Abstract
Myelination of the CNS requires the migration of oligodendrocyte precursors throughout the CNS from restricted regions within the ventricular and subventricular zones. In light of the significant effects of cell–extracellular matrix (ECM) interactions on cell migration in other developing systems, we have analyzed the role of integrins in oligodendrocyte precursor migration. We have shown previously that oligodendrocyte precursors in vitroexpress a limited repertoire of integrins, including α6β1, αvβ1, and αvβ3, and that differentiation is associated with downregulation of αvβ1 and upregulation of αvβ5. Using a migration assay based on the movement of cells away from an agarose drop containing a high-density cell suspension, we find that RGD peptides (that block αv but not α6 integrins) and anti-β1 antibodies block migration on an astrocyte-derived ECM, whereas anti-β3 antibodies have little effect. These re- sults suggest that αvβ1 but not α6β1 plays a role in oligodendrocyte precursor migration, and this is confirmed by the use of blocking monoclonal antibodies that distinguish these two integrins. In keeping with the results of others, we find that differentiated oligodendrocytes lose migratory potential and that the timing of this loss correlates with downregulation of αvβ1. Taken together with the work of others showing that ECM ligands for αvβ1 are expressed within the CNS, we propose that this integrin plays a significant role in the migration of oligodendrocyte precursors in vivoand that its downregulation during differentiation could be an important factor regulating the migratory phenotype of these cells.
Keywords: oligodendrocyte, integrin, extracellular matrix, platelet-derived growth factor, differentiation, migration, astroglial matrix, vitronectin
The importance of cell migration during development is well illustrated in the CNS, where neurons arise in the ventricular zone and then migrate away to their final destinations (Rakic, 1972). Another cell type that shows extensive migration is the oligodendrocyte precursor, or O-2A progenitor cell. This cell type arises in the ventricular and subventricular zones of the developing CNS (Paterson et al., 1973) and migrates during development to produce the widespread distribution of differentiated oligodendrocytes seen in the mature animal. Direct evidence for this migration has come from two sets of in vivo studies. First, cells labeled with alac-Z reporter gene while in the subventricular zone subsequently migrate into the developing cortex and subcortical white matter and form oligodendrocytes (Levison and Goldman, 1993). Second, migration is seen when oligodendrocyte precursors are transplanted into myelin-deficient or normal mice (Lachapelle et al., 1984, 1994).
Previous studies examining the molecular mechanisms regulating oligodendroglial migration have focused on the role of growth factors. These studies have demonstrated that platelet-derived growth factor (PDGF) promotes oligodendrocyte precursor migration in culture and that withdrawal of PDGF is accompanied by differentiation and loss of migratory activity (Small et al., 1987; Noble et al., 1988; Armstrong et al., 1990). Interactions between cells and the extracellular matrix (ECM), however, can also provide instructive signals for many aspects of cell behavior, including survival, proliferation, differentiation, and migration (Streuli et al., 1991; Adams and Watt, 1993), and may therefore play a role in regulating oligodendrocyte precursor migration. Integrins are one important family of ECM receptors. These are cell-surface heterodimeric molecules consisting of α and β subunits made from at least 14 α and 9 β mammalian integrin subunits, which may combine in various ways to confer distinct ligand binding and cell signaling properties (Hemler, 1990; Ruoslahti, 1991;Hynes, 1992; Hynes and Lander, 1992; Diamond and Springer, 1994). Within the nervous system, integrins play a role in the migration of both neural crest cells (Bronner-Fraser, 1986) and neuroblasts in the developing chick tectum (Galileo et al., 1992), but their role in glial migration remains unknown.
To investigate the roles of cell–ECM interactions in regulating oligodendrocyte behavior, we characterized the integrins expressed by oligodendrocytes and their precursors in vitro. These studies show that oligodendroglial cells express a limited repertoire of integrins comprising α6β1 and several αv integrins (Milner and ffrench-Constant, 1994; Shaw et al., 1996). αv integrin expression is developmentally regulated: αvβ1 is lost and αvβ5 upregulated with differentiation (Milner and ffrench-Constant, 1994). In the current study, we have investigated the function of these integrins in oligodendrocyte precursor migration. We show that ECM substrates recognized by oligodendroglial integrins, including laminin, fibronectin, and vitronectin, promote migration. whereas collagen, not recognized by oligodendroglial integrins, does not. In addition, we find that blocking integrin function inhibits migration on an astrocyte-derived ECM and that the αvβ1 integrin, but not α6β1 or αvβ3, plays the dominant role in integrin-mediated migration of oligodendrocyte precursor cells on this substrate.
