Abstract
The Runx family of transcription factors regulate cell growth and differentiation, and control the expression of target genes involved in cell fate decisions. We examined the role of the bone-related member of this family, Runx2, in regulating apoptosis via modulation of the Bcl2 family of genes in the osteosarcoma cell line Saos2. Our data demonstrate that Runx2 directly binds to two Runx-specific regulatory elements on the human bax promoter thereby inducing Bax expression. Furthermore, bone morphogenetic protein-induced or vector-mediated expression of Runx2 resulted in upregulation of Bax expression, and subsequent increased sensitivity of Saos2 cells to apoptosis. Finally, the observed upregulation of Bax expression and increased apoptosis were Runx2 dependent as Runx2 loss of function abrogated these effects. Our study provides the first evidence for Bax as a direct target of Runx2, suggesting that Runx2 may act as a proapoptotic factor in osteosarcoma cells.
Keywords: Runx2, Bax, osteosarcoma, apoptosis
Introduction
The mammalian homologues of the drosophila Runt gene constitute a small family of transcription factors recently named RUNX (van Wijnen et al., 2004). All three members of this family, Runx1, 2 and 3, have been extensively shown to be key regulators of cell growth and differentiation in various tissues during organogenesis and pathogenesis (Coffman, 2003). These transcriptional regulators recognize the same DNA-biding motif and form multiple interactions with other proteins including their cofactor CBFβ that stabilizes their DNA–protein complexes (Kagoshima et al., 1993). The effects of RUNX–CBFβ regulation were initially shown to be tissue specific. Thus, while Runx1 is required for definitive hematopoiesis (Okuda et al., 1996), Runx2 regulates skeletal development (Komori, 2003), and Runx3 is required for nerve cell differentiation (Inoue et al., 2002).
In addition to their role in normal development and differentiation, Runx factors are implicated in carcinogenesis. However, their exact role in this process remains ambiguous. The most common classification of genes implicated in cancer describes them either as tumor suppressors or oncogenes. Evidence has been presented to suggest that Runx2 may have both biological functions (Blyth et al., 2005). The earliest cases of oncogenic functions of Runx proteins were associated with hematopoietic tumors where chromosomal translocations of the RUNX1 gene were responsible for various types of acute myeloid leukemias (Miyoshi et al., 1991). On the other hand, the presence of inactivating mutations in the Runx3 gene demonstrated its tumor suppressor function in gastric cancer in mice (Li et al., 2002). In humans, mutated Runx3 proteins were also identified in various cases of gastric cancer (Li et al., 2002). Furthermore, restoration of Runx3 function in an animal model exhibited a dramatic inhibitory effect on cancer cells (Guo et al., 2002; Torquati et al., 2004).
The initial association of Runx2 with pathogenesis was exclusively related to skeletal dysplasia such as cleidocranial dysplasia in humans (Mundlos et al., 1997). Recently, a role for Runx2 in the control of the cell cycle as well as its possible involvement in tumor development was postulated (Galindo et al., 2005). Runx2 was also shown to increase the metastatic potential of breast and prostate cancer cells via upregulation of expression of genes involved in metastasis (Barnes et al., 2003; Brubaker et al., 2003). However, in osteosarcoma Thomas et al. (2004) reported that Runx2 function as an inducer of osteoblast differentiation is disrupted. They further showed that restoration of Runx2 function in these tumors results in their growth arrest. Furthermore, activation of bone morphogenetic protein (BMP)/SMAD signaling in skeletal tissues ultimately induces Runx2 expression and activation (Ducy et al., 1997) and leads to terminal differentiation and growth arrest (Pratap et al., 2003; Noda, 2006). We believe that in the context of BMP/SMAD signaling, Runx2 functions as a growth suppressor and apoptosis inducer in both normal osteoblasts and osteosarcoma.
One of the most important regulators of programmed cell death is the Bax protein. Bax is a proapoptotic member of the Bcl2 family of proteins (Adams and Cory, 1998) that counterregulate the permeability of the outer mitochondrial membrane to cytochrome c, a cofactor in activation of caspases (Reed, 1997), and to Smac/Diablo, endoG, AIF and others (Cande et al., 2002; van Gurp et al., 2003). Antiapoptotic Bcl2 family proteins, such as Bcl2 itself permanently reside in the mitochondrial membrane and function by limiting its permeability (Cory and Adams, 2002). Proapoptotic Bax and Bak are cytosolic but translocate to the outer mitochondrial membrane upon induction of apoptosis and induce permeabilization of the membrane and release of the intermembrane apoptogenic factors (Martinou and Green, 2001; Willis and Adams, 2005; Antignani and Youle, 2006; Reed, 2006). Gene knockout studies indicate that Bax/Bak double knockout mice display a lack of apoptosis and therefore Bax and Bak proteins are crucial for execution of programmed cell death (Wei et al., 2001). Bax is ubiquitously expressed in all tissues, however in many cancer cells, Bax is downregulated (Rampino et al., 1997; Meijerink et al., 1998) while Bcl2 is upregulated (Nunez et al., 1989) leading to the pronounced imbalance between the proapoptotic and antiapoptotic cellular systems and to a dramatic increase in Bcl2 to Bax ratio, a major determinant of cell sensitivity to apoptosis (Cory and Adams, 2002).
Several studies have demonstrated a role for Runx family members during programmed cell death (Bae and Choi, 2004; Vogiatzi et al., 2006). Recently, Yano et al. (2006) showed that Runx3 specifically upregulates the levels of the Bcl2 proapoptotic family member, Bim, in gastric epithelial cells. Pratap et al. (2003) demonstrated that Runx2 inhibits cell proliferation and mediates osteoblast differentiation and terminal maturation. Furthermore, Runx2-mediated osteoblast differentiation was shown to be disrupted in osteosarcoma evidenced by enhanced growth inhibition and exit from the cell cycle in a Runx2 gain of function model (Thomas et al., 2004). These reports clearly implicate Runx2 as a tumor suppressor in osteosarcoma cells. Here we examined the role of Runx2 in regulating apoptosis through modulation of the Bcl2 family of genes in osteosarcoma. Our data demonstrate that Runx2 directly binds to two Runx-specific regulatory elements within the 5′ upstream region of the human bax gene thereby activating Bax transcription. Furthermore, BMP-induced or vector-mediated expression of Runx2 leads to upregulation of Bax and increased sensitivity of the osteosarcoma cells Saos2 to apoptosis.
