Abstract
The identification of cell-free circulating mitochondrial DNA (ccf-mtDNA) in early stage Alzheimer’s disease (AD) raised the possibility that the same neurodegenerative effect could be seen in Parkinson’s disease (PD). Here and for the first time, we investigated the role of ccf-mtDNA in PD, identifying a significant reduction of ccf-mtDNA in PD patient cerebrospinal fluid (CSF) when compared to controls.
Our data demonstrates that CSF ccf-mtDNA is not only a powerful biomarker for PD but, given that the effect is also seen in AD, is likely a biomarker for neurodegeneration.
1. Introduction
The identification of early clinical biomarkers of Parkinson’s disease (PD) is increasingly important to developing neuroprotective strategies in a rapidly ageing population. Currently, the typical clinical diagnosis of PD is based on the observation of several cardinal indicators, including tremor, bradykinesia, rigidity and postural instability,[1] however pathological process leading to PD can begin decades before these symptoms develop. Advances in imaging technologies and diagnostic algorithms are helping, but translating into understanding neurodegenerative predisposition is critical in efficient early detection and differential diagnosis.
The links between mitochondria and the pathoetiology of PD are well studied, beginning in the 1970s when the potent respiratory chain inhibitor MPTP was shown to cause parkinsonism in illicit drug users,[2] through the identification of complex-I mediated reactive oxygen species formation in PD brains [3] and continuing recently, where inherited mitochondrial DNA (mtDNA) variants have been shown to affect PD risk.[4]
The role of circulating cell-free (ccf) mtDNA in plasma, has been used to discriminate between breast tumor cases and controls [5] and recent work indicates that ccf-mtDNA levels increase in response to injury related immune activation.[6] Moreover, cerebrospinal fluid (CSF) investigations in pre-clinical Alzheimer’s disease (AD) patients indicate that ccf-mtDNA levels are decreased when compared to matched controls, suggesting that a reduction in ccf-mtDNA may be a biomarker for early stage neurodegenerative disease.[7] Given the pathological similarities between PD and AD (neuronal cell death), we hypothesized that ccf-mtDNA could also be an early indicator of PD pathology.
2. Materials and Methods
Subjects
56 CSF samples, from a UK community-based PD study (males=34 and females=19, mean ages=66.5 StDev=10.5 and =62.7 StDev=7.8 years respectively), [8] were selected alongside 10 age-matched asymptomatic UK control CSF samples (males=6 and females=4, mean ages=61.2 StDev=7.6 and =60.1 StDev=2.7 years respectively). All PD cases were assessed locally by a neurologist using UK-PD Society brain bank criteria for the diagnosis of PD.[9] Disease duration < 1 year (disease duration mean=5.4 months, StDev=4.9 months).
At baseline, all PD cases underwent cognitive assessment; global cognitive function was assessed using the mini-mental state examination (MMSE)[10] and the Montreal Cognitive Assessment (MoCA).[11] Attention was measured using Cognitive Drug Research (CDR, Goring-on-Thames, UK) computer testing.[12] Memory was assessed using Pattern Recognition Memory (PRM) and Spatial Recognition Memory (SRM) from the computerized Cambridge Neuropsychological Test Automated Battery (CANTAB) battery.[8, 13] Mild cognitive impairment (MCI) was determined using published criteria (using 1.5 SD as a cut-off).[8] Testing was repeated after 18 months.
Additionally, samples were genotyped for variants known to affective cognitive function: rs9468 (defining H1/H2, MAPT) and rs429358/rs7142 (defining APOE1-4).[14] Genotyping was performed by KASPtm genotyping (LGC, Middlesex, UK).
CSF Samples
Lumbar puncture (LP) was performed on consenting PD cases (n=56) (lateral or sitting position, depending on the flexibility of the patient and presence of tremor) as part of the ICICLE-PD study,[8] within a four month period after initial cognitive assessment (t=0 months) and was repeated in a subset of cases (n=30) after 18 months (t=18 months), again within a four month period of the repeat cognitive assessment.