MATERIALS AND METHODS
Cell culture. Purified oligodendrocyte precursors were obtained as described previously (Milner and ffrench-Constant, 1994), using a technique modified from McCarthy and De Vellis (1980). Briefly, primary cultures were established from rat or mouse neonatal forebrain cells obtained by dissociating cortices in papain, and they were grown for ∼10 d in DMEM supplemented with 10% fetal calf serum (FCS) (Globepharm) before being shaken overnight to separate the loosely attached oligodendrocyte precursors (“top cells”). The cells were purified further by removing contaminating microglia using selective adhesion to nontissue culture-treated plastic. The remaining cell suspension was then centrifuged and resuspended in Sato media [DMEM supplemented with bovine insulin (Sigma, St. Louis, MO) (5 μg/ml), human transferrin (Sigma) (50 μg/ml), BSA V (Sigma) (100 μg/ml), progesterone (Sigma) (6.2 ng/ml), putrescine (Sigma) (16 μg/ml), sodium selenite (Sigma) (5 ng/ml), T3 (Sigma) (400 ng/ml), T4 (Sigma) (400 ng/ml), l-glutamine (Sigma) (4 mm), penicillin and streptomycin (Sigma)] containing 0.5% FCS, and secondary cultures were established by plating the cells onto poly-ornithine-coated six-well plates (Nunc, Naperville, IL) or 100 mm petri dishes (Nunc). The purity of the resulting cell suspension was assessed by morphology, with process-bearing oligodendroglia distinguished from fibroblast-like astrocytes and microglia, and was >95% oligodendroglial cells (oligodendrocytes and precursors) at day 1. In these secondary cultures, precursor cells constitutively differentiate into oligodendrocytes. This was confirmed by immunostaining with a monoclonal antibody (Ranscht et al., 1982) against the oligodendrocyte-specific marker galactocerebroside (GalC) (Raff et al., 1978). By immunocytochemistry, the oligodendroglial cells in these cultures were <10% GalC+ after 4 hr (i.e., >90% precursor cells) but >80% GalC+ at day 7 and >95% GalC+ by day 10. Astrocytes were obtained by removing all top cells from a wholebrain flask and using the basal layer of astrocytes left behind. Mouse fibroblasts were obtained from meninges, as described previously (Milner and ffrench-Constant, 1994), and then cultured in DMEM supplemented with 10% FCS and l-glutamine (Sigma)(4 mm) and penicillin and streptomycin (Sigma).
Antibodies. The antibodies used in immunoprecipitations and adhesion and migration assays were generous gifts of the following: (1) monoclonals: GoH3 (rat IgG1, anti-α6) from Dr. Arnoud Sonnenberg, Amsterdam, Holland (Sonnenberg et al., 1987), 9EG7 (rat IgG2a, anti-β1) from Dr. Dietmar Vestweber, Freiburg, Germany (Lenter et al., 1993), and F11 (mouse IgG1, anti-β3) from Dr. Michael Horton, University College London, London, UK (Helfrich et al., 1992b); and (2) polyclonals: anti-ECMR (GP140) from Dr. Caroline Damsky, San Francisco, CA (Knudsen et al., 1981; Damsky et al., 1982), and anti-αv from Dr. Guido Tarone, Torino, Italy (Hirsch et al., 1994). The anti-αvβ3 antiserum and RGD and RGE peptides were obtained from Life Technologies (Paisley, Scotland). Additional purified GoH3 was obtained from Serotec (Oxford, UK), and 9EG7 was obtained from PharMingen (San Diego, CA).
