Abstract
Abstract. The liver is normally proliferatively quiescent, but hepatocyte loss through partial hepatectomy, uncomplicated by virus infection or inflammation, invokes a rapid regenerative response from all cell types in the liver to perfectly restore liver mass. Moreover, hepatocyte transplants in animals have shown that a certain proportion of hepatocytes in foetal and adult liver can clonally expand, suggesting that hepatoblasts/hepatocytes are themselves the functional stem cells of the liver. More severe liver injury can activate a potential stem cell compartment located within the intrahepatic biliary tree, giving rise to cords of bipotential transit amplifying cells (oval cells), that can ultimately differentiate into hepatocytes and biliary epithelial cells. A third population of stem cells with hepatic potential resides in the bone marrow; these haematopoietic stem cells may contribute to the albeit low renewal rate of hepatocytes, but can make a more significant contribution to regeneration under a very strong positive selection pressure. In such instances, cell fusion rather than transdifferentiation appears to be the underlying mechanism by which the haematopoietic genome becomes reprogrammed.
INTRODUCTION
Perhaps born out of necessity from the plethora of potentially cell‐damaging xenobiotics that assail the liver, plus a myriad of other cellular insults, e.g. hepatotropic viruses, the mammalian liver can invoke not just one, but at least three apparently distinct cell lineages to contribute to regenerative growth after damage (Fig. 1).
Figure 1.

Current understanding of the origin and inter‐relationships of the cells involved in liver development and regeneration. Foetal liver contains bipotential hepatoblasts capable of differentiating into hepatocytes and cholangiocytes. These cells are capable of self‐renewal after loss, but when hepatocyte renewal is compromised, bipotential oval cells are activated from the canal of Hering cells (potential stem cell niche) to take over the burden of regenerative growth. The bone marrow also harbours cells with liver potential, but the factors determining their durable ingress are poorly understood: in the Fah null mouse, fusion between bone marrow cells and deficient hepatocytes occurs. Evidence that bone marrow can transdifferentiate into biliary cells is weak.
HEPATOCYTES
In response to parenchymal cell loss, the hepatocytes are the cells that normally restore the liver mass, rapidly re‐entering the cell cycle from the G0 phase (Fig. 2). However, even after a two‐thirds partial hepatectomy, the remaining cells have to cycle on average only 1.4 times to restore the pre‐operative cell number, as all remaining hepatocytes traverse the cell cycle at least once in young adult rats. This seemingly modest response lead to the incorrect assumption that hepatocytes had only limited division potential, and thus were not true stem cells. A crucial property that defines a stem cell is its ability to give rise to a large family of descendants, i.e. be clonogenic (Alison et al. 2002), and, importantly, at least some hepatocytes can do this. Hepatocyte transplantation models have shown that the transplanted cells are capable of significant clonal expansion within the diseased livers of experimental animals and probably humans (see below).
Figure 2.
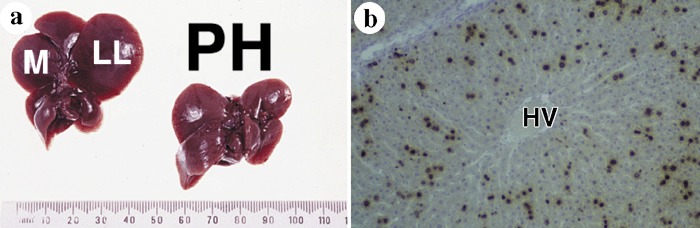
(a) Partial hepatectomy (PH) in a rat involves resection of the left lateral (LL) and median (M) lobes comprising two‐thirds of the liver mass and the pre‐operative mass is restored within 10 days. (b) Twenty‐four hours after PH, many hepatocytes are in DNA synthesis as indicated by bromodeoxyuridine labelling, but note the relative absence of labelling around the hepatic vein (HV) at this time, although within 48 h after PH almost all hepatocytes will have traversed the cell cycle at least once.
In the diseased human liver there may not be the substantial selective growth advantage for transplanted cells that is operative in many of the rodent models, and it therefore becomes of interest to determine if it is possible to enrich for true stem cells that would continue to expand in the recipient liver in the absence of a major growth stimulus. The foetal rat liver is potentially a rich source of bipotential stem cells for liver transplantation (Shafritz & Dabeva 2002; 2003a, 2003b) and has been the focus of considerable attention. For example, Kubota & Reid (2000) have described a population of bipotential progenitors from ED13 foetal rat liver that lacked expression of major histocompatability complex (MHC) class I and had modest ICAM‐1 expression, features that may allow hepatoblasts to escape from the immune system when transplanted into an MHC‐incompatible host. These cells were clonogenic in culture with some descendants expressing phenotypic markers of hepatocytes [α‐fetoprotein (AFP) and albumin] and others of cholangiocytes [cytokeratin (CK)‐19].
In a similar vein, Shafritz and colleagues (Sandhu et al. 2001) have clearly demonstrated that foetal liver epithelial progenitors (FLEPs) from ED14 rats are more clonogenic than normal adult hepatocytes; when wild‐type FLEPs are injected into recently hepatectomized syngeneic dipeptidyl peptidase (DPPIV–)‐deficient F344 rats they proliferate for at least 6 months and constitute 7% of the recipient liver at this time compared with colonization of only 0.06% of the liver by wild‐type adult hepatocytes. DPPIV is an exopeptidase expressed in the bile canalicular surface of hepatocytes in contrast to diffuse cytoplasmic expression in bile duct epithelia. Thus, DPPIV‐positive hepatocytes are readily detected in the recipient mutant liver either by enzyme histochemistry or immunohistochemistry (IHC). Much greater colonization of the mutant liver was observed when the recipient rats were given prior administration of the DNA‐binding pyrrolizidine alkaloid retrorsine (usually two injections of 30 mg/kg each, 2 weeks apart); at 6 months after transplantation 60–80% of the recipient liver was occupied by DPPIV+ hepatocytes (Dabeva et al. 2000). Likewise, when these FLEPs were transduced with lentiviral vectors expressing green fluorescent protein (GFP) under the control of the albumin promoter, the colonizing cells co‐expressed DPPIV and GFP (Oertel et al. 2003). Progress is being made to identify the genes that are exclusively expressed by these FLEPs with a view to their specific identification and isolation (Petkov et al. 2000).
Clonogenic cells can also be isolated from the foetal mouse liver (ED13.5); hepatocytes expressing the integrins α6 (CD49f) and β1 (CD29), but not c‐kit, CD45 or Ter119 (erythroid precursor antigen) had the greatest colony forming ability (Suzuki et al. 2000). Designated hepatic colony forming units in culture (H‐CFU‐C) this sorting achieved a 35‐fold enrichment of H‐CFU‐C over total foetal liver cells. In a recent development, further selection based on c‐Met‐positivity, enriched for H‐CFU‐C and these cells, could produce both hepatocytes (albumin‐positive) and biliary cells (cytokeratin‐19‐positive) in culture (Suzuki et al. 2002). EGFP‐marked cells from these clonally derived H‐CFU‐C also produced hepatocytes and biliary cells when injected into mice, and more surprisingly were found to apparently differentiate into pancreatic ducts and acini and duodenal mucosal cells when injected directly into these organs.
Many other studies have examined the transplantation potential of adult hepatocytes in the DPPIV– mutant rat combining retrorsine treatment with a mitogenic stimulus such as partial hepatectomy or triidothyronine (T3) leading to rapid replacement of DPPIV– cells by DPPIV+ donor cells (Laconi et al. 1998; Oren et al. 1999); even in the absence of a mitogenic stimulus near total replacement by donor cells occurs within 12 months (Laconi et al. 2001).
Intriguingly, when retrorsine‐treated adult rats are given a two‐thirds partial hepatectomy, regeneration is accomplished by the activation, expansion and differentiation of so‐called small hepatocyte‐like progenitors (SHPCs) (Gordon et al. 2000). These cells showed phenotypic traits of foetal hepatoblasts, oval cells and fully differentiated hepatocytes, but they were morphologically and phenotypically distinct from all three. Cytochrome (CYP) P450 enzymes have a pivotal role in hepatocyte biology (Mugford & Kedderis 1998), but typically these cell clusters lacked CYP enzymes that are usually readily induced by retrorsine, and this probably accounted for their resistance to the anti‐proliferative effects of retrorsine. When such cells (H4‐positive) were isolated, established in short‐term culture and then transplanted into syngeneic rats, they gave rise to differentiated hepatocytes as evidenced by expression of albumin and transferrin, but lack of AFP (Gordon et al. 2002).