Results
BMP2 induces Bax expression and sensitizes cells to apoptosis
BMPs are known to induce terminal differentiation and apoptosis in a variety of tissues including bone and cartilage. Although increasingly investigated, the mechanisms of BMP-induced apoptosis are not fully elucidated. To assess the effects of BMP2 on proapoptotic vs antiapoptotic factors in osteosarcoma cells we treated the Saos2 cell line with recombinant human BMP2 and measured the expression of Bcl2 and Bax. Figure 1 shows that while BMP2 has no effect on Bcl2 transcripts, it significantly induced Bax mRNA levels in Saos2 cells (Figure 1a). Concomitantly, Bax protein levels were upregulated by BMP2 treatment while Bcl2 levels remained unchanged (Figures 1b and c). This BMP2-induced upregulation of Bax expression was also observed in the human osteosarcoma cell line 143B as well as in nonmalignant human osteoblasts hFOB (data not shown). The induction of Bax expression in Saos2 cells resulted in 50% decrease in the Bcl2 to Bax ratio (Figure 1d), a major determinant of cell sensitivity to apoptosis (Cory and Adams, 2002). Subsequent induction of apoptosis in the BMP2-treated cells was confirmed with caspase-3 activation (Figure 2a) and chromatin condensation (Figure 2b) assays. To determine whether Bax plays a key role in BMP2-induced apoptosis in Saos2 cells, we performed shRNA-mediated knockdown of Bax expression. Figure 2c shows that the pKD-Bax shRNA vector led to 70% decrease in Bax mRNA levels when transfected into Saos2 cells indicating that this vector is effective in inhibiting Bax expression. Furthermore, Figures 2a and b show that loss of Bax function completely inhibited BMP2-induced apoptosis as assessed by both caspase activity and chromatin condensation assays, respectively. Thus, this data show the ability of BMP2 to induce apoptotic signals in the human osteosarcoma cell line Saos2 primarily via upregulation of Bax expression.
Figure 1.
Bone morphogenetic protein 2 (BMP2) induces expression of Bax. Human Saos2 cells were incubated with 100ngml−1 of human recombinant BMP2 for 48h, total RNA extracts and protein lysates were prepared and various assays were performed. (a) Levels of Bcl2 and Bax mRNA were measured using real time RT-PCR analysis as described in ‘Materials and methods’, normalized to levels of GAPDH) mRNA and expressed as fold change over untreated controls; (b) western blotting of protein lysate samples using monoclonal antibodies against Bcl2 or Bax was performed as described in ‘Materials and methods’. The blots were reprobed for β-actin to verify equal loading. The blots are representatives of four experiments; (c) band intensities were measured by densitometry and normalized to β-actin; (d) Bcl2 to Bax ratios were determined by measuring densities of corresponding bands on western blots and normalizing to β-actin. Data are means±s.d. (n=4). Asterisk (*) indicates P<0.05 when compared to control.
Figure 2.
Bone morphogenetic protein 2 (BMP2) induces differentiation and apoptosis in Saos2 cells. (a) Caspase-3 activity in cell lysates was measured using fluorogenic substrate Ac-DEVD-amc as described in detail in ‘Materials and methods’; (b) cells were stained with Hoechst 33342 fluorescent nuclear stain (inset) and apoptotic nuclei showing condensation of chromatin (marked with arrowheads in the inset picture) were counted; (c) real-time RT-PCR analysis of Bax expression normalized to GAPDH) after pKD-Bax shRNA vector transfection; (d) alkaline phosphatase (ALP), an osteoblast differentiation marker, was assayed in cell lysates enzymatically using colorgenic substrate; (e) levels of Runx2 mRNA were measured using real time RT-PCR analysis and normalized to levels of GAPDH mRNA. Data are means±s.d. (n=3). Asterisk (*) indicates P<0.05 when compared to control.
We concomitantly characterized the phenotype of these cells in response to BMP2. We found that BMP2 treatment of Saos2 cells for 48h is capable of inducing both alkaline phosphatase (ALP) enzymatic activity (Figure 2d) and Runx2 gene expression (Figure 2e). Together, these results show that activation of BMP2 signaling promotes Saos2 cell differentiation and may subsequently induce apoptosis through induction of Bax expression in these osteosarcoma cells.
The effect of BMP2 on Bax expression and apoptosis is Runx2 dependent
To determine whether the BMP2 upregulation of Bax expression and subsequent induction of apoptosis is Runx2 dependent, we performed Runx2 knockdown using small interfering RNA (siRNA) in Saos2 cells followed by their treatment with BMP2. We first verified that the Runx2 transcripts are inhibited by the siRNA. Figure 3a shows that transfection of Saos2 cells with Runx2 siRNA resulted in at least 70% inhibition of its expression (Figure 3a). Furthermore, Runx2 knockdown resulted in a significant downregulation of Bax expression in the untreated controls while it abrogated the effect of BMP2 on Bax expression (Figure 3b). The inhibitory effects of Runx2 loss of function on BMP2-induced apoptosis were also observed by caspase-3 activation (Figure 3c) and chromatin condensation (Figure 3d). These results indicate that BMP2-induced Bax expression and apoptosis are at least partly mediated through Runx2 in Saos2 osteosarcoma cells.
Figure 3.
Loss of Runx2 function downregulates Bax and inhibits apoptosis. Saos2 cells were either treated with Runx2 siRNA (Rx2 siRNA) or control oligonucleotides (Ctrl). Runx2 (a) and Bax (b) expression levels were measured using real time RT-PCR, normalized to GAPDH) expression and plotted as fold change over controls. In (b), (c) and (d) cells were incubated in the presence or absence of bone morphogenetic protein 2 (BMP2) for 48h. Caspase-3 activity (c) and apoptotic chromatin condensation (d) were measured as described in Figure 2. Data are means±s.d. (n=4 (a, b) or 3 (c, d)). Asterisk (*) indicates P<0.05 when compared to control.
