Abstract
The broadening field of microbiome research has led to a substantial reappraisal of the gut–liver axis and its role in chronic liver disease. The liver is a central immunologic organ that is continuously exposed to food and microbial-derived antigens from the gastrointestinal tract. Mucosal-associated invariant T (MAIT) cells are enriched in the human liver and can be activated by inflammatory cytokines and microbial antigens. In chronic inflammatory liver disease, MAIT cells are depleted suggesting an impaired MAIT cell-dependent protection against bacterial infections.
Keywords: mucosal-associated invariant T cells, hepatitis, inflammation, cirrhosis, liver metastases, gut–liver axis
The liver is an important immunologic organ, which receives >75% of its blood supply via the portal vein from the gastrointestinal tract. This antigen-rich blood passes through a network of fenestrated sinusoids with a unique repertoire of innate and adaptive immune cells, before it returns via the hepatic veins into the systemic circulation. Thus, the healthy liver can be regarded as a vascular firewall that prevents the entry of gut-derived antigens and bacteria into the systemic circulation.1 Advanced liver disease often presents with spontaneous bacterial peritonitis and hepatic encephalopathy. Gastrointestinal dysbiosis, impairment of the intestinal barrier function, and increased bacterial translocation have been reported in advanced liver disease and may contribute to inflammation via disturbed host–microbial interactions.2
Keeping the close relationship of the liver and the gastrointestinal tract in mind, it is important to decipher mechanisms of intrahepatic immune cell activation and their role in liver inflammation and disease progression. In this review, we will introduce and explore the role of MAIT cells, an innate-like T cell population, in chronic inflammatory liver disease in humans.
Phenotypic and Functional Characteristics of MAIT Cells
MAIT cells are called “mucosal-associated” because they have originally been described as an abundant population of T cells in the lamina propria of the small intestine.3 However, MAIT cells are also enriched at other barrier sites, such as liver and lung (►Table 1).4 They constitute up to 40% of intrahepatic and up to 10% of peripheral blood T cells.4,5 Thus, MAIT cells represent the most abundant subset of all nonconventional T cells. The majority of MAIT cells in both liver and blood are CD8+ (most are CD8αα+, few are CD8αβ+), a small proportion is CD8−CD4− double negative, and only a few MAIT cells are CD4+.6–8
Table 1.
Distribution of MAIT cells in human tissues
| Tissue | MAIT cells/CD3+ T cells [%] | References |
|---|---|---|
| Peripheral blood | 0.1–10% | Dusseaux et al 20114 Bolte et al 201729 Franciszkiewicz et al 20165 |
| Liver | 10–40% | Dusseaux et al 20114 Bolte et al 201729 Jeffery et al 20168 Tang et al 201320 |
| Lung | 1–10% | Franciszkiewicz et al 20165 Hinks et al 201654 Le Bourhis et al 201018 |
| Intestine | 2–10% | Dusseaux et al 20114 Serriari et al 201455 Howson et al 201556 |
| Lymphoid tissue | <1% | Dusseaux et al 20114 |
Abbreviation: MAIT, mucosal-associated invariant T.