The clonogenic potential of transplanted adult hepatocytes has been most impressively demonstrated in the Fah null mouse, a model of hereditary type 1 tyrosinaemia, where there exists a profoundly strong positive selection pressure on the transplanted wild‐type cells, as Fah‐deficient mice will die as neonates unless rescued by 2‐(2‐nitro‐4‐trifluoro‐methylbenzoyl)‐1,3‐cyclohexanedione (NTBC), a compound that prevents the accumulation of toxic metabolites in the tyrosine catabolic pathway. When 104 normal hepatocytes from congenic male wild‐type mice are intrasplenically injected into mutant female mice, these cells will quickly colonize the mutant liver (Overturf et al. 1997). Moreover, serial transplantations from the colonized liver to other mutant livers indicated that at least 69 doublings would have been necessary from the original hepatocytes for six rounds of liver re‐population. This estimate is likely to be a minimal figure as it assumes that all injected hepatocytes migrate to the liver from the spleen and take part equally in the cycles of regeneration. In fact, probably at best only 15% of intrasplenically transplanted hepatocytes migrate to the liver, and if all these participated equally in repopulation, a minimum of 86 doublings would be required. This figure may be even higher if not all the cells that migrated to the liver actually took part in re‐population, and the authors suggested that maybe there is a subpopulation of hepatocyte stem cells which they designated as regenerative transplantable hepatocytes (RTHs). We could speculate that perhaps these RTHs are analogous to the SHPCs described by Gordon et al. (2000). The Fah null mouse can also be rescued by pancreatic cells; though most Fah‐deficient mice withdrawn from NTBC treatment and transplanted with pancreatic cells will die, a small proportion do survive with 50–90% replacement of the diseased liver by pancreatic cell‐derived hepatocytes (Wang et al. 2001). Given that animals fed a copper‐deficient diet undergo pancreatic exocrine cell atrophy and re‐feeding induces the surviving ducts to give rise to hepatocytes (Rao & Reddy 1995), it was surprising that pancreatic cell suspensions enriched for pancreatic ducts were poorer than unfractionated pancreatic cells at reconstituting the diseased Fah‐deficient liver with functional hepatocytes.
Other cell lines with hepatocyte potential can also be isolated from adult rat liver (Nagai et al. 2002); these non‐hepatocyte cells were AFP‐, albumin‐ and CK19‐negative, but after co‐culture with hepatic stellate cells they expressed albumin, transferrin and α ‐1 anti‐trypsin. It is well known that the WB‐F344 rat liver diploid epithelial cell line readily differentiates into hepatocytes when transplanted into syngeneic rats, but biliary differentiation [CK19+, BDS7+, gamma glutamyl transpepetidase (GGT)+] can be induced by in vitro culture on Matrigel (Couchie et al. 2002). Tateno & Yoshizato (1996) have defined the conditions for the long‐term expansion of cells isolated from adult Fisher 344 rats.
Hepatic stem cells, from whatever source, may be therapeutically useful for treating a variety of diseases that affect the liver. This would include a number of genetic diseases that produce liver disease such as Wilson's disease (copper accumulation), Crigler Najjar syndrome (lack bilirubin conjugation activity) and tyrosinemia, and cases where there is extrahepatic expression of the disease, e.g. Factor IX deficiency. In terms of therapeutic potential, we have already noted the rescue of the Fah null mouse and the DPPIV‐negative rat by hepatocytes, and there are now other examples. Transplantation of adult rat hepatocytes has also been effective in normalizing bilirubin levels and improving bilirubin conjugation activity in Gunn rats (a model of Crigler–Najjar syndrome) (Tada et al. 1998; Guha et al. 2002). These cells were reversibly immortalized and transduced with the bilirubin‐uridine 5′‐diphosphoglucuronate glucuronsyltransferase gene (UGT1A1) and engraftment was improved by prior irradiation and partial hepatectomy of the recipient rats. Following on from this, an infusion of isolated hepatocytes through the portal vein equivalent to 5% of the parenchymal mass to a patient with Crigler Najjar syndrome, achieved a medium‐term reduction in serum bilirubin and increased bilirubin conjugate levels in the bile (Fox et al. 1998). Hepatocyte transplantation has also been successful in the treatment of human glycogen storage disease type 1a (Muraca et al. 2002), but not in the treatment of severe ornithine transcarbamylase deficiency, where rejection of the transplanted cells was thought to be the reason for only temporary (11 days) relief (Horslen et al. 2003).
CHOLANGIOCYTES
When either massive damage is inflicted upon the liver or regeneration after damage is compromised, a potential stem cell compartment located within the smallest branches of the intrahepatic biliary tree is activated. This so‐called ‘oval cell’ or ‘ductular reaction’ amplifies the biliary population before these cells differentiate into either hepatocytes or cholangiocytes (1998, 1996a, 1997a, 1997b; Alison et al. 1998). Careful studies in rats indicate that oval cells are pre‐dominantly derived from the canal of Hering, and thus this is the location of a stem cell niche (Paku et al. 2001). In rats and mice, the canals of Hering barely extend beyond the limiting plate, but the resultant oval cell proliferation can result in arborizing ducts that express AFP (Fig. 3a), that stretch to the midzonal areas (Fig. 3b) before these cells differentiate into hepatocytes (Fig. 3c). A wide range of markers has been used to identify ovals cells including GGT and glutathione‐S‐transferase (GST‐P) activity along with a host of monoclonal antibodies raised against cytoskeletal proteins and unknown surface antigens (see Table 1) (Agelli et al. 1997; Hixson et al. 1997; Hixson et al. 2000). Moreover, antigens traditionally associated with haematopoietic cells can also be expressed by oval cells, including c‐kit, flt‐3, Thy‐1 and CD34 (Omori et al. 1997; Lemmer et al. 1998; Petersen et al. 1998; Baumann et al. 1999). This may be no more than coincidental, but it has given support to the notion that at least some hepatic oval cells are directly derived from a precursor of bone marrow origin, particularly when the biliary tree is damaged (Petersen 2001), though other studies indicate that many/most oval cells are derived from the direct intrahepatic proliferation of cells already located within the biliary tree (Alison et al. 1996b). Regarding the contribution of bone marrow (see below), Hatch et al. (2002) have suggested that only when the rat liver is severely damaged do hepatocytes up‐regulate the chemokine, stromal‐derived factor‐1 alpha (SDF‐1α), and both oval cells are activated and bone marrow cells recruited through SDF−1α/CXCR4 inter‐actions.
Figure 3.

Oval cell behaviour in the rat liver treated by the AAF/PH protocol. (a) AFP expression (IHC staining) is typically observed in the migrating oval cells; note absence of staining in the inter‐lobular duct in the portal tract (PT). (b) The oval cell response can be visualized by CK8 immunostaining, with cords of cells emanating from the portal tract (PT). (c) At later times the cords of oval cells differentiate into small hepatocytes (SH) but with a notable lack of CYP immunoexpression (brown staining). Note occasional residual oval cell ductules expressing CK19 (purple staining).
Table 1.
Markers that have aided in the identification of oval cells in liver (see text and references therein for details)
| OV‐6 |
| OC.2, OC.3, OC.4, OC.5, OC.10 |
| BDS7 |
| Thy‐1 |
| c‐kit |
| CD34 |
| ABCG2/BRCP1 |
| Connexin 43 |
| CK7, CK19, CK14 |
| AFP |
| Gamma‐glutamyltranspeptidase (GGT) |
| Placental form of glutathione‐S‐transferase (GST‐P) |
| flt‐3 ligand/flt‐3 |
| DMBT1 |
The identification of molecular markers that best facilitate the isolation and characterization of stem cell populations has long been a challenge. In studies of the haematopoietic system, both experimental and clinical, the expression of the sialomucin CD34 has traditionally been exploited to enrich for cells with long‐term marrow re‐population capacity. However, in 1996, Goodell et al. reported on a new method for the isolation of haematopoietic stem cells (HSCs) based on the ability of the HSCs to efflux a fluorescent dye. Like the activity of the P‐glycoprotein (encoded by the mdr1 gene), this activity was verapamil‐sensitive. Cells subjected to Hoechst 33342 dye staining that actively efflux the Hoechst dye appeared as a distinct population of cells on the side of a flow cytometry profile, hence the name the ‘side population’ (SP) has been given to these cells (Alison 2003). Numerous studies now point to the fact that the SP phenotype of HSCs in mice and humans is largely determined by the expression of a protein known as the ABCG2 transporter [ATP‐binding cassette (ABC) subfamily G member 2, also known as BCRP1] (Scharenberg et al. 2002). Perhaps not surprisingly, we now have reports of the up‐regulation of several ABC transport proteins in damaged human liver, particularly in regenerating ductules (Ros et al. 2003) and of ABCG2/BCRP1 in rat oval cells (Shimano et al. 2003), adding support to the belief that ABC transporter proteins are intimately involved in the biology of stem/progenitor cells in many tissues.