Control CSF (n=10) was taken from asymptomatic age-matched controls in the same manner. To standardise collections, all LPs were performed between 8 and 10am after an overnight fast and whilst withholding Parkinson’s mediations, as some biomarkers may be altered by circadian rhythms and by medication use.[15] Samples that were free of visual contamination by blood were centrifuged within 15 minutes of collection at 2000g at 4°C for 10 minutes, with the supernatant then divided into aliquots of 0.25mL and frozen at -80°C in labelled polypropylene cryovials which do not influence biomarker outcome.[15] CSF-tau, -phosphorylated-tau and –synuclein were measured in CSF using previously published methodology.[8]
mtDNA copy number determination
Quantification of mitochondrial DNA was performed in triplicate by multiplex Taqman qPCR amplification of the mitochondrial genes MTND1 and MTND4 and the nuclear encoded gene B2M, using serial dilutions of cloned vectors to ensure reaction linearity and for standard curve quantification [16]. Similar to previous work, samples containing >1 copies B2M per sample (n=2) were rejected, ruling out the possibility of CSF contamination with DNA from peripheral cells [7]. mtDNA copy number is expressed copies per microliter of using both MTDN1 and MTDN4. Case and control samples were randomly assigned to each run to limit run-specific stratification.
Validation of qPCR results
A random proportion (10%) of samples were replicated from source DNA, with a coefficient or repeat variance estimated as 0.35% (indicating that qPCR results are reproducible).
Statistical Analysis
Data were analyzed using SPSS v22 with data appropriate tests (detailed in text). Statistical significance was set at P=<0.05.
3. Results
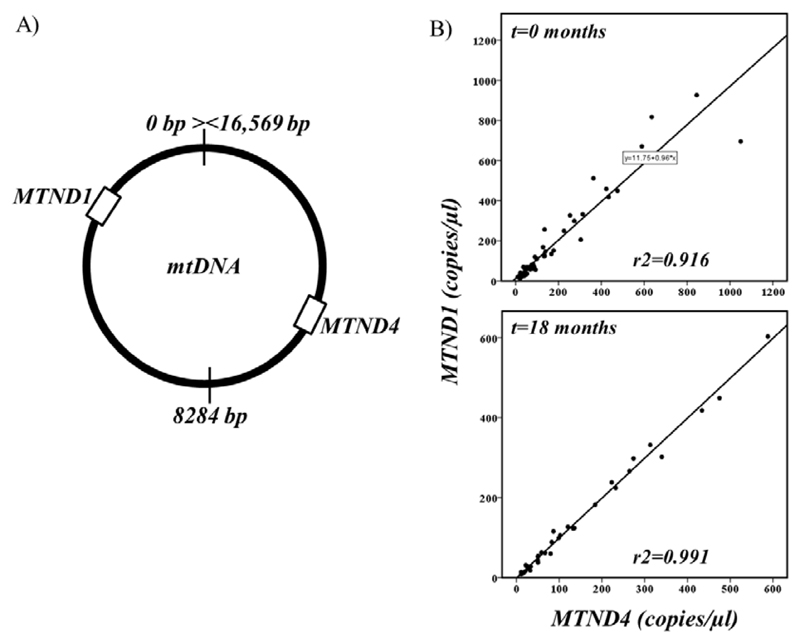
Similar to a previous study [7], we were able to detect and accurately quantify ccf-mtDNA from low volumes of prepared CSF with high, whole, mtDNA target specificity. High correlation of both opposing mtDNA regions (Figure 1) indicates that the mtDNA molecule is likely intact and comparative assessment of amplicon ratios [17] did not indicate the presence of mtDNA deletions or the presence of nDNA.
Figure 1.
(A) Cartoon showing the relative position of both ccf-mtDNA real-time PCR targets (MTND1 and MTND4). (B) Correlation of MTND1 and MTND4 copy-number from PD cases and controls at t=0 months (n=64) and cases at t=18 months (n=30).
