Chronic wasting disease (CWD), a fatal neurodegenerative prion disease of cervids, has spread across North America and has been detected in The Republic of Korea, Finland, and Norway. CWD appears to spread by horizontal transmission, and prions shed in saliva, feces, and urine are thought to contribute.
KEYWORDS: CWD, PMCA, RT-QuIC, deer, detection, prion, saliva
ABSTRACT
Chronic wasting disease (CWD), a fatal neurodegenerative prion disease of cervids, has spread across North America and has been detected in The Republic of Korea, Finland, and Norway. CWD appears to spread by horizontal transmission, and prions shed in saliva, feces, and urine are thought to contribute. However, studies investigating the rapid spread of CWD have been hampered by assay inhibitors and a lack of consistent and sensitive means to detect the relatively low levels of prions in these samples. Here we show that saliva frequently contains an inhibitor of the real-time quaking-induced conversion assay (RT-QuIC) and that the inhibitor is a member of the mucin family. To circumvent the inhibitor, we developed a modified protein misfolding cyclic amplification (PMCA) method to amplify CWD prions in saliva that were undetectable or ambiguous by RT-QuIC. Our results reinforce the impact of saliva in horizontal CWD transmission and highlight the importance of detection optimization.
INTRODUCTION
Chronic wasting disease (CWD) is a uniformly fatal neurodegenerative disease affecting wild and captive cervid populations. CWD is rapidly spreading across North America and has been detected in The Republic of Korea and Scandinavia (1–3). While horizontal transmission appears to be responsible for CWD spread in both wild and captive cervid populations, the sources of infectious prions remain unclear (4). Infectious prions have been detected in excreta, including saliva, urine, and feces, which likely contribute to horizontal spread, but inconsistent prion detection in these sample types makes temporal shedding patterns difficult to assess (5–10).
Development of amplification assays, including protein misfolding cyclic amplification (PMCA) and real-time quaking-induced conversion (RT-QuIC), has increased the sensitivity of prion detection in tissues and fluids with titers too low for detection by traditional immunoassay methods such as Western blotting (WB) or immunohistochemistry (IHC) (11). PMCA and RT-QuIC rely on conversion of a normal prion protein substrate, PrPC (derived from transgenic mouse brain homogenate and recombinant bacterial protein, respectively), by a prion-containing sample. Prion conversion in PMCA has traditionally been detected by WB of amplified products, whereas conversion in RT-QuIC is detected by thioflavin T intercalation in amyloid fibrils and subsequent fluorescence. Despite the advances in sensitivity of prion detection by RT-QuIC and PMCA, interpretation can still be complicated by falsely negative results.
RT-QuIC has been used to detect prions in a variety of samples, including saliva, feces, blood, cerebrospinal fluid (CSF), and urine (6, 7, 12–15). Some samples have near-perfect detection sensitivity and specificity, but others have lower sensitivity, which has been attributed to the presence of reaction inhibitors. Hoover et al. observed that the highest concentrations of brain homogenate inhibited amyloid formation in RT-QuIC and that the expected dilutional response does not commence until the brain homogenate has been diluted to approximately 0.01% (wt/vol) (16). The removal of polar lipids from brain samples enabled detection of seeding activity in more concentrated samples and restored a linear dilution response (16). Other compelling evidence for RT-QuIC inhibitors comes from studies of prions in “inhibitor-laden” samples, such as whole blood and plasma (17, 18). For example, Orrú et al. used immunoaffinity capture to trap prions from plasma and aid in escaping the putative inhibitor(s) (14). Likewise, in previous work with saliva and urine, we used phosphotungstic acid (PTA) precipitation or iron oxide magnetic extraction (IOME) to increase RT-QuIC sensitivity (6, 19, 20). Although these methods increased prion detection, we continued to observe (i) fewer positive saliva samples than predicted and (ii) a pattern wherein saliva samples appeared negative until diluted (6, 20). Because dilution was often required for prion detection in saliva samples, we suspected that RT-QuIC may fail to detect prions in saliva samples due to the presence of assay inhibitors.
PMCA has also been used to amplify and detect CWD prions in a variety of tissue types where other assays have failed, including in skeletal muscle and whole blood (21, 22). In contrast, PMCA was unable to detect CWD prions in urine and saliva samples that induced infection in transgenic mouse bioassays (23). However, an optimized version of PMCA could detect prions in saliva from cows experimentally infected with bovine spongiform encephalopathy (BSE) (24, 25). To improve the detection of prions in saliva, which is necessary to understand CWD transmission, we endeavored to both identify the assay inhibitor(s) in saliva and develop a sensitive method to detect CWD prions despite the presence of such inhibitors.
In ruminants and in fallow and roe deer (Cervus dama dama and Capreolus capreolus) in particular, saliva is basic (pH 8.2 to 8.6) and buffered, principally by Na+, K+, inorganic phosphate, and Ca2+ (26, 27). Mixed saliva (from all salivary gland tissue) contains 0.7 to 6.7 mg/ml of protein (26). The most common protein family in saliva is the mucin family, which is comprised of complex glycoproteins that lend saliva its viscosity and provide lubrication (28–30). Mucins have cysteine-rich termini to facilitate disulfide bonds between and within monomers and serine/threonine-rich cores to facilitate the addition of O- and N-linked oligosaccharides, including sialic acids and acetylhexosamine (30). Due to their complex structure, mucins have been hypothesized to be “traps” for smaller molecules, rendering them inaccessible (31). In our investigations, we examined the major components of saliva, including the mucin protein family, for their potential to inhibit RT-QuIC.
In the present study, we (i) confirmed the presence of RT-QuIC inhibitors in saliva and have concluded that the RT-QuIC inhibitor is a member of the mucin family, (ii) introduced a modified PMCA technique wherein saliva successfully seeds PrPC in brain homogenate and assay sensitivity is enhanced by using RT-QuIC versus WB as the PMCA readout, and (iii) used the modified PMCA approach to detect prions in saliva samples that were inhibited in RT-QuIC. Together, the data suggest that prion shedding in saliva may be more frequent than previously appreciated and that the combination of these two powerful prion amplification assays can optimize sensitivity in complex biological samples.
MATERIALS AND METHODS
Saliva samples.
White-tailed deer were sourced from the University of Georgia Warnell School of Forestry and Natural Resources (a region where CWD is not endemic; see Acknowledgments) and were housed in an indoor CWD research facility at Colorado State University. We maintained all deer in accordance with protocols approved by the Colorado State University Institutional Animal Care and Use Committee. Saliva was obtained from anesthetized deer by inserting a 3-ml syringe into the cheek pouch closest to the ground and aspirating. Samples were aliquoted and frozen at −80°C until testing. Deer were inoculated as follows: deer 121, 1.0 g of CWD+ mule deer brain intracerebrally; deer 139, 0.25 g of CWD+ transgenic 1536 mouse brain intravenously (32); deer 142, 0.25 g of CWD+ transgenic 1536 mouse brain intracerebrally (32); deer 372, 250 ml of whole blood from CWD+ deer intravenously (10, 33); deer 4116, 300 ml of whole blood from CWD+ deer intravenously (10, 33); deer 777, 783, and 786, 1.0 g of CWD+ white-tailed deer brain per os (52); and deer 813, 815, 816, and 817, 0.1 g of CWD+ white-tailed deer brain via aerosolization (34).
Western blotting and Coomassie stain.
We pooled replicate RT-QuIC wells and combined 20 μl of RT-QuIC product or saliva with 20 μl of 2× Laemmli sample buffer (LSB)–β-mercaptoethanol (β-ME) (Novagen) and boiled the samples for 5 min. We loaded 30 μl of each sample to a 12-well, 12% bis-Tris, precast polyacrylamide gel (Novagen) and electrophoresed it at 150 V for 1 h. For Coomassie-stained gels, we added Coomassie stain (0.25% [wt/vol] Coomassie brilliant blue R250, 7.5% [vol/vol] glacial acetic acid, 50% [vol/vol] methanol) to the gel and rocked it at room temperature for 2 h, then removed the stain, rinsed the gel, and destained (7.5% [vol/vol] glacial acetic acid, 50% [vol/vol] methanol) it for 2 h at room temperature. For Western blots, we transferred the proteins to polyvinylidene fluoride (PVDF) membranes for 1 h at 80 V on ice. We blocked the membranes in 5% nonfat dry milk and then exposed the membranes to anti-PrP antibody Bar224 (Cayman Chemicals) overnight at 4°C, followed by secondary antibody (goat anti-mouse IgG2a-horseradish peroxidase [HRP] [SeraCare]) for 1 h at room temperature. We developed the Western blots with Pierce ECL substrate (Thermo Scientific). For both Western blots and Coomassie-stained gels, we collected images with an ImageQuant LS (GE).