BMP2-induced Bax transcription is mediated through direct interaction of Runx2 with its promoter in osteosarcoma cells
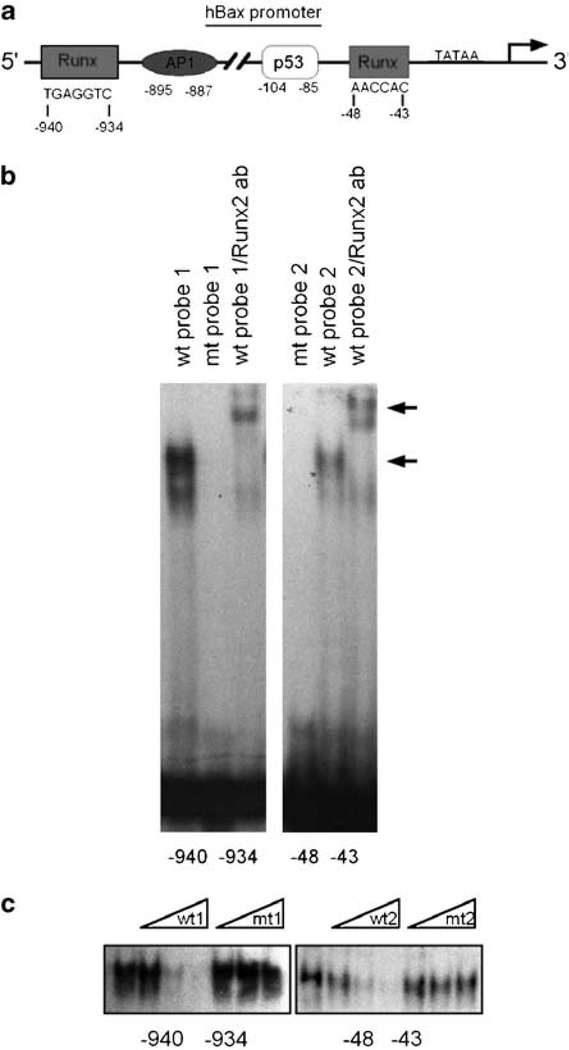
Our analysis of the 5′ upstream region of the human bax gene shows that in addition to the previously characterized p53, and Myc sites, two 100% matching putative response elements for Runx proteins located between nucleotides –940 to –934 and –48 to –43bp were also identified (Figure 4a). However, no Smad1, 5 or 8 response elements were shown in our computerized search using the Web-based Transcription Element Search System.
Figure 4.
In vitro binding of Runx2 to Runx motives found in human bax gene promoter. (a) Shown are the positions of a distal and proximal Runx-binding motives relative to the transcription initiation site, TATAA element and neighboring transcription element-binding sites within the human bax gene promoter; (b) a representative image (n=3) of gel shift and supershift assays in Saos2 nuclear lysates using oligonucleotides containing the Runx-binding elements from the human bax gene promoter (–940 to –934bp, left; and –48 to –43bp, right). The lower arrow indicates the specific DNA–protein complex, and the upper arrow indicates a supershift with Runx2 antibody. Incubation with the mutant probe did not result in gel shift, confirming the specificity of binding; (c) electrophoretic mobility shift (EMSA) competition assay performed with increasing amounts (25-, 50- and 100-fold molar excess) of either cold wild-type (wt) or mutant (mt) probes showing the specificity of DNA–protein complex. Each gel is representative of three experiments.
To determine whether Runx2 can directly bind to the human bax promoter, we performed electrophoretic mobility shift (EMSA) and chromatin immunoprecipitation (ChIP) DNA-binding assays. For our EMSA experiments we designed two probes spanning the distal and proximal Runx sites at positions –940 to –934 and –48 to –43, respectively. Figure 4b shows that using nuclear extracts from Saos2 osteosarcoma cells, we observed protein/DNA complex formation with both oligonucleotides indicating that these Runx sites are capable of binding Runx2 proteins. Furthermore, mutation of the consensus sequence for Runx failed to show any protein/DNA complex. We further confirmed the binding of Runx2 to both sites by performing gel shift immunoassays using Runx2-specific antibodies. Protein/DNA complexes were completely supershifted by the Runx2 monoclonal antibody (Figure 4b). The specificity of this protein/DNA interaction was also confirmed by competition experiments using cold wild-type (wt) and mutated Runx oligonucleotides. Figure 4c shows that while the wt probe increasingly competes for Runx2 binding, the mutant probe is unable to compete even at 100-fold molar excess. To further examine the occupancy of the human bax gene promoter by Runx2 in vivo, we performed ChIP analyses using chromatin extracts from Saos2 cells treated or not with BMP2. Figure 5 shows recruitment of Runx2 to both distal (Figure 5a) and proximal (Figure 5b) regions of the human bax gene promoter containing the Runx2-binding sites. This Runx2 occupancy on the Bax promoter was dramatically enhanced in the BMP2-treated samples. Together, these data demonstrate direct binding of Runx2 to both Runx regulatory elements within the human bax gene promoter.
Figure 5.
In vivo occupancy of the human bax gene promoter by Runx2. Chromatin immunoprecipitation (ChIP) assay of nuclei from Saos2 cells incubated in the presence or absence of 100ngml−1 of bone morphogenetic protein 2 (BMP2) for 48h. (a) PCR analysis of the ChIP assay was performed using primers designed to amplify a region (nt –997 to nt –824) containing the distal Runx regulatory element on the human bax promoter; (b) PCR analysis of the ChIP assay was performed using primers designed to amplify the proximal Runx regulatory element within a region between nt –167 and nt –5 on the human bax promoter. The arrows in diagrams indicate the positions of the forward and reverse primers. The shown agarose gels are representative of three experiments and show selective amplification of regions of the human bax gene promoter containing distal (a) or proximal (b) Runx regulatory elements in the input and Runx2 immunoprecipitate lanes, while negative control did not show any products. BMP treatment significantly enhanced the occupancy of the Runx regulatory elements within the human bax gene promoter by Runx2.
