Abstract
Background:
Fatigue and depression are closely associated. The purpose of this secondary analysis was to understand the relationships between depression and improvements in specific depression domains on the anti-fatigue effects of ketamine, which we previously reported.
Methods:
This secondary analysis re-evaluated data collected longitudinally from 39 patients with treatment-resistant Major Depressive Disorder (MDD) enrolled in a double-blind, randomized, placebo-controlled, crossover trial using a single intravenous infusion of ketamine hydrochloride (0.5mg/kg over 40 minutes) or placebo. A mediation model assessed the effect of depression on the anti-fatigue effects of a single dose of intravenous ketamine versus placebo at Day 1 post-infusion. Fatigue was measured using the National Institutes of Health — Brief Fatigue Inventory (NIH-BFI), and depression was assessed by the Montgomery-Ǻsberg Depression Rating Scale (MADRS).
Results:
Compared to placebo, ketamine significantly improved fatigue (p=.0003) as measured by the NIH-BFI, but the anti-fatigue effects of ketamine disappeared (p=.47) when controlling for depression as measured by MADRS total score. In this study sample, the anti-fatigue effects of ketamine were mostly accounted for by the changes in amotivation and depressed mood scores.
Conclusions:
In this study, ketamine did not have a unique effect on fatigue outside of its general antidepressant effects in patients with treatment-resistant depression. Specifically, the anti-fatigue effects of ketamine observed in this study seem to be explained by the effects of ketamine on two symptom domains of depression: amotivation and depressed mood. The study findings suggest that the anti-fatigue effects of ketamine should be assessed by fatigue-specific measures other than the NIH-BFI or future studies should enroll fatigued patients without depression.
Fatigue and depression are complex constructs, but their association is well documented (Bakshi et al., 2000; Jacobsen et al., 2003; Brown & Kroenke, 2009). Both significantly influence the capacity to perform everyday actions and endorse greater functional disability (Lin et al., 2013; Milanovic et al., 2018). They have been strongly correlated in multiple reports (Bakshi et al., 2000; Jacobsen et al., 2003; Brown & Kroenke, 2009). However, some evidence suggests that depression and fatigue are distinct constructs, for example, it was observed that fatigue symptoms persist even after the remission of depression (Ferguson et al., 2014). Nevertheless, separating fatigue from depression, especially when evaluating for treatment outcomes, continues to be a challenge because of the limits of available tools to distinguish one construct from the other.
Amotivation is a common symptom of depression, as well as in fatigue (Hegerl & Ulke, 2016). There is a dearth of information related to the relationship of amotivation and fatigue, especially in the clinical population. Most of the studies investigating the association of amotivation and fatigue used pre-clinical models. Amotivation is associated with a constellation of negative symptoms such as apathy characterized by reduced interest in participation in purposeful behavior, lack of initiative, problems in initiation or sustaining an activity to completion, lack of concern or indifference, and a flattening of affect (Pluck & Brown, 2002). A previous study proposed a shared biological mechanism to explain amotivation and fatigue in a subset of depressed patients (Raison & Miller, 2011). Further investigations to explore this relationship are warranted.
Previous work from our team observed that a single dose of ketamine (0.5 mg/kg intravenous dose) had rapid and sustained anti-fatigue effects 40 minutes after infusion, which lasted until 2 days post infusion (Saligan et al., 2016). In fact, the effect size of the ketamine-placebo difference was greatest at day 2 (d=0.59). What is unknown is whether the anti-fatigue effects of ketamine are markedly linked by the association of depression with fatigue. Knowing the extent of the association of depression on the anti-fatigue effects of ketamine is important, in order to identify potential shared or distinct biological pathways that distinguish fatigue from depression. Currently, the etiology of fatigue is unknown.
