observations, suggesting that the probes entered, bound and inhibited intracellular legumain with a similar potency (Figure 6B). Whether the methylated probes were demethylated by intracellular esterases before legumain binding is probable but there is no formal proof.
Figure 6. MP-L01, -L02 and –L03 bind and inhibit legumain in living cells.
Legumain over-expressing M38L cells (25.000 cell/well; 48-well plate) were incubated with the probes (0–100 μM) for 24 h before parallel wells were harvested in either 50 μl legumain lysis buffer or SDS-PAGE sample buffer (Figure S5) and analyzed by immunoblotting. A. One representative immunoblot of lysates in legumain lysis buffer from cells incubated with MP-L01 is shown detecting probe binding (left panel, green in right overlay panel), legumain (middle panel, red in the right overlay panel) and overlay panel (right). Equal amount of lysates (13.3 μl) were loaded per lane. Blots for all probes in both buffers, see Figure S5. Lane 1, control; lane 2, 1 μM probe; lane 3, 10 μM probe; lane 4, 25 μM probe; lane 5, 50 μM probe; lane 6, 75 μM probe; lane 7, 100 μM probe; L, Broad Range SDS-PAGE Standard Ladder (BioRad). B. Legumain activity per μg total protein in all probe-treated cell lysates. Normalized values ± SEM are presented. n=3–6.
Table 1. Selectivity of MP-L01 towards legumain compared to caspases.
Blank entries (−) designate no observable inhibition. The enzymes were incubated with MP-L01 probe for 30 min at 37°C. Optimal enzymes buffers (pH 5.8 for legumain, and pH 7.4 for caspases) were used. The experiment was performed in triplicate and average results are presented (S.D. < 10%).
| MP-L01, nM | % enzyme inhibition (enzyme concentration 10 nM) | |||||||
|---|---|---|---|---|---|---|---|---|
| Legumain | Casp-2 | Casp-3 | Casp-6 | Casp-7 | Casp-8 | Casp-9 | Casp-10 | |
| 320 | 100 | - | 14 | - | - | 9 | 7 | 28 |
| 160 | 100 | - | 6 | - | - | - | - | 21 |
| 80 | 100 | - | - | - | - | - | - | 6 |
| 40 | 99 | - | - | - | - | - | - | - |
| 20 | 94 | - | - | - | - | - | - | - |
| 10 | 95 | - | - | - | - | - | - | - |
| 5 | 53 | - | - | - | - | - | - | - |
To investigate the minimum time needed for probe cell entrance and detection, a time-course (0–24 h) was conducted, showing maximal detection of the MP-probes (MP-L01, -L02, -L03) after 8–24 h of incubation (Figure S6). The P1 methylated probes seemed to be detectable in the cells slightly earlier than the non-methylated one. Overall, the selected 24 h incubation for the dose-response experiments was reasonable and practical, although 8–12 h incubation seemed to be sufficient. In this experiment, the caspase-3/-7 activity based probe biotin-6-ahx-DEVD-AOMK (DEVD) was included and incubation of M38L cells with DEVD-probe and harvesting in SDS-sample buffer showed that this probe did not bind any intracellular proteins in these cells or was not cell permeable. Low permeability was probably due to three carboxylic groups which are deprotonated and making the probe hydrophilic. Surprisingly, cells incubated with DEVD-probe and harvested in legumain lysis buffer showed that this probe bound to and inhibited active legumain (36 kDa), probably due to probe attachment to the well plastic or cell membrane and binding after lysis (not shown). Thus, the inhibition rate constants (kobs/I) for DEVD-probe was measured and calculated (Table S4), showing that this probe was inhibiting recombinant legumain approximately 10-fold better than the methylated legumain probes (MP-L02 and -L03) but with 20-fold lower efficiency than the best and non-methylated legumain probe (MP-L01).
The use of MP-L01 in apoptosis models
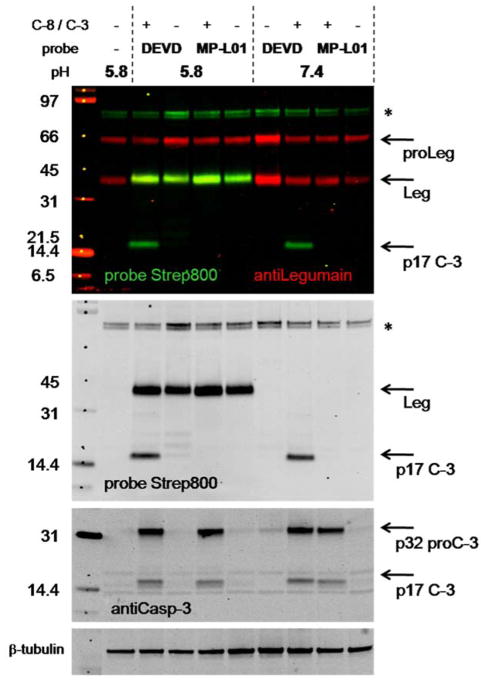
To investigate in detail the MP-L01 selectivity for legumain versus caspases and comparing with the caspase-3 biotin-6-ahx-DEVD-AOMK probe (DEVD), we used two different apoptosis models. In the first model, M38L cells were cotransfected with caspase-8 and its physiological substrate caspase-3 to obtain a maximal apoptotic signal. After 24 hours cells were harvested in legumain lysis buffer (pH 5.8) or caspases lysis buffer (pH 7.4), incubating with MP-L01 or DEVD-probes and immunoblotting (Figure 7). MP-L01 only binds legumain, while DEVD-probe labels both legumain and caspase-3. Legumain binding of MP-L01 was only detected at pH 5.8 and not at 7.4, which is in agreement with decreased activity of legumain above pH 6 (Chen et al., 1997). In control cells (non cotransfected) only legumain activity was detected. As a second model we employed cytochrome c-mediated programmed in vitro apoptosis (Denault et al., 2007; Poreba et al., 2014a). Hypotonic extracts from HEK293 FreeStyle cells were activated by adding 10 mM cytochrome c and 1 mM dATP at 37°C, driving caspase-9, -3 and -7 activation. Subsequently, aliquots of the activated cell extract were directly incubated with MP-L01 or DEVD-probe (0–1 μM) in either legumain or caspase activity buffer (pH 5.8 and pH 7.2, respectively) for 5 or 30 min at 30°C. Immunoblotting showed that procaspase-3 (35 kDa) was activated to the 17 and 12 kDa forms (Figure S7). Whereas the DEVD-probe strongly bound active caspase-3 both at pH 5.8 and 7.2 at all concentrations tested, MP-L01 only weakly bound to caspase-3 (Figure S7). When incubating the probes (10 nM) with 50 nM recombinant auto-activated legumain (46 kDa) or active caspase-3 (17 kDa), legumain was bound by MP-L01 at pH 5.8 and not at pH 7.2, whereas the DEVD probe bound caspase-3 at both pH values (Figure S7).
