Abstract
Background
The optimal target in the dorsolateral prefrontal cortex (DLPFC) for treating depression with repetitive transcranial magnetic stimulation (rTMS) remains unknown. Better efficacy has been associated with stimulation sites that are 1) more anterior and lateral and 2) more functionally connected to the subgenual cingulate. Here we prospectively test whether these factors predict response in individual patients.
Methods
A primary cohort (Boston, N = 25) with medication-refractory depression underwent conventional open-label rTMS to the left DLPFC. A secondary cohort (Michigan, N = 16) underwent 4 weeks of sham followed by open label rTMS for non-responders (N = 12). In each patient, the location of the stimulation site was recorded with frameless stereotaxy. Connectivity between each patient’s stimulation site and the subgenual cingulate was assessed using resting state fcMRI from a cohort of healthy subjects (N = 1000), and confirmed using connectivity from depression patients (N = 38).
Results
In our primary cohort, antidepressant efficacy was predicted by stimulation sites that were both more antero-lateral (r = .51, p < .01) and more negatively correlated with the subgenual cingulate (r = −.55, p < .005). However, subgenual connectivity was the only independent predictor of response and the only factor to predict response to active (r = −.52, p < .05) but not sham rTMS in our secondary cohort.
Conclusions
This study provides prospective validation that functional connectivity between an individual’s rTMS cortical target and the subgenual cingulate predicts antidepressant response. Implications for improving the cortical rTMS target for depression are discussed.
Keywords: depression, dorsolateral prefrontal cortex, resting state functional connectivity, subgenual cingulate, TMS, transcranial magnetic stimulation
Introduction
Neuroimaging studies suggest that activity in the dorsolateral prefrontal cortex (DLPFC) is decreased in patients with major depression and increases with antidepressant treatment (1–9). Consistent with these findings, repetitive transcranial magnetic stimulation (rTMS) applied over the left DLPFC for multiple weeks is an effective treatment for medication-resistant depression (10–13). However, antidepressant efficacy of rTMS varies greatly across individual patients (14–16).
Patient characteristics such as age, degree of treatment-resistance, illness duration, and baseline neuroimaging findings likely contribute to this variability (17–22). However, these factors cannot be easily modified to improve TMS response. Factors that are modifiable include stimulation site, frequency, intensity, and number of pulses (23). Of these, the location of stimulation site has received particular attention (24–27). Based on the first rTMS studies for depression (28; 29), and subsequent randomized controlled trials (12; 13), most clinics identify the left DLPFC stimulation site by identify the site over the motor cortex that produces a finger twitch, then moving 5–6 cm anterior to this site along the scalp surface (24; 28–31). Due to individual differences in anatomy, this method leads to different patients being stimulated at different brain locations and heterogeneity in clinical response (24; 25; 32; 33). Other targeting methods have been proposed (31; 33–36), but are limited by the fact that the optimal stimulation site within the DLPFC remains unknown.
To this end, two properties of the stimulation site have been associated with improved antidepressant response. First, patients are more likely to respond if stimulated at sites more anterior and lateral within the DLPFC (24; 25; 33). This observation led to an equation to predict antidepressant response based on the anatomical coordinates of a patient’s stimulation site (25). Second, patients are more likely to respond if stimulated at sites more functionally connected to the subgenual cingulate (27). This limbic region is thought to be hyperactive in depression and decreases in subgenual activity have been associated with antidepressant response across a range of different therapies (1; 4; 9; 37–42). Consistent with these findings, rTMS sites resulting in higher clinical efficacy are more negatively correlated (i.e. anticorrelated) with the subgenual cingulate based on resting state functional connectivity (23). This functional relationship is present independent of whether one uses connectome data from normal subjects or patients with depression (27; 43), although connectivity differences between groups have been reported (20; 44–46).
These two observations regarding stimulation site have led to suggestions that the rTMS site for depression should be moved more antero-lateral within the DLPFC or to a site more anticorrelated with the subgenual cingulate (24–27; 35; 47). These suggestions are not mutually exclusive and, depending on the distribution of the stimulation sites, can be correlated (27). However, before either can be considered, these retrospective observations must be confirmed prospectively. Further, it remains unknown whether these factors are independent predictors, predictive of all or only some antidepressant symptoms, and specific to active (versus sham) stimulation.
In the present study, we used neuronavigation to record the precise patient-specific location of stimulation across two independent cohorts undergoing rTMS for treatment of depression. The first cohort (Boston, N = 25) was prospectively collected specifically to test two existing hypotheses regarding the relationship between stimulation site and antidepressant response (25; 27). A second cohort (Michigan, N = 16) was retrospectively analyzed to evaluate the relationship to sham stimulation.
Methods and Materials
Full methodological details can be found in the online supplemental information. Briefly, data from two cohorts of patients with medication-resistant major depressive disorder treated with 4–7 weeks of daily rTMS applied over the left DLPFC were included. The primary cohort was prospectively enrolled and received conventional open-label rTMS at the Berenson-Allen Center at Beth Israel Deaconess Medical Center (BIDMC), Boston. Outcome was measured using the Beck Depression Inventory (BDI)-II. The secondary cohort was treated at the Department of Psychiatry at University of Michigan, Ann Arbor and underwent 4 weeks of blinded, sham stimulation. Some of these patients then received open-label active rTMS similar to our primary cohort. These patients were the sham arm of a larger study collected for a different purpose (NCT01900314), the results of which will be published separately. Outcome was assessed using the Montgomery-Asberg Depression Rating Scale (MADRS).
In both cohorts, the precise location of individual stimulation sites was recorded using a frameless neuronavigation system (Fig. 1). In the first cohort, the stimulation site was identified using the 5.5 cm rule per routine clinical practice at our center (30). Specifically, the site over motor cortex that evokes a maximal finger twitch was identified then the coil was moved 5.5 cm anterior to this site as measured along the scalp surface (also see S1). In the second cohort, the stimulation site was identified based on perfusion changes during a working memory task. Regions of interest reflecting the electric field induced by TMS at these sites were generated based on a prior model (26; 43). Resting state functional connectivity of each subject’s stimulation site with the subgenual cingulate was assessed using a normative connectome dataset from 1000 healthy subjects (48). Because functional connectivity differences can be seen in depression (20; 44–46), results were replicated using connectome dataset from 38 patients with medication-refractory depression (N = 38) (43).
