Abstract
Purpose of review
To examine the results of the Action to Control Cardiovascular Risk in Diabetes (ACCORD)-Lipid trial, particularly the subgroup analyses. It is important, when a study fails to meet its overall primary endpoint, to ensure that interpretation of the results include analyses of subgroups that might benefit from the treatment tested. The goal of this review, therefore, is to provide insight and advice to physicians and healthcare workers treating patients similar to those enrolled in ACCORD.
Recent findings
The recently published results of ACCORD-Lipid trial will be presented upon the background of previous trials that have tested the ability of fibrates to lower cardiovascular risk.
Summary
Although ACCORD-Lipid trial did not provide support for the general addition of fenofibrate to statin-treated patients with type 2 diabetes mellitus (T2DM), it added significantly to the results from fibrate monotherapy trials indicative of benefit from such treatment in subgroups of patients who present with significant dyslipidemia. In particular, ACCORD-Lipid trial, in our view, supports the addition of fenofibrate to statin therapy in patients with T2DM and optimal low-density lipoprotein cholesterol levels but persistent, significant hypertriglyceridemia (>200 mg/dl) and low high-density lipoprotein cholesterol levels (<35–40 mg/dl).
Keywords: coronary heart disease, diabetes, fenofibrate, HDL-cholesterol, triglyceride
Introduction
Fibrates have been used for the treatment of hypertriglyceridemia, with or without concomitant low levels of HDL cholesterol (HDL-C), for more than 40 years. Their efficacy, particularly in individuals with significant elevations in plasma triglycerides, has been well defined. The mechanism underlying the triglyceride-lowering effect of the fibrates has been carefully studied but is less well characterized. Increased fractional catabolism of very low-density lipoprotein (VLDL) triglycerides secondary to increased lipoprotein lipase activity is thought to be the major mechanism of triglyceride lowering, although reduced VLDL secretion (measured by appearance of VLDL apolipoprotein B in the circulation) has also been observed [1–4]. HDL-C increases with fibrate treatment but often in the absence of changes in apolipoprotein A-I. The mechanisms underlying these effects on HDL are also poorly defined; both increased apolipoprotein A-I production and increased fractional clearance have been reported during fibrate treatment [4]. Effects of fibrates on LDL metabolism and LDL-C levels are complex and dependent on the pretreatment levels of VLDL triglycerides [1,5].
Fibrate therapy and cardiovascular outcomes before ACCORD
There is a long history of studies examining the effects of fibrates on cardiovascular disease (CVD) events. Indeed, six major randomized clinical trials examined the impact of fibrate monotherapy on CVD events prior to Action to Control Cardiovascular Risk in Diabetes (ACCORD) [6–11] (Table 1). The impact of fibrate therapy on the primary study outcome, major CVD events, was variable, with three of the studies demonstrating a significant impact on CVD events in the study population as a whole and three showing no significant overall effect. In reviewing these studies, it is apparent that there were significant differences with regard to the population studied in each of these studies. The Helsinki Heart Study (HHS) and the Veterans Administration HDL Intervention Trial (VA-HIT) were conducted exclusively in males and included a low percentage of minority participants, whereas the Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) included a significant number of females. Both HHS and VA-HIT used entry criteria that enhanced recruitment of participants with low HDL-C and hypertriglyceridemia. Additionally, although VA-HIT and HHS included some participants with type 2 diabetes mellitus (T2DM), FIELD was conducted exclusively in patients with T2DM; T2DM was an exclusion criterion in the Bezafibrate Infarction Prevention trial (BIP). On the contrary, VA-HIT, BIP, and HHS included a high percentage of participants with metabolic syndrome. Of course, none of these trials was conducted on the background of statin treatment.
Table 1.
Cardiovascular disease prevention with fibrate therapy
| Trial | Year reported | Drug | CVD risk reduction (primary endpoint) |
|---|---|---|---|
| Coronary Drug Project (CDP) [6] | 1975 | Clofibrate | 9% (NS) |
| World Health Organization (WHO) [7] | 1978 | Clofibrate | 20% (P < 0.05) |
| Helsinki Heart Study (HHS) [8] | 1987 | Gemfibrozil | 34% (P < 0.02) |
| VA HDL Intervention Trial (VA-HIT) [9] | 1999 | Gemfibrozil | 22% (P < 0.006) |
| Bezafibrate Infarction Prevention Study (BIP) [10] | 2000 | Bezafibrate | 7.3% (P = 0.26) |
| Fenofibrate Event lowering and Intervention in Diabetes (FIELD) [11] | 2005 | Fenofibrate | 11% (P = 0.16) |
Randomized Clinical Trials Prior to ACCORD-Lipid. These are the major cardiovascular endpoint trials prior to ACCORD-Lipid that tested the efficacy of fibrate monotherapy to reduce cardiovascular (CVD) events.
