Abstract
Background
Electromechanical and robotic‐assisted gait training devices are used in rehabilitation and might help to improve walking after stroke. This is an update of a Cochrane Review first published in 2007.
Objectives
To investigate the effects of automated electromechanical and robotic‐assisted gait training devices for improving walking after stroke.
Search methods
We searched the Cochrane Stroke Group Trials Register (last searched April 2012), the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2012, Issue 2), MEDLINE (1966 to November 2012), EMBASE (1980 to November 2012), CINAHL (1982 to November 2012), AMED (1985 to November 2012), SPORTDiscus (1949 to September 2012), the Physiotherapy Evidence Database (PEDro, searched November 2012) and the engineering databases COMPENDEX (1972 to November 2012) and INSPEC (1969 to November 2012). We handsearched relevant conference proceedings, searched trials and research registers, checked reference lists and contacted authors in an effort to identify further published, unpublished and ongoing trials.
Selection criteria
We included all randomised and randomised cross‐over trials consisting of people over 18 years old diagnosed with stroke of any severity, at any stage, or in any setting, evaluating electromechanical and robotic‐assisted gait training versus normal care.
Data collection and analysis
Two review authors independently selected trials for inclusion, assessed methodological quality and extracted the data. The primary outcome was the proportion of participants walking independently at follow‐up.
Main results
In this update of our review, we included 23 trials involving 999 participants. Electromechanical‐assisted gait training in combination with physiotherapy increased the odds of participants becoming independent in walking (odds ratio (OR) (random effects) 2.39, 95% confidence interval (CI) 1.67 to 3.43; P < 0.00001; I² = 0%) but did not significantly increase walking velocity (mean difference (MD) = 0.04 metres/s, 95% CI ‐0.03 to 0.11; P = 0.26; I² = 73%) or walking capacity (MD = 3 metres walked in six minutes, 95% CI ‐29 to 35; P = 0.86; I² = 70%). The results must be interpreted with caution because (1) some trials investigated people who were independent in walking at the start of the study, (2) we found variations between the trials with respect to devices used and duration and frequency of treatment, and (3) some trials included devices with functional electrical stimulation. Our planned subgroup analysis suggests that people in the acute phase may benefit but people in the chronic phase may not benefit from electromechanical‐assisted gait training. Post hoc analysis showed that people who are non‐ambulatory at intervention onset may benefit but ambulatory people may not benefit from this type of training. Post hoc analysis showed no differences between the types of devices used in studies regarding ability to walk, but significant differences were found between devices in terms of walking velocity.
Authors' conclusions
People who receive electromechanical‐assisted gait training in combination with physiotherapy after stroke are more likely to achieve independent walking than people who receive gait training without these devices. Specifically, people in the first three months after stroke and those who are not able to walk seem to benefit most from this type of intervention. The role of the type of device is still not clear. Further research should consist of a large definitive, pragmatic, phase III trial undertaken to address specific questions such as the following: What frequency or duration of electromechanical‐assisted gait training might be most effective? How long does the benefit last?
Keywords: Humans, Orthotic Devices, Stroke Rehabilitation, Walking, Combined Modality Therapy, Combined Modality Therapy/instrumentation, Combined Modality Therapy/methods, Equipment Design, Exercise Therapy, Exercise Therapy/methods, Gait, Randomized Controlled Trials as Topic, Robotics, Robotics/instrumentation
Electromechanical‐assisted training for walking after stroke
Many people who have had a stroke have difficulties with walking, and improving walking is one of the main goals of rehabilitation. Electromechanical‐assisted gait training uses specialist machines to assist walking practice. This review of 23 trials, which included 999 participants, found evidence that electromechanical‐assisted gait training combined with physiotherapy may improve recovery of independent walking in people after stroke. Specifically, people in the first three months after stroke and those who are not able to walk appear to benefit most from this type of intervention. The importance of the type of device is still not clear. Further research should address what frequency or duration of walking training might be most effective and how long the benefit can last. Also it is still not clear how such devices should be used in routine rehabilitation.
Background
Description of the condition
A stroke is a sudden, non‐convulsive loss of neurological function due to an ischaemic or haemorrhagic intracranial vascular event (WHO 2006). In general, cerebrovascular accidents are classified by anatomic location in the brain, vascular distribution, aetiology, age of the affected individual, and haemorrhagic versus non‐haemorrhagic nature (Adams 1993). Stroke is a leading cause of death and of serious, long‐term disability in adults. Three months after stroke, 20% of people remain wheelchair bound, and approximately 70% walk at reduced velocity and capacity (Jorgensen 1995). Restoration of walking ability and gait rehabilitation are therefore highly relevant for people who are unable to walk independently after stroke (Bohannon 1991), as well as for their relatives. To restore gait, modern concepts of rehabilitation favour a repetitive task‐specific approach (Carr 2003; French 2007). In recent years it has also been shown that higher intensities of walking practice (resulting in more repetitions trained) result in better outcomes for people after stroke (Kwakkel 1999; Van Peppen 2004).
Description of the intervention
As an adjunct to overground gait training (States 2009), in recent years treadmill training has been introduced for the rehabilitation of people after stroke (Moseley 2005). Treadmill training with and without partial body weight support enables the repetitive practice of complex gait cycles for these people. However, one disadvantage of treadmill training might be the effort required by therapists to set the paretic limbs and to control weight shift, thereby possibly limiting the intensity of therapy, especially in more severely disabled people. Automated electromechanical gait machines were developed to reduce dependence on therapists. They consist of either a robot‐driven exoskeleton orthosis (Colombo 2000) or an electromechanical solution, with two driven foot plates simulating the phases of gait (Hesse 1999).
One example of automated electromechanical gait rehabilitation is the 'Lokomat' (Colombo 2000). A robotic gait orthosis combined with a harness‐supported body weight system is used together with a treadmill. However, the main difference from treadmill training is that the patient's legs are guided by the robotic device according to a preprogrammed gait pattern. A computer‐controlled robotic gait orthosis guides the patient, and the process of gait training is automated.
A second example is the 'Gait Trainer', which is based on a double crank and rocker gear system (Hesse 1999). In contrast to a treadmill, the electromechanical 'Gait Trainer' consists of two foot plates positioned on two bars, two rockers and two cranks, which provide the propulsion. The harness‐secured patient is positioned on the foot plates, which symmetrically simulate the stance and swing phases of walking (Hesse 1999). A servo‐controlled motor guides the patient during walking exercise. Vertical and horizontal movements of the trunk are controlled in a phase‐dependent manner. Again, the main difference from treadmill training is that the process of gait training is automated and is supported by an electromechanical solution.
Other similar, more recently developed electromechanical devices include the 'Haptic Walker' (Schmidt 2005), the 'Anklebot' (MIT 2005) and the 'LOPES' (Lower Extremity Powered Exoskeleton) (Veneman 2005).
How the intervention might work
Electromechanical devices (such as those previously described) can be used to give non‐ambulatory patients intensive practice (in terms of high repetitions) of complex gait cycles. The advantage of these electromechanical devices, compared with treadmill training with partial body weight support, may be the reduced effort required of therapists, as they no longer need to set the paretic limbs or assist trunk movements (Hesse 2003).
Why it is important to do this review
Scientific evidence for the benefits of the above‐mentioned technologies may have changed since our Cochrane Review was first published in 2007 (Mehrholz 2007); therefore an update of the review is required to justify the large equipment and human resource costs needed to implement electromechanical‐assisted gait devices, as well as to confirm the safety and acceptance of this method of training.
Therefore, the aim of this review was to provide an update of the best available evidence about the above‐mentioned approach.
Objectives
To investigate the effects of automated electromechanical and robotic‐assisted gait training devices for improving walking after stroke.
Methods
Criteria for considering studies for this review
Types of studies
We searched for all randomised controlled trials (RCTs) and randomised controlled cross‐over trials for inclusion in this review. If we included randomised controlled cross‐over trials, we analysed only the first period as a parallel‐group trial.
Types of participants
We included studies with participants of any gender over 18 years of age after stroke, using the World Health Organization (WHO) definition of stroke (WHO 2006) or a clinical definition of stroke if the WHO definition was not specifically stated.
Types of interventions
We included all trials that evaluated electromechanical and robotic‐assisted gait training plus physiotherapy versus physiotherapy (or usual care) for regaining and improving walking after stroke. We also included automated electromechanical devices that were used in combination with therapies such as functional electrical stimulation applied to the legs during gait training (compared with therapies not using electromechanical devices). We defined an automated electromechanical device as any device with an electromechanical solution designed to assist stepping cycles by supporting body weight and automating the walking therapy process in people after stroke. This category included any mechanical or computerised device designed to improve walking function. We also searched for electromechanical devices such as robots for gait training after stroke (MIT 2005; Schmidt 2005; Veneman 2005).
Electromechanical devices can principally be differentiated into end‐effector and exoskeleton devices. Examples of end‐effector devices are the 'Lokohelp' (Freivogel 2009), the 'Haptic Walker' (Schmidt 2005) and the 'Gait Trainer GT 1' (Hesse 1999). The definition of an end‐effector principle is that a patient's feet are placed on foot plates, whose trajectories simulate the stance and swing phases during gait training (Hesse 2010). Examples of the exoskeleton type of device are the 'Lokomat' (Colombo 20003). Such exoskeletons are outfitted with programmable drives or passive elements, which move the knees and hips during the phases of gait (Hesse 2010).
We did not include non‐weight‐bearing interventions such as non‐interactive devices that deliver continuous passive motion only (Nuyens 2002). We excluded trials testing the effectiveness of treadmill training or other approaches, such as repetitive task training in physiotherapy or electrical stimulation alone, to prevent duplication with other Cochrane Reviews and protocols (e.g. Moseley 2005).
Types of outcome measures
Primary outcomes
Regaining the ability to walk is a very important goal for people after stroke (Bohannon 1988). Therefore, we defined the primary outcome as the ability to walk independently. We measured the ability to walk with the Functional Ambulation Category (FAC) (Holden 1984). A FAC score of 4 or 5 indicated independent walking over a 15‐metre surface, irrespective of aids used, such as a cane. A FAC score less than 4 indicates dependency in walking (supervision or assistance, or both, must be given in performing walking).
If FAC scores were not reported in the included studies, we used alternative indicators of independent walking such as:
a score of 3 on the ambulation item of the Barthel Index (BI) (Wade 1988); or
a score of 6 or 7 for the walking item of the Functional Independence Measure (FIM) (Hamilton 1994); or
a 'yes' response to the item 'walking inside, with an aid if necessary (but with no standby help)' or 'yes' to 'walking on uneven ground' in the Rivermead Mobility Index (RMI) (Collen 1991).
Secondary outcomes
We defined secondary outcomes as measures of activity limitations. As relevant measures of activity limitations, we used walking speed (in metres per second), walking capacity (metres walked in six minutes) and the RMI score, if stated by the trialists. Additionally, as a secondary outcome, we used death from all causes.
Adverse outcomes
We investigated the safety of electromechanical‐assisted gait training devices with the incidence of adverse outcomes such as thrombosis, major cardiovascular events, injuries, pain and any other reported adverse events. To measure the acceptance of electromechanical‐assisted gait training devices in walking therapies, we used visual analogue scales or withdrawal from the study for any reason (drop‐out rates), or both, during the study period, depending on data provided by the study authors.
Depending on the above‐stated categories and the availability of variables used in the included trials, we discussed and reached consensus on which outcome measures should be included in the analysis.
Search methods for identification of studies
See the 'Specialized register' section in the Cochrane Stroke Group module. We searched for trials in all languages and arranged translation of relevant papers published in languages other than English.
Electronic searches
We searched the Cochrane Stroke Group Trials Register (last searched April 2012), the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library, Issue 2, 2012), MEDLINE (1966 to November 2012), EMBASE (1980 to November 2012), CINAHL (1982 to November 2012), AMED (1985 to November 2012), SPORTDiscus (1949 to September 2012), the Physiotherapy Evidence Database (PEDro, searched November 2012) and the engineering databases COMPENDEX (1972 to November 2012) and INSPEC (1969 to November 2012) (Appendix 1).
We developed the search strategies with the help of the Cochrane Stroke Group Trials Search Co‐ordinator and adapted the MEDLINE search strategy for the other databases.
We identified and searched the following ongoing trials and research registers:
International Standard Randomised Controlled Trial Number Register at http://www.controlled‐trials.com/isrctn/ (searched December 2012);
Clinical trials.gov at www.clinicaltrials.gov (searched December 2012); and
Stroke Trials Register at www.strokecenter.org (searched December 2012).
Searching other resources
We also:
-
handsearched the following relevant conference proceedings:
World Congress of NeuroRehabilitation (2002, 2006, 2008, 2010 and 2012);
World Congress of Physical Medicine and Rehabilitation (2001, 2003, 2005, 2007, 2009 and 2011);
World Congress of Physical Therapy (2003, 2007 and 2011);
Deutsche Gesellschaft für Neurotraumatologie und Klinische Neurorehabilitation (2001 to 2012);
Deutsche Gesellschaft für Neurologie (2000 to 2012);
Deutsche Gesellschaft für Neurorehabilitation (1999 to 2012); and
Asian Oceania Conference of Physical and Rehabilitation (2008 to 2012).
screened reference lists of all relevant articles; and
contacted trialists, experts and researchers in our field of study.
Data collection and analysis
Selection of studies
Two review authors (JM, BE) independently read the titles and abstracts of the identified references and eliminated obviously irrelevant studies. We obtained the full text for the remaining studies. Based on our inclusion criteria (types of studies, participants, aims of interventions, outcome measures), the same two review authors independently ranked these studies as relevant, irrelevant or possibly relevant. We excluded all trials ranked initially as irrelevant but included all other trials at this stage. We excluded all trials of specific treatment components, such as electrical stimulation as stand‐alone treatment, treadmill training and continuous passive motion treatment, because these have been the subject of other Cochrane Reviews (e.g. Moseley 2005). We resolved any disagreements through discussion between all four review authors. If further information was necessary to reach consensus, we contacted trialists in an effort to obtain the missing information.
Data extraction and management
Two review authors (JM, BE) independently extracted trial and outcome data from the selected trials. We established the characteristics of unpublished trials through correspondence with the trial co‐ordinator or principal investigator. If any review author was involved in any of the selected studies, another member of our review author group not involved in the study extracted the study information. If there was any doubt whether the study should be excluded, we retrieved the full text of the article. In cases of disagreement between the two review authors, a third member of the review author group (JK) reviewed the information to decide on inclusion or exclusion of a study. We used checklists to record the following details independently.
Methods of generating the randomisation schedule.
Method of concealment of allocation.
Blinding of assessors.
Use of an intention‐to‐treat analysis (all participants initially randomly assigned were included in the analyses as allocated to groups).
Adverse events and drop‐outs for all reasons.
Important imbalance in prognostic factors.
Participants (country, number of participants, age, gender, type of stroke, time from stroke onset to entry to the study, inclusion and exclusion criteria).
Comparison (details of the intervention in treatment and control groups, details of co‐intervention(s) in both groups, duration of treatment).
Outcomes and time points of measures (number of participants in each group and outcome, regardless of compliance).
The two review authors checked all of the extracted data for agreement, with a third review author (JK) arbitrating any items for which consensus could not be reached. If necessary, we contacted trialists to request more information, clarification and missing data.
Assessment of risk of bias in included studies
Two review authors (JM, MP) independently evaluated the methodological quality of the included trials using the Cochrane risk of bias tool, as described in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
We checked all methodological quality assessments for agreement between review authors. We resolved disagreements by discussion. If one of the review authors was a co‐author of an included trial, another review author (BE or JK) conducted the methodological quality assessment for this trial in this case.
Measures of treatment effect
We planned to compare electromechanical and robotic‐assisted gait training plus physiotherapy versus physiotherapy (or usual care) for primary and secondary outcome parameters. We analysed binary (dichotomous) outcomes with an odds ratio (OR) random‐effects model with 95% confidence intervals (CIs). We analysed continuous outcomes with mean differences (MDs), using the same outcome scale. We quantified inconsistency across studies by using the I² statistic. We used a random‐effects model for all analyses. For all statistical comparisons, we used the current version of the Cochrane Review Manager software, RevMan 5 (RevMan 2012).
Subgroup analysis and investigation of heterogeneity
As planned in our protocol (Mehrholz 2006), we did a formal subgroup analysis according to the suggestions of the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2011), comparing participants treated in the acute and subacute phases of their stroke (within three months) with participants treated in the chronic phase (longer than three months).
Sensitivity analysis
As planned in our protocol, we performed a sensitivity analysis of methodological quality for each included study.
We carried out the following sensitivity analyses by including only those studies:
with an adequate sequence generation process;
with adequate concealed allocation;
with blinded assessors for the primary outcome; and
without incomplete outcome data.
We believed it was necessary to do a further sensitivity analysis by removing the largest study (Pohl 2007) because some of the review authors (JM, MP and CW) were investigators in this large trial. We carried out this sensitivity analysis by including all studies without the largest study (Pohl 2007).
We did two further (post hoc) sensitivity analyses.
Ambulatory status at start of study (including only studies that included an independent walker, including only studies that included dependent and independent walkers and including only studies that included a dependent walker.
Type of device used in trials (including only studies that used end‐effector devices and including only studies that used exoskeleton devices).
Results
Description of studies
See the Characteristics of included studies, Characteristics of excluded studies and Characteristics of ongoing studies tables.
Results of the search
Figure 1 shows the flow diagram for the selection of studies. Searches of the electronic databases and of trials registers generated 4747 unique references for screening. After excluding non‐relevant citations, we obtained the full text of 136 papers, and from these, we identified and included 23 trials in the review.
Figure 1.
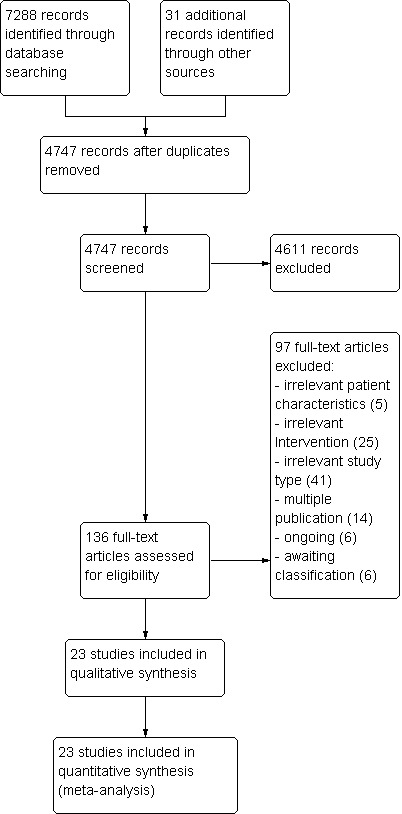
Study flow diagram.
Included studies
We included in this update of the review 23 trials involving a total of 999 participants (see the Characteristics of included studies, Figure 1, Table 6 and Table 7). All included studies investigated the effects of automated electromechanical or robotic‐assisted gait training devices in improving walking after stroke.
Table 1.
