Abstract
Immune-based therapies for cancer are generating substantial interest because of the success of immune checkpoint inhibitors. This study aimed to enhance anticancer immunity by exploiting the capacity of dendritic cells (DCs) to initiate T cell immunity by efficient uptake and presentation of endocytosed material. Delivery of tumor-associated antigens to DCs using receptor-specific monoclonal antibodies (mAbs) in the presence of DC-activating agents elicits robust antigen-specific immune responses in preclinical models. DEC-205 (CD205), a molecule expressed on DCs, has been extensively studied for its role in antigen processing and presentation. CDX-1401 is a vaccine composed of a human mAb specific for DEC-205 fused to the full-length tumor antigen NY-ESO-1. This phase 1 trial assessed the safety, immunogenicity, and clinical activity of escalating doses of CDX-1401 with the Toll-like receptor (TLR) agonists resiquimod (TLR7/8) and Hiltonol (poly-ICLC, TLR3) in 45 patients with advanced malignancies refractory to available therapies. Treatment induced humoral and cellular immunity to NY-ESO-1 in patients with confirmed NY-ESO-1–expressing tumors across various dose levels and adjuvant combinations. No dose-limiting or grade 3 toxicities were reported. Thirteen patients experienced stabilization of disease, with a median duration of 6.7 months (range, 2.4+ to 13.4 months). Two patients had tumor regression (~20% shrinkage in target lesions). Six of eight patients who received immune-checkpoint inhibitors within 3 months after CDX-1401 administration had objective tumor regression. This first-in-human study of a protein vaccine targeting DCs demonstrates its feasibility, safety, and biological activity and provides rationale for combination immunotherapy strategies including immune checkpoint blockade.
INTRODUCTION
Generation of both humoral and cellular immune responses is a desired goal of protective vaccines against cancer and certain pathogens. Although protein-based vaccines have several potential advantages in terms of ease of manufacture and the presence of multiple antigenic epitopes, they have suffered in the past from weak immunogenicity, inefficient uptake by antigen-presenting cells (APCs) for presentation to T and B cells, and lack of targeting to appropriate APCs. DCs are highly specialized APCs that play a central role in initiating and regulating immunity. Pioneering preclinical studies from Steinman and Nussenzweig demonstrated that antibody-mediated targeting of proteins to surface receptors on DCs leads to a marked increase in immunogenicity of protein antigens in vivo (1–3). One of the first DC receptors targeted via this approach is deca-lectin, DEC-205 (CD205), which is highly expressed by both human and murine DCs and can mediate antigen uptake, processing, and presentation (4). Targeting antigens to DEC-205–expressing DCs in steady state has been shown to induce tolerance in mice (5, 6). However, this can be overcome by coadministration of an adjuvant providing DC activation, leading to induction of immune responses (7, 8).
Ligands for Toll-like receptors (TLRs) are attractive adjuvants because they not only promote maturation of DCs to an immunogenic state but also lead to release of cytokines and chemokines to create an inflammatory milieu (9). Differences between specific TLR ligands may relate not only to the pattern of TLR expression in DC subsets and other immune cells (10, 11) but also to MyD88-dependent (TLR8) or MyD88-independent (TLR3) downstream signaling aided by their intracellular distribution in the early (TLR3) and late (TLR7 and TLR8) endosomes (12).
Cancer cells express several antigens that can be targeted by the immune system. The cancer-testis antigen NY-ESO-1 is expressed in diverse cancer types but is not detected in nonmalignant tissues with the exceptions of germ cells and trophoblasts (13). Although the function of NY-ESO-1 is unknown, it has been speculated that its expression by cancers might reflect acquisition of properties that cancers find useful, such as immortality, self-renewal, migratory ability, and capacity to invade (14). Spontaneous immunity to NY-ESO-1, consisting of both cellular and humoral responses, is often detected in patients with advanced cancers, attesting to the immunogenicity of the molecule (15–17). These characteristics make NY-ESO-1 an attractive target for cancer vaccine development, and the antigen has been extensively studied in many clinical trials. NY-ESO-1 has been confirmed to be immunogenic in melanoma (13), and its expression in many cancers suggests its relevance in the generation of humoral and cellular responses (18–21). With regard to NY-ESO-1–specific therapy alone, much progress is reflected in the art of protein-based therapeutics that have focused on delivery using lipid matrices (22, 23), harnessing innate and adaptive immunity with nonspecific bacterial products like Coley’s toxin (24) or specific TLR-targeted agonists (25, 26), novel NY-ESO-1–derived promiscuous human leukocyte antigen–DR (HLA-DR) and HLA-DP epitopes (27), and antigen-specific and engineered T cell receptors for adoptive immunotherapy (28).
CDX-1401 is a fully human anti–DEC-205 monoclonal antibody (mAb) (3G9) genetically fused to the full-length NY-ESO-1 tumor antigen. In preclinical studies, NY-ESO-1 was more efficiently cross-presented to T cells when fused to 3G9 than NY-ESO-1 protein alone (29). In a human DEC-205 transgenic mouse model, the administration of 3G9-HIV-Gag together with poly-ICLC (polyinosinic-polycytidylic acid complexed with poly-L-lysine and carboxymethylcellulose) and anti-CD40 antibody led to robust anti-Gag cellular and humoral immunity (30). The combination of a DC-targeted vaccine, CDX-1401, with resiquimod (TLR7/8 agonist) and Hiltonol (poly-ICLC; TLR3 agonist) was shown to enhance antigen-specific T cell immunity in nonclinical studies (9, 31). Together, these data suggest that the DEC-205–targeted protein vaccines, in combination with TLR agonists, may be an effective platform for testing the induction of immunity against pathogens as well as cancers.
RESULTS
NY-ESO-1 expression screening
Immunohistochemistry (IHC) and polymerase chain reaction (PCR) testing of archived tumor tissue for 280 screened patients showed that melanoma and synovial sarcoma frequently expressed NY-ESO-1, and results were highly concordant between the two assays (Table 1). However, colorectal, ovarian, breast, and bladder cancers were rarely strongly positive and showed poor correlation between the assays for the target antigen based on the relatively small number of samples tested.
Table 1. NY-ESO-1 tissue analysis.
