Abstract
Type 1 diabetes is an autoimmune disease resulting from the destruction of pancreatic-beta cells by the immune system involving innate and adaptive immune cells. Mucosal-associated invariant T (MAIT) cells are innate-like T-cells recognizing bacterial riboflavin-precursor derivatives presented by the MHC-I related molecule, MR1. Since T1D is associated with gut microbiota modification, we investigated MAIT cells in this pathology. In T1D patients and non-obese diabetic mice, we detected MAIT cell alterations, including increased granzyme B production, which occur before disease onset. Analysis of NOD mice deficient for MR1 and therefore lacking MAIT cells revealed a loss of gut integrity, increased anti-islet responses associated with exacerbated diabetes. Altogether our data highlight the role of MAIT cells in the maintenance of gut integrity and the control of anti-islet autoimmune responses. MAIT cell monitoring could represent a new biomarker in T1D while their manipulation may open new therapeutic strategies.
Introduction
Type 1 Diabetes (T1D) is an auto-immune disease characterized by the selective destruction of pancreatic islet β cells producing insulin. The consecutive lack of insulin results in hyperglycemia and requires a life-long insulin therapy1. The physiopathology of T1D involves both innate and adaptive immune systems that are inappropriately activated inducing a loss of self-tolerance and islet destruction2–5. T1D is characterized by the presence of anti-islet autoantibodies and autoreactive T cells. Innate immune cells are involved at various stages of the disease and are particularly important for the initiation of the local immune response in the pancreas and the pancreatic lymph nodes2,4. Recent data have highlighted the role of the intestinal microbiota in T1D by transfer experiments in NOD mice6–9 and gut microbiota differences in children associated with T1D development10–12. Several studies also described gut mucosa alterations in NOD mice and T1D patients13–17.
MAIT cells are innate-like T cells recognizing bacterial metabolites, derived from the synthesis of riboflavin, presented by the monomorphic major-histocompatibility-complex-class-I-related protein MR118–20. MAIT cells typically express an invariant TCRα chain, Vα7.2-Jα33 in humans and Vα19-Jα33 in mice, and produce various cytokines and granzyme B (GzB) that could participate to tissue inflammation and cell death18,21–31. The near absence of MAIT cells in germ-free mice18,32 and their physiological localization at mucosal sites including the gut18,23 suggest a strong interaction with the microbiota. Here for the first time we described MAIT cell alteration in T1D patients and our mouse data reveal the protective role of MAIT cells against T1D. The localization and the function of MAIT cells highlight their key role in the maintenance of gut integrity, thereby controlling the development of autoimmune responses against pancreatic β cells.
Results
Alteration of blood MAIT cell frequency and phenotype in children with recent onset T1D
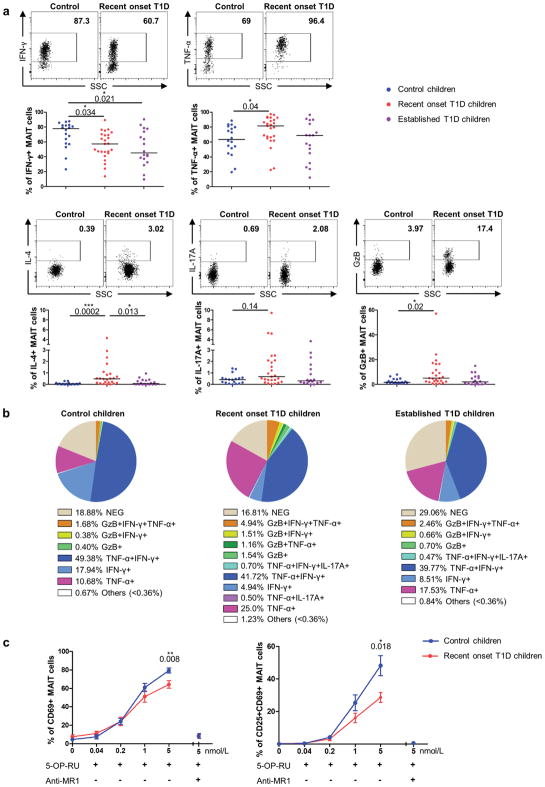
We first began the investigation of MAIT cells in T1D by analyzing MAIT cell frequency and phenotype in fresh peripheral blood samples from children with recent onset T1D and children with established T1D as compared to age-matched control children (Supplementary Tables 1 and 2). MAIT cells can be identified in human blood as CD4− T lymphocyte expressing Vα7.2 TCRα gene segment and CD161high 19,20,24,33,34 (Fig. 1a). MAIT cell frequency and number was decreased (3-fold) in the blood of recent onset T1D children whereas no significant difference was observed in children with established disease as compared to control children (Fig. 1a and Supplementary Fig. 1a). Decreased frequency was observed in both CD8+ and double negative (DN) MAIT cell subsets (Supplementary Fig. 1b). Of note there was no difference in the frequencies of conventional CD4 and CD8 T cells, and of Vα7.2+CD161− T cells between the three children populations confirming that the decrease of MAIT cell frequency at the onset of T1D was not consecutive of changes in other T cell populations nor to down-regulation of the CD161 marker (Supplementary Fig. 2a–b). Analysis of MAIT cell phenotype showed a decreased frequency of MAI T cells expressing tissue recruitment/adhesion molecules (CCR6, CD56) at the onset of the disease, an increased frequency of MAIT cells expressing the activation/exhaustion markers CD25 and PD1, and a decreased frequency of MAIT cells expressing the anti-apoptotic molecule Bcl-2 (Fig. 1b–c). Multi-parametric analysis of MAIT cells in the children with established T1D highlighted the intermediate phenotype of MAIT cells between those from recent onset T1D and control children (Fig. 1c). Interestingly in recent onset children the frequency of MAIT cells expressing migratory CCR6+ or anti-apoptotic Bcl-2 molecules were positively correlated with the frequency of MAIT cells (Supplementary Fig.3). These data suggest that decreased blood MAIT cell frequency could reflect their migration to inflamed tissues and/or their death by apoptosis subsequent to their activation.
Figure 1.