MAIT cells are selected in the thymus, but remain at low frequency with an immature naïve phenotype (CD45RAhi, CD45ROlo, CD27hi) after thymic egress and then expand preferentially in barrier tissues.7,9 In humans, MAIT cells have been detected as early as the second fetal trimester in the small intestine, lung, and liver.9 They display an effector memory phenotype (CD45RA−, CD45RO+, CD62lo, CD122int, CD127hi, CD95hi) with a high proliferative capacity in these tissues as indicated by expression of the proliferation marker Ki67 and high level expression of CD127, which enables homeostatic proliferation in response to IL-7.9 Their maturation and functionality are dependent on the commensal flora, cytokines, and the transcription factor promyelocytic leukemia zinc finger (PLZF).10–12 In mice, postnatal MAIT cell development in the intestine has been shown to depend on the commensal flora of the gut and on B cells as demonstrated by the absence of MAIT cells in germfree mice and mice that lack B cells.3 MAIT cells remain rare and immature in lymphoid tissues such as spleen and mesenteric lymph nodes.4
MAIT cells are strikingly different from conventional T cells. First, MAIT cells express a conserved invariant T cell receptor (TCR) α chain (Vα7.2-Jα33)13,14 and a limited repertoire of β chains (predominantly Vβ6 and Vβ20).15 Second, MAIT cells are not reactive to peptides. They recognize vitamin B (riboflavin) metabolites from microorganisms that are presented on major histocompatibility complex (MHC) class I-like molecules, called MR1.16,17 Microorganisms that possess the riboflavin-synthesizing pathway include enterobacteriaceae, staphylococci, mycobacteria, and fungi. All have been shown to activate MAIT cells.18,19 This appears to be an evolutionary conserved mechanism because the sequence of the antigen-presenting MHC class I-like molecule is shared among mammals.20,21 MR1 is expressed at mRNA level in multiple tissues,22 but its surface expression is tightly regulated and depends on antigen exposure.23–25 This contrasts with classical MHC class I molecules, which are highly expressed on cells even in the absence of antigens. Stimulation by diverse pathogen-derived ligands results in antigen-dependent expansion of individual MAIT cell clones and in a distinct TCR repertoire of the MAIT cell population in each individual.26 Third, MAIT cells express high levels of the IL-18 receptor along with receptors for IL-12, IL-23, IL-2,4,12 and the C-type lectin-like receptor CD161, which they share with liver-infiltrating T cells.27 Fourth, as mentioned above, MAIT cells display an effector memory phenotype and rapidly respond to antigen (TCR dependent) and cytokine (TCR independent) stimulation in an innate-like fashion. Their effector response includes a mixed TH1/TH17 profile with the production of proinflammatory cytokines including IFN-γ, TNF-α, and IL-17.4,20,27 In addition, MAIT cells exert cytotoxic effector functions by releasing granzyme B and perforin upon activation.28