NY-ESO-1 analysis was performed on all archived paraffin-fixed tumor tissues submitted for screening. N/A, not applicable.
| n | IHC n (% positive)* |
PCR, n (% positive)† | Concordance, n (% positive)‡ |
|||
|---|---|---|---|---|---|---|
| >5% | >30% | >5% | >30% | |||
| All | 280 | 69 (25) | 44 (16) | 55 (20) | 43 (62) | 32 (73) |
| Melanoma | 55 | 17 (31) | 11 (20) | 19 (35) | 16 (94) | 10 (91) |
| Colorectal | 41 | 12 (29) | 7 (17) | 6 (15) | 4 (33) | 1 (14) |
| Sarcoma | 41 | 16 (39) | 15 (37) | 18 (44) | 15 (94) | 14 (93) |
| Synovial | 14 | 9 (64) | 9 (64) | 9 (64) | 8 (89) | 8 (89) |
| Leiomyosarcoma | 6 | 0 (0) | 0 (0) | 0 (0) | N/A | N/A |
| Not specified/other | 21 | 7 (33) | 6 (29) | 9 (43) | 7 (100) | 6 (100) |
| Lung | 38 | 8 (21) | 7 (18) | 4 (11) | 4 (50) | 4 (57) |
| Ovarian | 23 | 5 (22) | 1 (4) | 3 (13) | 2 (40) | 1 (100) |
| Bladder/urothelial | 13 | 3 (23) | 1 (8) | 1 (8) | 1 (33) | 1 (100) |
| Breast | 13 | 1 (8) | 1 (8) | 1 (8) | 1 (100) | 1 (100) |
| Other | 56 | 7 (13) | 1 (2) | 3 (5) | 0 (0) | 0 (0) |
Results are shown for NY-ESO-1 expression either at ≥5% of cells with ≥1+ intensity (cutoff used for study eligibility) or at >30% of cells with ≥1+ intensity.
Threshold for positive was determined on the basis of negative and positive control samples.
Concordance between PCR and IHC positivity was determined at each IHC cutoff.
Patient enrollment and disposition
A total of 45 patients were enrolled and treated on study. Figure 1 illustrates the allocation of patients to each treatment cohort. In cohorts 1 to 3, 23 patients received escalating doses of CDX-1401 (0.1, 1, or 3 mg) in combination with topical resiquimod. A dose of 1 mg was subsequently chosen for cohorts 4 to 6, in which 23 patients received CDX-1401 with subcutaneously administered adjuvant, either poly-ICLC, resiquimod, or both.
Fig. 1. Patient allocation to treatment cohorts. i.c., intracutaneous; sc, subcutaneous.

As shown in Table 2, nearly half of the enrolled patients had melanoma. Additional cancer types included ovarian, sarcoma, non–small cell lung, and colorectal cancers. Distant metastases were noted in 87% of the treated patients, consistent with the presence of advanced disease, as required for enrollment. Expression of the NY-ESO-1 antigen in tumor specimen was confirmed by IHC or PCR in 27 of 42 (64%) of the treated patients with available tissue. In cohorts 1 to 4, 12 of 30 (40%) had confirmed tumor NY-ESO-1 expression, whereas revised entry criteria required that all patients in cohorts 5 and 6 had confirmed tumor NY-ESO-1 expression.
As shown in Table 2, nearly half of the enrolled patients had melanoma. Additional cancer types included ovarian, sarcoma, non–small cell lung, and colorectal cancers. Distant metastases were noted in 87% of the treated patients, consistent with the presence of advanced disease, as required for enrollment. Expression of the NY-ESO-1 antigen in tumor specimen was confirmed by IHC or PCR in 27 of 42 (64%) of the treated patients with available tissue. In cohorts 1 to 4, 12 of 30 (40%) had confirmed tumor NY-ESO-1 expression, whereas revised entry criteria required that all patients in cohorts 5 and 6 had confirmed tumor NY-ESO-1 expression.
Table 2.
Pretreatment patient characteristics.
| All patients (N = 45) | |
|---|---|
| Male, n (%) | 23 (51) |
| Age (years), median (range) | 64 (38–90) |
| Cancer type, n (%) | |
| Melanoma | 21 (47) |
| Ovarian | 6 (13) |
| Sarcoma* | 5 (11) |
| Non–small cell lung cancer | 4 (9) |
| Colorectal | 4 (9) |
| Other† | 5 (11) |
| Distant metastases, n (%) | 39 (87) |
| Previous anticancer treatment regimens, median (range) | 3 (0–10) |
| NY-ESO-1+ tumor, n (%) | 27 (64)‡ |
Sarcoma subtypes were leiomyosarcoma (n = 2), endometrial stromal sarcoma, adipocytic, and round cell liposarcoma.
Other tumor types included cholangiocarcinoma; carcinoid tumor of liver, anal, and bladder; and myeloma.
Tumor NY-ESO-1 expression status is unknown for three patients.
Dosing, toxicity, and pharmacokinetics
Dose escalation proceeded as planned with no dose-limiting toxicities (DLTs). Of the 45 enrolled patients, 41 completed at least one cycle of CDX-1401 treatment, defined as a 6-week course of treatment with CDX-1401 and applicable adjuvant(s). Ten patients with stable disease were re-treated, with a median of 10 CDX-1401 doses (range, 6 to 20).
There were no cases of treatment discontinuation due to toxicity, and treatment-related toxicities were all of grade 1 or 2 severity. As shown in Table 3, the most frequent treatment-related adverse events were administration site reaction, fatigue, nausea, and chills.
Table 3. Adverse events considered related to study treatment (CDX-1401, resiquimod, and/or poly-ICLC).
Table includes all adverse events reported with a possible, probable, or definite relationship to any of the study treatments, occurring in more than one patient. No grade 3, 4, or 5 events have been reported as potentially related to study treatment.
| CDX-1401plus topical resiquimod (cohorts 1–3; n = 23), n (%) | CDX-1401 plus poly-ICLC (cohort 4; n = 7), n (%) | CDX-1401 plus subcutaneous resiquimod (cohort 5; n = 7), n (%) | CDX-1401 plus poly-ICLC and subcutaneous resiquimod (cohort 6; n = 8), n (%) | |
|---|---|---|---|---|
| Administration site reaction | 18 (78) | 5 (71) | 5 (71) | 7 (88) |
| Fatigue | 5 (22) | 3 (43) | 2 (29) | 1 (13) |
| Nausea | 2 (9) | 0 (0) | 0 (0) | 2 (25) |
| Chills | 1 (4) | 1 (14) | 0 (0) | 2 (25) |
| Influenza-like illness | 2 (9) | 1 (14) | 0 (0) | 0 (0) |
| Decreased appetite | 3 (13) | 0 (0) | 0 (0) | 0 (0) |
| Arthralgia | 2 (9) | 0 (0) | 0 (0) | 1 (13) |
| Myalgia | 0 (0) | 0 (0) | 2 (29) | 1 (13) |
| Pyrexia | 1 (4) | 1 (14) | 0 (0) | 0 (0) |
| Hemoglobin decreased | 2 (9) | 0 (0) | 0 (0) | 0 (0) |
| Dizziness | 1 (4) | 0 (0) | 0 (0) | 1 (13) |
Table 3. Adverse events considered related to study treatment (CDX-1401, resiquimod, and/or poly-ICLC).
As expected from these relatively small doses (up to 3 mg), pharmacokinetic analysis revealed no detectable levels of circulating CDX-1401 (limit of detection, 0.08 μg/ml) in samples obtained at 2, 4, or 24 hours after administration of CDX-1401.
