Abstract
Platinum-based regimens have not been proved to increase survival from advanced prostate cancer (PCa). Incontrovertible evidence that a proportion of prostate cancers have homologous recombination DNA (HRD) repair defects, and that such genomic aberrations are synthetically lethal with both poly(ADP)-ribose polymerase inhibition and platinum, has increased interest in the utilisation of these drugs against a subset of these diseases. Here in we report three patients with advanced castration-resistant PCa with HRD defects having exceptional responses to carboplatin.
Keywords: Prostate cancer, Homologous recombination defects, Platinum, DNA repair genes
1. Introduction
A distinct subgroup of patients with metastatic castration-resistant prostate cancer (mCRPC) [1] carry germline or somatic defects in DNA repair genes. Some of these alterations associate with significant and long-lasting responses to poly (ADP-ribose) polymerase (PARP) inhibitors [2]. In particular, homologous recombination defects (HRD) are also known to confer sensitivity to platinum derivatives in diverse cancers [3], [4]. Nevertheless, platinum has not been routinely used for the care of advanced prostate cancer (PCa) as the only randomised phase III trial with a platinum compound in molecularly unselected mCRPC patients failed to show overall survival benefit [5]. Here we report on three patients with metastatic PCa with strong family histories and different DNA repair defects who have had remarkable and highly durable responses to platinum-based treatment and unequivocal clinical benefit. Interestingly, no specific DNA repair defects were detected in the first patient but whole exome sequencing revealed a mutational signature with a high homologous recombination repair deficiency score. In the second and third patient, deleterious germline BRCA2 and ATM mutations, respectively, were identified.
Patient 1 was aged 52 yr when he presented in January 2014 with adenocarcinoma of the prostate with extensive nodal, lung, and bone metastatic disease and a relatively low prostate-specific antigen (PSA: 8.8 ng/ml). His family history was highly suspicious for a cancer syndrome (Fig. 1). He was treated initially with androgen deprivation therapy (ADT) and radiotherapy (RT) to the thoracic spine but progressed after 4 mo with multiple liver metastases and worsening of the lung lesions. Docetaxel, cabazitaxel (four cycles each), and enzalutamide (two cycles) administered in sequence failed to provide a response. A liver biopsy at this time confirmed the presence of prostate adenocarcinoma (Fig. 2). Having exhausted all the standard treatment options, single-agent carboplatin was initiated at the target dose of area under the curve (AUC) 6 (total dose of 1100 mg) based on ethylenediamine tetra acetic acid. The first restaging after the second cycle showed a dramatic radiological response (70% reduction of the measurable disease) with further regression until cycle 11 when treatment was discontinued due to prolonged neutropenia. After 10 mo, he presented with symptomatic liver disease and a solitary cerebellar lesion. Re-challenge with carboplatin was once again successful, with regression of the cerebellar lesion indicating carboplatin delivery to the brain (Fig. 3). CyberKnife of his solitary brain lesion was administered after two cycles of carboplatin, which was resumed 2 mo after this RT, for four additional cycles but then again stopped due to persistent neutropenia. Restaging in October 2017, 2 mo after carboplatin interruption, indicated progression of the liver metastases and stable disease elsewhere. In February 2018, after participation in a phase I clinical trial, he was re-challenged for the third time with carboplatin. Three years after commencing single-agent carboplatin, he is still very well and responding to the same agent and continuous ADT (Fig. 4).
Fig. 1.
Family tree of patient 1.
H&N cancer = head and neck cancer.
Fig. 2.
Liver biopsy of patient 1 showing poorly differentiated adenocarcinoma with minor degree of neuroendocrine differentiation. There is strong ERG and androgen receptor expression, focal positivity for prostate-specific antigen, and some neuroendocrine features: focal expression of CD56, synaptophysin, chromogranin, and neuron-specific enolase (not shown).
AR = androgen receptor; PSA = prostate-specific antigen.