The anatomic location of MAIT cells along with their evolutionary conserved semi-invariant TCR and their rapid innate-like responses to bacterial products suggest that they play an essential role in the host defense at the gut–liver interface.
MAIT Cells in Chronic Liver Disease
MAIT cells can be identified in human blood and liver samples by co-staining with an antibody against CD161 and with either an antibody against the TCR Vα7.2 chain or an MR1 tetramer loaded with a vitamin B derivate (6-hydroxymethyl-8-d-ribityllumazine, rRL-6-CH2OH).15
MAIT cells are recruited to the liver based on their expression of the tissue-homing chemokine receptors CCR6, CXCR6, and the integrin αEβ7. They lack expression of the lymph node homing receptor CCR7.4,8 During liver inflammation, additional recruitment occurs via the IFN-inducible chemokines CXCL9 and CXCL10 that bind to the CXCR3 receptor on MAIT cells.8 This is consistent with greater activation and cytotoxicity of MAIT cells in the liver than in the blood of HCV-infected patients.29 Within the liver, MAIT cells have been shown to localize around the bile ducts in the portal tracts.8 They express the multidrug resistance transporter ABCB1 and thus are more resistant to bacterial-derived xenobiotics, drugs, and chemotherapeutic agents than other lymphocytes.4
MAIT cells can be activated in an antigen-dependent manner and in a cytokine-dependent manner in chronic inflammatory liver disease (►Fig. 1). As regards antigen-dependent activation, MAIT cells from the liver produce IFN-γ and degranulate upon in vitro culture with Escherichia coli-exposed biliary epithelial cells.8 Their protective role is supported by experimental studies that demonstrated a higher bacterial burden following intraperitoneal injection of E. coli in MR1–/– mice lacking MAIT cells.18,30 Moreover, in a large human study Grimaldi et al showed a higher incidence of severe bacterial infections in critically ill patients with a depletion of MAIT cells in the peripheral blood.31
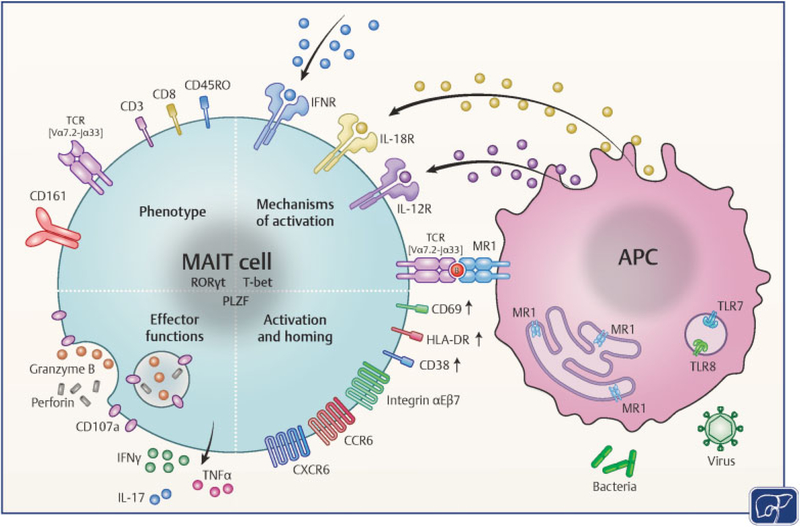
Fig. 1.
Mechanisms of MAIT cell activation in the liver. MAIT cells are characterized by their semi-invariant T cell receptor, TCR Vα7.2-Jα33, the C-type lectin receptor, CD161, and the transcription factors T-bet, RORγt and PLZF. They express the chemokine receptors CCR6, CXCR-6, and integrin αEβ7 to home close to the bile ducts in the portal tracts. MAIT cell activation in a TCR-dependent manner by riboflavin-synthesizing bacteria and in a TCR-independent manner by cytokines is dependent on APC and results in IFN-γ, TNF-α, IL-17, granzyme B, and perforin expression. (Modified with permission from Kurioka A, Walker LJ, Klenerman P, Willberg CB. MAIT cells: new guardians of the liver. Clin Transl Immunology 2017;6(2):e132.) APC, antigen-presenting cells; B, vitamin-B metabolites; IFNR, interferon receptor; IL-17; interleukin-17; MAIT, mucosal-associated invariant T; PLZF, promyelocytic leukemia zinc finger; RORγt, retinoid acid-related orphan receptor gamma; TCR, T cell receptor; T-bet, T-box transcription factor; TNF-α, tumor necrosis factor-alpha.