Induction of humoral immunity to NY-ESO-1
As shown in Fig. 2, most patients (79%) had NY-ESO-1–specific immunoglobulin G (IgG) titers after vaccination, with high titers (≥1:10,000) in 52% and very high titers (≥1:100,000) in 33% of patients. Similarly, strong humoral immunity developed in each cohort and in patients with or without confirmed NY-ESO-1 expression in their tumor. Patients with NY-ESO-1+ tumors frequently (54%) had anti–NY-ESO-1 antibodies present at baseline, including 5 patients with titers above 1:100,000. Isotype analysis of patient’s samples with anti–NY-ESO-1 responses after treatment revealed a predominant IgG1 response, with several patients also showing IgG2, IgG3, and IgG4 anti–NY-ESO-1 antibodies (table S1).
Fig. 2. Induction of anti–NY-ESO-1 IgG titers in patients vaccinated with CDX-1401.

Pre- and posttreatment sera were tested for anti–NYESO-1 IgG responses by enzyme-linked immunosorbent assay (ELISA) using full-length recombinant NY-ESO-1. The titer was established by determining the endpoint dilution with the peak response shown by color map as indicated in the legend. Patients with archival tumor that demonstrated NY-ESO-1 expression by IHC or PCR are indicated with green shading. Patients who experienced stable disease are indicated by asterisk.
Induction of cellular immunity to NY-ESO-1
Cellular immunity to NY-ESO-1 was determined by interferon-γ (IFN-γ) enzyme-linked immunospot (ELISpot) on purified blood mononuclear cells from patient samples collected before and after CDX-1401 administration. Patient samples were qualified by demonstrating responsiveness to CD3 stimulation and to a pool of major histocompatibility complex class I peptides derived from human viruses (fig. S1). We detected NY-ESO-1–specific T cell responses in 56% patients with evaluable pre- and posttreatment samples (Fig. 3A). As seen with humoral immunity, significant NY-ESO-1–specific T cell responses were observed in most cohorts, suggesting that even low doses of CDX-1401 were immunogenic and both poly-ICLC and resiquimod can provide appropriate immune activation to drive T and B cell responses. Durability of the T cell response was demonstrated in two patients from whom samples from additional cycles of CDX-1401 treatment were available. In these patients, the induction of NY-ESO-1–specific T cells was maintained through three cycles (about 7 months) of treatment (Fig. 3B).
Fig. 3. Induction of NY-ESO-1–specific T cell responses in patients vaccinated with CDX-1401.
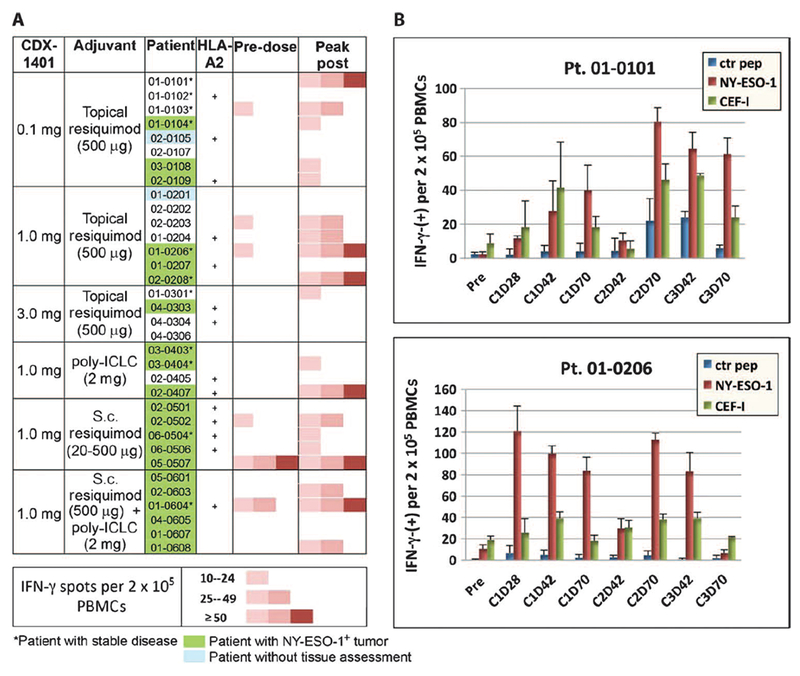
Cell-mediated immune responses as evaluated by IFN-g ELISpot assay. PBMCs were re-stimulated in vitrowithNYESO-1 peptide pool for 7 days with low-dose interleukin-2 (IL-2) and tested in an IFN-g ELISpot assay. Effector lymphocytes were incubated with T-depleted APCs (5:1) loaded with an NY-ESO-1 peptide pool or a control peptide pool and plated in anti–IFN-γ–coated ELISpot plates and incubated overnight at 37°C. HLA class I– and class II–binding CEF peptide pools served as positive controls. (A) Colormap of IFN-γ–producing NYESO- 1–specific T cells (spot counts) detected in a pre- and a selected post-vaccinated sample. (B) Selected patients, NY-ESO-1– (top) and NY-ESO-1+ (bottom), with sustained NY-ESO-1 specific IFN-γ response through multiple cycles of treatment.
In a few patients with sufficient lymphocytes, it was possible to further characterize NY-ESO-1 T cell responses by intracellular cytokine and HLA pentamer staining. Figure 4A shows a patient sample with an NY-ESO-1–specific response by both CD4+ and CD8+ cells as demonstrated by intracellular cytokine staining for IFN-γ and TNF-α (tumor necrosis factor–α). Of the five patients tested, three had both CD8+ and CD4+ NY-ESO-1 T cell responses, whereas two patients only showed a dominant CD4+ response (Fig. 4B).
Fig. 4. Detection of NY-ESO-1–specific IFN-γ production by circulating T cell subsets assessed by intracellular cytokine staining assay.

Patient PBMC samples were treated as described in Fig. 3. After stimulation with NY-ESO-1 or control peptide pools, cells were stained for surface markers and then permeabilized for detection of IFN-g and TNF-α before analysis by flow cytometry. (A) PBMCs from patient 01-0101 at cycle 3 day 70 were analyzed for IFN-γ and TNF-α. (B) PBMCs from patient 02-0206 from cycle 2 day 70 and cycle 3 day 42 were analyzed for IFN-γ.
Peripheral blood mononuclear cells (PBMCs) from HLA-A2.1+ patients were stained directly (that is, no in vitro stimulations) with an NY-ESO-1 peptide–specific pentamer or irrelevant control pentamer. Of the 16 patients stained with the pentamers, only 3 showed NY-ESO-1 pentamer–positive CD8 T cells that increased in numbers after vaccination (fig. S2), suggesting a low abundance of circulating T cells reactive to the well-known immunodominant epitope, NY-ESO157–165, and possible reactivity against epitopes presented by other HLA class I/II alleles.
Clinical activity
Stable disease was noted for 13 patients, distributed among treatment cohorts (Fig. 2). Seven of these patients had melanoma, two had colorectal cancer, and the remaining four had myeloma, bladder cancer, cholangiocarcinoma, and non–small cell lung cancer. Median duration of stable disease was 6.7 months (range, 2.4+ to 13.4 months). Remarkably, two of the melanoma patients were also observed to have some tumor shrinkage after one cycle of CDX-1401 treatment, with both patients having about 20% shrinkage in the sum of the diameters of target lesions. One patient with regression of a tonsil lesion subsequently showed clinical progression before additional treatment with CDX-1401, but was still alive at the 2-year follow-up. The second patient had regression in a lung lesion and received a second cycle of treatment before progression indicated by the appearance of a new lesion.