The preparation and characterization of the function-blocking anti-β1 antibody will be described elsewhere (G. Edwards and C. Streuli, unpublished observations) and is summarized briefly here. α4β1 integrin was affinity-purified under nondenaturing conditions from whole mouse embryos using sepharose-conjugated PS/2, a rat monoclonal antibody specific for mouse α4 integrin. The purified integrin was used to immunize rabbits, and IgG was isolated from the resulting sera. This antibody specifically recognized both α4 and β1 integrin subunits in Western blotting and immunoprecipitation experiments.
Cell-surface labeling and immunoprecipitation. Cell-surface molecules were labeled with biotin by removing growth media, washing the cell layer twice with PBS, and then labeling with 0.1 mg/ml NHS-LC-Biotin in PBS (Pierce, Rockford, IL) at 37°C in 7.5% CO2 for 30 min. Cell monolayers were then washed three times with cell wash buffer (50 mm Tris-HCL, pH 7.5, 0.15 m NaCl, 1 mm CaCl2, 1 mm MgCl2) and harvested with a cell scraper before being washed twice more in suspension. Cells were then lysed in 1% NP40 extraction buffer (cell wash buffer plus 300 μg/ml PMSF, 1 μg/ml pepstatin A, 2 μg/ml aprotinin, and 4 μg/ml leupeptin) for 30 min on ice, followed by trituration and centrifugation at 14,000 rpm at 4°C to remove the insoluble fraction. The supernatants then were precleared by two sequential 2 hr incubations with 30 μl of protein A-sepharose (Pharmacia, Piscataway, NJ) and 4 μl of nonimmune rabbit serum/ml of cell lysate. Immunoprecipitations were carried out overnight at 4°C on a rotating platform using 1 μl of rabbit antisera/250 μl of cell lysate. Where rat monoclonal antibodies were used (GoH3 and 9EG7), rabbit anti-rat antisera (Nordic, Capistrano Beach, CA) was also added at 1:250 to the tube. The immune complexes were collected by incubation with 30 μl of protein A-sepharose beads for 2 hr, after which time the beads were washed five times in immunoprecipitation wash buffer [identical to the cell wash buffer except for a higher salt concentration (0.5 m NaCl) and the addition of 0.1% Tween 20]. Integrins then were separated from the beads by boiling in nonreducing SDS sample buffer for 5 min before being analyzed by SDS-PAGE on a 7.5% resolving gel and 4% stacking gel under nonreducing conditions. Proteins were then electroblotted for 3 hr onto nitrocellulose (Hybond-C, Amersham, Arlington Heights, IL), blocked overnight with 3% BSA in TBS (10 mm Tris-HCL, 0.15 NaCl, pH 8.0) containing 0.1% Tween 20, and detected with streptavidin-HRP (ECL detection system, Amersham) for 1 hr according to manufacturer’s instructions.
Adhesion assays. All substrates were prepared by coating small areas of a 90 mm bacteriological grade plastic petri dish with 50 μl of ECM solution (10 μg/ml) or poly-dl-ornithine (5 μg/ml) for 2 hr at 37°C. Before the addition of cells, all substrates were blocked with 50 μl of heat-inactivated (5 min at 80°C) 0.3% BSA (BSA-fraction V, Sigma) for 30 min to prevent nonspecific binding to substrates. Substrates were then washed twice with Sato media immediately before addition of the cells. Oligodendrocyte precursors, freshly isolated from rat or mouse primary forebrain cultures as described above, were centrifuged, resuspended in Sato media, and applied to the substrates in a 50 μl drop for 30 min at 37°C. The adhesion assay was stopped by adding 10 ml of DMEM to the petri dish and washing off loosely attached cells. The attached cells were then fixed with 4% paraformaldehyde in PBS for 20 min. Adhesion was quantified by counting all attached cells under phase microscopy. Murine laminin, bovine fibronectin, and bovine vitronectin were all obtained from Sigma and diluted to the required concentration in PBS. In antibody-blocking experiments, the antibody was added to the medium used to resuspend the cells after their final wash and therefore was present when the cells were added to the substrate and throughout the experiment. The polyclonal anti-ECMR antibody was used at a dilution of 1:400. The monoclonal 9EG7 antibody was used at a concentration of 5 μg/ml, and GoH3 was used at a range of concentrations from 0 to 10 μg/ml.