Our research team is pursuing prospects to identify potential therapeutic targets for both debilitating conditions of fatigue and depression. Moreover, because they seem to be clinically interrelated, it is of more importance to find a way to understand the influence of one over the other, especially in evaluating treatment outcomes. For this purpose, we have incorporated independent measures of fatigue and depression in our clinical trials. We developed the National Institutes of Health-Brief Fatigue Inventory (NIH-BFI) as a distinct measure of fatigue within the context of depression (Saligan, et al., 2015), and administered the Montgomery-Asberg Depression Rating Scale (MADRS), a commonly used unidimensional measure of depressive symptoms (Montgomery & Asberg, 1979). Our published reports on the anti-depressant and anti-fatigue effects of ketamine provide the unique possibility of advancing our understanding on how fatigue and depression are closely related (Zarate et al., 2006; Saligan et al., 2016). More importantly, new data collected from the National Institute of Mental Health ketamine clinical trials meant to assess ketamine’s mechanism of action are poised to identify which improvements in specific domains of depression are related to the anti-fatigue effects of ketamine (Ballard et al., 2018).
This study investigated the association of depression on the rapid anti-fatigue effects of a single intravenous dose of ketamine. Further, the study identified the improvements of specific symptom domains of depression that are related to the anti-fatigue effects of ketamine. Findings from this study can advance our understanding of the tapestry of symptoms that can co-occur or are closely bound to this multidimensional fatigue construct. This information is clinically useful to refine the conceptual definition of fatigue, and to identify shared and distinct biologic pathways to develop optimal therapy.
Methods
Design and subjects:
This is a secondary analysis of data collected from an original study (NCT00088699) (Nugent et al., 2018). This study was a double-blind, randomized, placebo-controlled, crossover clinical study exploring the efficacy of ketamine as an intervention in reducing depressive symptoms in patients with Major Depressive Disorder (MDD). This analysis includes the 35 MDD patients described in the Nugent et al., publication as well as four additional participants who were collected for additional biomarker/sleep analyses(Nugent et al., 2018). Informed consent was obtained for all study participants. The study was conducted at the NIH Clinical Center, Bethesda, Maryland. Participants with treatment-resistant MDD (DSM-IV criteria) received a single infusion of ketamine hydrochloride intravenously at a dose of 0.5mg/kg over 40 minutes or placebo on 2 experimental days separated by 2 weeks.
Men and women, ages 18 to 65 years with diagnosis of recurrent MDD without psychotic features as diagnosed using the Structured Clinical Interview for Axis I DSM-IV Disorders-Patient Version (First MB, 2001), and with an age of onset ≤40 years were eligible participants. Potential participants were enrolled if they had a score of ≥20 on the MADRS (Montgomery and Asberg, 1979) at screening and before each infusion. In addition, each subject had to have failed to respond to at least one prior adequate antidepressant trial, as assessed by the Antidepressant Treatment History Form, and to be experiencing a current major depressive episode of at least four weeks duration (Sackeim, 2001). Subjects were either un-medicated or tapered off medications for a minimum of two weeks before randomization (5 weeks for fluoxetine, 3 weeks for aripiprazole). Individuals with a DSM-IV diagnosis of drug or alcohol dependence or abuse within the past three months, serious or unstable illness, or uncorrected hypo- or hyperthyroidism were excluded. Additional study details have been previously published (Nugent al. 2018).
Measures:
Fatigue was assessed using the seven-item, clinician-administered NIH-BFI validated to measure fatigue separate from depression in the context of depressive disorders (Saligan et al., 2015). All items were scored using a descending order of Likert-type of response from none or normal to worst symptoms. Concentration difficulties and lassitude were scored from 0 to 6, while fatigability, work and activities, retardation were scored from 0 to 4. Irritability was scored from 0 to 8, and general somatic symptoms were rated from 0 to 2. All items except for retardation asked the responder to recall their experiences in the past week, while retardation was rated in real time by the clinician conducting the interview. Total NIH-BFI scores ranged from 0–34, where a higher NIH-BFI score suggests severity of fatigue symptoms. Depression was assessed using the 10-item MADRS. The MADRS is a reliable and valid unidimensional instrument to assess six core depressive symptoms such as apparent sadness, reported sadness, inner tension, lassitude, inability to feel, and pessimistic thoughts (Montgomery & Asberg, 1979). All MADRS items were also scored using a descending order of Likert-type of response from 0 (none or normal) to 6 (worst symptom). Similarly, MADRS rates the participant’s experience over the past week. The MADRS total score ranges from 0 to 60 points, with higher total scores suggesting greater depressive symptoms. Both measures were administered by clinical interview from trained clinical research staff. Both questionnaires were administered in the mornings at baseline and Day 1 (24 hrs) post-ketamine infusion.
