Abstract
Normal tissue damages induced by radiation therapy remain dose-limiting factors in radiation oncology and this is still true despite recent advances in treatment planning and delivery of image-guided radiation therapy. Additionally, as the number of long-term cancer survivors increases, unacceptable complications emerge and dramatically reduce the patients’ quality of life. This means that patients and clinicians expect discovery of new options for the therapeutic management of radiation-induced complications. Over the past four decades, research has enhanced our understanding of the pathophysiological, cellular and molecular processes governing normal tissue toxicity. Those processes are complex and involve the cross-talk between the various cells of a tissue, including fibroblasts, endothelial, immune and epithelial cells as well as soluble paracrine factors including growth factors and proteases. We will review the translatable pharmacological approaches that have been developed to prevent, mitigate, or reverse radiation injuries based upon the targeting of cellular and signalling pathways. We will summarize the different steps of the research strategy, from the definition of initial biological hypotheses to preclinical studies and clinical translation. We will also see how novel research and therapeutic hypotheses emerge along the way as well as briefly highlight innovative approaches based upon novel radiotherapy delivery procedures.
Introduction
Cancer causes 8.2 million deaths each year globally. Today, most of the anti-cancer therapeutic improvements are achieved by combined treatment modalities, in which radiation therapy remains a cornerstone and is delivered with curative intent in 50% of cancer patients. During the past 20 years, ballistics and imaging improvements have enabled the individualized treatment of patients with a precise and conformal delivery of the dose to the tumor. These technological improvements have greatly enhanced the irradiation therapeutic index. Nevertheless, the level of dose that can be delivered, and accordingly, the possibility of achieving local control over the tumor, is still limited by the toxicity induced to normal (surrounding) tissues. In addition, the combined treatment protocols currently used against cancer are associated with an enhanced risk of toxicity. As the number of cancer survivors increase, preventing and reducing the treatment’s side effects is a priority. The present review provides a list of validated strategies as exhaustive as possible that have been validated to prevent, mitigate, or reverse radiation-induced toxicity in preclinical and clinical studies, together with several new options provided by novel types of radiation therapy.
Cancer causes 8.2 million deaths each year globally. Today, most of the anticancer therapeutic improvements are achieved by combined treatment modalities, in which radiation therapy remains a cornerstone and is delivered with curative intent in 50% of cancer patients. During the past twenty years, ballistics and imaging improvements have enabled the individualised treatment of patients with a precise and conformal delivery of the dose to the tumor. These technological improvements have greatly enhanced the irradiation therapeutic index. Nevertheless, the level of dose that can be delivered, and, accordingly, the possibility of achieving local control over the tumour, is still limited by the toxicity induced to normal (surrounding) tissues. In addition, the combined treatment protocols currently used against cancer are associated with an enhanced risk of toxicity. As the number of cancer survivors increase, preventing and reducing the treatment’s side effects is a priority. The present review provides a list of validated strategies as exhaustive as possible that have been validated to prevent, mitigate, or reverse radiation-induced toxicity in preclinical and clinical studies, together with several new options provided by novel types of radiation therapy.
Post-radiotherapeutic normal tissue injury
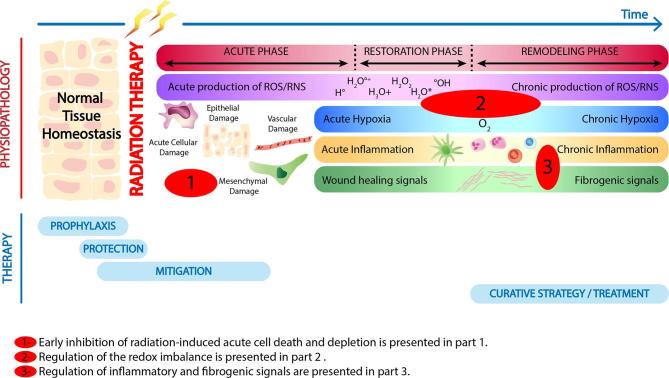
Typical side effects are systemic in the case of drug therapies, whereas radiation-induced normal tissue damages are local or locoregional and can be divided into early and late side-effects. Typically, in the clinic, early effects occur during the time-course of the treatment or within a few weeks of the completion of a fractionated radiotherapy schedule. These effects include skin erythema, dry or moist desquamation of the skin, mucositis, nausea, diarrhea, edema or headaches. Late effects are expressed after latent periods of months to years, and include radiation-induced fibrosis, atrophy, and vascular damage (Figure 1). Likewise, in pre-clinical models, radiation-induced normal tissue injury can be dichotomized into an acute inflammatory phase followed by a late chronic phase characterized by both chronic inflammation and fibrosis. The complications varies from undetectable to highly disabling levels for the patient, inducing a loss of function of the altered organ1 depending upon extrinsic factors such as variations in the dose delivered, changes in the treatment volume or dose fractionation as well as intrinsic factors such as individual radiation sensitivity and the presence of comorbidity factors.2–5
Figure 1.
Biology-driven strategy to identify therapeutic approaches. RNS, reactive nitrogen species; ROS, reactive oxygen species.
In order to reduce toxicities, it is important to understand the mechanisms underlying the radiation injury of normal tissues. While cell death is the primary and intended effect of ionizing radiation on tumor cells, the deleterious effects of irradiation on normal tissues comprise a cascade of molecular, cellular, and tissue events that spans for a long time after exposure,6 and has been compared to a “complex wound”.7 However, whereas wound repair has three distinct stages that include a clotting/coagulation phase, a restoration phase with fibroblast migration/proliferation, and a final remodeling phase where the normal tissue architecture is restored (Figure 1), this cascade of finely-tuned processes is disrupted post-radiation therapy. Tissue response to irradiation depends upon the intrinsic sensitivity of the various cellular compartments (direct cell death) that compose the organ and the complex crosstalk established in between all these compartments (indirect functional effects). For instance, rapid renewing compartments, like the epithelial layers and bone marrow, show an acute response to irradiation. This is due to a severe depletion of actively dividing upstream progenitor compartments, ultimately resulting in a loss of replenishment of downstream mature cell pools.8, 9
In contrast, in connective tissues where cellular turnover is low, radiation injury may be expressed months or even years after exposure if cell death occurs when the cellular division is attempted. The functional consequences are a result of non-lethal effects on different intra- and extracellular molecules and changes in gene expression in irradiated cells, synthesis of paracrine factors leading, e.g. to direct inactivation of anticoagulant molecules, activation of latent growth factors such as transforming growth factor, TGFβ1, and activation of proteases. The various steps involved in this cascade can constitute valuable therapeutic targets to modify normal tissue injury and enhance the outcome of radiation therapy. In the following sections of this review, we will focus our attention on selected targets, explain their pathophysiological relevance and report the therapeutic strategies that have been used over the past decades. We also discuss how the selection of the timing of administration depends upon the pathway targeted and precludes the selection of the appropriate drug.
Treatment of normal tissue injury- terminology
Before reviewing available treatment options developed to modulate radiation-induced toxicity, it is important to precisely define the possible types of intervention that depend on the time of administration relative to the time of radiation exposure and the appearance of symptoms.6 Prophylactic agents or protectors are administered before radiation exposure, mitigators are administered shortly after exposure, but before symptoms arise, while curative treatments are given after the appearance of symptoms as shown in Figure 1.
Inhibiting radiation-induced acute cell death and depletion
To prevent and inhibit radiation-induced toxicity, the early prevention of epithelial, endothelial, and stem cell deaths is one of the primary options that can be proposed. These interventions imply the administration of drugs delivered prophylactically (Table 1) including inhibitors of pro-apoptotic molecules, such as the transient blockade of p53 (Pifithrin);10, 11 the inhibition of p-53 upregulated modulator of apoptosis (Puma Inhibitors) or the inhibition of the ceramide pathway in endothelial cells.16, 17 Alternatively, stimulation of antiapoptotic molecules such as NF-ҡB by the flagellin-derivative, CBLB50218, 19 protects microvascular endothelial cells from radiation-induced death. Similarly, the enhancement of endothelial cell radiation resistance can be achieved by blocking the TSP1/CD47 pathway.20 Other cytoprotective therapies such as keratinocyte growth factor, fibroblast growth factor and glucagon-like peptide-2 treatment15, 21,22 also have proven efficacy, and prevent the development of normal tissue toxicity. For instance, keratinocyte growth factor administration has been validated and shown to stimulate cell proliferation and promote epithelial cell survival along with the differentiation of oral mucosa, both in pre-clinical and clinical trials.23, 24 Furthermore, it displayed a beneficial off-target effect by decreasing reactiveoxygen species (ROS) levels and stimulating DNA repair.23, 24
Table 1.
Therapeutic strategies that inhibit acute cell death and depletion
| Substance |
Intervention/
administration route |
Function |
Pre-clinical results/
clinical use |
References |
| Pifithrin-α pifithrin-μ | Prophylaxis/protection Oral application of drugs |
Inhibition of p53-induced apoptosis | Radioprophylaxis/protection in mice after total body irradiation | 10, 11 |
| KGF-1 (palifermin), KGF-2 (repifermin), FGF-20 (velafermin) | Prophylaxis/protection Oral application of recombinant proteins |
Suppression of apoptosis | Reduced mucositis by KGF-1 in patients after whole body irradiation, failure of KGF-2 and FGF-20 in clinical studies | 10 |
| Synthetic triterpenoids (bardoxolone methyl (BARD) and 2-cyano-3,12-dioxooleana-1,9 (11)-dien-28-oic acid-ethylamide) | Prophylaxis/protection Oral application |
Reduced apoptosis by activation of transcription factor NRF2 | Radioprophylaxis/protection in mice after total body irradiation | 10, 12 |
| PUMA inhibitors | Prophylaxis/protection Lentiviral vector |
Suppression of apoptosis by inhibition of PUMA | Radioprophylaxis/protection in vitro in germ cell tumor line NCCIT, murine hematopoietic progenitor cell line 32D cl 3, and human lymphoblast cell line TK6 | 10, 13 |
| Recombinant MDR1 | Prophylaxis/Protection Lentiviral vector |
Suppression of apoptosis | Radioprophylaxis/protection in vitro in human hematopoietic stem cells | 10, 14 |
| CBLB502/entolimod | Prophylaxis/protection Oral application |
Reduced apoptosis by activation of transcription factor NF-κB | Radioprophylaxis/protection in mice and rhesus macaques; clinical study terminated by financial sponsor | 10 |
| GLP-2 | Prophylaxis/protection | Trophic factor | RIF gut in rats | 15 |
FGF, fibroblast growth factor; GLP, glucagon-like peptide; KGF, keratinocyte growth factor; MDR, multiple drug resistance; NRF, nuclearre spiratory factor; PUMA, p-53 upregulated modulator of apoptosis; RIF, radiation-induced fibrosis.