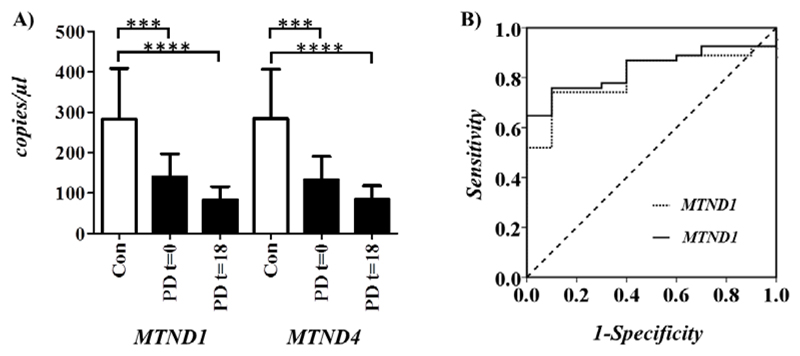
Comparison of ccf-mtDNA showed a significant reduction in PD cases compared to controls, when analyzing either MTND1 (Mann-Whitney P=2.0x10-3) or MTND4 (Mann-Whitney P=1.0x10-3) copy-number (Figure 2A). This is supported by receiver operating characteristic curve analysis, which indicates that MTND1 and MTND4 calculated ccf-mtDNA are strong predictors of PD status (MTND1 P= 2x10-2 and area under curve = 0.81 and MTND4 P= 1x10-2 and area under curve = 0.84), Figure 2B) [18]. Subsequent assessment of t=18 month CSF ccf-mtDNA confirmed the previous reduction in PD cases when compared to controls in both MTND1 (Mann-Whitney P=1.2x10-4) or MTND4 (Mann-Whitney P=5.9x10-4) (Figure 2A), but was not significantly reduced between t=0 and t=18 months (MTND1, Mann-Whitney P=2.4x10-1 and MTND4 Mann-Whitney P=5.1x10-1) in PD cases.
Figure 2.
A) Analysis of cell free circulating mtDNA (ccf-mtDNA) in CSF, analysed using two mtDNA targets: MTND1 and MTDN4 showing mean mtDNA copy number per microliter CSF (with 95% CI) for PD cases at t=0 /t=18 months (shaded) and matched controls (unshaded). Asterisks indicate statistical significance (***P=10-3 and ***P=10-4). B) Receiver operating characteristic curve analysis of MTND1 (dotted line) and MTND4 (solid line) calculated ccf-mtDNA; where area under curve (AUC) =0.81 =0.84 and P=2x10-2 P=1x10-2, respectively. Dashed line indicates reference line.
Phenotypic analysis found no association between patient age or gender to either t=0 or t=18 month ccf-mtDNA (both MTND1 or MTND4) (Table 1). In addition we found no significant correlation between PD patient ccf-mtDNA when analysed as individual cardinal measurements of cognitive function or when stratified as mild-cognitive impairment/cognitively normal (Table 1). Moreover, we found no significant association when stratifying PD cases as either ‘tremor dominant’ or ‘postural instability gait difficulty’, phenotypic sub-types (Table 1) [19]. To investigate further, we correlated ccf-mtDNA to CSF-tau, -phosphorylated tau (pTau) and -α-synuclein, but failed to find a significant correlation (Table 1). At a genetic level, we found no direct correlation between MAPT (H1/H2) or APOE (E1/2/3/4) genotypes and ccf-mtDNA (Table1). Although not significant (P=7.2x10-1) we did observe an inverse trend when correlating APOE genotype to t=0 month ccf-mtDNA, with ccf-mtDNA paradoxically increasing with APOE risk alleles.
Table 1.
Comparative statistical analysis of ccf-mtDNA copy-number in PD cases to (A) age, gender and age of onset, (B) assessments of cognitive function (Montreal cognitive assessment score (MoCA), the mini-mental state examination score (MMSE) the unified Parkinson's disease rating scale (UPDRS), mild cognitive impairment (MCI), cognitively normal (CN),power of attention (PoA), pattern recognition memory (PRM),spatial recognition Memory (SRM)), (C) assessments of PD/dementia markers (stratified ‘postural instability gait difficulty’/’tremor dominant’ or (PIGD v TD), CSF-total tau (CSF-ttau), CSF-phosphorylated tau (CSF-ptau) and CSF alpha synuclein (CSF-α-synuclein) concentrations) and (D) MAPT and APOE genotypes.