Rationale and design of ACCORD-Lipid trial
The ACCORD study examined the effect of intensive treatments of blood glucose, blood pressure, and lipids on CVD events in a population of patients with T2DM who were at high risk for such events [12]. ACCORD-Lipid trial was one of three interventions in the overall ACCORD trial; the results of each intervention were published separately [13,14,15••]. ACCORD-Lipid trial tested the hypothesis that in the context of good glycemic control of patients with T2DM, a strategy that used combination therapy with a fibrate and statin to increase HDL-C and decrease triglyceride in addition to LDL-C lowering would reduce CVD risk more than a strategy that used statins alone to lower LDL-C [15••]. The fibrate used in ACCORD-Lipid trial was fenofibrate and the statin was simvastatin.
In ACCORD-Lipid trial, 5518 patients with T2DM who were treated with simvastatin, 20 or 40 mg/day, were also randomly assigned to receive fenofibrate or placebo. The average follow-up was 4.7 years, and the primary cardiovascular endpoint was the difference in fatal and nonfatal coronary heart disease and stroke between the fenofibrate and placebo groups. There were a number of secondary endpoints as well as 10 predetermined subgroup analyses based on baseline characteristics [15••].
The rationale for ACCORD-Lipid included the high incidence of atherosclerotic CVD in patients with T2DM [16–18] that remains significant despite treatment with either statin or fibrate monotherapy [19–22]. The high prevalence of hypertriglyceridemia and low HDL-C among patients with T2DM [23,24] and the efficacy of combination therapy with a fibrate and statin for this dyslipidemia [25] were additional considerations.
ACCORD-Lipid also allowed a detailed examination of the safety of combined fenofibrate–simvastatin therapy. Reports of myositis and rhabdomyolysis in patients treated with combined statin–fibrate therapy, particularly the combination of statin and gemfibrozil, have led to concerns over the safety of this combination [26,27]. With more than 5000 patients participating, half of whom were assigned to combination treatment with simvastatin and fenofibrate, ACCORD-Lipid represented more than 25 000 patient-years of exposure, allowing for a detailed assessment of the safety of this combination of lipid-altering medications.
Effect of fenofibrate on plasma lipoproteins in ACCORD-Lipid trial
Fenofibrate therapy was associated with a fall in plasma triglyceride levels from 189.0 to 149.0 mg/dl between baseline and the first annual visit, a 21% decrease [15••]. Triglyceride levels remained in this range throughout the follow-up period in the fenofibrate group. Triglyceride levels in the placebo group were only 1.0% lower at the end of the first year, but trended lower over the remainder of the trial such that by the final visit triglycerides were 8.7% lower than baseline. Thus, there was a 13.5% relative difference between placebo and fenofibrate groups at the end of the study. HDL-C increased from 38.0 to 40.4 mg/dl during the first year of treatment, an increase of 6.3% (2.4 mg/dl absolute change) in the fenofibrate treatment group [15••], and this was maintained throughout the follow-up period (range 40.2–41.2 mg/dl). In the placebo group, HDL-C levels increased from 38.2 to 39.4 mg/dl by 1 year, a 3.1% increase from baseline (1.2 mg/dl absolute increase). However, HDL-C levels continued to increase during the course of the full trial and the relative difference in HDL-C between the fenofibrate and placebo groups had narrowed to 1.7% (0.7 mg/dl absolute difference) by the end of the trial [15••]. The differences between fenofibrate and placebo were highly significant for both triglyceride and HDL-C concentrations. Plasma levels of LDL-C were 10.8% lower in both groups at the first annual visit and progressively decreased as statin therapy was intensified over the remainder of the trial (range 77.7–83.0 mg/dl) during the third to seventh annual visits; LDL-C levels did not differ between the fenofibrate and the placebo groups [15••].