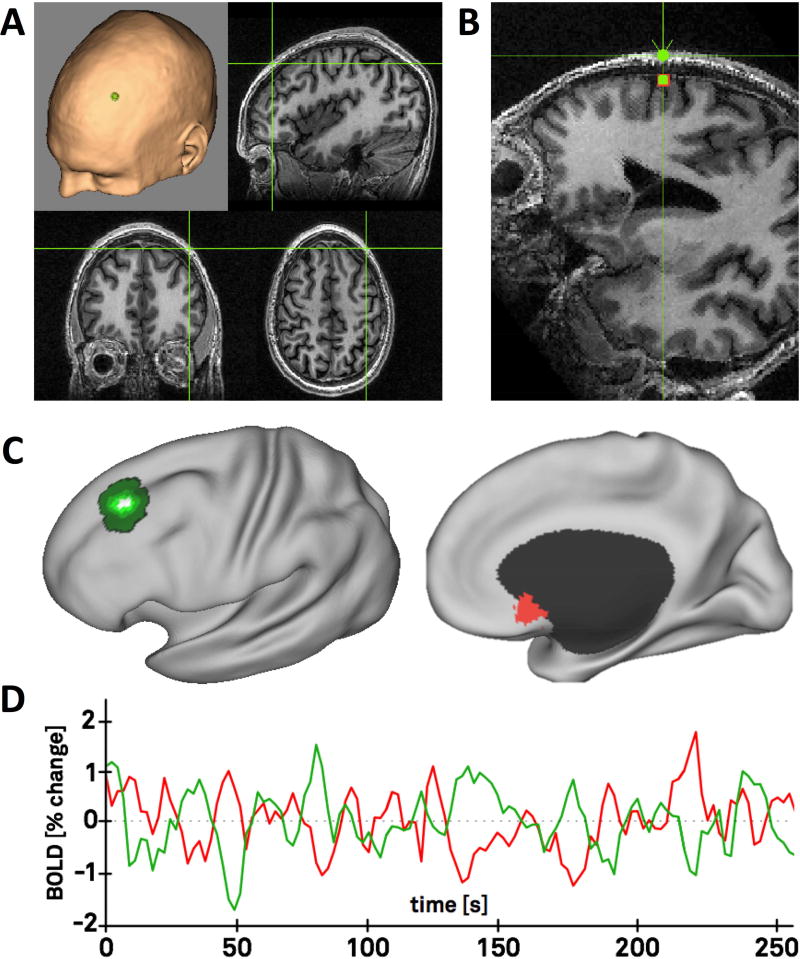
Figure 1. Analysis approach.
Each patient’s transcranial magnetic stimulation site was recorded using their MRI and a frameless neuronavigation system (A). Stimulation sites on the scalp surface were projected to the nearest location on the brain surface using an automated algorithm (B). Brain coordinates were transformed into standard atlas space and the volume of stimulated tissue was approximated using an existing TMS model (C, green). Functional connectivity between the stimulation site (C, green) and the subgenual cingulate (C, red) was assessed using fMRI data from a large normative cohort of 1000 subjects. Representative time courses from a single subject are shown (D).
Results
Patient and treatment characteristics
Our primary cohort included 25 right-handed patients with medication resistant major depression (17 female) with a mean age of 54.8 years (SD = 9.9, range = 28–67; for details of patient flow, see Fig. S1). Mean BDI score at baseline was 38.6 (SD = 9.3) and significantly decreased to 21.2 (SD = 13.0) after the course of rTMS treatment (t(24) = 10.05, p < .0001). 13 of 25 patients were identified as responders (defined as ≥ 50% reduction in BDI score). On average, the treatment consisted of 28.5 sessions (SD = 3.4), administered over a period of 4–7 weeks (mean = 6.0, SD = 0.8). There were no significant correlations between percent change in BDI scores and gender (rpb = .01, p = 0.96), age (r = −.05, p = .82), number of treatment sessions (r = −.11, p = .62), applied TMS frequency (rpb = .16, p = .46), or current medications, including antidepressants (r = −.20, p = .34), mood stabilizer (r = −.07, p = .74), antipsychotics (r = .19, p = .38) and stimulants (r = −.13, p = .53). Trend-wise correlations were found between treatment outcome and baseline BDI scores (r = −.34, p = .09 with a better response for less severely depressed patients), TMS device (rpb = .37, p = .07 with a better response for patients receiving treatment with the Magstim device) and benzodiazepines (r = −.38, p = .06 with a better response for patients taking this medication). However, none of these variables was a significant predictor of response when correcting for multiple comparisons or in a multivariate analysis (including gender, age, all medications, TMS device and BDI baseline).
Predicting clinical response based on the stimulation site
The cortical stimulation site identified by the 5.5 cm technique (Boston Cohort) was highly variable across different patients (Fig. 2A). Average MNI coordinates were x=−33 ±7, y=30 ±9, z=50 ±9. Repeated markings of the stimulation site within one of these patients suggests that within subject variability of the stimulation site was low relative to inter-subject variability (4.4 ± 1.4 mm vs 12.7 ± 6.1 mm, T = 3.01, p = 0.005, Figure S2).
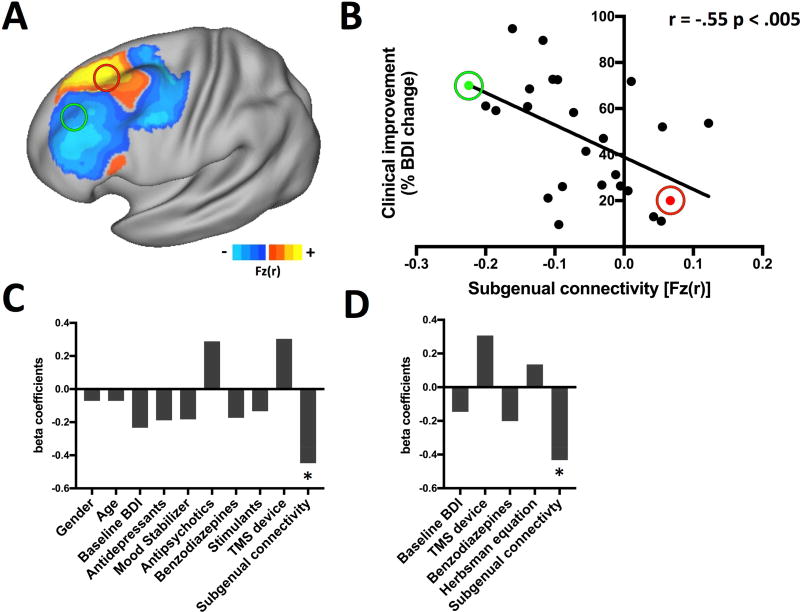
Figure 2. Anatomical location of the TMS site predicts antidepressant response.
The location of the stimulation site in standard atlas space is shown for each patient in our primary cohort (Boston, N = 25) including examples of a responder (green) and a non-responder (red) (A). Clinical improvement (% change in the Beck Depression Inventory) was predicted based anatomical coordinates of each patient’s stimulation site and a previously published equation (16) (B). There was a significant correlation between measured and predicted clinical improvement (r = .51, p < .01).
Based on a linear combination of the anatomical coordinates from each patient’s stimulation site (25), the Herbsman equation significantly predicted antidepressant response in the Boston cohort (r = .51, p < .01, Fig. 2B). As previously hypothesized (25), more anterior and lateral stimulation sites were more effective.
Functional connectivity between each patient’s stimulation site and the subgenual cingulate was also a significant predictor of antidepressant response in the Boston cohort (r = −.55, p < .005, Fig. 3A and B). As previously hypothesized (27), more effective rTMS sites were more strongly anticorrelated with the subgenual cingulate. Repeating this analysis using connectome data from depression patients produced similar results (r = −0.51, p = 0.01). Functional connectivity estimates from the normative (1000 healthy subjects) and depression (38 patients) connectomes were nearly identical (R = 0.96, p < 10−14) thus only the larger connectome was used for subsequent analyses. The peak subgenual anticorrelation within the left DLPFC (based on N = 1000) was located at x = −42, y = 44, z = 30 mm.