Figure 7. MP-L01 does not bind caspases in caspases-cotransfected M38L cells.
Apoptosis was induced in M38L cells (500.000/6-well) by cotransfection of caspase-8 and caspase-3 DNA (C-8/C-3) for 24h before harvesting in legumain (pH 5.8) or caspase (pH 7.4) lysis buffer. Aliquots of lysates were incubated with MP-L01 or biotin-6-ahx-DEVD-AOMK (DEVD) probes (1 μM) at pH 5.8 or 7.4 for 30 min at 30°C before addition of SDS-PAGE sample buffer and immunoblotting. Equal amount of lysates (20 μl) were loaded per lane. The immunoblots show legumain (red) and probe (green) overlay (top panel), as well as individual blots of MP-L01 and DEVD probes (detected by streptavidin). Full-length (p32) and active caspase-3 (p17) were detected with caspase-3 antibody. β-tubulin serves as loading control (n=2). Asterisks indicate background bands of unknown identity.
DISCUSSION
Unnatural (non-proteinogenic) amino acids offer an excellent platform for the design of new substrates and activity based probes for protease investigations. The broad structural and chemical diversity of these amino acids is especially useful when analyzing proteases displaying overlapping substrate specificity. Recently we reported that the HyCoSuL approach (substrate libraries employing natural and unnatural amino acids) is suitable for development of tetrapeptide substrates distinguishing apoptotic caspases within the group (Poreba et al., 2014a). In the present study we took advantage of the HyCoSuL to determine the substrate specificity profile of human legumain. We found that the best recognized amino acid in the P2 position is natural Thr, however other small amino acids were also well tolerated. The P3 position was more liberal recognizing most amino acids, however, the best were cysteine-benzyl derivatives. Importantly, the P4 position displayed no preferences, tolerating almost all amino acids, including those with D-stereochemistry. Based on these results we concluded that the first two positions in substrates (P2 and P1) are crucial for legumain activity (Table S1), and the P3 position could be important for legumain selectivity. Because P4 had a wide tolerance we were able to employ the counter selection strategy (CoSeSuL) to select for probes that would not react with caspases and showed exquisite selectivity for legumain. To test this hypothesis, in the next step we used the P4 and P3 legumain specificity matrixes to obtain the first P1-Asp legumain selective substrates that were not recognized by apoptotic caspases (Figure 3), and to design a sequence-based legumain-selective biotin-labeled activity based probe (MP-L01) (Figure 4). This probe was equipped with a biotin tag (N-termini) and electrophilic acyloxymethyl group (AOMK) that inhibits and labels active legumain by forming the covalent bond with the active site cysteine residue. We also synthesized two other probes (MP-L02, and MP-L03), with the methylated P1 aspartic acid residue to make the compounds more cell permeable. Using the lysates from the stable monoclonal legumain over-expressing M38L cell line we demonstrated that all three probes bind effectively to active legumain, and do not label neither the prolegumain form, nor cysteine cathepsins. To further explore the utility of our probes in biological studies, we then used them to detect the active legumain in living cells. We demonstrated that all three probes bind and inhibit legumain in M38L cells (Figure 6), however the most potent was the non-P1-Asp-methylated MP-L01, which was thought to be less cell-permeable that MP-L02 and MP-L03. It must be stressed here that the probes’ potency toward legumain in living cells strongly depends on two factors: (1) the probes’ potency and (2) their cell-permeability. Even if P1-Asp-methylated derivatives are more cell-permeable, once they reach the cytosol, they must undergo the P1-Asp demethylation carried out by esterases to function as legumain inhibitors.
Finally, we demonstrated MP-L01 selectivity for legumain versus caspases following in vitro and in cellulo models of apoptotic caspase induction (Figure 7 and Figure S7). After cotransfection of M38L cells with caspase-8 and full length caspase-3 DNA we observed robust activation of caspase-3 using a DEVD-probe, however MP-L01 could not detect such activity even after adding 1μM directly to the lysate. We validated the MP-L01 selectivity by employing a well-established cell-free model of apoptosis, in which executioner caspases are activated through cytochrome c addition. The comparison of MP-L01 and biotin-6-ahx-DEVD-AOMK probes revealed that MP-L01 displays high selectivity toward legumain over caspases.