Safety and tolerability of combination fenofibrate–simvastatin therapy in ACCORD-Lipid trial
In ACCORD-Lipid, more than 40% of participants reported muscle symptoms during the trial; however, the number reporting such symptoms was essentially identical in both the fenofibrate and placebo groups (40.1 vs 40.5%, respectively). Importantly, such complaints were very rarely associated with elevation in creatine phosphokinase (CPK) in either group (0.04 vs 0.07%, respectively). Myositis reported as an adverse event was also extremely infrequent in both groups (0.1%), as were elevations of CPK greater than 10× upper limit of normal (0.4 and 0.3%, respectively). These data indicate that combination therapy with fenofibrate and simvastatin did not increase the risk of myopathy. There were no signals for other potential adverse effects associated with fibrate therapy. In particular, reports of gallbladder-related events did not differ between the groups and there were no reported thromboembolic events. Elevations of liver enzymes did occur more frequently in the fenofibrate group, but the overall incidence was low (0.6 vs 0.2%).
Moderate, reversible increases in serum creatinine levels, particularly in patients with moderate renal insufficiency, can occur during fenofibrate therapy. The mechanism underlying the increase in plasma creatinine is unknown. In ACCORD-Lipid trial, serum creatinine elevations occurred approximately twice as often in fenofibrate-treated patients compared to patients receiving placebo [15••]. This effect of fenofibrate was also observed in the FIELD trial [11]. However, when creatinine levels were measured 8 weeks after completion of fenofibrate treatment in FIELD, they had returned to levels comparable to those seen in placebo-treated controls [11]. Thus, the effect of fenofibrate upon serum creatinine appeared to be reversible. Similar analyses are being conducted in ACCORD. In addition, in ACCORD-Lipid trial, the postrandomization incidence of microalbuminuria and macroalbuminuria was lower in the fenofibrate treatment group (38.2 and 10.5% vs 41.6 and 12.3% in fenofibrate and placebo-treated participants, respectively; P = 0.01 and 0.03).
Effect of combination therapy on cardiovascular outcomes in ACCORD-Lipid trial
The primary outcome of ACCORD was the occurrence of fatal CVD events, nonfatal myocardial infarction, and stroke. Over an average of 4.7 years of treatment the rate of these CVD events was 2.24% per year in the fenofibrate group vs 2.41% per year in the placebo group (hazard ratio 0.92, 95% confidence 0.79–1.08, P = 0.32) [15••]. Outcomes were similar in the several secondary endpoints, including total mortality. It must be concluded, therefore, that the widespread use of fenofibrate as an adjunct to statin therapy in patients with T2DM at high risk for CVD events is not beneficial.
ACCORD, however, studied a very heterogeneous population of patients, including significant numbers of women, minorities, and participants with a wide range of baseline lipid levels; response to fenofibrate may have differed within each of these groups. In addition, age, the presence of prior CVD, or assignment to intensive vs standard glycemic therapy may have affected outcomes in the fenofibrate–simvastatin group. Therefore, the primary CVD outcome was examined for heterogeneity in response to therapy in 10 prespecified subgroups based on these baseline characteristics [15••]. There was no significant heterogeneity of response in terms of the primary endpoint to fenofibrate treatment in subgroups defined by age, prior CVD, or glycemic therapy arm assignment. Nor was there an indication of heterogeneity of response when the primary outcome was examined by tertiles of baseline LDL-C, or HDL-C, or triglyceride levels, or by baseline HbA1c above and below the median for the entire study cohort. In contrast, there was evidence of heterogeneity of response by gender and race, with men and whites appearing more likely to benefit compared to women and nonwhites [15••] (Table 2). Furthermore, there was a strong suggestion of heterogeneity in response to treatments when participants with dyslipidemia, defined by the combination of baseline plasma triglyceride concentrations in the top third (≥204 mg/dl) and HDL-C in the bottom third (≤34 mg/dl), were compared to all other participants. The CVD event rate was very high in dyslipidemic patients receiving placebo compared to placebo-treated patients who did not have dyslipidemia (17.3 vs 10.1%) and that rate was reduced by 32% with fenofibrate treatment [15••] (Table 2). Thus, in this dyslipidemic subgroup, comprising 17% of the ACCORD-Lipid cohort, combination therapy with fenofibrate and simvastatin appeared to reduce CVD events. Of note, the male–female dichotomy in CVD events was not apparent within the hypertriglyceridemia–low HDL-C subgroup [15••].
Table 2.