Patient characteristics in studies
| Study ID |
Experimental: age, mean (SD) |
Control: age, mean (SD) |
Experimental: time post‐stroke |
Control: time post‐stroke |
Experimental: sex |
Control: sex |
Experimental: side paresis |
Control: side paresis |
| Aschbacher 2006 | 57 years | 65 years | ≤ 3 months after stroke | ≤ 3 months after stroke | 2 female | 4 female | Not provided by the authors | Not provided by the authors |
| Brincks 2011 | 61 (median) years | 59 (median) years | 56 (median) days | 21 (median) days | 5 male, 2 female | 4 male, 2 female | 5 right, 2 left | 1 right, 5 left |
| Chang 2012 | 56 (12) years | 60 (12) years | 16 (5) days | 18 (5) days | 13 male, 7 female | 10 male, 7 female | 6 right, 14 left | 6 right, 11 left |
| Dias 2006 | 70 (7) years | 68 (11) years | 47 (64) months | 48 (30) months | 16 male, 4 female | 14 male, 6 female | Not provided by the authors | Not provided by the authors |
| Fisher 2008 | Not stated by the authors | Not stated by the authors | Less than 12 months | Less than 12 months | Not stated by the authors | Not stated by the authors | Not stated by the authors | Not stated by the authors |
| Geroin 2011 | 63 (7) years | 61 (6) years | 26 (6) months | 27 (6) months | 14 male, 6 female | 9 male, 1 female | Not stated by the authors | Not stated by the authors |
| Hidler 2009 | 60 (11) years | 55 (9) years | 111 (63) days | 139 (61) days | 21 male, 12 female | 18 male, 12 female | 22 right, 11 left | 13 right, 17 left |
| Hornby 2008 | 57 (10) years | 57 (11) years | 50 (51) months | 73 (87) months | 15 male, 9 female | 15 male, 9 female | 16 right, 8 left | 16 right, 8 left |
| Husemann 2007 | 60 (13) years | 57 (11) years | 79 (56) days | 89 (61) days | 11 male, 5 female | 10 male, 4 female | 12 right, 4 left | 11 right, 3 left |
| Kyung 2008 | 48 (8) years | 55 (16) years | 22 (23) months | 29 (12) months | 9 male, 8 female | 4 male, 4 female | 9 right, 8 left | 4 right, 4 left |
| Mayr 2008 | Not provided by the authors | Not provided by the authors | Between 10 days and 6 months | Between 10 days and 6 months | Not provided by the authors | Not provided by the authors | Not provided by the authors | Not provided by the authors |
| Morone 2011 | 62 (11) years | 62 (14) years | 19 (11) days | 20 (14) days | 15 male, 9 female | 13 male, 11 female | 13 right, 11 left | 15 right, 9 left |
| Noser 2012 | 67 (9) years | 64 (11) years | 1354 days | 525 days | 7 male, 4 female | 6 male, 4 female | Not provided by the author | Not provided by the author |
| Peurala 2005 | 52 (8) years | 52 (7) years | 2.5 (2.5) years | 4.0 (5.8) years | 26 male, 4 female | 11 male, 4 female | 13 right, 17 left | 10 right, 5 left |
| Peurala 2009 | 67 (9) years | 68 (10) years | 8 (3) days | 8 (3) days | 11 male, 11 female | 18 male, 16 female | 11 right, 11 left | 14 right, 20 left |
| Pohl 2007 | 62 (12) years | 64 (11) years | 4.2 (1.8) weeks | 4.5 (1.9) weeks | 50 male, 27 female | 54 male, 24 female | 36 right, 41 left | 33 right, 45 left |
| Saltuari 2004 | 62 (13) years | 60 (19) years | 3.6 (4.6) months | 1.9 (0.8) months | 4 male, 4 female | 2 male, 6 female | Not provided by the authors | Not provided by the authors |
| Schwartz 2006 | 62 (9) years | 65 (8) years | 22 (9) days | 24 (10) days | 21 male, 16 female | 20 male, 10 female | 17 right, 20 left | 8 right, 22 left |
| Tanaka 2012 | 63 (10) years | 60 (9) years | 55 (37) months | 65 (67) months | 10 male, 2 female | 9 right, 3 left | ||
| Tong 2006 | 71 (14) years | 64 (10) years | 2.5 (1.2) weeks | 2.7 (1.2) weeks | 19 male, 11 female | 12 male, 8 female | 13 right, 17 left | 7 right, 13 left |
| Van Nunen 2012 | 53 (10) years | 2.1 (1.3) months | 16 male, 14 female | Not provided by the author | Not provided by the author | |||
| Werner 2002 | 60 (9) years | 60 (9) years | 7.4 (2.0) weeks | 6.9 (2.1) weeks | 8 male, 7 female | 5 male, 10 female | 8 right, 7 left | 8 right, 7 left |
| Westlake 2009 | 59 (17) years | 55 (14) years | 44 (27) months | 37 (20) months | 6 male, 2 female | 7 male, 1 female | 4 right, 4 left | 3 right, 5 left |
SD: standard deviation
Table 2.
Demographics of studies including drop‐outs and adverse events
| Criteria | Stroke severity | Electromechanical device used | Duration of study intervention | Aetiology (ischaemic/haemorrhage) | Intensity of treatment per day | Description of the control intervention | Drop‐outs |
Reasons for drop‐out and adverse events in the experimental group |
Reasons for drop‐out and adverse events in the control group |
Source of information |
| Aschbacher 2006 | Not provided by the authors | Lokomat | 3 weeks | Not provided by the authors | 5 times a week 30 minutes | Described as task‐oriented physiotherapy, 5 times a week for 3 weeks (2.5 hours a week) | 4 of 23 | Not provided by the authors | Not provided by the authors | Unpublished information provided as a presentation at a conference |
| Brincks 2011 | Mean FIM 92 of 126 points | Lokomat | 3 weeks | Not provided by the authors | Not provided by the authors | Physiotherapy | 0 of 13 | ‐ | ‐ | Unpublished and published information provided by the authors |
| Chang 2012 | Not provided by the authors | Lokomat | 10 days | Not provided by the authors | 30 minutes daily for 10 days | Conventional physiotherapy, same sessions of conventional gait training by physical therapist as compared with experimental group | 3 of 40 | Not described by group (3 participants dropped out: 1 due to aspiration pneumonia; 2 were not able to co‐operate well with the experimental procedure) |
Unpublished and published information provided by the authors | |
| Dias 2006 | Mean Barthel Index 75 points | Gait Trainer | 4 weeks | Not provided by the authors | 5 times a week 40 minutes | Bobath method, 5 times a week for 5 weeks | 0 of 40 | ‐ | Unpublished and published information provided by the authors | |
| Fisher 2008 | Not provided by the authors | AutoAmbulator | 24 sessions | Not provided by the authors | Minimum of 3 sessions a week up to 5; however, unclear for how many minutes | 'Standard' physical therapy, 3 to 5 times a week for 24 consecutive sessions | 0 of 20 | 14 adverse events, no details about adverse events described |
11 adverse events, no details about adverse events described |
Unpublished and published information provided by the authors |
| Geroin 2011 | Mean European Stroke Scale, 80 points | Gait Trainer | 2 weeks | Not provided by the authors | 5 times a week for 50 minutes | Walking exercises according to the Bobath approach | 0 of 30 | ‐ | ‐ | Unpublished and published information provided by the authors |
| Hidler 2009 | Not stated by the authors | Lokomat | 8 to 10 weeks (24 sessions) | 47/16 | 3 days a week for 45 minutes | Conventional gait training, 3 times a week for 8 to 10 weeks for 24 sessions, each session lasted 1.5 hours | 9 of 72 | Not described by group (9 withdrew or were removed because of poor attendance or a decline in health, including one death, which was unrelated to study according to the authors) |
Unpublished and published information provided by the authors | |
| Hornby 2008 | Not stated by the authors | Lokomat | 12 sessions | 22/26 | 12 sessions, 30 minutes | Therapist‐assisted gait training, 12 sessions, each session lasted 30 minutes | 14 of 62 | 4 participants dropped out (2 discontinued secondary to leg pain during training, 1 experienced pitting oedema and 1 experienced travel limitations) |
10 participants dropped out (4 discontinued secondary to leg pain, 1 experienced an injury outside therapy, 1 reported fear of falling during training, 1 presented with significant hypertension, 1 experienced travel limitations, and 2 discontinued because of subjective exercise intolerance) |
Published information provided by the authors |
| Husemann 2007 | Median Barthel Index, 35 points | Lokomat | 4 weeks | 22/8 | 5 times a week, 30 minutes | Conventional physiotherapy, 30 minutes per day for 4 weeks | 2 of 32 | 1 participant enteritis | 1 participant pulmonary embolism | Information as provided by the authors |
| Kyung 2008 | Not provided by the authors | Lokomat | 4 weeks | 18/7 | 3 days a week, 45 minutes | Conventional physiotherapy, received equal time and sessions of conventional gait training | 10 of 35 | 1 participant dropped out for private reasons (travelling); adverse events not described |
9 participants refused after randomisation (reasons not described); adverse events not described |
Unpublished and published information provided by the authors |
| Mayr 2008 | Not provided by the authors | Lokomat | 8 weeks | Not provided by the authors | Not provided by the authors | Add‐on conventional physiotherapy, received equal time and sessions of conventional gait training | 7 of 72 | 1 participant dropped out (reasons not described); adverse events not described | 6 participants dropped out (reasons not described) | |
| Morone 2011 | Canadian Neurological Scale, 6 points | Gait Trainer | 4 weeks | 41/7 | 5 times a week, 40 minutes | Focused on trunk stabilisation, weight transfer to the paretic leg and walking between parallel bars or on the ground. If necessary, the participant was helped by 1 or 2 therapists and walking aids | 21 of 48 | 12 (hypotension, referred weakness, knee pain, urinary infection, uncontrolled blood pressure, fever, absence of physiotherapist) | 9 (hypotension, referred weakness, knee pain, ankle pain, uncontrolled blood pressure, fever, absence of physiotherapist) | Information as provided by the authors |
| Noser 2012 | Not provided by the authors | Lokomat | Unclear | Not provided by the author | Not provided by the author | Not provided by the author | 1 of 21 | No drop‐outs 2 serious adverse events (1 skin breakdown as a result of therapy, 1 second stroke during the post‐treatment phase) |
1 drop‐out due to protocol violation 2 serious adverse events (1 sudden drop in blood pressure at subject's home leading to brief hospitalisation, 1 sudden chest pain before therapy leading to brief hospitalisation) |
Information as provided by the authors |
| Peurala 2005 | Scandinavian Stroke Scale, 42 points | Gait Trainer | 3 weeks | 25/20 | 5 times a week, 20 minutes for 3 weeks, in addition to rehabilitation treatment | Walking overground; all participants practised gait for 15 sessions over 3 weeks (each session lasting 20 minutes) |
0 of 45 | None | None | Information as published by the authors |
| Peurala 2009 | Not described by the authors | Gait Trainer | 3 weeks | 42/14 | 5 times a week, 20 minutes for 3 weeks, in addition to rehabilitation treatment | Overground walking training; in the other control group, 1 or 2 physiotherapy sessions daily but not at the same intensity as in the other groups | 9 of 56 | 5 drop‐outs (2 worsening the situation after 1 to 2 treatment days; 1 had 2 unsuccessful attempts in device; 1 had scheduling problems; 1 felt protocol too demanding) |
4 drop‐outs (1 felt protocol too demanding; 2 worsening the situation after 1 to 2 treatment days; 1 death) |
Information as published by the authors |
| Pohl 2007 | Mean Barthel Index, 37 points | Gait Trainer | 4 weeks | 124/31 | 5 times a week, 20 minutes | Physiotherapy every weekday for four weeks | 11 of 155 | 2 participants refused therapy, 1 increased cranial pressure, 1 relapsing pancreas tumour, 1 cardiovascular unstable |
4 participants refused therapy, 1 participant died, 1 myocardial infarction | Information as published by the authors |
| Saltuari 2004 | Not described by the authors | Lokomat | 2 weeks | 13/3 | ABA study: in phase A, 5 days a week, 30 minutes | Physiotherapy every weekday for 3 weeks (phase B) | 0 of 16 | None | None | Unpublished and published information provided by the authors |
| Schwartz 2006 | Mean NIHSS, 11 points | Lokomat | 6 weeks | 49/67 | 3 times a week, 30 minutes | Physiotherapy with additional gait training 3 times a week for 6 weeks | 6 of 46 | 2 participants with leg wounds, 1 participant with recurrent stroke, 1 refused therapy |
1 participant with recurrent stroke, 1 with pulmonary embolism |
Unpublished and published information provided by the authors |
| Tanaka 2012 | Mean FIM, 79 points | Gait Master4 | 4 weeks | Not provided by the authors | 2 or 3 times a week, 20 minutes a day (12 sessions) | Non‐intervention (non‐training) | 0 of 12 | ‐ | ‐ | Information as published by the authors |
| Tong 2006 | Mean Barthel Index, 51 points | Gait Trainer | 4 weeks | 39/11 | 5 times a week, 20 minutes | Conventional physiotherapy alone, based on Bobath concept | 4 of 50 | None | 2 participants discharged before study end, 1 participant readmitted to an acute ward, 1 participant deteriorating condition |
Information as published by the authors |
| Van Nunen 2012 | Not described by the author | Lokomat | 8 weeks | Not described by the author | Twice a week, 30 minutes per session | Overground therapy | 0 of 30 | ‐ | ‐ | Unpublished and published information provided by the author |
| Werner 2002 | Mean Barthel Index, 38 points | Gait Trainer | 2 weeks | 13/12 | 5 times a week, 20 minutes | Gait therapy including treadmill training with body weight support | 0 of 30 | None | None | Information as published by the authors |
| Westlake 2009 | Not described by the authors | Lokomat | 4 weeks (12 sessions) | 8/8 | 3 times a week,30 minutes | 12 physiotherapy sessions including manual guided gait training (3 times a week over 4 weeks) | 0 of 16 | None | None | Information as published by the authors |
FIM: Functional Independence Measure
One of the included studies has been published only as an abstract (Mayr 2008), but we obtained at least some results through correspondence with the trial co‐ordinator or principal investigator. Another study is not yet published, but the results of the trial were presented as an oral presentation, and a handout with information about the study was provided by the principal investigator (Aschbacher 2006).
A detailed description of all participant characteristics can be found in Table 6 and Table 7 (see also the Characteristics of included studies). Mean age in the included studies ranged from 48 years (Kyung 2008) to 71 years (Tong 2006; Table 6). More males than females were included (approximately 60% males), as were more participants with ischaemic stroke than haemorrhagic stroke lesions (approximately 70% ischaemic stroke), and almost as many participants with left‐sided hemiparesis compared with participants with right‐sided hemiparesis (approximately 50% left‐sided) were included in the studies (for additional details, see Table 6 and Table 7).
Ten studies provided information about baseline stroke severity (Table 7): four of them used the Barthel Index score, which ranged from 35 Barthel Index points (Husemann 2007) to 75 of 100 Barthel Index points (Dias 2006; Table 7). Details of all inclusion and exclusion criteria used in the studies can be found in the Characteristics of included studies table.
The duration of study intervention (time frame during which experimental interventions were applied) was heterogeneous, ranging from 10 days (Chang 2012) to eight weeks (Mayr 2008). Most studies used a three‐ or four‐week study intervention period (Table 7). Nine of the 23 studies included participants who could walk independently at the start of the study, a further nine studies included participants who were dependent and independent walkers (Analysis 4.1) and five studies included only non‐ambulatory participants (Analysis 4.1). Thirteen studies investigated the robotic‐assisted device 'Lokomat' as the experimental intervention (Table 7), eight studies investigated the electromechanical‐assisted device 'Gait Trainer' (Table 7), one study the 'Gait Master4' (Tanaka 2012) and one study the robotic‐assisted device 'AutoAmbulator' (Fisher 2008).
Analysis 4.1.

Comparison 4 Post‐hoc sensitivity analysis: ambulatory status at study onset, Outcome 1 Recovery of independent walking: ambulatory status at study onset.
Frequency (in terms of therapy provided per week) of treatment ranged from two or three times a week (Tanaka 2012) to five times a week (Table 7). Intensity (in terms of duration of experimental therapy provided) of treatment ranged from 20 minutes (Werner 2002) to 50 minutes (Geroin 2011). In many studies, details of the interventions provided by study authors were not clear; for example, for some studies, details about the intensity of the experimental treatment remain unclear (Table 7). Except for Tanaka 2012, in none of the included studies did the gait training time differ between control and experimental groups. Seven included studies used a follow‐up assessment after study end (Dias 2006; Hidler 2009; Hornby 2008; Peurala 2005; Peurala 2009; Pohl 2007; Schwartz 2006). Most studies investigated improvement in walking function as a primary outcome for analysis and used the Functional Ambulation Category (FAC) or comparable scales to assess independent walking (Characteristics of included studies). Furthermore, frequently investigated outcomes included assessment of walking function using gait velocity in metres per second. A more detailed description of the primary and secondary outcomes for each trial can be found in the Characteristics of included studies table.
The highest drop‐out rates for all reasons at the end of the treatment phase were found to be 23% (Hornby 2008) and 29% (Kyung 2008). Six trialists reported no drop‐outs at scheduled follow‐up (Dias 2006; Fisher 2008;Geroin 2011; Peurala 2005; Werner 2002; Westlake 2009).
Excluded studies
We excluded 12 studies (see Characteristics of excluded studies and Figure 1 for further information).
Ongoing studies and studies awaiting assessment
We identified five ongoing studies (see Characteristics of ongoing studies). Six studies are still awaiting assessment because we were unable to make contact with the trialists (see Characteristics of studies awaiting classification; Globokar 2005; Golyk 2006; Jang 2005; Kim 2001; Koeneman 2004; Mehrberg 2001).
Risk of bias in included studies
We have provided details about methodological quality for each included study in Characteristics of included studies. We wrote to the trialists of all included studies and studies awaiting assessment to request clarification of some design features or missing information to complete the quality ratings. The correspondence was sent via email or letter, and we sent reminders every month if we received no response. Most trialists provided at least some of the requested data, but we did not receive all required data.
Using the 'Risk of bias' assessment tool to assess the adequacy of methods for random sequence generation, allocation concealment, blinding of outcome assessment and incomplete outcome data, two review authors (JM, MP) independently assessed the methodological quality of all included trials except two (Pohl 2007; Werner 2002), which were rated by other review authors (BE and JK) in an interview with the trialists. The review authors discussed all disagreements and sought arbitration by another author (JK or BE) if necessary.
Of the 23 included studies, 13 described adequate random sequence generation, 13 described adequate allocation concealment, seven reported blinding of the primary outcome assessment and nine reported incomplete outcome data (attrition bias). The risk of bias of included studies is described in greater detail in Characteristics of included studies and in Figure 2.
Figure 2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Four out of 23 included trials (Saltuari 2004; Brincks 2011; Tanaka 2012; Werner 2002) used a cross‐over design with random allocation to the order of treatment sequences. We analysed only the first intervention period as a parallel group trial in this review. All other included studies used a parallel group design with true randomisation to group allocation.
Three studies used two experimental groups and one control group (Geroin 2011; Peurala 2005; Tong 2006), and one study used one experimental and two control groups (Peurala 2009). In three of these studies (Geroin 2011; Peurala 2005; Tong 2006), additional functional electrical stimulation of leg muscles (or transcranial stimulation of the brain Geroin 2011) during gait training was applied in one of the treatment groups. Because functional electrical stimulation or transcranial stimulation of the brain was done as an adjunct during electromechanical‐assisted gait training, and because the results in these experimental groups did not differ significantly, we combined the results of both experimental groups into one (collapsed) group and compared this with results from the control group. In one study (Peurala 2009), an electromechanical‐assisted device was used in the experimental group and was compared with two control groups (CT‐group and WALK‐group) that did not use a device. Because we were interested in the effects of electromechanical and robotic‐assisted gait training devices for improving walking after stroke, we combined the results of both control groups without devices together in one (collapsed) group and compared this with results of the CT‐Group.
Effects of interventions
Comparison 1.1: Independent walking at the end of intervention phase, all electromechanical devices used
Twenty‐three trials with a total of 999 participants measured independent walking at study end (Aschbacher 2006; Brincks 2011; Chang 2012; Dias 2006; Fisher 2008; Geroin 2011; Hidler 2009; Hornby 2008; Husemann 2007; Kyung 2008; Mayr 2008; Morone 2011; Noser 2012; Peurala 2005; Peurala 2009; Pohl 2007; Saltuari 2004; Schwartz 2006; Tanaka 2012; Tong 2006; Van Nunen 2012; Werner 2002; Westlake 2009), but for 10 included trials, no effect estimate (OR) was feasible because no events (e.g. no participant reached the ability to walk) or only events (e.g. all participants regained walking) were reported (Deeks 2011) (Analysis 1.1).
Analysis 1.1.

Comparison 1 Electromechanical and robotic‐assisted gait training plus physiotherapy versus physiotherapy (or usual care), Outcome 1 Independent walking at the end of intervention phase, all electromechanical devices used.
The use of electromechanical devices in gait rehabilitation for people after stroke increased the chance of walking independently (OR 2.39, 95% CI 1.67 to 3.43; P < 0.00001; level of heterogeneity I² = 0%). However, nine out of 23 studies investigated at least some participants who were already independent in walking at the start of the study. A further nine studies included participants who were dependent and independent walkers, and five studies included only non‐ambulatory participants (Analysis 4.1).
Of the total population of 999 participants, approximately 45% were independent walkers at the start of the study.