Specific symptom domains were assessed using previous results from an Exploratory Factor Analysis (EFA) of the MADRS, the Hamilton Depression Rating Scale (HAMD), the Snaith-Hamilton Pleasure Rating Scale (SHAPS), and the Beck Depression Inventory (BDI) (Ballard et al., 2018). EFA depression subscales included Depressed Mood, Tension, Negative Cognition, Impaired Sleep, Suicidal Thoughts, Reduced Appetite, and Amotivation. Ratings for the eighth available subscale, anhedonia, was obtained from only about half of the sample and was therefore excluded from analysis. Ratings from eight assessments were used in this analysis (−60 minutes before ketamine infusion, then 40 minutes, 80 minutes, 120 minutes, 230 minutes, Day 1, Day 2, Day 3 after ketamine infusion), but Day 1 was selected as the timepoint of interest as it correlates with peak ketamine response.
Data analyses.
Descriptive statistics characterized the demographic and clinical attributes of the study participants. A mediation model was generated to determine whether the effect of ketamine on depression symptoms accounted for its effect on fatigue. Because a proposed mediator variable must be correlated with treatment (Kraemer et al., 2002), we confirmed, as previously reported (Nugent et al., 2018), a significant effect of ketamine versus placebo on MADRS total score. Next, we evaluated the effect of ketamine versus placebo on the NIH-BFI total score. Finally, we entered the MADRS total score (the putative mediator) into the model and documented whether it was statistically significant and whether the effect of ketamine versus placebo remained statistically significant in its presence. This process was repeated in further analyses using the EFA depression subscales as potential mediators.
General linear mixed models were used. A repeated effect of (categorical) time was entered for each drug nested within subject, with an unstructured covariance matrix. A random intercept for each subject was used to account for nesting of drug within subject. Degrees of freedom were calculated using the Kenward-Roger approximation. Baseline assessments were modeled, and the effect of ketamine was estimated using a contrast between the baseline — Day 1 difference for each drug. Cohen’s d effect size was calculated using the estimated difference, standard error, and degrees of freedom from this contrast. Infusion was entered as a covariate. Both drug and putative mediator were centered at the sample mean prior to analysis. Given the exploratory nature of these secondary analyses, alpha was unadjusted (α = .05, two-tailed).
Results
Sample.
Responses from the 39 study participants with MDD were included in this secondary analysis. The mean age was 36.26 (± 10.06) years, and 59% of the sample was female.
Depression as a mediator of the Anti-Fatigue Effects of Ketamine.
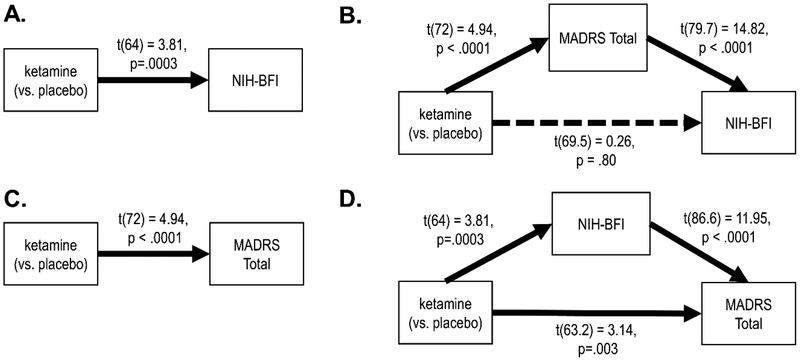
NIH-BFI scores were significantly improved under ketamine compared to placebo (d = 0.95, 95%CI: 0.45 – 1.45). However, this effect was completely mediated by the MADRS Total Score; when the MADRS Total Score was entered in the model, there was no unique effect of ketamine on the NIH-BFI score (d = 0.06, 95%CI: −0.42 – 0.54). The converse was not true; while NIH-BFI was a partial mediator of the effect of ketamine on the MADRS Total Score (see Figure 1), it accounted for only a small proportion of the effect (d = 1.16, 95%CI: 0.69 – 1.64 versus d = 0.79, 95% CI: 0.29 – 1.29).