Alteration of blood MAIT cell function in children with recent onset T1D
Cytokine and GzB production by fresh blood MAIT cells was analyzed after PMA-ionomycin stimulation. MAIT cells from children with recent onset T1D produced less IFN-γ, whereas their production of TNF-α, IL-4, and GzB was increased as compared with MAIT cells from control children (Fig. 2a–b). Multi-parametric analysis of cytokines and GrzB production by MAIT cells also showed an intermediate status of blood MAIT cells from children with established T1D, between those from control children and recent onset T1D children, as already observed for MAIT cell surface phenotype (Fig. 2b). We next analyzed the ability of MAIT cells to respond to specific TCR activation by the ligand 5-OP-RU20. Upon stimulation, MAIT cells from control children up-regulated CD69 and CD25 activation markers. Addition of blocking MR1 mAb confirms that this activation was TCR-dependent. Interestingly, MAIT cell activation was significantly reduced in children with recent onset T1D (Fig. 2c). Together, these results highlight functional alteration of MAIT cells in children with recent onset T1D.
Figure 2.
Association between MAIT cell alterations and clinical characteristics
We next investigated potential links between phenotype and functional alterations of MAIT cells and clinical characteristics of children with recent onset T1D (Supplementary Tables 1 and 2). Interestingly, the frequency of GzB+ MAIT cells was negatively associated (r=−0.71, P<0.0001) with children’s age at diagnosis (Fig. 3a), which is in agreement with the current view that T1D is more aggressive in the youngest children35,36. We speculate that production of GzB by MAIT cells, reflecting their cytotoxic potential, could be involved in the physiopathology of T1D. Other MAIT cell parameters, such as their frequency, CCR6 and Bcl-2 expression, were also associated with the age at diagnosis (Supplementary Fig. 4). Such correlations between MAIT cell parameters and age were not observed in controls and children with established T1D (data not shown). Production of GzB by MAIT cells inversely correlated with HbA1c level at the onset of the disease (Fig. 3a). Indeed, a more aggressive disease associated with sustained MAIT cell abnormalities suggests a shorter time of hyperglycemia before the onset thereby lower levels of HbA1c36.
Figure 3.
To further explore the link between MAIT cell parameters and clinical characteristics, 15 of the children with recent onset T1D were further analyzed one year later. Although MAIT cell frequency among T cells remain to a similar level after one year of insulin, both CCR6+ and Bcl-2+ MAIT cell frequencies significantly increased. Conversely the frequencies of CD25+, PD1+, IL-17A+, and to some extent GzB+, MAIT cells decreased to levels observed in the control children (Fig. 3b). This longitudinal analysis strengthened the data obtained in the transversal analysis showing that MAIT cell phenotype was more similar to controls in the children under insulin therapy than at disease onset.
MAIT cells as a new biomarker of T1D
Canonical analysis was performed to compare MAIT cell alterations in the three groups of children (controls, recent onset and established T1D). This analysis revealed that MAIT cell parameters were sufficient to discriminate the three groups of children analyzed (Fig. 3c). Moreover a statistical regression analysis identified four surface markers that define a predictive model for the diagnosis of the disease tested on the ROC curve (Fig. 3d).
Importantly, we confirmed in another cohort of children from Milano that frequency and phenotype alteration of MAIT cell was observed in recent onset T1D as compared to age-matched control children (Supplementary Table 3 and supplementary Fig. 5). However technical difficulties impacting CD56 analysis on these frozen cells did not allow to applying the predictive model.
Finally, to test whether MAIT cell alterations could be detected before the onset of diagnosis, we characterized MAIT cells in adults at risk to develop T1D defined as direct relatives of T1D patients with at least two positive autoantibodies (Supplementary Table 4). As compared to the control group, there were increased frequencies of CD25+ and PD1+ MAIT cells and a trend toward a lower CCR6+ MAIT cell frequency, even though the number of individuals analyzed were limited (Fig. 3e). Altogether our data in patients suggest that MAIT cells represent a new biomarker in T1D and they could play a role in the pathogenesis of T1D. Therefore we investigated MAIT cells in mouse models, which allow analysis in tissues at different stages of disease development and could be manipulated to determine whether MAIT cells are involved in T1D physiopathology.
MAIT cell characterization in NOD mice
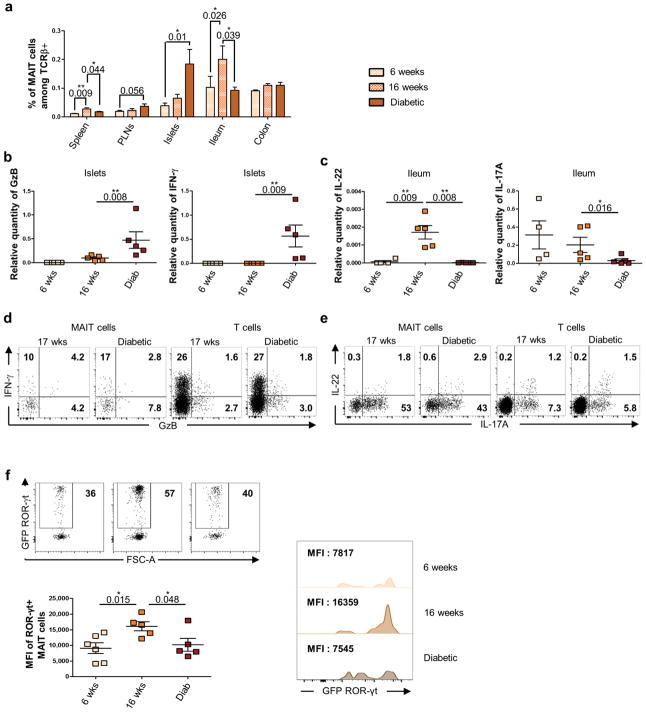
The NOD mouse is the most studied animal model of T1D because it shares many features with the human disease3,4, thus we investigated whether any of the MAIT cell alterations observed in T1D patients were evident in this model. MAIT cells were characterized, in 10 weeks old NOD and C57BL/6 mice, using mouse-MR1 tetramers loaded with the riboflavin derivative 5-OP-RU, whereas control tetramers were loaded with the non-agonist folate derivative Ac-6-FP20,27,32,37 (Fig. 4a). The frequency and absolute number of MAIT cells was lower in NOD than C57BL/6 mice; 3 to 10-fold lower in spleen and pancreatic lymph nodes (PLN). However MAIT cells were present in pancreatic islets of NOD mice and were more abundant in the ileum of NOD than C57BL/6 mice (Fig. 4b). Of note circulating MAIT cells were below detection limit in the blood from NOD mice. Mouse MAIT cells can be divided in three subsets, CD4+, CD8+ or DN37 (Fig. 4c–d). As previously shown, MAIT cells in peripheral tissues of C57BL/6 mice were mainly DN and expressed CD44 (Fig. 4e supplementary Fig. 6), corresponding to functionally mature MAIT cells32. In contrast, the proportion of DN MAIT cells was lower in NOD than C57BL/6 mice. Analysis of activation markers on MAIT cells from NOD mice revealed that CD69 was highly expressed in pancreatic islets and ileum, and CD25 was elevated in PLN and islets (Fig. 4e–f). While CD69 was also elevated on ileum MAIT cells from C57BL/6 mice, CD25 was not up-regulated in PLN from C57BL/6 mice (Supplementary Fig. 7). Of note CD44 was more expressed on ileal MAIT cells from NOD than C57BL/6 mice. Regarding cytokine production, in all tissues MAIT cells are strong producers of IL-17A and TNF-α, whereas IFN-γ and IL-22 was detected in only few cells (Fig. 4g–h and supplementary Fig. 8). Interestingly, a majority of MAIT cells in PLN and ileum express CD44 and produce large amount of cytokines. As in C57BL/6 mice34,37,38, most MAIT cells in NOD mice express PLZF, ROR-γt and T-bet, transcription factors characteristic of this innate-like T cell lineage23 (Fig. 4i and supplementary Fig. 9). Altogether these data show that spleen MAIT cells are less frequent and mature in NOD than in C57BL/6 mice, however they express an activated phenotype in PLN, islets and ileum from NOD mice.