The detection of NY-ESO-1 expression in tumor tissue did not appear to correlate with patient outcome. Stable disease was seen in 7 of 27 (26%) of the patients with NY-ESO-1 expression, and in 5 of 15 (33%) of those lacking NY-ESO-1 expression. Note that these tested tissue samples represented archival specimens obtained at a median of 17.5 months (range, 2.3 months to 10.2 years) before study entry. Similarly, the rate of disease stabilization was similar for most patients who developed or maintained NY-ESO-1–specific humoral response (10 of 34, 29%) and for the few patients who did not (3 of 8, 38%).
However, the proportion of patients with stable disease was higher for those who maintained or developed NY-ESO-1–specific T cell responses (Fig. 3). Stable disease was seen in three of six (50%) patients who entered the study with preexisting cellular immunity to NY-ESO-1, and all three had increased responses while on study. For the remaining 13 patients who developed cellular immunity while on treatment, 6 (46%) experienced stable disease. In contrast, stable disease was seen in only 2 (13%) of the 15 patients who did not develop cellular immunity to NY-ESO-1. Four of six (67%) patients who displayed the strongest responses (>50 IFN-γ spots per 2 × 105 PBMCs) also experienced stable disease. The association of cellular response and stable disease does not appear to be a consequence of extended duration of therapy. Peak responses were observed in the first treatment cycle for seven of the nine patients with stable disease who developed cellular immunity.
Eleven patients completed study follow-up at 2 years, whereas one patient remains in follow-up. During study follow-up, six melanoma patients went on to receive anti–CTLA-4 mAb within 3 months of the last CDX-1401 dose, and four of these patients were reported to experience a partial response (PR) or complete response (CR) by the Response Evaluation Criteria in Solid Tumors version 1.1 (RECIST 1.1) (32) or irResponse (33) criteria (table S2). In addition, two non–small cell lung cancer patients who received investigational checkpoint blockade within 2 months of discontinuing CDX-1401 were also reported to experience PR. All six of these responding patients had tumors confirmed to express NY-ESO-1. Five also developed NY-ESO-1–specific cellular response, whereas four also developed or maintained NY-ESO-1–specific humoral response, by the end of treatment with CDX-1401.
DISCUSSION
Biological therapy of cancer with tumor-associated antigens delivered directly to APCs in vivo represents an advancement in the field of immunotherapy. Pivotal studies from Steinman and Nussenzweig provided convincing proof-of-concept data in preclinical models showing that an antigen bearing anti–DEC-205 antibody, when targeted to DCs in the presence of activating agents, could induce enhanced immunity in vivo, which was superior to other approaches including adoptive transfer of antigen-loaded DCs (1–3). We have previously reported on a vaccine construct that delivers antigen to a broader population of APCs through targeting the mannose receptor (34), which helped establish the feasibility of antibody-based vaccines. Here, we present a translational study in humans targeting the cancer testis antigen NY-ESO-1 directly to the DC DEC-205 receptor with an anti–DEC-205 antibody (CDX-1401). The approach does not require previous knowledge of specific NY-ESO-1–derived peptide antigens or individual HLA, providing a potential vaccine for all patients with NY-ESO-1–expressing tumors. Our study demonstrates that treatment with CDX-1401, in combination with TLR ligands (poly-ICLC or resiquimod), provides a well-tolerated and practical approach that results in integrated humoral and cellular NY-ESO-1–specific immunity in most patients including those with tumors that tested negative for this antigen.
The magnitude of the humoral response was particularly impressive, with a third of the patients achieving titers greater than 1:100,000. NY-ESO-1–specific T cells were observed in more than half of the patients using an in vitro restimulation assay. We demonstrated evidence of both CD8 and CD4 responses, suggesting that this protein-based vaccination approach can generate a broad spectrum of immunity in advanced cancer patients.
Overall, we observed similar induction of NY-ESO-1 immune responses using various dosing regimens. No clear dose response of CDX-1401 was observed, which is consistent with preclinical studies of DEC-205 vaccines. In addition, no particular advantage of the subcutaneous formulation of resiquimod or the combination of resiquimod and poly-ICLC was noted compared to topical resiquimod or poly-ICLC as single adjuvants. However, given the small number of patients and the variability of responses among patients in each cohort, further confirmatory studies would be required. On the basis of the preclinical data supporting poly-ICLC as the most potent immune activator for combination with DEC-205–targeted vaccines (35) together with the current data, we plan to use the 1-mg dose of CDX-1401 with poly-ICLC for further clinical development.
Our study confirmed the frequent and more robust expression of NY-ESO-1 in melanoma and various types of sarcoma using validated centralized assays for IHC and PCR. Expression was observed in other cancer types, but with less frequency, lower levels, and less concordance between assays. However, some of these cancer types were only represented by a small number of samples.
Despite the induction of measurable T cell responses, tumor regression was not observed in most patients. This suggests the need to consider earlier-stage patients or to combine this approach with strategies that overcome the immunosuppressive effects in the tumor bed, including combination with new antibodies that block T cell inhibitory checkpoints such as anti-PD1 or anti-PDL1. Follow-up of patients who went on to receive such therapies indicates an overall response rate of 6 of 8 (75%) for patients receiving checkpoint inhibitors after CDX-1401 treatment. Of those patients with melanoma who went on to receive anti–CTLA-4 mAb, four of six (67%) had responses, which is potentially interesting when compared to the overall response rate of 11% previously reported in metastatic melanoma patients treated with single-agent anti–CTLA-4 mAb (36).
In recent years, a variety of immunotherapeutic approaches have been tested for the induction of humoral and cellular immune responses to NY-ESO-1. In particular, studies using recombinant vaccinia and fowlpox vectors expressing NY-ESO-1 antigen (37) and synthetic long peptides derived from NY-ESO-1 (38, 39) have helped document the ability of cancer patients to develop antibodies as well as CD4 and CD8 T cell responses to NY-ESO-1. A recent study has also demonstrated that some vaccines may inadvertently lead to increases in NY-ESO-1–specific regulatory T cells that could limit clinical benefit (40). Differences in patient populations and immune monitoring preclude direct comparisons between these studies. Vaccines against NY-ESO1 may, in principle, also lead to development of immune responses against other antigens because of epitope spreading. Further studies are needed to better evaluate the possibility of epitope spreading with this vaccine (41).
This specific in vivo targeting of DCs using CDX-1401 has advantages over alternate technologies. The collection of autologous DCs with ex vivo manipulation has shown efficacy as evidenced by the survival advantage shown with sipuleucel-T (42); however, the practical complexity and limited quantity of vaccine product may be limiting to the therapeutic effect. The off-the-shelf nature of antibody-mediated DC targeting offers a well-tolerated and practical approach to generating protein-specific immunity that does not need to be restricted to HLA subtypes and can be readily combined with other treatment strategies. This study has shown that CDX-1401, when given in various regimens, can effectively generate NY-ESO-1 immunity, even in patients with antigen-expressing tumor, and the treatment was associated with clinical benefit in some patients.