In the liver, bile secretion is driven largely by ABC‐type proteins that are located in the canalicular membrane and effect ATP‐dependent transport of bile acids, phospholipids and non‐bile acid organic ions. Canalicular ABC‐type proteins belong to two subfamilies (see http://www.nutrigene.4t.com/humanabc.htm); members of subfamily B including MDR1 (ABCB1), MDR3 (ABCB4) and SPGP (sister of P‐glycoprotein, ABCB11) and subfamily C including MRP1‐3 (ABCC1‐3). Subfamily C also contains the cystic fibrosis transmembrane conductance regulator (CFTR, ABCC7) that is expressed by bile duct epithelia (cholangiocytes). However, is the expression of these ABC transporters in liver ductules a genuine marker of stem cells/progenitors or merely a reflection of the protective role these proteins undoubtedly perform within the biliary tree against toxic bile constituents (Scheffer et al. 2002)? The correct answer is probably the latter as it is highly unlikely that all the reactive ductular cells are stem cells or even progenitors, and many are imminently going to differentiate into hepatocytes and cholangiocytes. Nevertheless, the expression of ABC transporters adds incrementally to the battery of already established markers for this stem cell response (Table 1). A further factor that might afford oval cells protection in a hostile environment is the expression of ‘deleted in malignant brain tumour 1’ (DMBT1), a molecule significantly up‐regulated in rat oval cells (Bisgaard et al. 2002); DMBT1 is a putative receptor for the mucus‐associated trefoil peptides that may have a general role of cytoprotection throughout the gastrointestinal tract (Taupin & Podolsky 2003).
Most models of oval cell activation have employed potential carcinogens to inhibit hepatocyte replication in the face of a regenerative stimulus; these have been reviewed elsewhere (1996a, 1997b, 1998). In the rat, protocols have included administering 2‐acetylaminofluorene (2‐AAF) prior to a two‐thirds partial hepatectomy (AAF/PH) or a necrogenic dose of carbon tetrachloride, feeding a choline‐deficient diet supplemented with ethionine (CDE), or simply treating animals with the likes of 3′‐methyl‐diaminobenzidine (3′‐Me‐DAB), galactosamine or furan. Cells derived by such procedures are clearly not relevant to human studies, but Sell and co‐workers (1999, 2002) have demonstrated that it is possible to derive bipotential liver progenitor cells (LPCs) from rat livers without resorting to mutagenic chemicals. Allyl alcohol causes periportal necrosis, and the resultant oval cells can be isolated and propagated, producing a number of clonally derived cell lines, capable of at least 100 doublings in the presence of a feeder layer of lethally irradiated fibroblasts. These cell lines were able to differentiate into hepatocytes after removal of the feeder cells; supplementation with either a combination of oncostatin M and dexamethasone or bFGF‐promoted differentiation. Similarly, such factors have proved instrumental in causing the transdifferentation of pancreatic acinar cells to hepatocytes (oncostatin M and dexamethasone) (Shen et al. 2000) and multipotent adult progenitor cells (MAPCs) to hepatocytes (bFGF) (Schwartz et al. 2002).
We have little idea of the precise cues that promote the in vivo hepatocytic differentiation of oval cells. GGT is a useful oval/biliary cell marker, however, GGT gene expression is driven by a number of different promoters and in the rat, differentiation down either the hepatocytic or biliary lineages is associated with specific promoter extinction (Holic et al. 2000). Under the CDE protocol, oval cell differentiation towards hepatocytes (expression of albumin, CYP4A1 and AFP) can be promoted by an activator of the transcription factor ‘peroxisome proliferator activated receptor (PPARα) (Kaplanski et al. 2000). In terms of therapeutic potential, one of the most impressive demonstrations of the hepatocytic potential of oval cells has come from experiments in which oval cells were isolated from Long Evans Cinnamon (LEC) rats (a model of Wilson's disease); transduced ex vivo with a reporter gene, β‐galactosidase, and then transplanted into LEC/Nagase analbuminemic double mutant rats, these cells differentiated into albumin‐expressing hepatocytes (Yasui et al. 1997).
Oval cells also occur in human liver. Elegant 3‐dimensional reconstructions of serial sections of human liver immunostained for cytokeratin‐19 have shown that the smallest biliary ducts, the canals of Hering, unlike those in rodents normally extend into the proximate third of the lobule (Theise et al. 1999), and it is envisaged that these canals react to massive liver damage (akin to a trip‐wire), proliferating and then differentiating into hepatocytes (Fig. 4). Oval cell numbers in human liver rise with increasing severity of liver disease (Lowes et al. 1999) and this ductular reaction is widely accepted to be a stem cell response rather than a ductular metaplasia of ‘damaged’ hepatocytes. Notwithstanding, Falkowski et al. (2003) have recently still felt compelled to formally dispel such a notion in human liver, showing that ‘cholestatic’ hepatocytes were very largely not associated with ductular reactions and moreover, that in cirrhosis most (94%) intraseptal buds of hepatocytes were associated with ductular reactions. Non‐parenchymal epithelial cells from adult porcine liver, essentially ductal epithelia, are also capable of differentiating into both biliary epithelia and hepatocytes in vitro (Kano et al. 2000).
Figure 4.

The canals of Hering (green) extend from the portal areas into the proximate third of the hepatic lobule in the human liver (see Theise et al. 1999) and major hepatocyte damage activates the lining cells to divide and probably differentiate into hepatocytes.
BONE MARROW
Within an adult tissue, the locally resident stem cells were formerly considered to be capable of only giving rise to the cell lineage(s) normally present. However, adult haematopoietic stem cells (HSCs) in particular appear to be much more flexible: removed from their usual niche they are capable of differentiating into all manner of tissues including skeletal and cardiac muscle, endothelia, and a variety of epithelia including neuronal cells, pneumocytes and hepatocytes. Some oval cells/hepatocytes were first revealed to be derived from circulating bone marrow cells in the rat (Table 2a): Petersen et al. (1999) followed the fate of syngeneic male bone marrow cells transplanted into lethally irradiated female recipient animals whose livers were subsequently injured by a regime of 2‐acetylaminofluorene (which blocks hepatocyte regeneration) and carbon tetrachloride (which causes hepatocyte necrosis) designed to cause oval cell activation. Y chromosome‐positive oval cells were found at 9 days after liver injury and some Y chromosome‐positive hepatocytes were seen at 13 days when oval cells were differentiating into hepatocytes. Additional evidence for hepatic engraftment of bone marrow cells was forthcoming from a rat whole liver transplant model. Lewis rats expressing the MHC class II antigen L21‐6 were recipients of livers from Brown Norway rats that were negative for L21‐6. Subsequently, ductular structures in the transplants contained both L21‐6‐negative and L21‐6‐positive cells indicating that some biliary epithelium was of in‐situ derivation and some was of recipient origin, presumably from circulating bone marrow cells.
Table 2.