Inhibitory saliva pools.
We made a large pool of CWD− saliva to be used for the experiments in this study. The pool was comprised of 1 ml of baseline saliva samples (preinoculation) from each of the following deer: 1201, 1204, 1205, 1211, and 1218.
Protease treatment.
We treated saliva samples with proteinase K (PK; final concentration of 4 μg/ml) at 45°C for 1 h at 900 rpm. We added 10 mM phenylmethylsulfonyl fluoride (PMSF) to each sample and boiled the samples for 5 min to inactivate the PK. Next, we spiked 1 μl of 0.01% brain homogenate into 9 μl of treated saliva.
Protease inhibitor treatment.
To test whether the proteases present in saliva were destroying the recombinant PrP (rPrP) upon which the RT-QuIC assay relies, we preincubated saliva with rPrP, with or without protease inhibitors. We substituted one-third of the water in the RT-QuIC mastermix (1 mM EDTA, 0.1 mg/ml of rPrP, 320 mM NaCl, 10 μM thioflavin T [ThT], H2O) with saliva (for a final concentration of 20% [vol/vol]). We added protease inhibitor cocktail (EDTA-free protease inhibitor cocktail; Thermo Scientific; 78437) and incubated the mixture for 1 h, 2 h, 6 h, or overnight at 42°C with 1 min of shaking (500 rpm) alternating with 1 min of rest. We used these samples as the substrate for RT-QuIC or for electrophoresis and then Western blotting or Coomassie staining.
Mucin treatment.
We made fresh mucin daily (25 mg/ml of bovine submaxillary mucin [Sigma] in 10 mM HEPES buffer, pH 7.6) and diluted it in phosphate-buffered saline (PBS) or saliva electrolytes for final concentrations of 0.5 mg/ml, 1.25 mg/ml, 2.5 mg/ml, 5.0 mg/ml, 6.25 mg/ml, and 12.5 mg/ml. The use of 10 mM HEPES as a diluent for bovine submaxillary mucin was published previously (35).
Saliva electrolyte treatment.
We created a solution with the electrolyte concentrations of ruminant saliva based on a 1948 report by McDougall (36). The solution composition is as follows: 117 mM NaHCO3, 26 mM Na2HPO4·12H2O, 8 mM NaCl, 8 mM KCl, 0.2 mM CaCl2 anhydrous, 0.3 mM MgCl2 anhydrous. We dissolved these salts in deionized water and filtered the solution. We added 2% SDS for a final concentration of 0.1% SDS and diluted brain homogenate into the SDS-electrolyte solution.
Saliva screen.
To determine the frequency with which negative saliva samples contain inhibitors of RT-QuIC, we devised a screening protocol. First, we diluted positive brain to a final concentration of 0.01% or 0.001% in saliva and 0.01% SDS and tested for inhibition in triplicate in RT-QuIC. To test the dilutional response of the inhibitor, we added 0.001% positive brain in 30% saliva or 60% saliva, added SDS for a final concentration of 0.01%, and added the sample to triplicate wells in RT-QuIC.
RT-QuIC.
We purified Syrian hamster rPrP (codons 90 to 231) as previously described (37). We expressed the cDNA in E. coli BL21-Star cells overnight under the following inducing conditions: LB medium, kanamycin and chloramphenicol, 20× NPS [0.5 M (NH4)2SO4, 1 M KH2PO4, 1 M Na2HPO4], 50× 5052 [0.5% glycerol, 0.05% glucose, 0.2% lactose], and 1 mM MgSO4. We harvested inclusion bodies with lysozyme (0.25 mg/ml), DNase (1 μg/ml), and MgCl2 (5 mM) in 1× Bugbuster (Novagen) by following the manufacturer's protocol (Novagen). We solubilized inclusion body pellets in 8 M guanidine hydrochloride (GdnHCl) overnight and then bound the solubilized protein to Ni-agarose resin, rotating at room temperature for 45 min (GE Healthcare Life Sciences). We refolded the rPrP with a gradient from 6 M GdnHCl in 100 mM Na2HPO4 and 10 mM Tris to the same buffer with no GdnHCl on a GE fast-performance liquid chromatograph (FPLC; AktaPure; GE Healthcare Life Sciences). We eluted with a gradient from 0.0 M to 0.5 M imidazole and dialyzed the rPrP in two changes of 20 mM NaH2PO4 overnight and then it stored at 4°C.
We added 0.1 mg/ml of rPrP to 10 μM ThT, 320 mM NaCl, 1 mM EDTA, and 1× PBS in 1 well of a 96-well plate (Nunc black, optical-bottom, 96-well plates; Thermo Scientific). Sample preparation was variable, depending on the treatment, and is described in the previous sections, but we always added 2 μl of the sample to each well. We either prepared the sample in 0.1% SDS or added 2 μl of 0.1% SDS to the plate separately. For controls, we included brain (positive and negative) diluted into PBS and into inhibitory saliva on every plate. We used a BMG Fluostar Omega microplate reader to shake the plates for 1 min (double orbital shaking at 700 rpm), followed by 1 min of rest, for 62.5 h at 42°C. The fluorescence was recorded every 15 min with a 450-nm excitation wavelength, a 480-nm emission wavelength, and a gain of 1,700.
To test PMCA products by RT-QuIC, we added 0.09 mg/ml of rPrP to 10 μM ThT, 320 mM NaCl, 1 mM EDTA, and 1× PBS in 1 well of a 96-well plate. We diluted PMCA products 1,000-fold in 0.1% SDS–1× PBS and then added 2 μl of PMCA product to each well.
The RT-QuIC data are displayed as lag phases. To calculate the lag phase, we established a threshold fluorescence (5 standard deviations above the average baseline fluorescence on the plate) and determined the time that the fluorescence signal crosses the threshold (lag phase). For samples that did not cross the threshold during the 62.5-h experiment, we assigned a lag phase of 70 h.
Sonication.
We added saliva (undiluted or diluted) to 0.2-ml tubes and sonicated the tubes in a horn sonicator (QSonica LLC) in a 37°C incubator. We used a program consisting of 29.5 min of rest, followed by 30 s of sonication with an amplitude of 30. For an experiment lasting 72 h, the samples were typically exposed to ∼1,000,000 J.
PMCA.
Our PMCA reaction was adapted from previous publications (9, 22, 38). We used a substrate of 10% (wt/vol) brain homogenate from transgenic mice that overexpress elk PrPC (Tg5037 mice [39]), homogenized in 1× PBS, 150 mM NaCl, and 1% Triton X-100. Brain homogenate was stored at −80°C until use. We seeded 90 μl of brain homogenate substrate with 10 μl of saliva in 0.2-ml Eppendorf tubes containing three 1.59-mm-diameter and two 2.38-mm-diameter polytetrafluoroethylene beads. In the first round, samples were subjected to cycles of 29.5 min of rest followed by 30 s of pulse sonication for 72 h in a Qsonica microplate horn sonicator housed within a 37°C incubator and received energy levels of approximately 1,000,000 J. All subsequent rounds used 50 μl of 10% brain homogenate as the substrate and 20 μl of PMCA product from the previous round. Rounds 2 to 5 were also subjected to cycles of 29.5 min of rest and 30 s of pulse sonication for 24 h, receiving energy levels of approximately 300,000 J. PMCA reactions were continued for 5 rounds. A temperature probe measuring the water temperature of the sonicator indicated that the operating temperature was between 48 and 50°C during the experiment. PMCA samples were evaluated for PrPSc amplification by Western blotting or RT-QuIC.
Western blot detection of PMCA products.