Cell migration assay. Cell migration was quantified by measuring the extent of migration from agarose drops using a modification of the method described by Varani et al. (1978). Oligodendrocyte precursors were obtained as described above and resuspended at 40 × 106 cells/ml in Sato media containing 10% FCS and 0.3% low melting point agarose (Sigma) maintained at 37°C to prevent setting of the agarose; 1.5 μl drops of the cell suspension were applied to the center of wells within a 24-well tissue culture dish (Nunc), which was then placed at 4°C for 15 min to allow the agarose to solidify. Three different sets of substrates were used in these experiments. For experiments using astroglial matrix (AGM), the matrix was prepared in 24-well plates before the addition of the drop by removing growth media from mixed glial cultures and adding 1 ml of water per well for 2 hr at 37°C. The lysed cell material was then removed with three washes of PBS, before the AGM was stored in PBS at 37°C. Agarose drops were placed onto this matrix after removal of the PBS and covered with 0.4 ml of serum-free Sato media after cooling. For experiments using poly-dl-ornithine substrates, the plastic was coated as described above, washed with water, and dried, after which the agarose drop was added and covered with 0.4 ml of serum-free Sato media after cooling. For purified ECM substrates, drops were plated directly onto tissue-culture plastic, and the cooled drop was surrounded by 50 μl of Sato media containing 10 μg/ml of the chosen ECM molecule and incubated for 2 hr at 37°C. After this incubation, 0.35 ml of media was added. With the exception of the first set of experiments to examine the effect of PDGF on oligodendrocyte precursor migration, PDGF was always present at 5 ng/ml. Cell migration was measured at daily intervals for 1–5 d using a phase microscope with a calibrated graticule in the eyepiece, in which the width of one grid square represented 100 μm actual distance at a magnification of 10×. Cells migrate out to form a uniform corona around the drop. At any one time point, the distance between the edge of the drop and the leading edge of migrating cells within the corona was recorded on four sides of the drop. The few individual cells that had migrated ahead of the corona were not included in the measurement. Within single experiments, each condition was tested in duplicate or triplicate. The mean migration was calculated for each experiment, and results were expressed as mean ± SEM. Statistical significance was assessed by using the Students paired t test, in which p < 0.05 was defined as statistically significant. Blocking antibodies and peptides were added to the wells immediately after addition of the surrounding media. Antibodies were added only once, whereas RGE and RGD peptides were added at daily intervals. Antibodies were used at the following dilutions/concentrations: anti-ECMR antiserum, 1:400; anti-αvβ3 antiserum, 1:100; anti-β1 antisera, 60 μg/ml; 9EG7, 5 μg/ml; and GoH3, 5 μg/ml. RGE and RGD peptides were used at a concentration of 0.1 mg/ml. Photomicrographs of the agarose drop assays were taken on a Nikon Diaphot inverted microscope using phase optics.
RESULTS
Oligodendrocyte precursors migrate radially from an agarose drop in response to PDGF
To measure the migration of oligodendrocyte precursors, we have modified the technique described by Varani et al. (1978). This assay measures cell migration away from a high-density population of cells contained in an agarose drop. Purified populations of oligodendrocyte precursor cells were resuspended in a 1.5 μl drop containing 0.3% low melting point agarose and plated onto a poly-dl-ornithine substrate, as described in Materials and Methods. A defined media of serum-free Sato medium (see Materials and Methods) was then added to the culture. Within 2 hr of plating, oligodendrocyte precursors had begun to migrate out of the drop, and they continued to migrate radially for a number of days, producing a uniform corona of cells (Fig. 1.). Migration was quantified daily by measuring the distance between the leading edge of the corona and the edge of the agarose drop. As expected from previous studies using different assays (Noble et al., 1988; Armstrong et al., 1990), PDGF promoted oligodendrocyte precursor migration in this assay. Indeed PDGF seemed to be required for migration; in the presence of PDGF, oligodendrocyte precursors migrated distances in excess of 1 mm during a 5 d period, whereas in the absence of PDGF there was no migration (Fig. 1).
Fig. 1.