EXPERIMENTAL PROCEDURES
Preparation of recombinant caspases and legumain
The detailed description of expression and purification of caspases can be found elsewhere (Stennicke and Salvesen, 1999). The detailed protocol for cloning, expression and purification of human legumain can be found in Supplemental Information.
Enzymatic kinetic studies
These studies were carried out using a fMax spectrofluorimeter (Molecular Devices, Sunnyvale, CA, USA) operating in the kinetic mode in 96-well Corning plates (Corning, NY, USA). The excitation wavelength for ACC-labeled substrates was 355 nm, and the emission wavelength was 460 nm (cutoff at 455 nm). Prolegumain was first activated in the following buffer: 100 mM sodium acetate, 10 mM DTT, 1 mM EDTA, pH 4.5. The legumain autoactivation was measured using the fluorogenic substrate Ac-DGTN-ACC. After 150 min legumain was fully active. The active legumain concentration was determined by titration using Z-ATN-AOMK irreversible inhibitor. In the next step active legumain was diluted in legumain assay buffer containing 40 mM citric acid, 1 mM EDTA, 120 mM Na2HPO4, 10 mM DTT, pH 4.5 (buffer 1) or pH 5.8 (buffer 2). To determine whether legumain substrate specificity depended on the pH this enzyme was examined in both buffers. First, legumain substrate specificity was determined using two tetrapeptide libraries (of only natural amino acids, a traditional Positional Scanning Substrate Combinatorial Library (PS-SCL) approach) containing Asp or Asn in P1 position. Kinetic measurements were performed at 37°C. The library concentration in each well was 100 μM, the reaction volume was 100 μL, and the legumain concentration was 80 nM for the P1-Asn library and 350 nM for the P1-Asp library. The total assay time was 15–30 min, and the linear portion of each progress curve (around 10 min) was used to calculate substrate hydrolysis velocity. All experiments were performed at least three times and the results presented as mean values. The standard error for each sample measurement was less than 10%. Finally, the substrate specificity matrix was made based on the RFU/s value for each substrate, setting the highest value from each sublibrary to 100% and adjusting the other substrates accordingly. The precise procedure for the determination of protease substrate specificity profile using the PS-SCL approach can be found elsewhere (Poreba et al., 2014b).
Characterization of legumain specificity using HyCoSuL
Since legumain displayed the same specificity at pH 4.5 and pH 5.8, its full substrate specificity using the Hybrid Combinatorial Substrate Library (HyCoSuL) approach was determined at pH 5.8 (optimal pH for legumain activity). The synthesis and structural characteristic of HyCoSuL has been described previously (Poreba et al., 2014a). This library contains three sublibraries (P4, P3 and P2), and into each sublibrary 109 unnatural amino acids were incorporated. Prolegumain was autoactivated as described in the previous section. The legumain assay buffer was 40 mM citric acid, 1 mM EDTA, 120 mM Na2HPO4, and 10 mM DTT, pH 5.8. The reaction volume was 100 μL, the total final substrate mixture concentration was 100 μM and the legumain concentration in the assay was 350 nM. Kinetic measurements were performed at 37°C and the data analysis was carried out as described above.
Determination of kinetic parameters (kcat, Km, kcat/Km) for new legumain-selective substrates
To determine whether the obtained substrates were legumain-selective and not also hydrolyzed by caspases, their kinetic parameters was determined toward legumain, caspase 3, 6, 7, 8, 9, and 10. The detailed protocol for the determination of these kinetic parameters can be found in Supplemental Information.
Determination of direct legumain binding and inhibition by MP-L01, MP-L02 and MP-L03 in cell lysates
The HEK293 monoclonal legumain over-expressing M38L cell line was used in this study and is thoroughly characterized elsewhere (Smith et al., 2012). Cells were cultured in Dulbecco’s modified essential medium (DMEM) supplemented with 10% fetal bovine serum, L-glutamine and antibiotics (100 U/ml penicillin, 100 μg/ml streptomycin, and 800 μg/ml G418). To obtain conditioned media (CM) for prolegumain autoactivation (see below) the cells were cultured for 4 days in serum-free DMEM before CM harvesting. Cells were washed in PBS before total cell lysates were obtained in a lysis buffer maintaining legumain activity (100 mM sodium citrate, 1 mM disodium-EDTA, 1 % n-octyl-β-glucopyranoside, pH 5.8), three cycles of freezing (−70°C) and thawing (30°C) before centrifugation at 10,000 g for 5 min. Total protein concentrations in the lysates were measured by Pierce BCA Protein Assay Kit and performed according to the manufacturer in a microplate reader (Spectra Max 190, Molecular Devices) measuring absorbance at 562 nm. Bovine gamma globulin (Pierce) was used to establish a standard curve for the calculation of the total protein concentrations.First, aliquots of M38L cell lysate (3.5 μg total protein) was directly added various concentrations (0–10 μM) of the MP-L01, -L02 or –L03 probes and incubated in legumain assay buffer (39.5 mM citric acid, 121 mM Na2HPO4, 1 mM Na2EDTA, 0.1 % CHAPS and 1 mM DTT, total volume 50 μl) at 30°C for 30 min before legumain activity measurements and immunoblotting. Legumain activity measurements were performed on cell lysates using the peptide substrate Z-Ala-Ala-Asn-AMC (Bachem) and a kinetic protocol described elsewhere (Johansen et al., 1999). Increase in fluorescence over 15 min at 30°C was determined in a microplate spectrofluorimeter (Spectra Max Gemini EM, Molecular Devices) using excitation wavelength 355 nm and emission wavelength 460 nm (cut off 455 nm). Gel