First, 10-μl volumes of PMCA products (10% brain homogenate) were digested with 1 μl of solution containing 50 μg of proteinase K (Invitrogen) and incubated at 45°C for 1 h with shaking. Following PK digestion, samples were mixed with a reducing agent (10× LDS–4× sample buffer; Invitrogen) at a final concentration of 1× and boiled for 10 min. A total of 15 μl of sample was run on a 26-well 12% bis-Tris gel (Bio-Rad) at 125 V for 1.5 h. Proteins were transferred to a PVDF membrane at 80 V for 1 h on ice. The PVDF membrane was blocked in 5% nonfat dry milk for 1 h at room temperature and then incubated with anti-PrP antibody Bar224 (Cayman Chemicals) diluted in Tris-buffered saline–0.01% Tween (TBS-T) to 0.2 μg/ml overnight at 4°C, followed by goat anti-mouse secondary antibody conjugated to HRP (goat anti-mouse IgG2a-HRP [SeraCare]) diluted in TBS-T to 0.4 μg/ml for 1 h at room temperature. We developed the Western blots with Pierce ECL substrate (Thermo Scientific) and captured images with an ImageQuant LS (GE).
RESULTS
Saliva samples often require dilution to seed RT-QuIC.
We previously demonstrated that prions in saliva are infectious and have used RT-QuIC to quantify prions in saliva and other excreta (5, 6, 10, 13, 19, 20, 38). In RT-QuIC experiments, some saliva samples appeared negative until they were diluted at least 10-fold (examples in Fig. 1A); this suggested that saliva contained an inhibitor of RT-QuIC which must be diluted to allow prion seeding.
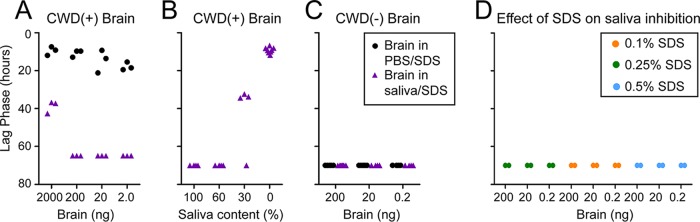
FIG 1.
Saliva inhibits seeding activity in RT-QuIC. (A) Points indicate the lag phase for each replicate, which indicates the efficiency of amyloid formation. Briefly, the lag phase is the time at which the fluorescence crosses the threshold (5 SDs above baseline fluorescence). If a sample did not cross the threshold during the 62.5-h experiment, it was assigned a value of 70. Three saliva samples that had detectable seeding activity only upon dilution are shown. (B to D) We tested 39 saliva samples from CWD− deer for their ability to inhibit prion seeding activity in RT-QuIC. (B) First, we tested the ability of saliva to inhibit seeding from 200 ng of CWD+ brain or 20 ng of CWD+ brain. Saliva samples prevented seeding in either 100%, 66%, 33%, or 0% of the three spiked replicates. The numbers of saliva samples in the categories are indicated by the bars. (C) Next, we tested whether dilution of saliva resulted in a dilutional response of seeding inhibition. More saliva samples completely abolished RT-QuIC seeding by 20 ng of CWD+ brain (inhibited 4/4 replicates) as the percentage of saliva in the diluent increased. (D) Many saliva samples did not completely abolish seeding but still showed an inhibitory effect by delaying the RT-QuIC lag phase. The shaded area represents the normal range of lag phases for 20 ng of CWD+ brain without saliva. Each point represents the lag phase for an individual replicate for an individual saliva sample (3 replicates per sample; 39 samples).
First, we investigated how commonly saliva inhibited RT-QuIC. We performed a series of spiking experiments, in which CWD+ brain was diluted into 39 CWD− saliva samples, and examined the ability of the mixture to seed RT-QuIC. Roughly 25% of the saliva samples completely abolished seeding when 200 ng of CWD+ brain homogenate was added. That percentage increased to nearly 50% when only 20 ng of CWD+ brain homogenate was used as the seed (Fig. 1B). Next, we added 200 ng of brain to serial dilutions of saliva in 1× PBS. More saliva samples completely inhibited RT-QuIC at higher saliva concentrations and less inhibition was observed at lower saliva concentrations, confirming a dilutional response (Fig. 1C). Even in samples that displayed some seeding activity, it was common for the lag phase (an indication of seeding efficiency) to be prolonged in the presence of increasing saliva concentrations (Fig. 1D). These data confirmed the presence of an RT-QuIC reaction inhibitor in saliva.
Saliva inhibits seeding activity in RT-QuIC.
We created a pool of inhibitory saliva from CWD-naive deer (n = 5) to ensure that we had a consistent sample of saliva for subsequent experiments. First, we confirmed that the CWD− saliva pool inhibited RT-QuIC by repeating the CWD+ brain spiking experiments described above. We diluted CWD+ brain in 100% saliva plus 0.1% SDS or in PBS plus 0.1% SDS, a required component of the RT-QuIC assay. Seeding for all but the most concentrated dilution of brain homogenate was completely blocked by dilution in saliva but not PBS. The dilution containing the highest concentration of brain had an increased lag phase compared to that of the PBS-SDS control, confirming that our CWD− saliva pool inhibited RT-QuIC even in the presence of high concentrations of brain prions (Fig. 2A).
FIG 2.
Negative saliva pool inhibits seeding activity in RT-QuIC. We created a pool of negative saliva and confirmed its ability to inhibit seeding activity of CWD+ brain homogenate. (A) Pooled CWD− saliva was used to dilute CWD+ brain homogenate, and 2 μl of each dilution was added to seed the RT-QuIC assay. (B) Next 200 ng of brain homogenate was added to a series of CWD− saliva dilutions (100%, 60%, 30%, and 0% saliva, diluted in PBS) and 2 μl of each sample was added to RT-QuIC. (C) CWD− brain was diluted in CWD− saliva to ensure that saliva did not cause spontaneous conversion of rPrP to amyloid. (D) The SDS concentration of the sample is indicated in the color key; the SDS concentration in the final RT-QuIC reaction is 2% of the concentration indicated in the key. Samples in panels A to D are from the same experiment, with positive controls (PBS as diluent) shown in panel A.
Next, we repeated the saliva dilutional series by adding 200 ng of CWD+ brain to serial dilutions of saliva in 1× PBS. Again, there was a dilutional response to saliva inhibition; seeding activity was completely inhibited in the presence of 100% saliva and 60% saliva, delayed at 30% saliva, and uninhibited at 0% saliva (Fig. 2B). We also tested dilutions of CWD− brain in PBS–0.1% SDS or saliva–0.1% SDS to ensure that our saliva pool did not cause spontaneous misfolding of the rPrP substrate (Fig. 2C). We did not observe any spontaneous misfolding under these conditions. Finally, to ensure that saliva did not inhibit RT-QuIC by rendering SDS ineffective, we examined the impact of increased SDS concentrations in the reaction (Fig. 2D). Increased SDS concentrations failed to rescue the inhibition by saliva.
Saliva contains proteases that degrade rPrP but do not affect the RT-QuIC reaction.
We hypothesized that the RT-QuIC inhibitor in saliva may damage one of the essential components of the RT-QuIC assay, especially rPrP or ThT. To test whether saliva impeded the RT-QuIC assay by degrading rPrP, we incubated saliva with rPrP for 1 h and assessed degradation by Western blotting. The addition of large concentrations of saliva (20 to 33% total volume, 10 to 15 times the volume normally used in an RT-QuIC experiment) resulted in degradation of the rPrP substrate, which confirmed that salivary proteases are active under typical experimental conditions (Fig. 3A). Not surprisingly, when we used the rPrP samples preincubated with saliva as the RT-QuIC substrate with a CWD+ brain seed, we observed no amyloid formation (Fig. 3B, QuIC products D to F).
FIG 3.