Cardiovascular event rates in ACCORD-lipid by sex, race, and baseline lipoproteins
| Subgroup | Fenofibrate [% (N)] | Placebo [% (N)] | Hazard ratio (95% CI) | P value for interaction |
|---|---|---|---|---|
| Overall | 10.5 (2765) | 11.3 (2753) | 0.92 (0.79–1.08) | |
| Sex | ||||
| Female | 9.1 (851) | 6.6 (843) | 1.38 (0.98–1.95) | 0.01 |
| Male | 11.2 (1914) | 13.3 (1910) | 0.82 (0.69–0.99) | |
| Race | ||||
| Nonwhite | 9.7 (856) | 8.2 (888) | 1.15 (0.84–1.57) | 0.09 |
| White | 10.9 (1909) | 12.7 (1865) | 0.84 (0.70–1.02) | |
| Lipoproteins | ||||
| HTG/L-HDLa | 12.4 (485) | 17.3 (456) | 0.69 (0.49–0.97) | 0.06 |
| All Others | 10.1 (2264) | 10.1 (2284) | 1.00 |
Data are percentage of participants experiencing major cardiovascular events and number in group.
HTG/L-HDL = combined hypertriglyceridemia (triglyceride ≥204 mg/dl) and low HDL-C (≤34 mg/dl). Reproduced with permission from ACCORD-Lipid trial [15••].
Subgroup analyses examining interactions or heterogeneity of response to an intervention must always be interpreted with great caution, even when they have been specified at the start of the study. First, there is often insufficient statistical power to observe such interactions. Additionally, the large number of subgroup analyses that are typically performed, when added to the primary and several secondary analyses, requires significant statistical penalties that can negate nominally significant P values. It is clear, therefore, that any interactions observed in such analyses should raise hypotheses that could be tested in future studies. However, in the absence of data from additional studies designed specifically to test hypotheses raised by subgroup analyses, one can potentially gain insights by examining subgroup analyses performed in similar trials. In the case of ACCORD-Lipid trial, and the suggestion that dyslipidemic patients did benefit from combined fenofibrate–simvastatin therapy, we focused on HHS, VA-HIT, BIP, and FIELD.
In HHS, a placebo-controlled primary prevention trial conducted in 4081 asymptomatic Finnish male postal and railway workers, gemfibrozil treatment resulted in a significant 34% reduction in the occurrence of the primary endpoint of myocardial infarction and coronary heart disease death [8]. Post hoc analyses indicated that the benefit of gemfibrozil treatment was greatest among those with hypertriglyceridemia [8,28], particularly in a subgroup of participants with hypertriglyceridemia, low HDL-C, and BMI greater than 26 [29] (Fig. 1, Table 3).
Figure 1. Influence of pretreatment HDL and triglyceride on cardiovascular endpoint response in The Helsinki Heart Study.
Data are incidence of cardiac endpoints by combined categories of triglyceride and HDL-cholesterol at baseline. The P value was derived using Cox regression models with age as a covariate.  , gemfibrozil;
, gemfibrozil;  , placebo. Adapted with permission from [29]. TG, triglycerides.
, placebo. Adapted with permission from [29]. TG, triglycerides.
Table 3.
Comparison of lipid subgroup analyses in fibrate trials
| Study (drug) | CVD reduction (P) entire cohort |
Lipid subgroup criteria | CVD reduction (P) subgroup |
|---|---|---|---|
| Helsinki Heart Study (Gemfibrozil) [8,28,29] | −34% (0.02) | TG ≥204 mg/dl HDL-C <42 mg/dla | −78% (0.002) |
| BIP (Bezafibrate) [10] | −7.3% (0.24) | TG >200 mg/dl | −39.5% (0.02) |
| FIELD (Fenofibrate) [11,30•] | −11% (0.16) | TG ≥204 mg/dl HDL-C = <40 mg/dlb | −27% (0.005) |
| ACCORD (Fenofibrate) [15••] | 8% (0.26) | TG ≥204 mg/dl HDL-C = <34 mg/dl | −31% |
Data are percentage reduction in the occurrence of the primary cardiovascular endpoint of each study respectively in the entire cohort and in a subgroup defined by the presence of low HDL-C and/or hypertriglyceridemia upon entry into the study. TG, triglycerides.
HHS subgroup further defined by the presence of BMI >26 kg/m2.
In FIELD, low HDL-C defined as <40 mg/dl in men and <50 mg/dl for women.