Figure 4.
MAIT cell accumulation and dual function during diabetes development in NOD mice
We next analyzed MAIT cells in NOD mice, at three stages of disease development, early stage (6–7 weeks old), prediabetic (15–17 weeks old) and diabetic mice (Fig. 5a) These three stages correspond respectively to mild peri-insulitis characterized by moderate infiltration of inflammatory cells around pancreatic islets, to insulitis associated to partial beta-cell destruction and to severe destructive insulitis resulting in insufficient insulin production4,5. During disease progression, MAIT cell frequency increased in pancreatic islets and transiently in spleen and ileum (Fig. 5a). These data show that disease progression is associated to modification of MAIT cell tissue distribution.
Figure 5.
To further explore the function of MAIT cells in T1D, their cytokine production was analyzed in various tissues at the three stages of disease development. MAIT cells were purified by cell-sorter after MR1-tetramer staining, and level of GzB, IFN-γ, IL-17A and IL-22 mRNA was analyzed by RT-qPCR (Fig. 5b–c). Interestingly, in pancreatic islets and ileum, MAIT cell cytokine profiles evolved with disease progression. In islets, MAIT cell production of IFN-γ was detected in diabetic mice, and GzB was already detectable at prediabetic stage and further increased in diabetic mice (Fig. 5b). Such increase of GzB production was not observed in other tissues (data not shown). Both GzB and IFN-γ from MAIT cells could participate to pancreatic β-cell death4. This increased production of IFN-γ and GzB in islets was confirmed at protein levels (Fig. 5d). In ileum from NOD mice, T1D development was associated with decreased level of IL-17A and IL-22 mRNA in MAIT cells at the diabetic stage (Fig. 5c). Diminished IL-17A production by ileal MAIT cells from diabetic NOD mice was confirmed by intra-cellular staining (Fig. 5e). Since ROR-γt is the master transcription factor for IL-17A39 and is involved in IL-22 production40,41, we analyzed ROR-γt expression in MAIT cells using ROR-γt-GFP NOD mice (Fig. 5f). According to the decreased level of IL-17A and IL-22 mRNA in diabetic mice, ROR-γt expression was also impacted at the onset of the disease. Altogether these results suggest that MAIT cells could play a dual role in NOD mice according to their tissue localization, promoting β-cell death in the pancreas whereas participating in gut mucosa homeostasis.
Absence of MAIT cells impacts diabetes development and intestinal homeostasis
To determine the role of MAIT cells in the pathogenesis of T1D, we generated MR1−/− NOD mice, since MR1 is required for thymic development of MAIT cells18,32,33,42. As expected MR1−/− NOD mice lacked MAIT cells, whereas there were no significant differences in the frequency of Foxp3+ CD4 Treg, iNKT and γδT cells in the spleen and PLN from both lines (Fig. 6a and Supplementary Fig. 10). Most interestingly, MR1−/− NOD mice developed exacerbated diabetes compared to their littermate MR1+/− controls indicating a protective role of MAIT cells in T1D development (Fig. 6b). Corroborating incidence results, we observed an elevated frequency of anti-islet CD8+ T cells specific for islet-specific glucose-6-phosphatase catalytic subunit-related protein (IGRP)43 and IFN-γ+ anti-IGRP CD8 T cells, respectively in the PLN and pancreatic islets, from MR1−/− NOD mice compared to their littermate controls (Fig. 6c–d). Since PLN dendritic cells (DC) play a key role in anti-islet T cell priming44,45, DC phenotype was analyzed in both lines of NOD mice. Supporting increased anti-islet T cell response, CD11c+CD11b+CD103+ DC46–48 frequency was elevated in PLN from MR1−/− NOD mice and these DC expressed higher level of MHC class II molecules and CD86 reflecting their more activated status (Fig. 6e). Since gut mucosa integrity controls immune cell activation49–51 and MAIT cells are abundant and activated in this tissue (Fig. 4), we explored gut mucosa of MR1−/− NOD mice. The lack of MAIT cells resulted in a significant increased intestinal permeability evaluated by FITC-dextran level in the blood after oral gavage (Fig. 6f). Reduced mRNA expression of occludin and mucin-2 confirmed defective gut epithelial cell function and histology analysis showed an abnormal mucus accumulation in goblet cells of MR1−/− NOD mice (Fig. 6g–h). These abnormalities were associated with increased lymphoid cell infiltration in the lamina propria (Fig. 6i). Taken together these data highlight the role of MAIT cells in the maintenance of the gut integrity that could control DC activation, the priming of anti-islet autoreactive T cells and thereby the development of T1D.
Figure 6.