Comparison 1.2: Recovery of independent walking at follow‐up after study end
Five trials with a total of 390 participants measured recovery of independent walking with follow‐up after the study end (Hidler 2009; Hornby 2008; Peurala 2009; Pohl 2007; Tong 2006), but for two included trials (with 125 participants), no effect estimate (OR) was feasible because no events (e.g. no participant reached ability to walk) or only events (e.g. all participants regained walking) were reported (Analysis 1.2). The use of electromechanical devices for gait rehabilitation of people after stroke increased the chance of walking independently at follow‐up after study end (OR 3.16, 95% CI 1.76 to 5.65; P < 0.0001; level of heterogeneity I² = 17%). However, some included trials investigated participants who were already independent in walking at the start of the study. No definitive conclusion can be drawn for a longer‐lasting effect of the use of electromechanical devices.
Analysis 1.2.

Comparison 1 Electromechanical and robotic‐assisted gait training plus physiotherapy versus physiotherapy (or usual care), Outcome 2 Recovery of independent walking at follow‐up after study end.
Comparison 1.3: Walking velocity (metres per second) at the end of intervention phase
Seventeen trials with a total of 690 participants (Analysis 1.3) provided data for walking velocity (metres per second, m/s) at study end. The use of electromechanical devices for gait rehabilitation did not significantly increase the walking velocity. The pooled mean difference (random‐effects model) for walking velocity was 0.04 m/s (95% CI ‐0.03 to 0.11; P = 0.26; level of heterogeneity I² = 73%). Participants who were unable to walk were regarded as having a walking velocity of zero metres per second. No definitive conclusion can be drawn for a longer‐lasting effect of the use of electromechanical devices for walking velocity.
Analysis 1.3.

Comparison 1 Electromechanical and robotic‐assisted gait training plus physiotherapy versus physiotherapy (or usual care), Outcome 3 Walking velocity (metres per second) at the end of intervention phase.
Comparison 1.4: Walking velocity (metres per second) at follow‐up
Six trials with a total of 398 participants provided data for walking velocity (metres per second, m/s) at follow‐up after study end (Hidler 2009; Hornby 2008; Noser 2012; Kyung 2008; Pohl 2007; Tong 2006). The use of electromechanical devices for gait rehabilitation did not significantly increase the walking velocity at follow‐up after study end. The pooled mean difference (random‐effects model) for walking velocity was 0.04 m/s (95% CI ‐0.11 to 0.20; P = 0.59; level of heterogeneity I² = 86%) (Analysis 1.4). Participants who were unable to walk were regarded as having a walking velocity of zero metres per second. No definitive conclusion can be drawn for a longer‐lasting effect of the use of electromechanical devices for walking velocity.
Analysis 1.4.

Comparison 1 Electromechanical and robotic‐assisted gait training plus physiotherapy versus physiotherapy (or usual care), Outcome 4 Walking velocity (metres per second) at follow‐up.
Comparison 1.5: Walking capacity (metres walked in six minutes) at the end of intervention phase
Seven trials with a total of 386 participants provided data for walking capacity (metres walked in six minutes) at study end (Hidler 2009; Hornby 2008;Noser 2012; Peurala 2005; Pohl 2007; Saltuari 2004; Westlake 2009). The use of electromechanical devices in gait rehabilitation did not increase the walking capacity of people after stroke. The pooled mean difference (random‐effects model) for walking capacity was 2.91 metres walked in six minutes (95% CI ‐29.16 to 34.99; P = 0.86; level of heterogeneity I² = 70%) (Analysis 1.5).
Analysis 1.5.
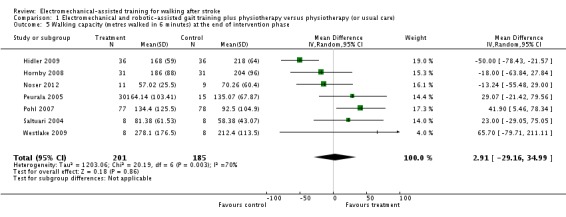
Comparison 1 Electromechanical and robotic‐assisted gait training plus physiotherapy versus physiotherapy (or usual care), Outcome 5 Walking capacity (metres walked in 6 minutes) at the end of intervention phase.
Comparison 1.6: Walking capacity (metres walked in six minutes) at follow‐up
Four trials with a total of 309 participants (Hidler 2009; Hornby 2008; Noser 2012; Pohl 2007) provided data for walking capacity (metres walked in six minutes) at follow‐up after study end. The use of electromechanical devices for gait rehabilitation did not increase the walking capacity at follow‐up after study end for people after stroke. The pooled mean difference (random‐effects model) for walking capacity was ‐8.26 metres walked in six minutes (95% CI ‐54.17 to 37.65; P = 0.72; level of heterogeneity I² = 76%).
Comparison 1.7: Acceptability of electromechanical‐assisted gait training devices during intervention phase: drop‐outs
All trialists provided information about participants who dropped out from all causes during the trial period, but for nine included trials, no effect estimate (OR) was feasible because no events/drop‐outs or only events/drop‐outs were reported (Analysis 1.7). The use of electromechanical devices for gait rehabilitation of non‐ambulatory people after stroke did not increase the risk of participants dropping out (OR (random‐effects model) 0.62, 95% CI 0.33 to 1.15, P = 0.13; level of heterogeneity I² = 38%). The reasons for drop‐outs and all adverse events are described in detail for each trial in Table 7.
Analysis 1.7.

Comparison 1 Electromechanical and robotic‐assisted gait training plus physiotherapy versus physiotherapy (or usual care), Outcome 7 Acceptability of electromechanical‐assisted gait training devices during intervention phase: drop‐outs.
Comparison 1.8: Death from all causes until the end of intervention phase
Only two larger trials reported any deaths during the intervention period (Hidler 2009; Pohl 2007). In Pohl 2007 one participant in the control group died as the result of aspiration pneumonia, and one participant in the treatment group died because of recurrent stroke. In Hidler 2009, the group in which the death occurred was not further described. We therefore used a worst‐case (conservative) scenario and counted the one death for the experimental group. The use of electromechanical devices for gait rehabilitation of non‐ambulatory people after stroke did not increase the risk of participants dying during the intervention period (risk difference (random) 0.00, 95% CI ‐0.02 to 0.02, P = 0.86; level of heterogeneity I² = 0%) (Analysis 1.8).
Analysis 1.8.
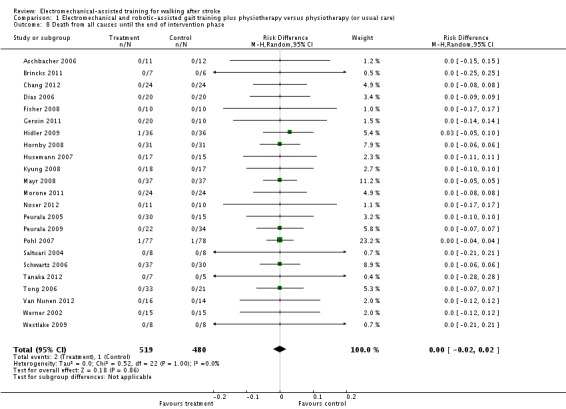
Comparison 1 Electromechanical and robotic‐assisted gait training plus physiotherapy versus physiotherapy (or usual care), Outcome 8 Death from all causes until the end of intervention phase.
Comparison 2: Regaining independent walking ability: planned sensitivity analysis by trial methodology
To examine the robustness of the results, we specified variables in a sensitivity analysis that we believed could influence the size of effect observed (adequate sequence generation process, adequate concealed allocation, blinded assessors for primary outcome, incomplete outcome data and excluding the largest study).
As stated in Comparison 1 above, for some of the included trials, no effect estimate (OR) was feasible (Analysis 2.1).
Analysis 2.1.

Comparison 2 Planned sensitivity analysis by trial methodology, Outcome 1 Regaining independent walking ability.
Studies with adequate sequence generation process
We included 13 trials with a total of 625 participants with an adequate sequence generation process (Figure 2). The use of electromechanical devices for gait rehabilitation of people after stroke increased the chance of walking independently (OR (random‐effects model) 2.12, 95% CI 1.09 to 4.11l P = 0.03; level of heterogeneity, I² = 39%).
Studies with adequate concealed allocation
We included 13 trials with a total of 611 participants with adequate concealed allocation (Figure 2). The use of electromechanical devices for gait rehabilitation of people after stroke increased the chance of walking independently (OR (random‐effects model) 2.21, 95% CI 1.20 to 4.08; P = 0.01; level of heterogeneity, I² = 37%).
Studies with blinded assessors for the primary outcome
Seven trials with a total of 360 participants had blinded assessors for the primary outcome (Figure 2). The use of electromechanical devices for gait rehabilitation of people after stroke increased the chance of walking independently (OR (random) 3.29, 95% CI 1.94 to 5.58, P < 0.00001; level of heterogeneity, I² = 0%).
Studies with complete outcome data
Nine trials with a total of 368 participants adequately described complete outcome data (Figure 2). The use of electromechanical devices for gait rehabilitation of people after stroke increased the chance of walking independently (OR (random) 3.84, 95% CI 2.15 to 6.85; P < 0.00001; level of heterogeneity, I² = 0%).
Excluding the largest study (Pohl 2007)
After excluding the largest study (Pohl 2007), 22 trials with a total of 844 participants remained in this analysis. The use of electromechanical devices for gait rehabilitation of people after stroke increased the chance of walking independently (OR (random) 1.97, 95% CI 1.30 to 3.00; P = 0.001; level of heterogeneity, I² = 0%).
Comparison 3: Subgroup analysis comparing participants in the acute and chronic phases of stroke
Independent walking at the end of intervention phase, all electromechanical devices used
In our planned subgroup analysis comparing independent walking at the end of the intervention phase in people in the acute and chronic phases of stroke, we tried to arrange all included studies in one of two subgroups (acute and chronic phases). Fourteen trials with a total of 718 participants investigated people in the acute or subacute phase defined as less than or equal to three months after stroke (Analysis 3.1).
Analysis 3.1.

Comparison 3 Subgroup analysis comparing patients in acute and chronic phases of stroke, Outcome 1 Independent walking at the end of intervention phase, all electromechanical devices used.
As stated in the comparisons above, for some of the included trials, no effect estimate (OR) was feasible (Analysis 3.1). The use of electromechanical devices for gait rehabilitation of participants after stroke increased the chance of walking independently (OR (random) 2.75, 95% CI 1.86 to 4.08; P < 0.00001; level of heterogeneity, I² = 0%).
Nine trials with a total of 241 participants investigated people in the chronic phase, defined as more than three months after stroke (Analysis 3.1). The use of electromechanical devices for gait rehabilitation of people after stroke did not increase the chance of walking independently (OR (random) 1.20, 95% CI 0.40 to 3.65; P = 0.74; level of heterogeneity, I² = 29%).
In a formal subgroup analysis, we did not find statistically significant differences in regaining independent walking between participants treated in the acute/subacute phase compared with participants treated in the chronic phase after stroke (Chi² = 1.90, df = 1; P = 0.17).
Comparison 4: Post hoc sensitivity analysis by ambulatory status at study onset
Independent walking at the end of intervention phase
To examine the robustness of the results and to explore the relationship between the main effect and walking status at the start of the study, we compared independent walking rates at the end of the intervention phase by ambulatory status at start of study.
Ambulatory participants at start of study
Nine trials, with a total of 340 participants, investigated independent walkers (Analysis 4.1).
As stated in the comparisons above, for some of the included trials, no effect estimate (OR) was feasible (Analysis 4.1), and the conclusions therefore are based on one trial. The use of electromechanical devices for gait rehabilitation of people after stroke did not increase the chance of walking independently (OR (random) 1.38, 95% CI 0.45 to 4.20; P = 0.57; level of heterogeneity, I² = not applicable).
Ambulatory and nonambulatory participants at start of study
Nine trials, with a total of 340 participants, investigated a mixed population of dependent and independent walkers (Analysis 4.1). The use of electromechanical devices for gait rehabilitation of people after stroke did increase the chance of walking independently (OR (random) 1.90, 95% CI 1.11 to 3.25; P = 0.02; level of heterogeneity, I² = 0%).
Nonambulatory participants at start of study
Five trials, with a total of 319 participants, investigated dependent walkers (Analysis 4.1). The use of electromechanical devices for gait rehabilitation of people after stroke did increase the chance of walking independently (OR (random) 3.43, 95% CI 2.00 to 5.86; P < 0.00001; level of heterogeneity, I² = 0%).
In a subgroup analysis, we did not find statistically significant differences in regaining independent walking between people who were dependent or independent walkers at the start of the study (Chi² = 3.36, df = 2; P = 0.19).
Walking speed at the end of the intervention phase
To examine the robustness of the results and to explore the relationship between walking velocity and ambulatory status at the start of the study, we compared achieved walking velocity at the end of the intervention phase by ambulatory status at the start of the study.
Ambulatory participants at start of study
Seven trials, with a total of 225 participants, investigated independent walkers at the start of the study and provided data for walking velocity (metres per second, m/s) at study end (Analysis 4.2). The use of electromechanical devices for gait rehabilitation did not significantly increase the walking velocity. The pooled mean difference (random‐effects model) for walking velocity was ‐0.04 m/s (95% CI ‐0.15 to 0.07; P = 0.45; level of heterogeneity I² = 61%).
Analysis 4.2.

Comparison 4 Post‐hoc sensitivity analysis: ambulatory status at study onset, Outcome 2 Walking velocity: ambulatory status at study onset.
Ambulatory and nonambulatory participants at start of study
Five trials, with a total of 146 participants, investigated dependent and independent walkers at the start of the study and provided data for walking velocity (metres per second, m/s) at study end (Analysis 4.2). The use of electromechanical devices for gait rehabilitation did not significantly increase the walking velocity. The pooled mean difference (random‐effects model) for walking velocity was 0.03 m/s (95% CI ‐0.05 to 0.11; P = 0.44; level of heterogeneity I² = 0%).
Nonambulatory participants at start of study
Five trials, with a total of 319 participants, investigated dependent walkers at the start of the study and provided data for walking velocity (metres per second, m/s) at study end (Analysis 4.2). The use of electromechanical devices for gait rehabilitation did significantly increase the walking velocity. The pooled mean difference (random‐effects model) for walking velocity was 0.12 m/s (95% CI 0.02 to 0.22; P = 0.02; level of heterogeneity I² = 77%).
In a subgroup analysis, we did not find statistically significant differences in regaining independent walking between participants who were dependent or independent walkers at the start of the study (Chi² = 4.63, df = 2; P = 0.10).
Comparison 5: Post hoc sensitivity analysis by type of electromechanical device
Independent walking at the end of intervention phase
To examine the robustness of the results, we compared independent walking rates at the end of the intervention phase as well as the effects between end‐effector devices followed by exoskeleton devices for gait rehabilitation.
End‐effector devices
Nine trials, with a total of 470 participants, used an end‐effector device as the experimental intervention (Table 7). As stated in the comparisons above, for some of the included trials, no effect estimate (OR) was feasible (Analysis 5.1). The use of electromechanical devices for gait rehabilitation of people after stroke increased the chance of walking independently (OR (random) 2.17, 95% CI 1.07 to 4.43; P = 0.03; level of heterogeneity, I² = 48%).
Analysis 5.1.

Comparison 5 Post‐hoc sensitivity analysis: type of device, Outcome 1 Different devices for regaining walking ability between devices.
Exoskeleton devices
Fourteen trials, with a total of 529 participants, used an exoskeleton device as the experimental intervention (Table 7). The use of electromechanical devices for gait rehabilitation of people after stroke increased the chance of walking independently (OR (random) 2.26, 95% CI 1.28 to 3.99; P = 0.005; level of heterogeneity, I² = 0%).
We did not find statistically significant differences in regaining independent walking between participants treated with end‐effector or exoskeleton devices (Chi² = 0.01, df = 1; P = 0.93).
Walking speed at the end of the intervention phase
To examine the robustness of the results, we compared the walking speed at the end of the intervention phase as well as the effects between electromechanical devices used (end‐effector devices versus exoskeleton devices).
End‐effector devices
Seven trials, with a total of 374 participants, used an end‐effector device as the experimental intervention and provided data for walking velocity (metres per second, m/s) at study end (Analysis 4.2). The use of electromechanical devices for gait rehabilitation significantly increased the walking velocity. The pooled mean difference (random‐effects model) for walking velocity was 0.15 m/s (95% CI 0.07 to 0.23; P = 0.003; level of heterogeneity I² = 57%).
Exoskeleton devices
Ten trials, with a total of 316 participants, used an exoskeleton device as the experimental intervention and provided data for walking velocity (metres per second, m/s) at study end (Analysis 4.2). The use of electromechanical devices for gait rehabilitation significantly decreased the walking velocity. The pooled mean difference (random‐effects model) for walking velocity was ‐0.05 m/s (95% CI ‐0.10 to 0.00; P = 0.05; level of heterogeneity I² = 13%).
In a formal subgroup analysis, we found statistically significant differences in improvement in walking velocity between participants treated with an end‐effector device or an exoskeleton device (Chi² = 16.68, df = 1; P < 0.0001).
Discussion
The aim of this review was to evaluate the effects of electromechanical and robotic‐assisted gait training devices (with body weight support) for improving walking after stroke. Our aim was to estimate the likelihood or chance of becoming independent in walking as a result of these interventions, which is a main rehabilitation goal for patients after stroke (Bohannon 1988; Bohannon 1991). We included in this review 23 trials with a total of 999 participants and found evidence that the use of electromechanical‐assisted devices in combination with physiotherapy in rehabilitation settings may improve walking function after stroke. Furthermore, adverse events, drop‐outs and deaths do not appear to be more frequent in participants who received electromechanical or robotic‐assisted gait training. This indicates that the use of electromechanical‐assisted gait training devices was safe and acceptable to most patients included in the trials analysed by this review.
A risk of publication bias is present in all systematic reviews. However, we searched extensively for relevant literature in electronic databases and handsearched conference abstracts. Additionally, we contacted and asked authors, trialists and experts in the field for other unpublished and ongoing trials. Upon visual inspection, we did not detect graphical evidence of publication bias (see Figure 3).
Figure 3.
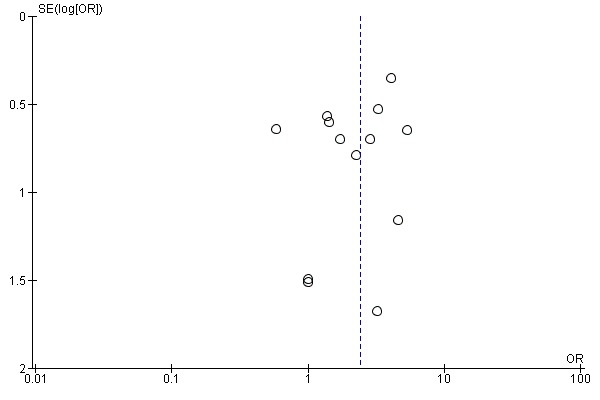
Funnel plot of comparison: 1 Electromechanical‐ and robotic‐assisted gait training plus physiotherapy versus physiotherapy (or usual care), outcome: 1.1 Independent walking at the end of intervention phase, all electromechanical devices used.
Methodological issues
There was heterogeneity between the trials in terms of trial design (two arms, three arms, parallel‐group or cross‐over trial, duration of follow‐up, and selection criteria for participants), characteristics of the therapy interventions (especially duration of the intervention) and participant characteristics (length of time since stroke onset and stroke severity at baseline). Methodological differences in the mechanisms of randomisation and in the allocation concealment methods used were noted, as were blinding of primary outcomes and the presence or use of intention‐to‐treat analysis.
After examining the effects of methodological quality on the odds of independence in walking, we found that the benefits were relatively robust when we removed trials with an inadequate sequence generation process, inadequate concealed allocation, no blinded assessors for the primary outcome and incomplete outcome data (Analysis 2.1). However, we found that the odds of independence in walking were slightly lower after the largest included study (Pohl 2007, N = 155) was removed, but a statistically significant and clinically relevant benefit for participants can still be observed.
Although the methodological quality of the included trials seemed generally moderate (Figure 2), trials investigating electromechanical and robotic‐assisted gait training devices are subject to potential methodological limitations. These limitations include inability to blind the therapist and participants, so‐called contamination (provision of the intervention to the control group) and co‐intervention (when the same therapist unintentionally provides additional care to either treatment or comparison group). All these potential methodological limitations introduce the possibility of performance bias. However, as discussed previously, this was not supported in our sensitivity analyses by methodological quality.