electrophoresis and immunoblotting of cell lysates and CM were performed using Bolt 4–12 % Bis-Tris Plus Gels (Novex, Life Tech.) and nitrocellulose membranes (0.2 μM; BioRad). Ponceau staining verified equal loading. Membranes were blocked with 2% bovine serum albumin (BSA) in TBST (vol/vol). Legumain was detected by subsequent goat anti-human legumain (1:1000; R&D Systems) and fluorescent donkey anti-goat IRDye 680LT (1:10.000, LI-CORE Biosciences; red) in TBS-T. Fluorescent streptavidin IRDye 800CW (1:10.000, LI-CORE; green) was used to visualize the biotinylated probes and yellow bands indicated legumain and probe comigration. Fluorescence was scanned at 700 nm (red) and 800 nm (green) in an Odyssey fluorescence imaging system (LI-CORE). Secondly, M38L cells were cultured for 4 days in serum-free media and the conditioned medium (CM) containing large amounts of secreted prolegumain was collected and centrifuged for 5 min at 1000 g to remove cell debris contaminations. The supernatant was put on a PD-10 column for sampling in legumain activation buffer (200 mM acetic acid, 4 mM Na2-EDTA, pH 4.0) as previously described (Smith et al., 2012). After incubation and autoactivation at 30°C for 4–6 h, aliquots was added various concentrations of MP-L01, -L02 or -L03 (0–1 μM) and incubated for additional 30 min at 30°C. The final incubates were analyzed by legumain activity measurements and immunoblotting as above. Third, recombinant human prolegumain (2.045 μM; R&D Systems) was autoactivated by incubation in legumain activation buffer (pH 4.0) at 37°Cfor 2 h according to the manufacturer and aliquots (200 nM) was further incubated for 20 min with and without MP-L01 (0–1 μM), before legumain activity measurements and immunoblotting were performed as described.
Determination of inhibition kinetics (kobs/I) for the activity based probes MP-L01, MP-L02 and MP-L03
For the following studies recombinant human prolegumain (rhLeg) was purchased from R&D Systems and autoactivated in legumain activation buffer (pH 4.0) as described above. A constant amount of 2.5 nM enzyme was added various concentrations of probes (at least 5-fold excess) and 100 mM Z-Ala-Ala-Asn-AMC substrate. Probes and substrate were incubated together in legumain assay buffer (pH 5.8) at 30°C for 10 min before addition to the enzyme and fluorescence measurements every 2nd sec for 30 min. The reactions were repeated three times for each probe and kobs/I calculated as previously described (Kasperkiewicz et al., 2014). In addition, Km for legumain cleavage of the legumain substrate Z-Ala-Ala-Asn-AMC was measured using 2.5 nM autoactivated rhLeg and various concentrations of substrate (1.565–200 nM) at the same conditions as above.
Entrance of activity based probes into living cells
M38Lcells (100.000 or 25.000) were seeded in 12- or 48-well plates, respectively, and incubated with various concentrations (0–100 μM) of MP-L01, -L02 or -L03 for 0–24 h. In addition, the caspase activity based probe biotin-6-ahx-DEVD-AOMK was included for comparison. DMSO was added to control cells. The cells were washed in PBS and parallel treated wells were lysed in either legumain lysis buffer (pH 5.8) or SDS-PAGE sample buffer. The cell lysates in lysis buffer were subjected to three circles of freezing-thawing before centrifugation and legumain activity measurements. To ensure complete solubilization in SDS-PAGE sample buffer, these lysates were sonicated before 10 min boiling. All lysate samples were analyzed by immunoblotting as above.
Caspase-8 and full-length caspase-3 cotransfection into M38L cells
To investigate the MP-L01 selectivity toward legumain over caspase-3 and-8 in competitive manner, we cotransfected legumain over-expressing M38L cells with full-length caspase-8 in pcDNA3 (Boatright et al., 2003) and caspase-3 cloned into pcDNA3 with 5′ Ecorl and 3′ Xbal. We seeded M38L cells (500 000 cells/well, 2mL) on 6-well plates. After 24 hours we mixed 2.5μg of caspase-8 plasmid, 2.5μg of caspase-3 plasmid, and 25 μg of Lipofectamine® 2000 (Invitrogen) in 300μL Opti-MEM (OM, Reduced Serum Media, ThermoFisher) buffer and incubated the mixture for 30 min at room temperature. After 30 min, we dropwised the mixture into the cells (300μL per well). We also included a negative control (25 μg of Lipofectamine® 2000 in 300μL Opti-MEM buffer without caspase DNA plasmids). After 24 hours, we harvested cells and labeled the lysates with MP-L01 and DEVD-probes as described in The use of MP-L01 in apoptosis models section. Legumain lysis/reaction buffer: 40mM citric acid, 120 mM Na2HPO4, 1mM EDTA, 1% IGEPAL, 10mM DTT, pH 5.8; caspase lysis/reaction buffer: 10% w/v sucrose, 20mM Pipes, 10mM NaCl, 1mM EDTA, 1% IGEPAL, 10mM DTT, pH 7.4
Selectivity of MP-L01 and DEVD-probe for legumain versus caspases, respectively
To finally demonstrate that MP-L01 was not binding to cellular caspases, cytosolic extracts of FreeStyle 293-F cells (Life Technologies, Grand Island, NY, USA) were obtained in a hypotonic buffer as described previously (Denault et al., 2007). Each 100 μl portion of FreeStyle293-F cytosolic extract was activated by addition of 1 μl of dATP (12mM in water) and 2 μl of horse cytochrome c (1mM in water). Activated extract was then incubated with MP-L01 or DEVD-probe (0–1 μM) for 5 or 30 min in legumain (pH 5.8) or caspase (pH 7.2) activity buffer, respectively, before the incubates were analyzed by immunoblotting using caspase-3 antibody (1:1000; Cell Signaling #9662) and fluorescent donkey anti-rabbit IRDye 680LT (1:10.000, LI-CORE Biosciences; red), in addition to streptavidin IRDye 800CW. Recombinant autoactivated legumain (50 nM) and recombinant human active caspase-3 (50 nM) were incubated with 10 nM probes and included as controls.