Hepatocyte differentiation of haematopoietic cells in rats (a), mice (b), man (c) and human umbilical cord blood (h.UCB) cells in immunodeficient mice (d)
| (a) | |||||
|---|---|---|---|---|---|
| Authors | Procedure | Injury | Evidence | Haematopoietic contribution to hepatocyte population (%) | Comments |
| Petersen et al. 1999 | Male BMTx to females Male wild‐type to DPPIV‐null | 2‐AAF/CCL4 | Y+ cells in female DPPIV+ hepatocytes in DPPIV‐ liver | 0.16% | Also noted Y+ oval cells |
| Avital et al. 2001 | Strain‐mismatch liver transplantation | Organ rejection | C3 antigen not detected in cells integrated into hepatic plates | NS | No positive evidence that cells were hepatocytes |
| Dahlke et al. 2003 | CD45 mismatch BMTx | Retrorsine and CCL4 | Donor MHC antigens (IHC) | None | Hepatocyte hypertrophy responsible for restoration of liver mass |
| (b) | |||||
|---|---|---|---|---|---|
| Authors | Procedure | Injury | Evidence | Haematopoietic contribution to hepatocyte population (%) | Comments |
| Theise et al. 2000a | Male BMTx to female mice | None | Y+/albumin mRNA+ | Up to 2.2% | |
| Lagasse et al. 2000 | Male BMTx to female Fah null mice | Liver failure | Y+/Fah+ hepatocytes | 30–50% | |
| Wang et al. 2002 | Male BMTx to female Fah null mice | Liver failure | Y+/Fah+ hepatocytes | > 30% | Without liver failure got same initial engraftment but no clonal expansion |
| Mallet et al. 2002 | Male Bcl‐2 BMTx to female mice | 8 × Jo2 antibody | Y+/Bcl‐2+/CK 8, 18, 19+ | 0.8% | Each Jo2 injection destroyed 50% liver |
| Fujii et al. 2002 | GFP BMTx | 2/3 PH | GFP+ hepatocytes | None | Major contribution of GFP+ cells to endothelia and Kupffer cells |
| Wang et al. 2003b | Female BMTx to male Fah null mice | Liver failure | Genotype of Fah+ cells Fah+/Y+ cells | ∼50% | Fah+ cells had mixture of donor and recipient Genotype and were Y+: fusion occurring |
| Vassilopoulos et al. 2003 | Male BMTx to female Fah null mice | Liver failure | Genotype of Fah+ cells | NS | Mixed genotype (as above) = cell fusion |
| Alvarez‐Dolado et al. 2003 | Cre recombinase + BMTx to conditional Cre reporter mice | None | LacZ+ hepatocytes, albumin+, with bile canaliculi | < 0.01% | Cell fusion occurring. |
| Also fusion of BM with Purkinje cells and cardiomyocytes | |||||
| Terai et al. 2003 | GFP+ BMTx | CCL4‐induced cirrhosis | GFP cells in cords, mostly Liv2+ | ∼25% | Periportal location of GFP+ cells. Rise in serum albumin |
| Kanazawa & Verma 2003 | Marked (EGFP or LacZ) BMTx | 3 models of chronic liver injury | Y+ or GFP+ or LacZ+ | None | |
| (c) | |||||
|---|---|---|---|---|---|
| Authors | Procedure | Injury | Evidence | Haematopoietic contribution to hepatocyte population (%) | Comments |
| Alison et al. 2000 | Male BMTx to female Female allograft in male | Variable | Y+/CK8+ cells | 1–2% | Some small clusters |
| Theise et al. 2000b | Male BMTx to female Female allograft in male | Variable | Y+/CAM5.2+ cells | 4–43%* | *Recurrent HCV |
| Kleeberger et al. 2002 | Allografted liver | Variable | Genotype chimerism | NS | Cholangiocyte chimerism also seen |
| Korbling et al. 2002 | Male PBSC Tx to female | Variable | Y+/CAM5.2+ cells | 4–7% | Also Y+ cells in skin and gastrointestinal tract |
| Fogt et al. 2002 | Sex‐mismatched liver transplant | Variable | X and Y, CD45 | None | Followed up to 12 years |
| Ng et al. 2003 | Sex‐mismatched liver transplant | Variable | Genotype chimerism | 0.62% | Most Y+ cells in liver were macrophages |
| Wu et al. 2003 | Female allograft in male | Variable | Y+ hepatocytes | Infrequent or non‐existent | Followed for up to 16 years |
| (d) | |||||
|---|---|---|---|---|---|
| Authors | Procedure | Injury | Evidence | Haematopoietic contribution to hepatocyte population (%) | Comments |
| Danet et al. 2002 | CD34+/−, C1qRp+ h.UCB to NOD/SCID mice | None | h.albumin (RT‐PCR) c‐Met (IHC) | 0.05–0.1% | Illustrated cells were commonly binucleate – fusion? |
| Ishikawa et al. 2003 | CD34+ or CD45+ h.UCB to NOD/SCID/BMG‐ mice | None | h.albumin (RT‐PCR) HepPar1 (IHC) | 1–2% | FISH with human and murine centromeric probes found no fusion |
| Newsome et al. 2003 | h.UCB to NOD/SCID mice | None | HepPar1 (IHC) h.DNA sequences (FISH) | NS | No evidence for fusion |
| Wang et al. 2003a | CD34+ h.UCB to NOD/SCID and NOD/SCID/BMG‐ | CCL4 | h.Alu sequences h.albumin mRNA | 1–10% are human in liver, but only 1 in 20 express albumin | rhHGF increased h.albumin expression |
| Kakinuma et al. 2003 | h.UCB to SCID mice | 2‐AAF/ PH | h. X chromosome (FISH) h.albumin (IHC) | 0.1–1% | Claimed albumin+ cells in clusters (data not shown) |
| Kollet et al. 2003 | h.UCB (CD34+) to NOD/SCID | CCL4 | h.albumin | ∼50–175/1.5 × 106 | Occasional clusters. Human cells adjacent to SDF‐1+ bile ducts. HGF + SDF induced lamellipodia on CD34+ cells |
2‐AAF/PH, acetylaminofluorene followed by partial hepatectomy; 2‐AAF/CCL4, acetylaminofluorene followed by carbon tetrachloride; BMTx, bone marrow transplant; DPPIV, dipeptidyl peptidase IV; EGFP, enhanced green fluorescent protein; Fah, fumarylacetoacetate hydrolase; FISH, fluorescence in situ hybridization; GFP, green fluorescent protein; h.UCB, human umbilical cord blood; IHC, immunohistochemistry; NOD/SCID/BMG‐, non‐obese/severe combined immunodeficient/β2 microglobulin negative; NS, not stated; PBSC, peripheral blood stem cell; RT‐PCR, reverse transcription‐polymerase chain reaction.
Using a similar gender mismatch bone marrow transplantation approach in mice to track the fate of bone marrow cells, Theise et al. (2000a) reported that, over a 6‐month period, 1–2% of hepatocytes in the murine liver may be derived from bone marrow in the absence of any obvious liver damage, suggesting bone marrow contributes to normal ‘wear and tear’ renewal (Table 2b). In two contemporaneous papers, Alison et al. (2000) and Theise et al. (2000b) demonstrated that hepatocytes can also be derived from bone marrow cell populations in humans (see Table 2c). Two approaches were adopted – firstly, the livers of female patients who had previously received a bone marrow transplant from a male donor were examined for cells of donor origin using a DNA probe specific for the Y chromosome, localized using in situ hybridization. Secondly, Y‐positive cells were sought in female livers engrafted into male patients but which were later removed for recurrent disease. In both sets of patients, Y‐chromosome‐positive hepatocytes were readily identified, but the degree of hepatic engraftment of HSCs into human liver was highly variable; most likely related to the severity of parenchymal damage, up to 40% of hepatocytes and cholangiocytes appeared to be derived from bone marrow in a liver transplant recipient with recurrent hepatitis (Theise et al. 2000b). Subsequent human investigations with G‐CSF mobilized peripheral blood CD34+ stem cells have shown that these cells are also apparently able to transdifferentiate into hepatocytes, with 4–7% of hepatocytes in female livers being Y chromosome‐positive after the cell transplant from male donors (Korbling et al. 2002). However, it is worth noting that some other studies, examining the contribution of recipient cells to liver allografts in humans, have failed to register any real engraftment in the allografted liver (Fogt et al. 2002; Ng et al. 2003; Wu et al. 2003; see Table 2c).
In undoubtedly the most convincing ‘proof of principle’ demonstration of the potential therapeutic utility of bone marrow, mice with a metabolic liver disease have been cured (Lagasse et al. 2000). Female mice deficient in the enzyme fumarylacetoacetate hydrolase (fah–/–, a model of fatal hereditary tyrosinaemia type 1), a key component of the tyrosine catabolic pathway, can be rescued biochemically by a million unfractionated bone marrow cells that are wild‐type for Fah. Moreover, only purified HSCs (c‐kithighThylowLin−Sca‐1+) were capable of this functional repopulation, with as few as 50 of these cells being capable of hepatic engraftment when haematopoiesis was supported by 2 × 105 fah–/– congenic adult female bone marrow cells. The salient point to arise from this powerful demonstration of the therapeutic potential of bone marrow cells was that, though the initial engraftment was low, approximately one bone marrow cell for every million indigenous hepatocytes, the strong selection pressure exerted thereafter on the engrafted bone marrow cells resulted in their clonal expansion to eventually occupy almost half the liver (Fig. 5). This positive selection was achieved by cycles of withdrawal of NTBC, the compound that blocks the breakdown of tyrosine to fumarylacetoacetate (FAA) in the Fah‐deficient mice, so protecting against liver failure. In the absence of NTBC, FAA accumulates and destroys the hepatocytes; thus the ensuing regenerative stimulus promotes the growth of the engrafted cells. Furthermore, in the absence of NTBC, no engraftment was seen (Wang et al. 2002).
Figure 5.