Figure 1.
Ketamine significantly improved NIH-BFI (Panel A), but the anti-fatigue effects of ketamine were completely accounted for when controlling for the MADRS Total Score (Panel B). The effect of ketamine on the MADRS Total Score (Panel C) was not accounted for by its effect on NIH-BFI (Panel D). In each panel, the effect of ketamine on the outcome is the difference in baseline — Day 1 comparisons between conditions, and the effect of the mediator on the outcome is the slope of the mediator at Day 1.
Specific depression symptom domains as mediators of the Anti-Fatigue Effect of Ketamine.
Most of the specific symptom domains of depression accounted for only a very small amount of the effect of ketamine on fatigue as measured by NIH-BFI, decreasing the effect size only slightly (Table 1). The exceptions were Depressed Mood and Amotivation, which accounted for most of the effect of ketamine on NIH-BFI.
Table 1.
Specific depression symptom domains as mediators of the effect of ketamine on fatigue
| Mediator | Effect Ketamine vs. Placebo on NIH-BFI at Day 1 | Cohen’s d for Ketamine vs. Placebo on NIH-BFI at Day 1 | Effect of mediator on NIH-BFI at Day 1 |
|---|---|---|---|
| Depressed mood | t(66.8) = 1.34, p=.18 | 0.33 (−0.16 – 0.82) | t(75.9) = 12.20, p<.0001 |
| Negative cognitions | t(64.8) = 2.48, p=.02 | 0.62 (0.12 – 1.11) | t(103) = 5.10, p<.0001 |
| Tension | t(62.4) = 3.09, p=.003 | 0.78 (0.28 – 1.29) | t(106) = 6.42, p<.0001 |
| Impaired sleep | t(67.3) = 3.41, p=.001 | 0.83 (0.34 – 1.32) | t(69.7) = 1.62, p=.11 |
| Suicidal thoughts | t(61.7) = 3.47, p=.001 | 0.88 (0.37 – 1.39) | t(121) = 5.33, p<.0001 |
| Amotivation | t(39.6) = 1.87, p=.07 | 0.59 (−0.04 – 1.24) | t(42.9) = 5.38, p<.0001 |
Note: The effect of ketamine versus placebo at Day 1 on NIH-BFI (fatigue) without a mediator in the model, against which values in the third column should be compared, is d = 0.95 (95%CI: 0.45 – 1.45). Note that the sample size is smaller for the Amotivation subscale, on which only 25 participants had data; the effect of ketamine versus placebo on NIH-BFI in that subsample was d = 1.07 (95% 0.41 – 1.72 (Ballard et al., 2018).
Discussion
In these patients with treatment-resistant MDD, ketamine can rapidly improve fatigue symptoms. However, this study suggests that in this population, this improvement is mostly explained by its broader antidepressant effects, rather than a unique anti-fatigue effect. More specifically, effects of ketamine on amotivation and depressed mood, both symptom domains of depression, fully explained the anti-fatigue effects of ketamine. The study findings are clinically valuable for two reasons: (1) ketamine may be effective in treating affective subtypes of fatigue, and (2) fatigue and depression may potentially share common biological networks.