Runx2 transactivates the human bax gene promoter
To assess the functionality of these Runx2-binding sites, we subcloned the human bax promoter into a luciferase reporter gene construct and generated two deletion mutants, the –0.25kb promoter fragment that includes the proximal Runx motif, and the –1.2kb fragment that includes both distal and proximal Runx sites (Figure 6a). Figure 6b shows that basal promoter activity is significantly increased between the –0.25 and the –1.2kb fragments of the Bax promoter by two and fivefold, respectively compared to the empty reporter control. Over-expression of Runx2 significantly and dose dependently enhanced Bax promoter activity of both the –0.25 and the –1.2kb fragments (Figure 6c). To further evaluate the functional relevance of these two Runx sites, we performed site-directed mutagenesis of either the –940 to –934 or the –48 to –43 sites within the –1.2kb of the Bax promoter. Figure 6d shows that the basal activities of both mutants are significantly decreased. Furthermore, while over-expression of Runx2 strongly induced Bax promoter activity in the wt control, mutagenesis of either Runx sites inhibited Runx2 ability to transactivate the Bax promoter. Together these results show that both Runx sites are functional and capable of mediating the effects of Runx2 on the human bax promoter.
Figure 6.
Transactivation of the human bax gene promoter by Runx2. Luciferase reporter vectors contained either –1.2 or –0.25kb regions of the human bax gene promoter. (a) A diagram of the constructed reporter vectors. The –1.2kb reporter contains both Runx regulatory elements while the –0.25kb reporter contains only a proximal Runx regulatory element; (b) basal activities of the –1.2 and –0.25kb reporter constructs vs pGL4.10 empty vector transfected into Saos2 cells; (c) effect of Runx2 expression on the –0.25 and –1.2kb promoter construct activities. Saos2 cells were cotransfected with the reporter and the increasing amounts of pCMV-Runx2 expression vector. In (b) and (c) data are means±s.d. (n=5). In (b) asterisk (*) indicates P<0.05 when compared to basal activity of pGL4.10 vector. In (c) * indicates P<0.05 when compared to basal activity of the corresponding constructs; (d) the effect of point mutations in –934 and –48 Runx sites within the –1.2kb promoter construct on basal or Runx2-stimulated promoter activity. Data are means±s.d. (n=3). Asterisk indicates P<0.05 when compared to basal activity of –1.2kb reporter construct. Numbers shown are actual mean values.
Overexpression of Runx2 leads to upregulation of Bax and increased sensitivity to apoptosis
To evaluate the role of Runx2 as a regulator of Bax expression and apoptosis, we overexpressed Runx2 protein in Saos2 cells using the Runx2-pCMV expression construct. Overexpression of Runx2 verified by western blot analyses (Figures 7a and b) did not alter the levels of Bcl2 protein but upregulated the levels of Bax protein by twofold (Figures 7a and b). This enhanced Bax expression also resulted in a significant shift in the Bcl2 to Bax ratio toward proapoptotic Bax in Saos2 cells transfected with the Runx2 expression vector when compared to the empty vector controls (Figure 7c). Similar results were observed when Runx3 was over-expressed indicating that this effect may also be mediated by other members of the Runx family (data not shown).
Figure 7.
Overexpression of Runx2 increases Bax expression and Saos2 cells sensitivity to apoptosis. Saos2 cells were transfected with the Runx2-pCMV expression vector (Runx2) or with the control pCMV vector (EV) and after 48h various assays were performed. (a) Cell lysates were prepared as described in ‘Materials and methods’ and subjected to western blotting using antibodies against Runx2, Bax, Bcl2 and β-actin. When compared to empty vector-transfected controls, Runx2 transfectants showed marked increase in Runx2 expression and a corresponding increase in Bax expression but not Bcl2 expression. The blots are representatives of three experiments; (b) band intensities were measured by densitometry and normalized to b-actin; (c) Bax and Bcl2 lane densities were analysed with densitometry, normalized to β-actin and Bcl2 to Bax ratios were calculated. Cells transfected with Runx2-pCMV or the empty vector were induced with 10μM etoposide or vehicle control for 3h, and apoptosis was detected by measuring activity of caspase-3 in cell lysates (d) and chromatin condensation (e) as described in ‘Materials and methods’. Data are means±s.d. (n=3). Asterisk (*) indicates P<0.05 when compared to control.
To determine whether the Runx2-induced upregulation of Bax had an effect on sensitivity to apoptosis, we measured caspase-3 activation and chromatin condensation in cells induced or not with 10μM etoposide. Figures 7d and e show that while no significant activation of caspase-3 and condensation of chromatin were observed in empty vector-transfected controls and following etoposide treatment, Runx2 overexpression significantly induced both caspase-3 activity and chromatin condensation by at least twofold over control. Furthermore, 3h etoposide treatments together with Runx2 overexpression show a cumulative effect resulting in a threefold increase in caspase-3 activity (Figure 7d) and fivefold increase in chromatin condensation (Figure 7e). Together, these results demonstrate that the increase in Saos2 cell sensitivity to apoptosis is due to decreased Bcl2 to Bax ratio as a result of Runx2-mediated upregulation of Bax in these cells.
Discussion
There is a body of evidence implementing the role of apoptosis in the control of carcinogenesis and tumor metastasis in various tissues (Reed, 1999). A significant number of these studies focused on the role of Bax and Bcl2. However, the majority of them examined the counterregulation of permeability of the outer mitochondrial membrane by Bax and Bcl2 while the transcriptional pathways utilized by tumor cells to manipulate apoptosis-related genes are still underinvestigated. Recent evidence has emerged describing a role for the bone-related transcription factor Runx2 in the control of cell growth (Pratap et al., 2003; Thomas et al., 2004). Runx2 regulates several genes responsible for growth control and differentiation, and is a downstream target of BMP/SMAD signaling. BMP2 is known to induce differentiation and also increases cell sensitivity to apoptosis in skeletal tissues (Pratap et al., 2003). However, the mechanisms underlying BMP-induced apoptosis are not fully elucidated.