| t=0 months | t=18 months | ||||
|---|---|---|---|---|---|
| Variable | MTND1 (P) | MTND4 (P) | MTND1 (P) | MTND4 (P) | |
| (A) | Age** | 9.00x10-2 | 1.70x10-1 | 6.44x10-1 | 8.48x10-1 |
| Gender* | 9.00x10-1 | 9.11x10-1 | 8.17x10-1 | 9.26x10-1 | |
| Age of Onset** | 1.67x10-1 | 2.11x10-1 | 3.14x10-1 | 3.01x10-1 | |
| (B) | MoCa** | 2.84x10-1 | 3.90x10-1 | 4.72x10-1 | 4.52x10-1 |
| MMSE** | 2.73x10-1 | 3.39x10-1 | 8.67x10-1 | 9.43x10-1 | |
| UPDRSII (on)** | 3.77x10-1 | 4.68x10-1 | 2.57x10-1 | 6.51x10-1 | |
| UPDRSIII (on)** | 4.22x10-1 | 9.97x10-1 | 7.57x10-1 | 8.19x10-1 | |
| MCI v CN* | 5.97x10-1 | 3.09x10-1 | 1.75x10-1 | 1.21x10-1 | |
| POA** | 6.93x10-1 | 4.67x10-1 | 4.39x10-1 | 4.37x10-1 | |
| PRM** | 6.98x10-1 | 7.77x10-1 | 1.77x10-1 | 1.18x10-1 | |
| SRM** | 7.41x10-1 | 6.32x10-1 | 5.93x10-1 | 5.45x10-1 | |
| (C) | PIGD v TD* | 8.20 x10-2 | 7.30x10-2 | 6.18x10-1 | 6.83x10-1 |
| CSF-ttau (pg/ml)** | 9.69x10-1 | 8.95x10-1 | - | - | |
| CSF-ptau (pg/ml)** | 5.33x10-1 | 8.04x10-1 | - | - | |
| CSF-α-synuclein (pg/ml) ** | 1.03x10-1 | 1.64x10-1 | - | - | |
| (D) | MAPT*** | 8.80x10-2 | 5.40x10-2 | 7.26x10-2 | 4.89x10-2 |
| APOE*** | 8.44x10-1 | 7.20x10-1 | 8.41x10-2 | 8.21x10-2 | |
Where * is testing by Mann-Whitney U of mtDNA copy-number versus binary variable, ** is testing by Spearman correlation of mtDNA copy-number versus linear variable and *** is ANOVA testing of
Inspection of t=0 and t=18 month ccf-mtDNA measurements showed that in some instances (>10%, n=11, 36.6%) ccf-mtDNA increased over time, although not to comparative control levels. 50% of PD cases showed a >10% decrease (n=15) and a small proportion remained static (within 10%, n=4, 13.4%). However, stratification by these three categories failed to find an association to previously defined phenotypes.
4. Discussion
In the only study in PD to date, our investigations indicate that circulating cell-free mtDNA (ccf-mtDNA) is associated with PD. Moreover the early-disease stage in our cohort indicates that ccf-mtDNA may be an important early biomarker for PD onset.
Our results are in keeping with a similar study in Alzheimer’s disease (AD),[7] which showed strikingly similar ccf-mtDNA levels when comparing AD patients to controls, indicating that ccf-mtDNA may indeed be a marker for broad neurodegeneration. A reduction in cellular mtDNA level is a hallmark of several neurological disorders, including a PBC-copy number reduction in Huntington’s disease [20] and a reduction of mtDNA in pyramidal neurons in Alzheimer's disease hippocampi.[21] However, the exact role of reduced ccf-mtDNA is unknown. In cancer, elevated ccf-mtDNA level is thought to correlate to increased apoptosis, with high copy-number cells releasing mtDNA upon death [22]. Although cell death is a hallmark of PD, we observed paradoxically lower ccf-mtDNA in cases, suggesting that neuronal mtDNA levels in patients are compromised prior to cell death. This seem likely, as neuronal mtDNA copy number is reduced in a number of neurodegenerative diseases [23, 24] and corresponds with a reduction in cellular energy. In addition, elevated plasma ccf-mtDNA is a hallmark of ICU mortality, rising as a direct response to inflammatory stimuli, [25] and cognitive impairment is associated with elevated pro-inflammatory markers in PD CSF [26], raising the possibility that elevated ccf-mtDNA may correlate with cognitive decline. However, we found no significant association between non-motor phenotypes and CSF ccf-mtDNA. This could be a limitation of the early stage of disease in our cohort, where cognitive decline has yet to reach develop, or may indicate that rising ccf-mtDNA is a feature restricted to PD motor-phenotypes. In this regard, further studies, including longitudinal studies of PD and cognitive decline, are recommended to more fully assess this. Finally, further study as to the origin of the ccf-mtDNA is warranted. Although a neuronal origin seems probable, given the phenotypic differences we observed, it is possible the ccf-mtDNA is representative of broader neuronal loss throughout the brain.