The study findings revealed that the anti-fatigue effect of ketamine was uniquely independent of the effect of ketamine on reduced sleep. The relationship of fatigue and sleep impairment has been established (Tinajero et al., 2018; Åkerstedt et al., 2018). Nonrestorative sleep was significantly associated with daily fatigue in healthy young adults without insomnia (Tinajero et al., 2018). Similarly, changes on fatigue and sleep related to aging were closely linked in a longitudinal study that followed three age groups for eight years (Åkerstedt et al., 2018). In this study, the lack of association of fatigue and sleep impairment is intriguing and worth investigating.
The relationships between variables observed in this secondary analysis may be influenced by how patients with MDD were specifically selected to be included in the analysis. Different relationships between variables may be observed if patients with fatigue without depression were selected. Further, the single dose ketamine used in this clinical trial has a short half-life and its effects are transient; hence, the relationships between variables observed in this study may be different if repeated doses of ketamine or other treatments with more sustained anti-depressant effects were used.
The study findings also raise the possibility that the NIH-BFI may not be a sufficient measure to capture fatigue as a separate construct from depression; this may explain why we were unable to detect the specific anti-fatigue effects of ketamine, above and beyond its anti-depressant effects. After all, MADRS items were used to develop the NIH-BFI, and its psychometric properties were established using patients with MDD (Saligan et al., 2015). Future studies should consider using other fatigue instruments that are known to distinctly measure fatigue separate from depression or consider complementing NIH-BFI with other measures that can capture the specific domains of fatigue, such as behavioral tasks that can assess the cognitive and motivational aspects of fatigue. Diurnal variability in fatigue severity has been established, where fatigue severity is expected to gradually worsen during the course of the day (Dodge, 1982). Future studies should consider using fatigue measures that can capture these circadian variations. To address these concerns related to the clinical population used in the secondary analysis and the possible limitation of the NIH-BFI to measure fatigue on its own, future studies should explore the unique anti-fatigue effects of ketamine by enrolling non-depressed patients with clinically significant fatigue. This secondary analysis is limited by the small sample size of treatment-resistant MDD. Hence, the results of this analysis cannot be generalized to all MDD patients.
Conclusions
This study revealed that ketamine did not have a unique effect on fatigue independent of its general antidepressant effects using the NIH-BFI, in patients with depression. Further, the anti-fatigue effects of ketamine can be explained by its localized effects on amotivation and depressed mood. This finding provides clinically relevant information on the utility of ketamine as a potential therapy for specific subtypes of fatigue (e.g., affective fatigue), agnostic of disease condition. The study highlights the need to use measures that assess specific or several dimensions of fatigue within a considerable period of time that would capture the duration of the desired treatment effect. Several of these measures are listed in a past review (Hjollund et al., 2007).
Highlights.
In patients with treatment-resistant depression, ketamine does not have a unique effect on fatigue outside of its general antidepressant effects.
The anti-fatigue effects of ketamine were mostly accounted for by the changes in amotivation and depressed mood scores.
The anti-fatigue effects of ketamine should be assessed by fatigue-specific measures other than the National Institutes of Health - Brief Fatigue Inventory.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Åkerstedt T, Discacciati A, Miley- Åkerstedt A, Westerlund H (2018). Aging and the change in fatigue and sleep — a longitudinal study across 8 years in three age groups. Front Psychol, 9:234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakshi R, Shaikh ZA, Miletich RS, Czarnecki D, Dmochowski J, Henschel K, Janardhan V, Dubey N, Kinkel PR: Fatigue in multiple sclerosis and its relationship to depression and neurologic disability. Mult Scler 2000;6:181–185. [DOI] [PubMed] [Google Scholar]
- Ballard ED, Yarrington JS, Farmer CA, Lener MS, Kadriu B, Lally N, Williams D, Machado-Vieira R, Niciu MJ, Park L, Zarate CA Jr. Parsing the heterogeneity of depression: An exploratory factor analysis across commonly used depression rating scales. J Affect Disord. 2018. April 15;231:51–57. doi: 10.1016/j.jad.2018.01.027. Epub 2018 Feb 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown LF, Kroenke K: Cancer-related fatigue and its associations with depression and anxiety: A systematic review. Psychosomatics 2009;50:440–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodge R Circadian rhythms and fatigue: a discrimination of their effects on performance. Aviat Space Environ Med. 1982;53:1131–1137. [PubMed] [Google Scholar]
- Ferguson M, Dennehy EB, Marangell LB, Martinez J, Wisniewski SR: Impact of fatigue on outcome of selective serotonin reuptake inhibitor treatment: Secondary analysis of STAR*D. Curr Med Res Opin DOI: 10.1185/03007995.2014.936553. [DOI] [PubMed] [Google Scholar]
- First MB, S. R, Gibbon M, Williams AR. (2001). Structured clinical interview for DSM-IV TR axis I disorders, research version, patient edition (SCID-I/P).