The effect of PDGF on the migration of oligodendrocyte precursors. Cells were resuspended at high density in agarose, as described in Materials and Methods, and then plated as small drops onto poly-dl-ornithine-coated plastic and cultured in the absence (A) or presence (B) of PDGF (5 ng/ml). Note that PDGF promotes the migration of oligodendrocyte precursors, with no migration observed in the absence of this growth factor.
PDGF also acts as a mitogen for oligodendrocyte precursor cells (Noble et al., 1988; Raff et al., 1988), and time-lapse analyses showed cell division as oligodendrocyte precursors migrated away from the drop, confirming that PDGF has both mitogenic and migration-enhancing properties. This raises the possibility that the movement of oligodendrocyte precursors away from the drop may be a consequence of cell division, with increasing cell density causing passive movement, rather than being attributable to active cell migration. To separate these two effects of PDGF, migration assays were performed in the presence of the mitotic inhibitor aphidicholin, which inhibits oligodendroglial cell division (McKinnon et al., 1993). The maximal extent of oligodendrocyte precursor migration under these conditions was the same as that seen in the absence of aphidicholin (data not shown). This confirms that the dispersal of oligodendrocyte precursors in the agarose drop assay reflects cell migration.
Differentiation of oligodendrocyte precursors reduces migratory potential
Previous studies show that the oligodendrocyte precursor is a migratory cell, whereas differentiated oligodendrocytes possess no migratory potential (Small et al., 1987; Noble et al., 1988). To examine the timing of this loss of migratory ability, we took advantage of the observation that oligodendrocyte precursors grown in defined medium without added mitogens differentiate constitutively into oligodendrocytes (Temple and Raff, 1985). This differentiation occurred over a 7 d period in our cultures, after which the majority of cells in our cultures have stopped dividing and have differentiated into GalC+ oligodendrocytes (Milner and ffrench-Constant, 1994). Populations of oligodendrocyte precursors in agarose drops were allowed to differentiate for various times before the addition of PDGF. On successive days after plating, PDGF was then added to the media to stimulate migration, and the extent of migration was measured for the following 5 d. As shown in Figure 2, oligodendrocyte precursors maintained from the start (day 0) in PDGF show a high level of migration throughout the 5 d period. Cell populations cultured in defined medium alone without PDGF for 3 d (day 3) showed reduced migratory ability once PDGF was added. Cell populations cultured in defined medium alone without PDGF for 7 d (day 7), allowing almost complete differentiation, showed virtually no migration on addition of PDGF. Therefore, as expected from previous studies (Small et al., 1987; Noble et al., 1988), there was an inverse correlation between the extent of differentiation within the population and the ability to migrate, with no migration seen in differentiated oligodendrocytes. PDGF was included in all further studies at the time of plating so that the assay could be used to measure migration of undifferentiated oligodendrocyte precursors.
Fig. 2.
The relationship between differentiation and migration in oligodendrocyte precursor cells. Cells were resuspended at high density in agarose, as described in Materials and Methods, and then plated as small drops onto poly-dl-ornithine-coated plastic and grown in defined medium alone, without PDGF. Cells were then allowed to differentiate for 0–7 d before PDGF was added to promote migration, and migration was measured for the following 5 d. Data from three experiments are shown. Note that the more the cell populations are allowed to differentiate before the addition of PDGF (most differentiated at day 7, least at day 0), the less they migrate in response to PDGF.
ECM molecules promote oligodendrocyte precursor migration by integrin-dependent mechanisms
To assess the ability of oligodendrocyte precursor integrins to promote migration, we examined migration on three ECM ligands—laminin, fibronectin, and vitronectin—recognized by these integrins (Cheresh and Spiro, 1987; Cheresh et al., 1989; Bodary and McLean, 1990), and on one ECM ligand, type-1 collagen, for which oligodendrocyte precursors lack any recognized integrin receptors. An example of these assays is shown in Figure 3, which shows that laminin and fibronectin promote migration after 2 d, whereas collagen inhibits migration relative to the tissue-culture plastic control. Combined data from four separate experiments demonstrate that laminin, fibronectin, and vitronectin all increased the rate of migration as compared with uncoated plastic. Laminin and fibronectin were the most effective, promoting the extent of migration after 2 d to 191.9 ± 18.4% (p < 0.002) and 188.5 ± 23.6% (p < 0.02) of the uncoated plastic control, respectively, with vitronectin promoting migration to 132.2 ± 12.6% of the uncoated plastic control. In contrast, migration on a collagen substrate was reduced to 48.4 ± 4.8% (p < 0.002) of the control. On uncoated tissue-culture plastic, migration of cells is initially slower than on laminin or fibronectin, but the cells then increase speed and migrate at a constant rate for the duration of the assay.