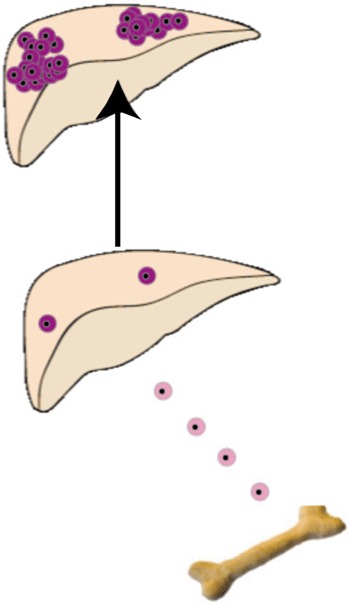
The Fah null mouse can be rescued by wild‐type bone marrow. Even in the severely damaged Fah null liver, low levels of bone marrow engraftment are found (perhaps one for every million indigenous hepatocytes), but following prolonged application of a positive selection pressure for the engrafted cells, achieved by NTBC withdrawal, clonal expansion of the bone marrow cells that have fused with host hepatocytes occurs – leading to the correction of a potentially fatal metabolic liver disease (see text for further details).
However, it now turns out that the new healthy liver cells in the fah–/– mouse contain chromosomes from both the recipient and donor cells, with presumably the donor haematopoietic cell nuclei being reprogrammed when they fused with the unhealthy fah–/– hepatocyte nuclei to become functional hepatocytes (Vassilopoulos et al. 2003; Wang et al. 2003b). The key to this discovery was to perform the gender mismatch bone marrow transplantation experiments the other way round from the usual, i.e. instead of male to female and looking for the Y chromosome in apparently transdifferentiated, say epithelial cells, bone marrow transplants were performed between female donors and male recipients – a cell with a donor‐specific marker and a putative transdifferentiated marker is acceptable, but if it also has a Y chromosome then you know it is cell fusion (Fig. 6). In one experiment, a million donor bone marrow cells (fah+/+) from Fanconi anaemia group C (fancc–/–) homozygous mutant mice were serially transplanted into lethally irradiated fah–/– recipients (Wang et al. 2003b). The usual repopulation (∼50%) of the mutant liver by Fah‐positive hepatocytes was noted, but Southern blot analysis of the purified repopulating cells revealed that they were heterozygous for alleles (fah+/+; fancc–/–) that were unique to the donor marrow – fusion with host liver cells must have occurred. To confirm this conclusion, in a second experiment, fah+/+ bone marrow from ROSA26 female mice was transplanted into male fah knockout mice. Cytogenetic analysis of the LacZ‐positive, sorted bone marrow‐derived hepatocytes revealed that most, if not all had a Y chromosome, thus confirming fusion (Fig. 6). Before bone marrow transplant, most host hepatocytes had a karyotype of either 40,XY or 80,XXYY, but after transplantation with fah+/+ bone marrow, the commonest karyotype of Fah‐positive heptocytes was either 80,XXXY suggesting fusion between a diploid female donor cell and a diploid male host cell, or 120,XXXXYY, suggesting fusion between a female donor diploid blood cell and a tetraploid male host hepatocyte. However, a substantial proportion of bone marrow‐derived hepatocytes were aneuploid, suggesting fusion had created some sort of genetic instability with the hybrid cells randomly shedding chromosomes. In the companion paper (Vassilopoulos et al. 2003) to that of Grompe and colleagues, lineage‐depleted wild‐type bone marrow was transplanted into lethally irradiated female fah–/– mice, and, following withdrawal of NTBC, the usual Fah‐positive nodules emerged 4–5 months later. When restriction enzyme‐digested genomic DNA from these nodules was probed for fah sequences, then the mean level of donor DNA was found to be only 26%, again leading to the conclusion that the donor haematopoietic cells had fused with the host fah–/–, generating polyploid hepatocytes. Fusion of bone marrow cells has also been found to occur in the normal mouse, not only with hepatocytes, but also with Purkinje cells and cardiomyocytes (Alvarez‐Dolado et al. 2003). These were very elegant studies in vivo and in vitro in which a reporter gene was activated only when cells fused. However, unlike the Fah null mouse, no selection pressure (liver damage) was operative, and even after 10 months only 9–59 fused cells/5.5 × 105 hepatocytes were found: importantly, they also found evidence that with time either donor genes had been inactivated or eliminated, again suggestive of genetic instability in heterokaryons.
Figure 6.
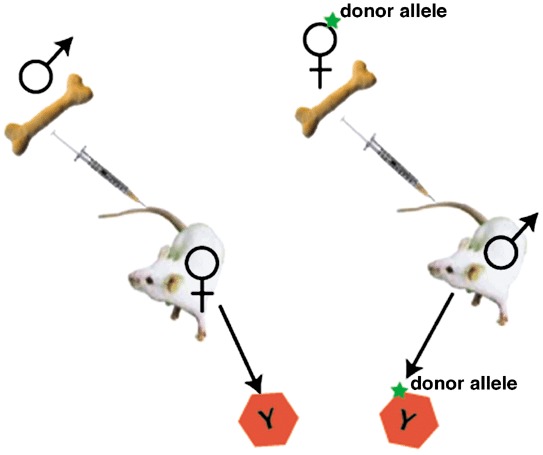
Tracking bone marrow by gender mismatch bone marrow transplantation. Male to female transplantation has traditionally been used for detecting transdifferentiation by detection of the Y chromosome. However, cell fusion may be missed as not all chromosomes might be visible in the tissue section. Female to male transplantation is more suitable for detecting fusion events; marked bone marrow‐derived parenchymal cells with a Y chromosome have definitely been formed by fusion with host cells.
Mouse bone marrow‐derived hepatocytes can also be expanded selectively if they are engineered to over‐express Bcl‐2, and then the indigenous cells are targeted for destruction by an anti‐Fas antibody (Mallet et al. 2002); it will be interesting to know whether cell fusion operates in this model. One could also add that if fusion was responsible for all these observations made in the liver, then clearly these hybrids have a selective growth advantage turning unhealthy hepatocytes into metabolically competent hepatocytes and would not negate the therapeutic potential of bone marrow cells in the liver. Expressing a similar sentiment, Blau (2002) has suggested that if cell fusion was responsible for the apparent reprogramming of certain adult cells, then there is something ‘exciting’ about rescuing damaged cells through fusion, with, for example, bone marrow‐derived cells providing a healthy and entire genetic complement, even one that has been manipulated for gene therapy. As in the human arena, not all murine studies are in complete accord, e.g. Terai et al. (2003) report an impressive 25% contribution of bone marrow to the parenchyma after CCL4, but Kanazawa & Verma (2003) failed to find any evidence for bone marrow engraftment in three models of chronic liver injury, including CCL4 (see Table 2b). However, many studies have testified as to the ability of human cord blood cells to transdifferentiate into hepatocytes in the liver of the immunodeficient mouse (Table 2d), albeit at a low level.
While it seems logical to believe that parenchymal damage is a stimulus to hepatic engraftment by HSCs, the molecules that mediate this homing reaction to the liver are not well understood. Petrenko et al. (1999) speculated that in mice the molecule AA4 (murine homologue of the C1q receptor protein) may be involved in the homing of haematopoietic progenitors to the foetal liver – maybe this receptor protein is expressed on HSCs that engraft to the damaged liver? Another alternative is that cells in the liver express the stem cell chemoattractant ‘stromal derived factor‐1’ (SDF‐1) for which HSCs have the appropriate receptor known as CXCR4 (Whetton & Graham 1999). Hatch et al. (2002) have persuasive evidence that SDF‐1 is involved in oval cell activation, furthermore speculating that this chemokine may secondarily recruit bone marrow to the injured liver. More definitive proof was provided by Kollet et al. (2003) who observed increased SDF‐1 expression (particularly in biliary epithelia) after parenchymal damage, and concomitant with such damage was increased HGF production, a cytokine that was very effective in promoting protrusion formation and CXCR4 up‐regulation in human CD34+ haematopoietic progenitors.
CONCLUSIONS
This review has highlighted recent progress in identifying cells with hepatic potential in rodents and humans. The chronic shortage of livers for orthotopic liver transplantation has provided a strong impetus for the search for alternative sources of cellular therapy, in particular hepatocyte or even bone marrow transplants. The clinical need for healthy functioning hepatocytes is very clear, not only for the correction of inherited metabolic liver disease, but also for acute liver failure, hepatocellular carcinoma, cirrhosis, bioartificial liver support, hepatotropic viral studies and drug toxicity testing. Hepatocyte transplants have been moderately successful in humans, but clearly there is a need to enrich for cells with potent clonogenic potential; studies in rodents have gone some way to the identification of such cells in these species. Clearly, the key to the success of hepatocyte transplants will be to selectively enhance the growth of the transplanted cells, but unfortunately treatments such as retrorsine (used with conspicuous success in rats) are not clinically acceptable.