Saliva contains proteases, which degrade rPrP in RT-QuIC but do not alter seeding at experimental saliva concentrations. (A) To test whether saliva alters RT-QuIC rPrP substrate, we incubated rPrP with variable concentrations of saliva and then assessed protein degradation by Western blot. When rPrP was combined with a final saliva concentration of 33%, 25%, or 20% for 1 h at room temperature, there was a noticeable degradation product (indicated by the red arrow). (B) Lane A, 20 ng of CWD+ brain diluted in saliva, added at 2 μl; lane B, 2 ng of CWD+ brain diluted in saliva, added at 2 μl; lane C, 2 μl of saliva added; lane D, 20 ng of CWD+ brain added to 33% saliva–rPrP mixture; lane E, 20 ng of CWD+ brain added to 25% saliva–rPrP mixture; lane F, 20 ng of CWD+ brain added to 20% saliva–rPrP mixture; lane G, 20 ng of CWD− brain added to rPrP (no saliva). (C) We treated saliva (20% [vol/vol]) and/or rPrP (0.1 mg/ml) with a protease inhibitor cocktail (PIC) and collected samples during a 16-h incubation period. We used the treated samples as the substrate for RT-QuIC and compared pre- and post-RT-QuIC samples by Western blotting.
To evaluate the effect of proteases in normal RT-QuIC conditions, we diluted CWD+ brain homogenate in the inhibitory saliva pool and added 2 μl to the RT-QuIC assay with fresh rPrP substrate (this constituted a final saliva concentration in the RT-QuIC reaction of approximately 2%). In this case, we observed the previously described inhibition of amyloid formation without substantial degradation of the rPrP (Fig. 3B, QuIC products A and B). Excess rPrP remained at the end of the experiment. These results supported the tenet that RT-QuIC inhibition was not due to degradation of rPrP; the concentration of salivary proteases was too low in the face of excess rPrP. To confirm this hypothesis, we treated saliva and rPrP with a protease inhibitor cocktail, sampled the mixture over a 16-h incubation, and then used the incubated samples as the substrate for the RT-QuIC assay. The addition of protease inhibitors reduced the minor degradation of rPrP by saliva proteases, but seeding was not rescued (Fig. 3C). We concluded that saliva did not inhibit RT-QuIC by degrading the rPrP substrate.
To assess whether saliva interfered with the function of ThT, which is required for detection of amyloid in the RT-QuIC assay, we compared the baseline fluorescence and the maximum fluorescence in positive wells with or without saliva (Table 1). Neither the baseline nor the maximum fluorescence values differed significantly among wells with and without saliva, suggesting that saliva had no effect on ThT function (P = 0.8229 and 0.119, respectively; two-sided, unpaired t test).
TABLE 1.
Saliva does not affect thioflavin T fluorescencea
| Parameter | Value (mean ± SEM) for sample type |
P value (two-sided, unpaired t test, α = 0.05) | |
|---|---|---|---|
| Not containing saliva (n = 20) | Containing 2% (vol/vol) saliva (n = 54) | ||
| Avg baseline fluorescence | 7,994 ± 761 | 8,195 ± 465 | 0.8229 (not significant) |
| Avg maximum fluorescence of positive sample | 140,475 ± 12,328 | 121,842 ± 5,585 | 0.1196 (not significant) |
We compared the baseline fluorescence from RT-QuIC wells containing saliva or no saliva with a two-sided, unpaired t test. We compared the final fluorescence (250 cycles, 62.5 h) from positive wells (those with amyloid formation) that contained saliva or did not contain saliva. Wells that contained saliva contained 2 μl of saliva in a 100-μl reaction volume for a 2% (vol/vol) concentration.
Proteinase K removes the RT-QuIC inhibitor in CWD− saliva but only occasionally rescues seeding in CWD+ saliva samples.
Based on the high protein content of ruminant saliva, we suspected that the inhibitor may be a protein, so we treated our CWD− saliva pool with several concentrations of PK (26). This produced changes in the total protein content in saliva samples treated with 1.0 to 5.0 μg/ml of PK (Fig. 4A). We tested the ability of PK-treated saliva to inhibit RT-QuIC (after PMSF treatment to stop the PK activity). Upon treatment with ≥1.0 μg/ml of PK, the inhibitory saliva pool was no longer inhibitory (Fig. 4B). We also PK treated PBS and stopped the reaction with PMSF to (i) ensure that we were removing all PK activity and (ii) ensure that PMSF did not alter the RT-QuIC reaction. PMSF eliminated all detectable PK activity and had no discernible effect on the RT-QuIC reaction. Based on the effects of PK on total protein composition (Fig. 4A), we examined the effect of 4 μg/ml of PK on the inhibitory saliva pool. PK treatment, but not sham treatment, rescued seeding (Fig. 4C).
FIG 4.
Protease treatment rescues seeding in spiked negative saliva and in some positive saliva samples. (A) To evaluate the effect of proteinase K (PK) concentration on the protein population of saliva, we treated the inhibitory saliva pool with PK concentrations ranging from 0.5 μg/ml to 5.0 μg/ml, stopped the PK with phenylmethylsulfonyl fluoride (PMSF), and assessed the total protein content by Coomassie staining. PBS at 1× was treated in parallel as a negative control. (B) We treated saliva with PK, stopped the reaction with PMSF, then spiked in 200 ng CWD+ brain homogenate and added the solution to RT-QuIC. Points indicate the lag phase for each replicate. C. We chose a concentration of 4 μg/ml PK and compared treatment with PK to sham treatment (heat and PMSF). Points indicate the lag phase for each replicate. 20 ng CWD+ brain were added to each treated saliva sample. D. Finally, we tested the effect of PK on CWD+ saliva samples that inhibit RT-QuIC and require dilution for seeding. PK treatment rescued seeding activity in 2/3 samples analyzed. In the sample that failed to rescue (number 121), we diluted the PK-treated saliva to examine if inhibitors were still present in the sample (bright green). There were 8 replicates of each sample.
We next examined the effect of 4 μg/ml of PK on individual potentially inhibited saliva samples. PK rescued seeding in two of the three saliva samples from CWD+ deer that are normally inhibited in RT-QuIC (Fig. 4D). For the sample (121) in which seeding was not rescued by PK, we hypothesized that if the inhibitor remained unaffected by PK, dilution would result in positive seeding (as it did when sample 121 was not PK treated). However, dilution did not enable seeding, suggesting that the prions in sample 121 may have been less PK resistant than “typical” prions (Fig. 4D). Together, these data support the notion that the RT-QuIC inhibitor is a protein and imply that prions in some saliva samples may be relatively protease sensitive.
Electrolytes in saliva are compatible with RT-QuIC.
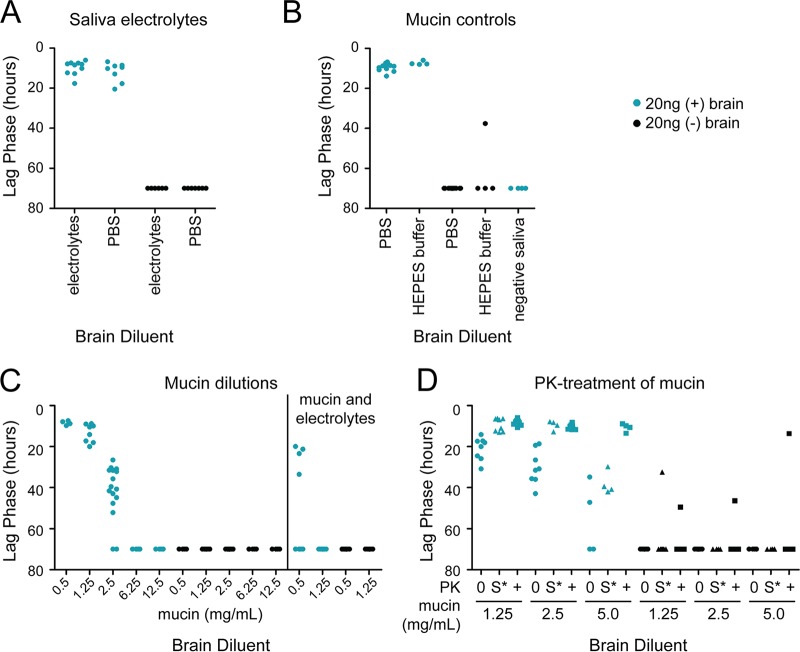
The major components of saliva besides proteins are electrolytes. We created a solution (117 mM NaHCO3, 26 mM Na2HPO4·12H2O, 8 mM NaCl, 8 mM KCl, 0.2 mM CaCl2 anhydrous, 0.3 mM MgCl2 anhydrous) to mimic the electrolyte milieu of ruminant saliva and diluted brain homogenate into the solution instead of saliva (36). This electrolyte solution did not inhibit RT-QuIC (Fig. 5A).