Discussion
This study reveals MAIT cell alteration in T1D patients as well as in NOD mice. MAIT cell frequency is lower in the peripheral blood of patients compared to healthy children. Such decreased frequency could reflect their migration from the blood to inflamed tissues as described in other inflammatory and autoimmune diseases24–26,29,52–54 and as supported by their increased frequency in the pancreas of NOD mice during T1D development. Lower frequency of blood MAIT cells could also result from their sustained activation leading to cell exhaustion as indicated by their expression of Bcl-2, CD25 and PD1 and by their defective in vitro response. Of note MAIT cell defect in T1D might not only be a consequence of diabetes development but also might reflect intrinsic differences. Indeed in NOD mice, as compared to C57BL/6 mice, MAIT cells are less frequent and express a less mature phenotype, based on CD44 expression.
Our study in NOD mice points out the different phenotype and function of MAIT cells according to their tissue localization. This heterogeneity of MAIT cells observed in mice might explain the mosaic of MAIT cell populations detected in the blood from control individuals and T1D patients. It would be interesting to further investigate the recirculation of MAIT cells between peripheral tissues and the blood. However study of blood MAIT cells in NOD mice was not performed due to their extremely low frequency.
The analysis of peripheral tissues from NOD mice highlights the role of MAIT cells in two tissues, the pancreas and the gut mucosa. In the pancreas, not only MAIT cell frequency increased with diabetes development but also their production of GzB and IFN-γ, which could participate to the destruction of beta cells4,5. Most interestingly, in the youngest children with recent onset T1D, usually associated to a more aggressive disease35,36, circulating MAIT cells express the highest level of GzB. This parameter has the strongest correlations with clinical characteristics, age at diagnosis and % of HbA1c. Of note, increased GzB production was not observed in other T cell populations (data not shown).
In contrast to the pancreas, in the gut mucosa MAIT cells might play a protective role through their production of IL-17A and IL-22, two key cytokines in intestinal homeostasis40,41,55,56. Moreover the lack of MAIT cells in MR1−/− NOD mice promotes T1D development in association to increased anti-islet pathogenic T cell responses and local DC activation in PLN. This increased pathogenic response is associated with a loss of gut integrity shown by FITC-dextran assay, decreased expression of tight junction proteins and abnormal mucus distribution. These data are reminiscent of recent reports showing a critical link between the gut mucosa and the development of inflammatory and autoimmune diseases including T1D49–51. MAIT cells could act as a sensor of environmental changes by responding to alteration of gut microbiota by modulating the host immune system. Their abundance and activation status (CD69, cytokine production) in the gut could explain the prominent regulatory role of MAIT cells in the intestinal mucosa of NOD mice.
Overall the present study reveals MAIT cell alteration in T1D patients as well as in NOD mice and highlights the protective role of MAIT cells against diabetes, despite their infiltration in the pancreas of NOD mice and their production of GzB and IFN-γ. These data lead us to propose that MAIT cells are at the crossroad between microbiota, inflammation and T1D (Supplementary Fig. 11). Interestingly, MAIT cell alteration in both patients and mice has been detected before the onset of diabetes, indicating that MAIT cell could represent a new biomarker of T1D development. Moreover our data suggesting the protective role of MAIT cells in NOD mice at the level of the gut mucosa could pave the way for the development of new therapeutic strategies based on their local triggering in this tissue at early phase of disease development.
Online Methods
Human samples
Peripheral blood samples were obtained from control children and from T1D children admitted in the Pediatric Endocrinology department of Necker hospital, Paris, France at T1D onset (i.e. within 10 days from first insulin injection), or with established disease. None of the control children had a personal or familial history of T1D or autoantibodies associated with T1D. Non-inclusion clinical following parameters contain: infection during the admission and associated others autoimmune disease. The Ethics Committee (Comité de protection des personnes (CPP) Ile-de-France) approved the clinical investigations and written informed consent was obtained from all the parents. For the Milan cohort, peripheral blood from healthy control subjects and patients at T1D onset (i.e., within 10 days from first insulin injection) were collected. The study was approved by the San Raffaele Hospital Ethic Committee (protocol: DRI-003). At risk subjects were enrolled in the Type 1 Diabetes TrialNet Pathway to Prevention Trial (TN01 trial, former TrialNet Natural History Study)57,58. The overall objective of this study is to perform baseline and repeated assessments over time of the immunologic and metabolic status of individuals who are at risk for T1D (first/second degree relatives of patients with T1D). The study was approved by the San Raffaele Hospital Ethics Committee (protocol: NHPROTOCOL32803 TN01). Our local study was approved by the TrialNet Ancillary Studies Subcommittee. All subjects included in this study signed the informed consent prior to blood donation.
Mice
MR1−/+ NOD mice was generated by backcrosses (>15 times) of MR1−/− C57BL/6J18,33 on the NOD background, 17 insulin-dependent diabetes (Idd) loci associated with susceptibility to T1D have been checked on all chromosomes and particularly on the chromosome 1 in proximity of the mr1 gene as indicated on supplementary Fig. 15. As previously described there is a protective locus close to mr159, which could explain the lower incidence of diabetes in MR1−/+ NOD compared to NOD mice. MR1−/− NOD mice were generated by intercross of MR1−/+ NOD mice and the breeding was performed between MR1−/− and MR1−/+ NOD littermates. Transgenic ROR-γt-GFP NOD mouse was generated by microinjection of ROR-γt-GFP BAC construct60 into NOD embryos. All mice were bred under specific pathogen-free conditions and the study was performed on females. Mice were tested weekly and considered diabetic after two consecutive positive urine glucose tests (DIABUR-TEST 5000, Roche), confirmed by a glycemia > 200 mg/Dl (ACCU-CHEK, Roche). This study was approved by the ethics committee on animal experimentation (APAFIS N°2015102016444419).