MATERIALS AND METHODS
Patient population
The study was open to men and women at least 18 years of age, with cancer types known to express NY-ESO-1, including, but not limited to, bladder, breast, ovary, non–small cell lung, myeloma, sarcoma, and melanoma. Eligible patients had advanced malignancies that had progressed after any standard curative or salvage therapies. Confirmation of tumor NY-ESO-1 expression (>5% positive cells) by PCR and/or IHC at a central laboratory was required for cohorts 5 and 6. For cohorts 1 to 4, this analysis was performed retrospectively. Exclusionary conditions included Eastern Cooperative Oncology Group (ECOG) performance status >1, active central nervous system metastases or potential alternate malignancy, autoimmune disease, and concurrent treatment with immunosuppressive or immunomodulatory agents. Before the conduct of any protocol-specific procedures, all patients signed written informed consent after the nature and possible consequences of the studies were explained.
Study design and procedures
This phase 1 study was designed to examine the safety and tolerability profile of CDX-1401 in combination with the TLR agonists, resiquimod and/or poly-ICLC, in patients with malignancies known to express NY-ESO-1. All patients who received at least one dose of study drug were included in safety analyses. Additional objectives of this study included an evaluation of CDX-1401–induced immune responses, antitumor activity, and pharmacokinetics.
The study consisted of six sequentially enrolled (nonrandomized) open-label treatment cohorts (Fig. 1). An initial dose-escalation phase (cohorts 1 to 3; CDX-1401 in combination with topical resiquimod) was conducted to select a CDX-1401 dose level of interest for further study. Subsequent cohorts evaluated CDX-1401 in combination with poly-ICLC, escalating doses of parenteral resiquimod, or both to assess an optimal adjuvant regimen.
Safety parameters assessed at baseline and study visits included physical examination, vital signs, ECOG performance status, hematology, blood chemistry, urinalysis, thyroid function, and the incidence and severity of adverse events. Toxicity was graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 3.0. DLT was defined as any treatment-related grade ≥3 nonhematologic adverse event excluding alopecia, or persistent grade 4 hematologic adverse event. Assessment of immunological activity included measurements of cellular and humoral response to NY-ESO-1. Standard disease-specific tumor evaluation was performed according to RECIST 1.1 within 28 days before each re-treatment cycle and at a minimum frequency of 12 weeks. Patients were followed for up to 2 years for assessment of safety, response to subsequent therapy, and survival.
Study drugs and dosing
In all cohorts, a treatment cycle was defined as a 6-week course of treatment with CDX-1401 and applicable adjuvant(s), followed by a 6-week rest. Additional 12-week cycles were allowed for patients who continued to meet all eligibility criteria and who had not experienced DLT/intolerance or progression of disease.
CDX-1401 (Celldex Therapeutics) was administered via intracutaneous injection (a combination of intradermal and subcutaneous injection, with about half of the planned dose administered by each route) within a 5 χ 5–cm area on the extremities or the abdomen. CDX-1401 was given biweekly for a total of four administration sessions over the ~6-week treatment period (Fig. 1). Dose levels were 0.1, 1.0, and 3.0 mg. Topical resiquimod (3M Pharmaceuticals; also known as R-848; S-28463; 4-amino-2-ethoxymethyl-α, α-dimethyl-1H-imidazo[4,5-C]quinoline-1-ethanol) was applied topically at a dose of 500 μg (250 mg of 0.2% gel) immediately after CDX-1401 vaccination and again about 24 hours later. Each topical resiquimod application was to remain on the treatment site for about 8 hours, unless local toxicity warrants premature removal. Resiquimod for injection (0.02 to 0.5 mg in a 0.25-ml volume) was administered as a subcutaneous injection after CDX-1401 vaccination. Poly-ICLC (Oncovir; Hiltonol) was administered subcutaneously at a dose of 2 mg (1.0 ml) after CDX-1401 vaccination and again 24 hours later.
Pharmacokinetics
Serum samples for assessment of circulating CDX-1401 were obtained before vaccination and at 2, 4, and 24 hours after the first vaccination, and before vaccination for all other vaccinations in the first cycle. A functional ELISA was developed to test serum and plasma samples on a 96-well microtiter plate coated with a fragment of DEC-205. Bound CDX-1401 was detected with anti–NY-ESO-1 antibody followed by goat anti-human IgG-HRP (horseradish peroxidase) and visualized with a 3,3’,5,5’-tetramethylbenzidine (TMB) substrate. The assay has a lower detection limit of 0.08 μg/ml.
NY-ESO-1 expression
The IHC method was optimized, and expression of NY-ESO-1 was verified with control sections from breast cancer, melanoma, and ovarian carcinoma sections at Clarient Inc. Briefly, 4- to 5-μm cuts of standard formalin-fixed, paraffin-embedded tissue specimens were mounted on charged glass slides, deparaffinized, and washed per standard methods. After heat-induced antigen retrieval at 100°C, samples were stained for 30 min with a murine mAb specific for NY-ESO-1 (E978, Invitrogen). Known positive cell line HT-1080 fibrosarcoma and negative cell line SiHa squamous cell carcinoma were used for staining verification. Expression of NY-ESO-1 was visualized with the Leica Bond Refine-HRP detection system and DAB as chromagen and hematoxylin as counterstain. Tumors were manually evaluated on the basis of the percentage of positive cells with a standard bright-field microscope (0 to 100%, intensity 0 to 3+). A sample with greater than or equal to 5% positive cells was considered positive for NY-ESO-1 protein.
For reverse transcription PCR (RT-PCR), total RNA was prepared from 10-μm-thick, formalin-fixed, paraffin-embedded tumor sections and from control tumor cell lines with the in-house methods (Clarient Inc.) Quantitative RT-PCR was performed with a TaqMan assay and an ABI Prism 7900 HT system (Applied Biosystems) with a TaqMan RNA-to-CT 1-Step kit (Life Technologies). TaqMan gene expression primers and probes for NY-ESO-1 (Hs00265824_m1) and human GUSB (HS99999908_m1) were purchased from Life Technologies. As a positive control, total RNA extracted from HT-1080 cells transformed with NY-ESO-1, and a negative control from HL-60 cell line extracts, determined not to express the target, were used in every run, as well as a negative template control to test for contamination. GUSB expression was included as a sample amplification control. For inclusion in the trial CDX1401-01, any sample with an NY-ESO-1 signal greater than the threshold was considered positive.