Supplementary Material
SIGNIFICANCE.
Using the HyCoSuL approach we determined the substrate specificity of human legumain and in parallel used CoSeSuL to develop new fluorogenic substrates and activity-based probes that selectively target this protease. The utility of the new activity based probe was confirmed by labeling active legumain in lysates from M38L cells and also in living cells, providing a clear path towards investigating legumain activity in cell-based studies. Finally, we showed that our combined HyCoSuL/CoSeSuL approach led to the generation of a legumain probe not binding to caspases, which is of great significance as these enzymes display overlapping substrate specificity. The new compounds developed in this study can be used for tracking active legumain in various biological models in which caspases are also active, and provide for a novel paradigm in the development of highly selective protease probes.
Acknowledgments
This work was supported by Ministry of Science and Higher Education in Poland (grant Iuventus Plus IP2012 040172 to MP, a statutory activity subsidy for the Faculty of Chemistry at Wroclaw University of Technology to MP and MD), University of Oslo, Norway (RS and NNL),the grants P1-140 and P1-0048 from the Research Agency of Slovenia (BT and DT), and by the grant 2011/03/B/ST5/01048 (National Science Center) and FOCUS (Foundation for Polish Science) to MD. MP and PK are beneficiaries of START scholarship from Foundation for Polish Science. This project has received founding from the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No 661187.
Footnotes
Supplemental Information includes Supplemental Experimental Procedures (cloning and expression of legumain; synthesis of substrate library, individual substrates, inhibitors, and activity-based probes), seven figures and four tables can be found with this article online.
AUTHOR CONTRIBUTIONS
M.P., R.S., W.R., G.S.S., and M.D. designed research; M.P., R.S., W.R., N.N.L., P.K., and S.J.S. performed research; M.M., D.T., and B.T. contributed new reagents/analytic tools; M.P., R.S., W.R., N.N.L., P.K., S.J.S., G.S.S., and M.D. analyzed data; and M.P., R.S., G.S.S., and M.D. wrote the paper.
References
- Alvarez-Fernandez M, Barrett AJ, Gerhartz B, Dando PM, Ni J, Abrahamson M. Inhibition of mammalian legumain by some cystatins is due to a novel second reactive site. J Biol Chem. 1999;274:19195–19203. doi: 10.1074/jbc.274.27.19195. [DOI] [PubMed] [Google Scholar]
- Asgian JL, James KE, Li ZZ, Carter W, Barrett AJ, Mikolajczyk J, Salvesen GS, Powers JC. Aza-peptide epoxides: a new class of inhibitors selective for clan CD cysteine proteases. J Med Chem. 2002;45:4958–4960. doi: 10.1021/jm025581c. [DOI] [PubMed] [Google Scholar]
- Bajjuri KM, Liu Y, Liu C, Sinha SC. The legumain protease-activated auristatin prodrugs suppress tumor growth and metastasis without toxicity. ChemMedChem. 2011;6:54–59. doi: 10.1002/cmdc.201000478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boatright KM, Renatus M, Scott FL, Sperandio S, Shin H, Pedersen IM, Ricci JE, Edris WA, Sutherlin DP, Green DR, et al. A unified model for apical caspase activation. Molecular cell. 2003;11:529–541. doi: 10.1016/s1097-2765(03)00051-0. [DOI] [PubMed] [Google Scholar]
- Briggs JJ, Haugen MH, Johansen HT, Riker AI, Abrahamson M, Fodstad O, Maelandsmo GM, Solberg R. Cystatin E/M suppresses legumain activity and invasion of human melanoma. BMC Cancer. 2010;10:17. doi: 10.1186/1471-2407-10-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan CB, Abe M, Hashimoto N, Hao C, Williams IR, Liu X, Nakao S, Yamamoto A, Zheng C, Henter JI, et al. Mice lacking asparaginyl endopeptidase develop disorders resembling hemophagocytic syndrome. Proc Natl Acad Sci U S A. 2009;106:468–473. doi: 10.1073/pnas.0809824105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JM, Dando PM, Rawlings ND, Brown MA, Young NE, Stevens RA, Hewitt E, Watts C, Barrett AJ. Cloning, isolation, and characterization of mammalian legumain, an asparaginyl endopeptidase. J Biol Chem. 1997;272:8090–8098. doi: 10.1074/jbc.272.12.8090. [DOI] [PubMed] [Google Scholar]
- Chen JM, Fortunato M, Barrett AJ. Activation of human prolegumain by cleavage at a C-terminal asparagine residue. Biochem J. 2000;352(Pt 2):327–334. [PMC free article] [PubMed] [Google Scholar]
- Chen JM, Fortunato M, Stevens RA, Barrett AJ. Activation of progelatinase A by mammalian legumain, a recently discovered cysteine proteinase. Biol Chem. 2001;382:777–783. doi: 10.1515/BC.2001.093. [DOI] [PubMed] [Google Scholar]