Figure 3. Functional connectivity between the TMS site and the subgenual cingulate is an independent predictor of antidepressant response.
The stimulation sites for an example responder (green) and non-responder (red) are shown overlaid on a map of functional connectivity with the subgenual cingulate from 1000 healthy subjects, masked to highlight the DLPFC (A). Functional connectivity between each patient’s stimulation site and subgenual cingulate was a significant predictor of antidepressant response (B, r = −.55, p < .005). Subgenual connectivity was a significant independent predictor of antidepressant response in multivariate analyses of baseline clinical variables (C) or all variables with signs of predictive utility, including the coordinate-based Herbsman equation (D).
Next, we tested whether these two factors (coordinates and connectivity) were independent predictors of antidepressant response in the Boston cohort. When combined in a multivariate analysis with baseline clinical variables (gender, age, all medications, TMS device and BDI baseline) subgenual connectivity was a significant independent predictor of response (β = −1.24, p < .02, Fig. 3C). When this same analysis was run using the Herbsman equation, it just missed significance (β = 0.68, p = .054). When all variables with signs of predictive utility in the present study were combined (subgenual connectivity, Herbsman equation, baseline BDI scores, TMS device, and benzodiazepines), subgenual connectivity was the only independent predictor of clinical response (β = −1.17, p < .05; Fig. 3D).
Symptom Specificity
To explore whether subgenual connectivity predicted improvement in all or only some antidepressant symptoms in the Boston cohort, we segmented BDI scores into cognitive, affective, and somatic symptoms based on an established three-factor model (49). Subgenual connectivity was a significant predictor of improvement in cognitive (r = −.58, p < .005) and affective symptoms (r = −.63, p < .001), but not somatic symptoms (r = −.15, p = .48).
In an exploratory analysis of 21 individual symptoms described by items in the BDI-II, subgenual connectivity was associated with improvement in sadness, loss of pleasure, self-dislike, self-criticalness, suicidal thoughts, loss of interest, and worthlessness (Table 1). However, there was no association with improvement in irritability, appetite, or fatigue. Further, subgenual connectivity was associated with LACK of improvement in sleep symptoms and interest in sex. In other words, the more anticorrelated a patient’s stimulation site was to the subgenual, the better the improvement in sadness but the worse the improvement in interest in sex.
Table 1. Improvement in only some depression symptoms is related to connectivity with the subgenual cingulate.
Mean improvement (raw score change) for each question in the Beck Depression Inventory is shown along with the correlation between this improvement and subgenual connectivity to the stimulation site for all patients (all). Patients with zero scores at baseline and after treatment were excluded in the last column (no zero scores).
| Mean Improvement (raw scores) (all) |
Correlation with subgenual connectivity (r) (all) |
Correlation with subgenual connectivity (r) (no zero values) |
|
|---|---|---|---|
| 1 - Sadness | 1.36 | −0.35 | −0.35 |
| 2 - Pessimism | 1.04 | −0.32 | −0.32 |
| 3 - Past Failure | 0.72 | −0.11 | −0.07 |
| 4 - Loss of Pleasure | 0.76 | −0.36 | −0.36 |
| 5 - Guilty Feelings | 0.80 | −0.21 | −0.20 |
| 6 - Punishment Feelings | 0.52 | −0.15 | −0.28 |
| 7 - Self-Dislike | 1.00 | −0.44 | −0.44 |
| 8 - Self-Criticalness | 0.96 | −0.34 | −0.33 |
| 9 - Suicidal Thoughts or Wishes | 0.36 | −0.44 | −0.57 |
| 10 - Crying | 1.08 | −0.29 | −0.25 |
| 11 - Agitation | 0.52 | −0.13 | −0.15 |
| 12 - Loss of Interest | 1.04 | −0.59 | −0.59 |
| 13 - Indecisiveness | 1.00 | −0.13 | −0.15 |
| 14 - Worthlessness | 0.76 | −0.37 | −0.37 |
| 15 - Loss of Energy | 1.00 | −0.18 | −0.18 |
| 16 - Changes in Sleeping Pattern | 0.44 | 0.47 | 0.41 |
| 17 - Irritability | 0.92 | −0.06 | −0.15 |
| 18 - Changes in Appetite | 0.52 | −0.07 | −0.10 |
| 19 - Concentration Difficulty | 0.76 | −0.38 | −0.38 |
| 20 - Tiredness or Fatigue | 0.96 | 0.08 | 0.08 |
| 21 - Loss in Interest in Sex | 0.96 | 0.32 | 0.55 |
Predicting sham stimulation
A limitation of the above analyses is that results could be driven by placebo rather than active rTMS. We therefore retrospectively examined data from a separate cohort in which 16 patients were treated with sham stimulation for 4 weeks (Fig. 4A). There was no association between placebo response and subgenual connectivity (r = .01, p(1) = .49; Fig. 4B). Twelve of these 16 patients remained depressed after sham stimulation and subsequently received open-label active rTMS at the same stimulation site (Fig. 4C). Antidepressant response to active stimulation was predicted by subgenual connectivity similar to our primary cohort (r = −.52, p(1) < .05; Fig. 4D)
Figure 4. Subgenual connectivity predicts response to active but not sham stimulation.
The anatomical location of the stimulation site is shown for each patient in our secondary cohort (A, N = 16), all of whom received sham stimulation. Subgenual connectivity did not predict sham rTMS response (B, r = .01, p(1) = .49). A subset of these patients went on to get active stimulation at the same stimulation sites (C, N = 12). Subgenual connectivity was a predictor of clinical response to active stimulation, matching results from our primary cohort (D, r = −.52, p(1) < 0.05).
Repeating this same analysis using the Herbsman equation returned different results. Surprisingly, the Herbsman equation (i.e. more anterolateral coil position) was associated with response to sham stimulation (r = .53, p(1) < .05) but not active stimulation (r = −.09, p(1) = .39).
This dataset also controlled for a second concern, which is that subgenual connectivity is only a predictor of response when using the 5-cm method to identify the stimulation site. This second cohort targeted stimulation based on perfusion changes during a working memory task, resulting in stimulation locations that were significantly more anterior, lateral, and inferior compared to those in our primary cohort (Michigan MNI coordinates x = −33 ± 4.7, y = 50 ± 7.9, z = 30 ± 9.4 mm, all coordinates p < 0.05). Due to this difference in targeting, this cohort also provided better separation between the Herbsman equation and subgenual connectivity, as the two variables were correlated in the Boston cohort (r = −.67, p < .001) but not in the Michigan cohort (r = .08, p = .80).
Discussion
There are three important findings in the present study. First, stronger anticorrelation between a patient’s cortical rTMS site and the subgenual cingulate predicts clinical outcome in individual patients. Second, subgenual connectivity is an independent predictor of clinical response and specific to active compared to sham stimulation. Third, subgenual connectivity predicts improvement in cognitive and affective but not somatic symptoms of depression. These findings each have implications for identifying the optimal rTMS site for treatment of patients with medication-resistant depression.