Although Runx2 was established as a downstream mediator of BMP/SMAD-induced osteoblast differentiation (Komori, 2003), the role of Runx2 in BMP-induced apoptosis is not understood. We therefore hypothesized that BMP2-induced apoptosis in osteosarcoma cells requires Runx2-mediated activation of the proapoptotic gene Bax. Our data show that treatment of Saos2 human osteosarcoma cells with recombinant BMP2 induces their differentiation through enhancement of Runx2 gene expression. Furthermore, increased sensitivity to apoptosis was also observed as a result of BMP2 treatment in these cells. Here, we determined the mechanisms through which BMP2 induces this increased sensitivity to apoptosis by measuring the expression of antiapoptotic Bcl2 and proapoptotic Bax in Saos2 cells upon BMP2 treatment. We found that although the expression of antiapoptotic Bcl2 did not change, a significant increase in the expression of proapoptotic Bax was observed. This led to a marked decrease in Bcl2 to Bax ratio, a key determinant of cell sensitivity to apoptosis (Cory and Adams, 2002). These data are in agreement with findings recently reported by Fukuda et al. (2006) showing that BMP4-induced upregulation of Bax in mouse hybridoma HS-72 cells leads to enhanced cell death through a p53-dependent mechanism. Our study was performed in a p53-null osteosarcoma cell line, Saos2, indicating the existence of a novel, p53-independent mechanism for Bax regulation by BMP2. The loss of function experiment using a specific siRNA-mediated knockdown of Runx2 in Saos2 cells showed that the effect of BMP2 on Bax expression and subsequent apoptosis is Runx2 dependent. Our analysis of the human bax gene promoter demonstrates the involvement of Runx2 in regulating Bax expression. To our knowledge, this is the first evidence for a Runx2-mediated regulation of Bax transcription in osteosarcoma cells. Our results complement the initial characterization of the Bax promoter by Miyashita and Reed (1995) demonstrating the induction of Bax transcription by p53. Thus, we established Runx2 as a key positive regulator of Bax transcription in the absence of p53 in cancer cells. This role of Runx2 is novel and differs from its previously described function as an antiproliferative agent via its cooperation with the tumor-suppressing protein pRb (Lee et al., 2006) and the cyclin kinase inhibitor p27 (Thomas et al., 2004).
Here we present a novel mechanism of growth inhibition by Runx2 directed against cell survival mechanisms rather than cell proliferation. Our data clearly show that incubation of Runx2-transfected cells with a well-known inducer of apoptosis, etoposide, leads to further activation of caspase-3. Therefore, our results provide evidence supporting a role for Runx2 as a proapoptotic factor in osteosarcoma cells. We have also assessed the effects of BMP2 on the other proapoptotic genes and found that only Bim is upregulated in Saos2 cells (data not shown). This is in agreement with the previously reported study by Yano et al. (2006) showing that Runx3 specifically upregulates the levels of the Bcl2 proapoptotic family member, Bim, in gastric epithelial cells. Furthermore, the presence of Runx responsive elements on the Bim promoter was also postulated and shown to interact with Runx3. Interestingly, when Runx3 was overexpressed in Saos2 cells (data not shown), Bax expression was upregulated suggesting that Runx3 may also play a role in promoting osteosarcoma cell apoptosis in combination with Runx2.
Loss of osteoblastic differentiation is a characteristic feature of osteosarcomas (Wang, 2005), while gain of Runx2 function restores differentiation and inhibits growth of osteosarcoma cells (Thomas et al., 2004). Thus, despite the presence of Runx2 in osteosarcoma cells at levels comparable to that found in nonmalignant osteoblasts, Runx2 activity in these cancer cells is inhibited (Thomas et al., 2004 and our unpublished data). One could speculate that the proapoptotic function of Runx2 in osteosarcoma cells must somehow be specifically suppressed to support cell cycle progression in normal osteoblasts. Similarly, this proapoptotic function of Runx2 would also need to be suppressed in osteosarcoma cells in a significant subset of the cells to support tumor growth. It is also important to note that fundamental differences may exist between osteosarcoma and normal osteoblasts as forced expression of Runx2 in normal osteoblast progenitors does not affect apoptosis (Galindo et al., 2005) while our data clearly demonstrate the ability of Runx2 to be proapoptotic in malignant cells.
Although the cancer-related mutations of Runx2 similar to those found in Runx1 and Runx3 have not yet been identified, posttranslational modifications of Runx2 have been reported, such as deacetylation and phosphorylation. The tumor suppressor protein, p300 acetylates and consequently stabilizes Runx2 while the factors that phosphorylate Runx2, such as Smurf1 and others, target it for ubiquitination and degradation (Bae and Lee, 2006). It is, therefore possible that in tumor cells derived from tissues that are largely dependent on Runx2 signaling, such as bone, the tumor suppressor function of Runx2 is inhibited by such posttranslational modifications. Therefore, targeting negative regulators of Runx2, such as HDACs, Smurf1 and others may unmask the tumor suppressor potential of Runx2 leading not only to inhibition of proliferation but, according to our data, to the direct activation of the bax gene and increased sensitivity to apoptosis. Overall, our findings establish Runx2 as a novel transcriptional regulator of Bax expression. We also provide evidence for a possible mechanism underlying BMP2-induced apoptosis via Runx2-mediated upregulation of Bax in osteosarcoma cells.
Materials and methods
Materials
Most chemicals were from Sigma (St Louis, MO, USA) unless otherwise noted. Cell culture media and components were from Gibco (Carlsbad, CA, USA). All oligonucleotides were custom made by IDT (Coralville, IA, USA). Primary antibodies were from Epitomics (Burlingame, CA, USA) (anti-Bcl2, anti-Bax,), Santa Cruz (Santa Cruz, CA, USA) (anti-Runx2) and Sigma (anti-β-actin). Horseradish peroxidase-conjugated secondary antibodies and Precision Plus molecular weight markers for western blotting were from Bio-Rad (Hercules, CA, USA).
Cell culture and treatments
Human osteosarcoma Saos2 cells obtained from American Type Cell Culture were cultured in Dulbecco’s modified Eagle’s medium media supplemented with 10% fetal bovine albumin and 1% penicillin/streptomycin mixture and treated with 100 ng ml−1 of human recombinant BMP2 (Peprotech, Rocky Hill, NJ, USA) or phosphate-buffered saline (PBS). Cells were harvested after 48h of treatment for further analysis. A subset of these cells was transfected with pCMV-Runx2 construct or an empty vector as a control. After 48h, cells were treated with etoposide at 10μM or with dimethyl sulfoxide as a vehicle control. To achieve Runx2 knockdown, we used an anti-sense siRNA specific for human Runx2 (5′-CAGACAGAAGCUUGAUGACUGUAAA-3′). A non-specific oligonucleotide was used as a control. To achieve knockdown of Bax, we used pKD-Bax shRNA vector (Upstate Biotechnologies, Lake Placid, NY, USA) or an empty vector as a control. Transfections were performed using Fugene-HD liposome reagent (Roche, Basel, Switzerland).