As regards to antigen-independent activation, type I IFN32 and IL-12/IL-1833 are among the cytokines that activate MAIT cells in chronic viral hepatitis. Type I IFN is secreted by plasmacytoid dendritic cells sensing virus-infected hepatocytes34,35 and IL-12 and IL-18 are released by monocytes/macrophages in response to single-stranded RNA binding to intracellular toll-like receptor-8 (TLR-8).36,37 Indeed, it has been shown that MAIT cells along with natural killer (NK) cells are the main producers of IFN-γ after TLR-8 stimulation of monocytes.38 This monocyte-dependent activation pathway extends beyond the liver and has also been described in infections with dengue virus, influenza virus, and human immunodeficiency virus (HIV).39
Importantly, the frequency of MAIT cells is decreased in both liver and blood in a variety of chronic inflammatory liver diseases. These include alcoholic steatohepatitis and NASH, primary sclerosing cholangitis, and primary biliary cholangitis8 as well as chronic HBV and HCV infec-tion.29,40–42 In chronic HCV infection, MAIT cells have been extensively characterized, and the residual MAIT cells show an activated phenotype with increased expression of the activation markers CD69, HLA-DR, and CD38. Their response to TCR-mediated but not cytokine-mediated stimulation is impaired when compared with uninfected controls.29,42 As for conventional T cells, this impaired function is associated with an upregulation of the exhaustion markers PD-1, Tim-3, and CTLA-4.41 Impairment of MAIT cell function depends on the severity of liver fibrosis as demonstrated in a crosssectional study of HCV-infected patients.43 However, a reduced frequency of MAIT cells is not solely found in liver disease and has also been reported in HIV infection.6,44 Patients with HIV/HCV co-infection have the lowest frequency of peripheral MAIT cells.45
The frequency of MAIT cells in the liver correlates inversely with liver inflammation, as assessed by the histologic activity index (HAI score), in chronic HCV infection.29 Thus, MAIT cells may be recruited from the blood to the liver where they are activated by inflammatory cytokines and undergo activation-induced cell death. This is consistent with the observation of a selective loss of CD8+ MAIT cells of HCV-infected patients upon in vitro stimulation with either IL-12/IL-18 or E. coli/IL-15.29 Moreover, activation-induced death of MAIT cells has been shown by Cosgrove et al after in vitro stimulation of MAIT cells with E. coli44 and a pro-apoptotic phenotype of MAIT cells with increased expression of caspase 3 and 7 has been reported by Gérart et al.46
With the introduction of highly effective direct acting antivirals (DAA), HCV infection has become an invaluable disease model to study immune cell alterations before and after viral clearance. HCV titers typically decrease to undetectable levels within 4 weeks of treatment along with an effective reduction in liver inflammation. This results in a decrease in MAIT cell activation and an increase in MAIT cell frequency in the liver.29 These changes parallel a decrease in intrahepatic monocyte activation and plasma IL-18 levels.29 Overall, this underlines the predominant role of virus-induced cytokines in MAIT cell activation in chronic HCV infection.
However, despite the rapid decrease in viral titer and intrahepatic inflammation within 4 weeks of antiviral therapy, the intrahepatic MAIT cell frequency remains significantly lower than in uninfected controls.29 In the blood, the MAIT cell frequency remains reduced for at least 72 weeks after successful antiviral therapy, which is the longest follow-up of sustained virologic responders to date.42 Importantly, successful antiviral therapy did not restore the impaired antigen-dependent effector function of MAIT cells.29,42 Collectively, these findings indicate that HCV infection leaves a lasting imprint on the MAIT cell population.
MAIT Cells in Metastatic Liver Disease
MAIT cells have been recognized as an attractive immunotherapeutic target in patients with colorectal cancer because they are enriched in the intestine, the primary site of colorectal cancer, and the liver, a common site of metastases, and respond rapidly to cytokine and antigen stimulation. MAIT cells infiltrate colorectal liver metastases but are less abundant in liver metastases than in healthy liver tissue.47 Furthermore, MAIT cell function decreases with increasing proximity to metastatic liver lesions. This is not a result of decreased cytokine receptor expression on MAIT cells or prior chemotherapy.47 Tumor-infiltrating MAIT cells are functionally impaired to produce IFN-γ in response to both cytokine (IL-12/IL-18) and antigen (staphylococcus enterotoxin B, Klebsiella lysate) stimulation.47 Collectively, these findings indicate that tumor-infiltrating MAIT cells are profoundly impaired in their function by the tumor microenvironment.