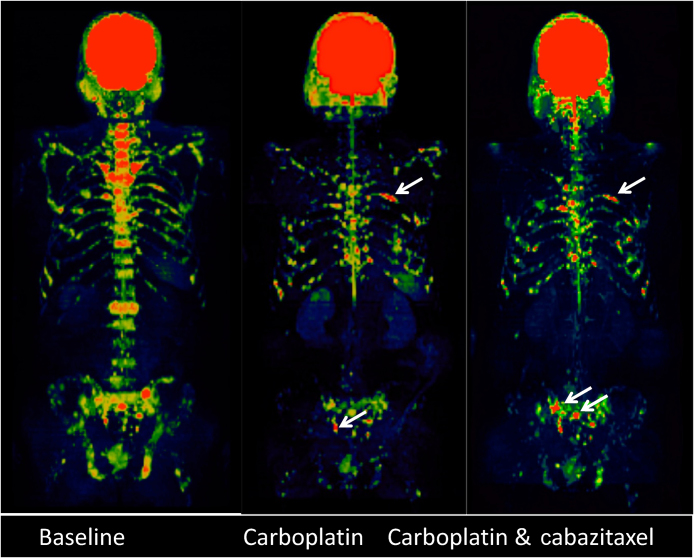
Fig. 3.
A male patient aged 54 yr with metastatic castration- resistant prostate cancer showing incremental radiological response after two cycles (middle column) and six cycles (right column) of carboplatin. (A) Coronal computed tomography (CT)-enhanced images showing incremental radiological response with almost complete resolution of the soft tissue disease associated with lytic right iliac bone metastasis (black circle). (B) Coronal CT-enhanced images showing dramatic reduction in the total liver metastatic burden and resolution of the hepatomegaly. Note the intralesional calcification associated with response. (C) Axial-enhanced CT brain images showing good radiological response to carboplatin challenge of the solitary left cerebellar metastasis after two cycles of treatment (middle column) with further improvement after CyberKnife and further carboplatin; note intralesional calcification.
Fig. 4.
Prostate-specific antigen responses on treatments in patient 1.
We analysed germline and somatic cancer DNA with next-generation sequencing (NGS) using a targeted panel of DNA repair genes at more than 5000-fold depth, as well as with the BROCA panel (which also covers intronic and flanking regions of genes documented to predispose to cancer) at the University of Washington (Dr. Colin Pritchard), and finally whole exome sequencing (WES) of his liver biopsy at two separate laboratories (Ann Arbor, Michigan and The ICR, London). None of these assays were able to identify pathogenic defects in canonical DNA repair genes (Fig. 5). Immunohistochemistry showed no mismatch repair deficiency or loss of ataxia telangiectasia mutation (ATM) expression. WES data analysis, however, using multiple bioinformatic approaches, confirmed a mutational signature and genome scars associated with HRDs (Fig. 6) [6]. Since ATM loss does not cause an HRD signature, these data indicate a different genomic alteration [7].
Fig. 5.
Circos plot of the genetic alterations in patient 1 based on the data generated from tumour-normal paired exome sequencing. Inner track indicates the type of the mutations and the genes that have the mutation. The middle and outer track indicate the B-allele frequency and logR ratio, respectively, which is derived from copy number alteration analysis.