- Chen JM, Rawlings ND, Stevens RA, Barrett AJ. Identification of the active site of legumain links it to caspases, clostripain and gingipains in a new clan of cysteine endopeptidases. FEBS Lett. 1998;441:361–365. doi: 10.1016/s0014-5793(98)01574-9. [DOI] [PubMed] [Google Scholar]
- Choi SJ, Reddy SV, Devlin RD, Menaa C, Chung H, Boyce BF, Roodman GD. Identification of human asparaginyl endopeptidase (legumain) as an inhibitor of osteoclast formation and bone resorption. J Biol Chem. 1999;274:27747–27753. doi: 10.1074/jbc.274.39.27747. [DOI] [PubMed] [Google Scholar]
- Clerin V, Shih HH, Deng N, Hebert G, Resmini C, Shields KM, Feldman JL, Winkler A, Albert L, Maganti V, et al. Expression of the cysteine protease legumain in vascular lesions and functional implications in atherogenesis. Atherosclerosis. 2008;201:53–66. doi: 10.1016/j.atherosclerosis.2008.01.016. [DOI] [PubMed] [Google Scholar]
- Dall E, Brandstetter H. Activation of legumain involves proteolytic and conformational events, resulting in a context- and substrate-dependent activity profile. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2012;68:24–31. doi: 10.1107/S1744309111048020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dall E, Brandstetter H. Mechanistic and structural studies on legumain explain its zymogenicity, distinct activation pathways, and regulation. Proc Natl Acad Sci U S A. 2013;110:10940–10945. doi: 10.1073/pnas.1300686110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dall E, Brandstetter H. Structure and function of legumain in health and disease. Biochimie. 2016;122:126–150. doi: 10.1016/j.biochi.2015.09.022. [DOI] [PubMed] [Google Scholar]
- Denault JB, Eckelman BP, Shin H, Pop C, Salvesen GS. Caspase 3 attenuates XIAP (X-linked inhibitor of apoptosis protein)-mediated inhibition of caspase 9. Biochem J. 2007;405:11–19. doi: 10.1042/BJ20070288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgington LE, Verdoes M, Ortega A, Withana NP, Lee J, Syed S, Bachmann MH, Blum G, Bogyo M. Functional imaging of legumain in cancer using a new quenched activity-based probe. J Am Chem Soc. 2013;135:174–182. doi: 10.1021/ja307083b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekici OD, Gotz MG, James KE, Li ZZ, Rukamp BJ, Asgian JL, Caffrey CR, Hansell E, Dvorak J, McKerrow JH, et al. Aza-peptide Michael acceptors: a new class of inhibitors specific for caspases and other clan CD cysteine proteases. J Med Chem. 2004;47:1889–1892. doi: 10.1021/jm049938j. [DOI] [PubMed] [Google Scholar]
- Garcia-Calvo M, Peterson EP, Rasper DM, Vaillancourt JP, Zamboni R, Nicholson DW, Thornberry NA. Purification and catalytic properties of human caspase family members. Cell Death Differ. 1999;6:362–369. doi: 10.1038/sj.cdd.4400497. [DOI] [PubMed] [Google Scholar]
- Gawenda J, Traub F, Luck HJ, Kreipe H, von Wasielewski R. Legumain expression as a prognostic factor in breast cancer patients. Breast Cancer Res Treat. 2007;102:1–6. doi: 10.1007/s10549-006-9311-z. [DOI] [PubMed] [Google Scholar]
- Gotz MG, James KE, Hansell E, Dvorak J, Seshaadri A, Sojka D, Kopacek P, McKerrow JH, Caffrey CR, Powers JC. Aza-peptidyl Michael acceptors. A new class of potent and selective inhibitors of asparaginyl endopeptidases (legumains) from evolutionarily diverse pathogens. J Med Chem. 2008;51:2816–2832. doi: 10.1021/jm701311r. [DOI] [PubMed] [Google Scholar]
- Hashimoto S, Suzuki T, Dong HY, Yamazaki N, Matsushima K. Serial analysis of gene expression in human monocytes and macrophages. Blood. 1999;94:837–844. [PubMed] [Google Scholar]
- Haugen MH, Johansen HT, Pettersen SJ, Solberg R, Brix K, Flatmark K, Maelandsmo GM. Nuclear legumain activity in colorectal cancer. PLoS One. 2013;8:e52980. doi: 10.1371/journal.pone.0052980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James KE, Gotz MG, Caffrey CR, Hansell E, Carter W, Barrett AJ, McKerrow JH, Powers JC. Aza-peptide epoxides: potent and selective inhibitors of Schistosoma mansoni and pig kidney legumains (asparaginyl endopeptidases) Biol Chem. 2003;384:1613–1618. doi: 10.1515/BC.2003.179. [DOI] [PubMed] [Google Scholar]
- Johansen HT, Knight CG, Barrett AJ. Colorimetric and fluorimetric microplate assays for legumain and a staining reaction for detection of the enzyme after electrophoresis. Analytical biochemistry. 1999;273:278–283. doi: 10.1006/abio.1999.4221. [DOI] [PubMed] [Google Scholar]