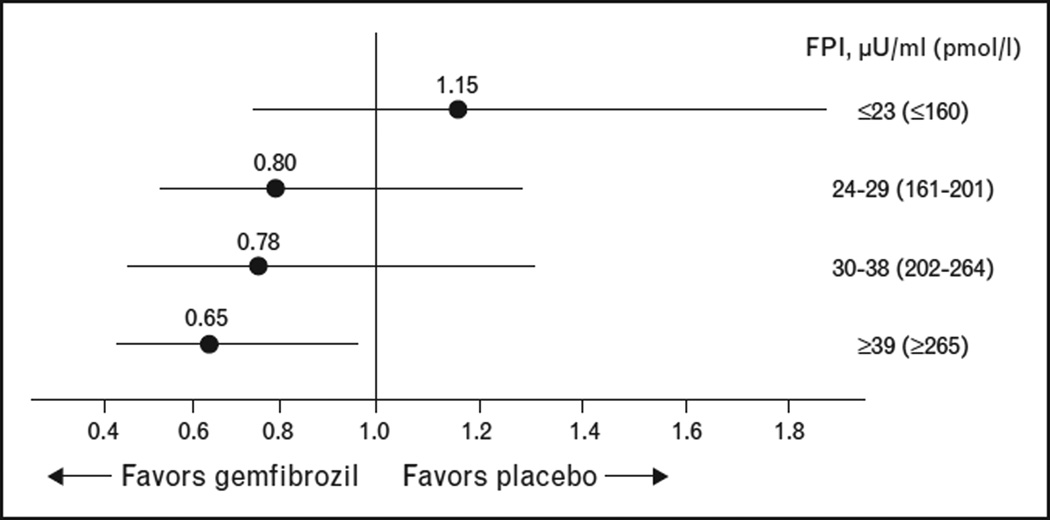
VA-HIT was a placebo-controlled secondary prevention trial of gemfibrozil treatment conducted in 2531 men with low HDL-C as their primary lipid abnormality [9]. Gemfibrozil treatment was associated with a reduction of 22% in the combined primary endpoint of myocardial infarction, coronary heart disease death and stroke following 5 years of gemfibrozil treatment. Post hoc analyses suggested both increased risk and greater response to gemfibrozil among those with extremely low HDL-C (≤33.5 mg/dl) and among those with high triglyceride (>180 mg/dl) [31]. The 769 VA-HIT participants with T2DM experienced a roughly two-fold higher occurrence of CVD events and gemfibrozil use resulted in a significant 32% reduction in major CVD events in those with T2DM [22]; the greatest reduction in CVD events occurred among those within the highest quartile of fasting plasma insulin [22] (Fig. 2). Thus, in VA-HIT, even in the presence of uniformly low HDL-C, the presence of insulin resistance and hypertriglyceridemia identified a subgroup with higher risk and greater risk reduction with fibrate therapy [32].
Figure 2. Fasting plasma insulin as a predictor of risk reduction with gemfibrozil treatment in VA-HIT.
Hazard ratios for major cardiovascular events with gemfibrozil compared to placebo by fasting plasma insulin (FPI quartiles in VA-HIT patients without diabetes (n = 1733). Adapted with permission from [22].
In BIP, which was a secondary prevention trial conducted in 3090 patients (90% men), bezafibrate therapy over 6.2 years resulted in a nonsignificant 7.3% reduction in nonfatal and fatal myocardial infarction and sudden cardiac death. However, those entering the trial with a triglyceride greater than 200 mg/dl experienced a significant 39% reduction in the primary study endpoint [10] (Table 3). Significant benefit was also seen in BIP participants meeting the definition of metabolic syndrome [33]. Thus, in BIP the presence of hypertriglyceridemia and other components of the metabolic syndrome predicted CVD event reduction with bezafibrate therapy.
FIELD was a placebo-controlled trial using fenofibrate in 9795 patients with T2DM, most of whom were classified as primary prevention [11]. In the overall cohort, fenofibrate treatment was associated with a nonsignificant 11% overall reduction in the primary outcome of first myocardial infarction or coronary heart disease death [11]. In subsequent post hoc analyses of the influence of various components of the metabolic syndrome on CHD outcomes in FIELD a subset (20.6%) of participants with the baseline characteristic of combined low HDL-C (<40 mg/dl in men and <50 mg/dl in women) and hypertriglyceridemia (>204 mg/dl) experienced 27% fewer CVD events with fenofibrate treatment [30•] (Table 3).
These post hoc analyses are supported by recent publication-based meta-analyses of fibrate studies which suggested that fenofibrate was significantly more efficacious in individuals with high baseline triglycerides and low HDL levels [34,35].