Real-time RT-PCR
Total cellular RNA was prepared using RNeasy Mini Kit (Qiagen, Valencia, CA, USA) and reverse transcribed into cDNA using SuperScript First-Strand Synthesis Kit (Invitrogen, Carlsbad, CA, USA) and oligo-dT primers according to the manufacturer’s protocol. cDNA per sample (1μl) was subjected to real-time PCR using following sets of primers: Bax (5′-CACCAGCTCTGAGCAGATCATGAAG-3′ and 5′-GCGGCAATCATCCTCTGCAG-3′), Bcl2 (5′-GTACGACAACCGGGAGATAGTGATG-3′ and 5’-GTGGACCACAGGTGGCACC-3′), Runx2 (5-CCGGAATGCCTCTGCTGTTA TGA-30 and 50-ACTGAGGCGGTCAGAGAACAAACT-3′), and GAPDH) (5′-GAGTCAACGGATTTGGTCGT-3′ and 5′-GACAAGCTTCCCGTTCTCAG-3′). Real-time PCR was performed using the RotorGene real-time DNA amplification system (Corbett Research, Sydney, Australia). SYBR Green (ABgene, Epsom, UK) was used to monitor DNA synthesis. The expression of proteins of interest was normalized to the expression of GAPDH.
Western blotting
Cells were lysed and 25μg of total protein per sample was mixed 1:1 with 2×Laemmli buffer, boiled, electrophoresed and then electroblotted onto polyvinylidene difluoride membranes. Blots were developed using standard technique, visualized using SuperSignal WestPico chemiluminescent substrate (Pierce, Rockford, IL, USA) and photographed. Blots were then stripped in Re-Blot Plus stripping buffer (Chemicon, Billerica, MA, USA) and reprobed with anti-β-actin antibody to verify equal loading. Blot images were scanned and lane densities were measured using Adobe Photoshop.
Caspase-3 activity assay
Caspase-3 activity was measured using fluorogenic substrate cleavage assay (Nicholson, 1999). The reaction mixture contained 200μl of PBS, 10μg of cell lysate and 20μM of the caspase-3 fluorogenic substrate Ac-DEVD-amc (Calbiochem, San Diego, CA, USA). The reactions were loaded into 96-well plates and incubated at 37°C for 30min. Fluorescence at 440nm (excitation 380nm) was measured using a Hitachi plate reader.
Chromatin condensation assay
Cells plated on 24-well plates were treated as described above. A nuclear stain Hoechst 33342 was added to the media at 1μM. Nuclear morphology was assessed using Zeiss AxioVert inverted fluorescence microscope and the number of apoptotic nuclei showing condensed chromatin vs total number of nuclei was counted.
Alkaline phosphatase activity assay
Alkaline phosphatase activity in cell lysates was measured enzymatically using p-nitrophenylphosphate as a substrate. The ALP activity was determined spectrophotometrically at 410nm by comparison with standard solutions of p-nitrophenol using Beckman Coulter DU640 spectrometer.
Gel shift, supershift and competition assays
Nuclear fractions were prepared using the NE-PER nuclear extraction kit (Pierce) according to the manufacturer’s protocol. EMSA was performed as described previously (Drissi et al., 2002). Briefly, reactions were incubated at room temperature for 30min prior to electrophoresis. For competition experiments, wt or mutated oligonucleotides were added to nuclear extracts for 20min at room temperature and then incubated with labeled probe for 30min before separation in nondenaturing polyacrylamide gel. The oligonucleotides corresponding to –934 (1) and –48 (2) Runx sites were as follows: wt1 5′-ATCACTTGAGGTCAGGAGC-3′ and 5′-GCTCCTGACCTCAAGTGAT-3′; wt2 5′-AAACAAAACCACTCAGTT-3′ and 5′-AACTGAGTGGTTTTGTTT-3′; mt1 5′-ATCACTTaAGaTCAGGAGC-3′ and 5′-GCTCCTGatCTtAAGTGAT-3′; and mt2 5′-AAACAAAtCtACTCAGTT-3′ and 5′-AACTGAGTaGaTTTGTTT-3′. Gels were visualized by autoradiography.
Chromatin immunoprecipitation assay
Chromatin immunoprecipitation assay was performed using ChIP-IT kit (Active Motif, Carlsbad, CA, USA) according to the manufacturer’s instructions. Briefly, cells were fixed with formaldehyde and homogenized. Nuclei were pelleted and DNA was sheared enzymatically and immunoprecipitated with either anti-Runx2 antibody or negative control immunoglobulin G or positive control pol II antibody using protein G beads. DNA was released from immunoprecipitate complexes using proteinase K and purified. The purified DNA was used as a template for PCR with primers designed to amplify either –997 to –824 region or –167 to –5 region within the human bax gene promoter. The corresponding primers were as follows: (5′-ACAGTGGCTCACGCCTGTAAT-3′ and 5′-AGCCTCCCAAGTAGCTGGAATTG-3′) and (5′-TCATGCCTGTAATCCCAGCGCTTT-3′ and 5′-TGCTTCCAGGCAGGACGTTATAGA-3′).