- Kasperkiewicz P, Poreba M, Snipas SJ, Parker H, Winterbourn CC, Salvesen GS, Drag M. Design of ultrasensitive probes for human neutrophil elastase through hybrid combinatorial substrate library profiling. Proc Natl Acad Sci U S A. 2014;111:2518–2523. doi: 10.1073/pnas.1318548111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato D, Boatright KM, Berger AB, Nazif T, Blum G, Ryan C, Chehade KA, Salvesen GS, Bogyo M. Activity-based probes that target diverse cysteine protease families. Nat Chem Biol. 2005;1:33–38. doi: 10.1038/nchembio707. [DOI] [PubMed] [Google Scholar]
- Kembhavi AA, Buttle DJ, Knight CG, Barrett AJ. The two cysteine endopeptidases of legume seeds: purification and characterization by use of specific fluorometric assays. Arch Biochem Biophys. 1993;303:208–213. doi: 10.1006/abbi.1993.1274. [DOI] [PubMed] [Google Scholar]
- Lecaille F, Muno D, Kominami E, Ishidoh K. Proteinases participating in the processing and activation of prolegumain in primary cultured rat macrophages. Biol Chem. 2004;385:511–516. doi: 10.1515/BC.2004.060. [DOI] [PubMed] [Google Scholar]
- Lee J, Bogyo M. Development of near-infrared fluorophore (NIRF)-labeled activity-based probes for in vivo imaging of legumain. ACS Chem Biol. 2010;5:233–243. doi: 10.1021/cb900232a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Bogyo M. Synthesis and evaluation of aza-peptidyl inhibitors of the lysosomal asparaginyl endopeptidase, legumain. Bioorg Med Chem Lett. 2012;22:1340–1343. doi: 10.1016/j.bmcl.2011.12.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Liu Q, Su Q, Wei C, Lan B, Wang J, Bao G, Yan F, Yu Y, Peng B, et al. Effects of legumain as a potential prognostic factor on gastric cancers. Med Oncol. 2013;30:621. doi: 10.1007/s12032-013-0621-9. [DOI] [PubMed] [Google Scholar]
- Liu C, Sun C, Huang H, Janda K, Edgington T. Overexpression of legumain in tumors is significant for invasion/metastasis and a candidate enzymatic target for prodrug therapy. Cancer Res. 2003;63:2957–2964. [PubMed] [Google Scholar]
- Loak K, Li DN, Manoury B, Billson J, Morton F, Hewitt E, Watts C. Novel cell-permeable acyloxymethylketone inhibitors of asparaginyl endopeptidase. Biol Chem. 2003;384:1239–1246. doi: 10.1515/BC.2003.136. [DOI] [PubMed] [Google Scholar]
- Luo Y, Zhou H, Krueger J, Kaplan C, Lee SH, Dolman C, Markowitz D, Wu W, Liu C, Reisfeld RA, et al. Targeting tumor-associated macrophages as a novel strategy against breast cancer. J Clin Invest. 2006;116:2132–2141. doi: 10.1172/JCI27648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoury B, Mazzeo D, Li DN, Billson J, Loak K, Benaroch P, Watts C. Asparagine endopeptidase can initiate the removal of the MHC class II invariant chain chaperone. Immunity. 2003;18:489–498. doi: 10.1016/s1074-7613(03)00085-2. [DOI] [PubMed] [Google Scholar]
- Mathieu MA, Bogyo M, Caffrey CR, Choe Y, Lee J, Chapman H, Sajid M, Craik CS, McKerrow JH. Substrate specificity of schistosome versus human legumain determined by P1-P3 peptide libraries. Mol Biochem Parasitol. 2002;121:99–105. doi: 10.1016/s0166-6851(02)00026-9. [DOI] [PubMed] [Google Scholar]
- Mattock KL, Gough PJ, Humphries J, Burnand K, Patel L, Suckling KE, Cuello F, Watts C, Gautel M, Avkiran M, et al. Legumain and cathepsin-L expression in human unstable carotid plaque. Atherosclerosis. 2010;208:83–89. doi: 10.1016/j.atherosclerosis.2009.07.022. [DOI] [PubMed] [Google Scholar]
- McLuskey K, Mottram JC. Comparative structural analysis of the caspase family with other clan CD cysteine peptidases. Biochem J. 2015;466:219–232. doi: 10.1042/BJ20141324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G, Matthews SP, Reinheckel T, Fleming S, Watts C. Asparagine endopeptidase is required for normal kidney physiology and homeostasis. FASEB J. 2011;25:1606–1617. doi: 10.1096/fj.10-172312. [DOI] [PubMed] [Google Scholar]
- Morita Y, Araki H, Sugimoto T, Takeuchi K, Yamane T, Maeda T, Yamamoto Y, Nishi K, Asano M, Shirahama-Noda K, et al. Legumain/asparaginyl endopeptidase controls extracellular matrix remodeling through the degradation of fibronectin in mouse renal proximal tubular cells. FEBS Lett. 2007;581:1417–1424. doi: 10.1016/j.febslet.2007.02.064. [DOI] [PubMed] [Google Scholar]
- Niestroj AJ, Feussner K, Heiser U, Dando PM, Barrett A, Gerhartz B, Demuth HU. Inhibition of mammalian legumain by Michael acceptors and AzaAsn-halomethylketones. Biol Chem. 2002;383:1205–1214. doi: 10.1515/BC.2002.133. [DOI] [PubMed] [Google Scholar]
- Ohno Y, Nakashima J, Izumi M, Ohori M, Hashimoto T, Tachibana M. Association of legumain expression pattern with prostate cancer invasiveness and aggressiveness. World J Urol. 2013;31:359–364. doi: 10.1007/s00345-012-0977-z. [DOI] [PubMed] [Google Scholar]