MAIT Cells in Metabolic Disease
Nonalcoholic fatty liver disease (NAFLD), a disorder ranging from accumulation of fat in the liver to NASH and liver cirrhosis, is the hepatic manifestation of the metabolic syndrome.48 With a rising prevalence, NAFLD has become the most common cause of chronic liver disease in developed countries.49,50 A critical factor in the progression of NAFLD to NASH is oxidative stress perpetuating liver inflammation.51 This raises the questions whether MAIT cells are activated and contribute to the disease pathogenesis. In our own studies, we found an activated MAIT cell phenotype with increased cytotoxicity but reduced cytokine production in patients with NASH.52 Magalhaes et al investigated MAIT cells in obesity and type 2 diabetes, which are features of the metabolic syndrome and commonly associated with NAFLD. The authors found that MAIT cells are present in adipose tissue, but they are significantly reduced in their frequency in the peripheral blood of obese and type 2 diabetic patients.21 The peripheral blood MAIT cells displayed an activated phenotype and, in contrast to NASH patients, enhanced cytokine production.21 Of note, there was also a striking increase in IL-17 production in the adipose tissue of obese patients. Both, the peripheral MAIT cell frequency and the functional alterations, were restored after bariatric surgery.21
In conclusion, MAIT cells represent a major T cell population in the human liver, which is capable of exerting antimicrobial and antiviral functions. Their enrichment in the liver and their location in the portal tracts close to bile ducts suggest an important role of MAIT cells in liver immune surveillance. In chronic inflammatory liver diseases, the frequency of MAIT cells is significantly reduced and their function in response to bacteria is impaired. This may impact liver immune surveillance and result in increased susceptibility to bacterial infections which is a major cause of mortality in patients with decompensated liver cirrhosis.53 Further studies will help to define the role of MAIT cells in healthy and diseased livers and to identify mechanisms that contribute to both progression and complications of chronic liver disease.
Main Concepts and Learning Points
The liver comprises an abundant population of mucosal-associated invariant T (MAIT) cells that are preferentially localized to bile ducts in the portal tracts.
MAIT cells are innate-like T cells with an effector memory phenotype that can rapidly respond to cytokines and bacteria by production of interferon-gamma (IFN-γ), tumor necrosis factor-alpha (TNF-α), and interleukin-17 (IL-17).
MAIT cells are severely reduced in frequency in chronic inflammatory liver diseases including chronic hepatitis B and C virus (HBV and HCV) infection, alcoholic and non-alcoholic steatohepatitis (NASH), primary sclerosing cholangitis, and primary biliary cholangitis.
Alterations in the MAIT cell compartment in chronic liver disease may impact liver immune surveillance, resulting in increased susceptibility to infections.
References
- 1.Balmer ML, Slack E, de Gottardi A, et al. The liver may act as a firewall mediating mutualism between the host and its gut commensal microbiota. Sci Transl Med 2014;6(237):237ra66 [DOI] [PubMed] [Google Scholar]
- 2.Heymann F, Tacke F. Immunology in the liver–from homeostasis to disease. Nat Rev Gastroenterol Hepatol 2016;13(02):88–110 [DOI] [PubMed] [Google Scholar]
- 3.Treiner E, Duban L, Bahram S, et al. Selection of evolutionarily conserved mucosal-associated invariant T cells by MR1. Nature 2003;422(6928):164–169 [DOI] [PubMed] [Google Scholar]
- 4.Dusseaux M, Martin E, Serriari N, et al. Human MAIT cells are xenobiotic-resistant, tissue-targeted, CD161hi IL-17-secreting T cells. Blood 2011;117(04):1250–1259 [DOI] [PubMed] [Google Scholar]