Predicting response based on subgenual connectivity
The strongest finding in the current study is confirmation that connectivity between the rTMS targeted cortical site and the subgenual cingulate predicts antidepressant response. This hypothesis was originally based on retrospective analysis of existing data (27). Confirmation of this hypothesis in a prospective cohort specifically collected for this purpose is important, especially given the poor reproducibility of most neuroimaging findings (50–53) and the potential clinical implications. Post-hoc analyses further suggest that subgenual connectivity is an independent predictor of response when combined with other variables, is not related to sham response, predicts improvement in only certain depressive symptoms, and predicts active rTMS response across different rTMS cohorts.
It is important to note that unlike our primary Boston cohort, the Michigan cohort was not specifically collected to test our hypothesis. Further, the Michigan cohort differed in many ways from the Boston cohort such that results are not directly comparable between cohorts (e.g. determining which cohort had a better TMS response). However, variance in outcome within a cohort, and the factors responsible for that variance, are comparable. For example, while the Michigan cohort can’t be used a sham control for the Boston cohort, we can conclude that subgenual connectivity within the Michigan cohort is unrelated to the response to sham stimulation. Further, the differences between cohorts is also an advantage with respect to generalizability of our results. It is remarkable that despite differences in center (Boston versus Michigan), targeting method (5.5 cm versus task-activation), target distribution in the DLPFC (Fig. 2A versus Fig. 4), outcome measure (BDI versus MADRS), average duration of daily active rTMS (6 versus 4 weeks), and preceding treatments (none versus sham), subgenual connectivity predicts similar variance in clinical outcome during open-label TMS in both groups.
The current results add to accumulating data that connectivity to subcortical limbic regions plays a role in mediating rTMS antidepressant response (12; 20; 26; 27; 43; 54–56). In particular, the subgenual cingulate has been linked to sadness in normal subjects (3; 57; 58) and antidepressant response across a wide range of therapies (e.g. 1; 2; 59–62).
Subgenual connectivity predicted rTMS-induced improvement in some but not all depression symptoms. The strong association with affective symptoms is expected given data implicating the subgenual in sadness and mood (3; 57; 58). The strong association with cognitive symptoms is less intuitive, however the “cognitive” factor is less about cognitive function such as attention or working memory and more about negative thoughts such as guilt, pessimism, and self-dislike (49). Subgenual connectivity may identify the specific part of the DLPFC mediating such thoughts. Whether improvement in “cognitive” symptoms relates to any objective improvement in cognitive function remains unknown, as there is a known dissociation between the two (59).
Finally, subgenual connectivity was not related to improvement in somatic symptoms. This is not surprising, as these symptoms of depression are likely mediated by other brain systems (2; 60; 61). Interestingly, subgenual connectivity predicted less improvement in sexual interest, consistent with decreased libido observed in response to many SSRIs (62), which are also drugs that suppress subgenual activity (4; 38).
Predicting response based on anatomical coordinates
The current study is the also the first to show that the Herbsman equation, based on more anterior and lateral stimulation coordinates, predicts antidepressant response in a prospective cohort (Boston cohort). An interesting question is why anterolateral coil position was predictive in our Boston cohort but failed to predict response in our Michigan cohort or when previously applied to other TMS cohorts (24; 27; 63). The most likely explanation is that in our Boston cohort, there was a strong correlation between the Herbsman equation and subgenual connectivity. When the variance related to subgenual connectivity was controlled for in a linear model, the Herbsman equation was no longer predictive. In datasets using alternative methods to target rTMS such as task-activation (Michigan cohort) or PET (63), the correlation between the Herbsman equation and subgenual connectivity is reduced, and only subgenual connectivity remains predictive.
More anterolateral coil position also failed to predict outcome in a prior (and much larger) study using rTMS targeting similar to that used in our Boston cohort (24). Differences that may have contributed to the positive finding in our study include frameless stereotaxy to record the stimulation site, nonlinear transformation of MRIs into standard atlas space, 4–7 weeks of open-label rTMS in accordance with current clinical practice, and analysis of antidepressant response as a continuous rather than discrete variable.
An unexpected result in the current study is the finding that more anterolateral coil position (per the Herbsman equation) was associated with response to sham stimulation (Michigan cohort). This result highlights the fact that no similar association was seen between sham response and subgenual connectivity. However, why anterolateral coil position would lead to higher placebo response is unclear and this result should be interpreted with caution.
Other predictive factors
It should be noted that other factors other than the location and connectivity of the stimulation are likely to play a role in TMS response (17–19). The only clinical variables with even trend-level predictive utility in the present study were baseline BDI, TMS device, and benzodiazepines. However, none of these variables survived correction for multiple comparisons or was significant in our multivariate analysis. Further, some variables, such as TMS device, have been specifically evaluated in larger trials and found to not impact TMS response (64).
Individualized versus group connectivity
It is worth highlighting that our study was based primarily on normative connectome data from a large cohort of healthy subjects, as was prior work on this topic including the 2012 paper whose hypotheses this study was designed to address (27; 43). This is a major practical advantage, as MRI-based connectivity data is not routinely acquired in TMS patients. If the stimulation site is recorded, our technique can be used to predict patient response. Further, normative connectome datasets are acquired with specialized MRI hardware and cohort sizes in the thousands, leading to extremely robust connectivity estimates. Such normative connectome datasets have proven valuable in predicting stroke symptoms from patient-specific lesions (65–69), clinical response from patient-specific deep brain stimulation sites (70), and we now show that it can predict clinical response from patient-specific TMS sites. Although differences in connectivity have been reported in patients with depression (1–9), using a disease-matched connectome had almost no effect on our results, and if anything, results were slightly better using the normative connectome. This is consistent with prior work from our group (65; 70) and suggests that the signal to noise benefits of large normative connectomes may outweigh small differences in connectivity associated with a disease state.
An important question is how results of the current study, based entirely on group connectome data, relate to prior work emphasizing the importance of individual differences in connectivity for identifying TMS targets (26; 47; 71). Single-subject connectivity estimates are inherently noisy compared to group connectome estimates, especially in brain regions with poor signal to noise like the subgenual cingulate (26; 71). Advanced processing strategies are required to generate reproducible individualized connectivity maps and TMS targets (26; 72). Whether individualized connectivity with the stimulation site can improve on predictions of TMS response based on group connectome data is an important question for future work.
Clinical implications
The current results provide the strongest evidence to date that subgenual connectivity with the stimulation site predicts antidepressant response, raising the question of whether this should change clinical practice. At a minimum, these results suggest that recording the location of the rTMS site with neuronavigation has prognostic value. If the planned stimulation site predicts a poor response, one could justify moving stimulation to a site predictive of a better response. Indeed, this strategy was used in one of the large randomized clinical trials of rTMS (12). When the 5 cm technique identified a site over the premotor cortex it was moved anterior and lateral (24). The current data suggests that subgenual connectivity, rather than antero-lateral location, may better guide this correction.