Fig. 3.

The migration of oligodendrocyte precursors on different ECM substrates. Cells were resuspended at high density in agarose, as described in Materials and Methods, and then plated as small drops onto uncoated tissue-culture plastic. The ECM substrate was then added, as described in Materials and Methods. A, Control with no ECM; B, laminin; C, fibronectin; D, collagen. Note that after 2 d, cells on laminin and fibronectin had migrated further than the uncoated plastic control. Cells on collagen, in contrast, had migrated less than the control and have lined up around the periphery of the agarose drop.
Having established that ECM molecules recognized by oligodendroglial integrins promote migration, we next confirmed the role of integrins in this migration by blocking the function of all oligodendroglial integrins using the anti-αv and anti-β1 function-blocking antibody anti-ECMR (Knudsen et al., 1981; Damsky et al., 1982). Immunoprecipitations performed with this antibody on oligodendrocyte precursors grown in secondary culture for 2 d show that anti-ECMR immunoprecipitates the same pattern of bands as a combined αv and β1 immmunoprecipitation, with bands running at 150 (α6), 140 (αv), 110 (β1), and 80 kDa (β3/β80k) (Fig.4A). This confirms that the anti-ECMR antibody recognizes all of the integrins expressed by oligodendrocyte precursors (Milner and ffrench-Constant, 1994). As shown in Figure5, the anti-ECMR antibody significantly inhibited oligodendrocyte precursor migration on all substrates tested, including laminin (36.7 ± 1.93% of control), fibronectin (25.26 ± 6.00% of control), and vitronectin (11.78 ± 9.20% of control) (n = 3; p < 0.001 for all substrates).
Fig. 4.
Immunoprecipitations of integrins from rat oligodendrocyte precursors to illustrate the specificity of the polyclonal antibodies used. Immunoprecipitations of biotin-labeled cell-surface proteins were performed, as described in Materials and Methods, with the anti-ECMR antiserum (A), anti-β1 antiserum (B, lane 1), or anti-αv antisera (B, lane 2). After this, the proteins were separated on nonreducing gels. Note that the anti-ECMR antiserum immunoprecipitates a repertoire of bands corresponding to all of the integrins expressed by oligodendrocyte precursors (α6β1, αvβ1, and αvβ80k). The anti-β1 antiserum recognizes the β1 subunit in association with two α subunits corresponding to α6 and αv, but it does not cross-react with β80k.
Fig. 5.

Effect of the anti-ECMR antiserum on oligodendrocyte precursor migration on different substrates. Cell migration away from agarose drops (prepared as described in Materials and Methods) after 2 d on poly-dl-ornithine (A, B), laminin (C, D), fibronectin (E, F), or vitronectin (G, H) is shown in the presence of either normal goat serum (A, C, E, G) or anti-ECMR antiserum (B, D, F, H), both at 1:400. Note that the anti-ECMR antiserum reduces oligodendrocyte precursor migration on all substrates tested but does not alter the bipolar morphology of the cells.
An astrocyte-derived ECM promotes oligodendrocyte precursor migration
In addition to examining migration on purified ECM substrates, oligodendrocyte precursor migration was also examined on astroglial matrix (AGM), an ECM secreted by cortical astrocytes that may mimic the physiological ECM within CNS white matter (Cardwell and Rome, 1988b;Malek-Hedayat and Rome, 1994). This substrate provides a model of that encountered by migrating oligodendrocyte precursors in vivobecause astrocytes form the normal neighbors of the precursors and axons within white matter tracts. In this assay, the AGM substrate also promoted extensive migration of oligodendrocyte precursors (Fig.6). All subsequent experiments examining integrin function in oligodendrocyte precursor migration over ECM substrates were therefore performed on this substrate.