Conclusion
The post hoc subgroup analyses summarized above, together with the data from a dyslipidemic subgroup defined a priori in ACCORD-Lipid trial, provide consistent evidence supporting the hypothesis that individuals with hypertriglyceridemia and low HDL-C levels can benefit from fibrate monotherapy or fibrate–statin combination therapy. Additional characteristics predictive of benefit from fibrate treatment may be insulin resistance/metabolic syndrome. Thus, in contrast to statins, which effectively reduce CVD risk across a wide range of LDL values, the cardiovascular benefit of fibrates may be concentrated in a specific population of patients, that is those with hypertriglyceridemia and low HDL-C. We believe, therefore, that although ACCORD-Lipid did not provide support for the general addition of fenofibrate to statin-treated patients with T2DM, it added significantly to the results from fibrate monotherapy trials indicative of benefit from such treatment in a significant subgroup of T2DM who present with significant dyslipidemia. In particular, ACCORD-Lipid trial, in our view, supports the addition of fenofibrate to statins in patients with optimal LDL-C levels but persistent significant hypertriglyceridemia (>200 mg/dl) and low HDL-C levels (<35–40 mg/dl).
Key points.
The ACCORD-Lipid trial tested the hypothesis that the addition of a fibrate to lower plasma triglycerides and increase plasma HDL cholesterol levels in statin-treated patients with type 2 diabetes mellitus would provide benefit compared to the use of statin alone to lower LDL cholesterol.
The overall result of ACCORD-Lipid trial was negative; the combination of fenofibrate and simvastatin did not lower the rate of the primary composite endpoint of cardiovascular death, nonfatal myocardial infarction, nonfatal stroke, compared to treatment with simvastatin plus placebo.
A prespecified subgroup analysis indicated that participants with triglyceride levels in the upper third and HDL cholesterol levels in the lower third at baseline (dyslipidemic group) had a much higher rate for the primary outcome than did all other individuals and fenofibrate treatment reduced the primary outcome by 32% in this group.
Acknowledgements
The ACCORD study was supported by the National Heart Lung and Blood Institute (contracts N01-HC-95178, N01-HC-95179, N01-HC-95180, N01-HC-95181, N01-HC-95182, N01-HC-95183, N01-HC-95184, IAAY1-HC-9035, and IAAY1-HC-1010), the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute on Aging, the National Eye Institute, the Centers for Disease Control and Prevention, and General Clinical Research Centers at many sites. The following companies provided study medications, equipment, or supplies: Abbott Laboratories, Amylin Pharmaceutical, AstraZeneca Pharmaceuticals, Bayer Health-Care, Closer Healthcare, GlaxoSmith-Kline Pharmaceuticals, King Pharmaceuticals, Merck, Novartis Pharmaceuticals, Novo Nordisk, Omron Healthcare, Sanofi-Aventis, and Takeda Pharmaceuticals.
Footnotes
A full list of participating ACCORD institutions and investigators is available in Section 20 of Supplementary Appendix 1 of [15••], available with the full text of this article at www.NEJM.org.
M.E.: Consultant (Pfizer, Solvay), Speakers Bureau (Abbott, Merck- Schering Plough).
L.L.: None.
H.G.: Advisory Board (Merck, Merck Schering Plough, BMS/AstraZeneca), Consultant (Merck, Abbott/Astra Zeneca, BMS, Roche, Isis/Genzyme, GlaxoSmithKline, Novatis, Pfizer, Regeneron/SanofiAventis), Research Grants (Merck, ISI/Genzyme, Roche, Astrazeneca), Speakers Bureau (Pfizer).
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Ginsberg HN. Changes in lipoprotein kinetics during therapy with fenofibrate and other fibric acid derivatives. Am J Med. 1987;83:66–70. doi: 10.1016/0002-9343(87)90873-4. [DOI] [PubMed] [Google Scholar]
- 2.Grundy SM, Vega GL. Fibric acids: effects on lipids and lipoprotein metabolism. Am J Med. 1987;83:9–20. doi: 10.1016/0002-9343(87)90866-7. [DOI] [PubMed] [Google Scholar]
- 3.Watts GF, Barrett PH, Ji J, et al. Differential regulation of lipoprotein kinetics by atorvastatin and fenofibrate in subjects with the metabolic syndrome. Diabetes. 2003;52:803–811. doi: 10.2337/diabetes.52.3.803. [DOI] [PubMed] [Google Scholar]