Cell preparations
Human peripheral blood mono-nucleated cells (PBMC) of patients from Necker Hospital were isolated from fresh blood samples by Ficoll-Paque (Leucosep) or samples from San Raffaele hospital were defrosted in RPMI with 10% FCS (Fetal Cow Serum). Mouse cells were prepared from different tissues as described below. Mice were sacrificed and pancreas was perfused with 3 mL of a collagenase P solution (0.8 mg/mL, Roche), which was then isolated and set free from surrounding tissues and lymph nodes. Digestion of the pancreas was performed at 37°C for 10 min and was stopped by adding large volume of cold 5% FCS RPMI before extensive washes. Pancreatic islets were then purified on a Ficoll discontinuous gradient. For immunofluorescence analysis, islets were dissociated with a non-enzymatic cell-dissociation solution (Sigma). Miltenyi Lamina Propria Dissociation Kit for mouse was used to prepare epithelial and lamina propria cells. Mice were sacrificed and intestine was removed from mice. Intestine was cleared of feces, fat tissue and Peyer’s patches with HBSS without Ca++ and Mg++ containing 10 mM HEPES solution. After passage through 100 μm filter, the cell suspension was subjected to Percol (GE Hehcare) density gradient of 40% and 80% and the interface between the layers containing lamina propria lymphocytes were collected and suspended in PBS containing 2% FCS and 0.1% azide. For the preparation of mouse dendritic cells from lymph nodes and spleen, mice were sacrificed and organ were cut in small pieces and then digested at 37°C for 30 min with 2 mL of collagenase D (1.0 mg/mL, Roche) solution. Digestion was stopped by adding a large volume of cold 5% FCS RPMI, and cells were washed and filtered on cell strainer (40 μm, BD Falcon) before staining.
Flow cytometry and antibodies
Cells were stained in PBS containing 5% FCS and 0.1% azide. For human PBMC the following antibodies were used: CD3 (OKT3), CD4 (OKT4), Vα7.2 (3C10), CD161 (HP-3G10), CCR6 (G034E3), CD56 (HCD56), CD69 (FN50), Bcl-2 (100), IFN-γ (4S B3), IL17A (BL168), TNF-α (MAb11) mAbs from BioLegend; CD8 (SK1), PD1 (MIH4), CD25 (M-A251), IL4 (8D4-8), granzyme B (GB11) mAbs from BD Biosciences; CD3 (REA), CD161 (REA) mAbs from Miltenyi and CD4 (RPA-T4) mAb from eBiosciences. Data acquisition was performed using BD Biosciences LSRFortessa cytometer or FACS ARIA III cytometer for cells from patients from Necker hospital and Beckman Coulter Gallios for cells from patients from San Raffaele hospital.
Staining of mouse cells was performed with the following antibodies: TCRβ (H57), TCR-γδ (GL3), CD45 (30F11), CD8α (53–6.7), CD103 (M290), CD44 (IM7), I-Ak (10-3.6CD45), CD86 (GL1), IL-17A (TC11-18H10) and IFN-γ (XMG1.2) mAbs, were from BD Biosciences; CD19 (6D5), CD4 (GK1.5), CD11b (M1/70), PLZF (9E12), T-bet (4B10), CD25(PC61), CD69 (HI-2F3), CD304 (3E12), and CXCR3 (SA011F11) mAbs, were from Biolegend; CD11c (N418), CD170 (1RNM44N), F4/80 (BM8), ROR-γt (B2D) and FOXP3 (FJK-16s) mAbs were from eBioscience. Alpha-Galactosylceramide-CD1d tetramer was prepared by the laboratory and coupled to streptavidin-BV421 (Biolegend). NRP-V7 tetramer (IGRP206-214 (KYNKANVFL) reactive T cell) and TUM tetramer (TUM (KYQAVTTTL) reactive T cell, were provided by the National Institutes of Health tetramer core facility. Biotinylated mouse MR1 tetramers loaded with the active ligand (5-OP-RU) were used to specifically identify MAIT cells and biotinylated MR1 tetramers loaded with the non-activating ligand 6-formyl-pterin (6-FP) were used as a negative control. MR1 tetramers were coupled to streptavidin-PE (BD Biosciences) or Streptavidin-BV421 (Biolegend) and cells were stained with tetramers for 45 min at RT before surface staining with mAbs. Data acquisition was performed using a BD Biosciences LSRFortessa or FACS ARIA III cytometer. Flow cytometric analyses were performed with the FlowJo analysis software V10.1 (Tree Star) and the Spice software V5.3 was used for studying the multiparametric combination of cellular staining.
In vitro cell stimulation
For ligand stimulation of human MAIT cells, 5-OP-RU solution was obtained after incubating 1 molar equivalent of 5-ARU with 2 molar equivalent of methylglyoxal (Sigma-Aldrich). Hela cells and PBMC from children controls and children with recent onset T1D were plated at a final concentration of 500 000 cells/mL each on 24-well plate, in RPMI supplemented with 10% FCS from children controls and children with recent onset T1D were plated at a final concentration of 106 cells/mL in RPMI supplemented with 10% FCS. PBMC were stimulated ON with various concentration of 5-OP-RU (0–5 nmol/L). Blocking MR1 (26.5 mAb) from Biolegend was added when indicated at 10 μg/mL. MAIT cell activation was analyzed by flow cytometry.
Intra-cellular staining
For human Bcl-2 and mouse transcription factor staining, after surface staining lymphocytes were resuspended in fixation/permeabilization buffer (Foxp3 staining kit from eBioscience) and incubated at 4°C in the dark then, cells were washed with PERM Wash buffer (eBioscience) and labeled with appropriate mAbs. For cytokine and granzyme B analysis of human MAIT cells, PBMC obtained from fresh samples, were analyzed after stimulation for 6 h at 37°C in RPMI medium supplemented with 10% FCS with PMA (25 ng/ml) and ionomycin (1 μg/ml), in the presence of Brefeldin A (10 μg/ml). For cytokine staining of mouse lymphocytes, cells were stimulated with PMA (10 ng/mL) and ionomycine (1 μg/mL) in the presence of Brefeldin A (10 μg/mL) for 4 h at 37°C (all reagents from Sigma-Aldrich). After surface staining, cells were fixed and permeabilized with Cytofix/Cytoperm kit (BD Biosciences) washed and incubated at 4°C in the dark for 30 min with anti-cytokines.
Isolation of RNA and real-time reverse PCR analysis
Mouse cells were lysed in RLT buffer with 1% β-mercaptoethanol, and mRNA was purified from lysed cells using RNeasy Mini Kit (Qiagen). cDNA was produced using the Superscript III reverse transcriptase (Invitrogen). Quantitative-PCR analysis was performed with SYBR Green (Roche) and analyzed with a LightCycler 480 (Roche). Data were normalized to GAPDH. The stability of GAPDH expression was confirmed by comparison with HPRT mRNA. The primers used for qPCR are described in Supplementary Table 5.