Subgenual connectivity may also help inform the ongoing debate of how to best target rTMS. Although the 5 cm technique was used in the large randomized trials (10–13), other work has suggested using 5.5 or 6 cm (30; 31). Recent consensus guidelines recommend targeting based on the EEG F3 coordinate, which may better account for head size (31; 33; 34). It is possible to convert target MNI coordinates into scalp measurements that could allow for targeting of TMS without neuronavigation (73). However, the accuracy and utility of such an approach remains to be tested in patients.
MNI coordinates of our peak subgenual anticorrelation in the left DLPFC (x=−42, y=44, z=30), fall directly between two peak coordinates we reported previously (x=−44, y=38, z=34) and (x=−38, y=44, z=26) (27). The current coordinates should be considered an update of this prior work, as the current study utilized functional connectivity from 1000 subjects rather than 98. Whether intentionally targeting this coordinate improves antidepressant response remains unknown. A small randomized trial targeting rTMS to a site near our anticorrelation peak showed some benefit over the 5 cm approach, but failed to meet its primary endpoint (35). To this end, it is important to note that the anticorrelation peak may not be best for all depression symptoms. The current data regarding which symptoms are most related to subgenual connectivity could prove useful in the design of a future clinical trial.
Limitations
There are several limitations to this study. First, although replication of an a priori hypothesis across two independent cohorts is strongly supportive, only one cohort was prospectively collected for this purpose and both cohorts were relatively small (N=25, N=16). Results will benefit from further replication in larger and ideally multi-center studies. Second, two different TMS devices and frequencies were used in our primary cohort. This does not significantly impact outcome in our current data or in prior literature (64; 74; 75), but introduces heterogeneity that could potentially lead to false negatives. Similarly, there was significant heterogeneity between cohorts, precluding direct cross-cohort comparisons. However, as noted earlier, reproducibility within cohorts in the face of this heterogeneity is a major strength of the present study. Third, neuronavigation was only used to record the stimulation site during a single session for most patients in our primary cohort, raising questions regarding within-subject variability. However, repeated measurements in one of our patients (see Figure S2) and prior work on the reliability of neuronavigation (76; 77) suggests this variability is small relative to the variability across subjects. Further, any within subject variability should bias us against the current findings. Fourth, our study was designed as a prospective test of a specific hypothesis, and thus focused on connectivity between the rTMS targeted cortical site and the subgenual cingulate. Connectivity to other brain regions may also be relevant to antidepressant response (20; 54; 55). Fifth, although we corrected for several clinical variables, we collected limited information on some factors that may influence TMS response, such as duration of the current depressive episode or genetic polymorphisms (17–19; 78). Finally, and perhaps most importantly, this was a prospective observational trial, not a randomized controlled trial. Whether directly targeting the peak site of subgenual anticorrelation improves antidepressant response beyond conventional targeting remains to be tested.
Supplementary Material
Acknowledgments
We thank Antigoni Sinanis, Sara Pedersen and Chester Pearlman for assistance with data collection, Elissa Wilker for statistical help, David Alsop for assistance with MRI acquisition and the Brain Genomics Superstruct Project for contributing data.
This work was supported by the NIH (R21MH099196, K23NS083741 and R21MH098174), by Harvard Clinical and Translational Science Center/ Harvard Catalyst (National Center for Advancing Translational Sciences, NIH Award UL1RR025758 and by Clinical Sciences Translational Award (Michigan) UL1TR000433; Additional assistance came from financial contributions from Harvard University and its affiliated academic health care centers), the Brain and Behavior Research Foundation, and by the Sidney R. Baer Jr. Foundation, the Nancy Lurie Marks Foundation. Research support was also provided by Neuronetics, which did not have a role in the design of the study. Work on this study was also supported, and by the resources provided by Shared Instrumentation Grants (1S10RR023043 and 1S10RR023401). APL serves on the scientific advisory boards for Starlab Neuroscience, Neuroelectrics, Constant Therapy, and Neosync; and is listed as an inventor on several issued and pending patents on the real-time integration of transcranial magnetic stimulation with electroencephalography and magnetic resonance imaging. MDF is listed as an inventor on submitted or pending patents using brain imaging to guide brain stimulation. AH received funding from Stiftung Charité; Berlin Institute of Health and Prof. Klaus Thiemann Foundation, as well as travel stipends from Movement Disorders Society and Ipsen Pharma. SFT has received research support from Neuronetics and St.Jude Medical.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
All other authors report no biomedical financial interests or potential conflicts of interest. The authors declare no competing interests.
The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic health care centers, the NIH, Brain and Behavior Research Foundation, or the Sidney R. Baer Jr. Foundation.
References
- 1.Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, et al. Deep brain stimulation for treatment-resistant depression. 2005;45:651–660. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 2.Brody AL, Saxena S, Mandelkern MA, Fairbanks LA, Ho ML, Baxter LR. Brain metabolic changes associated with symptom factor improvement in major depressive disorder. BPS. 2001;50:171–178. doi: 10.1016/s0006-3223(01)01117-9. [DOI] [PubMed] [Google Scholar]
- 3.Mayberg HS, Liotti M, Brannan SK, McGinnis S, Mahurin RK, Jerabek PA, et al. Reciprocal limbic-cortical function and negative mood: converging PET findings in depression and normal sadness. Am J Psychiatry. 1999;156:675–682. doi: 10.1176/ajp.156.5.675. [DOI] [PubMed] [Google Scholar]
- 4.Mayberg HS, Brannan SK, Tekell JL, Silva JA, Mahurin RK, McGinnis S, Jerabek PA. Regional metabolic effects of fluoxetine in major depression: serial changes and relationship to clinical response. BPS. 2000;48:830–843. doi: 10.1016/s0006-3223(00)01036-2. [DOI] [PubMed] [Google Scholar]
- 5.Biver F, Goldman S, Delvenne V, Luxen A, De Maertelaer V, Hubain P, et al. Frontal and parietal metabolic disturbances in unipolar depression. BPS. 1994;36:381–388. doi: 10.1016/0006-3223(94)91213-0. [DOI] [PubMed] [Google Scholar]