Potential benefit
The exclusion of certain patient groups, such as older people (over 80 years of age), people with unstable cardiovascular conditions, people with cognitive and communication deficits and people with a limited range of motion in the lower limb joints at the start of the intervention, may limit the general applicability of the findings. However, using the results from the primary outcomes, it is possible to explore the apparent effectiveness of electromechanical‐assisted devices for regaining walking ability. Of 519 participants in the treatment group, 299 (58%) were independently walking at the end of the intervention phase. We used the primary outcome of independently walking at the end of the intervention phase for all included patients (OR 2.39) to calculate the number needed to treat to benefit (NNTB). Together with our control event rate of 51% (214 out of 480 control participants were independently walking), we calculated an NNTB of 5 (with a 95% CI 4 to 6) (Sackett 1996). This means that every fifth dependency in walking ability after stroke could be avoidable if electromechanical‐assisted devices are used. However, what remains unclear is the optimum amount of electromechanical‐assisted gait training (optimal frequency, optimal duration in the use of assistive technologies and timing of application).
It appears that people in the acute and subacute phases after stroke profit more than people treated more than three months post‐stroke from this type of therapy (Analysis 3.1). This means that people may benefit more from electromechanical and robotic‐assisted gait training in the first three month after stroke than after three months.
We argue that 444 (44%) of the 999 included participants were independently walking at baseline (see Description of studies and the Characteristics of included studies table). Because people who are already ambulatory cannot regain or recover independent walking, our effect estimate could have been biased by performance bias. We therefore performed two further sensitivity analyses by ambulatory status at the start of the study (Analysis 4.1 and Analysis 4.2).
We found that studies that included mainly dependent walkers (i.e. participants who were non‐ambulatory at the start of the study) were more likely to report that these participants were able to walk at study end (Analysis 4.1) and to reach greater walking velocities at the end of the intervention phase (Analysis 4.2) compared with participants who were already ambulatory at the start of the study. This means that ambulatory people do not benefit from electromechanical and robotic‐assisted gait training.
We found that the ability to walk at study end was not dependent on the type of device used in the studies (Analysis 5.1). However, walking velocities at the end of the intervention phase (Analysis 5.2) were higher when end‐effector devices were used (compared with participants who received training by an exoskeleton device). Furthermore, participants who received gait training by an exoskeleton device had significantly decreased walking velocities at the end of the intervention phase. That means that the type of device used as the intervention could play a role in improving walking function after stroke. This is in line with another review that compared the effects of different types of devices and their effects on walking ability after stroke (Mehrholz 2012a). However, in the absence of a direct empirical comparison between electromechanical‐assisted gait training devices, this point warrants further investigation.
Analysis 5.2.

Comparison 5 Post‐hoc sensitivity analysis: type of device, Outcome 2 Different devices for regaining walking speed.
It is not clear whether the observed differences depend on the intensity of therapy, in terms of repetitions of gait practice. Time devoted to therapy is a crude measure of intensity. A 30‐minute therapy session could include no walking practice or high‐intensity walking practice with lots of steps taken. Reviews of the effectiveness of arm robotic therapy suggest that the positive benefit of robotic therapy may be lost when the intensity of practice is matched between experimental and control groups (Mehrholz 2012b). However, the numbers of repetitions in the experimental and control groups were not exactly counted in any of the included studies. Further studies should therefore ascertain whether the benefits described here are still visible when the intensity of gait practice (e.g. step repetitions) is exactly matched between groups.
It should be mentioned that we do not know yet whether these devices provide any cost benefit. Further studies should investigate, under the premise that gait practice is matched in terms of objective measures of intensity, the long‐term costs of regaining walking ability and the cost‐effectiveness of these devices.
Authors' conclusions
This systematic review provides evidence that the use of electromechanical‐assisted gait training devices in combination with physiotherapy increases the chance of regaining independent walking ability for people after stroke. These results could be interpreted as preventing one participant from remaining dependent in walking after stroke for every five (95% CI 4 to 6) treated. However, this apparent benefit for patients is not supported by our secondary outcomes. Gait training devices were not associated with improvements in walking velocity nor walking capacity. It appears that the greatest benefits with regard to independence in walking and walking speed can be achieved in participants who are non‐ambulatory at the start of the study and in those for whom the intervention is applied early post‐stroke.
There is still a need for well‐designed, large‐scale, multicentre studies to evaluate the benefits and harms of electromechanical‐assisted gait training for walking after stroke, including only non‐ambulatory people in the very early stages after stroke. Currently, comparisons between different devices are also lacking. Future research should include estimates of the costs (or savings) associated with electromechanical gait training. Further analyses should investigate whether non‐ambulatory or ambulatory people benefit most, and trials should include outcome measures in the activities of daily living and quality of life domains. Future updates of this review will consider investigating the effects of different control interventions using subgroup analysis. Additionally, in the next update, the effects of different duration and intensity of treatment (e.g. less than versus more than four weeks; five days per week versus less than five days) will be compared.
Feedback
Feedback, 30 June 2010
Summary
It appears that the P value for the walking capacity outcome is incorrect in your abstract. The P value is reported as P = 0.073 in the abstract but is reported as P = 0.73 in the results section and in the forest plot.
Reply
The feedback from Meghan Malone‐Moses, above, is accurate. I am sorry for this error which occurred in the abstract. The printed P value in the abstract (P = 0.073) was not correct and has now been changed to P = 0.73 as reported correctly in the Results section and in the forest plot. There is no change to the conclusions because the P value for the walking capacity outcome remains non‐significant.
Contributors
Commenter: Meghan Malone‐Moses, MPH, Medical Writer, DynaMed Responder: Jan Mehrholz
Acknowledgements
We thank Brenda Thomas for help with developing the search strategy and for providing us with relevant trials and systematic reviews from CINAHL, AMED, SPORTDISCUS and INSPEC; Hazel Fraser for providing us with relevant information about trials and systematic reviews from the Cochrane Stroke Group Trials Register and Gabi Voigt for conducting research and for providing us with many helpful studies. We thank Stanley Fisher, Carmen Krewer, Jorge Lians, Andreas Mayer, Stefan Hesse, Joseph Hidler, George Hornby, Yun‐Hee Kim, Zeev Meiner, Sinnika Peurala, Leopold Saltuari, Isabella Schwartz, Raymond Tong, John Brincks, Michael van Nunen and Naoki Tanaka for providing additional information or unpublished data about their trials.
Appendices
Appendix 1. MEDLINE, EMBASE, AMED and INSPEC search strategy (via OvidSP)
The following search strategy was used for MEDLINE, EMBASE, AMED and INSPEC and was modified for the other databases.
1. exp cerebrovascular disorders/ or brain injuries/ or brain injury, chronic/ 2. (stroke$ or cva or poststroke or post‐stroke).tw. 3. (cerebrovasc$ or cerebral vascular).tw. 4. (cerebral or cerebellar or brain$ or vertebrobasilar).tw. 5. (infarct$ or isch?emi$ or thrombo$ or emboli$ or apoplexy).tw. 6. 4 and 5 7. (cerebral or brain or subarachnoid).tw. 8. (haemorrhage or hemorrhage or haematoma or hematoma or bleed$).tw. 9. 7 and 8 10. hemiplegia/ or exp paresis/ 11. (hempar$ or hemipleg$ or brain injur$).tw. 12. Gait Disorders, Neurologic/ 13. 1 or 2 or 3 or 6 or 9 or 10 or 11 or 12 14. physical therapy modalities/ or exercise therapy/ or motion therapy, continuous passive/ or musculoskeletal manipulations/ 15. *exercise/ or *exercise test/ 16. robotics/ or automation/ or orthotic devices/ or man‐machine systems/ or self‐help devices/ or therapy, computer‐assisted/ 17. body weight/ or weight‐bearing/ 18. ((gait or locomot$) adj5 (train$ or therapy or rehabilitat$ or re‐educat$ or machine$ or powered or device$)).tw. 19. (electromechanical or electro‐mechanical or mechanical or mechanised or mechanized or driven or assistive device$).tw. 20. ((body‐weight or body weight) adj3 (support$ or relief)).tw. 21. (robot$ or orthos$ or orthotic or automat$ or computer aided or computer assisted or power‐assist$).tw. 22. (bws or harness or treadmill or exercise$ or fitness train$ or Lokomat or Locomat or GaiTrainer or GT1 or Kinetron or Haptic Walker or Anklebot or LOPES or AutoAmbulator).tw. 23. ((continuous passive or cpm) adj3 therap$).tw. 24. or/14‐23 25. gait/ or exp walking/ or locomotion/ 26. "Range of Motion, Articular"/ 27. recovery of function/ 28. (walk$ or gait$ or ambulat$ or mobil$ or locomot$ or balanc$ or stride).tw. 29. 25 or 26 or 27 or 28 30. 13 and 24 and 29 31. Randomized Controlled Trials as Topic/ 32. random allocation/ 33. Controlled Clinical Trials as Topic/ 34. controlgroups/ 35. clinical trials as topic/ or clinical trials, phase i as topic/ or clinical trials, phase ii as topic/ or clinical trials, phase iii as topic/ or clinical trials, phase iv as topic/ 36. double‐blind method/ 37. single‐blind method/ 38. Placebos/ 39. placebo effect/ 40. cross‐over studies/ 41. Therapies, Investigational/ 42. Research Design/ 43. evaluation studies as topic/ 44. randomized controlled trial.pt. 45. controlled clinical trial.pt. 46. (clinical trial or clinical trial phase i or clinical trial phase ii or clinical trial phase iii or clinical trial phase iv).pt. 47. (evaluation studies or comparative study).pt. 48. random$.tw. 49. (controlled adj5 (trial$ or stud$)).tw. 50. (clinical$ adj5 trial$).tw. 51. ((control or treatment or experiment$ or intervention) adj5 (group$ or subject$ or patient$)).tw. 52. (quasi‐random$ or quasi random$ or pseudo‐random$ or pseudo random$).tw. 53. ((multicenter or multicentre or therapeutic) adj5 (trial$ or stud$)).tw. 54. ((control or experiment$ or conservative) adj5 (treatment or therapy or procedure or manage$)).tw. 55. ((singl$ or doubl$ or tripl$ or trebl$) adj5 (blind$ or mask$)).tw. 56. (coin adj5 (flip or flipped or toss$)).tw. 57. versus.tw. 58. (cross‐over or cross over or crossover).tw. 59. placebo$.tw. 60. sham.tw. 61. (assign$ or alternate or allocat$ or counterbalance$ or multiple baseline).tw. 62. or/31‐61 63. 30 and 62 64. exp animals/ not humans.sh. 65. 63 not 64
Number of hits MEDLINE: 1928 Number of hits EMBASE: 2315 Number of hits AMED: 383 Number of hits INSPEC: 81
Appendix 2. CINAHL search strategy (via EBSCOHost)
s1 (MH "CerebrovascularDisorders+") s2 (MM "Brain Injuries") s3 (MH "Brain Damage, Chronic") s4 stroke* or cva or poststroke or post‐stroke s5 cerebrovasc* orcerebralvascular s6 cerebral or cerebellar or brain* or vertebrobasilar s7 infarct* or isch?emi* or thrombo* or emboli* or apoplexy s8 s6 and s7 s9 cerebralorbrainorsubarachnoid s10 haemorrhage or hemorrhage or haematoma or hematoma or bleed* s11 s9 and s10 s12 (MM "Hemiplegia") s13 "paresis" s14 hempar* or hemipleg* or brain injur* s15 (MM "GaitDisorders, Neurologic") s16 s1 or s2 or s3 or s4 or s5 or s8 or s11 or s12 or s13 or s14 or s15 s17 (MM "PhysicalTherapy") s18 (MM "TherapeuticExercise") s19 (MM "Motion Therapy, Continuous Passive") s20 (MM "Manipulation, Chiropractic") OR (MM "Manipulation, Orthopedic") s21 *exerciseor (*exercisetest) s22 (MM "Robotics") s23 (MM "Automation") s24 (MM "Orthoses") s25 "man‐machinesystems" s26 (MM "Assistive Technology Devices") s27 (MM "Therapy, Computer Assisted") s28 (MM "Body Weight") s29 (MM "Weight‐Bearing") s30 ((gait or locomot*) N5 (train* or therapy or rehabilitat* or re‐educat* or machine* or powered or device*)) s31 electromechanical or electro‐mechanical or mechanical or mechanised or mechanized or driven or assistive device* s32 ((body‐weight or body weight) N3 (support* or relief)) s33 robot* or orthos* or orthotic or automat* or computer aided or computer assisted or power‐assist* s34 bws or harness or treadmill or exercise* or fitness train* or Lokomat or Locomat or GaiTrainer or GT1 or Kinetron or Haptic Walker or Anklebot or LOPES or AutoAmbulator s35 (continuous passive or cpm) N3 therap* s36 s17 or s18 or s19 or s20 or s21 or s22 or s23 or s24 or s25 or s26 or s27 or s28 or s29 or s30 or s31 or s32 or s33 or s34 or s35 s37 (MM "Gait") s38 (MH "Walking+") s39 (MM "Locomotion") s40 (MM "Range of Motion") s41 (MM "Recovery") s42 walk* or gait* or ambulat* or mobil* or locomot* or balanc* or stride s43 s37 or s38 or s39 or s40 or s41 or s42 s44 s16 and s36 and s43 s45 (MM "RandomizedControlled Trials") s46 (MM "Random Assignment") s47 (MM "Clinical Trials") s48 (MM "Control Group") s49 (MM "Double‐Blind Studies") s50 (MM "Single‐Blind Studies") s51 (MM "Crossover Design") s52 "Therapies, Investigational" s53 (MM "Study Design") s54 (MM "Evaluation Research") s55 (PT "randomized controlled trial") s56 (PT "controlled clinical trial") s57 (PT "controlledclinicaltrial") s58 (PT "evaluation studies or comparative study") s59 (PT "evaluation studies or comparative study") s60 random* s61 controlled N5 (trial* or stud*) s62 clinical* N5 trial* s63 (control or treatment or experiment* or intervention) N5 (group* or subject* or patient*) s64 quasi‐random* or quasi random* or pseudo‐random* or pseudo random* s65 (multicenter or multicentre or therapeutic) N5 (trial* or stud*) s66 (control or experiment* or conservative) N5 (treatment or therapy or procedure or manage*) s67 (singl* or doubl* or tripl* or trebl*) N5 (blind* or mask*) s68 coin N5 (flip or flipped or toss*) s69 versus s70 (cross‐over or cross over or crossover) s71 assign* or alternate or allocat* or counterbalance* or multiple baseline s72 s45 or s46 or s47 or s48 or s49 or s50 or s51 or s52 or s53 or s54 or s55 or s56 or s57 or s58 or s59 or s60 or s61 or s62 or s63 or s64 or s65 or s66 or s67 or s68 or s69 or s70 or s71 s73 s44 and s72
Number of hits: 787
Appendix 3. CENTRAL search strategy
#1 MeSH descriptor Cerebrovascular Disorders explode all trees #2 MeSH descriptor Brain Injuries, this term only #3 MeSH descriptor Brain Injury, Chronic, this term only #4 stroke* or cva or poststroke or post‐stroke #5 cerebrovasc* orcerebralvascular #6 cerebral or cerebellar or brain* or vertebrobasilar #7 infarct* or isch?emi* or thrombo* or emboli* or apoplexy #8 (#6 AND #7) #9 cerebral or brain or subarachnoid #10 haemorrhage or hemorrhage or haematoma or hematoma or bleed* #11 (#9 AND #10) #12 MeSH descriptor Hemiplegia, this term only #13 MeSH descriptor Paresis explode all trees #14 hempar* or hemipleg* or brain injur* #15 MeSH descriptor Gait Disorders, Neurologic, this term only #16 (#1 OR #2 OR #3 OR #4 OR #5 OR #8 OR #11 OR #12 OR #13 OR #14 OR #15) #17 MeSH descriptor Physical Therapy Modalities, this term only #18 MeSH descriptor Exercise Therapy, this term only #19 MeSH descriptor Motion Therapy, Continuous Passive, this term only #20 MeSH descriptor Musculoskeletal Manipulations, this term only #21 MeSH descriptor Exercise, this term only #22 MeSH descriptor Exercise Test, this term only #23 MeSH descriptor Robotics, this term only #24 MeSH descriptor Automation, this term only #25 MeSH descriptor Orthotic Devices, this term only #26 MeSH descriptor Man‐Machine Systems, this term only #27 MeSH descriptor Self‐Help Devices, this term only #28 MeSH descriptor Therapy, Computer‐Assisted, this term only #29 MeSH descriptor Body Weight, this term only #30 MeSH descriptor Weight‐Bearing, this term only #31 ((gait or locomot*) NEAR/5 (train* or therapy or rehabilitat* or re‐educat* or machine* or powered or device*)) #32 (electromechanical or electro‐mechanical or mechanical or mechanised or mechanized or driven or assistive device*) #33 ((body‐weight or body weight) NEAR/3 (support* or relief)) #34 (robot* or orthos* or orthotic or automat* or computer aided or computer assisted or power‐assist*) #35 (bws or harness or treadmill or exercise* or fitness train* or Lokomat or Locomat or GaiTrainer or GT1 or Kinetron or Haptic Walker or Anklebot or LOPES or AutoAmbulator) #36 (continuous passive or cpm) NEAR/3 therap* #37 (#17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR ( #29 AND or#30 ) OR #31 OR #32 OR #33 OR #34 OR #35 OR #36) #38 MeSH descriptor Gait, this term only #39 MeSH descriptor Walking explode all trees #40 MeSH descriptor Locomotion, this term only #41 MeSH descriptor Range of Motion, Articular, this term only #42 MeSH descriptor Recovery of Function, this term only #43 walk* or gait* or ambulat* or mobil* or locomot* or balanc* or stride #44 (#38 OR #39 OR #40 OR #41 OR #42 OR #43) #45 (#16 AND #37 AND #44) #46 MeSH descriptor Animals explode all trees #47 (#45 AND NOT #46)
Number of hits: 1140
Appendix 4. PEDro search strategy
Abstract&Title: Stroke AND gait Method: Clinical Trial All other fields not mentioned here have been left blank.