These strategies prevent the alteration of an organ’s structure and function, and/or enable the rapid restoration of the tissue, markedly when stem cells are protected and activated. Activation of deleterious cascades will be interrupted, avoiding the excessive release of inflammatory mediators and dramatic tissue injury. However, two major drawbacks are associated with these strategies: primarily, there is a high risk of tumor protection and secondly, the acute rescue of heavily damaged cells could have a delayed detrimental impact on normal tissue structure and function, including the occurrence of a secondary cancers.
Restoring the redox equilibrium in tissue after radiotherapy
Another important strategy that has been extensively explored to counteract radiation injury involves antioxidant molecules and scavengers aiming at restoring the redox equilibrium of the tissue, immediately after irradiation or at later time points (Table 2).
Table 2.
Therapeutic strategies that prevent, mitigate, and reverse normal tissue injury by modulation of the redox equilibrium
| Substance |
Intervention/
administration route |
Mechanism | Pre-clinical results/clinical use | References |
| Amifostine | Prophylaxis/protection Oral application of prodrug, mainly activated in normal cells |
Scavenger of free radicals | Prophylaxis/protection against xerostomia during radiotherapy of head and neck cancer | 25–27 |
| Curcumin, ellagic acid, and bixin | Prophylaxis/protection, mitigation | ROS scavenger | RIF lung in rats and mice | 28 |
| Dietary flaxseed | Prophylaxis/protection, mitigation | ROS scavenger | RIF lung in rats and mice | 29, 30 |
| Glutathione (GSH) | Prophylaxis/protection Oral application of GSH esters or reduced GSH |
Scavenger of free radicals (hydroxyl) | Conflicting results in animal models regarding radioprotective effects | 10 |
| Genistein | Mitigation | ROS scavenger | RIF lung in rats | 31, 32 |
| Soy Isoflavone (83.3% genistein, 14.6% daidzein and 0.26% glycitein) | Mitigation | ROS scavenger | RIF lung in mice | 33 |
| SOD therapy | Mitigation treatment | Detoxification of superoxide | 5, 31,34,35 | |
| Pentoxyfilline + vitamin E+/clodronatePentoxyfilline +γ-tocotrienol | Treatment | Antioxidants, improves blood flow, anti-inflammatory, TNF-α and TGF-β1 inhibition |
Clinical evidence but lack of randomized trialClinical trials starting (e.g. NCT02230800) | 36–40 |
RIF, radiation-induced fibrosis; ROS, reactive oxygen species; SOD, super oxide dismutase; TGF, transforming growth factor; TNF, tumor necrosis factor.
Direct interactions of ionizing radiation with biological matter induces excitations and ionizations resulting in the ejection of electrons from biomolecules. In addition, indirect interactions occur through ionization of the water and represent the major part of the radiation’s effects on the biological matter. Both effects lead to free radical formation. In addition to this rapid burst of free radicals that occurs immediately following radiation, persistent and prolonged increase in reactive oxygen species/reactive nitrogen species (ROS/RNS) is also observed after irradiation. While ROS/RNS in physiological conditions do perform useful functions such as cell proliferation and differentiation41, 42 and are involved in homeostatis processes such as wound healing,43 when ROS production escalates beyond a certain threshold and becomes persistent, the antioxidant response is not sufficient to reset the system to the original level of redox homeostasis. These high levels of ROS/RNS result in pathological stress to tissues and cells2, 44 by acting as messenger molecules in cytoplasmic signalling pathways, and by direct effects on transcription.45 These elevated concentrations of ROS/RNS not only cause DNA damage, but also alter proteins, lipids, carbohydrates, and complex molecules.
At acute time point post-irradiation, early hypoperfusion occurs due to vascular changes, endothelial cell damages16, 46 and escalated oxygen consumption, a consequence of increased cellular metabolism. It generates tissue hypoxia, which further exacerbates the injury.47 Then, as time passes, the system may still reach an equilibrium associated with higher ROS concentrations called chronic oxidative stress,48 which does not really involve a loss of homeostasis but rather a chronic shift in the level of homeostasis. The high level of ROS/RNS are immediate activators of the fibrogenic signals including transcriptional activation of one of the most potent fibrogenic growth factor TGFβ1,49, 50 that subsequently upregulates collagen synthesis and perpetuates self-induction and autocrine induction of another potent fibrogenic growth factor, connective tissue growth factor (CTGF).51 Activation of inflammation mediators has also been described in the case of a high ROS level status, leading to deleterious chronic inflammation in the irradiated tissue. These observations support the fact that radiation-induced late effects are partially propelled by a chronic oxidative stress48 induced at late stages by the redox imbalance that occurs in the tissue as a result of intrinsic hypoxia52 which further enhance the redox imbalance.
Given the central and persistent role played by the loss of redox equilibrium in the tissue response to irradiation, targeting redox imbalance, ROS/RNS, and hypoxia is an obvious therapeutic option with applications during any of the steps of the cascade as shown in Table 2. Treatment with hyperbaric oxygen (HBO)53 and antioxidant therapy54–56 were both successfully used despite their apparent antagonistic mechanism of action. HBO induces transient tissue hyperoxia (typically ~2 h day-1) that should not overcome natural antioxidant defenses,57 but may help to remobilize tissue remodelling by activating signaling molecules in transduction cascades (see the review)58 and stimulate angiogenesis.59 In addition, the results from recent clinical trial show no benefice of HBO on lymphoedema.60, 61 Antioxidant therapies are based on a different mode of action and aim at scavenging ROS. Initial studies with amifostine25–27 and bovine liposomal Cu/Zn superoxide dismutase showed antifibrotic efficacy associated with TGFβ inhibition.62 More recent trials investigated the benefits of tocol isoforms (Vitamin E analogs) such as high-dose alpha-tocopherol combined with pentoxifylline and clodronate,36, 37 and γ-tocotrienol (GT3).39 In addition to their antioxidant action, both strategies have displayed off-target benefits with protective endothelial activity63, 64 and miRNA regulation.65 Interestingly, the efficacy of GT3 is enhanced when combined with pentoxifylline.66 Lastly, hypoxia-regulating molecules such as 2-methoxyestradiol have been shown to downregulate HIF1α-mediated Smad activation and inhibit radiation-induced lung fibrosis in mice.67
Targeting inflammatory and fibrogenic signals induced by radiotherapy
The third type of strategy is based upon the understanding of the pathophysiological processes (cellular and molecular) governing normal tissue toxicity. This knowledge has provided us with tools to improve the therapeutic ratio of radiation therapy, and biology-driven efforts have enabled the development of translatable therapeutic approaches to prevent, mitigate, and even reverse radiation injury based upon the targeting of signalling pathways. The relevance of these various signalling pathways to the pathogenesis and maintenance of radiation injury has been extensively and recently reviewed in several articles.40, 51,68,69 Therefore, we will focus on recent results that highlight the relevance of immune cells in response to irradiation. Elucidating the impact of radiotherapy on the immune compartment and subsequent immunomodulation is nowadays one of the most promising strategies for improving anticancer treatment,70 and recent studies suggest that it may also enhance the differential effect of radiotherapy.
The importance of myeloid cells in the radiation-induced response has been proposed and the role of macrophage reprogramming by radiotherapy has been demonstrated.71–73 Macrophage phenotypes are highly dependent upon the microenvironment and recent publications have revealed their complexity.74 In fibrotic tissue, macrophages do display immunosuppressive properties, secrete large amounts of the fibrogenic mediator TGF-β175 that activates the Smad pathway, and stimulate downstream fibrogenic genes such as CTGF and PAI-1.76 The macrophages isolated from bronchoalveolar fluid from patients undergoing thoracic irradiation spontaneously released platelet-derived growth factor, another important fibrogenic growth factor.77 Several older studies have suggested possible benefits of macrophage depletion using clodronate liposomes.78 The reduction of the number of macrophages by clodronate in wounded tissue indeed reduced excessive scar formation and delayed cutaneous wound healing.79 Froom and colleagues80 showed that the oral administration of clodronate (bisphosphonate) significantly reduced bone marrow fibrosis, and in the early 2000’s Delanian and Lefaix successfully administered clodronate in combination with a pentoxifylline–vitamin E treatment, and showed improved efficacy in the treatment of radiation-induced fibronecrosis.81, 82
More recent data validate and refine this strategy bringing molecular highlights and a biological rationale for macrophage targeting in the management of radiation-induced normal tissue complications.83, 84 Recently, the P Huber group showed that blocking CTGF with a specific antibody (FG-3019) was able to attenuate radiation-induced pulmonary remodeling and reverse fibrosis. Interestingly, they showed that this treatment was associated with the abrogation of M2-like macrophages influx.83 We extended these findings and recently characterized the contribution of pulmonary macrophages to radiation-induced pulmonary fibrosis.84 We showed that the populations of pulmonary macrophages are heterogeneous and their contribution to fibrosis is complex. A differential phenotype for alveolar and interstitial macrophages was indeed shown along with a specific fibrogenic contribution of interstitial macrophages but not alveolar macrophages. Ultimately, selective targeting of interstitial macrophages with CSF1R mAb was shown to display antifibrotic action.
Lastly, an overview of the drugs that have been used to prevent and mitigate radiation damages as well as the drugs that have been successfully used to reverse radiation-induced complications, fibrosis in particular, are provided in Tables 3 and 4. The impressive list of compounds shows the vitality of the research in this field with an impressive rate of translational/clinical studies (Table 4) using curative strategies. Three parameters have probably fostered this progress: first, the irreversibility of fibrosis was challenged by many cellular and experimental studies; second, curative strategies are clinically interesting as they do not interfere with anticancer treatments through possible tumor protection; third, they can be delivered to the targeted population of patients that need it.
Table 3.