Turning to the vexacious subject of bone marrow stem cells as a source of hepatocytes, then the jury is still very much out. Impressive functional re‐population has been achieved in the Fah‐deficient mouse liver, though this has been achieved through cell fusion between bone marrow cells and deficient hepatocytes. If deficient cells are re‐programmed in this way then that per se is not necessarily a bad thing, but if the formed heterokaryons are genetically unstable, then this has significant pathological implication. It also begs the question whether this process is going on all the time in healthy individuals without experimental manipulation such as irradiation and bone marrow transplantation? Like hepatocyte transplants, the key to success will be the selective amplification of bone marrow‐derived hepatocytes; studies in the Fah‐deficient mouse indicate that the level of initial engraftment is independent of damage, though subsequent clonal expansion is very much dependent on the induction of liver failure. Alternatively, other models of chronic liver injury have failed to detect bone marrow‐derived hepatocytes; such conundrums will exercise investigators in this exciting field over the next few years – watch this space.
REFERENCES
- Agelli M, Dello Sbarba P, Halay ED , Faris RA, Hixson DE, Reid LM (1997) Putative liver progenitor cells: conditions for long‐term survival in culture. Histochem. J. 29, 205. [DOI] [PubMed] [Google Scholar]
- Alison MR (1998) Liver stem cells: a two‐compartment system. Curr. Opin. Cell Biol. 10, 710. [DOI] [PubMed] [Google Scholar]
- Alison MR (2003) Tissue‐based stem cells: ABC transporter proteins take centre stage. J. Pathol. 200, 547. [DOI] [PubMed] [Google Scholar]
- Alison MR, Golding MH, Sarraf CE (1996a) Pluripotential liver stem cells: facultative stem cells located in the biliary tree. Cell Prolif. 29, 373. [DOI] [PubMed] [Google Scholar]
- Alison MR, Golding M, Sarraf CE, Edwards RJ, Lalani EN (1996b) Liver damage in the rat induces hepatocyte stem cells from biliary epithelial cells. Gastroenterology 110, 1182. [DOI] [PubMed] [Google Scholar]
- Alison M, Golding M, Lalani EN, Nagy P, Thorgeirsson S, Sarraf C (1997a) Wholesale hepatocytic differentiation in the rat from ductular oval cells, the progeny of biliary stem cells. J. Hepatol. 26, 343. [DOI] [PubMed] [Google Scholar]
- Alison MR, Golding M, Sarraf CE (1997b) Liver stem cells: when the going gets tough they get going. Int. J. Exp. Path. 78, 365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alison MR, Golding M, Lalani E‐N, Sarraf CE (1998) Wound healing in the liver with particular reference to stem cells. Phil. Trans. R. Soc. Lond. B. 353, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alison MR, Poulsom R, Jeffery R, Dhillon AP, Quaglia A, Jacob J, Novelli M, Prentice G, Williams J, Wright NA (2000) Hepatocytes from non‐hepatic adult stem cells. Nature 406, 257. [DOI] [PubMed] [Google Scholar]
- Alison MR, Poulsom R, Forbes S, Wright NA (2002) An introduction to stem cells. J. Pathol. 197, 419. [DOI] [PubMed] [Google Scholar]
- Alvarez‐Dolado M, Pardal R, Garcia‐Verdugo JM , Fike JR, Lee HO, Pfeffer K, Lois C, Morrison SJ, Alvarez‐Buylla A (2003) Fusion of bone‐marrow‐derived cells with Purkinje neurons, cardiomyocytes and hepatocytes. Nature 425, 968. [DOI] [PubMed] [Google Scholar]
- Avital I, Inderbitzin D, Aoki T, Tyan DB, Cohen AH, Ferraresso C, Rozga J, Arnaout WS, Demetriou AA (2001) Isolation, characterization, and transplantation of bone marrow‐derived hepatocyte stem cells. Biochem. Biophys. Res. Commun. 288, 156. [DOI] [PubMed] [Google Scholar]
- Baumann U, Crosby HA, Ramani P, Kelly DA, Strain AJ (1999) Expression of the stem cell factor receptor c‐kit in normal and diseased pediatric liver: identification of a human hepatic progenitor cell? Hepatology 30, 112. [DOI] [PubMed] [Google Scholar]
- Bisgaard HC, Holmskov U, Santoni‐Rugiu E, Nagy P, Nielsen O, Ott P, Hage E, Dalhoff K, Rasmussen LJ, Tygstrup N (2002) Heterogeneity of ductular reactions in adult rat and human liver revealed by novel expression of deleted in malignant brain tumor 1. Am. J. Pathol. 161, 1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blau HM (2002) A twist of fate. Nature 419, 437. [DOI] [PubMed] [Google Scholar]
- Couchie D, Holic N, Chobert MN, Corlu A, Laperche Y (2002) In vitro differentiation of WB‐F344 rat liver epithelial cells into the biliary lineage. Differentiation 69, 209. [DOI] [PubMed] [Google Scholar]
- Dabeva MD, Petkov PM, Sandhu J, Oren R, Laconi E, Hurston E, Shafritz DA (2000) Proliferation and differentiation of fetal liver epithelial progenitor cells after transplantation into adult rat liver. Am. J. Pathol. 156, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlke MH, Popp FC, Bahlmann FH, Aselmann H, Jager MD, Neipp M, Piso P, Klempnauer J, Schlitt HJ (2003) Liver regeneration in a retrorsine/CCl4‐induced acute liver failure model: do bone marrow‐derived cells contribute? J. Hepatol. 39, 365. [DOI] [PubMed] [Google Scholar]
- Danet GH, Luongo JL, Butler G, Lu MM, Tenner AJ, Simon MC, Bonnet DA (2002) C1qRp defines a new human stem cell population with hematopoietic and hepatic potential. Proc. Natl Acad. Sci. USA 99, 10441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkowski O, An HJ, Ianus IA, Chiriboga L, Yee H, West AB, Theise ND (2003) Regeneration of hepatocyte ‘buds’ in cirrhosis from intrabiliary stem cells. J. Hepatol. 39, 357. [DOI] [PubMed] [Google Scholar]
- Fiegel HC, Kluth J, Lioznov MV , Holzhuter S, Fehse B, Zander AR, Kluth D (2003a) Hepatic lineages isolated from developing rat liver show different ways of maturation. Biochem. Biophys. Res. Commun. 305, 46. [DOI] [PubMed] [Google Scholar]
- Fiegel HC, Park JJ, Lioznov MV , Martin A, Jaeschke‐Melli S, Kaufmann PM, Fehse B, Zander AR, Kluth D (2003b) Characterization of cell types during rat liver development. Hepatology 37, 148. [DOI] [PubMed] [Google Scholar]
- Fogt F, Beyser KH, Poremba C, Zimmerman RL, Khettry U, Ruschoff J (2002) Recipient‐derived hepatocytes in liver transplants: a rare event in sex‐mismatched transplants. Hepatology 36, 173. [DOI] [PubMed] [Google Scholar]
- Fox IJ, Chowdhury JR, Kaufman SS, Goertzen TC, Chowdhury NR, Warkentin PI, Dorko K, Sauter BV, Strom SC (1998) Treatment of the Crigler–Najjar syndrome type I with hepatocyte transplantation. N. Engl. J. Med. 338, 1422. [DOI] [PubMed] [Google Scholar]
- Fujii H, Hirose T, Oe S, Yasuchika K, Azuma H, Fujikawa T, Nagao M, Yamaoka Y (2002) Contribution of bone marrow cells to liver regeneration after partial hepatectomy in mice. J. Hepatol. 36, 653. [DOI] [PubMed] [Google Scholar]
- Goodell MA, Brose K, Paradis G, Conner AS, Mulligan RC (1996) Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo . J. Exp. Med. 183, 1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon GJ, Coleman WB, Hixson DC, Grisham JW (2000) Liver regeneration in rats with retrorsine‐induced hepatocellular injury proceeds through a novel cellular response. Am. J. Pathol. 156, 607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon GJ, Butz. GM, Grisham JW, Coleman WB (2002) Isolation, short‐term culture, and transplantation of small hepatocyte‐like progenitor cells from retrorsine‐exposed rats. Transplantation 73, 1236. [DOI] [PubMed] [Google Scholar]
- Guha C, Parashar B, Deb NJ, Garg M, Gorla GR, Singh A, Roy‐Chowdhury N, Vikram B, Roy‐Chowdhury J (2002) Normal hepatocytes correct serum bilirubin after repopulation of Gunn rat liver subjected to irradiation/partial resection. Hepatology 36, 354. [DOI] [PubMed] [Google Scholar]