- 4.Shah A, Rader DJ, Millar JS. The effect of PPAR-alpha agonism on apolipoprotein metabolism in humans. Atherosclerosis. 2010;210:35–40. doi: 10.1016/j.atherosclerosis.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 5.Kissebah AH, Alfarsi S, Evans DJ. Low density lipoprotein metabolism in familial combined hyperlipidemia: mechanism of the multiple lipoprotein phenotypic expression. Arteriosclerosis. 1984;4:614–624. doi: 10.1161/01.atv.4.6.614. [DOI] [PubMed] [Google Scholar]
- 6.Clofibrate and niacin in coronary heart disease. JAMA. 1975;231:360–381. [PubMed] [Google Scholar]
- 7.A co-operative trial in the primary prevention of ischaemic heart disease using clofibrate. Report from the Committee of Principal Investigators. Br Heart J. 1978;40:1069–1118. doi: 10.1136/hrt.40.10.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frick MH, Elo O, Haapa K, et al. Helsinki Heart Study: primary-prevention trial with gemfibrozil in middle-aged men with dyslipidemia. Safety of treatment, changes in risk factors, and incidence of coronary heart disease. N Engl J Med. 1987;317:1237–1245. doi: 10.1056/NEJM198711123172001. [DOI] [PubMed] [Google Scholar]
- 9.Rubins HB, Robins SJ, Collins D, et al. Gemfibrozil for the secondary prevention of coronary heart disease in men with low levels of high-density lipoprotein cholesterol. Veterans Affairs High-Density Lipoprotein Cholesterol Intervention Trial Study Group. N Engl J Med. 1999;341:410–418. doi: 10.1056/NEJM199908053410604. [DOI] [PubMed] [Google Scholar]
- 10.Secondary prevention by raising HDL cholesterol and reducing triglycerides in patients with coronary artery disease: the Bezafibrate Infarction Prevention (BIP) study. Circulation. 2000;102:21–27. doi: 10.1161/01.cir.102.1.21. [DOI] [PubMed] [Google Scholar]
- 11.Keech A, Simes RJ, Barter P, et al. Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomised controlled trial. Lancet. 2005;366:1849–1861. doi: 10.1016/S0140-6736(05)67667-2. [DOI] [PubMed] [Google Scholar]
- 12.Buse JB, Bigger JT, Byington RP, et al. Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial: design and methods. Am J Cardiol. 2007;99:21i–33i. doi: 10.1016/j.amjcard.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 13.Gerstein HC, Miller ME, Byington RP, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545–2559. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cushman WC, Evans GW, Byington RP, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362:1575–1585. doi: 10.1056/NEJMoa1001286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15••.Ginsberg HN, Elam MB, Lovato LC, et al. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med. 2010;362:1563–1574. doi: 10.1056/NEJMoa1001282. This paper reports on the results of the ACCORD-Lipid trial which compared treatment of people with type 2 diabetes and high risk for cardiovascular events with either simvastatin alone or simvastatin plus fenofibrate. In the entire cohort of 5518 participants, the addition of fenofibrate to simvastatin was associate with 8% fewer events, a nonsignificant difference compared to the group receiving simvastatin alone. A prespecified subgroup analysis (one of 19) that examined combination therapy in participants with baseline TG greater than 204 and HDL less than 34 mg/dl suggested that fenofibrate plus simvastatin was beneficial compared to simvastatin alone. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Almdal T, Scharling H, Jensen JS, Vestergaard H. The independent effect of type 2 diabetes mellitus on ischemic heart disease, stroke, and death: a population-based study of 13,000 men and women with 20 years of follow-up. Arch Intern Med. 2004;164:1422–1426. doi: 10.1001/archinte.164.13.1422. [DOI] [PubMed] [Google Scholar]
- 17.Stamler J, Vaccaro O, Neaton JD, Wentworth D. Diabetes, other risk factors, and 12-yr cardiovascular mortality for men screened in the Multiple Risk Factor Intervention Trial. Diabetes Care. 1993;16:434–444. doi: 10.2337/diacare.16.2.434. [DOI] [PubMed] [Google Scholar]
- 18.Haffner SM, Lehto S, Ronnemaa T, et al. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998;339:229–234. doi: 10.1056/NEJM199807233390404. [DOI] [PubMed] [Google Scholar]
- 19.Collins R, Armitage J, Parish S, et al. MRC/BHF Heart Protection Study of cholesterol-lowering with simvastatin in 5963 people with diabetes: a randomised placebo-controlled trial. Lancet. 2003;361:2005–2016. doi: 10.1016/s0140-6736(03)13636-7. [DOI] [PubMed] [Google Scholar]