Therapeutic strategies that prevent and mitigate normal tissue injury by modulation of inflammatory and fibrogenic signals
| Substance | Intervention/ administration route | Mechanisms | Pre-clinical results/clinical use | References |
| Ambroxol | Prophylaxis/protection | TNF-α and TGF-β1 inhibition | Clinical trial | 85 |
| Taurine | Prophylaxis/protection | TGF-β1 and collagen inhibition | RIF lung in mice | 86, 87 |
| IL-11 (targeted administration) | Prophylaxis/protection | TGF-β1 and collagen inhibition | RIF gut in mice | 88 |
| Hirudine | Prophylaxis/protection | Thrombine inhibition | RIF gut in rats | 89 |
| Halofunginone | Prophylaxis/protection | TGF-β1 inhibition | RIF skin in mice | 90 |
| Octreotide | Prophylaxis/protection | Somatostatin analogue | RIF gut in rats | 91 |
| Soluble TGF-β type II receptor | Prophylaxis/protection | TGF-β1 inhibition | RIF gut in mice | 92 |
| ACE inhibitors angiotensin II blockers | Prophylaxis/protection and mitigation | Angiotensin II modulator and inhibition of TGF-β | Clinical trial | 93 |
| Methylprednisolone, dexamethasone, ibuprofen) | Prophylaxis/protection and mitigation | Anti-inflammatory | RIF kidney and heart in rats and rabbits |
94–96
|
| Gefinitinib | Mitigation | EGFR inhibition-TKi | RIF lung in rats nhances inflammation but decreases fibrosis |
97 |
| LY2109761 | Mitigation | TGF-βR1 inhibition- S/TKi | RIF lung in mice Reduces inflammation and fibrosis |
98 |
| Chitosan/DsiRNA targeting TNF-α | Mitigation | TNF-α inhibition | RIF subcutaneous in mice | 99 |
ACE, angiotensin converting enzyme; EGFR, epidermal growth factor; RIF, radiation-induced fibrosis; TGF, transforming growth factor;TKi, tyrosine kinase inhibitor; TNF, tumor necrosis factor.
Table 4.
Therapeutic strategies that reverse normal tissue injury by modulation of inflammatory and fibrogenic signals
| Substance | Intervention/administration route | Mechanisms | Pre-clinical results/clinical use | References |
| All –trans-retinoic acid | Prophylaxis/Protection and treatment | TGF-β1, IL-6 and collagen inhibition | RIF lung and gut in mice | 100, 101 |
| Angelica sinensis | Prophylaxis/Protection and treatment | TGF-β1 inhibition | RIF lung in mice | 102 |
| Antibody against CTGF | Prophylaxis/protection and treatment | CTGF inhibition and macrophages depletion | RIF lung in mice | 83 |
| CSFR1 inhibition | Treatment | Macrophages depletion | RIF lung in mice | 84 |
| Interferon gamma (low dose) | Treatment | Collagen production inhibition | Small Clinical trial | 103 |
| Pirfenidone | Treatment | TGF-β1, PDGF, b-FGF, EGF, TNF-α inhibition | Clinical trial open | 104 |
| Heparine and Wwarfarine | Treatment | Anticoagulant | Small clinical trial open | 105 |
| Colchicine | Treatment | Collagen production inhibition | Clinical trial | 106 |
| Statins | Treatment | Vascular protector, anti-inflammatory, TGF-β1 inhibition |
Clinical evidence but lack of randomized trial |
93, 107
|
| Pentoxyfilline + vitamin E+/- clodronatePentoxyfilline +γ-tocotrienol |
Treatment | Antioxidants, improves blood flow, anti-inflammatory, TNF-α and TGF-β1 inhibition Macrophages depletion |
Clinical evidence but no randomized trialClinical trials starting(e.g., NCT02230800) |
36–40 |
CSF, colony stimulating factor; CTGF, connective tissue growth factor; EGF, epidermal growth factor; FGF, fibroblast growth factor; PDGF, platelet-derived growth factor; RIF, radiation-induced fibrosis; TGF, transforming growth factor; TNF, tumor necrosis factor.
Use of novel RT approaches to protect normal tissues
Aside from novel biological interventions, improvements in physics have been critical for protecting normal tissue, enhancing differential effects, and making progress on tumor control. Novel technologies based upon sophisticated instrumentation used in conjunction with imaging, such as stereotactic body radiotherapy, have helped to protect normal tissue by reducing the irradiated volume and more accurately targeting the tumor. More complex and expensive technologies, such as proton and carbon ion therapies, take advantage of their specific pattern of energy deposition in the biological matter and are already implemented in clinical practice. Other novel ideas and approaches have also been recently proposed such as dose rate escalation.108, 109 This last approach should be translatable into clinical application soon, which illustrates the broad range of opportunities that exist in radiation therapy, and highlight the need for interdisciplinary working teams composed of biologists, physicists, and physicians.
IGRT and Stereotactic body radiotherapy
Image-guided radiation therapy (IGRT) has been a major advancement in radiotherapy and makes it possible to visualize a target volume (tumor + margins) and the surrounding organs at risk before treatment and during the treatment course. Modifications in the targeted volume as well as movements are taken into consideration, and can be compensated for and even halted in the case of percussion-assisted RT (PART) recently described by Péguret et al.110 This innovative technique induces a long-lasting apnea-like suppression of respiratory motion for up to 10–11 min without inducing any physiological side effect.111 The pilot clinical study reported an interesting advantage of percussion-assisted RT compared to free-breathing or maximal-inspiration techniques coupled with three-dimensional conformal RT, SBRT or VMAT irradiations, in breast cancer, lung cancer, and lung metastases patients. IGRT combined with motion management is associated with the prescription of highly conformational doses (intensity modulatedradiotherapy, IMRT) which are meant to drastically decrease the irradiated volume and the dose delivered to the normal tissue. Different techniques may be used to follow the anatomical structures, with or without the addition of fiducial markers, starting from fluoroscopy, to CT, MRI, PET-CT, ultrasounds or optical tracking.
Studies have shown an improvement in toxicity prevention on several tumor sites112 and especially in prostate cancer patients,113 even if these results can sometimes be controversial.114 It is difficult, however, to differentiate the IGRT from the IMRT effects, and several studies have exposed this limitation115, 116 through their explanation of the reduced occurrence of xerostomia due to PTV margin reductions.
Proton therapy
Ballistic features of protons prevent normal tissue toxicities
Due to the protons’ in-depth dose deposition curves, one can expect to prevent normal tissue toxicity and therefore, decrease normal tissue injury. By now, more than 100,000 patients have been treated worldwide with proton beam therapy (PBT) in approximately 20 centers. This means that evaluating PBT superiority in terms of normal tissue protection is now feasible.117
Proton therapy has been largely used to treat ocular tumors such as uveal carcinoma, making it an alternative to enucleation or ocular brachytherapy thus sparing visual acuity.118–121 Concerning skull base tumor treatments, different Swiss and American studies have shown that the percentage of patients treated with PBT and suffering from temporal lobe injury is reduced compared to conventional X-Ray radiation therapy and IMRT.122–124
In pediatrics, the large volume of tissue exposed to low-doses induces severe and non-acceptable long-term injuries such as neurocognitive impairments, hearing loss, hormonal dysfunctions, infertility and secondary malignancies. Therefore, PBT seems to be ideal for treating pediatric patients. Dosimetry reconstruction comparing X-Rays, IMRT and PBT treatment plans show an improved dose distribution125 with PBT which, according to modeling, could be responsible for at least a 2-fold, and up to a 15-fold reduction in secondary malignancies because of normal tissue irradiation.126 Interestingly, a Chinese study estimated that IMRT is the technique that displays the highest risk of secondary malignancy (30%), while PBT was linked to only a 4% risk of developing a radio-induced cancer.127
PBT has also shown encouraging results concerning the neurocognitive toxicity of whole and partial brain irradiation in children. The reduction of the irradiated normal brain volume enables a drastic reduction of the deleterious effects in the early cognitive outcome 1 year post-RT compared to photon therapy. These results were observed for IQ, verbal comprehension, and working memory.128
Lung cancer treatments with PBT show a possibility of increasing dose and subsequent anti-tumor efficacy with reduced adverse effects—especially dermatitis, esophagitis, and pneumonitis—compared to classical X-ray treatments. For breast cancer treatments, PBT plans decrease by 71–81% and 75–99% of lung and heart irradiation respectively. Moreover, dose to the contralateral breast and dose to the whole body were also drastically reduced.129–134 All of these results suggest a decrease in radio-induced lung dysfunctions and cardiopathies, but also for the occurrence of secondary malignancies, showing that proton therapy, by decreasing dose to the normal tissue, reduces the occurrence of radiation-induced injury, and can improve the therapeutic ratio of radiation therapy.
Carbon ion therapy
Increase the relative biological effectiveness and the therapeutic ratio
The efficacy of high linear energy transfer (LET) particles in radiation therapy has been investigated since the late 70s.135 The first clinical trial was conducted in Japan in 1994,136 and since then, more than 13,000 patients have been treated with carbon ion radiation therapy.137 Carbon ions display the same pattern of dose deposition as protons, with a Bragg peak deposition of the dose, low entry and exit doses as well as a fall-off after the Bragg peak that is significantly steeper compared to protons. The dose deposited beyond the Bragg peak is higher compared to protons because of the nuclear interaction and fragmentation. One other additional interesting property of Carbon ions is their high LET, which is directly linked to their RBE that ranges between 2.0 and 3.5. The high RBE is certainly beneficial for tumor control, but can be detrimental to the normal tissue and therefore increase complications. Still, exploring the differential effects of Carbon ions is of great interest, and pioneering work performed more than 20 years ago by the Denekamp team has shown that reducing the number of fractions lowers the RBE for both normal tissue but not for the tumor.138 Hypofractionation in carbon ion radiation therapy would increase the therapeutic ratio, and provides a strong biological rationale for Carbon ion use.138–142
FLASH-RT
Opportunities to improve the efficacy of radiation therapy via the development of new irradiation techniques may have been under explored. Today, modern radiation therapy devices still use the same technology of electron acceleration in waveguides as half a century ago. However, the recent development of proton therapy facilities and the use of high LET ions exemplify some of the possibilities that are currently opened. Previous experiments conducted with short pulses of X-rays on lymphocytes,143 or more recently, conducted with protons on human–hamster hybrid cells and skin cells,144–146 including microchannel radiotherapy that operates at 200 Gy.s−1 dose-rate,147 showed fewer cytogenetic damages and significantly protected normal tissue from radiation-induced acute and long-term damages.