- Ovat A, Muindi F, Fagan C, Brouner M, Hansell E, Dvorak J, Sojka D, Kopacek P, McKerrow JH, Caffrey CR, et al. Aza-peptidyl Michael acceptor and epoxide inhibitors--potent and selective inhibitors of Schistosoma mansoni and Ixodes ricinus legumains (asparaginyl endopeptidases) J Med Chem. 2009;52:7192–7210. doi: 10.1021/jm900849h. [DOI] [PubMed] [Google Scholar]
- Poreba M, Drag M. Current strategies for probing substrate specificity of proteases. Curr Med Chem. 2010;17:3968–3995. doi: 10.2174/092986710793205381. [DOI] [PubMed] [Google Scholar]
- Poreba M, Kasperkiewicz P, Snipas SJ, Fasci D, Salvesen GS, Drag M. Unnatural amino acids increase sensitivity and provide for the design of highly selective caspase substrates. Cell Death Differ. 2014a;21:1482–1492. doi: 10.1038/cdd.2014.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poreba M, Szalek A, Kasperkiewicz P, Drag M. Positional scanning substrate combinatorial library (PS-SCL) approach to define caspase substrate specificity. Methods Mol Biol. 2014b;1133:41–59. doi: 10.1007/978-1-4939-0357-3_2. [DOI] [PubMed] [Google Scholar]
- Powers JC, Asgian JL, Ekici OD, James KE. Irreversible inhibitors of serine, cysteine, and threonine proteases. Chem Rev. 2002;102:4639–4750. doi: 10.1021/cr010182v. [DOI] [PubMed] [Google Scholar]
- Qiu J, Ai L, Ramachandran C, Yao B, Gopalakrishnan S, Fields CR, Delmas AL, Dyer LM, Melnick SJ, Yachnis AT, et al. Invasion suppressor cystatin E/M (CST6): high-level cell type-specific expression in normal brain and epigenetic silencing in gliomas. Lab Invest. 2008;88:910–925. doi: 10.1038/labinvest.2008.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawlings ND, Barrett AJ, Bateman A. Using the MEROPS Database for Proteolytic Enzymes and Their Inhibitors and Substrates. Curr Protoc Bioinformatics. 2014;48:1 25 21–21 25 33. doi: 10.1002/0471250953.bi0125s48. [DOI] [PubMed] [Google Scholar]
- Rozman-Pungercar J, Kopitar-Jerala N, Bogyo M, Turk D, Vasiljeva O, Stefe I, Vandenabeele P, Bromme D, Puizdar V, Fonovic M, et al. Inhibition of papain-like cysteine proteases and legumain by caspase-specific inhibitors: when reaction mechanism is more important than specificity. Cell Death Differ. 2003;10:881–888. doi: 10.1038/sj.cdd.4401247. [DOI] [PubMed] [Google Scholar]
- Sadaghiani AM, Verhelst SH, Bogyo M. Tagging and detection strategies for activity-based proteomics. Curr Opin Chem Biol. 2007;11:20–28. doi: 10.1016/j.cbpa.2006.11.030. [DOI] [PubMed] [Google Scholar]
- Salvesen GS, Nagase H. Inhibition of proteolytic enzymes. In: Bonf JS, Beynon RJ, editors. Proteolytic Enzymes: A Practical Approach. 2. Oxford University Press; Oxfors: 2001. pp. 105–130. [Google Scholar]
- Schwarz G, Brandenburg J, Reich M, Burster T, Driessen C, Kalbacher H. Characterization of legumain. Biol Chem. 2002;383:1813–1816. doi: 10.1515/BC.2002.203. [DOI] [PubMed] [Google Scholar]
- Sexton KB, Witte MD, Blum G, Bogyo M. Design of cell-permeable, fluorescent activity-based probes for the lysosomal cysteine protease asparaginyl endopeptidase (AEP)/legumain. Bioorganic & medicinal chemistry letters. 2007;17:649–653. doi: 10.1016/j.bmcl.2006.10.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirahama-Noda K, Yamamoto A, Sugihara K, Hashimoto N, Asano M, Nishimura M, Hara-Nishimura I. Biosynthetic processing of cathepsins and lysosomal degradation are abolished in asparaginyl endopeptidase-deficient mice. J Biol Chem. 2003;278:33194–33199. doi: 10.1074/jbc.M302742200. [DOI] [PubMed] [Google Scholar]
- Smith R, Johansen HT, Nilsen H, Haugen MH, Pettersen SJ, Maelandsmo GM, Abrahamson M, Solberg R. Intra- and extracellular regulation of activity and processing of legumain by cystatin E/M. Biochimie. 2012;94:2590–2599. doi: 10.1016/j.biochi.2012.07.026. [DOI] [PubMed] [Google Scholar]
- Snipas SJ, Stennicke HR, Riedl S, Potempa J, Travis J, Barrett AJ, Salvesen GS. Inhibition of distant caspase homologues by natural caspase inhibitors. Biochem J. 2001;357:575–580. doi: 10.1042/0264-6021:3570575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stennicke HR, Salvesen GS. Biochemical characteristics of caspases-3, -6, -7, and -8. J Biol Chem. 1997;272:25719–25723. doi: 10.1074/jbc.272.41.25719. [DOI] [PubMed] [Google Scholar]
- Stennicke HR, Salvesen GS. Caspases: preparation and characterization. Methods. 1999;17:313–319. doi: 10.1006/meth.1999.0745. [DOI] [PubMed] [Google Scholar]
- Wang L, Chen S, Zhang M, Li N, Chen Y, Su W, Liu Y, Lu D, Li S, Yang Y, et al. Legumain: a biomarker for diagnosis and prognosis of human ovarian cancer. J Cell Biochem. 2012;113:2679–2686. doi: 10.1002/jcb.24143. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.