- 5.Franciszkiewicz K, Salou M, Legoux F, et al. MHC class I-related molecule, MR1, and mucosal-associated invariant T cells. Immunol Rev 2016;272(01):120–138 [DOI] [PubMed] [Google Scholar]
- 6.Leeansyah E, Ganesh A, Quigley MF, et al. Activation, exhaustion, and persistent decline of the antimicrobial MR1-restricted MAIT-cell population in chronic HIV-1 infection. Blood 2013;121(07):1124–1135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martin E, Treiner E, Duban L, et al. Stepwise development of MAIT cells in mouse and human. PLoS Biol 2009;7(03):e54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jeffery HC, van Wilgenburg B, Kurioka A, et al. Biliary epithelium and liver B cells exposed to bacteria activate intrahepatic MAIT cells through MR1. J Hepatol 2016;64(05):1118–1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leeansyah E, Loh L, Nixon DF, Sandberg JK. Acquisition of innate-like microbial reactivity in mucosal tissues during human fetal MAIT-cell development. Nat Commun 2014;5:3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang H, Hogquist KA. How MAIT cells get their start. Nat Immunol 2016;17(11):1238–1240 [DOI] [PubMed] [Google Scholar]
- 11.Savage AK, Constantinides MG, Han J, et al. The transcription factor PLZF directs the effector program of the NKT cell lineage. Immunity 2008;29(03):391–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fergusson JR, Smith KE, Fleming VM, et al. CD161 defines a transcriptional and functional phenotype across distinct human T cell lineages. Cell Reports 2014;9(03):1075–1088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Porcelli S, Yockey CE, Brenner MB, Balk SP. Analysis of T cell antigen receptor (TCR) expression by human peripheral blood CD4–8-alpha/beta T cells demonstrates preferential use of several V beta genes and an invariant TCR alpha chain. J Exp Med 1993;178(01):1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lantz O, Bendelac A. An invariant T cell receptor alpha chain is used by a unique subset of major histocompatibility complex class I-specific CD4+ and CD4–8-T cells in mice and humans. J Exp Med 1994;180(03):1097–1106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reantragoon R, Corbett AJ, Sakala IG, et al. Antigen-loaded MR1 tetramers define T cell receptor heterogeneity in mucosal-associated invariant T cells. J Exp Med 2013;210(11):2305–2320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kjer-Nielsen L, Patel O, Corbett AJ, et al. MR1 presents microbial vitamin B metabolites to MAIT cells. Nature 2012;491 (7426):717–723 [DOI] [PubMed] [Google Scholar]
- 17.Patel O, Kjer-Nielsen L, Le Nours J, et al. Recognition of vitamin B metabolites by mucosal-associated invariant T cells. Nat Commun 2013;4:2142. [DOI] [PubMed] [Google Scholar]
- 18.Le Bourhis L, Martin E, Péguillet I, et al. Antimicrobial activity of mucosal-associated invariant T cells. Nat Immunol 2010;11(08):701–708 [DOI] [PubMed] [Google Scholar]
- 19.Dias J, Leeansyah E, Sandberg JK. Multiple layers of heterogeneity and subset diversity in human MAIT cell responses to distinct microorganisms and to innate cytokines. Proc Natl Acad Sci U S A 2017;114(27):E5434–E5443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tang XZ, Jo J, Tan AT, et al. IL-7 licenses activation of human liver intrasinusoidal mucosal-associated invariant T cells. J Immunol 2013;190(07):3142–3152 [DOI] [PubMed] [Google Scholar]
- 21.Magalhaes I, Pingris K, Poitou C, et al. Mucosal-associated invariant T cell alterations in obese and type 2 diabetic patients. J Clin Invest 2015;125(04):1752–1762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Riegert P, Wanner V, Bahram S. Genomics, isoforms, expression, and phylogeny of the MHC class I-related MR1 gene. J Immunol 1998;161(08):4066–4077 [PubMed] [Google Scholar]