- 6.Baxter LR, Schwartz JM, Phelps ME, Mazziotta JC, Guze BH, Selin CE, et al. Reduction of prefrontal cortex glucose metabolism common to three types of depression. Arch Gen Psychiatry. 1989;46:243–250. doi: 10.1001/archpsyc.1989.01810030049007. [DOI] [PubMed] [Google Scholar]
- 7.Galynker II, Cai J, Ongseng F, Finestone H, Dutta E, Serseni D. Hypofrontality and negative symptoms in major depressive disorder. Journal of Nuclear Medicine. 1998;39:608–612. [PubMed] [Google Scholar]
- 8.Fitzgerald PB, Oxley TJ, Laird AR, Kulkarni J, Egan GF, Daskalakis ZJ. An analysis of functional neuroimaging studies of dorsolateral prefrontal cortical activity in depression. Psychiatry Research: Neuroimaging. 2006;148:33–45. doi: 10.1016/j.pscychresns.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 9.Koenigs M, Grafman J. The functional neuroanatomy of depression: distinct roles for ventromedial and dorsolateral prefrontal cortex. Behavioural brain research. 2009;201:239–243. doi: 10.1016/j.bbr.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carpenter LL, Janicak PG, Aaronson ST, Boyadjis T, Brock DG, Cook IA, et al. Transcranial magnetic stimulation (TMS) for major depression: a multisite, naturalistic, observational study of acute treatment outcomes in clinical practice. Depress Anxiety. 2012;29:587–596. doi: 10.1002/da.21969. [DOI] [PubMed] [Google Scholar]
- 11.Connolly KR, Helmer A, Cristancho MA, Cristancho P, O'Reardon JP. Effectiveness of transcranial magnetic stimulation in clinical practice post-FDA approval in the United States: results observed with the first 100 consecutive cases of depression at an academic medical center. J Clin Psychiatry. 2012;73:e567–73. doi: 10.4088/JCP.11m07413. [DOI] [PubMed] [Google Scholar]
- 12.George MS, Lisanby SH, Avery D, McDonald WM, Durkalski V, Pavlicova M, et al. Daily left prefrontal transcranial magnetic stimulation therapy for major depressive disorder: a sham-controlled randomized trial. Arch Gen Psychiatry. 2010;67:507–516. doi: 10.1001/archgenpsychiatry.2010.46. [DOI] [PubMed] [Google Scholar]
- 13.O’Reardon JP, Solvason HB, Janicak PG, Sampson S, Isenberg KE, Nahas Z, et al. Efficacy and Safety of Transcranial Magnetic Stimulation in the Acute Treatment of Major Depression: A Multisite Randomized Controlled Trial. Biol Psychiatry. 2007;62:1208–1216. doi: 10.1016/j.biopsych.2007.01.018. [DOI] [PubMed] [Google Scholar]
- 14.Loo CK, Mitchell PB. A review of the efficacy of transcranial magnetic stimulation (TMS) treatment for depression, and current and future strategies to optimize efficacy. J Affect Disord. 2005;88:255–267. doi: 10.1016/j.jad.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 15.Padberg F, George MS. Repetitive transcranial magnetic stimulation of the prefrontal cortex in depression. Exp Neurol. 2009;219:2–13. doi: 10.1016/j.expneurol.2009.04.020. [DOI] [PubMed] [Google Scholar]
- 16.Slotema CW, Blom JD, Hoek HW, Sommer IE. Should we expand the toolbox of psychiatric treatment methods to include Repetitive Transcranial Magnetic Stimulation (rTMS)? A meta-analysis of the efficacy of rTMS in psychiatric disorders. J Clin Psychiatry. 2010;71:873–884. doi: 10.4088/JCP.08m04872gre. [DOI] [PubMed] [Google Scholar]
- 17.Brakemeier E-L, Luborzewski A, Danker-Hopfe H, Kathmann N, Bajbouj M. Positive predictors for antidepressive response to prefrontal repetitive transcranial magnetic stimulation (rTMS) Journal of Psychiatric Research. 2007;41:395–403. doi: 10.1016/j.jpsychires.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 18.Fregni F, Marcolin MA, Myczkowski M, Amiaz R, Hasey G, Rumi DO, et al. Predictors of antidepressant response in clinical trials of transcranial magnetic stimulation. Int J Neuropsychopharmacol. 2006;9:641–654. doi: 10.1017/S1461145705006280. [DOI] [PubMed] [Google Scholar]
- 19.Lisanby SH, Husain MM, Rosenquist PB, Maixner D, Gutierrez R, Krystal A, et al. Daily left prefrontal repetitive transcranial magnetic stimulation in the acute treatment of major depression: clinical predictors of outcome in a multisite, randomized controlled clinical trial. Neuropsychopharmacology. 2009;34:522–534. doi: 10.1038/npp.2008.118. [DOI] [PubMed] [Google Scholar]
- 20.Liston C, Chen AC, Zebley BD, Drysdale AT, Gordon R, Leuchter B, et al. Default mode network mechanisms of transcranial magnetic stimulation in depression. Biol Psychiatry. 2014;76:517–526. doi: 10.1016/j.biopsych.2014.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kimbrell TA, Little JT, Dunn RT, Frye MA, Greenberg BD, Wassermann EM, et al. Frequency dependence of antidepressant response to left prefrontal repetitive transcranial magnetic stimulation (rTMS) as a function of baseline cerebral glucose metabolism. BPS. 1999;46:1603–1613. doi: 10.1016/s0006-3223(99)00195-x. [DOI] [PubMed] [Google Scholar]
- 22.Paillère Martinot M-L, Martinot J-L, Ringuenet D, Galinowski A, Gallarda T, Bellivier F, et al. Baseline brain metabolism in resistant depression and response to transcranial magnetic stimulation. Neuropsychopharmacology. 2011;36:2710–2719. doi: 10.1038/npp.2011.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nahas Z, Teneback CC, Kozel A, Speer AM, DeBrux C, Molloy M, et al. Brain Effects of TMS Delivered Over Prefrontal Cortex in Depressed Adults. J Neuropsychiatry Clin Neurosci. 2001;13:459–470. doi: 10.1176/jnp.13.4.459. [DOI] [PubMed] [Google Scholar]
- 24.Johnson KA, Baig M, Ramsey D, Lisanby SH, Avery D, McDonald WM, et al. Prefrontal rTMS for treating depression: location and intensity results from the OPT-TMS multi-site clinical trial. Brain Stimul. 2013;6:108–117. doi: 10.1016/j.brs.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Herbsman T, Avery D, Ramsey D, Holtzheimer P, Wadjik C, Hardaway F, et al. More lateral and anterior prefrontal coil location is associated with better repetitive transcranial magnetic stimulation antidepressant response. Biol Psychiatry. 2009;66:509–515. doi: 10.1016/j.biopsych.2009.04.034. [DOI] [PubMed] [Google Scholar]
- 26.Fox MD, Liu H, Pascual-Leone A. Identification of reproducible individualized targets for treatment of depression with TMS based on intrinsic connectivity. NeuroImage. 2013;66:151–160. doi: 10.1016/j.neuroimage.2012.10.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fox MD, Buckner RL, White MP, Greicius MD, Pascual-Leone A. Efficacy of transcranial magnetic stimulation targets for depression is related to intrinsic functional connectivity with the subgenual cingulate. Biol Psychiatry. 2012;72:595–603. doi: 10.1016/j.biopsych.2012.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.George MS, Wassermann EM, Williams WA, Callahan A, Ketter TA, Basser P, et al. Daily repetitive transcranial magnetic stimulation (rTMS) improves mood in depression. Neuroreport. 1995;6:1853–1856. doi: 10.1097/00001756-199510020-00008. [DOI] [PubMed] [Google Scholar]