Humoral immune response analysis
The magnitude and isotype of the anti–NY-ESO-1 antibody response were determined from patient serum samples. Sample time points were day 0 (before dosing), day 28 (2 weeks after the second CDX-1401 dose in cycle 1 only), day 42 (2 weeks after the third CDX-1401 dose), and days 70 to 77 (about 1 month after the fourth CDX-1401 dose). NY-ESO-1–specific antibodies were detected by adding serially diluted serum to ELISA plates coated with NY-ESO-1 and developed with a goat anti-human IgG (H&L) peroxidase and TMB staining. A posttreatment sample was considered positive for the presence of anti–NY-ESO-1 antibodies if the mean absorbance value was greater than the mean value of the predose sample, plus 3 SDs or +0.1, whichever was greater. The titer of the sample was defined as the reciprocal of the dilution with an absorbance value closest to a preset background of 0.1. Anti–NY-ESO-1 antibody isotypes were determined with isotype-specific HRP-conjugated goat anti-human IgG1, IgG2, IgG3, IgG4, and IgM. Analysis was carried out on posttreatment samples (1:50 dilution) from all patients with positive NY-ESO-1 titers (n = 26).
Cellular immune response analysis of NY-ESO-1 synthetic peptide library
A library of HLA class I–binding peptides derived from NY-ESO-1 protein sequence previously described (29) was used to prepare high-quality and 95% pure synthetic peptides (ProImmune). Peptides were resuspended in anhydrous dimethyl sulfoxide at 10 mg/ml, aliquoted, flushed with nitrogen, sealed, and stored at −40°C until use.
ELISpot assay
ELISpot assay was used to screen the patient blood samples for immune responses. For every patient in the clinical trial, pre- and post-dose blood samples were collected, and multiple post-dose, in addition to the predose, samples were tested for NY-ESO-1–specific IFN-γ secretion. In each ELISpot assay, PBMCs were first presensitized in a 7-day in vitro sensitization culture in the presence of low-dose IL-2 (10 ng/ml). The NY-ESO-1 peptide pool comprised 28 overlapping peptides covering the full length of the NY-ESO-1 protein (29). In the assay, APCs (T-depleted PBMCs) were pulsed with NY-ESO-1 peptides and negative control peptides (WRKY47, ProImmune). Anti-CD3 mAb and CEF-I peptide pool served as positive controls in the assay. The CEF-I pool contains 32 HLA class I peptides from human cytomegalovirus, Epstein-Barr virus, and influenza virus (PANATecs GmbH). ELISpot plates were washed as per kit instructions. Spot counts were evaluated with the Zeiss system (ZellNet Consulting). Ambiguities arising from confluence issues were resolved with the following formula: total spot number = spot count + 2 × [spot count × % confluence/(100% − % confluence)].
Intracellular cytokine staining
Patient PBMCs were cultured for 7 days as described above and stained for intracellular cytokines. Briefly, presensitized cells were first restimulated with T-depleted APCs pulsed with various peptides and then treated with GolgiPlug (BD Biosciences). Cells were then stained with anti-CD3, anti-CD4, and anti-CD8 mAbs, fixed, and permeabilized followed by staining with fluorochrome-conjugated antibodies to IFN-γ (clone B27) and TNF-α (clone mAb 11) (BD Biosciences). Stained cells were then analyzed by flow cytometry with FACSCanto II (BD Biosciences).
HLA-A2–NY-ESO-1 pentamer staining
For pentamer staining, the A*0201/NY-ESO-1157–165 (SLLMWITQV) pentamer-RPE (retinal pigment epithelial) and control A*0201/[negative] pentamer-RPE were purchased from ProImmune. The patient PBMCs were thawed and stained directly with the pentamers without culturing. About 2 χ 106 cells were resuspended in 500 μl of phosphate-buffered saline and stained with 5 μl of the pentamers for 30 min and further stained for CD3 and CD8. A CD19-positive gate was used to exclude nonspecific binding by B cells with CD19 antibody (clone HIB19, BD Biosciences).
Statistical analysis
For this phase 1 trial, the statistical analysis consisted of descriptive summaries of the safety and immunologic data. Adverse events were tabulated on the basis of the standardized terms assigned by the Medical Dictionary for Regulatory Activities (MedDRA). Tumor responses were evaluated and reported in accordance with RECIST (version 1.1) (32).
Supplementary Material
Relevant patents and patent applications:
Title Patent/application serial number:
Antibodies that bind human dendritic and epithelial cell 205 (DEC-205) International Patent Application No. PCT/US2008/082745 (WO2009061996): now in national and regional phases
Antibodies that bind human dendritic and epithelial cell 205 (DEC-205) US Patent No. 8,236,318
Antibodies that bind human dendritic and epithelial cell 205 (DEC-205) US Patent No. 8,362,214
Antibodies that bind human dendritic and epithelial cell 205 (DEC-205) US Patent No. 8,586,720
Antibodies that bind human dendritic and epithelial cell 205 (DEC-205) US Patent Application No. 13/716,973
Acknowledgments:
We dedicate this paper to the memory of Ralph Steinman and his pioneering work on DCs and improving protein-based vaccination. We also thank A. M. Salazar (Oncovir Inc.) for supply of cGMP (current Good Manufacturing Practice)–grade poly-ICLC, L. Pilja (Celldex Therapeutics Inc.) for clinical trial management, and M. Calcamuggio (The Write Company) for assistance with manuscript writing. ClinicalTrials.gov identifier: NCT00948961. Funding: This study was funded by Celldex Therapeutics Inc.
Footnotes
Competing interests: B.Z., J.G., V.R., A.C., L.V., M.Y., T.D., and T.K. have equity interest in and are employees of Celldex Therapeutics Inc., which develops CDX-1401. The other authors have no competing interests.