Number of Hits: 165
Appendix 5. COMPENDEX search strategy (via DIALOG)
1. CEREBROVASCULAR (W) DISORDER? ?/TI,AB,DE 2. BRAIN (W) INJUR???/TI,AB,DE 3. CHRONIC (W) BRAIN (W) INJUR???/TI,AB,DE 4. (STROKE? OR CVA OR POSTSTROKE? OR CEREBROVASC?)/TI,AB,DE 5. CEREBRAL (W) VASCULAR/TI,AB,DE 6. (CEREBRAL OR CEREBELLAR OR BRAIN? OR 7. (INFARCT? OR ISCHAEMI? OR ISCHEMI? OR THROMBO? OR 8. S6 AND S7 9. (CEREBRAL OR BRAIN OR SUBARACHNOID)/TI,AB,DE 10. (HAEMORRHAGE OR HEMORRHAGE OR HAEMATOMA OR HEMATOMA OR 11. S9 AND S10 12. (HEMIPLEG? OR HEMIPAR? OR PARESIS)/TI,AB,DE 13. BRAIN (W) INJUR???/TI,AB,DE 14. GAIT (W) DISORDER? ?/TI,AB,DE 15. NEUROLOGIC??/TI,AB,DE 16. S14 AND S15 17. S1 OR S2 OR S3 OR S4 OR S5 OR S8 OR S16 OR S11‐S13 18. PHYSICAL (W) THERAPY (W) MODALIT???/TI,AB,DE 19. EXERCISE (W) THERAP?/TI,AB,DE 20. MOTION (W) THERAP? /TI,AB,DE 21. CONTINUOUS (3W) PASSIVE (3W) MOTION (3W) THERAP? 22. EXERCISE?/TI,AB,DE 23. (ROBOTICS OR AUTOMATION) /TI,AB,DE 24. ORTHOTIC (W) DEVICE? ?/TI,AB,DE 25. BODY (W) WEIGHT/TI,AB,DE 26. WEIGHT (W) BEARING/TI,AB,DE 27. GAIT (5N) TRAIN???/TI,AB,DE 28. GAIT (5N) THERAP?/TI,AB,DE 29. GAIT (5N) REHABILITAT?/TI,AB,DE 30. GAIT (5N) EDUCAT?/TI,AB,DE 31. LOCOMOT? (5N) TRAIN?/TI,AB,DE 32. LOCOMOT? (5N) THERAP?/TI,AB,DE 33. LOCOMOT? (5N) REHABILITAT?/TI,AB,DE 34. LOCOMOT? (5N) EDUCAT?/TI,AB,DE 35. (ELECTROMECHANICAL OR MECHANICAL OR MECHANI?ED OR 36. ((BODY (W) WEIGHT (3N) SUPPORT?))/TI,AB,DE 37. ((BODY (W) WEIGHT (3N) RELIEF))/TI,AB,DE 38. (ROBOT? OR ORTHOS? OR ORTHOTIC OR AUTOMAT?)/TI,AB,DE 39. COMPUTER (W) AIDED/TI,AB,DE 40. (COMPUTER (W) ASSISTED)/TI,AB,DE 41. (BWS OR HARNESS OR TREADMILL OR LO?OMAT OR GAITRAINER 42. FITNESS (W) TRAIN?/TI,AB,DE 43. CONTINUOUS (W) PASSIVE/TI,AB,DE 44. THERAP?/TI,AB,DE 45. S43 AND S44 46. CPM (3N) THERAP?/TI,AB,DE 47. S18‐S42 48. S48 OR S45 OR S46 49. (GAIT OR WALKING OR LOCOMOTION)/TI,AB,DE 50. RANGE (1W) MOTION/TI,AB,DE 51. ARTICULAR/TI,AB,DE 52. S51 AND S52 53. RECOVERY (3N) FUNCTION/TI,AB,DE 54. (WALK? OR GAIT? ? OR AMBULAT? OR MOBIL? OR LOCOMOT? OR 55. S50 OR S53 OR S54 OR S55 56. S17 AND S49 AND S56 57. S57 AND HUMAN
Number of Hits: 701
Appendix 6. SportDISCUS search strategy (via EBSCOHost)
S1 .DE "CEREBROVASCULAR disease" OR DE "BRAIN ‐‐ Hemorrhage" OR DE "CEREBRAL embolism & thrombosis" S2 .DE "CEREBROVASCULAR disease ‐‐ Patients" S3 .DE "HEMIPLEGIA" OR DE "HEMIPLEGICS" S4 .TI ( stroke* or poststroke* cva* or cerebrovascular* or cerebral vascular ) OR AB ( stroke* or poststroke* cva* or cerebrovascular* or cerebral vascular ) S5 .TI ( cerebral or cerebellar or brain* or vertebrobasilar ) OR AB ( cerebral or cerebellar or brain* or vertebrobasilar ) S6 .TI ( infarct* or ischemi* or ischaemi* or thrombo* or emboli* or apoplexy ) OR AB ( infarct* or ischemi* or ischaemi* or thrombo* or emboli* or apoplexy ) S7 .S5 and S6 S8 .TI ( cerebral or brain or subarachnoid ) OR AB ( cerebral or brain or subarachnoid ) S9 .TI ( haemorrhage or hemorrhage or haematoma or hematoma or bleed* ) OR AB ( haemorrhage or hemorrhage or haematoma or hematoma or bleed* ) S10 .S8 and S9 S11 .TI ( hempar$ or hemipleg* or brain injur* ) OR AB ( hempar$ or hemipleg* or brain injur* ) S12 .DE "GAIT disorders" S13 .S1 or S2 or S3 or S4 or S7 or S10 or S11 or S12 S14 .DE "PHYSICAL therapy" S15 .DE "EXERCISE" OR DE "LEG exercises" OR DE "STRENGTH training" OR DE "TREADMILL exercise" S16 .DE "EXERCISE therapy" S17 .DE "MANIPULATION (Therapeutics)" S18 .TI ( gait or locomot* ) OR AB ( gait or locomot* ) S19 .TI ( train* or therapy or rehabilitat* or re‐educat* or machine* or powered or device* ) OR AB ( train* or therapy or rehabilitat* or re‐educat* or machine* or powered or device* ) S20 .S18 and S19 S21 .TI ( electromechanical or electro‐mechanical or mechanical or mechanised or mechanized or driven or assistive device* ) OR AB ( electromechanical or electro‐mechanical or mechanical or mechanised or mechanized or driven or assistive device* ) S22 .TI ( body‐weight or body weight ) OR AB ( body‐weight or body weight ) S23 .TI ( support* or relief ) OR AB ( support* or relief ) S24 .S22 and S23 S25 .TI ( robot* or orthos* or orthotic or automat* or computer aided or computer assisted or power‐assist* ) OR AB ( robot* or orthos* or orthotic or automat* or computer aided or computer assisted or power‐assist* ) S26 .TI ( bws or harness or treadmill or exercise* or fitness train* or Lokomat or Locomat or GaiTrainer or GT1 or Kinetron or Haptic Walker or Anklebot or LOPES or AutoAmbulator ) OR AB ( bws or harness or treadmill or exercise* or fitness train* or Lokomat or Locomat or GaiTrainer or GT1 or Kinetron or Haptic Walker or Anklebot or LOPES or AutoAmbulator ) S27 .TI ( continuous passive or cpm ) OR AB ( continuous passive or cpm ) S28 .TI Therapy OR AB therapy S29 .S27 and S28 S30 .S14 or S15 or S16 or S17 or S20 or S21 or S24 or S25 or S26 or S29 S31 .DE "WALKING" OR DE "FITNESS walking" OR DE "GAIT in humans" S32 .DE "LOCOMOTION" OR DE "HUMAN locomotion" S33 .DE "JOINTS ‐‐ Range of motion" S34 .TI ( walk* or gait* or ambulat* or mobil* or locomot* or balanc* or stride ) OR AB ( walk* or gait* or ambulat* or mobil* or locomot* or balanc* or stride ) S35 .S31 or S32 or S33 or S34 S36 .S13 and S30 and S35 S37 .TI ( random* or RCT or trial* or placebo* or sham or double‐blind* or single‐blind or control or controls or assign* or allocat* ) OR AB ( random* or RCT or trial* or placebo* or sham or double‐blind* or single‐blind or control or controls or assign* or allocat* ) S38 .S36 and S37
Number of Hits: 461
Data and analyses
Comparison 1.
Electromechanical and robotic‐assisted gait training plus physiotherapy versus physiotherapy (or usual care)
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Independent walking at the end of intervention phase, all electromechanical devices used | 23 | 999 | Odds Ratio (M‐H, Random, 95% CI) | 2.39 [1.67, 3.43] |
| 2 Recovery of independent walking at follow‐up after study end | 5 | 390 | Odds Ratio (M‐H, Random, 95% CI) | 3.16 [1.76, 5.65] |
| 3 Walking velocity (metres per second) at the end of intervention phase | 17 | 690 | Mean Difference (IV, Random, 95% CI) | 0.04 [‐0.03, 0.11] |
| 4 Walking velocity (metres per second) at follow‐up | 6 | 398 | Mean Difference (IV, Random, 95% CI) | 0.04 [‐0.11, 0.20] |
| 5 Walking capacity (metres walked in 6 minutes) at the end of intervention phase | 7 | 386 | Mean Difference (IV, Random, 95% CI) | 2.91 [‐29.16, 34.99] |
| 6 Walking capacity (metres walked in 6 minutes) at follow‐up | 4 | 309 | Mean Difference (IV, Random, 95% CI) | ‐8.26 [‐54.17, 37.65] |
| 7 Acceptability of electromechanical‐assisted gait training devices during intervention phase: drop‐outs | 23 | 999 | Odds Ratio (M‐H, Random, 95% CI) | 0.62 [0.33, 1.15] |
| 8 Death from all causes until the end of intervention phase | 23 | 999 | Risk Difference (M‐H, Random, 95% CI) | 0.00 [‐0.02, 0.02] |
Analysis 1.6.

Comparison 1 Electromechanical and robotic‐assisted gait training plus physiotherapy versus physiotherapy (or usual care), Outcome 6 Walking capacity (metres walked in 6 minutes) at follow‐up.
Comparison 2.
Planned sensitivity analysis by trial methodology
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Regaining independent walking ability | 23 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 All studies with adequate sequence generation process | 13 | 625 | Odds Ratio (M‐H, Random, 95% CI) | 2.12 [1.09, 4.11] |
| 1.2 All studies with adequate concealed allocation | 13 | 611 | Odds Ratio (M‐H, Random, 95% CI) | 2.21 [1.20, 4.08] |
| 1.3 All studies with blinded assessors for primary outcome | 7 | 360 | Odds Ratio (M‐H, Random, 95% CI) | 3.29 [1.94, 5.58] |
| 1.4 All studies without incomplete outcome data | 9 | 368 | Odds Ratio (M‐H, Random, 95% CI) | 3.84 [2.15, 6.85] |
| 1.5 All studies excluding the largest study Pohl 2007 | 22 | 844 | Odds Ratio (M‐H, Random, 95% CI) | 1.97 [1.30, 3.00] |
Comparison 3.
Subgroup analysis comparing patients in acute and chronic phases of stroke
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Independent walking at the end of intervention phase, all electromechanical devices used | 23 | Odds Ratio (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Acute phase: less than or equal to 3 months after stroke | 14 | 718 | Odds Ratio (IV, Random, 95% CI) | 2.75 [1.86, 4.08] |
| 1.2 Chronic phase: more than 3 months after stroke | 9 | 281 | Odds Ratio (IV, Random, 95% CI) | 1.20 [0.40, 3.65] |
Comparison 4.
Post‐hoc sensitivity analysis: ambulatory status at study onset
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Recovery of independent walking: ambulatory status at study onset | 23 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Studies that included independent walkers | 9 | 340 | Odds Ratio (M‐H, Random, 95% CI) | 1.38 [0.45, 4.20] |
| 1.2 Studies that included dependent and independent walkers | 9 | 340 | Odds Ratio (M‐H, Random, 95% CI) | 1.90 [1.11, 3.25] |
| 1.3 Studies that included dependent walkers | 5 | 319 | Odds Ratio (M‐H, Random, 95% CI) | 3.43 [2.00, 5.86] |
| 2 Walking velocity: ambulatory status at study onset | 17 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Studies that included independent walkers | 7 | 225 | Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.15, 0.07] |
| 2.2 Studies that included dependent and independent walkers | 5 | 146 | Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.05, 0.11] |
| 2.3 Studies that included dependent walkers | 5 | 319 | Mean Difference (IV, Random, 95% CI) | 0.12 [0.02, 0.22] |
Comparison 5.
Post‐hoc sensitivity analysis: type of device
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Different devices for regaining walking ability between devices | 23 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 All studies using end‐effector devices | 9 | 470 | Odds Ratio (M‐H, Random, 95% CI) | 2.17 [1.07, 4.43] |
| 1.2 All studies using exoskeleton devices | 14 | 529 | Odds Ratio (M‐H, Random, 95% CI) | 2.26 [1.28, 3.99] |
| 2 Different devices for regaining walking speed | 17 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 All studies using end‐effector devices | 7 | 374 | Mean Difference (IV, Random, 95% CI) | 0.15 [0.07, 0.23] |
| 2.2 All studies using exoskeleton devices | 10 | 316 | Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.10, ‐.00] |
What's new
Last assessed as up‐to‐date: 23 January 2013.
| Date | Event | Description |
|---|---|---|
| 23 January 2013 | New citation required and conclusions have changed | The conclusions of the review have changed. The previous version of this review concluded that, for the primary outcome (walking), the number needed to treat was six patients to prevent one dependency; this updated version of our review concludes that five patients need to be treated to prevent one dependency in walking. |
| 14 January 2013 | New search has been performed | We have updated the searches to December 2012 and have revised the text as appropriate. We have included 23 trials with 999 participants in this update compared with 17 trials with 837 participants in the previous version of this review from 2009. |
History
Protocol first published: Issue 4, 2006 Review first published: Issue 3, 2007
| Date | Event | Description |
|---|---|---|
| 28 July 2010 | Feedback has been incorporated | Feedback and author response included in the Feedback section, and error corrected in the Abstract. |
| 16 October 2009 | New search has been performed | We have updated the searches to April 2009, and revised the text as appropriate. The conclusions of the review have not changed. We have included 17 trials with 837 participants in this update as compared with eight trials with 414 participants in the last version of this review from 2007. The previous version of this review concluded that, for the primary outcome (walking) the number needed to treat was four patients to prevent one dependency; this updated version of our review concludes that six patients need to be treated to prevent one dependency in walking. |
| 6 August 2008 | Amended | Converted to new review format. |
Differences between protocol and review
In our protocol we stated that we would use the PEDro Scale to assess the methodological quality of the included trials. However, in Chapter 8 of the latest edition of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), it is suggested that scales that yield a summary score should be avoided. In accordance with this suggestion, we no longer used the PEDro Scale to assess the methodological quality of the included trials. However, we used instead the Cochrane risk of bias tool to analyse trial methodology.
In our protocol we planned to quantify heterogeneity with the I² statistic and to use a cut‐off I² = 50% for all comparisons. Additonally, we planned to calculate the overall effects using a random‐effects model instead of a fixed‐effect model when substantial heterogeneity was found. However, In this update, we calculated the overall effects using a random‐effects model, regardless of the level of heterogeneity.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Aschbacher 2006
| Methods | RCT Method of randomisation: not stated Blinding of outcome assessors: stated as 'yes' by the investigator Adverse events: not stated Deaths: not stated Drop‐outs: 4 (1 in treatment group, 3 in control group) ITT: unclear | |
| Participants | Country: Switzerland 23 participants (12 in treatment group, 11 in control group) Not ambulatory at start of study Mean age: 57 to 67 years (control and treatment groups, respectively) Inclusion criteria: ≤ 3 months after stroke, ability to stand or walk 5 metres Exclusion criteria: orthopaedic problems, contractures, NYHA III‐IV | |
| Interventions | 2 arms:
|
|
| Outcomes | Outcomes were recorded at baseline and after 3 weeks and 6 months later Primary outcomes: walking velocity, step length, endurance, walking ability (FAC) Secondary outcomes: isometric knee extension strength, patient acceptance and satisfaction (Visual Analogue Scale) | |
| Notes | Unpublished data | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear |
Brincks 2011
| Methods | Randomised cross‐over trial Method of randomisation: shuffled envelopes Blinding of outcome assessors: no Adverse events: none Deaths: none Drop‐outs: none ITT: yes | |
| Participants | Country: Denmark 13 participants (7 in treatment group, 6 in control group) All participants were ambulatory at start of study Mean age: 59 to 61 years (control and treatment groups, respectively) Inclusion criteria: unknown Exclusion criteria: unknown | |
| Interventions | 2 arms:
|
|
| Outcomes | Outcomes were recorded at baseline, after 3 and 6 weeks later Primary outcomes: single support stance time in impaired extremity and gait asymmetry and swing time ratio Secondary outcomes: walking speed | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Shuffling envelopes |
| Allocation concealment (selection bias) | Low risk | Using sealed, shuffled envelopes |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding was done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT |
Chang 2012
| Methods | RCT Method of randomisation: not stated Blinding of outcome assessors: not stated Adverse events: not stated by the authors Deaths: not stated by the authors Drop‐outs: 3 (2 in experimental group, 1 in control group) ITT analysis: not described | |
| Participants | Country: Republic of Korea 48 allocated participants (24 in treatment group, 24 in control group) 38 participants were non‐ambulatory at start of study Mean age: 58 years Inclusion criteria: first‐ever stroke, stroke onset within 1 month, supratentorial lesion, age > 20 years and < 65 years, not an independent ambulator (FAC < 2) and ability to cooperate during exercise testing Exclusion criteria: people who met criteria for absolute and relative contraindications to exercise testing established by the American College of Sports Medicine (ACSM) were excluded. Also, people who met contraindications for Lokomat therapy or musculoskeletal disease involving the lower limbs, such as severe painful arthritis, osteoporosis, or joint contracture and other neurological diseases, were also excluded | |
| Interventions | 2 arms:
|
|
| Outcomes | Outcomes were recorded at baseline and after training
|
|
| Notes | This study describes the same study protocol and participants as described in the study Kim 2008 but provides some further explanations of participant characteristics; the ID Chang 2012 replaces therefore the ID Kim 2008 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation is unclear |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment is unclear |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear |
Dias 2006
| Methods | RCT Method of randomisation: permuted block randomisation Blinding of outcome assessors: stated as blinded Adverse events: none stated Deaths: none Drop‐outs: none ITT: not stated but probably done because there were no drop‐outs | |
| Participants | Country: Portugal 40 participants (20 in treatment group, 20 in control group) Ambulatory at start of study Mean age: 69 years Inclusion criteria: first‐ever stroke patients > 12 months after stroke; age > 18 and < 80 years; cognitive (Mini Mental State Examination > 19) and communication capacities of understanding the treatment; absence of cardiac, psychological and orthopaedic contraindications Exclusion criteria: not stated | |
| Interventions | 2 arms:
|
|
| Outcomes | Outcomes were recorded at baseline and after 4 weeks and 3 months later
After study end and at follow‐up, participants rated satisfaction with and efficiency of treatment in a self‐questionnaire (Likert scale) |
|
| Notes | Published and unpublished data provided by the authors | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
Fisher 2008
| Methods | RCT Method of randomisation: blocked randomisation Blinding of outcome assessors: stated as 'yes' Adverse events: control group 14; experimental group 11 Deaths: none Drop‐outs: none ITT: stated as 'yes' | |
| Participants | Country: USA 20 participants (10 in treatment group, 10 in control group) Initially 5 in treatment group and 7 in control group were ambulatory at start of study Mean age: not stated Inclusion criteria: subacute, < `2 months after stroke Exclusion criteria: not stated | |
| Interventions | 2 arms:
|
|
| Outcomes | Outcomes were recorded at baseline and after 24 sessions:
|
|
| Notes | This is described as an ongoing trial; the results of the first 20 participants were reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear |
| Allocation concealment (selection bias) | Unclear risk | Stated as concealed, but method not described |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Yes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT stated |
Geroin 2011
| Methods | RCT Method of randomisation: software‐generated randomisation scheme Blinding of outcome assessors: no Adverse events: control group none; experimental groups none Deaths: none Drop‐outs: none ITT: yes | |
| Participants | Country: Italy 30 participants (10 in treatment group 1, 10 in treatment group 2 and 10 in control group) Initially 5 in treatment group and 7 in control group were ambulatory at start of study Mean age: not stated Inclusion criteria: at least 12 months from their first unilateral ischaemic stroke; age < 75 years; European Stroke Scale score between 75 and 85; Mini Mental State Examination score ≧ 24; ability to maintain standing position without aids for at least 5 minutes; ability to walk independently for at least 15 metres with the use of walking aids (cane and orthoses) Exclusion criteria: preceding epileptic fits; an electroencephalography suspect of elevated cortical excitability; metallic implants within the brain and previous brain surgery; medications altering cortical excitability or with a presumed effect on brain plasticity; posterior circulation stroke; deficits of somatic sensation involving the paretic lower limb; presence of vestibular disorders or paroxysmal vertigo; presence of severe cognitive or communicative disorders; presence of other neurological or orthopaedic conditions involving the lower limbs; presence of cardiovascular comorbidity; performance of any type of rehabilitation treatment in the three months before start of study | |
| Interventions | 3 arms:
All participants received ten 50‐minute treatment sessions, 5 days a week, for 2 consecutive weeks |
|
| Outcomes | Outcomes were recorded at baseline and after 2 weeks:
|
|
| Notes | We combined the results of both robotic‐assisted groups (arms 1 and 2) as a single group and compared them with the results of the control group (arm 3) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Software‐generated list |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
Hidler 2009
| Methods | RCT Method of randomisation: randomisation table Blinding of outcome assessors: not described Adverse events: control group 14; experimental group 11 Deaths: 1, but which study arm not reported Drop‐outs: 9 ITT: no, described as analysis per protocol | |
| Participants | Country: USA 72 participants (36 in treatment group, 36 in control group); 63 participants completed all training sessions and were analysed as per protocol Initially all participants were ambulatory at start of study Mean age: 60 years Inclusion criteria: hemiparesis resulting from unilateral ischaemic or haemorrhagic stroke, time since stroke less than 6 months, no prior stroke, age > 18 years, ability to ambulate 5 metres without physical assistance and a self‐selected walking speed between 0.1 and 0.6 m/s, could not be receiving any other physical therapy targeting the lower limbs Exclusion criteria: severe osteoporosis, contractures limiting range of motion in the lower extremities, not ambulating before stroke; severe cardiac disease (New York Heart Association classification of II‐IV), uncontrolled hypertension (systolic > 200 mm Hg, diastolic > 110 mm Hg), stroke of the brainstem or cerebellar lesions, uncontrolled seizures, presence of lower limb non‐healing ulcers, lower limb amputation, uncontrolled diabetes, cognitive deficits (< 24 on the Mini Mental State Examination), symptoms of depression (≥ 16 on the Center for Epidemiological Studies Depression Scale) | |
| Interventions | 2 arms:
|
|