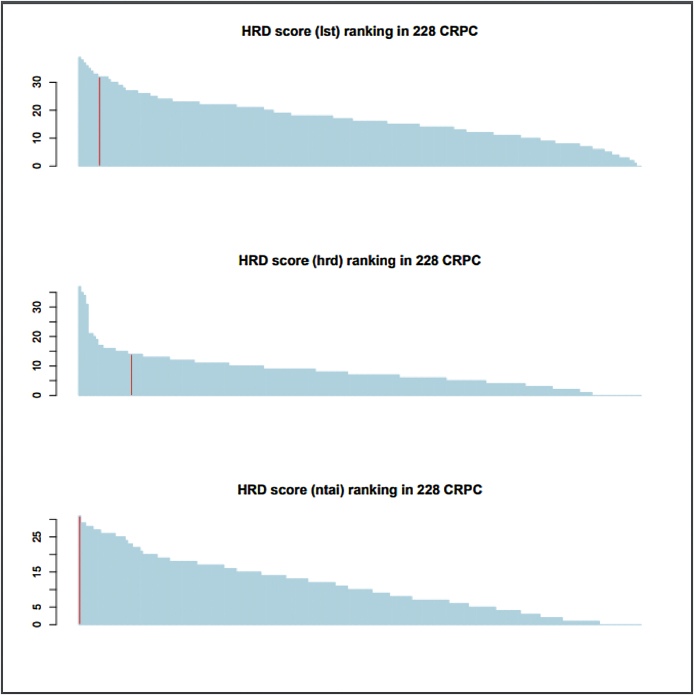
Fig. 6.
Distribution of three types of homologous recombination defect (HRD) score in a cohort of metastatic castration-resistant prostate cancer (n = 228). Red colour bar labels patient 1 HRD score.
CRPC = castration-resistant prostate cancer; HRD = homologous recombination defect.
Patient 2 was diagnosed in 2002 at the age of 37 yr with adenocarcinoma of the prostate, with a PSA at presentation of 293 ng/ml, a Gleason score of 8 in prostate biopsies, and metastatic bone disease. He was commenced on ADT and treated on a clinical trial regimen at Memorial Sloan Kettering Cancer Center with six cycles of carboplatin, paclitaxel, and estramustine, achieving a PSA nadir of 0.5 ng/ml. Following a rise in his PSA to 0.7 ng/ml 6 mo later, he received radical RT to the prostate and continued ADT until 7 yr later, when he developed castration-resistant disease. Subsequently, abiraterone was initiated for 2.5 yr with a good biochemical response and radiological stability. At this time point, he was referred to the Royal Marsden; as he had a strong family history of malignancy (Fig. 7), we performed NGS of his tumour tissue from a fresh bone biopsy. This demonstrated a BRCA2 frameshift truncating mutation (c.4876_4877delAA, p.Asn1626Serfs*12), confirmed to be of germline origin, with loss of the wild type allele. He entered a phase I trial of olaparib at 200 mg bid in combination with an ataxia telangiectasia and RAD3-related protein inhibitor but had no PSA decline; however, he did have radiological stability lasting for 4 mo. In light of the extraordinary previous response to carboplatin and paclitaxel, the patient was treated again with six cycles of carboplatin (AUC 5) and docetaxel (75 mg/m2), achieving a 44% PSA decline. On diffusion-weighted magnetic resonance imaging, the majority of his metastatic bone disease showed features consistent with disease regression (including disease at T6, T7, and T12); however, a large bone metastasis replacing his entire seventh right rib presented residual hypercellular disease activity. This seventh right rib became symptomatic with pain despite having been previously irradiated with a single fraction of 8 Gy (Fig. 8). He was, therefore, advised to pursue palliative metastatic seventh right rib resection by thoracotomy. In 2018, 15 yr after his original diagnosis, the patient remains well with on-going continuous ADT and oligorecurrent disease at T6 which has recently been treated with CyberKnife irradiation.
Fig. 7.
Family tree of patient 2.
Fig. 8.
Coronal diffusion-weighted imaging (DWI; b900 s/mm2), coronal fused T2W-DWI and axial apparent diffusion coefficient images (ADC) through the seventh rib showing a good response after six cycles of carboplatin (AUC5) and docetaxel with reduction in tumour diffusion value and increase in mean tumour ADC (>35%) of most of the metastatic bone disease but heterogeneous ADC values in the seventh rib in keeping with residual focal areas of low ADC (white arrows), suggestive of residual high tumour cellularity/active disease.
ADC = apparent diffusion coefficient; DWI = diffusion-weighted imaging.