REFERENCES AND NOTES
- 1.Steinman RM, Dendritic cells in vivo: A key target for a new vaccine science. Immunity 29, 319–324 (2008). [DOI] [PubMed] [Google Scholar]
- 2.Jiang W, Swiggard WJ, Heufler C, Peng M, Mirza A, Steinman RM, Nussenzweig MC, The receptor DEC-205 expressed by dendritic cells and thymic epithelial cells is involved in antigen processing. Nature 375, 151–155 (1995). [DOI] [PubMed] [Google Scholar]
- 3.Guo M, Gong S, Marie S, Misulovin Z, Pack M, Mahnke K, Nussenzweig MC, Steinman RM, A monoclonal antibody to the DEC-205 endocytosis receptor on human dendritic cells. Hum. Immunol 61,729–738 (2000). [DOI] [PubMed] [Google Scholar]
- 4.Bozzacco L, Trumpfheller C, Siegal FP, Mehandru S, Markowitz M, Carrington M, Nussenzweig MC, Piperno AG, Steinman RM, DEC-205 receptor on dendritic cells mediates presentation of HIV gag protein to CD8+ T cells in a spectrum of human MHC I haplotypes. Proc. Natl. Acad. Sci. U.S.A 104, 1289–1294 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonifaz L, Bonnyay D, Mahnke K, Rivera M, Nussenzweig MC, Steinman RM, Efficient targeting of protein antigen to the dendritic cell receptor DEC-205 in the steady state leads to antigen presentation on major histocompatibility complex class I products and peripheral CD8+ T cell tolerance. J. Exp. Med 196, 1627–1638 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hawiger D, Inaba K, Dorsett Y, Guo M, Mahnke K, Rivera M, Ravetch JV, Steinman RM, Nussenzweig MC, Dendritic cells induce peripheral T cell unresponsiveness under steady state conditions in vivo. J. Exp. Med 194, 769–779 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mahnke K, Guo M, Lee S, Sepulveda H, Swain SL, Nussenzweig M, Steinman RM, The dendritic cell receptor for endocytosis, DEC-205, can recycle and enhance antigen presentation via major histocompatibility complex class II–positive lysosomal compartments. J. Cell Biol 151, 673–684 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bonifaz LC, Bonnyay DP, Charalambous A, Darguste DI, Fujii S, Soares H, Brimnes MK, Moltedo B, Moran TM, Steinman RM, In vivo targeting of antigens to maturing dendritic cells via the DEC-205 receptor improves T cell vaccination. J. Exp. Med 199, 815–824 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Napolitani G, Rinaldi A, Bertoni F, Sallusto F, Lanzavecchia A, Selected Toll-like receptor agonist combinations synergistically trigger a T helper type 1–polarizing program in dendritic cells. Nat. Immunol 6, 769–776 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mittag D, Proietto AI, Loudovaris T, Mannering SI, Vremec D, Shortman K, Wu L, Harrison LC, Human dendritic cell subsets from spleen and blood are similar in phenotype and function but modified by donor health status. J. Immunol 186, 6207–6217 (2011). [DOI] [PubMed] [Google Scholar]
- 11.Ito T, Amakawa R, Fukuhara S, Roles of toll-like receptors in natural interferon-producing cells as sensors in immune surveillance. Hum. Immunol 63, 1120–1125 (2002). [DOI] [PubMed] [Google Scholar]
- 12.Akira S, Takeda K, Toll-like receptor signalling. Nat. Rev. Immunol 4, 499–511 (2004). [DOI] [PubMed] [Google Scholar]
- 13.Jungbluth AA, Chen YT, Stockert E, Busam KJ, Kolb D, Iversen K, Coplan K, Williamson B, Altorki N, Old LJ, Immunohistochemical analysis of NY-ESO-1 antigen expression in normal and malignant human tissues. Int. J. Cancer 92, 856–860 (2001). [DOI] [PubMed] [Google Scholar]
- 14.Nicholaou T, Ebert L, Davis ID, Robson N, Klein O, Maraskovsky E, Chen W, Cebon J, Directions in the immune targeting of cancer: Lessons learned from the cancer-testis Ag NY-ESO-1. Immunol. Cell Biol 84, 303–317 (2006). [DOI] [PubMed] [Google Scholar]
- 15.Jäger E, Gnjatic S, Nagata Y, Stockert E, Jäger D, Karbach J, Neumann A, Rieckenberg J, Chen YT, Ritter G, Hoffman E, Arand M, Old LJ, Knuth A, Induction of primary NY-ESO-1 immunity: CD8+ T lymphocyte and antibody responses in peptide-vaccinated patients with NY-ESO-1+ cancers. Proc. Natl. Acad. Sci. U.S.A 97, 12198–12203 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ebert LM, Liu YC, Clements CS, Robson NC, Jackson HM, Markby JL, Dimopoulos N, Tan BS, Luescher IF, Davis ID, Rossjohn J, Cebon J, Purcell AW, Chen W, A long, naturally presented immunodominant epitope from NY-ESO-1 tumor antigen: Implications for cancer vaccine design. Cancer Res 69, 1046–1054 (2009). [DOI] [PubMed] [Google Scholar]
- 17.Jäger E, Chen YT, Drijfhout JW, Karbach J, Ringhoffer M, Jäger D, Arand M, Wada H, Noguchi Y, Stockert E, Old LJ, Knuth A, Simultaneous humoral and cellular immune response against cancer–testis antigen NY-ESO-1: Definition of human histocompatibility leukocyte antigen (HLA)-A2–binding peptide epitopes. J. Exp. Med 187, 265–270 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hunder NN, Wallen H, Cao J, Hendricks DW, Reilly JZ, Rodmyre R, Jungbluth A, Gnjatic S, Thompson JA, Yee C, Treatment of metastatic melanoma with autologous CD4+ T cells against NY-ESO-1. N. Engl. J. Med 358, 2698–2703 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Rhee F, Szmania SM, Zhan F, Gupta SK, Pomtree M, Lin P, Batchu RB, Moreno A, Spagnoli G, Shaughnessy J, Tricot G, NY-ESO-1 is highly expressed in poor-prognosis multiple myeloma and induces spontaneous humoral and cellular immune responses. Blood 105, 3939–3944 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yuan J, Adamow M, Ginsberg BA, Rasalan TS, Ritter E, Gallardo HF, Xu Y, Pogoriler E, Terzulli SL, Kuk D, Panageas KS, Ritter G, Sznol M, Halaban R, Jungbluth AA, Allison JP, Old LJ, Wolchok JD, Gnjatic S, Integrated NY-ESO-1 antibody and CD8+ T-cell responses correlate with clinical benefit in advanced melanoma patients treated with ipilimumab. Proc. Natl. Acad. Sci. U.S.A 108, 16723–16728 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Odunsi K, Qian F, Matsuzaki J, Mhawech-Fauceglia P, Andrews C, Hoffman EW, Pan L, Ritter G, Villella J, Thomas B, Rodabaugh K, Lele S, Shrikant P, Old LJ, Gnjatic S, Vaccination with an NY-ESO-1 peptide of HLA class I/II specificities induces integrated humoral and T cell responses in ovarian cancer. Proc. Natl. Acad. Sci. U.S.A 104, 12837–12842 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davis ID, Chen W, Jackson H, Parente P, Shackleton M, Hopkins W, Chen Q, Dimopoulos N, Luke T, Murphy R, Scott AM, Maraskovsky E, McArthur G, MacGregor D, Sturrock S, Tai TY, Green S, Cuthbertson A, Maher D, Miloradovic L, Mitchell SV, Ritter G, Jungbluth AA, Chen YT, Gnjatic S, Hoffman EW, Old LJ, Cebon JS, Recombinant NY-ESO-1 protein with ISCOMATRIX adjuvant induces broad integrated antibody and CD4+ and CD8+ T cell responses in humans. Proc. Natl. Acad. Sci. U.S.A 101, 10697–10702 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gnjatic S, Nagata Y, Jager E, Stockert E, Shankara S, Roberts BL, Mazzara GP, Lee SY, Dunbar PR, Dupont B, Cerundolo V, Ritter G, Chen YT, Knuth A, Old LJ, Strategy for monitoring T cell responses to NY-ESO-1 in patients with any HLA class I allele. Proc. Natl. Acad. Sci. U.S.A 97, 10917–10922 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karbach J, Neumann A, Brand K, Wahle C, Siegel E, Maeurer M, Ritter E, Tsuji T, Gnjatic S, Old LJ, Ritter G, Jäger E, Phase I clinical trial of mixed bacterial vaccine (Coley’s toxins) in patients with NY-ESO-1 expressing cancers: Immunological effects and clinical activity. Clin. Cancer Res 18, 5449–5459 (2012). [DOI] [PubMed] [Google Scholar]