FIG 5.
The electrolytes in saliva are compatible with RT-QuIC, but mucin is not. Points indicate the lag phase for each RT-QuIC replicate. (A) We diluted CWD+ brain in an electrolyte solution designed to mimic the electrolyte composition of saliva and did not observe any effect on RT-QuIC seeding (36). (B) We tested the inhibitory effects of purified bovine submaxillary mucin in the RT-QuIC assay. We compared the mucin treatments to buffer only (HEPES) and to PBS as negative controls. (C) We tested multiple mucin concentrations within the physiological range (28, 29, 40, 41). (D) We treated mucin-spiked PBS with PK. 0, no PK and no treatment; S*, sham treatment (heat and PMSF but no PK); +, PK, heat, and PMSF treatment.
Mucin inhibits RT-QuIC.
The major protein component of saliva is the mucin family, a complex group of glycoproteins that provide the viscosity of saliva (40, 41). We compared several reported physiological concentrations of bovine submaxillary mucin with PBS as a diluent for CWD+ brain homogenate (28, 29, 40, 41). We observed a dose-dependent inhibition of RT-QuIC seeding activity with increasing mucin concentrations but not in the buffer-only controls (Fig. 5B and C). Interestingly, mucin inhibited the RT-QuIC assay at lower concentrations when combined with the saliva electrolyte solution described above (Fig. 5C). This is likely due to the effect of electrolytes on mucin structure; i.e., with increased ionic strength, mucin becomes more compact and may be a better “trap” for prions (42).
We tested the ability of PK to rescue the inhibition caused by mucin. Treatment with 4 μg/ml of PK removed the inhibition caused by 5 mg/ml of mucin, but the sham control was still inhibited (Fig. 5D). At low (but still physiologically relevant) mucin concentrations (≤2.5 mg/ml), the sham control (45°C for 1 h, followed by PMSF and boiling) was sufficient to eliminate the inhibition from mucin (Fig. 5D). Together, these data indicate that mucin inhibits the RT-QuIC assay and can be rescued by PK treatment.
Sonication removes RT-QuIC inhibitor in some CWD+ saliva samples but reduces detection specificity.
Because we suspected that mucin may be the inhibitor in the RT-QuIC assay, and that it may function by trapping prions, we sonicated the CWD− saliva pool for 72 h to fragment mucin, then spiked in CWD+ brain, and added it to the RT-QuIC assay. Sonication of the CWD− saliva removed inhibition (Fig. 6A, open circles). Encouraged by the results of sonication of the CWD− saliva pool, we sonicated inhibited CWD+ saliva samples. We hypothesized that sonication would sufficiently damage the mucin matrix to liberate the prions in CWD+ saliva and enable seeding in RT-QuIC. For two of three samples tested, sonication for 72 h did enable seeding (Fig. 6A). In the third sample (142, not the same sample that resisted PK treatment), sonication did not relieve inhibition, and the sample still required dilution for seeding activity to be detected. Importantly, we also found that CWD− saliva sonicated in parallel as assay negative controls was much more prone to induce spontaneous RT-QuIC seeding (Fig. 6B), which discouraged us from broader adoption of sonication alone to remove the inhibitor.
FIG 6.
Sonication rescues seeding in some samples but results in poor specificity. (A) Black circles indicate the RT-QuIC lag phase for unsonicated saliva samples, red circles indicate the RT-QuIC lag phase for sonicated samples (∼1,000,000 J for 3 days), and open circles indicate the RT-QuIC lag phase for control samples. (B) Black circles indicate the RT-QuIC lag phase for sonicated negative saliva samples. The box plot has a plus sign to indicate the mean and the box represents the 25% and 75% percentiles.
Modified PMCA protocol enables sensitive detection of CWD prions in saliva.
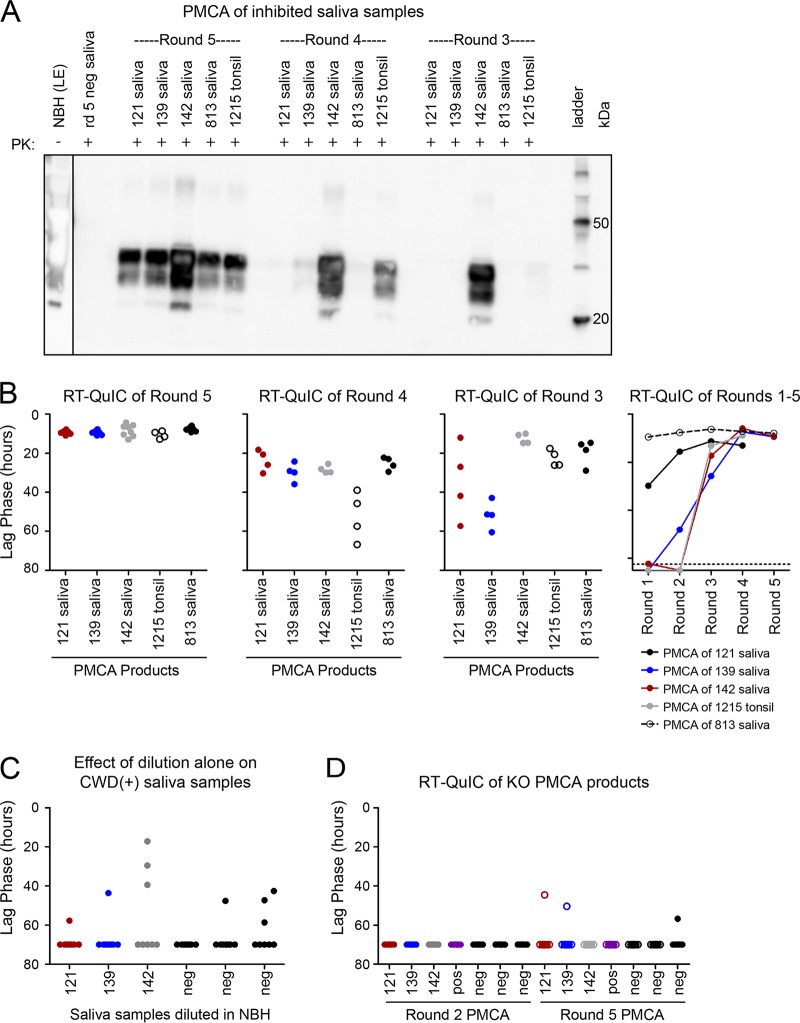
The effect of sonication on RT-QuIC inhibitors prompted us to test our inhibited saliva samples with PMCA, which uses sonication to break apart amyloid fibrils and form new reaction seeds (43). We used CWD+ saliva, which normally is inhibited in RT-QuIC, to seed transgenic mouse brain homogenate substrate in PMCA. In studies which detected BSE prions in saliva with PMCA, Murayama et al. suggested that a higher temperature and the subsequent increased energy output improved sensitivity (24, 25). We performed our PMCA reaction at 50°C with an average energy of 1,000,000 J in the first round over 3 days and 300,000 J in all subsequent 24-h rounds. PK-resistant prion-amplified material was detectable by WB at round 5 for all RT-QuIC-inhibited samples (Fig. 7A). We hypothesized that amplification would be consistently detectable before round 5 if we analyzed PMCA products by RT-QuIC. Indeed, we observed significant seeding activity by RT-QuIC in all saliva-seeded PMCA products starting at round 3, with lag phases ranging from 5 to 40 h. By comparison, Western blotting of the same round 3 PMCA products showed signal in only one of the three inhibited saliva samples. We detected seeding activity in the remaining saliva-seeded PMCA products at round 4, with lag phases less than 15 h (Fig. 7B). We did not observe any nonspecific RT-QuIC amplification in negative-control PMCA products (seeded with five individual CWD− saliva samples). Therefore, saliva samples with inhibited seeding in RT-QuIC could successfully seed PMCA, and RT-QuIC analysis of PMCA products provided detection of saliva-seeded prion amplification earlier than did Western blotting.
FIG 7.