In line with these experiments and with the objective of fostering innovation in radiation therapy, we have been the first to propose a completely novel modality of irradiation, named FLASH radiotherapy. It markedly increases the differential effect between tumors and normal tissues, and is able to destroy tumors with the same efficiency while providing better protection to the normal tissues and preventing side effects. Indeed, using several pre-clinical models,108, 109 we have shown that an ultrahigh dose rate delivery of irradiation was able to protect normal tissues in mice (lung, brain, gut and skin), in pigs (skin), and in a clinical trial performed in cats bearing spontaneous cancers for whom a major protection of normal tissues was observed, while maintaining a strong anti-tumor efficacy108 (Vozenin et al, under revision; Montay-Gruel et al. in prep This effect has been called the Flash effect. The Flash effect has been confirmed by another team from Stanford University (USA)148 and we found similar observations reported more than 40 years ago.149, 150 In addition to this radiobiological advantage, the ultrashort duration of dose deposition overcomes the potential problems associated with tumor motion, and can then enhance RT delivery accuracy. Currently, only a few devices are able to deliver an ultrahigh dose rate irradiation across a large volume of tissue; experimental electron Linacs of 4/6 MeV have limited therapeutic applications due to their energy profile and limited in-depth dose deposition (Kinetron, Orsay, Fr, PMB-Alcen, Pegnier, France; Oriatron, Lausanne, CH, PMB-Alcen, Pegnier, France; modified clinical accelerator, Stanford, USA ).109, 151 However, several technological improvements are ongoing to upgrade those systems and develop clinical transfer.152, 153
Conclusion
The selective protection of normal tissue function using modern targeted radiotherapy is a powerful approach to improve cure rates and simultaneously enhance the quality of life of long-term cancer survivors. Nowadays, high precision radiation therapy induces a drop in the rate of complications. In parallel, advancements in radiobiology have deciphered the complexity of the biological response induced by tissue exposure to ionizing radiation, and enabled the identification of therapeutic targets. These processes include profound microenvironment remodeling with alteration of the vascularization, perfusion/hypoxia, inflammation, modulation of immune compartments and stromal remodeling. Therefore, currently, well-selected combination strategies that target distinct pathogenic pathways induced by irradiation at specific time points of the pathogenic process can be proposed, and the next challenge will be to develop rational radiotherapy–drug combinations to maximize the therapeutic impact. The management of RT complication has also reach the era of personalized medicine and in many centers in Europe (France, UK, CH for instance), patients presenting with complications are managed in the frame of multidisciplinary consultations to adapt the best therapeutic answer for each specific situation. Simultaneously, novel radiotherapy approaches such as the ultra-high dose rate, Flash RT, have been developed, offering the potential to radically change the way radiation therapy is employed and delivered over the next few years.
Contributor Information
Pierre Montay-Gruel, Email: pierre.montay-Gruel@unil.ch.
Lydia Meziani, Email: lydia.meziani@gustaveroussy.fr.
Chakradhar Yakkala, Email: chakradhar.yakkala@chuv.ch.
Marie-Catherine Vozenin, Email: marie-catherine.vozenin@chuv.ch.
REFERENCES
- 1. Bentzen SM. Preventing or reducing late side effects of radiation therapy: radiobiology meets molecular pathology. Nat Rev Cancer 2006; 6: 702–13. doi: 10.1038/nrc1950 [DOI] [PubMed] [Google Scholar]
- 2. Delanian S, Lefaix J-L. The radiation-induced fibroatrophic process: therapeutic perspective via the antioxidant pathway. Radiother Oncol 2004; 73: 119–31. doi: 10.1016/j.radonc.2004.08.021 [DOI] [PubMed] [Google Scholar]
- 3. Alsner J, Andreassen CN, Overgaard J. Genetic markers for prediction of normal tissue toxicity after radiotherapy. Semin Radiat Oncol 2008; 18: 126–35. doi: 10.1016/j.semradonc.2007.10.004 [DOI] [PubMed] [Google Scholar]
- 4. Begg AC, Stewart FA, Vens C. Strategies to improve radiotherapy with targeted drugs. Nat Rev Cancer 2011; 11: 239–53. doi: 10.1038/nrc3007 [DOI] [PubMed] [Google Scholar]
- 5. Bourgier C, Levy A, Vozenin M-C, Deutsch E. Pharmacological strategies to spare normal tissues from radiation damage: useless or overlooked therapeutics? Cancer Metastasis Rev 2012; 31: 699–712. doi: 10.1007/s10555-012-9381-9 [DOI] [PubMed] [Google Scholar]
- 6. Denham JW, Hauer-Jensen M, Peters LJ. Is it time for a new formalism to categorize normal tissue radiation injury? Int J Radiat Oncol Biol Phys 2001; 50: 1105–6. doi: 10.1016/S0360-3016(01)01556-5 [DOI] [PubMed] [Google Scholar]
- 7. Denham JW, Hauer-Jensen M. The radiotherapeutic injury – a complex ‘wound’. Radiother Oncol 2002; 63: 129–45. doi: 10.1016/S0167-8140(02)00060-9 [DOI] [PubMed] [Google Scholar]
- 8. Hall EJ. Radiobiology for the radiobiologist. Fifth Edition Philadelphia: The British Institute of Radiology.; 2001. [Google Scholar]
- 9. Joiner MCE, Van der Kogel AE. Basic clinical radiobiology. 4th edition London; 2009. [Google Scholar]
- 10. Maier P, Wenz F, Herskind C. Radioprotection of normal tissue cells. Strahlenther Onkol 2014; 190: 745–52. doi: 10.1007/s00066-014-0637-x [DOI] [PubMed] [Google Scholar]
- 11. Sinn B, Schulze J, Schroeder G, Konschak R, Freyer D, Budach V, et al. Pifithrin-α as a potential cytoprotective agent in radiotherapy: protection of normal tissue without decreasing therapeutic efficacy in glioma cells. Radiat Res 2010; 174: 601–10. doi: 10.1667/RR2147.1 [DOI] [PubMed] [Google Scholar]
- 12. Kim SB, Pandita RK, Eskiocak U, Ly P, Kaisani A, Kumar R, et al. Targeting of Nrf2 induces DNA damage signaling and protects colonic epithelial cells from ionizing radiation. Proc Natl Acad Sci U S A 2012; 109: E2949–E2955. doi: 10.1073/pnas.1207718109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Maier P, Herskind C, Barzan D, Zeller WJ, Wenz F. SNAI2 as a novel radioprotector of normal tissue by gene transfer using a lentiviral bicistronic SIN vector. Radiat Res 2010; 173: 612–9. doi: 10.1667/RR1952.1 [DOI] [PubMed] [Google Scholar]
- 14. Maier P, Herskind C, Fleckenstein K, Spier I, Laufs S, Zeller WJ, et al. MDR1 gene transfer using a lentiviral SIN vector confers radioprotection to human CD34 +hematopoietic progenitor cells. Radiat Res 2008; 169: 301–10. doi: 10.1667/RR1067.1 [DOI] [PubMed] [Google Scholar]
- 15. Torres S, Thim L, Milliat F, Vozenin-Brotons M-C, Olsen UB, Ahnfelt-Rønne I, et al. Glucagon-like peptide-2 improves both acute and late experimental radiation enteritis in the rat. Int J Radiat Oncol Biol Phys 2007; 69: 1563–71. doi: 10.1016/j.ijrobp.2007.08.051 [DOI] [PubMed] [Google Scholar]
- 16. Pena LA, Fuks Z, Kolesnick RN. Radiation-induced apoptosis of endothelial cells in the murine central nervous system: protection by fibroblast growth factor and sphingomyelinase deficiency. Cancer Res 2000; 60: 321–7. [PubMed] [Google Scholar]
- 17. Rotolo J, Stancevic B, Zhang J, Hua G, Fuller J, Yin X, et al. Anti-ceramide antibody prevents the radiation gastrointestinal syndrome in mice. J Clin Invest 2012; 122: 1786–90. doi: 10.1172/JCI59920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Burdelya LG, Krivokrysenko VI, Tallant TC, Strom E, Gleiberman AS, Gupta D, et al. An agonist of toll-like receptor 5 has radioprotective activity in mouse and primate models. Science 2008; 320: 226–30. doi: 10.1126/science.1154986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Burdelya LG, Gleiberman AS, Toshkov I, Aygun-Sunar S, Bapardekar M, Manderscheid-Kern P, et al. Toll-like receptor 5 agonist protects mice from dermatitis and oral mucositis caused by local radiation: implications for head-and-neck cancer radiotherapy. Int J Radiat Oncol Biol Phys 2012; 83: 228–34. doi: 10.1016/j.ijrobp.2011.05.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Maxhimer JB, Soto-Pantoja DR, Ridnour LA, Shih HB, Degraff WG, Tsokos M, et al. Radioprotection in normal tissue and delayed tumor growth by blockade of CD47 signaling. Sci Transl Med 2009; 1: ra7. doi: 10.1126/scitranslmed.3000139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Booth C, Booth D, Williamson S, Demchyshyn LL, Potten CS, Teduglutide PCS. Teduglutide ([Gly2]GLP-2) protects small intestinal stem cells from radiation damage. Cell Prolif 2004; 37: 385–400. doi: 10.1111/j.1365-2184.2004.00320.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Winczura P, Jassem J. Combined treatment with cytoprotective agents and radiotherapy. Cancer Treat Rev 2010; 36: 268–75. doi: 10.1016/j.ctrv.2009.12.001 [DOI] [PubMed] [Google Scholar]