- 25.Karbach J, Neumann A, Atmaca A, Wahle C, Brand K, von Boehmer L, Knuth A, Bender A, Ritter G, Old LJ, Jäger E, Efficient in vivo priming by vaccination with recombinant NY-ESO-1 protein and CpG in antigen naive prostate cancer patients. Clin. Cancer Res 17, 861–870 (2011). [DOI] [PubMed] [Google Scholar]
- 26.Valmori D, Souleimanian NE, Tosello V, Bhardwaj N, Adams S, O’Neill D, Pavlick A, Escalon JB, Cruz CM, Angiulli A, Angiulli F, Mears G, Vogel SM, Pan L, Jungbluth AA, Hoffmann EW, Venhaus R, Ritter G, Old LJ, Ayyoub M, Vaccination with NY-ESO-1 protein and CpG in Montanide induces integrated antibody/Th1 responses and CD8 T cells through cross-priming. Proc. Natl. Acad. Sci. U S A. 104, 8947–8952 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mandic M, Castelli F, Janjic B, Almunia C, Andrade P, Gillet D, Brusic V, Kirkwood JM, Maillere B, Zarour HM, One NY-ESO-1-derived epitope that promiscuously binds to multiple HLA-DR and HLA-DP4 molecules and stimulates autologous CD4+ T cells from patients with NY-ESO-1-expressing melanoma. J. Immunol 174, 1751–1759 (2005). [DOI] [PubMed] [Google Scholar]
- 28.Zhao Y, Zheng Z, Robbins PF, Khong HT, Rosenberg SA, Morgan RA, Primary human lymphocytes transduced with NY-ESO-1 antigen-specific TCR genes recognize and kill diverse human tumor cell lines. J. Immunol 174, 4415–4423 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsuji T, Matsuzaki J, Kelly MP, Ramakrishna V, Vitale L, He LZ, Keler T, Odunsi K, Old LJ, Ritter G, Gnjatic S, Antibody-targeted NY-ESO-1 to mannose receptor or DEC-205 in vitro elicits dual human CD8+ and CD4+ T cell responses with broad antigen specificity. J. Immunol 186, 1218–1227 (2011). [DOI] [PubMed] [Google Scholar]
- 30.Cheong C, Choi JH, Vitale L, He LZ, Trumpfheller C, Bozzacco L, Do Y, Nchinda G, Park SH, Dandamudi DB, Shrestha E, Pack M, Lee HW, Keler T, Steinman RM, Park CG, Improved cellular and humoral immune responses in vivo following targeting of HIV Gag to dendritic cells within human anti-human DEC205 monoclonal antibody. Blood 116, 3828–3838 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ramakrishna V, Vasilakos JP, Tario JD Jr., Berger MA, Wallace PK, Keler T, Toll-like receptor activation enhances cell-mediated immunity induced by an antibody vaccine targeting human dendritic cells. J. Transl. Med 5, 5 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J, New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 45, 228–247 (2009). [DOI] [PubMed] [Google Scholar]
- 33.Wolchok JD, Hoos A, O’Day S, Weber JS, Hamid O, Lebbé C, Maio M, Binder M, Bohnsack O, Nichol G, Humphrey R, Hodi FS, Guidelines for the evaluation of immune therapy activity in solid tumors: Immune-related response criteria. Clin. Cancer Res 15, 7412–7420 (2009). [DOI] [PubMed] [Google Scholar]
- 34.Morse MA, Bradley DA, Keler T, Laliberte RJ, Green JA, Davis TA, Inman BA, CDX-1307: A novel vaccine under study as treatment for muscle-invasive bladder cancer. Expert Rev. Vaccines 10, 733–742 (2011). [DOI] [PubMed] [Google Scholar]
- 35.Longhi MP, Trumpfheller C, Idoyaga J, Caskey M, Matos I, Kluger C, Salazar AM, Colonna M, Steinman RM, Dendritic cells require a systemic type I interferon response to mature and induce CD4+ Th1 immunity with poly IC as adjuvant. J. Exp. Med 206, 1589–1602 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, Akerley W, van den Eertwegh AJ, Lutzky J, Lorigan P, Vaubel JM, Linette GP, Hogg D, Ottensmeier CH, Lebbé C, Peschel C, Quirt I, Clark JI, Wolchok JD, Weber JS, Tian J, Yellin MJ, Nichol GM, Hoos A, Urba WJ, Improved survival with ipilimumab in patients with metastatic melanoma. New. Engl. J. Med 363, 711–723 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Odunsi K, Matsuzaki J, Karbach J, Neumann A, Mhawech-Fauceglia P, Miller A, Beck A, Morrison CD, Ritter G, Godoy H, Lele S, duPont N, Edwards R, Shrikant P, Old LJ, Gnjatic S, Jäger E, Efficacy of vaccination with recombinant vaccinia and fowlpox vectors expressing NY-ESO-1 antigen in ovarian cancer and melanoma patients. Proc. Natl. Acad.Sci. U.S.A 109, 5797–5802 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sabbatini P, Tsuji T, Ferran L, Ritter E, Sedrak C, Tuballes K, Jungbluth AA, Ritter G, Aghajanian C, Bell-McGuinn K, Hensley ML, Konner J, Tew W, Spriggs DR, Hoffman EW, Venhaus R, Pan L, Salazar AM, Diefenbach CM, Old LJ, Gnjatic S, Phase I trial of overlapping long peptides from a tumor self-antigen and poly-ICLC shows rapid induction of integrated immune response in ovarian cancer patients. Clin. Cancer Res 18, 6497–6508 (2012). [DOI] [PubMed] [Google Scholar]
- 39.Kakimi K, Isobe M, Uenaka A, Wada H, Sato E, Doki Y, Nakajima J, Seto Y, Yamatsuji T, Naomoto Y, Shiraishi K, Takigawa N, Kiura K, Tsuji K, Iwatsuki K, Oka M, Pan L, Hoffman EW, Old LJ, Nakayama E, A phase I study of vaccination with NY-ESO-1f peptide mixed with Picibanil OK-432 and Montanide ISA-51 in patients with cancers expressing the NY-ESO-1 antigen. Int. J. Cancer 129, 2836–2846 (2011). [DOI] [PubMed] [Google Scholar]
- 40.Ebert LM, MacRaild SE, Zanker D, Davis ID, Cebon J, Chen W, A cancer vaccine induces expansion of NY-ESO-1-specific regulatory T cells in patients with advanced melanoma. PLOS One 7, e48424 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dhodapkar KM, Gettinger SN, Das R, Zebroski H, Dhodapkar MV, SOX2-specific adaptive immunity and response to immunotherapy in non-small cell lung cancer. Oncoimmunology 2, e25205 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Higano CS, Schellhammer PF, Small EJ, Burch PA, Nemunaitis J, Yuh L, Provost N, Frohlich MW, Integrated data from 2 randomized, double-blind, placebo-controlled, phase 3 trials of active cellular immunotherapy with sipuleucel-T in advanced prostate cancer. Cancer 115, 3670–3679 (2009). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