Saliva successfully seeds PMCA and prions can be observed at earlier rounds when detected by RT-QuIC (versus Western blotting). (A) We detected PK-resistant prions by Western blotting in the PMCA products of all three saliva samples (numbers 121, 139, and 143) and in a noninhibited, positive saliva control (number 813) in round 5. CWD+ tonsil diluted 1:100 was used as a PMCA positive control. NBH, normal 10% brain homogenate (the lane is derived from a lighter exposure [LE] of the same Western blot). (B) RT-QuIC of PMCA products from the same PMCA experiment. (C) We diluted saliva samples in NBH the same way we started round 1 of PMCA. Saliva and brain mixtures were then diluted 10−3 to seed the RT-QuIC reaction. (D) We performed the full PMCA experiment using brain homogenate from mice with PrPC knocked out (KO); we then used the products to seed RT-QuIC and saw no amplification.
Because the PMCA reaction involves dilution of saliva to seed the reaction and through subsequent rounds, we made the same dilution of saliva in normal brain homogenate that was used in round 1, but skipped the sonication and incubation, and used the dilution to seed RT-QuIC. None of the brain-diluted saliva samples displayed substantial seeding activity, indicating that the sonication and incubation components of PMCA are essential for the eventual detection of prions in saliva by RT-QuIC and that the effect is not due to dilution alone (Fig. 7C). Finally, we performed the full PMCA reaction with brain homogenate from a transgenic PrPC knockout (KO) mouse (44, 45). The presence of brain (without PrPC), sonication, time, and heat did not result in saliva seeding of RT-QuIC, which suggested that the combination of sonication, PrPC substrate, and the PMCA reaction conditions was necessary to overcome the inhibition (Fig. 7D).
Prions in inhibited saliva amplify in modified PMCA.
After demonstrating the efficacy of PMCA with samples we have characterized and know contain both prion seeding activity and an RT-QuIC inhibitor, we analyzed samples whose status was less clear. We identified 11 saliva samples with the following characteristics: (i) samples that produced ≤25% positive replicates in RT-QuIC (which would not be considered positive) and (ii) RT-QuIC negative saliva samples from deer in which both earlier and later collected samples were positive (Fig. 8A). We tested whether dilution of the ambiguous saliva samples alone would enable detection by RT-QuIC, and neither a 1:10 nor a 1:100 dilution resulted in consistent detection of prions in the saliva samples (Fig. 8B and C). We found that for 9 of the 11 ambiguous samples, seeding activity was detected by PMCA (Fig. 8D). We did not observe any prion-seeded amplification in the CWD-negative control saliva (Fig. 8D). Therefore, PMCA increased prion detection sensitivity over that of RT-QuIC alone.
FIG 8.
Prions in RT-QuIC-inhibited saliva amplify in PMCA. We selected 11 samples that we suspected to contain prions because the deer had an RT-QuIC-positive saliva sample in the month(s) before and after the sample collection but had limited seeding in RT-QuIC. Points indicate RT-QuIC lag phase. (A) Seeding activity in RT-QuIC (undiluted [neat] saliva). (B) Seeding activity was detected in 9 of the 11 saliva samples evaluated by PMCA. Points indicate individual replicates of round 5 PMCA products tested in RT-QuIC. (C) Samples were diluted 1:10 and tested in RT-QuIC. (D) Samples were diluted 1:100 and tested in RT-QuIC. mpi, months postinoculation (see saliva sample source in Materials and Methods).
DISCUSSION
Detection of prions in excreta from CWD+ cervids is an essential step for understanding horizontal transmission of CWD. Here we show that despite increased sensitivity of prion detection by RT-QuIC, saliva commonly contains a protein that inhibits amyloid formation, resulting in false negatives (6, 19, 20). We then adapted PMCA to demonstrate that a substantial fraction of such saliva samples do in fact contain prion seeding activity that is obscured by the presence of RT-QuIC inhibitors, likely members of the mucin family. Mucins have been implicated in obscuring in vitro detection assays, but the exact mechanism by which mucins trap seeding, binding, or other detection reactions remain incompletely elucidated (46–48).
We hypothesized that saliva could inhibit RT-QuIC by degrading rPrP, preventing ThT intercalation into amyloid fibrils, or blocking the seeding reaction and amyloid formation. We demonstrated that salivary proteases do degrade rPrP, but this activity does not prevent the RT-QuIC reaction, as rPrP substrate remains in excess, and that ThT is unaffected. We concluded that there must be a mechanism by which some component of saliva interferes with the prion-rPrP interaction. We demonstrated that a protein in saliva, specifically mucin, inhibits RT-QuIC. However, removal of mucin by PK was not an acceptable approach because a fraction of the prions in saliva appeared to be more protease sensitive than the prions in brain or lymphoid tissues.
Prompted by evidence that detection of prions in saliva may be inhibited by mucin, we were inspired to use a protocol that involved sonication, which would provide mechanical disruption of the sample. Influenced by the observations of Haley et al. and Murayama et al. (23–25), we modified our previous PMCA method by increasing both sonication time and temperature, and consequently total energy delivered, and observed prion amplification in saliva samples that inhibited in RT-QuIC. Previous publications reporting detection of BSE prions in saliva described additional steps, including the addition of sulfated dextrans and sodium phosphotungstic acid precipitation (24, 25), whereas we did not find those steps necessary. We also found that evaluation of PMCA products by RT-QuIC versus Western blotting increased sensitivity and enabled fewer PMCA amplification rounds and therefore less brain homogenate substrate.
Although saliva commonly inhibited the RT-QuIC assay, we did not observe such an effect when evaluating samples by PMCA. Potential reasons for this difference include (i) source of the PrP substrate and (ii) sonication versus shaking. RT-QuIC uses a bacterially produced recombinant rPrP substrate and thus lacks posttranslational modifications, such as the glycosylphosphatidylinositol (GPI) anchor and glycosylation (49). The source of PMCA substrate is brain homogenate containing the native cellular PrPC (50). Although the PrP substrates differ between the assays, this difference is not likely the source of inhibition in QuIC, as diluted saliva can convert rPrP. We suspect that assay methodology, sonication versus shaking, and differences in the rest periods between these steps (RT-QuIC has a rest of 1 min and PMCA has a rest of 29 min 30 s) account for the enhanced prion detection in saliva. We suspect that the sonication step in PMCA frees prions from mucin more efficiently than shaking and that the PMCA environment, including a longer rest period and higher temperature, facilitates prion amplification (based on the data in Fig. 7D).
CWD prions have been previously identified in excreta, including saliva, feces, and urine (7, 10, 13, 23). While the contribution of each of these shed prion sources remains unknown, comparisons of prion concentration in these samples suggest that saliva contains higher prion concentrations than do other excreta. Experimental oral inoculation of naive deer with saliva produced disease in 100% of animals (3/3) inoculated during the study period, while deer inoculated with urine and feces had no evidence of prion infection after 33 months of observation (10; unpublished data). This implies that saliva plays a greater role in horizontal transmission of CWD and environmental contamination than other excreta and body fluids. Nevertheless, our previous investigations found inconsistent detection of saliva prions throughout the course of disease (13, 51). Despite our improved PMCA method, we were still unable to detect prions in two samples. It remains unknown whether these samples were truly negative (due to sampling error or biological decrease) or whether the prion levels were simply undetectable, even with our enhanced methods. Optimized PMCA or other sample treatment steps before RT-QuIC may enable more sensitive detection of prions in saliva, which is essential to understand the effect of saliva on CWD transmission.
These results provide an example of prion detection optimization and the considerations for choosing one powerful amplification assay or another, or both. An in-series diagnostic paradigm may be able to capitalize on the ease and efficiency of RT-QuIC and the sensitivity of PMCA for saliva samples (6, 19). Efforts toward improved laboratory approaches will aid in untangling the mechanisms of prion shedding and transmission.