- Hegerl U, Ulke C. Fatigue with up- vs downregulated brain arousal should not be confused. Prog Brain Res 2016;229:239–254. [DOI] [PubMed] [Google Scholar]
- Hjollund NH, Andersen JH, Bech P. Assessment of fatigue in chronic disease: a bibliographic study of fatigue measurement scales. Health Qual Life Outcomes 2007;5:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen PB, Donovan KA, Weitzner MA: Distinguishing fatigue and depression in patients with cancer. Semin Clin Neuropsychiatry 2003;8:229–240. [PubMed] [Google Scholar]
- Kraemer HC, Wilson GT, Fairburn CG, & Agras WS (2002). Mediators and moderators of treatment effects in randomized clinical trials. Archives of general psychiatry, 59(10), 877–883. [DOI] [PubMed] [Google Scholar]
- Lin F, Chen DG, Vance DE, Ball KK, Mapstone M (2013). Longitudinal relationships between subjective fatigue, cognitive function, and everyday functioning in old age. Int Psychogeriatr, 25(2):275–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milanovic M, Holshausen K, Milev R, Bowie CR (2018). Functional competence in major depressive disorder: objective performance and subjective perceptions. J Affect Disor, 234:1–7. [DOI] [PubMed] [Google Scholar]
- Montgomery SA, Asberg M: A new depression scale designed to be sensitive to change. Br J Psychiatry 1979;134:382–389. [DOI] [PubMed] [Google Scholar]
- Nugent AC, Ballard ED, Gould TD, Park LT, Moaddel R, Brutsche NE, Zarate CA Jr (2018). Ketamine has distinct electrophysiological and behavioral effects in depressed and healthy subjects. Mol Psychiatry. doi: 10.1038/s41380-018-0028-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluck G, Brown R (2002). Apathy in Parkinson’s Disease. J Neurology, Neursurgery & Psychiatry, 73(6):636–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL, Miller AH (2011). Is depression an inflammatory disorder? Curr Psychiatry Rep 13: 467–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sackeim HA (2001). The definition and meaning of treatment-resistant depression. J Clin Psychiatry, 62 Suppl 16, 10–17. [PubMed] [Google Scholar]
- Saligan LN, Luckenbaugh DA, Slonena EE, Machado-Vieira R, Zarate CA Jr. (2015). Development of a clinician-administered National Institutes of Health – Brief Fatigue Inventory: a measure of fatigue in the context of depressive disorders. J Psychiatr Res 68, 99–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saligan LN, Luckenbaugh DA, Slonena EE, Machado-Vieira R, Zarate CA Jr. (2016). An assessment of the anti-fatigue effects of ketamine from a double-blind, placebo-controlled, cross-over study in bipolar disorder. J Affect Disord 194, 115–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinajero R, Williams PG, Cribbet MR, Rau HK, Bride DL, Suchy Y (2018). Nonrestorative sleep in healthy, young adults without insomnia: associations with executive functioning, fatigue, and pre-sleep arousal. Sleep Health, 4(3):284–291. [DOI] [PubMed] [Google Scholar]
- Zarate CA Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, Charney DS, Manji HK. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry 2006; 63: 856–864. [DOI] [PubMed] [Google Scholar]