- 23. Dörr W, Heider K, Spekl K. Reduction of oral mucositis by palifermin (rHuKGF): dose-effect of rHuKGF. Int J Radiat Biol 2005; 81: 557–65. doi: 10.1080/09553000500196136 [DOI] [PubMed] [Google Scholar]
- 24. Dörr W, Spekl K, Farrell CL. Amelioration of acute oral mucositis by keratinocyte growth factor: fractionated irradiation. Int J Radiat Oncol Biol Phys 2002; 54: 245–51. doi: 10.1016/S0360-3016(02)02918-8 [DOI] [PubMed] [Google Scholar]
- 25. Vujaskovic Z, Anscher MS, Feng Q-F, Rabbani ZN, Amin K, Samulski TS, et al. Radiation-induced hypoxia may perpetuate late normal tissue injury. Int J Radiat Oncol Biol Phys 2001; 50: 851–5. doi: 10.1016/S0360-3016(01)01593-0 [DOI] [PubMed] [Google Scholar]
- 26. Fleischer G, Dörr W. Amelioration of early radiation effects in oral mucosa (mouse) by intravenous or subcutaneous administration of amifostine. Strahlenther Onkol 2006; 182: 567–75. doi: 10.1007/s00066-006-1587-8 [DOI] [PubMed] [Google Scholar]
- 27. Koukourakis MI, Tsoutsou PG, Abatzoglou IM, Sismanidou K, Giatromanolaki A, Sivridis E. Hypofractionated and accelerated radiotherapy with subcutaneous amifostine cytoprotection as short adjuvant regimen after breast-conserving surgery: interim report. Int J Radiat Oncol Biol Phys 2009; 74: 1173–80. doi: 10.1016/j.ijrobp.2008.09.016 [DOI] [PubMed] [Google Scholar]
- 28. Lee JC, Kinniry PA, Arguiri E, Serota M, Kanterakis S, Chatterjee S, et al. Dietary curcumin increases antioxidant defenses in lung, ameliorates radiation-induced pulmonary fibrosis, and improves survival in mice. Radiat Res 2010; 173: 590–601. doi: 10.1667/RR1522.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Christofidou-Solomidou M, Tyagi S, Tan K-S, Hagan S, Pietrofesa R, Dukes F, et al. Dietary flaxseed administered post thoracic radiation treatment improves survival and mitigates radiation-induced pneumonopathy in mice. BMC Cancer 2011; 11: 269. doi: 10.1186/1471-2407-11-269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lee JC, Krochak R, Blouin A, Kanterakis S, Chatterjee S, Arguiri E, et al. Dietary flaxseed prevents radiation-induced oxidative lung damage, inflammation and fibrosis in a mouse model of thoracic radiation injury. Cancer Biol Ther 2009; 8: 47–53. doi: 10.4161/cbt.8.1.7092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mahmood J, Jelveh S, Calveley V, Zaidi A, Doctrow SR, Hill RP. Mitigation of lung injury after accidental exposure to radiation. Radiat Res 2011; 176: 770–80. doi: 10.1667/RR2562.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mahmood J, Jelveh S, Calveley V, Zaidi A, Doctrow SR, Hill RP. Mitigation of radiation-induced lung injury by genistein and EUK-207. Int J Radiat Biol 2011; 87: 889–901. doi: 10.3109/09553002.2011.583315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hillman GG, Singh-Gupta V, Runyan L, Yunker CK, Rakowski JT, Sarkar FH, et al. Soy isoflavones radiosensitize lung cancer while mitigating normal tissue injury. Radiother Oncol 2011; 101: 329–36. doi: 10.1016/j.radonc.2011.10.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Delanian S, Baillet F, Huart J, Lefaix J-L, Maulard C, Housset M. Successful treatment of radiation-induced fibrosis using liposomal Cu/Zn superoxide dismutase: clinical trial. Radiother Oncol 1994; 32: 12–20. doi: 10.1016/0167-8140(94)90444-8 [DOI] [PubMed] [Google Scholar]
- 35. Epperly M, Bray J, Kraeger S, Zwacka R, Engelhardt J, Travis E, et al. Prevention of late effects of irradiation lung damage by manganese superoxide dismutase gene therapy. Gene Ther 1998; 5: 196–208. doi: 10.1038/sj.gt.3300580 [DOI] [PubMed] [Google Scholar]
- 36. Delanian S, Balla-Mekias S, Lefaix J-L. Striking regression of chronic radiotherapy damage in a clinical trial of combined pentoxifylline and tocopherol. J Clin Oncol 1999; 17: 3283–90. doi: 10.1200/JCO.1999.17.10.3283 [DOI] [PubMed] [Google Scholar]
- 37. Boerma M, Roberto KA, Hauer-Jensen M. Prevention and treatment of functional and structural radiation injury in the rat heart by pentoxifylline and alpha-tocopherol. Int J Radiat Oncol Biol Phys 2008; 72: 170–7. doi: 10.1016/j.ijrobp.2008.04.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Delanian S, Lefaix J-L. Current management for late normal tissue injury: radiation-induced fibrosis and necrosis. Semin Radiat Oncol 2007; 17: 99–107. doi: 10.1016/j.semradonc.2006.11.006 [DOI] [PubMed] [Google Scholar]
- 39. Berbée M, Fu Q, Boerma M, Wang J, Kumar KS, Hauer-Jensen M. γ-Tocotrienol ameliorates intestinal radiation injury and reduces vascular oxidative stress after total-body irradiation by an HMG-CoA reductase-dependent mechanism. Radiat Res 2009; 171: 596–605. doi: 10.1667/RR1632.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Berbee M, Hauer-Jensen M. Novel drugs to ameliorate gastrointestinal normal tissue radiation toxicity in clinical practice: what is emerging from the laboratory? Curr Opin Support Palliat Care 2012; 6: 54–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Murrell GAC, Francis MJO, Bromley L. Modulation of fibroblast proliferation by oxygen free radicals. Biochem J 1990; 265: 659–65. doi: 10.1042/bj2650659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Allen RG. Oxygen-reactive species and antioxidant responses during development: the metabolic paradox of cellular differentiation. Proc Soc Exp Biol Med 1991; 196: 117–29. doi: 10.3181/00379727-196-43171A [DOI] [PubMed] [Google Scholar]
- 43. Gordillo GM, Sen CK. Revisiting the essential role of oxygen in wound healing. Am J Surg 2003; 186: 259–63. doi: 10.1016/S0002-9610(03)00211-3 [DOI] [PubMed] [Google Scholar]
- 44. Andersen JK. Oxidative stress in neurodegeneration: cause or consequence? Nat Med 2004; 10: S18–S25. doi: 10.1038/nrn1434 [DOI] [PubMed] [Google Scholar]
- 45. Bedard K, Krause K-H. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev 2007; 87: 245–313. doi: 10.1152/physrev.00044.2005 [DOI] [PubMed] [Google Scholar]
- 46. Paris F, Fuks Z, Kang A, Capodieci P, Juan G, Ehleiter D, et al. Endothelial apoptosis as the primary lesion initiating intestinal radiation damage in mice. Science 2001; 293: 293–7. doi: 10.1126/science.1060191 [DOI] [PubMed] [Google Scholar]
- 47. Fleckenstein K, Zgonjanin L, Chen L, Rabbani Z, Jackson IL, Thrasher B, et al. Temporal onset of hypoxia and oxidative stress after pulmonary irradiation. Int J Radiat Oncol Biol Phys 2007; 68: 196–204. doi: 10.1016/j.ijrobp.2006.12.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zhao W, Diz DI, Robbins ME. Oxidative damage pathways in relation to normal tissue injury. Br J Radiol 2007; 80: S23–S31. doi: 10.1259/bjr/18237646 [DOI] [PubMed] [Google Scholar]
- 49. Barcellos-Hoff MH, Dix TA. Redox-mediated activation of latent transforming growth factor-beta 1. Mol Endocrinol 1996; 10: 1077–83. [DOI] [PubMed] [Google Scholar]
- 50. Jobling MF, Mott JD, Finnegan MT, Jurukovski V, Erickson AC, Walian PJ, et al. Isoform-specific activation of latent transforming growth factor β (LTGF-β) by reactive oxygen species. Radiat Res 2006; 166: 839–48. doi: 10.1667/RR0695.1 [DOI] [PubMed] [Google Scholar]
- 51. Yarnold J, Vozenin Brotons M-C, Brotons MC. Pathogenetic mechanisms in radiation fibrosis. Radiother Oncol 2010; 97: 149–61. doi: 10.1016/j.radonc.2010.09.002 [DOI] [PubMed] [Google Scholar]
- 52. Vujaskovic Z, Feng Q-F, Rabbani ZN, Anscher MS, Samulski TV, Brizel DM. Radioprotection of lungs by amifostine is associated with reduction in profibrogenic cytokine activity. Radiat Res 2002; 157: 656–60. doi: 10.1667/0033-7587(2002)157[0656:ROLBAI]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- 53. Pasquier D, Hoelscher T, Schmutz J, Dische S, Mathieu D, Baumann M, et al. Hyperbaric oxygen therapy in the treatment of radio-induced lesions in normal tissues: a literature review. Radiother Oncol 2004; 72: 1–13. doi: 10.1016/j.radonc.2004.04.005 [DOI] [PubMed] [Google Scholar]
- 54. Benyahia B, Campana F, Perdereau B, Gez E, Fourquet A, Magdelenat H. Effects of SOD topical treatment on human skin radiofibrosis: a pathological study. The Breast 1996; 5: 75–81. doi: 10.1016/S0960-9776(96)90125-3 [DOI] [Google Scholar]
- 55. Lefaix J-L, Delanian S, Leplat J-J, Tricaud Y, Martin M, Nimrod A, et al. Successful treatment of radiation-induced fibrosis using Cu/Zn-SOD and Mn-SOD: an experimental study. Int J Radiat Oncol Biol Phys 1996; 35: 305–12. doi: 10.1016/0360-3016(96)00061-2 [DOI] [PubMed] [Google Scholar]
- 56. Liu R, Oberley TD, Oberley LW. Transfection and expression of MnSOD cDNA decreases tumor malignancy of human oral squamous carcinoma SCC-25 cells. Hum Gene Ther 1997; 8: 585–95. doi: 10.1089/hum.1997.8.5-585 [DOI] [PubMed] [Google Scholar]