In summary, the saliva of deer infected by CWD contains both prions and RT-QuIC inhibitors. This combination can produce false-negative results that are difficult to identify and can cause underestimation of shedding. We have introduced a modified PMCA method that enables sensitive detection of prions in saliva, including samples that are falsely negative by RT-QuIC. Prion shedding undoubtedly contributes to horizontal CWD transmission; our suggestion that mucin may shelter prions in some way prompts questions about the role of saliva in prion transmission and persistence. The inhibitor phenomenon likely extends beyond saliva, and protocols to bypass such inhibitors are essential for rapid in vitro identification of prions in the complex samples that are likely key vehicles for CWD transmission in nature.
ACKNOWLEDGMENTS
We thank our collaborators at the Warnell School of Forestry and Natural Resources, University of Georgia, for providing the hand- and indoor-raised CWD-naive white-tailed deer which were the source of the samples analyzed in these studies. We thank Nicholas Haley for helpful critique of the manuscript and Davin Henderson for feedback regarding the figures. We thank Erin McNulty for sample collection. We thank Nikki Buhrdorf and Sarah Cooper for assistance with saliva PMCA assays. Finally, we thank anonymous reviewers for their suggestions, which have certainly improved the clarity and impact of our data.
This work was supported by NIH grants R01-NS061902-09, P01-AI-077774 (E.A.H.), F30-OD021442 (K.A.D.), 9-T32OD010437 (C.E.H.), and R01-NS076894 (C.K.M.).
K.A.D. and C.E.H. designed research, performed research and data analysis, and wrote the paper. N.D.D. provided preliminary data. C.K.M. provided research administration support and contributed to research design. E.A.H. designed the research, wrote the paper, and provided funding.
REFERENCES
- 1.Haley NJ, Hoover EA. 2015. Chronic wasting disease of cervids: current knowledge and future perspectives. Annu Rev Anim Biosci 3:305–325. doi: 10.1146/annurev-animal-022114-111001. [DOI] [PubMed] [Google Scholar]
- 2.Williams ES, Young S. 1980. Chronic wasting disease of captive mule deer: a spongiform encephalopathy. J Wild Dis 16:89–98. doi: 10.7589/0090-3558-16.1.89. [DOI] [PubMed] [Google Scholar]
- 3.Norwegian Veterinary Institute. 8 March 2018. CWD in Finland is different from the Nordfjella CWD type. Norwegian Veterinary Institute, Oslo, Norway: https://www.vetinst.no/en/news/cwd-in-finland-is-different-from-the-nordfjella-cwd-type. [Google Scholar]
- 4.Miller MW, Williams ES. 2003. Prion disease: horizontal prion transmission in mule deer. Nature 425:35–36. doi: 10.1038/425035a. [DOI] [PubMed] [Google Scholar]
- 5.Henderson DM, Davenport KA, Haley NJ, Denkers ND, Mathiason CK, Hoover EA. 2015. Quantitative assessment of prion infectivity in tissues and body fluids by real-time quaking-induced conversion. J Gen Virol 96(Part 1):210–219. doi: 10.1099/vir.0.069906-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Henderson DM, Manca M, Haley NJ, Denkers ND, Nalls AV, Mathiason CK, Caughey B, Hoover EA. 2013. Rapid antemortem detection of CWD prions in deer saliva. PLoS One 8:e74377. doi: 10.1371/journal.pone.0074377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Henderson DM, Tennant JM, Haley NJ, Denkers ND, Mathiason CK, Hoover EA. 2017. Detection of chronic wasting disease prion seeding activity in deer and elk feces by real-time quaking-induced conversion. J Gen Virol 98:1953–1962. doi: 10.1099/jgv.0.000844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng YC, Hannaoui S, John TR, Dudas S, Czub S, Gilch S. 2016. Early and non-invasive detection of chronic wasting disease prions in elk feces by real-time quaking induced conversion. PLoS One 11:e0166187. doi: 10.1371/journal.pone.0166187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haley NJ, Mathiason CK, Zabel MD, Telling GC, Hoover EA. 2009. Detection of sub-clinical CWD infection in conventional test-negative deer long after oral exposure to urine and feces from CWD+ deer. PLoS One 4:e7990. doi: 10.1371/journal.pone.0007990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mathiason CK, Powers JG, Dahmes SJ, Osborn DA, Miller KV, Warren RJ, Mason GL, Hays SA, Hayes-Klug J, Seelig DM, Wild MA, Wolfe LL, Spraker TR, Miller MW, Sigurdson CJ, Telling GC, Hoover EA. 2006. Infectious prions in the saliva and blood of deer with chronic wasting disease. Science 314:133–136. doi: 10.1126/science.1132661. [DOI] [PubMed] [Google Scholar]
- 11.Saborio GP, Permanne B, Soto C. 2001. Sensitive detection of pathological prion protein by cyclic amplification of protein misfolding. Nature 411:810–813. doi: 10.1038/35081095. [DOI] [PubMed] [Google Scholar]
- 12.Haley NJ, Van de Motter A, Carver S, Henderson D, Davenport K, Seelig DM, Mathiason C, Hoover E. 2013. Prion-seeding activity in cerebrospinal fluid of deer with chronic wasting disease. PLoS One 8:e81488. doi: 10.1371/journal.pone.0081488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Henderson DM, Denkers ND, Hoover CE, Garbino N, Mathiason CK, Hoover EA. 2015. Longitudinal detection of prion shedding in saliva and urine by chronic wasting disease-infected deer by real-time quaking-induced conversion. J Virol 89:9338–9347. doi: 10.1128/jvi.01118-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Orrú CD, Wilham JM, Raymond LD, Kuhn F, Schroeder B, Raeber AJ, Caughey B. 2011. Prion disease blood test using immunoprecipitation and improved quaking-induced conversion. mBio 2(3):e00078-11. doi: 10.1128/mBio.00078-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elder AM, Henderson DM, Nalls AV, Wilham JM, Caughey BW, Hoover EA, Kincaid AE, Bartz JC, Mathiason CK. 2013. In vitro detection of prionemia in TSE-infected cervids and hamsters. PLoS One 8:e80203. doi: 10.1371/journal.pone.0080203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoover CE, Davenport KA, Henderson DM, Zabel MD, Hoover EA. 2017. Endogenous brain lipids inhibit prion amyloid formation in vitro. J Virol 91:e02162-16. doi: 10.1128/jvi.02162-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Orru CD, Wilham JM, Vascellari S, Hughson AG, Caughey B. 2012. New generation QuIC assays for prion seeding activity. Prion 6:147–152. doi: 10.4161/pri.19430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abdel-Haq H. 2015. Factors intrinsic and extrinsic to blood hamper the development of a routine blood test for human prion diseases. J Gen Virol 96(Part 3):479–493. doi: 10.1099/vir.0.070979-0. [DOI] [PubMed] [Google Scholar]
- 19.Denkers ND, Henderson DM, Mathiason CK, Hoover EA. 2016. Enhanced prion detection in biological samples by magnetic particle extraction and real-time quaking-induced conversion. J Gen Virol 97:2023–2029. doi: 10.1099/jgv.0.000515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davenport KA, Mosher BA, Brost BM, Henderson DM, Denkers ND, Nalls AV, McNulty E, Mathiason CK, Hoover EA. 2018. Assessment of chronic wasting disease prion shedding in deer saliva with occupancy modeling. J Clin Microbiol 56:e01243-. doi: 10.1128/JCM.01243-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Daus ML, Breyer J, Wagenfuehr K, Wemheuer WM, Thomzig A, Schulz-Schaeffer WJ, Beekes M. 2011. Presence and seeding activity of pathological prion protein (PrPTSE) in skeletal muscles of white-tailed deer infected with chronic wasting disease. PLoS One 6:e18345. doi: 10.1371/journal.pone.0018345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kramm C, Pritzkow S, Lyon A, Nichols T, Morales R, Soto C. 2017. Detection of prions in blood of cervids at the asymptomatic stage of chronic wasting disease. Sci Rep 7:17241. doi: 10.1038/s41598-017-17090-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haley