Given the severity of the reduction in CSF ccf-mtDNA in PD, and supported by both subsequent re-measurement and remarkably similar data seen in AD,[7] we conclude that ccf-mtDNA is a viable biomarker for the early detection of neurodegenerative disease.
Acknowledgments
GH is a Parkinson’s UK Senior Fellow (F-1202). The research was supported by the National Institute for Health Research (NIHR) Newcastle Biomedical Research Unit based at Newcastle upon Tyne Hospitals NHS Foundation Trust and Newcastle University. The research was also supported by NIHR Newcastle CRF Infrastructure funding. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR, or the Department of Health.
Footnotes
Author Contributions
AY, AT and DB were involved in the identification and preparation of critical patient and control CSF, analysis of patient cognitive function data and critical reading of the manuscript. BM performed the -tau, -phosphorylated-tau and –synuclein measurements in PD CSF. AP, RB and MKA performed the quantitative PCR and were all involved in the design of the experiments and subsequent analysis. PFC was involved in data analysis, design of experiments and assisted with manuscript preparation. GH provided the scientific questions, contributed to the experimental concept and design and supervised all experimental aspects of this study, analysis of results, writing and editing of the manuscript.
Potential Conflicts of Interest
The authors declare that they have no competing financial interests.
References
- 1.Jankovic J. Parkinson's disease: clinical features and diagnosis. J Neurol Neurosurg Psychiatry. 2008;79(4):368–76. doi: 10.1136/jnnp.2007.131045. [DOI] [PubMed] [Google Scholar]
- 2.Davis GC, Williams AC, Markey SP, et al. Chronic Parkinsonism secondary to intravenous injection of meperidine analogues. Psychiatry Res. 1979;1(3):249–54. doi: 10.1016/0165-1781(79)90006-4. [DOI] [PubMed] [Google Scholar]
- 3.Mullin S, Schapira A. alpha-Synuclein and mitochondrial dysfunction in Parkinson's disease. Mol Neurobiol. 2013;47(2):587–97. doi: 10.1007/s12035-013-8394-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hudson G. Recent mitochondrial DNA mutations increase the risk of developing common late-onset human diseases. PLoS Genet. 2014;10:e1004369. doi: 10.1371/journal.pgen.1004369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kohler C, Radpour R, Barekati Z, et al. Levels of plasma circulating cell free nuclear and mitochondrial DNA as potential biomarkers for breast tumors. Molecular Cancer. 2009;8 doi: 10.1186/1476-4598-8-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang Q, Raoof M, Chen Y, et al. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010;464(7285):104–7. doi: 10.1038/nature08780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Podlesniy P, Figueiro-Silva J, Llado A, et al. Low cerebrospinal fluid concentration of mitochondrial DNA in preclinical Alzheimer disease. Ann Neurol. 2013;74(5):655–68. doi: 10.1002/ana.23955. [DOI] [PubMed] [Google Scholar]
- 8.Yarnall AJ, Breen DP, Duncan GW, et al. Characterizing mild cognitive impairment in incident Parkinson disease: the ICICLE-PD study. Neurology. 2014;82(4):308–16. doi: 10.1212/WNL.0000000000000066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hughes AJ, Daniel SE, Kilford L, et al. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55(3):181–4. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roth M, Tym E, Mountjoy CQ, et al. CAMDEX. A standardised instrument for the diagnosis of mental disorder in the elderly with special reference to the early detection of dementia. Br J Psychiatry. 1986;149:698–709. doi: 10.1192/bjp.149.6.698. [DOI] [PubMed] [Google Scholar]