Anti-islet T cell response
Cells from mouse pancreatic islets were recovered and then cultured for 7 days in RPMI medium containing 10% FCS, 1% penicillin/streptomycin and 25 U/mL of human rIL-2. To evaluate peptide-specific reactive T cells, single cell suspensions from islet culture were stimulated for 5 h with IGRP206-214 peptide (the last 4 h in presence of Brefeldine A) and stained with CD45, CD19, TCRβ, CD8 and IFN-γ mAbs.
In vivo analysis of intestinal permeability
Intestinal permeability was determined in MR1−/− and MR1+/− NOD mice by measuring the level of FITC-dextran in the blood. Briefly, mice were water-starved overnight and in the morning, FITC-dextran (40 kDa; Sigma) was administered by oral gavages (44 mg/100 g body weight). Blood was collected 4 h later and centrifuged at 3000 g for 20 min at 4°C. Plasma (50 μL) was diluted volume to volume with PBS to determine fluorescence using a SPARK 10M (TECAN). The concentration was determined using FITC-dextran dilutions.
Alcian blue staining and analysis
Intestinal sections (1 cm in length) of ileum from mice were collected and immediately fixed in 4% paraformaldehyde PBS. Paraffin-embedded sections from ileum were cut into 4 μm slices and stained with Alcian blue periodic acid using standard techniques. Slices were visualized under a light microscope (Leica) and mean intensity of staining were evaluated with the software inForm 2.0.2 and image J.
Statistical analysis
For human studies, statistical tests between two groups were performed using two-tailed Mann-Whitney test and signed-rank Wilcoxon test with Graph Pad Prism. The Kruskal-Wallis test followed by the Wilcoxon rank sum test adjusted with the Holm method and the Spearman correlation test was applied for all the correlation analysis with Software R. A factorial discriminant analysis was performed using the XLSTAT 2016 Software. A logistic regression model was fitted with the PROC CANDISC software (SAS version p.3) then a backward elimination procedure was applied. Prognostic validity of the model was evaluated by the receiver operating characteristic (ROC) curve analysis and measured using the area under the ROC curve (AUC). Statistical analyses were performed using the GraphPad Prism software version 5.00.288 and the R software version 3.2.3. For mouse studies, statistical analysis was performed with Prism software (Graph Pad) using nonparametric tests one-tailed Mann-Whitney or Student’s t-test, as appropriate. Diabetes incidence was plotted according to the Kaplan–Meier method and statistical differences between groups were analyzed using the Gehan-Breslow-Wilcoxon test or the log-rank test. P values were considered statistically significant (*P<0.05, **P<0.01, ***P<0.001)
Supplementary Material
Acknowledgments
We thank all the children and their parents, the adult patients, their physicians, the nurse and the technical staff who helped for this study. We thank Cécile Godot, Annabelle Voltine and Magalie Viaud from Necker hospital for help in children recruitment and administrative tasks. We also thank Li Yu, Sandrine Olivre for technical help, Amine Toubal for critical reading of the manuscript, the mouse facility, Cybio and HistIM facility from Cochin Institute, the National Institutes of Health tetramer core facility for NRP-V7 tetramer, Hélène Fohrer-Ting for help to use SPICE software, Angela Stabilini from San Raffaele, Milano, Italy, for technical help and Raphael Porcher from Hotel Dieu, Paris, France for help to statistical analyses, Paris. Type 1 Diabetes TrialNet is a clinical trials network funded by the National Institutes of Health (NIH) through the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), the National Institute of Allergy and Infectious Diseases (NIAID), the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), the National Center for Research Resources (NCRR), the Juvenile Diabetes Research Foundation International (JDRF), and the American Diabetes Association (ADA). The laboratory is supported by funds from INSERM, CNRS, Université Paris Descartes, ANR-11-IDEX-0005-02 Laboratory of Excellence INFLAMEX, Fondation pour la Recherche Médicale (n° DEQ20140329520) and EFSD/JDRF/Lilly to A.L., Ministry of Research fellowship to O.R., Aide aux Jeunes Diabétiques fellowship to I.N., the Région Ile-de-France, and the Département Hospitalo-Universitaire (DHU) AutHors (Autoimmune and Hormonal Diseases).
Footnotes
Author contributions
OR, IN, JDS, LB performed most of the experiments and data analyses; CT, BK, LC performed experiments; JR, JMC provided mouse MR1 tetramers; OL provided MR1−/− C57BL/6 mice and reagents; MB, MP and JB characterized patients and provided human samples; OR, IN, JDS, LB, AL wrote the manuscript; AL supervised the work.