- 29.Pascual-Leone A, Rubio B, Pallardo F, Catala MD. Rapid-rate transcranial magnetic stimulation of left dorsolateral prefrontal cortex in drug-resistant depression. Lancet. 1996;348:233–237. doi: 10.1016/s0140-6736(96)01219-6. [DOI] [PubMed] [Google Scholar]
- 30.Horvath JC, Mathews J, Demitrack MA, Pascual-Leone A. The NeuroStar TMS device: conducting the FDA approved protocol for treatment of depression. J Vis Exp. 2010 doi: 10.3791/2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McClintock SM, Reti IM, Carpenter LL, McDonald WM, Dubin M, Taylor SF, et al. Consensus Recommendations for the Clinical Application of Repetitive Transcranial Magnetic Stimulation (rTMS) in the Treatment of Depression. J Clin Psychiatry. 2017 doi: 10.4088/JCP.16cs10905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Herwig U, Padberg F, Unger J, Spitzer M, Schonfeldt-Lecuona C. Transcranial magnetic stimulation in therapy studies: examination of the reliability of “standard” coil positioning by neuronavigation. BPS. 2001;50:58–61. doi: 10.1016/s0006-3223(01)01153-2. [DOI] [PubMed] [Google Scholar]
- 33.Herwig U, Satrapi P, Schonfeldt-Lecuona C. Using the international 10–20 EEG system for positioning of transcranial magnetic stimulation. Brain Topogr. 2003;16:95–99. doi: 10.1023/b:brat.0000006333.93597.9d. [DOI] [PubMed] [Google Scholar]
- 34.Beam W, Borckardt JJ, Reeves ST, George MS. An efficient and accurate new method for locating the F3 position for prefrontal TMS applications. Brain Stimul. 2009;2:50–54. doi: 10.1016/j.brs.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fitzgerald PB, Maller JJ, Hoy KE, Thomson R, Daskalakis ZJ. Exploring the optimal site for the localization of dorsolateral prefrontal cortex in brain stimulation experiments. Brain Stimul. 2009;2:234–237. doi: 10.1016/j.brs.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 36.Rusjan PM, Barr MS, Farzan F, Arenovich T, Maller JJ, Fitzgerald PB, Daskalakis ZJ. Optimal transcranial magnetic stimulation coil placement for targeting the dorsolateral prefrontal cortex using novel magnetic resonance image-guided neuronavigation. Hum Brain Mapp. 2010;31:1643–1652. doi: 10.1002/hbm.20964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dougherty DD, Weiss AP, Cosgrove GR, Alpert NM, Cassem EH, Nierenberg AA, et al. Cerebral metabolic correlates as potential predictors of response to anterior cingulotomy for treatment of major depression. J Neurosurg. 2003;99:1010–1017. doi: 10.3171/jns.2003.99.6.1010. [DOI] [PubMed] [Google Scholar]
- 38.Goldapple K, Segal Z, Garson C, Lau M, Bieling P, Kennedy S, Mayberg H. Modulation of cortical-limbic pathways in major depression: treatment-specific effects of cognitive behavior therapy. Arch Gen Psychiatry. 2004;61:34–41. doi: 10.1001/archpsyc.61.1.34. [DOI] [PubMed] [Google Scholar]
- 39.Mottaghy FM, Keller CE, Gangitano M, Ly J, Thall M, Parker JA, Pascual-Leone A. Correlation of cerebral blood flow and treatment effects of repetitive transcranial magnetic stimulation in depressed patients. Psychiatry Res. 2002;115:1–14. doi: 10.1016/s0925-4927(02)00032-x. [DOI] [PubMed] [Google Scholar]
- 40.Nobler MS, Oquendo MA, Kegeles LS, Malone KM, Campbell CC, Sackeim HA, Mann JJ. Decreased regional brain metabolism after ect. Am J Psychiatry. 2001;158:305–308. doi: 10.1176/appi.ajp.158.2.305. [DOI] [PubMed] [Google Scholar]
- 41.Malizia AL. The frontal lobes and neurosurgery for psychiatric disorders. J Psychopharmacol (Oxford) 1997;11:179–187. doi: 10.1177/026988119701100211. [DOI] [PubMed] [Google Scholar]
- 42.Drevets WC, Savitz J, Trimble M. The subgenual anterior cingulate cortex in mood disorders. CNS Spectr. 2008;13:663–681. doi: 10.1017/s1092852900013754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fox MD, Buckner RL, Liu H, Chakravarty MM, Lozano AM, Pascual-Leone A. Resting-state networks link invasive and noninvasive brain stimulation across diverse psychiatric and neurological diseases. Proceedings of the National Academy of Sciences. 2014;111:E4367–75. doi: 10.1073/pnas.1405003111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Greicius MD, Flores BH, Menon V, Glover GH, Solvason HB, Kenna H, et al. Resting-state functional connectivity in major depression: abnormally increased contributions from subgenual cingulate cortex and thalamus. BPS. 2007;62:429–437. doi: 10.1016/j.biopsych.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gudayol-Ferré E, Peró-Cebollero M, González-Garrido AA, Guàrdia-Olmos J. Changes in brain connectivity related to the treatment of depression measured through fMRI: a systematic review. Front Hum Neurosci. 2015;9:1–17. doi: 10.3389/fnhum.2015.00582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brakowski J, Spinelli S, Dörig N, Bosch OG, Manoliu A, Holtforth MG, Seifritz E. Resting state brain network function in major depression - Depression symptomatology, antidepressant treatment effects, future research. Journal of Psychiatric Research. 2017;92:147–159. doi: 10.1016/j.jpsychires.2017.04.007. [DOI] [PubMed] [Google Scholar]
- 47.Opitz A, Fox MD, Craddock RC, Colcombe S, Milham MP. An integrated framework for targeting functional networks via transcranial magnetic stimulation. NeuroImage. 2016;127:86–96. doi: 10.1016/j.neuroimage.2015.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yeo BTT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106:1125–1165. doi: 10.1152/jn.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Buckley TC, Parker JD, Heggie J. A psychometric evaluation of the BDI-II in treatment-seeking substance abusers. J Subst Abuse Treat. 2001;20:197–204. doi: 10.1016/s0740-5472(00)00169-0. [DOI] [PubMed] [Google Scholar]
- 50.Boekel W, Wagenmakers EJ, Belay L, Verhagen J, Brown S, Forstmann BU. A purely confirmatory replication study of structural brain-behavior correlations. Cortex. 2015;66:115–133. doi: 10.1016/j.cortex.2014.11.019. [DOI] [PubMed] [Google Scholar]
- 51.Button KS, Ioannidis JP, Mokrysz C, Nosek BA, Flint J, Robinson ES, Munafo MR. Power failure: why small sample size undermines the reliability of neuroscience. Nat Rev Neurosci. 2013;14:365–376. doi: 10.1038/nrn3475. [DOI] [PubMed] [Google Scholar]
- 52.Eklund A, Nichols TE, Knutsson H. Cluster failure: Why fMRI inferences for spatial extent have inflated false-positive rates. Proceedings of the National Academy of Sciences. 2016;113:7900–7905. doi: 10.1073/pnas.1602413113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nichols TE, Das S, Eickhoff SB, Evans AC, Glatard T, Hanke M, et al. Best practices in data analysis and sharing in neuroimaging using MRI. Nat Neurosci. 2017;20:299–303. doi: 10.1038/nn.4500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Baeken C, Marinazzo D, Wu GR, Van Schuerbeek P, De Mey J, Marchetti I, et al. Accelerated HF-rTMS in treatment-resistant unipolar depression: Insights from subgenual anterior cingulate functional connectivity. World J Biol Psychiatry. 2014;15:286–297. doi: 10.3109/15622975.2013.872295. [DOI] [PubMed] [Google Scholar]