Fig. 6.

The effect of integrin inhibitors on oligodendrocyte precursor migration on AGM. Cell migration away from agarose drops (prepared as described in Materials and Methods) after 2 d on AGM is shown in the presence of normal rabbit serum (A), RGE peptide (B), RGD peptide (C), anti-β1 antiserum (D), anti-αvβ3 antiserum (E), or anti-β1 and anti-αvβ3 antiserum (F). The peptides were present at 0.1 mg/ml. Note that cell migration was inhibited both by RGD peptides and anti-β1 antiserum without any change to the morphology of the cells.
RGD peptides and anti-β1 antibodies inhibit oligodendrocyte precursor migration
To establish whether any one of the integrins expressed by oligodendrocyte precursors plays a dominant role in the migration on AGM, we performed function-blocking experiments using RGD peptides, anti-β1 antiserum, and two different anti-β3 antibodies. As discussed in Materials and Methods, the anti-β1 antiserum was raised against purified α4β1 and recognizes both the α4 and β1 subunits. The α4 integrin is not expressed on oligodendroglial cells, and so the antiserum will only recognize β1-containing integrins on these cells. The specificity of the anti-β1 antiserum is shown in Figure 4B, which demonstrates that it immunoprecipitates the β1 subunit and the two associated α subunits (αv and α6) from oligodendrocyte precursor cells, but not β5 or β3/β80k.
For these blocking experiments, migration assays were carried out for 2 d. As shown in Figures 6 and 7, the control RGE peptide had no effect on the extent of migration, whereas the RGD peptide reduced migration to ∼55% of the control (p < 0.002). The anti-β1 antiserum reduced the extent of migration to ∼45% (p < 0.001). Two different antibodies against the β3 integrin subunit were used: an anti-αvβ3 antisera (Suzuki et al., 1986; Tawil et al., 1994), which had no significant effect on migration, and the function-blocking monoclonal antibody F11 (Helfrich et al., 1992a), which also had no effect. When both anti-β1 and anti-αvβ3 antisera were included, cell migration was reduced to ∼35% of the control (p < 0.01), which was not significantly greater than the blockade mediated by anti-β1 antiserum alone.
Fig. 7.
Quantification of the effect of integrin inhibitors on oligodendrocyte precursor migration on AGM. Cell migration away from agarose drops (prepared as described in Materials and Methods) after 2 d on AGM was measured in the presence of either normal rabbit serum, RGE peptide, RGD peptide, anti-β1 antiserum, anti-αvβ3 antiserum, F11 (monoclonal anti-β3), or anti-β1 and anti-αvβ3 antiserum in combination. The peptides were present at 0.1 mg/ml. Each point represents the mean ± SEM of three separate experiments. Note that cell migration was inhibited only by RGD peptides and anti-β1 antiserum.
Inhibition of migration by the RGD peptide or anti-β1 antiserum was not associated with any change in morphology of the oligodendrocyte precursors. This suggests that the block to migration is not a consequence of differentiation, which is associated with a loss of migratory potential, as shown earlier. To confirm this, cells were allowed to migrate from the agarose drops for 1 d without any blocking agent present and then blocked with either anti-β1 antiserum or RGD peptide for 2 d, after which time the block was removed (Fig. 8). The subsequent rate of migration (as assessed by the slope of the graph showing distance traveled vs time) of these cells was compared with cells under control conditions that had not been exposed to blocking agents. If differentiation was induced by the blocking agents, then migration after removal of the block would be slower than that under control conditions. As shown in Figure 8, however, cells whose migration is blocked with either RGD peptides or β1 antiserum migrate at a rate equal to the control cells once the block has been removed, showing them to be at equivalent stages of differentiation.
Fig. 8.
Migration of oligodendrocyte precursor cells after removal of integrin blockade. Cells were allowed to migrate away from agarose drops (prepared as described in Materials and Methods) for 1 d before the following reagents were introduced: RGE peptide (control), RGD peptide, or anti-β1 antiserum. After incubation for 2 d, the reagents were removed and cell migration was measured for an additional 3 d. Note that cells whose migration was blocked with either the RGD peptide or anti-β1 antiserum migrate at equal rates to the control cells once the blockade is removed.