Cloning of bax promoter into luciferase reporter and reporter activity assay
Human bax gene promoter fragments, –1.2kb (–1.2kbp to +42bp) and –0.25kb (–250 to +42bp), were PCR amplified from the BAC clone RP11–3N16 containing q3.3–3.4 region of the human chromosome 19 using the following pairs of primers: 5′-AAGGACTCGAGTCTCTGACAGGCCCAAA-3′ and 5′-TCCTTCTCGAGGCAGAAGGAATTAGCAAGTG-3′ (1.2kb); and 5′-AAGGACTCGAGAAATATGGCA TATTTGGGGC-3′ and 5′-TCCTTCTCGAGGCAGAAGGAATTAGCAAGTG-3′ (–0.25kb), respectively. These primers were designed to introduce CTCGAG XhoI 5′ and 3′ flanking sequences. The fragments were then subcloned into the XhoI site of the promoterless pGL4.10 luciferase reporter vector. The correct insert orientation of the resulting promoter reporters was verified by sequencing. To evaluate promoter activities, the constructed –1.2 and –0.25kb reporters were transfected into Saos2 cells at 2μg per well in six-well plates. The renilla luciferase vector pRL-TK (Promega, Madison, WI, USA) was cotransfected at 0.2μg per well as a reference. Firefly and renilla luciferase activities were measured using an Optocomp 1 luminometer and a Dual Luciferase Reporter Assay System (Promega) according to the manufacturer’s protocol. The firefly luciferase signal was normalized to renilla luciferase signal and expressed as relative luminescence units.
Site-directed mutagenesis
The putative Runx-binding sequences between nucleotides –940 and –934 and between –48 and –43 on the human Bax promoter in –1.2kb reporter were mutated using the QuickChange Site-Directed Mutagenesis Kit (Stratagene, La Jolla, CA, USA) according to the manufacturer’s instructions. Generated mutants were as follows: –940 –934 site 5′TGAGGTC-3′ was changed into 5′-TGATCTC-3′ and –48 –43 site 5′-AACCAC-3′ was changed into 5′-AAGAAC-3′. Mutants were cotransfected with pRL-TK vector and luciferase assay was performed as described above.
Statistical analysis
Experiments were repeated 3–5 times. Mean values and standard deviations were calculated, and the statistical significance was determined using Student’s t-test. Data with P<0.05 were considered statistically significant.
Acknowledgements
Financial support for this study was provided by the Wilmot Cancer Research Foundation and Karen D’Amico Foundation and an NIH RO-1 Grant (AR-052674–01) to Hicham Drissi. We thank Dr Do Yu Soung for reading the manuscript.
References
- Adams JM, Cory S. (1998). The Bcl-2 protein family: arbiters of cell survival. Science 281: 1322–1326. [DOI] [PubMed] [Google Scholar]
- Antignani A, Youle RJ. (2006). How do Bax and Bak lead to permeabilization of the outer mitochondrial membrane? Curr Opin Cell Biol 18: 685–689. [DOI] [PubMed] [Google Scholar]
- Bae SC, Choi JK. (2004). Tumor suppressor activity of RUNX3. Oncogene 23: 4336–4340. [DOI] [PubMed] [Google Scholar]
- Bae SC, Lee YH. (2006). Phosphorylation, acetylation and ubiquitination: the molecular basis of RUNX regulation. Gene 366: 58–66. [DOI] [PubMed] [Google Scholar]
- Barnes GL, Javed A, Waller SM, Kamal MH, Hebert KE, Hassan MQ et al. (2003). Osteoblast-related transcription factors Runx2 (Cbfa1/ AML3) and MSX2 mediate the expression of bone sialoprotein in human metastatic breast cancer cells. Cancer Res 63: 2631–2637. [PubMed] [Google Scholar]
- Blyth K, Cameron E, Neil J. (2005). The Runx genes: gain or loss of function in cancer. Nature Rev 5: 376–387. [DOI] [PubMed] [Google Scholar]
- Brubaker KD, Vessella RL, Brown LG, Corey E. (2003). Prostate cancer expression of runt-domain transcription factor Runx2, a key regulator of osteoblast differentiation and function. Prostate 56: 13–22. [DOI] [PubMed] [Google Scholar]
- Cande C, Cohen I, Daugas E, Ravagnan L, Larochette N, Zamzami N et al. (2002). Apoptosis-inducing factor (AIF): a novel caspase-independent death effector released from mitochondria. Biochimie 84: 215–222. [DOI] [PubMed] [Google Scholar]
- Coffman JA. (2003). Runx transcription factors and the developmental balance between cell proliferation and differentiation. Cell Biol Int 27: 315–324. [DOI] [PubMed] [Google Scholar]
- Cory S, Adams JM. (2002). The Bcl2 family: regulators of the cellular life-or-death switch. Nat Rev Cancer 2: 647–656. [DOI] [PubMed] [Google Scholar]
- Drissi H, Pouliot A, Stein JL, van Wijnen AJ, Stein GS, Lian JB. (2002). Identification of novel protein/DNA interactions within the promoter of the bone-related transcription factor Runx2/Cbfa1. J Cell Biochem 86: 403–412. [DOI] [PubMed] [Google Scholar]
- Ducy P, Zhang R, Geoffroy V, Ridall AL, Karsenty G. (1997). Osf2/ Cbfa1: a transcriptional activator of osteoblast differentiation. Cell 89: 747–754. [DOI] [PubMed] [Google Scholar]
- Fukuda N, Saitoh M, Kobayashi N, Miyazono K. (2006). Execution of BMP-4-induced apoptosis by p53-dependent ER dysfunction in myeloma and B-cell hybridoma cells. Oncogene 25: 3509–3517. [DOI] [PubMed] [Google Scholar]
- Galindo M, Pratap J, Young DW, Hovhannisyan H, Im H, Choi JY et al. (2005). The bone-specific expression of Runx2 oscillates during the cell cycle to support a G1-related antiproliferative function in osteoblasts. J Biol Chem 280: 20274–20285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo WH, Weng LQ, Ito K, Chen LF, Nakanishi H, Tatematsu M et al. (2002). Inhibition of growth of mouse gastric cancer cells by Runx3, a novel tumor suppressor. Oncogene 21: 8351–8355. [DOI] [PubMed] [Google Scholar]
- Inoue K, Ozaki S, Shiga T, Ito K, Masuda T, Okado N et al. (2002). Runx3 controls the axonal projection of proprioceptive dorsal root ganglion neurons. Nat Neurosci 5: 946–954. [DOI] [PubMed] [Google Scholar]