- Hatch HM, Zheng D, Jorgensen ML, Petersen BE (2002) SDF‐1α/CXCR4: a mechanism for hepatic oval cell activation and bone marrow stem cell recruitment to the injured liver of rats. Cloning Stem Cells 4, 339. [DOI] [PubMed] [Google Scholar]
- Hixson DC, Chapman L, McBride A et al. (1997) Antigenic phenotypes common to rat oval cells, primary hepatocellular carcinomas and developing bile ducts. Carcinogenesis 18, 1169. [DOI] [PubMed] [Google Scholar]
- Hixson DC, Brown J, McBride AC, Affigne S (2000) Differentiation status of rat ductal cells and ethionine‐induced hepatic carcinomas defined with surface‐reactive monoclonal antibodies. Exp. Mol. Pathol. 68, 152. [DOI] [PubMed] [Google Scholar]
- Holic N, Suzuki T, Corlu A, Couchie D, Chobert NM, Guguen‐Guillouzo C, Laperche Y (2000) Differential expression of the rat γ‐glutamyl transpeptidase gene promoters along with differentiation of hepatoblasts into biliary or hepatocytic lineage. Am. J. Pathol. 157, 537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horslen SP, McCowan TC, Goertzen TC, Warkentin PI, Cai HB, Strom SC, Fox IJ (2003) Isolated hepatocyte transplantation in an infant with a severe urea cycle disorder. Pediatrics 111, 1262. [DOI] [PubMed] [Google Scholar]
- Ishikawa F, Drake CJ, Yang S, Fleming P, Minamiguchi H, Visconti RP, Crosby CV, Argraves WS, Harada M, Key LL Jr, Livingston AG, Wingard JR, Ogawa M (2003) Transplanted human cord blood cells give rise to hepatocytes in engrafted mice. Ann. NY Acad. Sci. 996, 174. [DOI] [PubMed] [Google Scholar]
- Kakinuma S, Tanaka Y, Chinzei R, Watanabe M, Shimizu‐Saito K, Hara Y, Teramoto K, Arii S, Sato C, Takase K, Yasumizu T, Teraoka H (2003) Human umbilical cord blood as a source of transplantable hepatic progenitor cells. Stem Cells 21, 217. [DOI] [PubMed] [Google Scholar]
- Kanazawa Y, Verma IM (2003) Little evidence of bone marrow‐derived hepatocytes in the replacement of injured liver. Proc. Natl Acad. Sci. USA 100, 11850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kano J, Noguchi M, Kodama M, Tokiwa T (2000) The in vitro differentiating capacity of nonparenchymal epithelial cells derived from adult porcine livers. Am. J. Pathol. 156, 2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplanski C, Pauley CJ, Griffiths TG, Kawabata TT, Ledwith BJ (2000) Differentiation of rat oval cells after activation of peroxisome proliferator‐activated receptor α43. Cancer Res. 60, 580. [PubMed] [Google Scholar]
- Kleeberger W, Rothamel T, Glockner S, Flemming P, Lehmann U, Kreipe H (2002) High frequency of epithelial chimerism in liver transplants demonstrated by microdissection and STR‐analysis. Hepatology 35, 110. [DOI] [PubMed] [Google Scholar]
- Kollet O, Shivtiel S, Chen YQ, Suriawinata J, Thung SN, Dabeva MD, Kahn J, Spiegel A, Dar A, Samira S, Goichberg P, Kalinkovich A, Arenzana‐Seisdedos F, Nagler A, Harden I, Revel M, Shafritz DA, Lapidot T (2003) HGF, SDF‐1, and MMP‐9 are involved in stress‐induced human CD34+ stem cell recruitment to the liver. J. Clin. Invest. 112, 160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korbling M, Katz RL, Khanna A, Ruifrok AC, Rondon G, Albitar M, Champlin RE, Estrov Z (2002) Hepatocytes and epithelial cells of donor origin in recipients of peripheral‐blood stem cells. N. Engl. J. Med. 346, 738. [DOI] [PubMed] [Google Scholar]
- Kubota H, Reid LM (2000) Clonogenic hepatoblasts, common precursors for hepatocytic and biliary lineages, are lacking classical major histocompatibility complex class I antigen. Proc. Natl Acad. Sci. USA 97, 12132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laconi E, Oren R, Mukhopadhyay DK, Hurston E, Laconi S, Pani P, Dabeva MD, Shafritz DA (1998) Long‐term, near‐total liver replacement by transplantation of isolated hepatocytes in rats treated with retrorsine. Am. J. Pathol. 153, 319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laconi S, Pillai S, Porcu PP, Shafritz DA, Pani P, Laconi E (2001) Massive liver replacement by transplanted hepatocytes in the absence of exogenous growth stimuli in rats treated with retrorsine. Am. J. Pathol. 158, 771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagasse E, Connors H, Al‐Dhalimy M, Reitsma M, Dohse M, Osborne L, Wang X, Finegold M, Weissman IL, Grompe M (2000) Purified hematopoietic stem cells can differentiate into hepatocytes in vivo . Nature Med. 6, 1229. [DOI] [PubMed] [Google Scholar]
- Lemmer ER, Shepard EG, Blakolmer K, Kirsch RE, Robson SC (1998) Isolation from human fetal liver of cells co‐expressing CD34 haematopoietic stem cell and CAM 5.2 pancytokeratin markers. J. Hepatol. 29, 450. [DOI] [PubMed] [Google Scholar]
- Lowes KN, Brennan BA, Yeoh GC, Olynyk JK (1999) Oval cell numbers in human chronic liver diseases are directly related to disease severity. Am. J. Pathol. 154, 537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallet VO, Mitchell C, Mezey E, Fabre M, Guidotti JE, Renia L, Coulombel L, Kahn A, Gilgenkrantz H (2002) Bone marrow transplantation in mice leads to a minor population of hepatocytes that can be selectively amplified in vivo . Hepatology 35, 799. [DOI] [PubMed] [Google Scholar]
- Mugford CA, Kedderis GL (1998) Sex‐dependent metabolism of xenobiotics. Drug Metab. Rev. 30, 441. [DOI] [PubMed] [Google Scholar]
- Muraca M, Gerunda G, Neri D, Vilei MT, Granato A, Feltracco P, Meroni M, Giron G, Burlina AB (2002) Hepatocyte transplantation as a treatment for glycogen storage disease type 1a. Lancet 359, 317. [DOI] [PubMed] [Google Scholar]
- Nagai H, Terada K, Watanabe G, Aiba N, Shibuya T, Kawagoe M, Kameda T, Sato M, Senoo H, Sugiyama T (2002) Differentiation of liver epithelial (stem‐like) cells into hepatocytes induced by coculture with hepatic stellate cells. Biochem. Biophys. Res. Commun. 293, 1420. [DOI] [PubMed] [Google Scholar]
- Newsome PN, Johannessen I, Boyle S , Dalakas E, McAulay KA, Samuel K, Rae F, Forrester L, Turner ML, Hayes PC, Harrison DJ, Bickmore WA (2003) Human cord blood‐derived cells can differentiate into hepatocytes in the mouse liver with no evidence of cellular fusion. Gastroenterology 124, 1891. [DOI] [PubMed] [Google Scholar]
- Ng IO, Chan KL, Shek WH, Lee JM, Fong DY, Lo CM, Fan ST (2003) High frequency of chimerism in transplanted livers. Hepatology 38, 989. [DOI] [PubMed] [Google Scholar]
- Oertel M, Rosencrantz R, Chen YQ, Thota PN, Sandhu JS, Dabeva MD, Pacchia AL, Adelson ME, Dougherty JP, Shafritz DA (2003) Repopulation of rat liver by fetal hepatoblasts and adult hepatocytes transduced ex vivo with lentiviral vectors. Hepatology 37, 994. [DOI] [PubMed] [Google Scholar]
- Omori N, Omori M, Evarts RP, Teramoto T, Miller MJ, Hoang TN, Thorgeirsson SS (1997) Partial cloning of rat CD34 cDNA and expression during stem cell‐dependent liver regeneration in the adult rat. Hepatology 26, 720. [DOI] [PubMed] [Google Scholar]
- Oren R, Dabeva MD, Karnezis AN, Petkov PM, Rosencrantz R, Sandhu JP, Moss SF, Wang S, Hurston E, Laconi E, Holt PR, Thung SN, Zhu L, Shafritz DA (1999) Role of thyroid hormone in stimulating liver repopulation in the rat by transplanted hepatocytes. Hepatology 30, 903. [DOI] [PubMed] [Google Scholar]
- Overturf K, Al‐Dhalimy M, Ou CN, Finegold M, Grompe M (1997) Serial transplantation reveals the stem‐cell‐like regenerative potential of adult mouse hepatocytes. Am. J. Pathol. 151, 1273. [PMC free article] [PubMed] [Google Scholar]