| Outcomes | Outcomes were recorded at baseline and after 12 and 24 sessions, and at 3‐month follow‐up Primary outcome measures: self‐selected walking speed over 5 metres, walking distance in 6 minutes Secondary outcome measures: Berg Balance Scale, FAC, NIH Stroke Scale, Motor Assessment Scale, Rivermead Mobility Index, Frenchay Activities Index, SF‐36 Health Survey, cadence | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation table |
| Allocation concealment (selection bias) | Unclear risk | Not described in sufficient detail |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not done |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No ITT, analysis per protocol |
Hornby 2008
| Methods | RCT Method of randomisation: opaque sealed envelopes Blinding of outcome assessors: not done Adverse events: 8 events in control group and 3 events in experimental group Deaths: none Drop‐outs: 14 (10 in control group and 4 in experimental group) ITT: no ITT; analysis per protocol | |
| Participants | Country: USA 62 participants (31 in treatment group, 31 in control group), 48 participants completed all training sessions and were analysed as per protocol Initially all participants were ambulatory at start of study Mean age: 57 years Inclusion criteria: hemiparesis of longer than 6 months' duration after patients with unilateral, supratentorial, ischaemic or haemorrhage stroke were recruited; no evidence of bilateral or brain stem lesions; able to walk 10 metres overground without physical assistance at speeds of 0.8 m/s at self‐selected velocity, using assistive devices and bracing below the knee as needed Exclusion criteria: significant cardiorespiratory/metabolic disease or other neurological or orthopaedic injury that may limit exercise participation or impair locomotion, size limitations for the harness/counterweight system or robotic orthosis, botulinum toxin therapy in the lower limbs within 6 months before enrolment, scores lower than 23 on the Mini Mental Status Examination, patients could not receive concurrent physical therapy | |
| Interventions | 2 arms:
|
|
| Outcomes | Outcomes were recorded at baseline and after 12 sessions and at 6‐month follow‐up Primary outcome measures: self‐selected walking speed Secondary outcome measures: single‐limb stance time, step length asymmetry, 6‐minute walk test, modified Emory Functional Ambulation Profile, Berg Balance Scale, Frenchay Activities Index, physical component summary score of the Medical Outcomes Questionnaire Short Form 36, strength, Modified Ashworth Scale, Center for Epidemiological Studies‐Depression Scale | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | (Probably) shuffling envelopes |
| Allocation concealment (selection bias) | Low risk | Opaque sealed envelopes |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 'As‐treated' analysis done |
Husemann 2007
| Methods | RCT Method of randomisation: opaque envelopes, stratified by side of paresis and etiology Blinding of outcome assessors: yes Adverse events: 2 (1 in experimental group, 1 in control group) Deaths: none Drop‐outs: 2 (1 in experimental group, 1 in control group) ITT analysis: not provided for all drop‐outs | |
| Participants | Country: Germany 32 participants (17 in treatment group, 15 in control group) Non‐ambulatory at start of study Mean age: not provided by the authors Inclusion criteria: not provided by the authors Exclusion criteria: not provided by the authors | |
| Interventions | 2 arms:
Both groups received additional 30 minutes of physiotherapy daily |
|
| Outcomes | Outcomes were recorded at baseline and after 4 weeks
|
|
| Notes | Published and unpublished data, provided by the authors | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers generated by a computer program, block randomisation |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Evaluating therapist blinded for group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient reporting of attrition/exclusions to permit judgement of ‘Low risk’ or ‘High risk’ |
Kyung 2008
| Methods | RCT Method of randomisation: unclear Blinding of outcome assessors: unclear Adverse events: unclear Deaths: unclear Drop‐outs: 3 (2 in experimental group, 1 in control group) ITT analysis: unclear | |
| Participants | Country: Republic of Korea 35 participants (18 in treatment group, 17 in control group) 10 participants of the experimental group and 7 participants of the control group were ambulatory at start of study Mean age: not stated by the authors Inclusion criteria: not stated by the authors Exclusion criteria: not stated by the authors | |
| Interventions | 2 arms:
|
|
| Outcomes | Outcomes were recorded at baseline and after training
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear |
| Allocation concealment (selection bias) | Unclear risk | Method not described nor stated by the authors |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear |
Mayr 2008
| Methods | RCT Method of randomisation: unclear Blinding of outcome assessors: unclear Adverse events: not stated by the authors Deaths: unclear, probably none Drop‐outs: 3 (2 in experimental group, 1 in control group) ITT analysis: not stated by the authors | |
| Participants | Country: Austria 74 participants (37 in treatment group, 37 in control group) Unclear how many participants in the experimental and control groups were non‐ambulatory at start of study Mean age: not stated by the authors Inclusion criteria: primary ischaemic lesion of the medial cerebral artery, between 10 days and 6 weeks after stroke, stable cardiovascular system, ability to walk with assistance of one therapist Exclusion criteria: brain stem lesions, thrombosis, severe contractures, good walking ability with standing only with help by therapist | |
| Interventions | 2 arms:
|
|
| Outcomes | Outcomes were recorded at baseline and after training phase
|
|
| Notes | This trial is published only as a conference abstract | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear |
Morone 2011
| Methods | RCT Method of randomisation: by computer program Blinding of outcome assessors: stated as 'yes' Adverse events: control group 4; experimental group 3 Deaths: none Drop‐outs: (in this study defined as discontinued intervention) 12 in robotic groups and 9 in control groups ITT: yes | |
| Participants | Country: Italy 48 participants (12 in treatment group 1, 12 in treatment group 2, 12 in control group 1 and 12 in control group 2) All participants were non‐ambulatory at start of study Mean age: 62 years Inclusion criteria: hemiplegia/hemiparesis in the subacute phase with significant gait deficits (FAC < 3) caused by a first‐ever stroke, lesions that were confirmed by computed tomography or magnetic resonance imaging and age between 18 and 80 years Exclusion criteria: presence of subarachnoid haemorrhages, sequelae of prior cerebrovascular accidents or other chronic disabling pathologies, orthopaedic injuries that could impair locomotion, spasticity that limited lower extremity range of motion to less than 80%, sacral skin lesions, Mini Mental State Examination (MMSE) score < 24 and hemispatial neglect, as evaluated by a neuropsychologist |
|
| Interventions | 2 arms (including strata for motor function):
The standard physiotherapy, shared by both groups, was focused on facilitation of movement on the paretic side and upper limb exercises, as well as improving balance, standing, sitting and transferring |
|
| Outcomes | Outcomes were recorded by a physician, blinded to the treatment, at baseline, after 4 weeks of the intervention, and at hospital discharge
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Generated electronically by www.random.org |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Yes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT done; missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups |
Noser 2012
| Methods | RCT Method of randomisation: unclear Blinding of outcome assessors: stated as 'yes' Adverse events: 4 (2 in experimental and 2 in control group) Deaths: none stated Drop‐outs: 1 in the control group (protocol violation) ITT: stated as 'yes' | |
| Participants | Country: USA 21 participants (11 in treatment group, 10 in control group), 20 participants completed all training sessions and were analysed as per protocol All participants were ambulatory at start of study Mean age: unclear Inclusion criteria: people with ischaemic or haemorrhagic stroke confirmed by cerebral CT or MRI scan, age > 18, at least 3 months post‐stroke at time of enrolment into study, ability to walk at least 10 feet with maximum 1 person assist, but not to walk in the community independently; residual paresis in the lower extremity as defined by NIHSS lower extremity motor score 2 to 4, ability to perform Lokomat ambulation training with assistance of 1 therapist, ability to follow a 3‐step command, physician approval for patient participation, ability to give informed consent, completed rehabilitation services (i.e. receiving no concurrent physical, occupational or speech therapy) Exclusion criteria: serious cardiac condition, uncontrolled blood pressure defined as > 200 or diastolic > 100 at rest, history of serious chronic obstructive pulmonary disease or oxygen dependence, severe weight‐bearing pain, lower extremity amputation, claudication while walking, life expectancy < 1 year, history of deep vein thrombosis or pulmonary embolism within 6 months, severe orthopaedic problem, any medical or psychiatric condition that the investigators believe would make the patient unable to participate in this study | |
| Interventions | 2 arms:
|
|
| Outcomes | Outcomes were recorded at baseline and at post‐intervention, 3 months post‐Intervention
|
|
| Notes | NCT00975156 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Yes |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear if reasons for missing outcome data are unlikely to be related to true outcome |
Peurala 2005
| Methods | RCT Method of randomisation: an investigator not involved in the study randomly assigned participants to groups with the help of concealed envelopes Blinding of outcome assessors: no Adverse events: no Deaths: none Drop‐outs: none ITT analysis: not stated | |
| Participants | Country: Finland 45 participants (15 in treatment group A, 15 in treatment group B, 15 in control group) Ambulatory and non‐ambulatory at study onset Mean age: 52 years Inclusion criteria: first supratentorial stroke with duration of illness longer than 6 months, younger than 65 years of age, slow or difficult walking, no unstable cardiovascular disease, no severe malposition of joints, no severe cognitive or communicative disorders, written informed consent Exclusion criteria: not stated | |
| Interventions | 3 arms:
All participants practised gait for 15 sessions over 3 weeks (each session lasting 20 minutes) and received an additional 55 minutes daily physiotherapy |
|
| Outcomes | Outcomes were recorded at baseline, after 2 and 3 weeks and after 6 months
|
|
| Notes | Published and unpublished data provided by the authors | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | An investigator not involved in the study randomly assigned participants to groups with the help of concealed envelopes |
| Allocation concealment (selection bias) | Low risk | Concealed envelopes |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not done |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear if reasons for missing outcome data are unlikely to be related to true outcome |
Peurala 2009
| Methods | RCT Method of randomisation: sealed envelopes (stratified according ability to walk) Blinding of outcome assessors: no Adverse events: 2 in treatment group A, 3 in control group Deaths: 1 in control group Drop‐outs: 5 in treatment group A, 1 in treatment group B, 3 in control group ITT analysis: not stated | |
| Participants | Country: Finland 56 participants (22 in treatment group A, 21 in treatment group B, 13 in control group) Non‐ambulatory at start of study Mean age: 68 years Inclusion criteria: first supratentorial stroke or no significant disturbance from an earlier stroke (Modified Rankin Scale 0 to 2), acute phase after stroke with a maximum duration of 10 days, FAC 0 to 3, voluntary movement in the leg of the affected side, Barthel Index 25 to 75 points, age 18 to 85 years, no unstable cardiovascular disease, body mass index < 32, no severe malposition of joints, no severe cognitive or communicative disorders Exclusion criteria: not stated | |
| Interventions | Between June 2003 and December 2004, random allocation to 2 arms took place (2 walking exercise groups)
All participants received 55 minutes daily gait‐oriented physiotherapy and additional gait training for 15 sessions over 3 weeks (each session lasting maximum of 20 minutes of walking) Between January 2005 and February 2007, random allocation took place to 3 arms (3 walking exercise groups)
All participants received 55 minutes daily gait‐oriented physiotherapy and additional gait training for 15 sessions over 3 weeks (each session lasting maximum of 20 minutes of walking). However, CT‐Group received 1 or 2 physiotherapy sessions daily but not at the same intensity as in the other groups |
|
| Outcomes | Outcomes were recorded at baseline, after 3 weeks and after 6 months
|
|
| Notes | Because we were interested in the effects of automated electromechanical and robotic‐assisted gait training devices for improving walking after stroke, we combined the results of the control group and the WALK group as one group and compared them with results from the GT‐Group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | An investigator not involved in the study randomly assigned participants to groups with the help of concealed envelopes |
| Allocation concealment (selection bias) | Low risk | Allocation was performed by an independent person not otherwise involved with participants |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear whether reasons for missing outcome data are unlikely to be related to true outcome |
Pohl 2007
| Methods | RCT Method of randomisation: lots, indicating A or B, had been prepared in sealed envelopes Blinding of outcome assessors: primary outcomes were evaluated by blinded assessors Adverse events: 4 (3 in experimental group, 1 in control group) Deaths: 2 (1 in experimental group, 1 in control group) Drop‐outs: 11 (5 in experimental group, 6 in control group) ITT analysis: yes | |
| Participants | Country: Germany 155 participants (77 in treatment group, 78 in control group) Non‐ambulatory at study onset Mean age: 63 years Inclusion criteria: first supratentorial stroke (ischaemic or haemorrhagic), age between 18 and 79 years, interval between stroke and study onset less than 60 days, able to sit unsupported (i.e. without holding onto supports such as the edge of the bed), with feet supported, could not walk at all, or required the help of 1 or 2 therapists irrespective of the use of an ankle‐foot orthosis or a walking aid (FAC 3 or less), understanding the meaning of the study and following instructions, providing written informed consent to participation in the study approved by the local ethical committee Exclusion criteria: unstable cardiovascular condition after a 12‐lead electrocardiogram examined by a cardiologist, restricted passive range of motion in the major lower limb joints (extension deficit > 20° for the affected hip or knee joints, or a dorsiflexion deficit > 20° for the affected ankle, tested while lying supine and on the non‐affected side, prevalence of other neurological or orthopaedic diseases impairing walking ability | |
| Interventions | 2 arms:
|
|
| Outcomes | Outcomes were recorded at baseline, after 4 weeks and after 6 months Primary outcomes:
Responders to therapy were defined as ambulatory (Functional Ambulation Category 4 or 5) or reaching a Barthel Index > 75 Secondary outcomes:
|
|
| Notes | Published and unpublished data provided by the authors | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Lots, indicating A or B, had been prepared in sealed envelopes, a person not involved in the study allocated participants to groups with the help of concealed envelopes |
| Allocation concealment (selection bias) | Low risk | Concealed envelopes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded primary outcomes (a person not involved in the study rated video tapes of participants) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis done; missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups |
Saltuari 2004
| Methods | Cross‐over RCT Method of randomisation: by random numbers Blinding of outcome assessors: unclear Adverse events: none Deaths: none Drop‐outs: none ITT: yes | |
| Participants | Country: Austria 16 participants (8 in treatment group, 8 control group) Ambulatory and non‐ambulatory at study onset Mean age: 61 years Inclusion criteria: not provided by the authors Exclusion criteria: not provided by the authors | |
| Interventions | 2 arms (A: Lokomat, B: physiotherapy):
|
|
| Outcomes | Outcomes were recorded at baseline and after 3 weeks (additionally after 6 and 9 weeks, but only outcomes of the first phase were included in this review) | |
| Notes | Unpublished data provided by the authors | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | By random numbers |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
Schwartz 2006
| Methods | RCT Method of randomisation: block sampling method (each block contained 6 participants: 4 experimental group and 2 control group) Blinding of outcome assessors: no Adverse events: 5 (3 in experimental group, 2 in control group) Deaths: none Drop‐outs: 11 (8 in experimental group, 3 in control group) ITT: no (stated, but 2 participants from the control group were excluded from analysis) | |
| Participants | Country: Israel 67 participants (at October 2006) (37 in treatment group, 30 in control group) Non‐ambulatory at study onset Mean age: 60 years Inclusion criteria: first stroke, until 3 months after stroke Exclusion criteria: not provided by the authors | |
| Interventions | 2 arms:
|
|
| Outcomes | Outcomes were recorded at baseline and after 3, 6 and 9 weeks
|
|
| Notes | Published and unpublished data provided by the authors | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Block sampling |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not done |
| Incomplete outcome data (attrition bias) All outcomes | High risk | For dichotomous outcome data, the proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias into intervention effect estimate |
Tanaka 2012
| Methods | Cross‐over RCT Method of randomisation: computer‐generated randomisation Blinding of outcome assessors: no Adverse events: none described Deaths: none Drop‐outs: none ITT: no | |
| Participants | Country: Japan 12 participants (7 in treatment group, 5 in control group) All were ambulatory at study onset Mean age: 62 years Inclusion criteria: first stroke; more than six months had passed since stroke onset; slight to moderate motor deficit (Brunnstrom recovery stages III–VI) and could walk with or without walking aids Exclusion criteria: a higher brain function disorder or cognitive deficit affecting ability to understand and describe symptoms (< 24 on the Mini Mental State Examination), severe heart disorder affecting gait movement intensity or severe bone and joint disease affecting gait movement |
|
| Interventions | 2 arms (only the first 12 weeks before cross‐over are described here; A: no training, B: gait training with Gait Master 4, 1 session: 20 minutes):
|
|
| Outcomes | Outcomes were recorded weekly over a 24 week period:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear |
Tong 2006
| Methods | RCT Randomisation was done by computer‐generated random numbers Blinding of outcome assessors: no (except for Barthel Index and Functional Independence Measure Scores, which were performed by nurses who were blinded in this study) Adverse events: 2 (none in experimental group, 2 in control group) Deaths: none Drop‐outs: 4 (none in experimental group, 4 in control group) ITT: yes | |
| Participants | Country: Hong Kong, China 50 participants (15 in treatment group A, 15 in treatment group B, 20 in control group) Non‐ambulatory at study onset Mean age: 68 years Inclusion criteria: diagnosis of first ischaemic brain injury or intracerebral haemorrhage shown by magnetic resonance imaging or computed tomography less than 6 weeks after onset of stroke; sufficient cognition to follow simple instructions and understand the content and purpose of the study (Mini Mental State Examination score > 21); ability to stand upright, supported or unsupported, for 1 minute; significant gait deficit (FAC score < 3); no skin allergy to electrical stimulation Exclusion criteria: recurrent stroke; other neurological, medical or psychological deficit or condition that would affect ambulation ability or compliance with study protocol (such as Parkinson's disease, major depression, pain, cardiac arrhythmias); aphasia with an inability to follow 2 consecutive step commands or a cognitive deficit; or severe hip, knee, or ankle contracture that would preclude passive range of motion of the leg | |
| Interventions | 3 arms:
The study consisted of 1 training session per weekday for 4 weeks Experimental groups (1) and (2) underwent gait training for 20 minutes, with body weight support by an electromechanical gait trainer; Group (2) also received functional electrical stimulation to the paretic lower limb during gait training Participants in Group (3) received physiotherapy overground gait training based on the principles of proprioceptive neuromuscular facilitation and Bobath concepts |
|
| Outcomes |
|
|
| Notes | Published and unpublished data provided by the authors | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers |
| Allocation concealment (selection bias) | Low risk | Concealed |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Yes for primary outcome |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | ITT done; unclear |
Van Nunen 2012
| Methods | RCT Method of randomisation: stated as block randomisation Blinding of outcome assessors: no Adverse events: none described Deaths: none Drop‐outs: none ITT: unknown | |