NJ, Seelig DM, Zabel MD, Telling GC, Hoover EA. 2009. Detection of CWD prions in urine and saliva of deer by transgenic mouse bioassay. PLoS One 4:e4848. doi: 10.1371/journal.pone.0004848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murayama Y, Yoshioka M, Masujin K, Okada H, Iwamaru Y, Imamura M, Matsuura Y, Fukuda S, Onoe S, Yokoyama T, Mohri S. 2010. Sulfated dextrans enhance in vitro amplification of bovine spongiform encephalopathy PrP(Sc) and enable ultrasensitive detection of bovine PrP(Sc). PLoS One 5:e13152. doi: 10.1371/journal.pone.0013152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Okada H, Murayama Y, Shimozaki N, Yoshioka M, Masujin K, Imamura M, Iwamaru Y, Matsuura Y, Miyazawa K, Fukuda S, Yokoyama T, Mohri S. 2012. Prion in saliva of bovine spongiform encephalopathy-infected cattle. Emerg Infect Dis 18:2091–2092. doi: 10.3201/eid1812.120528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fickel J, Göritz F, Joest AB, Hildebrandt T, Hofmann RR, Breves G. 1998. Analysis of parotid and mixed saliva in Roe deer (Capreolus capreolus L.). J Comp Physiol B 168:257–264. [DOI] [PubMed] [Google Scholar]
- 27.Kay RNB. 1960. The rate of flow and composition of various salivary secretions in sheep and calves. J Physiol 150:515–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bartley EE, Yadava IS. 1961. Bloat in cattle IV: the role of bovine saliva, plant mucilages, and animal mucins. J Anim Sci 20:648–653. [Google Scholar]
- 29.Park MS, Chung JW, Kim YK, Chung SC, Kho HS. 2007. Viscosity and wettability of animal mucin solutions and human saliva. Oral Dis 13:181–186. doi: 10.1111/j.1601-0825.2006.01263.x. [DOI] [PubMed] [Google Scholar]
- 30.Tettamanti G, Pigman W. 1968. Purification and characterization of bovine and ovine submaxillary mucins. Arch Biochem Biophys 124:41–50. [DOI] [PubMed] [Google Scholar]
- 31.Habte HH, Mall AS, de Beer C, Lotz ZE, Kahn D. 2006. The role of crude human saliva and purified salivary MUC5B and MUC7 mucins in the inhibition of human immunodeficiency virus type 1 in an inhibition assay. Virol J 3:99. doi: 10.1186/1743-422X-3-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Angers R, Christiansen J, Nalls AV, Kang H-E, Hunter N, Hoover E, Mathiason CK, Sheetz M, Telling GC. 2014. Structural effects of PrP polymorphisms on intra- and interspecies prion transmission. Proc Natl Acad Sci U S A 111:11169–11174. doi: 10.1073/pnas.1404739111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mathiason CK, Hayes-Klug J, Hays SA, Powers J, Osborn DA, Dahmes SJ, Miller KV, Warren RJ, Mason GL, Telling GC, Young AJ, Hoover EA. 2010. B cells and platelets harbor prion infectivity in the blood of deer infected with chronic wasting disease. J Virol 84:5097–5107. doi: 10.1128/JVI.02169-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Denkers ND, Hayes-Klug J, Anderson KR, Seelig DM, Haley NJ, Dahmes SJ, Osborn DA, Miller KV, Warren RJ, Mathiason CK, Hoover EA. 2013. Aerosol transmission of chronic wasting disease in white-tailed deer. J Virol 87:1890–1892. doi: 10.1128/JVI.02852-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brayshaw DJ, Berry M, McMaster TJ. 2003. Optimisation of sample preparation methods for air imaging of ocular mucins by AFM. Ultramicroscopy 97:289–296. doi: 10.1016/S0304-3991(03)00054-8. [DOI] [PubMed] [Google Scholar]
- 36.McDougall EI. 1948. Studies on ruminant saliva. 1. The composition and output of sheep's saliva. Biochem J 43:99–109. [PMC free article] [PubMed] [Google Scholar]
- 37.Davenport KA, Henderson DM, Mathiason CK, Hoover EA. 2016. Assessment of the PrPc amino-terminal domain in prion species barriers. J Virol 90:10752–10761. doi: 10.1128/JVI.01121-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Haley NJ, Mathiason CK, Carver S, Zabel M, Telling GC, Hoover EA. 2011. Detection of chronic wasting disease prions in salivary, urinary, and intestinal tissues of deer: potential mechanisms of prion shedding and transmission. J Virol 85:6309–6318. doi: 10.1128/JVI.00425-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Angers RC, Seward TS, Napier D, Green M, Hoover E, Spraker T, O'Rourke K, Balachandran A, Telling GC. 2009. Chronic wasting disease prions in elk antler velvet. Emerg Infect Dis 15:696–703. doi: 10.3201/eid1505.081458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ionta FQ, Mendonca FL, de Oliveira GC, de Alencar CR, Honorio HM, Magalhaes AC, Rios D. 2014. In vitro assessment of artificial saliva formulations on initial enamel erosion remineralization. J Dent 42:175–179. doi: 10.1016/j.jdent.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 41.Langstroth GO, McRae DR, Stavraky GW. 1938. The secretion of protein material in the parasympathetic submaxillary saliva. Proc R Soc Lond B Biol Sci 125:335–347. [Google Scholar]
- 42.Feldötö Z, Pettersson T, Dėdinaitė A. 2008. Mucin-electrolyte interactions at the solid-liquid interface probed by QCM-D. Langmuir 24:3348–3357. doi: 10.1021/la703366k. [DOI] [PubMed] [Google Scholar]
- 43.Barria MA, Gonzalez-Romero D, Soto C. 2012. Cyclic amplification of prion protein misfolding. Methods Mol Biol 849:199–212. doi: 10.1007/978-1-61779-551-0_14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Büeler H, Aguzzi A, Sailer A, Greiner RA, Autenried P, Aguet M, Weissmann C. 1993. Mice devoid of PrP are resistant to scrapie. Cell 73:1339–1347. [DOI] [PubMed] [Google Scholar]
- 45.Büeler H, Fischer M, Lang Y, Bluethmann H, Lipp H-P, DeArmond SJ, Prusiner SB, Aguet M, Weissmann C. 1992. Normal development and behaviour of mice lacking the neuronal cell-surface PrP protein. Nature 356:577. [DOI] [PubMed] [Google Scholar]
- 46.Gustafsson A, Ajeti V, Ljunggren L. 2011. Detection of suPAR in the saliva of healthy young adults: comparison with plasma levels. Biomark Insights 6:BMI.S8326. doi: 10.4137/BMI.S8326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fung AO, Damoiseaux R, Grundeen S, Panes JL, Horton DH, Judy JW, Moore TB. 2012. Quantitative detection of Pf HRP2 in saliva of malaria patients in the Philippines. Malar J 11:175. doi: 10.1186/1475-2875-11-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gröschl M, Rauh M. 2006. Influence of commercial collection devices for saliva on the reliability of salivary steroids analysis. Steroids 71:1097–1100. doi: 10.1016/j.steroids.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 49.Kim JI, Cali I, Surewicz K, Kong Q, Raymond GJ, Atarashi R, Race B, Qing L, Gambetti P, Caughey B, Surewicz WK. 2010. Mammalian prions generated from bacterially expressed prion protein in the absence of any mammalian cofactors. J Biol Chem 285:14083–14087. doi: 10.1074/jbc.C110.113464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Castilla J, Saa P, Morales R, Abid K, Maundrell K, Soto C. 2006. Protein misfolding cyclic amplification for diagnosis and prion propagation studies. Methods Enzymol 412:3–21. doi: 10.1016/S0076-6879(06)12001-7. [DOI] [PubMed] [Google Scholar]
- 51.Davenport KA, Christiansen JR, Bian J, Young M, Gallegos J, Kim S, Balachandran A, Mathiason CK, Hoover EA, Telling GC. 2018. Comparative analysis of prions in nervous and lymphoid tissues of chronic wasting disease-infected cervids. J Gen Virol 99:753–758. doi: 10.1099/jgv.0.001053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Goni F, Mathiason CK, Yim L, Wong K, Hayes-Klug J, Nalls A, Peyser D, Estevez V, Denkers N, Xu J, Osborn DA, Miller KV, Warren RJ, Brown DR, Chabalgoity JA, Hoover EA, Wisniewski T. 2015. Mucosal immunization with an attenuated Salmonella vaccine partially protects white-tailed deer from chronic wasting disease. Vaccine 33:726–733. doi: 10.1016/j.vaccine.2014.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]