- 57. Dennog C, Radermacher P, Barnett YA, Speit G. Antioxidant status in humans after exposure to hyperbaric oxygen. Mutat Res 1999; 428: 83–9. doi: 10.1016/S1383-5742(99)00034-4 [DOI] [PubMed] [Google Scholar]
- 58. Valko M, Leibfritz D, Moncol J, Cronin MTD, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol 2007; 39: 44–84. doi: 10.1016/j.biocel.2006.07.001 [DOI] [PubMed] [Google Scholar]
- 59. O'Sullivan B, Levin W. Late radiation-related fibrosis: pathogenesis, manifestations, and current management. Semin Radiat Oncol 2003; 13: 274–89. doi: 10.1016/S1053-4296(03)00037-7 [DOI] [PubMed] [Google Scholar]
- 60. Gerlach NL, Barkhuysen R, Kaanders JHAM, Janssens GORJ, Sterk W, Merkx MAW. The effect of hyperbaric oxygen therapy on quality of life in oral and oropharyngeal cancer patients treated with radiotherapy. Int J Oral Maxillofac Surg 2008; 37: 255–9. doi: 10.1016/j.ijom.2007.11.013 [DOI] [PubMed] [Google Scholar]
- 61. Gothard L, Haviland J, Bryson P, Laden G, Glover M, Harrison S, et al. Randomised phase II trial of hyperbaric oxygen therapy in patients with chronic arm lymphoedema after radiotherapy for cancer. Radiother Oncol 2010; 97: 101–7. doi: 10.1016/j.radonc.2010.04.026 [DOI] [PubMed] [Google Scholar]
- 62. Vozenin-Brotons M-C, Sivan V, Gault N, Renard C, Geffrotin C, Delanian S, et al. Antifibrotic action of Cu/Zn SOD is mediated by TGF-β1 repression and phenotypic reversion of myofibroblasts. Free Radic Biol Med 2001; 30: 30–42. doi: 10.1016/S0891-5849(00)00431-7 [DOI] [PubMed] [Google Scholar]
- 63. Berbee M, Fu Q, Boerma M, Pathak R, Zhou D, Kumar KS, et al. Reduction of radiation-induced vascular nitrosative stress by the vitamin E analog gamma-tocotrienol: evidence of a role for Tetrahydrobiopterin. Int J Radiat Oncol Biol Phys 2011; 79: 884–91. doi: 10.1016/j.ijrobp.2010.08.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Berbée M, Fu Q, Boerma M, Sree Kumar K, Loose DS, Hauer-Jensen M. Mechanisms underlying the radioprotective properties of γ-tocotrienol: comparative gene expression profiling in tocol-treated endothelial cells. Genes Nutr 2012; 7: 75–81. doi: 10.1007/s12263-011-0228-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Hamama S, Noman MZ, Gervaz P, Delanian S, Vozenin M-C. MiR-210: a potential therapeutic target against radiation-induced enteropathy. Radiother Oncol 2014; 111: 219–21. doi: 10.1016/j.radonc.2013.10.030 [DOI] [PubMed] [Google Scholar]
- 66. Berbée M, Fu Q, Garg S, Kulkarni S, Kumar KS, Hauer-Jensen M. Pentoxifylline enhances the radioprotective properties of γ-tocotrienol: differential effects on the hematopoietic, gastrointestinal and vascular systems. Radiat Res 2011; 175: 297–306. doi: 10.1667/RR2399.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Choi S-H, Hong Z-Y, Nam J-K, Lee H-J, Jang J, Yoo RJ, et al. A hypoxia-induced vascular endothelial-to-mesenchymal transition in development of radiation-induced pulmonary fibrosis. Clin Cancer Res 2015; 21: 3716–26. doi: 10.1158/1078-0432.CCR-14-3193 [DOI] [PubMed] [Google Scholar]
- 68. Hauer-Jensen M, Denham JW, Andreyev HJN. Radiation enteropathy—pathogenesis, treatment and prevention. Nat Rev Gastroenterol Hepatol 2014; 11: 470–9. doi: 10.1038/nrgastro.2014.46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Montay-Gruel P, Boivin G, Vozenin MC. Novel strategies to prevent, mitigate or reverse radiation injury and fibrosis : Anscher MS V, Strategies to enhance the therapeutic ratio of radiation as a cancer treatment. Berlin: The British Institute of Radiology.; 2016. [Google Scholar]
- 70. Herrera FG, Bourhis J, Coukos G. Radiotherapy combination opportunities leveraging immunity for the next oncology practice. CA Cancer J Clin 2017; 67: 65–85. doi: 10.3322/caac.21358 [DOI] [PubMed] [Google Scholar]
- 71. De Palma M, Coukos G, Hanahan D. A new twist on radiation oncology: low-dose irradiation elicits immunostimulatory macrophages that unlock barriers to tumor immunotherapy. Cancer Cell 2013; 24: 559–61. doi: 10.1016/j.ccr.2013.10.019 [DOI] [PubMed] [Google Scholar]
- 72. Klug F, Prakash H, Huber PE, Seibel T, Bender N, Halama N, et al. Low-dose irradiation programs macrophage differentiation to an iNOS+/M1 phenotype that orchestrates effective T cell immunotherapy. Cancer Cell 2013; 24: 589–602. doi: 10.1016/j.ccr.2013.09.014 [DOI] [PubMed] [Google Scholar]
- 73. Groves AM, Johnston CJ, Misra RS, Williams JP, Finkelstein JN. Whole-lung irradiation results in pulmonary macrophage alterations that are subpopulation and strain specific. Radiat Res 2015; 184: 639–49. doi: 10.1667/RR14178.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Chevrier S, Levine JH, Zanotelli VRT, Silina K, Schulz D, Bacac M, et al. An immune atlas of clear cell renal cell carcinoma. Cell 2017; 169: 736–49. doi: 10.1016/j.cell.2017.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Roberts NJ, Prill AH, Mann TN. Interleukin 1 and interleukin 1 inhibitor production by human macrophages exposed to influenza virus or respiratory syncytial virus. Respiratory syncytial virus is a potent inducer of inhibitor activity. J Exp Med 1986; 163: 511–9. doi: 10.1084/jem.163.3.511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Ikushima H, Miyazono K. TGFβ signalling: a complex web in cancer progression. Nat Rev Cancer 2010; 10: 415–24. doi: 10.1038/nrc2853 [DOI] [PubMed] [Google Scholar]
- 77. Abdollahi A, Li M, Ping G, Plathow C, Domhan S, Kiessling F, et al. Inhibition of platelet-derived growth factor signaling attenuates pulmonary fibrosis. J Exp Med 2005; 201: 925–35. doi: 10.1084/jem.20041393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Thepen T, Van Rooijen N, Kraal G. Alveolar macrophage elimination in vivo is associated with an increase in pulmonary immune response in mice. J Exp Med 1989; 170: 499–509. doi: 10.1084/jem.170.2.499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. SW L, Zhang XM, Luo HM, YC F, MY X, Tang SJ. Clodronate liposomes reduce excessive scar formation in a mouse model of burn injury by reducing collagen deposition and TGF-beta1 expression. Mol Biol Rep 2014; 41: 2143–9. [DOI] [PubMed] [Google Scholar]
- 80. Froom P, Braester A, Aghai E, Quitt M, Elmalah I. Clodronate in myelofibrosis: a case report. Am J Med Sci 2002; 323: 115–6. doi: 10.1097/00000441-200202000-00012 [DOI] [PubMed] [Google Scholar]
- 81. Delanian S, Lefaix J-L. Complete healing of severe osteoradionecrosis with treatment combining pentoxifylline, tocopherol and clodronate. Br J Radiol 2002; 75: 467–9. doi: 10.1259/bjr.75.893.750467 [DOI] [PubMed] [Google Scholar]
- 82. Delanian S, Lefaix J-L, Maisonobe T, Salachas F, Pradat P-F. Significant clinical improvement in radiation-induced lumbosacral polyradiculopathy by a treatment combining pentoxifylline, tocopherol, and clodronate (Pentoclo). J Neurol Sci 2008; 275: 164–6. doi: 10.1016/j.jns.2008.08.004 [DOI] [PubMed] [Google Scholar]
- 83. Bickelhaupt S, Erbel C, Timke C, Wirkner U, Dadrich M, Flechsig P, et al. Effects of CTGF blockade on attenuation and reversal of radiation-Induced pulmonary fibrosis. J Natl Cancer Inst 2017; 109. doi: 10.1093/jnci/djw339 [DOI] [PubMed] [Google Scholar]
- 84. Meziani L, Mondini M, Petit B, Boissonnas A, Thomas de Montpreville V, Mercier O, et al. CSF1R inhibition prevents radiation pulmonary fibrosis by depletion of interstitial macrophages. Eur Respir J 2018; 51: 1702120. doi: 10.1183/13993003.02120-2017 [DOI] [PubMed] [Google Scholar]
- 85. Xia D-H, Xi L, Xv C, Mao W-D, Shen W-S, Shu Z-Q, et al. The protective effects of ambroxol on radiation lung injury and influence on production of transforming growth factor β1 and tumor necrosis factor α. Med Oncol 2010; 27: 697–701. doi: 10.1007/s12032-009-9271-3 [DOI] [PubMed] [Google Scholar]
- 86. Robb WB, Condron C, Moriarty M, Walsh TN, Bouchier-Hayes DJ. Taurine attenuates radiation-induced lung fibrosis in C57/Bl6 fibrosis prone mice. Ir J Med Sci 2010; 179: 99–105. doi: 10.1007/s11845-009-0389-2 [DOI] [PubMed] [Google Scholar]
- 87. Song L, Wang D, Cui X, Hu W. The protective action of taurine and L-arginine in radiation pulmonary fibrosis. J Environ Pathol Toxicol Oncol 1998; 17: 151–7. [PubMed] [Google Scholar]
- 88. Boerma M, Wang J, Burnett AF, Santin AD, Roman JJ, Hauer-Jensen M. Local administration of interleukin-11 ameliorates intestinal radiation injury in rats. Cancer Res 2007; 67: 9501–6. doi: 10.1158/0008-5472.CAN-07-0810 [DOI] [PubMed] [Google Scholar]
- 89. Xavier S, Pike E, Fujii M, Javelaud D, Mauviel A, Flanders KC, et al. Amelioration of radiation-induced fibrosis: inhibtion of transforming growth factor-b signaling by halofuginone. J Biol Chem 2004;. [DOI] [PubMed] [Google Scholar]