- Paku S, Schnur J, Nagy P, Thorgeirsson SS (2001) Origin and structural evolution of the early proliferating oval cells in rat liver. Am. J. Pathol. 158, 1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen BE (2001) Hepatic stem cells: coming full circle. Blood Cells, Molecules, Diseases 27, 590. [DOI] [PubMed] [Google Scholar]
- Petersen BE, Goff JP, Greenberger JS, Michalopoulos GK (1998) Hepatic oval cells express the hematopoietic stem cell marker Thy‐1 in the rat. Hepatology 27, 433. [DOI] [PubMed] [Google Scholar]
- Petersen BE, Bowen WC, Patrene KD, Mars MW, Sullivan AK, Murase N, Boggs SS, Greenberger JS, Goff JP (1999) Bone marrow as a potential source of hepatic oval cells. Science 284, 1168. [DOI] [PubMed] [Google Scholar]
- Petkov PM, Kim K, Sandhu J et al (2000) Identification of differentially expressed genes in epithelial stem/progenitor cells of fetal rat liver. Genomics 68, 197. [DOI] [PubMed] [Google Scholar]
- Petrenko O, Beavis A, Klaine M, Kittappa R, Godin I, Lemischka IR (1999) The molecular characterization of the fetal stem cell marker AA4. Immunity 10, 691. [DOI] [PubMed] [Google Scholar]
- Rao MS, Reddy JK (1995) Hepatic transdifferentiation in the pancreas. Semin. Cell Biol. 6, 151. [DOI] [PubMed] [Google Scholar]
- Ros JE, Libbrecht L, Geuken M, Jansen JL, Roskams TA (2003) High expression of MDR1, MRP1 and MRP3 in the hepatic progenitor cell compartment and hepatocytes in severe human liver disease. J. Pathol. 200, 553. [DOI] [PubMed] [Google Scholar]
- Sandhu JS, Petkov PM, Dabeva MD, Shafritz DA (2001) Stem cell properties and repopulation of the rat liver by fetal liver epithelial progenitor cells. Am. J. Pathol. 159, 1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharenberg CW, Harkey MA, Torok‐Storb B (2002) The ABCG2 transporter is an efficient Hoechst 33342 efflux pump and is preferentially expressed by immature human hematopoietic progenitors. Blood 99, 507. [DOI] [PubMed] [Google Scholar]
- Scheffer GL, Kool M, De Haas M, De Vree JM, Pijnenborg AC, Borman DK, Elferink RP, Van Der Valk P, Borst P, Scheper RJ (2002) Tissue distribution and induction of human multidrug resistant protein 3. Laboratory Invest. 82, 193. [DOI] [PubMed] [Google Scholar]
- Schwartz RE, Reyes M, Koodie L, Jiang Y, Blackstad M, Lund T, Levnik T, Johnson S, Hu WS, Verfailie CM (2002) Multipotent adult progenitor cells from bone marrow differentiate into functional hepatocyte‐like cells. J. Clin. Invest. 109, 1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafritz DA, Dabeva MD (2002) Liver stem cells and model systems for liver repopulation. J. Hepatol. 36, 552. [DOI] [PubMed] [Google Scholar]
- Shen CN, Slack JM, Tosh D (2000) Molecular basis of transdifferentiation of pancreas to liver. Nat. Cell Biol. 2, 879. [DOI] [PubMed] [Google Scholar]
- Shimano K, Satake M, Okaya A, Kitanaka J, Kitanaka N, Takemura M, Sakagami M, Terada N, Tsujimura T (2003) Hepatic oval cells have the side population phenotype defined by expression of ATP‐binding cassette transporter ABCG2/BCRP1. Am. J. Pathol. 163, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A, Zheng Y, Kondo R, Kusakabe M, Takada Y, Fukao K, Nakauchi H, Taniguchi H (2000) Flow‐cytometric separation and enrichment of hepatic progenitor cells in the developing mouse liver. Hepatology 32, 1230. [DOI] [PubMed] [Google Scholar]
- Suzuki A, Zheng YW, Kaneko S, Onodera M, Fukao K, Nakauchi H, Taniguchi H (2002) Clonal identification and characterization of self‐renewing pluripotent stem cells in the developing liver. J. Cell Biol. 156, 173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada K, Roy‐Chowdhury N, Prasad V, Kim BH, Manchikalapudi P, Fox IJ, Van Duijvendijk P, Bosma PJ, Roy‐Chowdhury J (1998) Long‐term amelioration of bilirubin glucuronidation defect in Gunn rats by transplanting genetically modified immortalized autologous hepatocytes. Cell Transplant. 7, 607. [DOI] [PubMed] [Google Scholar]
- Tateno C, Yoshizato K (1996) Long‐term cultivation of adult rat hepatocytes that undergo multiple cell divisions and express normal parenchymal phenotypes. Am. J. Pathol. 148, 383. [PMC free article] [PubMed] [Google Scholar]
- Taupin D, Podolsky DK (2003) Trefoil factors: initiators of mucosal healing. Nat. Rev. Mol. Cell Biol. 4, 721. [DOI] [PubMed] [Google Scholar]
- Terai S, Sakaida I, Yamamoto N, Omori K, Watanabe T, Ohata S, Katada T, Miyamoto K, Shinoda K, Nishina H, Okita K (2003) An in vivo model for monitoring trans‐differentiation of bone marrow cells into functional hepatocytes. J. Biochem. (Tokyo) 134, 551. [DOI] [PubMed] [Google Scholar]
- Theise ND, Saxena R, Portmann BC, Thung SN, Yee H, Chiriboga L, Kumar A, Crawford JM (1999) The canals of Hering and hepatic stem cells in humans. Hepatology 30, 1425. [DOI] [PubMed] [Google Scholar]
- Theise ND, Badve S, Saxena R, Henegariu O, Sell S, Crawford JM, Krause DS (2000a) Derivation of hepatocytes from bone marrow cells in mice after radiation induced myeloablation. Hepatology 31, 235. [DOI] [PubMed] [Google Scholar]
- Theise ND, Nimmakalu M, Gardner R, Illei PB, Morgan G, Teperman L, Henegariu O, Krause DS (2000b) Liver from bone marrow in humans. Hepatology 32, 11. [DOI] [PubMed] [Google Scholar]
- Vassilopoulos G, Wang PR, Russell DW (2003) Transplanted bone marrow regenerates liver by cell fusion. Nature 422, 901. [DOI] [PubMed] [Google Scholar]
- Wang X, Al‐Dhalimy M, Lagasse E, Finegold M, Grompe M (2001) Liver repopulation and correction of metabolic liver disease by transplanted adult mouse pancreatic cells. Am. J. Pathol. 158, 571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Montini E, Al‐Dhalimy M, Lagasse E, Finegold M, Grompe M (2002) Kinetics of liver repopulation after bone marrow transplantation. Am. J. Pathol. 161, 565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Ge S, Mcnamara G, Hao QL, Crooks GM, Nolta JA (2003a) Albumin‐expressing hepatocyte‐like cells develop in the livers of immune‐deficient mice that received transplants of highly purified human hematopoietic stem cells. Blood 101, 4201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Willenbring H, Akkari Y, Torimaru Y, Foster M, Al‐Dhalimy M, Lagasse E, Finegold M, Olson S, Grompe M (2003b) Cell fusion is the principal source of bone‐marrow‐derived hepatocytes. Nature 422, 897. [DOI] [PubMed] [Google Scholar]
- Whetton AD, Graham GJ (1999) Homing and mobilization in the stem cell niche. Trends Cell Biol. 9, 233. [DOI] [PubMed] [Google Scholar]
- Wu T, Cieply K, Nalesnik MA, Randhawa PS, Sonzogni A, Ballamy C, Abu‐Elmagd K, Michalopolous GK, Jaffe R, Kormos RL, Gridelli B, Fung JJ, Demetris AJ (2003) Minimal evidence of transdifferentiation from recipient bone marrow to parenchymal cells in regenerating and long‐surviving human allografts. Am. J. Transplant. 3, 1173. [DOI] [PubMed] [Google Scholar]
- Yasui O, Miura N, Terada K, Kawarada Y, Koyama K, Sugiyama T (1997) Isolation of oval cells from Long‐Evans Cinnamon rats and their transformation into hepatocytes in vivo in the rat liver. Hepatology 25, 329. [DOI] [PubMed] [Google Scholar]
- Yin L, Lynch D, Sell S (1999) Participation of different cell types in the restitutive response of the rat liver to periportal injury induced by allyl alcohol. J. Hepatol. 31, 497. [DOI] [PubMed] [Google Scholar]
- Yin L, Sun M, Ilic Z, Leffert HL, Sell S (2002) Derivation, characterization, and phenotypic variation of hepatic progenitor cell lines isolated from adult rats. Hepatology 35, 315. [DOI] [PubMed] [Google Scholar]