- 20.Shepherd J, Barter P, Carmena R, et al. Effect of lowering LDL cholesterol substantially below currently recommended levels in patients with coronary heart disease and diabetes: the Treating to New Targets (TNT) study. Diabetes Care. 2006;29:1220–1226. doi: 10.2337/dc05-2465. [DOI] [PubMed] [Google Scholar]
- 21.Colhoun HM, Betteridge DJ, Durrington PN, et al. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet. 2004;364:685–696. doi: 10.1016/S0140-6736(04)16895-5. [DOI] [PubMed] [Google Scholar]
- 22.Rubins HB, Robins SJ, Collins D, et al. Diabetes, plasma insulin, and cardiovascular disease: subgroup analysis from the Department of Veterans Affairs high-density lipoprotein intervention trial (VA-HIT) Arch Intern Med. 2002;162:2597–2604. doi: 10.1001/archinte.162.22.2597. [DOI] [PubMed] [Google Scholar]
- 23.Chahil TJ, Ginsberg HN. Diabetic dyslipidemia. Endocrinol Metab Clin North Am. 2006;35:491–510. doi: 10.1016/j.ecl.2006.06.002. vii–viii. [DOI] [PubMed] [Google Scholar]
- 24.Turner RC, Millns H, Neil HA, et al. Risk factors for coronary artery disease in noninsulin dependent diabetes mellitus: United Kingdom Prospective Diabetes Study (UKPDS: 23) BMJ. 1998;316:823–828. doi: 10.1136/bmj.316.7134.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grundy SM, Vega GL, Yuan Z, et al. Effectiveness and tolerability of simvastatin plus fenofibrate for combined hyperlipidemia (the SAFARI trial) Am J Cardiol. 2005;95:462–468. doi: 10.1016/j.amjcard.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 26.Pasternak RC, Smith SC, Jr, Bairey-Merz CN, et al. ACC/AHA/NHLBI clinical advisory on the use and safety of statins. Circulation. 2002;106:1024–1028. doi: 10.1161/01.cir.0000032466.44170.44. [DOI] [PubMed] [Google Scholar]
- 27.Ballantyne CM, Corsini A, Davidson MH, et al. Risk for myopathy with statin therapy in high-risk patients. Arch Intern Med. 2003;163:553–564. doi: 10.1001/archinte.163.5.553. [DOI] [PubMed] [Google Scholar]
- 28.Manninen V, Elo MO, Frick MH, et al. Lipid alterations and decline in the incidence of coronary heart disease in the Helsinki Heart Study. JAMA. 1988;260:641–651. [PubMed] [Google Scholar]
- 29.Tenkanen L, Manttari M, Manninen V. Some coronary risk factors related to the insulin resistance syndrome and treatment with gemfibrozil. Experience from the Helsinki Heart Study. Circulation. 1995;92:1779–1785. doi: 10.1161/01.cir.92.7.1779. [DOI] [PubMed] [Google Scholar]
- 30•.Scott R, O’Brien R, Fulcher G, et al. Effects of fenofibrate treatment on cardiovascular disease risk in 9,795 individuals with type 2 diabetes and various components of the metabolic syndrome: the Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) study. Diabetes Care. 2009;32:493–498. doi: 10.2337/dc08-1543. In this paper, several post hoc analyses of the subgroups of participants in the FIELD study were examined regarding effects of fenofibrate vs placebo on cardiovascular outcomes. Groups with low HDL, high triglycerides, and the combination of low HDL (<40 mg/dl) and high triglycerides (>200 mg/dl) had significantly fewer events on fenofibrate than placebo. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Robins SJ, Collins D, Wittes JT, et al. Relation of gemfibrozil treatment and lipid levels with major coronary events: VA-HIT: a randomized controlled trial. JAMA. 2001;285:1585–1591. doi: 10.1001/jama.285.12.1585. [DOI] [PubMed] [Google Scholar]
- 32.Robins SJ, Rubins HB, Faas FH, et al. Insulin resistance and cardiovascular events with low HDL cholesterol: the Veterans Affairs HDL Intervention Trial (VA-HIT) Diabetes Care. 2003;26:1513–1517. doi: 10.2337/diacare.26.5.1513. [DOI] [PubMed] [Google Scholar]
- 33.Tenenbaum A, Motro M, Fisman EZ, et al. Bezafibrate for the secondary prevention of myocardial infarction in patients with metabolic syndrome. Arch Intern Med. 2005;165:1154–1160. doi: 10.1001/archinte.165.10.1154. [DOI] [PubMed] [Google Scholar]
- 34.Jun M, Foote C, Lv J, et al. Effects of fibrates on cardiovascular outcomes: a systematic review and meta-analysis. Lancet. 2010;375:1875–1884. doi: 10.1016/S0140-6736(10)60656-3. [DOI] [PubMed] [Google Scholar]
- 35.Sacks FM, Carey VJ, Fruchart JC. Combination lipid therapy in type 2 diabetes. N Engl J Med. 2010;363:692–694. doi: 10.1056/NEJMc1006407. author reply 694–695. [DOI] [PubMed] [Google Scholar]