Patient 3 was aged 66 yr in June 2015 when he was found to have a slightly elevated PSA (5.38 ng/ml) and an enlarged prostate, with biopsies confirming the diagnosis of prostate adenocarcinoma with neuroendocrine differentiation and a Gleason score of 4 + 5 (Fig. 9). A choline positron emission tomography-computed tomography scan showed positive pelvic lymph nodes and multiple bone metastases. He was treated with ADT and six cycles of docetaxel but progressed on this after 6 mo despite achieving castrate levels of testosterone. At this stage, in consideration of his strong family history (Fig. 10), he underwent genetic testing which identified a pathogenic germline mutation in ATM (heterozygous, c.2T>C [p.M1T]). Lack of ATM expression in his tumour was confirmed by immunohistochemistry. Furthermore, NGS analysis detected a TSC2 mutation (splice site donor Ex39), with loss of the other copy of TSC2, which is highly likely to be pathogenic. He was ineligible for a PARP-inhibitor trial since he developed disseminated intravascular coagulation with G3 thrombocytopenia as well as cauda equina syndrome requiring external beam radiotherapy to T12 to L3. He was subsequently commenced on single-agent carboplatin at the initial target dose of AUC 4, which was then escalated to AUC 5 after two cycles as his platelets count normalised. His PSA significantly declined from 159 ng/ml to a nadir of 36 ng/ml but then increased despite two further cycles of treatment; radiologically, there was no evidence of progression (Fig. 11). We continued treatment with carboplatin but added cabazitaxel at a dose of 20 mg/m2 with a further PSA decline from 82 ng/ml to 7.6 ng/ml. He continues on the same treatment combined with continuous ADT with responding disease as of May 2018.
Fig. 9.
Histology of patient 3 showing prostate adenocarcinoma with significant neuroendocrine differentiation. Immunohistochemistry shows that the tumour cells are very focally positive for prostate-specific antigen and negative for PSPA. Of note, tumour cells were negative for CD56 and chromogranin and positive (90%) for synaptophysin. The tumour cells lack nucleoli, have fine granular “salt-and-pepper” chromatin, and form occasional loose rosettes.
Fig. 10.
Family tree of patient 3.
Fig. 11.
Colour-coded coronal maximum intensity projection diffusion-weighted imaging showing (b900 s/mm2) incremental improvement with reduction in the tumour diffusion volume accompanied by increase in apparent diffusion coefficient values. Of note are focal areas such as in the left-sided ribs or in the bony pelvis (white arrows) showing lack of improvement throughout the studies in keeping with tumour response heterogeneity.
2. Discussion
Platinum-based chemotherapy has demonstrated impressive anti-tumour activity in some mCRPC patients; however, little published data are available on the different tumour DNA repair defects associated with platinum sensitivity. These three patients shared some atypical clinical features: a strong family history for cancer, presentation with metastatic disease, young age at diagnosis, and low PSA levels relatively to the extent of their metastatic disease. Interestingly, the first two patients were given carboplatin based solely on these clinical characteristics. The availability of NGS subsequently allowed us to elucidate the underlying genomic characteristics of these tumours.
The first patient had exhausted all of the standard treatment options and had rapidly progressive symptomatic disease before initiation of carboplatin which has given him further more than 3 yr of disease control and excellent quality of life. We were unable to identify a specific genetic defect responsible for his remarkable platinum sensitivity although WES demonstrated a mutational signature of HRD. The second patient has a germline BRCA2 mutation previously reported to confer a poorer prognosis [8]; however, impressively, he is still alive 15 yr later which compares favourably to the median survival of 6 yr on the CHAARTED trial [9] and 6.4 yr on the STAMPEDE trial [10]. These data suggest that as with ovarian cancer, platinum treatment may convert this aggressive disease subtype into a better prognosis disease. Our third patient presented with a deleterious germline ATM mutation, and his response to carboplatin arguably supports the functional impact of this genomic aberration.