- 23.Chua WJ, Kim S, Myers N, et al. Endogenous MHC-related protein 1 is transiently expressed on the plasma membrane in a conformation that activates mucosal-associated invariant T cells. J Immunol 2011;186(08):4744–4750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McWilliam HE, Eckle SB, Theodossis A, et al. The intracellular pathway for the presentation of vitamin B-related antigens by the antigen-presenting molecule MR1. Nat Immunol 2016;17(05): 531–537 [DOI] [PubMed] [Google Scholar]
- 25.Ussher JE, van Wilgenburg B, Hannaway RF, et al. TLR signaling in human antigen-presenting cells regulates MR1-dependent activation of MAIT cells. Eur J Immunol 2016;46(07):1600–1614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gold MC, Napier RJ, Lewinsohn DM. MR1-restricted mucosal associated invariant T (MAIT) cells in the immune response to Mycobacterium tuberculosis. Immunol Rev 2015;264(01): 154–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Billerbeck E, Kang YH, Walker L, et al. Analysis of CD161 expression on human CD8+ T cells defines a distinct functional subset with tissue-homing properties. Proc Natl Acad Sci U S A 2010;107 (07):3006–3011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kurioka A, Ussher JE, Cosgrove C, et al. MAIT cells are licensed through granzyme exchange to kill bacterially sensitized targets. Mucosal Immunol 2015;8(02):429–440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bolte FJ, O’Keefe AC, Webb LM, et al. Intra-hepatic depletion of mucosal-associated invariant T cells in hepatitis C virus-induced liver inflammation. Gastroenterology 2017;153(05): 1392–1403.e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kurioka A, Walker LJ, Klenerman P, Willberg CB. MAIT cells: new guardians of the liver. Clin Transl Immunology 2016;5(08): e98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grimaldi D, Le Bourhis L, Sauneuf B, et al. Specific MAIT cell behaviour among innate-like T lymphocytes in critically ill patients with severe infections. Intensive Care Med 2014;40 (02):192–201 [DOI] [PubMed] [Google Scholar]
- 32.Spaan M, Hullegie SJ, Beudeker BJ, et al. Frequencies of circulating MAIT cells are diminished in chronic HCV, HIV and HCV/HIV co-infection and do not recover during therapy. PLoS One 2016;11 (07):e0159243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ussher JE, Bilton M, Attwod E, et al. CD161++ CD8+ T cells, including the MAIT cell subset, are specifically activated by IL-12+IL-18 in a TCR-independent manner. Eur J Immunol 2014;44 (01):195–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takahashi K, Asabe S, Wieland S, et al. Plasmacytoid dendritic cells sense hepatitis C virus-infected cells, produce interferon, and inhibit infection. Proc Natl Acad Sci U S A 2010;107(16):7431–7436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lau DT, Fish PM, Sinha M, Owen DM, Lemon SM, Gale M Jr. Interferon regulatory factor-3 activation, hepatic interferon-stimulated gene expression, and immune cell infiltration in hepatitis C virus patients. Hepatology 2008;47(03):799–809 [DOI] [PubMed] [Google Scholar]
- 36.Serti E, Werner JM, Chattergoon M, Cox AL, Lohmann V, Rehermann B. Monocytes activate natural killer cells via inflamma-some-induced interleukin 18 in response to hepatitis C virus replication. Gastroenterology 2014;147(01):209–220.e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chattergoon MA, Latanich R, Quinn J, et al. HIV and HCV activate the inflammasome in monocytes and macrophages via endosomal toll-like receptors without induction of type 1 interferon. PLoS Pathog 2014;10(05):e1004082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jo J, Tan AT, Ussher JE, et al. Toll-like receptor 8 agonist and bacteria trigger potent activation of innate immune cells in human liver. PLoS Pathog 2014;10(06):e1004210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van Wilgenburg B, Scherwitzl I, Hutchinson EC, et al. ; STOP-HCV consortium. MAIT cells are activated during human viral infections. Nat Commun 2016;7:11653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yong YK, Tan HY, Saeidi A, et al. Decrease of CD69 levels on TCR Vα7.2(+)CD4(+) innate-like lymphocytes is associated with impaired cytotoxic functions in chronic hepatitis B virus-infected patients. Innate Immun 2017;23(05):459–467 [DOI] [PubMed] [Google Scholar]