- Kagoshima H, Shigesada K, Satake M, Ito Y, Miyoshi H, Ohki M et al. (1993). The Runt domain identifies a new family of heteromeric transcriptional regulators. Trends Genet 9: 338–341. [DOI] [PubMed] [Google Scholar]
- Komori T (2003). Requisite roles of Runx2 and Cbfb in skeletal development. J Bone Miner Metab 21: 193–197. [DOI] [PubMed] [Google Scholar]
- Lee JC, Thomas DM, Gutierrez G, Carty SA, Yanagawa S, Hinds PW. (2006). HES1 cooperates with pRb to activate RUNX2-dependent transcription. J Bone Miner Res 21: 921–933. [DOI] [PubMed] [Google Scholar]
- Li QL, Ito K, Sakakura C, Fukamachi H, Inoue K, Chi XZ et al. (2002). Causal relationship between the loss of RUNX3 expression and gastric cancer. Cell 109: 113–124. [DOI] [PubMed] [Google Scholar]
- Martinou JC, Green DR. (2001). Breaking the mitochondrial barrier. Nat Rev Mol Cell Biol 2: 63–67. [DOI] [PubMed] [Google Scholar]
- Meijerink JPP, Mensink EJBM, Wang K, Sedlak TW, Sloetjes AW, De Witte T et al. (1998). Hematopoietic malignancies demonstrate loss-of-function mutations of BAX. Blood 91: 2991–2997. [PubMed] [Google Scholar]
- Miyashita T, Reed JC. (1995). Tumor suppressor p53 is a direct transcriptional activator of the human bax gene. Cell 80: 293–299. [DOI] [PubMed] [Google Scholar]
- Miyoshi H, Shimizu K, Kozu T, Maseki N, Kaneko Y, Ohki M. (1991). t(8;21) breakpoints on chromosome 21 in acute myeloid leukemia are clustered within a limited region of a single gene, AML1. Proc Natl Acad Sci USA 88: 10431–10434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundlos S, Otto F, Mundlos C, Mulliken JB, Aylsworth AS, Albright S et al. (1997). Mutations involving the transcription factor CBFA1 cause cleidocranial dysplasia. Cell 89: 773–779. [DOI] [PubMed] [Google Scholar]
- Nicholson DW. (1999). Caspase structure, proteolytic substrates, and function during apoptotic cell death. Cell Death Differ 6: 1028–1042. [DOI] [PubMed] [Google Scholar]
- Noda M (2006). Current topics in pharmacological research on bone metabolism: regulation of bone mass by the function of endogenous modulators of bone morphogenetic protein in adult stage. J Pharmacol Sci. 100: 211–214. [DOI] [PubMed] [Google Scholar]
- Nunez G, Seto M, Seremetis S, Ferrero D, Grignani F, Korsmeyer SJ et al. (1989). Growth- and tumor-promoting effects of deregulated BCL2 in human B-lymphoblastoid cells. Proc Natl Acad Sci USA 86: 4589–4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuda T, van Deursen J, Hiebert SW, Grosveld G, Downing JR. (1996). AML1, the target of multiple chromosomal translocations in human leukemia, is essential for normal fetal liver hematopoiesis. Cell 84: 321–330. [DOI] [PubMed] [Google Scholar]
- Pratap J, Galindo M, Zaidi SK, Vradii D, Bhat BM, Robinson JA et al. (2003). Cell growth regulatory role of Runx2 during proliferative expansion of preosteoblasts. Cancer Res 63: 5357–5362. [PubMed] [Google Scholar]
- Rampino N, Yamamoto H, Ionov Y, Li Y, Sawai H, Reed JC et al. (1997). Somatic frameshift mutations in the bax gene in colon cancers of the microsatellite mutator phenotype. Science 275: 967–969. [DOI] [PubMed] [Google Scholar]
- Reed JC. (1997). Cytochrome c: can’t live with it—can’t live without it. Cell 91: 559–562. [DOI] [PubMed] [Google Scholar]
- Reed JC. (1999). Dysregulation of apoptosis in cancer. J Clin Oncol 17: 2941–2953. [DOI] [PubMed] [Google Scholar]
- Reed JC. (2006). Proapoptotic multidomain Bcl-2/Bax-family proteins: mechanisms, physiological roles, and therapeutic opportunities. Cell Death Differ 13: 1378–1386. [DOI] [PubMed] [Google Scholar]
- Thomas DM, Johnson SA, Sims NA, Trivett MK, Slavin JL, Rubin BP et al. (2004). Terminal differentiation, mediated by runx2 and p27KIP1, is disrupted in osteosarcoma. J Cell Biochem 167: 925–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torquati A, O’Rear L, Longobardi L, Spagnoli A, Richards WO, Daniel BR. (2004). RUNX3 inhibits cell proliferation and induces apoptosis by reinstating transforming growth factor beta responsiveness in esophageal adenocarcinoma cells. Surgery 136: 310–316. [DOI] [PubMed] [Google Scholar]
- van Gurp M, Festjens N, van Loo G, Saelens X, Vandenabeele P. (2003). Mitochondrial intermembrane proteins in cell death. Biochem Biophys Res Commun 304: 487–497. [DOI] [PubMed] [Google Scholar]
- van Wijnen AJ, Stein GS, Gergen JP, Groner Y, Hiebert SW, Ito Y et al. (2004). Nomenclature for Runt-related (RUNX) proteins. Oncogene 23: 4209–4210. [DOI] [PubMed] [Google Scholar]
- Vogiatzi P, De Falco G, Claudio PP, Giordano A. (2006). How does the human RUNX3 gene induce apoptosis in gastric cancer? Latest data, reflections and reactions. Cancer Biol Ther 5: 371–374. [DOI] [PubMed] [Google Scholar]
- Wang LL. (2005). Biology of osteogenic sarcoma. Cancer J 11: 294–305. [DOI] [PubMed] [Google Scholar]
- Wei MC, Zong WX, Cheng EH, Lindsten T, Panoutsakopoulou V, Ross AJ et al. (2001). Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science 292: 727–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis SN, Adams JM. (2005). Life in the balance: how BH3-only proteins induce apoptosis. Curr Opin Cell Biol 17: 617–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano T, Ito K, Fukamachi H, Chi XZ, Wee HJ, Inoue K et al. (2006). The RUNX3 tumor suppressor upregulates Bim in gastric epithelial cells undergoing transforming growth factor beta-induced apoptosis. Mol Cell Biol 26: 4474–4488. [DOI] [PMC free article] [PubMed] [Google Scholar]