In conclusion, these case series suggest the efficacy of carboplatin in metastatic prostate cancer with DNA repair defects. Further clinical trials are warranted to support its use in this subset of patients. Our report indicates that treatment selection can be facilitated by family history records as well as NGS of germline and somatic cancer DNA.
Conflicts of interest: The authors have nothing to disclose.
Funding support: We would like to acknowledge support from Prostate Cancer UK and the Movember Foundation to the London Movember Prostate Cancer Centre of Excellence at The Institute of Cancer Research and Royal Marsden (grant number CEO013-2-002), The International PCF Dream Team, Experimental Cancer Medical Centre (ECMC) grant from Cancer Research UK and the Department of Health (Ref: C12540/A25128) and Cancer Research UK (Centre Programme Grant).
EU-ACME question
Please visit www.eu-acme.org/europeanurology to answer the following EU-ACME question online (the EUACME credits will be attributed automatically).
Question:
DNA repair defects are relatively common in advanced prostate cancer and can be heritable. Which of the following is incorrect:
-
A.
Approximately 10–12% of all patients with metastatic prostate cancer have deleterious and heritable germline mutations in DNA repair genes.
-
B.
Approximately 20–30% of advance prostate cancers have somatic, deleterious, genomic aberrations in DNA repair genes.
-
C.
BRCA1 is the commonest DNA repair gene mutation found in prostate cancer.
-
D.
Consideration must be given to germline DNA testing of all patients with metastatic prostate cancer.
-
E.
Patents with ATM loss prostate cancers can respond to platinum and PARP inhibitors.
Associate Editor: Giacomo Novara
References
- 1.Robinson D., Van Allen E.M., Wu Y.M. Integrative clinical genomics of advanced prostate cancer. Cell. 2015;161:1215–1228. doi: 10.1016/j.cell.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mateo J., Carreira S., Sandhu S. DNA-repair defects and olaparib in metastatic prostate cancer. N Engl J Med. 2015;373:1697–1708. doi: 10.1056/NEJMoa1506859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pennington K.P., Walsh T., Harrell M.I. Germline and somatic mutations in homologous recombination genes predict platinum response and survival in ovarian, fallopian tube, and peritoneal carcinomas. Clin Cancer Res. 2014;20:764–775. doi: 10.1158/1078-0432.CCR-13-2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheng H.H., Pritchard C.C., Boyd T., Nelson P.S., Montgomery B. Biallelic inactivation of BRCA2 in platinum-sensitive metastatic castration-resistant prostate cancer. Eur Urol. 2016;69:992–995. doi: 10.1016/j.eururo.2015.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sternberg C.N., Petrylak D.P., Sartor O. Multinational, double-blind, phase III study of prednisone and either satraplatin or placebo in patients with castrate-refractory prostate cancer progressing after prior chemotherapy: the SPARC trial. J Clin Oncol. 2009;27:5431–5438. doi: 10.1200/JCO.2008.20.1228. [DOI] [PubMed] [Google Scholar]
- 6.Marquard A.M., Eklund A.C., Joshi T. Pan-cancer analysis of genomic scar signatures associated with homologous recombination deficiency suggests novel indications for existing cancer drugs. Biomark Res. 2015;3:9. doi: 10.1186/s40364-015-0033-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Polak P., Kim J., Braunstein L.Z. A mutational signature reveals alterations underlying deficient homologous recombination repair in breast cancer. Nat Genet. 2017;49:1476–1486. doi: 10.1038/ng.3934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Castro E., Goh C., Olmos D. Germline BRCA mutations are associated with higher risk of nodal involvement, distant metastasis, and poor survival outcomes in prostate cancer. J Clin Oncol. 2013;31:1748–1757. doi: 10.1200/JCO.2012.43.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sweeney C.J., Chen Y.H., Carducci M. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer. N Engl J Med. 2015;373:737–746. doi: 10.1056/NEJMoa1503747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.James N.D., Sydes M.R., Clarke N.W. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): Survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet. 2016;387:1163–1177. doi: 10.1016/S0140-6736(15)01037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]