- 90. Wang J, Zheng H, Ou X, Albertson CM, Fink LM, Herbert J-M, et al. Hirudin ameliorates intestinal radiation toxicity in the rat: support for thrombin inhibition as strategy to minimize side-effects after radiation therapy and as countermeasure against radiation exposure. J Thromb Haemost 2004; 2: 2027–35. doi: 10.1111/j.1538-7836.2004.00960.x [DOI] [PubMed] [Google Scholar]
- 91. Wang J, Zheng H, Hauer-Jensen M. Influence of short-term octreotide administration on chronic tissue injury, transforming growth factor beta (TGF-beta) overexpression, and collagen accumulation in irradiated rat intestine. J Pharmacol Exp Ther 2001; 297: 35–42. [PubMed] [Google Scholar]
- 92. Zheng H, Wang J, Koteliansky VE, Gotwals PJ, Hauer–Jensen M. Recombinant soluble transforming growth factor β type II receptor ameliorates radiation enteropathy in mice. Gastroenterology 2000; 119: 1286–96. doi: 10.1053/gast.2000.19282 [DOI] [PubMed] [Google Scholar]
- 93. Wedlake LJ, Silia F, Benton B, Lalji A, Thomas K, Dearnaley DP, et al. Evaluating the efficacy of statins and ACE-inhibitors in reducing gastrointestinal toxicity in patients receiving radiotherapy for pelvic malignancies. Eur J Cancer 2012; 48: 2117–24. doi: 10.1016/j.ejca.2011.12.034 [DOI] [PubMed] [Google Scholar]
- 94. Geraci JP, Mariano MS, Jackson KL. Amelioration of radiation nephropathy in rats by dexamethasone treatment after irradiation. Radiat Res 1993; 134: 86–93. doi: 10.2307/3578505 [DOI] [PubMed] [Google Scholar]
- 95. Reeves WC, Stryker JA, Abt AB, Chung CK, Whitesell L, Zelis R. Early corticosteroid administration in experimental radiation-induced heart disease. Radiology 1980; 134: 533–5. doi: 10.1148/radiology.134.2.7352245 [DOI] [PubMed] [Google Scholar]
- 96. Reeves WC, Cunningham D, Schwiter EJ, Abt A, Skarlatos S, Wood MA, et al. Myocardial hydroxyproline reduced by early administration of methylprednisolone or ibuprofen to rabbits with radiation-induced heart disease. Circulation 1982; 65: 924–7. doi: 10.1161/01.CIR.65.5.924 [DOI] [PubMed] [Google Scholar]
- 97. Miyake K, Tani K, Kakiuchi S, Suzuka C, Toyoda Y, Kishi J, et al. Epidermal growth factor receptor-tyrosine kinase inhibitor (gefitinib) augments pneumonitis, but attenuates lung fibrosis in response to radiation injury in rats. J Med Invest 2012; 59: 174–85. doi: 10.2152/jmi.59.174 [DOI] [PubMed] [Google Scholar]
- 98. Flechsig P, Dadrich M, Bickelhaupt S, Jenne J, Hauser K, Timke C, et al. LY2109761 attenuates radiation-induced pulmonary murine fibrosis via reversal of TGF-beta and BMP-associated proinflammatory and proangiogenic signals. Clin Cancer Res 2012; 18: 3616–27. doi: 10.1158/1078-0432.CCR-11-2855 [DOI] [PubMed] [Google Scholar]
- 99. Nawroth I, Alsner J, Behlke MA, Besenbacher F, Overgaard J, Howard KA, et al. Intraperitoneal administration of chitosan/DsiRNA nanoparticles targeting TNFα prevents radiation-induced fibrosis. Radiother Oncol 2010; 97: 143–8. doi: 10.1016/j.radonc.2010.09.010 [DOI] [PubMed] [Google Scholar]
- 100. Tabata C, Kadokawa Y, Tabata R, Takahashi M, Okoshi K, Sakai Y, et al. All- trans -retinoic acid prevents radiation- or bleomycin-induced pulmonary fibrosis. Am J Respir Crit Care Med 2006; 174: 1352–60. doi: 10.1164/rccm.200606-862OC [DOI] [PubMed] [Google Scholar]
- 101. Okoshi K, Kubo H, Nagayama S, Tabata C, Kadokawa Y, Hisamori S, et al. All-trans-retinoic acid attenuates radiation-induced intestinal fibrosis in mice. J Surg Res 2008; 150: 53–9. doi: 10.1016/j.jss.2007.12.762 [DOI] [PubMed] [Google Scholar]
- 102. Han G, Zhou YF, Zhang MS, Cao Z, Xie CH, Zhou FX, et al. Angelica sinensis down-regulates hydroxyproline and Tgfb1 and provides protection in mice with radiation-induced pulmonary fibrosis. Radiat Res 2006; 165: 546–52. doi: 10.1667/RR3543.1 [DOI] [PubMed] [Google Scholar]
- 103. Gottlöber P, Steinert M, Bähren W, Weber L, Gerngross H, Peter RU. Interferon-gamma in 5 patients with cutaneous radiation syndrome after radiation therapy. Int J Radiat Oncol Biol Phys 2001; 50: 159–66. doi: 10.1016/S0360-3016(00)01542-X [DOI] [PubMed] [Google Scholar]
- 104. Simone NL, Soule BP, Gerber L, Augustine E, Smith S, Altemus RM, et al. Oral pirfenidone in patients with chronic fibrosis resulting from radiotherapy: a pilot study. Radiat Oncol 2007; 2: 19. doi: 10.1186/1748-717X-2-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Matsushita S, Ando K, Koike S, Grdina DJ, Furukawa S. Radioprotection by WR-151327 against the late normal tissue damage in mouse hind legs from gamma ray radiation. Int J Radiat Oncol Biol Phys 1994; 30: 867–72. doi: 10.1016/0360-3016(94)90362-X [DOI] [PubMed] [Google Scholar]
- 106. Percarpio B, Fischer JJ. Beta-aminopropionitrile as a radiation reaction preventive agent. Radiology 1976; 121: 737–40. doi: 10.1148/121.3.737 [DOI] [PubMed] [Google Scholar]
- 107. Bourgier C, Rivera S, Vozenin MC, Boisselier P, Azria D, Lassau N, et al. Pravastatin reverses established radiation-induced cutaneous and subcutaneous fibrosis in head and neck cancer patients: results of a biology-driven clinical trial, pravacur phase 2. Int J Radiat Oncol Biol Phys 2017; 99: S74–S. doi: 10.1016/j.ijrobp.2017.06.180 [DOI] [PubMed] [Google Scholar]
- 108. Favaudon V, Caplier L, Monceau V, Pouzoulet F, Sayarath M, Fouillade C, et al. Ultrahigh dose-rate FLASH irradiation increases the differential response between normal and tumor tissue in mice. Sci Transl Med 2014; 6: 245ra93. doi: 10.1126/scitranslmed.3008973 [DOI] [PubMed] [Google Scholar]
- 109. Montay-Gruel P, Petersson K, Jaccard M, Boivin G, Germond JF, Petit B, et al. Irradiation in a flash: Unique sparing of memory in mice after whole brain irradiation with dose rates above 100Gy/s. Radiother Oncol 2017; 124: 365–9. doi: 10.1016/j.radonc.2017.05.003 [DOI] [PubMed] [Google Scholar]
- 110. Péguret N, Ozsahin M, Zeverino M, Belmondo B, Durham AD, Lovis A, et al. Apnea-like suppression of respiratory motion: First evaluation in radiotherapy. Radiother Oncol 2016; 118: 220–6. doi: 10.1016/j.radonc.2015.10.011 [DOI] [PubMed] [Google Scholar]
- 111. Ogna A, Bernasconi M, Belmondo B, Long O, Simons J, Peguret N, et al. Prolonged apnea supported by high-frequency noninvasive ventilation: a pilot study. Am J Respir Crit Care Med 2017; 195: 958–60. doi: 10.1164/rccm.201608-1572LE [DOI] [PubMed] [Google Scholar]
- 112. Jaffray DA. Image-guided radiotherapy: from current concept to future perspectives. Nat Rev Clin Oncol 2012; 9: 688–99. doi: 10.1038/nrclinonc.2012.194 [DOI] [PubMed] [Google Scholar]
- 113. Zelefsky MJ, Kollmeier M, Cox B, Fidaleo A, Sperling D, Pei X, et al. Improved clinical outcomes with high-dose image guided radiotherapy compared with non-IGRT for the treatment of clinically localized prostate cancer. Int J Radiat Oncol Biol Phys 2012; 84: 125–9. doi: 10.1016/j.ijrobp.2011.11.047 [DOI] [PubMed] [Google Scholar]
- 114. Créhange G, Martin E, Supiot S, Chapet O, Mazoyer F, Naudy S, et al. Image-guided radiotherapy in prostate cancer: concepts and implications. Cancer Radiother 2012; 16: 430–8. doi: 10.1016/j.canrad.2012.07.183 [DOI] [PubMed] [Google Scholar]
- 115. Nutting CM, Morden JP, Harrington KJ, Urbano TG, Bhide SA, Clark C, et al . Parotid-sparing intensity modulated versus conventional radiotherapy in head and neck cancer (PARSPORT): a phase 3 multicentre randomised controlled trial. Lancet Oncol 2011; 12: 127–36. doi: 10.1016/S1470-2045(10)70290-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Chen AM, Li BQ, Farwell DG, Marsano J, Vijayakumar S, Purdy JA. Improved dosimetric and clinical outcomes with intensity-modulated radiotherapy for head-and-neck cancer of unknown primary origin. Int J Radiat Oncol Biol Phys 2011; 79: 756–62. doi: 10.1016/j.ijrobp.2009.11.020 [DOI] [PubMed] [Google Scholar]
- 117. Jermann M. Particle therapy statistics in 2014. International Journal of Particle Therapy 2015; 2: 50–4. doi: 10.14338/IJPT-15-00013 [DOI] [Google Scholar]
- 118. Rajendran R, Weinberg V, Daftari I, Castro J, Quivey J, Char D, et al. Decreased incidence of neovascular glaucoma by sparing anterior structures of the eye for proton beam therapy of ocular melanoma. Int J Radiat Oncol Biol Phys 2004; 60: S311–S312. doi: 10.1016/S0360-3016(04)01391-4 [DOI] [Google Scholar]
- 119. Munzenrider JE. Uveal melanomas. Conservation treatment. Hematol Oncol Clin North Am 2001; 15: 389–402. [DOI] [PubMed] [Google Scholar]
- 120. Dendale R, Lumbroso-Le Rouic L, Noel G, Feuvret L, Levy C, Delacroix S, et al. Proton beam radiotherapy for uveal melanoma: results of curie institut-orsay proton therapy center (ICPO). Int J Radiat Oncol Biol Phys 2006; 65: 780–7. doi: 10.1016/j.ijrobp.2006.01.020 [DOI] [PubMed] [Google Scholar]