References
- 1.Atkinson MA, Eisenbarth GS, Michels AW. Type 1 diabetes. Lancet. 2014;383:69–82. doi: 10.1016/S0140-6736(13)60591-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Diana J, et al. Crosstalk between neutrophils, B-1a cells and plasmacytoid dendritic cells initiates autoimmune diabetes. Nat Med. 2013;19:65–73. doi: 10.1038/nm.3042. [DOI] [PubMed] [Google Scholar]
- 3.Anderson MS, Bluestone JA. The NOD mouse: a model of immune dysregulation. Annu Rev Immunol. 2005;23:447–485. doi: 10.1146/annurev.immunol.23.021704.115643. [DOI] [PubMed] [Google Scholar]
- 4.Lehuen A, Diana J, Zaccone P, Cooke A. Immune cell crosstalk in type 1 diabetes. Nat Rev Immunol. 2010;10:501–513. doi: 10.1038/nri2787. [DOI] [PubMed] [Google Scholar]
- 5.Bluestone JA, Herold K, Eisenbarth G. Genetics, pathogenesis and clinical interventions in type 1 diabetes. Nature. 2010;464:1293–1300. doi: 10.1038/nature08933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wen L, et al. Innate immunity and intestinal microbiota in the development of Type 1 diabetes. Nature. 2008;455:1109–1113. doi: 10.1038/nature07336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Markle JGM, et al. Sex differences in the gut microbiome drive hormone-dependent regulation of autoimmunity. Science. 2013;339:1084–1088. doi: 10.1126/science.1233521. [DOI] [PubMed] [Google Scholar]
- 8.Yurkovetskiy L, et al. Gender bias in autoimmunity is influenced by microbiota. Immunity. 2013;39:400–412. doi: 10.1016/j.immuni.2013.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun J, et al. Pancreatic β-Cells Limit Autoimmune Diabetes via an Immunoregulatory Antimicrobial Peptide Expressed under the Influence of the Gut Microbiota. Immunity. 2015;43:304–317. doi: 10.1016/j.immuni.2015.07.013. [DOI] [PubMed] [Google Scholar]
- 10.Kostic AD, et al. The dynamics of the human infant gut microbiome in development and in progression toward type 1 diabetes. Cell Host Microbe. 2015;17:260–273. doi: 10.1016/j.chom.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alkanani AK, et al. Alterations in Intestinal Microbiota Correlate With Susceptibility to Type 1 Diabetes. Diabetes. 2015;64:3510–3520. doi: 10.2337/db14-1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vatanen T, et al. Variation in Microbiome LPS Immunogenicity Contributes to Autoimmunity in Humans. Cell. 2016;165:1551. doi: 10.1016/j.cell.2016.05.056. [DOI] [PubMed] [Google Scholar]
- 13.Alam C, et al. Inflammatory tendencies and overproduction of IL-17 in the colon of young NOD mice are counteracted with diet change. Diabetes. 2010;59:2237–2246. doi: 10.2337/db10-0147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alam C, et al. Effects of a germ-free environment on gut immune regulation and diabetes progression in non-obese diabetic (NOD) mice. Diabetologia. 2011;54:1398–1406. doi: 10.1007/s00125-011-2097-5. [DOI] [PubMed] [Google Scholar]
- 15.Bosi E, et al. Increased intestinal permeability precedes clinical onset of type 1 diabetes. Diabetologia. 2006;49:2824–2827. doi: 10.1007/s00125-006-0465-3. [DOI] [PubMed] [Google Scholar]
- 16.Sapone A, et al. Zonulin upregulation is associated with increased gut permeability in subjects with type 1 diabetes and their relatives. Diabetes. 2006;55:1443–1449. doi: 10.2337/db05-1593. [DOI] [PubMed] [Google Scholar]
- 17.Badami E, et al. Defective differentiation of regulatory FoxP3+ T cells by small-intestinal dendritic cells in patients with type 1 diabetes. Diabetes. 2011;60:2120–2124. doi: 10.2337/db10-1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Treiner E, et al. Selection of evolutionarily conserved mucosal-associated invariant T cells by MR1. Nature. 2003;422:164–169. doi: 10.1038/nature01433. [DOI] [PubMed] [Google Scholar]
- 19.Kjer-Nielsen L, et al. MR1 presents microbial vitamin B metabolites to MAIT cells. Nature. 2012;491:717–723. doi: 10.1038/nature11605. [DOI] [PubMed] [Google Scholar]
- 20.Corbett AJ, et al. T-cell activation by transitory neo-antigens derived from distinct microbial pathways. Nature. 2014;509:361–365. doi: 10.1038/nature13160. [DOI] [PubMed] [Google Scholar]
- 21.Birkinshaw RW, Kjer-Nielsen L, Eckle SBG, McCluskey J, Rossjohn J. MAITs, MR1 and vitamin B metabolites. Curr Opin Immunol. 2014;26:7–13. doi: 10.1016/j.coi.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 22.Dusseaux M, et al. Human MAIT cells are xenobiotic-resistant, tissue-targeted, CD161hi IL-17-secreting T cells. Blood. 2011;117:1250–1259. doi: 10.1182/blood-2010-08-303339. [DOI] [PubMed] [Google Scholar]
- 23.Franciszkiewicz K, et al. MHC class I-related molecule, MR1, and mucosal-associated invariant T cells. Immunol Rev. 2016;272:120–138. doi: 10.1111/imr.12423. [DOI] [PubMed] [Google Scholar]
- 24.Magalhaes I, et al. Mucosal-associated invariant T cell alterations in obese and type 2 diabetic patients. J Clin Invest. 2015;125:1752–1762. doi: 10.1172/JCI78941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Illés Z, Shimamura M, Newcombe J, Oka N, Yamamura T. Accumulation of Valpha7.2-Jalpha33 invariant T cells in human autoimmune inflammatory lesions in the nervous system. Int Immunol. 2004;16:223–230. doi: 10.1093/intimm/dxh018. [DOI] [PubMed] [Google Scholar]
- 26.Serriari NE, et al. Innate mucosal-associated invariant T (MAIT) cells are activated in inflammatory bowel diseases. Clin Exp Immunol. 2014;176:266–274. doi: 10.1111/cei.12277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen Z, et al. Mucosal-associated invariant T-cell activation and accumulation after in vivo infection depends on microbial riboflavin synthesis and co-stimulatory signals. Mucosal Immunol. 2016 doi: 10.1038/mi.2016.39. [DOI] [PubMed] [Google Scholar]
- 28.Le Bourhis L, et al. MAIT cells detect and efficiently lyse bacterially-infected epithelial cells. PLoS Pathog. 2013;9:e1003681. doi: 10.1371/journal.ppat.1003681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jeffery HC, et al. Biliary epithelium and liver B cells exposed to bacteria activate intrahepatic MAIT cells through MR1. J Hepatol. 2016;64:1118–1127. doi: 10.1016/j.jhep.2015.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kurioka A, et al. MAIT cells are licensed through granzyme exchange to kill bacterially sensitized targets. Mucosal Immunol. 2015;8:429–440. doi: 10.1038/mi.2014.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Walker LJ, et al. Human MAIT and CD8αα cells develop from a pool of type-17 precommitted CD8+ T cells. Blood. 2012;119:422–433. doi: 10.1182/blood-2011-05-353789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koay HF, et al. A three-stage intrathymic development pathway for the mucosal-associated invariant T cell lineage. Nat Immunol. 2016;17:1300–1311. doi: 10.1038/ni.3565. [DOI] [PubMed] [Google Scholar]