- 41.Barathan M, Mohamed R, Vadivelu J, et al. Peripheral loss of CD8 (+) CD161(++) TCRVα7·2(+) mucosal-associated invariant T cells in chronic hepatitis C virus-infected patients. Eur J Clin Invest 2016;46(02):170–180 [DOI] [PubMed] [Google Scholar]
- 42.Hengst J, Strunz B, Deterding K, et al. Nonreversible MAIT cell-dysfunction in chronic hepatitis C virus infection despite successful interferon-free therapy. Eur J Immunol 2016;46(09): 2204–2210 [DOI] [PubMed] [Google Scholar]
- 43.Beudeker BJB, van Oord GW, Arends JE, et al. MAIT-cell frequency and function in blood and liver of HCV mono- and HCV/HIV co-infected patients with advanced fibrosis. Liver Int 2017. 10.1111/liv.13544. [Epub ahead of print] [DOI] [PMC free article] [PubMed]
- 44.Cosgrove C, Ussher JE, Rauch A, et al. Early and nonreversible decrease of CD161++/MAIT cells in HIV infection. Blood 2013; 121(06):951–961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Eberhard JM, Kummer S, Hartjen P, et al. Reduced CD161(+) MAIT cell frequencies in HCV and HIV/HCV co-infection: is the liver the heart of the matter? J Hepatol 2016;65(06):1261–1263 [DOI] [PubMed] [Google Scholar]
- 46.Gérart S, Sibéril S, Martin E, et al. Human iNKT and MAIT cells exhibit a PLZF-dependent proapoptotic propensity that is counterbalanced by XIAP. Blood 2013;121(04):614–623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shaler CR, Tun-Abraham ME, Skaro AI, et al. Mucosa-associated invariant T cells infiltrate hepatic metastases in patients with colorectal carcinoma but are rendered dysfunctional within and adjacent to tumor microenvironment. Cancer Immunol Immun-other 2017 [DOI] [PMC free article] [PubMed]
- 48.Angulo P Nonalcoholic fatty liver disease. N Engl J Med 2002;346 (16):1221–1231 [DOI] [PubMed] [Google Scholar]
- 49.Browning JD, Szczepaniak LS, Dobbins R, et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology 2004;40(06):1387–1395 [DOI] [PubMed] [Google Scholar]
- 50.Kanwal F, Kramer JR, Duan Z, Yu X, White D, El-Serag HB. Trends in the burden of nonalcoholic fatty liver disease in a United States Cohort of Veterans. Clin Gastroenterol Hepatol 2016;14(02):301–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Arrese M, Cabrera D, Kalergis AM, Feldstein AE. Innate immunity and inflammation in NAFLD/NASH. Dig Dis Sci 2016;61(05): 1294–1303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Serti E, Werner J, Keane M, et al. Natural killer T cells and mucosal associated invariant T cells share phenotypic and functional alterations in patients with non-alcoholic fatty liver disease. Hepatology 2016;64:584A [Google Scholar]
- 53.Jalan R, Fernandez J, Wiest R, et al. Bacterial infections in cirrhosis: a position statement based on the EASL Special Conference 2013. J Hepatol 2014;60(06):1310–1324 [DOI] [PubMed] [Google Scholar]
- 54.Hinks TS, Wallington JC, Williams AP, Djukanović R, Staples KJ, Wilkinson TM. Steroid-induced deficiency of mucosal-associated invariant T cells in the chronic obstructive pulmonary disease lung. Implications for nontypeable Haemophilus influenzae infection. Am J Respir Crit Care Med 2016;194(10): 1208–1218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Serriari NE, Eoche M, Lamotte L, et al. Innate mucosal-associated invariant T (MAIT) cells are activated in inflammatory bowel diseases. Clin Exp Immunol 2014;176(02):266–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Howson LJ, Salio M, Cerundolo V. MR1-restricted mucosal-associated invariant T cells and their activation during infectious diseases. Front Immunol 2015;6:303. [DOI] [PMC free article] [PubMed] [Google Scholar]