| Participants | Country: Hong Kong, China 50 participants (15 in treatment group A, 15 in treatment group B, 20 in control group) Non‐ambulatory at study onset (but baseline FAC ≧ 3: intervention group: 3 out of 16 , control group: 2 out of 14) Mean age: 53 years Inclusion criteria: unknown Exclusion criteria: unknown |
|
| Interventions | 2 arms
|
|
| Outcomes | Outcomes were assessed at baseline and after 8 weeks:
|
|
| Notes | Published and unpublished data provided by the authors | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear |
| Allocation concealment (selection bias) | Low risk | A person not involved in the study was asked to draw one of the opaque envelopes in which group assignment was established each time a new participant entered the study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear |
Werner 2002
| Methods | Cross‐over RCT Method of randomisation: participants randomly assigned to groups (group allocation in envelopes that were drawn by an independent person) Blinding of outcome assessors: yes Adverse events: none Deaths: none Drop‐outs: none ITT: yes | |
| Participants | Country: Germany 30 participants (15 in treatment group, 15 in control group) Non‐ambulatory at study onset Mean age: 60 years Inclusion criteria: first stroke, supratentorial lesion 4 to 12 weeks post‐stroke, younger than 75 years of age, not able to walk (Functional Ambulation Category of 2 or less), able to sit unsupported on the edge of a bed, able to stand for at least 10 seconds with help, able to provide and did provide written informed consent Exclusion criteria: hip and knee extension deficit > 20 degrees; passive dorsiflexion of the affected ankle to less than a neutral position; severe impairment of cognition or communication; evidence of cardiac ischaemia, arrhythmia, decompression or heart failure; feeling of 'overexertion' or heart rate exceeding the age‐predicted maximum (i.e. 190 beats/minute minus age) during training; resting systolic blood pressure exceeding 200 mm Hg at rest or dropping by more than 10 mm Hg with increasing workload | |
| Interventions | 2 arms:
Treated as inpatients for 5 15‐ to 20‐minute sessions per week for 2 weeks A: treadmill training with body weight support: participants walked on a treadmill with partial body weight support provided by a harness B: gait trainer with body weight support: participants walked on a Gait Trainer with partial body weight support provided by a harness |
|
| Outcomes | Outcomes were recorded at baseline and after 2 weeks (additionally after 4 and 6 weeks, but only the first phase was included in this review):
|
|
| Notes | We used the first treatment phase only Published and unpublished data provided by the authors 0% drop‐outs at the end of first treatment phase (data were analysed as ITT) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | By envelopes |
| Allocation concealment (selection bias) | Low risk | Concealed envelopes that were drawn by an independent person |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Yes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
Westlake 2009
| Methods | RCT Method of randomisation: computer‐generated random order (stratified by fast or slow walking) Blinding of outcome assessors: not described Adverse events: 1 in control group Deaths: none Drop‐outs: none ITT: yes | |
| Participants | Country: USA 16 participants (8 in treatment group, 8 in control group) All were ambulatory at study onset Mean age: 57 years Inclusion criteria: hemiparesis resulting from a single cortical or subcortical stroke > 6 months before the study, categorised as at least unlimited household ambulatory, written informed consent Exclusion criteria: unstable cardiovascular, orthopaedic, or neurological conditions; uncontrolled diabetes that would preclude exercise of moderate intensity; significant cognitive impairment affecting ability to follow directions | |
| Interventions | 2 arms:
|
|
| Outcomes | Outcomes were recorded at, before and after 4 weeks
|
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear |
| Allocation concealment (selection bias) | Low risk | Randomisation list was overseen by one of the investigators, who had no contact with participants until group assignment was revealed. Group assignment was not revealed to study personnel until the participant consented and baseline testing was complete |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
CT: computerised tomography FAC: Functional Ambulation Category ITT: intention‐to‐treat MRI: magnetic resonance imaging NIHSS: National Institutes of Health Stroke Scale NYHA: New York Heart Association RCT: randomised controlled trial
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Caldwell 2000 | Did not investigate electromechanical and robotic‐assisted gait training devices as stated in the protocol of this review: Bicycle training versus treadmill walking versus variable surface training were investigated |
| David 2006 | Did not meet inclusion criteria of this review: not an RCT |
| Gong 2003 | Did not investigate electromechanical and robotic‐assisted gait training devices as stated in the protocol of this review: no electromechanical‐assisted devices were compared |
| Hesse 2001 | Did not meet inclusion criteria of this review: not an RCT |
| Mirelman 2009 | Did not meet inclusion criteria of this review: experimental and control groups received a kind of assisted stepping therapy in a seated position This study investigated the effects of virtual reality as an adjunct to stepping training After discussion, we reached consensus to exclude this study from our review |
| Page 2008 | Did not meet inclusion criteria of this review: the experimental group received a kind of assisted stepping therapy in a seated position This study investigated the effect of the NuStep apparatus After discussion, we reached consensus to exclude this study from our review |
| Patten 2006 | According to the information in ClinicalTrials.gov (NCT00125619), a 1‐arm, non‐randomised trial |
| Pitkanen 2002 | Did not meet inclusion criteria of this review: The study describes preliminary findings of an initial sample of 9 participants, the experimental group received treadmill training or gait training |
| Richards 1993 | Did not meet inclusion criteria of this review: The experimental group received a specialised locomotor training including early intensive physiotherapy with tilt table, limb load monitor, resistance exercises and treadmills to promote functional recovery After discussion, the review authors reached consensus to exclude this study |
| Richards 2004 | Did not meet inclusion criteria of this review: The experimental group received specialised locomotor training including early intensive physiotherapy with tilt table, limb load monitor, resistance exercises and treadmills to promote functional recovery After discussion, the review authors reached consensus to exclude this study |
| Shirakawa 2001 | Not an RCT |
| Skvortsova 2008 | Not an RCT, control groups were age and sex matched |
RCT: randomised controlled trial
Characteristics of studies awaiting assessment [ordered by study ID]
Globokar 2005
| Methods | Probably an RCT |
| Participants | People after stroke, unclear how many |
| Interventions | 2 arms:
|
| Outcomes | Unclear |
| Notes | This study was presented at the 5th World Congress of Physical Medicine and Rehabilitation |
Golyk 2006
| Methods | Probably an RCT |
| Participants | Unclear |
| Interventions | Unclear |
| Outcomes | Unclear |
| Notes | — |
Jang 2005
| Methods | Probably an RCT |
| Participants | 34 non‐ambulatory stroke survivors |
| Interventions | 2 arms:
|
| Outcomes | Unclear |
| Notes | This study was presented at the 5th World Congress of Physical Medicine and Rehabilitation |
Kim 2001
| Methods | Probably an RCT |
| Participants | Unclear |
| Interventions | Unclear |
| Outcomes | Unclear |
| Notes | Study was found in the Proceedings of the 1st International Congress of International Society of Physical and Rehabilitation Medicine (ISPRM), 7‐13 July 2001 |
Koeneman 2004
| Methods | Probably an RCT |
| Participants | Unclear |
| Interventions | Unclear |
| Outcomes | Unclear |
| Notes | — |
Mehrberg 2001
| Methods | Probably an RCT |
| Participants | Unclear |
| Interventions | Unclear |
| Outcomes | Unclear |
| Notes | — |
RCT: randomised controlled trial
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Efficacy of a mechanical gait repetitive training technique compared with a usual rehabilitation program on gait recovery in hemiparetic stroke patients |
| Methods | RCT with 2 arms |
| Participants | Country: France Inclusion criteria: men or women aged 18 years or older; hemiplegia secondary to stroke; interval between stroke and study inclusion of 2 months or less; first‐time supratentorial stroke; non‐ambulatory patient (FAC Stage 0); able to sit unsupported at the edge of the bed; no severe impairment of cognition or communication; written informed consent provided Exclusion criteria: orthopaedic or rheumatological disease impairing mobility, or both; other neurologically associated disease; history of myocardial infarction or deep venous embolism or pulmonary embolism within 3 months before study inclusion; chronic pulmonary disease; intolerance to standing up |
| Interventions | 4‐Week rehabilitation programme associating physiotherapy and gait trainer therapy or physiotherapy alone |
| Outcomes | Primary outcomes: walking speed (time needed to walk 10 metres) after the 4‐week rehabilitation programme Secondary outcomes: FAC; walking endurance (6‐minute walk); time to self‐sufficient gait recovery; spasticity (modified Ashworth score); Motricity Index; need for mobility and self‐assistance (Barthel score, PMSI‐SSR scores, need for physical assistance); economic evaluation (healthcare requirements, rehabilitation unit length of stay) |
| Starting date | March 2006 |
| Contact information | Principal investigator: Régine Brissot, MD, Service de Médecine Physique et Réadaptation, Hôpital Pontchaillou, Rennes, 35033, France Tel: +33 2 9928 4219 email: regine.brissot@chu‐rennes.fr |
| Notes | Expected total enrolment: 122 participants Sponsored by: Rennes University Hospital Information derived from: ClinicalTrials.gov Identifier: NCT00284115 |
| Trial name or title | Comparative study of GangTrainer GT1, Lokomat and conventional physiotherapy (GALOP) |
| Methods | RCT with 3 arms |
| Participants | Inclusion criteria: first supratentorial stroke (ischaemic, haemorrhagic or intracerebral haemorrhage) resulting in hemiparesis; interval from stroke: 3 to 12 weeks; non‐ambulatory (FAC < 3); free sitting on bedside for 1 minute, with both feet on the floor and holding onto bedside by hands, Barthel Index 25 to 65 Exclusion criteria: unstable cardiovascular system (in case of doubt, only after approval by a internist); manifested heart diseases like labile compensated cardiac insufficiency (NYHA III), angina pectoris, myocardial infarction 120 days before study onset, cardiomyopathy, severe cardiac arrhythmia, severe joint misalignment (severe constriction of movement for hip, knee or ankle, or any combination of the 3: more than 20° fixed hip and knee extension deficit, or more than 20° fixed plantar flexion of the ankle); severe cognitive dysfunction, which does not allow for comprehension of the aims of this study; severe neurological or orthopaedic diseases (like polio, Parkinson´s disease), which massively affect mobility; deep vein thrombosis; severe osteoporosis; or malignant tumour disease |
| Interventions | Group A: 30 minutes of treatment on the GangTrainer GT1 and 30 minutes of conventional physiotherapy every workday for 8 weeks Group B: 30 minutes of treatment on the Lokomat and 30 minutes of conventional physiotherapy every workday for 8 weeks Group C: 60 minutes of conventional physiotherapy every workday for 8 weeks |
| Outcomes | Primary outcomes: FAC, modified Emory Functional Ambulation Secondary outcomes: Barthel Index, 10‐metre walk test, 6‐minute walk test on the floor, Medical Research Council, Rivermead Visual Gait Assessment (RVGA), EuroQol 5 Dimensions (EQ‐5D) |
| Starting date | August 2010 |
| Contact information | Contact: Andreas Waldner, MD; +39 0471 471 471; waldner.andreas@villamelitta.it Contact: Christopher Tomelleri, MSc; +39 0471 471 471; christopher.tomelleri@villamelitta.it |
| Notes | Estimated enrolment: 120 Estimated study completion date: August 2013 Estimated primary completion date: August 2013 (final data collection date for primary outcome measure) |
| Trial name or title | A randomized controlled trial on hemiplegic gait rehabilitation: robotic locomotor training versus conventional training in subacute stroke |
| Methods | RCT with 2 arms |
| Participants | Country: Thailand Inclusion criteria: subacute first‐time stroke patients (haemorrhage and ischaemic), age 18 to 80 years, impaired FAC at initial score 0 to 2, cardiovascular stable, given signed informed consent Exclusion criteria: unstable general medical condition, severe malposition or fixed contracture of joint with an extension deficit > 30 degrees, any functional impairment before stroke, cannot adequately cooperate in training, severe communication problems, severe cognitive‐perceptual deficits |
| Interventions | Group A: conventional therapy means: 50 minutes individual physiotherapy and 60 minutes individual occupational therapy per workday (5 times per week) for 4 consecutive weeks Group B: conventional therapy plus robot‐assisted means: 30 minutes individual physiotherapy plus 20 minutes robotic‐assisted gait training (with Gait Trainer GT1) and 60 minutes individual occupational therapy per workday (5 times per week) for 4 consecutive weeks |
| Outcomes | Primary outcomes: FAC 0 to 5 and Barthel Index 0 to 100 Secondary outcome: Berg Balance Scale 0 to 56, REPAS‐Muscle tone 0 to 52 |
| Starting date | January 2011 |
| Contact information | Principal Investigator: Ratanapat Chanubol, MD, Rehabilitation Department, Prasat Neurological Institute, Mahidol University |
| Notes | Study Completion Date: July 2012 Enrollment: 60 |
| Trial name or title | Ankle robotics training after stroke: effects on gait and balance |
| Methods | RCT with 3 arms |
| Participants | Inclusion criteria: ischaemic or haemorrhagic stroke > 6 months prior in men or women aged 18 to 80 years, clear indications of hemiparetic gait by clinical observation, completed all conventional physical therapy, ability to walk on a treadmill with handrail support Exclusion criteria: cardiac history of (1) unstable angina, (2) recent (< 3 months) myocardial infarction, congestive heart failure (NYHA category II), (3) haemodynamically significant valvular dysfunction Major clinical depression: CESD score > 16 and judgment of clinical depression Medical history: (1) recent hospitalisation (< 3 months) for severe medical disease, (2) symptomatic peripheral arterial occlusive disease, (3) orthopaedic or chronic pain conditions that significantly alter gait function, (4) pulmonary or renal failure, (5) active cancer History of non‐stroke neuromuscular disorder restricting gait Aphasia or cognitive functioning that confounds participation, defined as unable to follow 2‐step commands. The Mini Mental State Examination will be administered with a cut‐off of < 23 (< 17 if education level at or below 8th grade), or judgment of the medical officer Hypertension that is a contraindication for a bout of treadmill training (greater than 160/100 on 2 assessments) Self‐report of pregnancy |
| Interventions | Experimental: Arm 1: seated robot training group. Participants at least 6 months post‐stroke will use the ankle robot in a seated visuo‐motor training paradigm. They will train on the robot 3 times per week for 6 weeks (18 sessions) by playing video games with the paretic ankle. They will be evaluated on outcomes at baseline, post‐6 weeks training and again after a 6‐week retention period with no training Experimental: Arm 2: treadmill training with ankle robot group. Participants at least 6 months post‐stroke will wear the ankle robot during treadmill locomotor training. They will walk on a treadmill with the ankle robot adjusted to promote paretic ankle engagement during 3 × weekly training sessions over 6 weeks (18 sessions). They will be evaluated on outcomes at baseline, post‐6 weeks training and again after a 6‐week retention period with no training Active comparator: Arm 3: treadmill‐only group. This group will consist of participants at least 6 months post‐stroke who engage in treadmill training 3 × weekly for 6 weeks without robotic support. They will be volunteers from another treadmill training study and will be evaluated on outcomes at baseline and post‐6 weeks training. They will not receive retention testing at 12 weeks because they will be continuing with regular treadmill training beyond the 6‐week period |
| Outcomes | Primary outcomes: self‐selected floor walking velocity, velocity and associated spatio‐temporal gait parameters from self‐selected; most comfortable and fastest floor walking over 10 metres Secondary outcomes: gait kinetics, anterior‐posterior and medio‐lateral ground reaction forces during walking to assess propulsive impulses from paretic and non‐paretic sides, Berg Balance Scale, Dynamic Gait Index, Anticipatory Postural Adjustments |
| Starting date | July 2011 |
| Contact information | Contact: Larry Forrester, PhD Larry.Forrester@va.gov |
| Notes | Estimated primary completion date: March 2013 (final data collection date for primary outcome measure) |
| Trial name or title | Robot walking rehabilitation in stroke patients |
| Methods | RCT with 3 arms |
| Participants | Inclusion criteria: between the ages of 18 and 95 years, able to walk 25 feet unassisted or with assistance, first acute event of cerebrovascular stroke, unilateral paresis, ability to understand and follow simple instructions, ability to walk without assistance before stroke, endurance sufficient to stand at least 20 minutes unassisted per participant report Exclusion criteria: unable to understand instructions required by the study (Informed Consent Test of Comprehension), medical or neurological comorbidities that might contribute to significant gait dysfunction, uncontrolled hypertension > 190/110 mm Hg, significant symptoms of orthostasis when standing up, circulatory problems, history of vascular claudication or significant (+ 3) pitting edema, lower extremity injuries or joint problems (hip or leg) that limit range of motion or function or cause pain with movement, bilateral impairment, severe sensory deficits in the paretic upper limb, cognitive impairment or behavioral dysfunction that would influence the ability to comprehend or participate in the study, women who are pregnant or lactating or both |
| Interventions | Experimental group: Robot G‐EO: each participant will be asked to perform 15 sessions (3 to 5 days a week for 4 up to 5 weeks) consisting of a treatment cycle using the GE‐O system device, according to individually tailored exercise scheduling Control group: Treadmill training: each participant will be asked to perform 15 sessions (3 to 5 days a week for 4 up to 5 weeks) consisting of a treatment cycle using the treadmill system device, according to individually tailored exercise scheduling Control group: Ground treatment: Ground Control Group (cCG): each participant will be asked to perform 15 sessions (3 to 5 days a week for 4 up to 5 weeks) of traditional lower limb physiotherapy |
| Outcomes | |
| Starting date | September 2012 |
| Contact information | Contact: Patrizio Sale, MD; patrizio.sale@gmail.com Contact: Marco Franceschini, MD; marco.franceschini@sanraffaele.it |
| Notes | Estimated enrolment: 90 Estimated study completion date: September 2015 Estimated primary completion date: August 2014 (final data collection date for primary outcome measure) |
FAC: Functional Ambulation Category RCT: randomised controlled trial
Contributions of authors
Jan Mehrholz (JM) contributed to the conception and to the design of the protocol and drafted the protocol. He searched electronic databases and conference proceedings, screened titles and abstracts of references identified by the search, selected and assessed trials, extracted trial and outcome data, guided the analysis and the interpretation of data and contributed to and approved the final manuscript of the review.
Joachim Kugler (JK) assessed and extracted trial and outcome data, assessed the methodological quality of selected trials, contributed to the interpretation of data and contributed to and approved the final manuscript of the review.
Cordula Werner (CW) screened titles and abstracts of references identified by the search; located, selected and assessed trials; extracted trial and outcome data; assessed the methodological quality of selected trials; contributed to the interpretation of data and contributed to and approved the final manuscript of the review.
Marcus Pohl (MP) contributed to the conception and design of the review, drafted the protocol and assessed the methodological quality of selected trials. Together with JM, he contacted trialists about unpublished data and also entered the data, carried out statistical analysis, helped with interpretation of the data, drafted the review and approved the final manuscript of the review.
Bernhard Elsner (BE) searched electronic databases and conference proceedings, screened titles and abstracts of references identified by the search, selected and assessed trials, guided analysis and interpretation of the data and contributed to and approved the final manuscript of the review.
Sources of support
Internal sources
Klinik Bavaria Kreischa, Wissenschaftliches Institut, Germany.
Technical University Dresden, Lehrstuhl Public Health, Germany.
SRH Fachhochschule Gera, Lehrstuhl Therapiewissenschaften, Germany.
External sources
No sources of support supplied
Declarations of interest
Marcus Pohl and Jan Mehrholz were authors of one included trial (Pohl 2007). Cordula Werner was an author of two included trials (Pohl 2007; Werner 2002) and of one excluded trial (Hesse 2001). They did not participate in quality assessment and data extraction of these studies.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
- Aschbacher B. Comparing gait training in patients after stroke with task oriented physiotherapy or robot‐assisted treadmill training a feasibility study. Unpublished2006.