- 33.Martin E, et al. Stepwise development of MAIT cells in mouse and human. PLoS Biol. 2009;7:e54. doi: 10.1371/journal.pbio.1000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reantragoon R, et al. Antigen-loaded MR1 tetramers define T cell receptor heterogeneity in mucosal-associated invariant T cells. J Exp Med. 2013;210:2305–2320. doi: 10.1084/jem.20130958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leete P, et al. Differential Insulitic Profiles Determine the Extent of β-Cell Destruction and the Age at Onset of Type 1 Diabetes. Diabetes. 2016;65:1362–1369. doi: 10.2337/db15-1615. [DOI] [PubMed] [Google Scholar]
- 36.Komulainen J, et al. Clinical, autoimmune, and genetic characteristics of very young children with type 1 diabetes. Childhood Diabetes in Finland (DiMe) Study Group. Diabetes Care. 1999;22:1950–1955. doi: 10.2337/diacare.22.12.1950. [DOI] [PubMed] [Google Scholar]
- 37.Rahimpour A, et al. Identification of phenotypically and functionally heterogeneous mouse mucosal-associated invariant T cells using MR1 tetramers. J Exp Med. 2015;212:1095–1108. doi: 10.1084/jem.20142110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cui Y, et al. Mucosal-associated invariant T cell-rich congenic mouse strain allows functional evaluation. J Clin Invest. 2015;125:4171–4185. doi: 10.1172/JCI82424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ivanov II, et al. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 40.Dudakov JA, Hanash AM, van den Brink MRM. Interleukin-22: immunobiology and pathology. Annu Rev Immunol. 2015;33:747–785. doi: 10.1146/annurev-immunol-032414-112123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rutz S, Wang X, Ouyang W. The IL-20 subfamily of cytokines--from host defence to tissue homeostasis. Nat Rev Immunol. 2014;14:783–795. doi: 10.1038/nri3766. [DOI] [PubMed] [Google Scholar]
- 42.Seach N, et al. Double positive thymocytes select mucosal-associated invariant T cells. J Immunol Baltim Md 1950. 2013;191:6002–6009. doi: 10.4049/jimmunol.1301212. [DOI] [PubMed] [Google Scholar]
- 43.Amrani A, et al. Progression of autoimmune diabetes driven by avidity maturation of a T-cell population. Nature. 2000;406:739–742. doi: 10.1038/35021081. [DOI] [PubMed] [Google Scholar]
- 44.Diana J, et al. Viral infection prevents diabetes by inducing regulatory T cells through NKT cell-plasmacytoid dendritic cell interplay. J Exp Med. 2011;208:729–745. doi: 10.1084/jem.20101692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Turley S, Poirot L, Hattori M, Benoist C, Mathis D. Physiological beta cell death triggers priming of self-reactive T cells by dendritic cells in a type-1 diabetes model. J Exp Med. 2003;198:1527–1537. doi: 10.1084/jem.20030966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fujimoto K, et al. A new subset of CD103+CD8alpha+ dendritic cells in the small intestine expresses TLR3, TLR7, and TLR9 and induces Th1 response and CTL activity. J Immunol Baltim Md 1950. 2011;186:6287–6295. doi: 10.4049/jimmunol.1004036. [DOI] [PubMed] [Google Scholar]
- 47.Cerovic V, Bain CC, Mowat AM, Milling SWF. Intestinal macrophages and dendritic cells: what’s the difference? Trends Immunol. 2014;35:270–277. doi: 10.1016/j.it.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 48.Murphy TL, et al. Transcriptional Control of Dendritic Cell Development. Annu Rev Immunol. 2016;34:93–119. doi: 10.1146/annurev-immunol-032713-120204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kawano Y, et al. Colonic Pro-inflammatory Macrophages Cause Insulin Resistance in an Intestinal Ccl2/Ccr2-Dependent Manner. Cell Metab. 2016;24:295–310. doi: 10.1016/j.cmet.2016.07.009. [DOI] [PubMed] [Google Scholar]
- 50.Garidou L, et al. The Gut Microbiota Regulates Intestinal CD4 T Cells Expressing RORγt and Controls Metabolic Disease. Cell Metab. 2015;22:100–112. doi: 10.1016/j.cmet.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 51.Costa FRC, et al. Gut microbiota translocation to the pancreatic lymph nodes triggers NOD2 activation and contributes to T1D onset. J Exp Med. 2016;213:1223–1239. doi: 10.1084/jem.20150744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cho YN, et al. Mucosal-associated invariant T cell deficiency in systemic lupus erythematosus. J Immunol Baltim Md 1950. 2014;193:3891–3901. doi: 10.4049/jimmunol.1302701. [DOI] [PubMed] [Google Scholar]
- 53.Magalhaes I, Kiaf B, Lehuen A. iNKT and MAIT Cell Alterations in Diabetes. Front Immunol. 2015;6:341. doi: 10.3389/fimmu.2015.00341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Toubal A, Lehuen A. Lights on MAIT cells, a new immune player in liver diseases. J Hepatol. 2016;64:1008–1010. doi: 10.1016/j.jhep.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 55.Maxwell JR, et al. Differential Roles for Interleukin-23 and Interleukin-17 in Intestinal Immunoregulation. Immunity. 2015;43:739–750. doi: 10.1016/j.immuni.2015.08.019. [DOI] [PubMed] [Google Scholar]
- 56.Lee JS, et al. Interleukin-23-Independent IL-17 Production Regulates Intestinal Epithelial Permeability. Immunity. 2015;43:727–738. doi: 10.1016/j.immuni.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mahon JL, et al. The TrialNet Natural History Study of the Development of Type 1 Diabetes: objectives, design, and initial results. Pediatr Diabetes. 2009;10:97–104. doi: 10.1111/j.1399-5448.2008.00464.x. [DOI] [PubMed] [Google Scholar]
- 58. [Accessed: 25th January 2017];TrialNet - Information for Patients. Available at: https://www.diabetestrialnet.org/PathwayToPrevention/
- 59.Hunter K, et al. Interactions between Idd5.1/Ctla4 and other type 1 diabetes genes. J Immunol Baltim Md 1950. 2007;179:8341–8349. doi: 10.4049/jimmunol.179.12.8341. [DOI] [PubMed] [Google Scholar]
- 60.Lochner M, et al. In vivo equilibrium of proinflammatory IL-17+ and regulatory IL-10+ Foxp3+ RORgamma t+ T cells. J Exp Med. 2008;205:1381–1393. doi: 10.1084/jem.20080034. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.