- 11.Nasreddine ZS, Phillips NA, Bedirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695–9. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 12.Wesnes KA, McKeith IG, Ferrara R, et al. Effects of rivastigmine on cognitive function in dementia with lewy bodies: a randomised placebo-controlled international study using the cognitive drug research computerised assessment system. Dement Geriatr Cogn Disord. 2002;13(3):183–92. doi: 10.1159/000048651. [DOI] [PubMed] [Google Scholar]
- 13.Robbins TW, James M, Owen AM, et al. Cambridge Neuropsychological Test Automated Battery (CANTAB): a factor analytic study of a large sample of normal elderly volunteers. Dementia. 1994;5(5):266–81. doi: 10.1159/000106735. [DOI] [PubMed] [Google Scholar]
- 14.Nombela C, Rowe JB, Winder-Rhodes SE, et al. Genetic impact on cognition and brain function in newly diagnosed Parkinson's disease: ICICLE-PD study. Brain. 2014;137(Pt 10):2743–58. doi: 10.1093/brain/awu201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Teunissen CE, Petzold A, Bennett JL, et al. A consensus protocol for the standardization of cerebrospinal fluid collection and biobanking. Neurology. 2009;73(22):1914–22. doi: 10.1212/WNL.0b013e3181c47cc2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giordano C, Iommarini L, Giordano L, et al. Efficient mitochondrial biogenesis drives incomplete penetrance in Leber's hereditary optic neuropathy. Brain. 2014;137(Pt 2):335–53. doi: 10.1093/brain/awt343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grady JP, Murphy JL, Blakely EL, et al. Accurate Measurement of Mitochondrial DNA Deletion Level and Copy Number Differences in Human Skeletal Muscle. Plos One. 2014;9(12) doi: 10.1371/journal.pone.0114462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thrifty genotype rendered detrimental by progress? Lancet. 1989;2(8667):839–40. doi: 10.1016/s0140-6736(89)93002-x. [DOI] [PubMed] [Google Scholar]
- 19.Selikhova M, Williams DR, Kempster PA, et al. A clinico-pathological study of subtypes in Parkinson's disease. Brain. 2009;132(Pt 11):2947–57. doi: 10.1093/brain/awp234. [DOI] [PubMed] [Google Scholar]
- 20.Petersen MH, Budtz-Jorgensen E, Sorensen SA, et al. Reduction in mitochondrial DNA copy number in peripheral leukocytes after onset of Huntington's disease. Mitochondrion. 2014;17:14–21. doi: 10.1016/j.mito.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 21.Rice AC, Keeney PM, Algarzae NK, et al. Mitochondrial DNA Copy Numbers in Pyramidal Neurons are Decreased and Mitochondrial Biogenesis Transcriptome Signaling is Disrupted in Alzheimer's Disease Hippocampi. Journal of Alzheimers Disease. 2014;40(2):319–30. doi: 10.3233/JAD-131715. [DOI] [PubMed] [Google Scholar]
- 22.Yu M. Circulating cell-free mitochondrial DNA as a novel cancer biomarker: opportunities and challenges. Mitochondr DNA. 2012;23(5):329–32. doi: 10.3109/19401736.2012.696625. [DOI] [PubMed] [Google Scholar]
- 23.Sheng B, Wang X, Su B, et al. Impaired mitochondrial biogenesis contributes to mitochondrial dysfunction in Alzheimer's disease. J Neurochem. 2012;120(3):419–29. doi: 10.1111/j.1471-4159.2011.07581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Keeney PM, Bennett JP. ALS spinal neurons show varied and reduced mtDNA gene copy numbers and increased mtDNA gene deletions. Molecular Neurodegeneration. 2010;5 doi: 10.1186/1750-1326-5-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakahira K, Kyung SY, Rogers AJ, et al. Circulating Mitochondrial DNA in Patients in the ICU as a Marker of Mortality: Derivation and Validation. Plos Medicine. 2013;10(12) doi: 10.1371/journal.pmed.1001577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lindqvist D, Hall S, Surova Y, et al. Cerebrospinal fluid inflammatory markers in Parkinson's disease--associations with depression, fatigue, and cognitive impairment. Brain Behav Immun. 2013;33:183–9. doi: 10.1016/j.bbi.2013.07.007. [DOI] [PubMed] [Google Scholar]