- 121. Egger E, Schalenbourg A, Zografos L, Bercher L, Boehringer T, Chamot L, et al. Maximizing local tumor control and survival after proton beam radiotherapy of uveal melanoma. Int J Radiat Oncol Biol Phys 2001; 51: 138–47. doi: 10.1016/S0360-3016(01)01560-7 [DOI] [PubMed] [Google Scholar]
- 122. Krishnan S, Foote RL, Brown PD, Pollock BE, Link MJ, Garces YI. Radiosurgery for cranial base chordomas and chondrosarcomas. Neurosurgery 2005; 56: 777–84. doi: 10.1227/01.NEU.0000156789.10394.F5 [DOI] [PubMed] [Google Scholar]
- 123. Ares C, Hug EB, Lomax AJ, Bolsi A, Timmermann B, Rutz HP, et al. Effectiveness and safety of spot scanning proton radiation therapy for chordomas and chondrosarcomas of the skull base: first long-term report. Int J Radiat Oncol Biol Phys 2009; 75: 1111–8. doi: 10.1016/j.ijrobp.2008.12.055 [DOI] [PubMed] [Google Scholar]
- 124. Munzenrider JE. Proton therapy for uveal melanomas and other eye lesions. Strahlenther Onkol 1999; 175(Suppl 2): 68–73. doi: 10.1007/BF03038893 [DOI] [PubMed] [Google Scholar]
- 125. Miralbell R, Lomax A, Russo M. Potential role of proton therapy in the treatment of pediatric medulloblastoma/primitive neuro-ectodermal tumors: spinal theca irradiation. Int J Radiat Oncol Biol Phys 1997; 38: 805–11. doi: 10.1016/S0360-3016(97)00005-9 [DOI] [PubMed] [Google Scholar]
- 126. Miralbell R, Lomax A, Cella L, Schneider U. Potential reduction of the incidence of radiation-induced second cancers by using proton beams in the treatment of pediatric tumors. Int J Radiat Oncol Biol Phys 2002; 54: 824–9. doi: 10.1016/S0360-3016(02)02982-6 [DOI] [PubMed] [Google Scholar]
- 127. Mu X, Björk-Eriksson T, Nill S, Oelfke U, Johansson KA, Gagliardi G, et al. Does electron and proton therapy reduce the risk of radiation induced cancer after spinal irradiation for childhood medulloblastoma? A comparative treatment planning study. Acta Oncol 2005; 44: 554–62. doi: 10.1080/02841860500218819 [DOI] [PubMed] [Google Scholar]
- 128. Pulsifer MB, Sethi RV, Kuhlthau KA, MacDonald SM, Tarbell NJ, Yock TI. Early cognitive outcomes following proton radiation in pediatric patients with brain and central nervous system tumors. Int J Radiat Oncol Biol Phys 2015; 93: 400–7. doi: 10.1016/j.ijrobp.2015.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Clarke M, Collins R, Darby S, Davies C, Elphinstone P, Evans V, et al . Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet 2005; 366: 2087–106. doi: 10.1016/S0140-6736(05)67887-7 [DOI] [PubMed] [Google Scholar]
- 130. Lomax AJ, Cella L, Weber D, Kurtz JM, Miralbell R. Potential role of intensity-modulated photons and protons in the treatment of the breast and regional nodes. Int J Radiat Oncol Biol Phys 2003; 55: 785–92. doi: 10.1016/S0360-3016(02)04210-4 [DOI] [PubMed] [Google Scholar]
- 131. Johansson J, Isacsson U, Lindman H, Montelius A, Glimelius B. Node-positive left-sided breast cancer patients after breast-conserving surgery: potential outcomes of radiotherapy modalities and techniques. Radiother Oncol 2002; 65: 89–98. doi: 10.1016/S0167-8140(02)00266-9 [DOI] [PubMed] [Google Scholar]
- 132. Weber DC, Ares C, Lomax AJ, Kurtz JM. Radiation therapy planning with photons and protons for early and advanced breast cancer: an overview. Radiat Oncol 2006; 1: 22. doi: 10.1186/1748-717X-1-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Taghian AG, Kozak KR, Katz A, Adams J, Lu HM, Powell SN, et al. Accelerated partial breast irradiation using proton beams: Initial dosimetric experience. Int J Radiat Oncol Biol Phys 2006; 65: 1404–10. doi: 10.1016/j.ijrobp.2006.03.017 [DOI] [PubMed] [Google Scholar]
- 134. Kozak KR, Smith BL, Adams J, Kornmehl E, Katz A, Gadd M, et al. Accelerated partial-breast irradiation using proton beams: initial clinical experience. Int J Radiat Oncol Biol Phys 2006; 66: 691–8. doi: 10.1016/j.ijrobp.2006.06.041 [DOI] [PubMed] [Google Scholar]
- 135. Tsuji T, Ibaragi S, Shima K, Hu MG, Katsurano M, Sasaki A, et al. Epithelial-mesenchymal transition induced by growth suppressor p12CDK2-AP1 promotes tumor cell local invasion but suppresses distant colony growth. Cancer Res 2008; 68: 10377–86. doi: 10.1158/0008-5472.CAN-08-1444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Okada T, Kamada T, Tsuji H, Mizoe JE, Baba M, Kato S, et al. Carbon ion radiotherapy: clinical experiences at National Institute of Radiological Science (NIRS). J Radiat Res 2010; 51: 355–64. doi: 10.1269/jrr.10016 [DOI] [PubMed] [Google Scholar]
- 137. Kramer D. Carbon-ion cancer therapy shows promise. Phys Today 2015; 68: 24–5. doi: 10.1063/PT.3.2812 [DOI] [Google Scholar]
- 138. Denekamp J, Waites T, Fowler JF. Predicting realistic RBE values for clinically relevant radiotherapy schedules. Int J Radiat Biol 1997; 71: 681–94. doi: 10.1080/095530097143699 [DOI] [PubMed] [Google Scholar]
- 139. Ando K, Kase Y. Biological characteristics of carbon-ion therapy. Int J Radiat Biol 2009; 85: 715–28. doi: 10.1080/09553000903072470 [DOI] [PubMed] [Google Scholar]
- 140. Ando K, Koike S, Uzawa A, Takai N, Fukawa T, Furusawa Y, et al. Biological gain of carbon-ion radiotherapy for the early response of tumor growth delay and against early response of skin reaction in mice. J Radiat Res 2005; 46: 51–7. doi: 10.1269/jrr.46.51 [DOI] [PubMed] [Google Scholar]
- 141. Battermann JJ, Mijnheer BJ. The Amsterdam fast neutron therapy project: a final report. Int J Radiat Oncol Biol Phys 1986; 12: 2093–9. doi: 10.1016/0360-3016(86)90007-6 [DOI] [PubMed] [Google Scholar]
- 142. Laramore GE. The use of neutrons in cancer therapy: a historical perspective through the modern era. Semin Oncol 1997; 24: 672–85. [PubMed] [Google Scholar]
- 143. Prempree T, Michelsen A, Merz T. The repair time of chromosome breaks induced by pulsed x-rays on ultra-high dose-rate. Int J Radiat Biol Relat Stud Phys Chem Med 1969; 15: 571–4. doi: 10.1080/09553006914550871 [DOI] [PubMed] [Google Scholar]
- 144. Auer S, Hable V, Greubel C, Drexler GA, Schmid TE, Belka C, et al. Survival of tumor cells after proton irradiation with ultra-high dose rates. Radiat Oncol 2011; 6: 139. doi: 10.1186/1748-717X-6-139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Schmid TE, Dollinger G, Beisker W, Hable V, Greubel C, Auer S, et al. Differences in the kinetics of gamma-H2AX fluorescence decay after exposure to low and high LET radiation. Int J Radiat Biol 2010; 86: 682–91. doi: 10.3109/09553001003734543 [DOI] [PubMed] [Google Scholar]
- 146. Schmid TE, Dollinger G, Hable V, Greubel C, Zlobinskaya O, Michalski D, et al. The effectiveness of 20 mev protons at nanosecond pulse lengths in producing chromosome aberrations in human-hamster hybrid cells. Radiat Res 2011; 175: 719–27. doi: 10.1667/RR2465.1 [DOI] [PubMed] [Google Scholar]
- 147. Zlobinskaya O, Girst S, Greubel C, Hable V, Siebenwirth C, Walsh DW, et al. Reduced side effects by proton microchannel radiotherapy: study in a human skin model. Radiat Environ Biophys 2013; 52: 123–33. doi: 10.1007/s00411-012-0450-9 [DOI] [PubMed] [Google Scholar]
- 148. Loo BW, Schuler E, Lartey FM, Rafat M, King GJ, Trovati S, et al. Delivery of ultra-rapid flash radiation therapy and demonstration of Normal tissue sparing after abdominal irradiation of mice. Int J Radiat Oncol Biol Phys 2017; 98(2 suppl): E16. doi: 10.1016/j.ijrobp.2017.02.101 [DOI] [Google Scholar]
- 149. Hendry JH, Moore JV, Hodgson BW, Keene JP. The constant low oxygen concentration in all the target cells for mouse tail radionecrosis. Radiat Res 1982; 92: 172–81. doi: 10.2307/3575852 [DOI] [PubMed] [Google Scholar]
- 150. Weiss H, Epp ER, Heslin JM, Ling CC, Santomasso A. Oxygen depletion in cells irradiated at ultra-high dose-rates and at conventional dose-rates. Int J Radiat Biol Relat Stud Phys Chem Med 1974; 26: 17–29. doi: 10.1080/09553007414550901 [DOI] [PubMed] [Google Scholar]
- 151. Schüler E, Trovati S, King G, Lartey F, Rafat M, Villegas M, et al. Experimental platform for ultra-high dose rate FLASH irradiation of small animals using a clinical linear accelerator. Int J Radiat Oncol Biol Phys 2017; 97: 195–203. doi: 10.1016/j.ijrobp.2016.09.018 [DOI] [PubMed] [Google Scholar]
- 152. Schüler E, Eriksson K, Hynning E, Hancock SL, Hiniker SM, Bazalova-Carter M, et al. Very high-energy electron (VHEE) beams in radiation therapy; treatment plan comparison between VHEE, VMAT, and PPBS. Med Phys 2017; 44: 2544–55. doi: 10.1002/mp.12233 [DOI] [PubMed] [Google Scholar]
- 153. Glinec Y, Faure J, Malka V, Fuchs T, Szymanowski H, Oelfke U. Radiotherapy with laser-plasma accelerators: monte carlo simulation of dose deposited by an experimental quasimonoenergetic electron beam. Med Phys 2006; 33: 155–62. doi: 10.1118/1.2140115 [DOI] [PubMed] [Google Scholar]