- Brincks J. The order of gait training, including Lokomat® and Physiotherapy, does not influence gait symmetry in subacute ambulatory persons with stroke. 16th World Congress of Physical Therapy, Amsterdam, 2011. [Google Scholar]
- Chang WH, Kim MS, Huh JP, Lee PK, Kim YH. Effects of robot‐assisted gait training on cardiopulmonary fitness in subacute stroke patients: a randomized controlled study. Neurorehabilitation and Neural Repair 2012;26(4):318–24. [DOI] [PubMed] [Google Scholar]; Kim M, Kim YH, Lee PKW, HGyong MK, Jung PH. Effect of robot‐assisted gait therapy on cardiopulmonary fitness in subacute stroke patients. Neurorehabilitation and Neural Repair 2008;22:594. [DOI] [PubMed] [Google Scholar]
- Dias D, Laíns J, Pereira A, Nunes R, Caldas J, Amaral C, et al. Partial body weight support in chronic hemiplegics: a randomized control trial. 6th Mediterranean Congress PRM 06, Vilamoura, 2006. [Google Scholar]; Dias D, Laíns J, Pereira A, Nunes R, Caldas J, Amaral C, et al. Partial body weight support in chronic hemiplegics: a randomized control trial. Europa Medicophysica 2007;43(4):499‐504. [PubMed] [Google Scholar]
- Fisher S. Use of autoambulator for mobility improvement in patients with central nervous system (CNS) injury or disease. Neurorehabilitation and Neural Repair 2008;22:556. [Google Scholar]; Fisher S, Lucas L, Thrasher TA. Robot‐assisted gait training for patients with hemiparesis due to stroke. Topics in Stroke Rehabilitation 2011;18(3):269‐76. [DOI] [PubMed] [Google Scholar]
- Geroin C, Picelli A, Munari D, Waldner A, Tomelleri C, Smania N. Combined transcranial direct current stimulation and robot‐assisted gait training in patients with chronic stroke: a preliminary comparison. Clinical Rehabilitation 2011;25(6):537‐48. [DOI] [PubMed] [Google Scholar]
- Hidler J, Nichols D, Pelliccio M, Brady K, Campbell DD, Kahn JH, et al. Multicenter randomized clinical trial evaluating the effectiveness of the Lokomat in subacute stroke. Neurorehabilitation and Neural Repair 2009;23(1):5‐13. [DOI] [PubMed] [Google Scholar]
- Hornby TG, Campbell DD, Kahn JH, Demott T, Moore JL, Roth HR. Enhanced gait‐related improvements after therapist‐ versus robotic‐assisted locomotor training in subjects with chronic stroke: a randomized controlled study. Stroke 2008;39(6):1786‐92. [DOI] [PubMed] [Google Scholar]
- Husemann B, Müller F, Krewer C, Laß A, Gille C, Heller S, et al. Effects of locomotion training with assistance of a driven gait orthosis in hemiparetic patients after stroke. Neurologie & Rehabilitation 2004;10(4):25. [Google Scholar]; Husemann B, Müller F, Krewer C, Laß A, Gille C, Heller S, et al. Effects of locomotion training with assistance of a robot‐driven gait orthosis in hemiparetic patients after stroke: a randomized controlled pilot study. Stroke 2007;38(2):349‐54. [DOI] [PubMed] [Google Scholar]
- Jung KH, Ha HG, Shin HJ, Ohn SH, Sung DH, Lee PKW, et al. Effects of robot‐assisted gait therapy on locomotor recovery in stroke patients. Journal of Korean Academy of Rehabilitation Medicine 2008;32:258‐66. [Google Scholar]; Kyung HJ, Kim YH. Effects of robot‐assisted gait therapy on locomotor recovery in stroke patients. Asian Oceania Conference of Physical and Rehabilitation Medicine, 16‐19 May 2008, Nanjing, China. 2008. [Google Scholar]
- Mayr A, Saltuari L, Quirbach E. Impact of Lokomat training on gait rehabilitation. Neurorehabilitation and Neural Repair 2008;22(5):596. [DOI] [PubMed] [Google Scholar]
- Morone G, Bragoni M, Iosa M, Angelis D, Venturiero V, Coiro P, et al. Who may benefit from robotic‐assisted gait training? A randomized clinical trial in patients with subacute stroke. Neurorehabilitation and Neural Repair 2011;25(7):636‐44. [DOI] [PubMed] [Google Scholar]
- Noser E. Improving ambulation post stroke with robotic training. ClinicalTrials.gov. NCT00975156 (accessed December 2012).
- Peurala S, Tarkka I, Pitkänen K, Sivenius J. The effectiveness of body weight‐supported gait training and floor walking in patients with chronic stroke. Archives of Physical Medicine and Rehabilitation 2005;86:1557‐64. [DOI] [PubMed] [Google Scholar]; Peurala SH, Pitkanen K, Sivenius J, Tarkka I. Body‐weight supported gait exercise compared with floor walking in chronic stroke patients. Archives of Physical Medicine and Rehabilitation 2004;85:E7. [DOI] [PubMed] [Google Scholar]; Peurala SH, Pitkanen K, Sivenius J, Tarkka IM. Body‐weight supported gait trainer exercises with or without functional electrical stimulation improves gait in patients with chronic stroke. Neurorehabilitation and Neural Repair 2006;20(1):98. [Google Scholar]
- Peurala SH, Airaksinen O, Huuskonen P, Jäkälä P, Juhakoski M, Sandell K, et al. Effects of intensive therapy using Gait Trainer or floorwalking exercises early after stroke. Journal of Rehabilitation Medicine 2009;41(3):166‐73. [DOI] [PubMed] [Google Scholar]
- Pohl M, Werner C, Holzgraefe M, Kroczek G, Mehrholz J, Wingendorf I, et al. Repetitive locomotor training and physiotherapy improve walking and basic activities of daily living in subacute, non‐ambulatory stroke patients: a single‐blind, randomised multi‐centre trial (DEutsche GAngtrainerStudie, DEGAS). Clinical Rehabilitation 2007;21(1):17‐27. [DOI] [PubMed] [Google Scholar]
- Saltuari L. Efficiency of Lokomat training in stroke patients. Neurologie & Rehabilitation 2004;10(4):S4. [Google Scholar]
- Schwartz I, Katz‐Leurer M, Fisher I, Sajin A, Shochina M, Meiner Z. The effectiveness of early locomotor therapy in patients with first CVA. Proceedings of the Collaborative Evaluation of Rehabilitation in Stroke Across Europe (CERISE) Congress. 10‐11 February 2006; Leuven, Belgium. 2006. [Google Scholar]
- Tanaka N, Saitou H, Takao T, Iizuka N, Okuno J, Yano H, et al. Effects of gait rehabilitation with a footpad‐type locomotion interface in patients with chronic post‐stroke hemiparesis: a pilot study. Clinical Rehabiilation 2012;26(8):686‐95. [DOI] [PubMed] [Google Scholar]
- Li LSW, Tong RYU, Ng MFW, So EFM. Effectiveness of gait trainer in stroke rehabilitation. Journal of the Neurological Sciences 2005;238(Suppl 1):S81. [Google Scholar]; Ng MFW, Tong RKY, Li LSW. A pilot study of randomized clinical controlled trial of gait training in subacute stroke patients with partial body‐weight support electromechanical gait trainer and functional electrical stimulation: six‐month follow‐up. Stroke 2008;39:154‐60. [DOI] [PubMed] [Google Scholar]; Ng MFW, Tong RKY, So EFM, Li LSW. The therapeutic effect of electromechanical gait trainer and functional electrical stimulation for patients with acute stroke. Neurorehabilitation and Neural Repair 2006;20(1):97 (Abstract F1D‐7). [Google Scholar]; Tong RKY, Ng MF, Li LS. Effectiveness of gait training using an electromechanical gait trainer, with and without functional electric stimulation, in subacute stroke: a randomized controlled trial. Archives of Physical Medicine and Rehabilitation 2006;87(10):1298‐304. [DOI] [PubMed] [Google Scholar]
- Nunen M. RCT evaluating the effectiveness of robot‐assisted treadmill training in restoring walking ability of stroke patients. 7th World Congress of Neurological Rehabilitation, Melbourne, 2012. [Google Scholar]
- Werner C, Frankenberg S, Treig T, Konrad M, Hesse S. Treadmill training with partial body weight support and an electromechanical gait trainer for restoration of gait in subacute stroke patients: a randomized crossover study. Stroke 2002;33(12):2895‐901. [DOI] [PubMed] [Google Scholar]
- Westlake K, Patten C. Pilot study of Lokomat versus manual‐assisted treadmill training for locomotor recovery post‐stroke. Journal of NeuroEngineering and Rehabilitation 2009;6:18. [DOI: 10.1186/1743-0003-6-18] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
- Caldwell C, Medley A. Effects of bicycling, treadmill, and variable surfaces on gait in people following a CVA. Neurology Report 2000;24(5):203. [Google Scholar]
- David D, Regnaux JP, Lejaille M, Louis A, Bussel B, Lofasso F. Oxygen consumption during machine‐assisted and unassisted walking: a pilot study in hemiplegic and healthy humans. Archives of Physical Medicine and Rehabilitation 2006;87:482‐9. [DOI] [PubMed] [Google Scholar]
- Gong S, Zhang J. Effect of early rehabilitation training on daily life activity of patients with hemiplegia after stroke. Chinese Journal of Clinical Rehabilitation 2003;7(5):848‐9. [Google Scholar]
- Hesse S, Werner C, Uhlenbrock D, Frankenberg S, Bardeleben A, Brandl‐Hesse B. An electromechanical gait trainer for restoration of gait in hemiparetic stroke patients: preliminary results. Neurorehabilitation and Neural Repair 2001;15:37‐48. [DOI] [PubMed] [Google Scholar]
- Mirelman A, Bonato P, Deutsch JE. Effects of training with a robot‐virtual reality system compared with a robot alone on the gait of individuals after stroke. Stroke 2009;40(1):169‐74. [DOI] [PubMed] [Google Scholar]
- Page SJ, Levine P, Teepen J, Hartman EC. Resistance‐based, reciprocal upper and lower limb locomotor training in chronic stroke: a randomized, controlled crossover study. Clinical Rehabilitation 2008;22(7):610‐7. [DOI] [PubMed] [Google Scholar]
- Patten C. Internally versus externally guided body weight supported treadmill training (BWSTT) for locomotor recovery post‐stroke, 2005. www.strokecenter.org/trials/ (accessed April 2009).
- Pitkanen K, Tarkka I, Sivenius J. Walking training with partial body weight support versus conventional walking training of chronic stroke patients: preliminary findings. Neurorehabilitation and Neural Repair 2001;15(4):312 (Abstract T7). [Google Scholar]; Pitkanen K, Tarkka IM, Sivenius J. Walking training with partial body weight support vs conventional walking training of chronic stroke patients: preliminary findings. Proceedings of the 3rd World Congress in Neurological Rehabilitation. Venice, Italy, 2002. [Google Scholar]
- Richards CL, Malouin F, Wood‐Dauphinee S, Williams JI, Bouchard JP, Brunet D. Task‐specific physical therapy for optimization of gait recovery in acute stroke patients. Archives of Physical Medicine and Rehabilitation 1993;74:612‐20. [DOI] [PubMed] [Google Scholar]
- Richards CL, Malouin F, Bravo G, Dumas F, Wood‐Dauphinee S. The role of technology in task‐oriented training in persons with subacute stroke: a randomized controlled trial. Neurorehabilitation and Neural Repair 2004;18(4):199‐211. [DOI] [PubMed] [Google Scholar]
- Shirakawa R, Uchida SU, Okajima YO, Sakaki TS, Shutou HS. Therapeutic effects of power‐assist training combined with biofeedback on hemiplegia by Therapeutic Exercise Machine (TEM). Proceedings of the 1st International Congress of International Society of Physical and Rehabilitation Medicine (ISPRM), Amsterdam, 7‐13 July 2001. [Google Scholar]
- Skvortsova VI, Ivanova GE, Kovrazhkina EA, Rumiantseva NA, Staritsyn AN, Suvorov AIu, et al. The use of a robot‐assisted Gait Trainer GT1 in patients in the acute period of cerebral stroke: a pilot study. Zhurnal nevrologii i psikhiatrii imeni S.S. Korsakova 2008;Suppl 23:28‐34. [PubMed] [Google Scholar]; Skvortsova VI, Ivanova GE, Kovrazhkina EA, Rumyantseva NA, Staritsin AN, Sogomonyan EK. The efficacy of gait rehabilitation after stroke training with assistance of a robotic device gait trainer: a pilot study. International Journal of Stroke 2008;3(Suppl 3):355. [Google Scholar]
References to studies awaiting assessment
- Globokar D. Gait trainer in neurorehabilitation of patients after stroke. 5th World Congress of Physical Medicine and Rehabilitation, Sao Paulo, Brazil, 2005:987‐1. [Google Scholar]
- Golyk VA, Pivnyk AP, Ipatov AV. Constraint‐induced movement therapy for walking improvement (comparison of two walking training machine modifications' efficacy) for stroke patients. European Journal of Neurology 2006;13(Suppl 2):263. [Google Scholar]
- Jang SJ, Park SW, Kim ES, Wee HM, Kim YH. Electromechanical gait trainer for restoring gait in hemiparetic stroke patients. 5th World Congress of Physical Medicine and Rehabilitation, Sao Paulo, Brazil, 2005:909‐1. [Google Scholar]
- Kim BO, Lee JJ, Cho KH, Kim SH. Gait training robot (gaiTrainer) in rehabilitation. Proceedings of the 1st International Congress of International Society of Physical and Rehabilitation Medicine (ISPRM), 7‐13 July 2001, Amsterdam (Abstract Th.139.P). [Google Scholar]
- Koeneman JB. Air muscle device for ankle stroke rehabilitation. http://crisp.cit.nih.gov/ (accessed May 2007).
- Mehrberg RD, Flick C, Dervay J, Carmody J, Carrington C, Jermer M. Clinical evaluation of a new over ground partial body weight support assistive device in hemiparetic stroke patients. Archives of Physical Medicine and Rehabilitation 2001;82:1293 (Abstract 10). [Google Scholar]
References to ongoing studies
- Brissot R, Laviolle B. Efficacy of a mechanical gait repetitive training technique in hemiparetic stroke patients. www.clinicaltrials.gov (last accessed December 2012).
- Waldner A. Robot assisted therapy for acute stroke patients: a comparative study of GangTrainer GT I, Lokomat system and conventional physiotherapy. www.clinicaltrials.gov (last accessed December 2012).
- Chanubol R. Robotic versus conventional training on hemiplegic gait. www.clinicaltrials.gov (last accessed December 2012).
- Forrester L. Ankle robotics training after stroke: effects on gait and balance. www.clinicaltrials.gov (last accessed December 2012).
- Sale P. Effect of robot assisted treatment on gait performance in stroke patients. www.clinicaltrials.gov (last accessed December 2012).
Additional references
- Adams HP Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, et al. Classification of subtype of acute ischemic stroke: definitions for use in a multicenter clinical trial (TOAST: Trial of Org 10172 in Acute Stroke Treatment). Stroke 1993;24(1):35‐41. [DOI] [PubMed] [Google Scholar]
- Bohannon R. Rehabilitation goals of patients with hemiplegia. International Journal of Rehabilitation Research 1988;11(2):181‐3. [Google Scholar]
- Bohannon RW, Horton MG, Wikholm JB. Importance of four variables of walking to patients with stroke. International Journal of Rehabilitation Research 1991;14:246‐50. [DOI] [PubMed] [Google Scholar]
- Carr J, Shepherd R. Stroke Rehabilitation: Guidelines for Exercises and Training. London: Butterworth Heinemann, 2003. [Google Scholar]
- Collen FM, Wade DT, Robb GF, Bradshaw CM. The Rivermead Mobility Index: a further development of the Rivermead Motor Assessment. International Disability Studies 1991;13(2):50‐4. [DOI] [PubMed] [Google Scholar]
- Colombo G, Joerg M, Schreier R, Dietz V. Treadmill training of paraplegic patients using a robotic orthosis. Journal of Rehabilitation Research and Development 2000;37(6):693‐700. [PubMed] [Google Scholar]
- Deeks JJ, Higgins JPT, Altman DG. Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
- Freivogel S, Schmalohr D, Mehrholz J. Improved walking ability and reduced therapeutic stress with an electromechanical gait device. Journal of Rehabilitation Medecine 2009;41:734–9. [DOI] [PubMed] [Google Scholar]
- French B, Thomas LH, Leathley MJ, Sutton CJ, McAdam J, Forster A. Repetitive task training for improving functional ability after stroke. Cochrane Database of Systematic Reviews 2007, Issue 4. [DOI: 10.1002/14651858.CD006073.pub2] [DOI] [PubMed] [Google Scholar]
- Hamilton BB, Laughlin JA, Fiedler RC, Granger CV. Interrater reliability of the 7‐level functional independence measure (FIM). Scandinavian Journal of Rehabilitation Medicine 1994;26(3):115‐9. [PubMed] [Google Scholar]
- Hesse S, Sarkodie‐Gyan T, Uhlenbrock D. Development of an advanced mechanised gait trainer, controlling movement of the centre of mass, for restoring gait in non‐ambulant subjects. Biomedizinische Technik [Biomedical Engineering] 1999;44(7‐8):194‐201. [DOI] [PubMed] [Google Scholar]
- Hesse S, Schmidt H, Werner C, Bardeleben A. Upper and lower extremity robotic devices for rehabilitation and for studying motor control. Current Opinion in Neurology 2003;16(6):705‐10. [DOI] [PubMed] [Google Scholar]
- Hesse S, Waldner A, Tomelleri C. Innovative gait robot for the repetitive practice of floor walking and stair climbing up and down in stroke patients. Journal of NeuroEngineering and Rehabilitation 2010;7:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
- Holden MK, Gill KM, Magliozzi MR, Nathan J, Piehl‐Baker L. Clinical gait assessment in the neurologically impaired: reliability and meaningfulness. Physical Therapy 1984;64(1):35‐40. [DOI] [PubMed] [Google Scholar]
- Jorgensen H, Nakayama H, Raaschou H, Olsen T. Recovery of walking function in stroke patients: the Copenhagen stroke study. Archives of Physical Medicine and Rehabilitation 1995;76:27‐32. [DOI] [PubMed] [Google Scholar]
- Kwakkel G, Wagenaar RC, Twisk JW, Lankhorst GJ, Koetsier JC. Intensity of leg and arm training after primary middle‐cerebral‐artery stroke: a randomised trial. Lancet 1999;354(9174):191‐6. [DOI] [PubMed] [Google Scholar]
- Mehrholz J, Pohl M. Electromechanical‐assisted gait training after stroke: a systematic review comparing end‐effector and exoskeleton devices. Journal of Rehabilitation Medicine 2012;44(3):193‐9. [DOI] [PubMed] [Google Scholar]
- Mehrholz J, Hädrich A, Platz T, Kugler J, Pohl M. Electromechanical and robot‐assisted arm training for improving generic activities of daily living, arm function, and arm muscle strength after stroke. Cochrane Database of Systematic Reviews 2012, Issue 6. [DOI: 10.1002/14651858.CD006876.pub3] [DOI] [PubMed] [Google Scholar]
- Massachusetts Institute of Technology. MIT develops Anklebot for stroke patients. http://web.mit.edu/newsoffice/2005/stroke‐robot.html (accessed 20 December 2005).
- Moseley AM, Stark A, Cameron ID, Pollock A. Treadmill training and body weight support for walking after stroke. Cochrane Database of Systematic Reviews 2005, Issue 4. [DOI: 10.1002/14651858.CD002840] [DOI] [PubMed] [Google Scholar]
- Nuyens GE, Weerdt WJ, Spaepen AJ Jr, Kiekens C, Feys HM. Reduction of spastic hypertonia during repeated passive knee movements in stroke patients. Archives of Physical Medicine and Rehabilitation 2002;83(7):930‐5. [DOI] [PubMed] [Google Scholar]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
- Sackett DL, Deeks JJ, Altman DG. Down with odds ratios!. Evidence‐based Medicine 1996;1:164‐6. [Google Scholar]
- Schmidt H, Hesse S, Bernhardt R, Krüger J. HapticWalker-a novel haptic foot device. ACM Transactions on Applied Perception 2005;2(2):166‐80. [Google Scholar]
- States RA, Pappas E, Salem Y. Overground physical therapy gait training for chronic stroke patients with mobility deficits. Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.CD006075.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peppen RP, Kwakkel G, Wood‐Dauphinee S, Hendriks HJ, Wees PJ, Dekker J. The impact of physical therapy on functional outcomes after stroke: what's the evidence?. Clinical Rehabilitation 2004;18(8):833‐62. [DOI] [PubMed] [Google Scholar]
- Veneman J, Kruidhof R, Helm FCT, Kooy H. Design of a Series Elastic‐ and Bowdencable‐based actuation system for use as torque‐actuator in exoskeleton‐type training robots. Proceedings of the International Conference on Rehabiitation Robotics, 28 June‐1 July 2005, Chicago, IL, USA. 2005. [Google Scholar]
- Wade DT, Collin C. The Barthel ADL Index: a standard measure of physical disability?. International Disability Studies 1988;10(2):64‐7. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Cerebrovascular accident, stroke. http://www.who.int/topics/cerebrovascular_accident/en/ (accessed 1 February 2006).
References to other published versions of this review
- Mehrholz J, Werner C, Kugler J, Pohl M. Electromechanical‐assisted training for walking after stroke [Protocol]. Cochrane Database of Systematic Reviews 2006, Issue 4. [DOI: 10.1002/14651858.CD006185] [DOI] [PubMed] [Google Scholar]
- Mehrholz J, Werner C, Kugler J, Pohl M. Electromechanical‐assisted training for walking after stroke. Cochrane Database of Systematic Reviews 2007, Issue 4. [DOI: 10.1002/14651858.CD006185.pub2] [DOI] [PubMed] [Google Scholar]