- 55.Salomons TV, Dunlop K, Kennedy SH, Flint A, Geraci J, Giacobbe P, Downar J. Resting-state cortico-thalamic-striatal connectivity predicts response to dorsomedial prefrontal rTMS in major depressive disorder. Neuropsychopharmacology. 2014;39:488–498. doi: 10.1038/npp.2013.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang JX, Rogers LM, Gross EZ, Ryals AJ, Dokucu ME, Brandstatt KL, et al. Targeted enhancement of cortical-hippocampal brain networks and associative memory. Science. 2014;345:1054–1057. doi: 10.1126/science.1252900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Phan KL, Wager T, Taylor SF, Liberzon I. Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. NeuroImage. 2002;16:331–348. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- 58.Smith R, Fadok RA, Purcell M, Liu S, Stonnington C, Spetzler RF, Baxter LC. Localizing sadness activation within the subgenual cingulate in individuals: a novel functional MRI paradigm for detecting individual differences in the neural circuitry underlying depression. Brain Imaging Behav. 2011;5:229–239. doi: 10.1007/s11682-011-9127-2. [DOI] [PubMed] [Google Scholar]
- 59.Shilyansky C, Williams LM, Gyurak A, Harris A, Usherwood T, Etkin A. Effect of antidepressant treatment on cognitive impairments associated with depression: a randomised longitudinal study. Lancet Psychiatry. 2016;3:425–435. doi: 10.1016/S2215-0366(16)00012-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cuthbert BN, Insel TR. Toward the future of psychiatric diagnosis: the seven pillars of RDoC. BMC Med. 2013;11:126. doi: 10.1186/1741-7015-11-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Woody ML, Gibb BE. Integrating NIMH Research Domain Criteria (RDoC) into Depression Research. Curr Opin Psychol. 2015;4:6–12. doi: 10.1016/j.copsyc.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Serretti A, Chiesa A. Treatment-emergent sexual dysfunction related to antidepressants: a meta-analysis. J Clin Psychopharmacol. 2009;29:259–266. doi: 10.1097/JCP.0b013e3181a5233f. [DOI] [PubMed] [Google Scholar]
- 63.Paillere Martinot ML, Galinowski A, Ringuenet D, Gallarda T, Lefaucheur JP, Bellivier F, et al. Influence of prefrontal target region on the efficacy of repetitive transcranial magnetic stimulation in patients with medication-resistant depression: a [(18)F]-fluorodeoxyglucose PET and MRI study. Int J Neuropsychopharmacol. 2010;13:45–59. doi: 10.1017/S146114570900008X. [DOI] [PubMed] [Google Scholar]
- 64.Oliveira-Maia AJ, Garcia-Guarniz AL, Sinanis A, Pascual-Leone A, Press D. Comparative Efficacy of Repetitive Transcranial Magnetic Stimulation for Treatment of Depression Using 2 Different Stimulation Devices: A Retrospective Open-Label Study. J Clin Psychiatry. 2016;77:e743–4. doi: 10.4088/JCP.15l10275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Boes AD, Prasad S, Liu H, Liu Q, Pascual-Leone A, Caviness VS, Jr, Fox MD. Network localization of neurological symptoms from focal brain lesions. Brain. 2015;138:3061–3075. doi: 10.1093/brain/awv228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Darby R, Laganiere S, Pascual-Leone A, Prasad S, Fox MD. Finding the imposter: brain connectivity of lesions causing delusional misidentifications. Brain. 2016:1–11. doi: 10.1093/brain/aww288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fasano A, Laganiere SE, Lam S, Fox MD. Lesions causing freezing of gait localize to a cerebellar functional network. Ann Neurol. 2017;81:129–141. doi: 10.1002/ana.24845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fischer DB, Boes AD, Demertzi A, Evrard HC, Laureys S, Edlow BL, et al. A human brain network derived from coma-causing brainstem lesions. Neurology. 2016 doi: 10.1212/WNL.0000000000003404-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Laganiere S, Boes AD, Fox MD. Network localization of hemichorea-hemiballismus. Neurology. 2016;86:2187–2195. doi: 10.1212/WNL.0000000000002741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Horn A, Reich M, Vorwerk J, Li N, Wenzel G, Fang Q, et al. Connectivity predicts deep brain stimulation outcome in Parkinson's disease. Ann Neurol. 2017 doi: 10.1002/ana.24974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mueller S, Wang D, Fox MD, Yeo BTT, Sepulcre J, Sabuncu MR, et al. Individual Variability in Functional Connectivity Architecture of the Human Brain. 2013;77:586–595. doi: 10.1016/j.neuron.2012.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang D, Buckner RL, Fox MD, Holt DJ, Holmes AJ, Stoecklein S, et al. Parcellating cortical functional networks in individuals. Nat Neurosci. 2015;18:1853–1860. doi: 10.1038/nn.4164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mir-Moghtadaei A, Caballero R, Fried P, Fox MD, Lee K, Giacobbe P, et al. Concordance Between BeamF3 and MRI-neuronavigated Target Sites for Repetitive Transcranial Magnetic Stimulation of the Left Dorsolateral Prefrontal Cortex. Brain Stimul. 2015;8:965–973. doi: 10.1016/j.brs.2015.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.DeBlasio KL, Tendler A. A Retrospective Chart Review of 10 Hz Versus 20 Hz Repetitive Transcranial Magnetic Stimulation for Depression. SAGE Open. (4) 2012;2 2158244012470109. [Google Scholar]
- 75.Shajahan PM, Glabus MF, Steele JD, Doris AB, Anderson K, Jenkins JA, et al. Left dorso-lateral repetitive transcranial magnetic stimulation affects cortical excitability and functional connectivity, but does not impair cognition in major depression. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26:945–954. doi: 10.1016/s0278-5846(02)00210-5. [DOI] [PubMed] [Google Scholar]
- 76.Gugino LD, Romero JR, Aglio L, Titone D, Ramirez M, Pascual-Leone A, et al. Transcranial magnetic stimulation coregistered with MRI: a comparison of a guided versus blind stimulation technique and its effect on evoked compound muscle action potentials. Clinical Neurophysiology. 2001;112:1781–1792. doi: 10.1016/s1388-2457(01)00633-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sparing R, Buelte D, Meister IG, Pauš T, Fink GR. Transcranial magnetic stimulation and the challenge of coil placement: a comparison of conventional and stereotaxic neuronavigational strategies. Hum Brain Mapp. 2008;29:82–96. doi: 10.1002/hbm.20360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cheeran B, Talelli P, Mori F, Koch G, Suppa A, Edwards M, et al. A common polymorphism in the brain-derived neurotrophic factor gene (BDNF) modulates human cortical plasticity and the response to rTMS. J Physiol. 2008;586:5717–5725. doi: 10.1113/jphysiol.2008.159905. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.