The αvβ1 but not the α6β1 integrin is involved in oligodendrocyte precursor migration on AGM
The preceding experiments show that oligodendrocyte precursor migration on AGM is blocked by both RGD peptides and anti-β1 integrin antibodies. Oligodendrocyte precursors express two different β1 integrins, α6β1 and αvβ1, either of which could be playing a role in precursor migration. Because the α6β1 integrin is not RGD-dependent (Hall et al., 1990), but all αv integrins presently characterized are RGD-dependent (Koivunen et al., 1993), the inhibition of cell migration by RGD peptides would favor a role for αvβ1 rather than α6β1 in the migratory process. We addressed this question directly by using blocking antibodies directed specifically against α6β1 or αvβ1 on oligodendrocyte precursors, GoH3 and 9EG7, respectively. To perform these experiments, it was necessary to use mouse rather than rat oligodendrocyte precursor cells because of the species specificity of these antibodies. GoH3 is a well characterized function-blocking anti-α6 monoclonal antibody (Sonnenberg et al., 1987) that we have used previously to immunoprecipitate α6β1 from mouse oligodendrocytes and their precursors (Milner and ffrench-Constant, 1994). Before the migration experiments, we performed adhesion assays confirming that GoH3 inhibited the function of α6β1 on mouse oligodendrocyte precursors. This showed that GoH3 inhibited cell adhesion to laminin in a dose-dependent manner (0–10 μg/ml) but had no effect on adhesion to fibronectin (not shown). In addition, longer-term assays in which blocking antibodies were introduced after oligodendrocyte precursors had been allowed to attach for 30 min showed that GoH3 inhibited process extension during a 3 hr time course on laminin but not fibronectin (R. Milner and C. ffrench-Constant, unpublished observations). 9EG7 is a monoclonal antibody directed against the β1 subunit and has been shown to recognize this subunit only in certain conformations thought to be related to activation state. As a result, it can be classified as a “reporter” antibody (Lenter et al., 1993). Two lines of evidence show that 9EG7 recognizes αvβ1 but not α6β1 on oligodendrocyte precursors. First, as shown in Figure9, 9EG7 immunoprecipitates the β1 subunit (running at 110 kDa) in association with an α subunit whose molecular weight corresponds to the dominant lower form of the αv subunit but not the α6 subunit immunoprecipitated by GoH3. We consistently see two αv subunits in these immunoprecipitation experiments on mouse but not rat oligodendroglia, as has also been described in Pleurodelescells (Alfandari et al., 1995). Second, when used as a blocking antibody in oligodendrocyte precursor adhesion assays, 9EG7 significantly reduced the extent of sprouting on fibronectin (a recognized ligand for αvβ1) but not on laminin (the α6β1 ligand) (Fig. 10). We conclude from this that 9EG7 specifically recognizes the αvβ1 integrin on oligodendrocyte precursors.
Fig. 9.
Specificity of the 9EG7 monoclonal antibody for oligodendroglial αvβ1 integrin. Immunoprecipitations of biotin-labeled cell-surface proteins from mouse oligodendrocyte precursors were performed as described in Materials and Methods, with either anti-αv antiserum (lane 1), the 9EG7 monoclonal antibody (lane 2), or the anti-α6-specific GoH3 monoclonal antibody (lane 3). The proteins were separated on a nonreducing gel. Lanes 1–3 represent three adjacent lanes on the gel that have been exposed for equal lengths of time, whereas lane 4 represents a longer exposure of lane 2. Note that the 9EG7 antibody immunoprecipitates the β1 subunit associated with an α subunit that comigrates with the dominant αv subunit but not the α6 subunit.
Fig. 10.
Specific function-blocking effect of the anti-αvβ1 9EG7 monoclonal antibody. Mouse oligodendrocyte precursors were plated onto either laminin (A, B) or fibronectin (C, D) in the absence (A, C) or presence (B, D) of the 9EG7 monoclonal antibody, and allowed to adhere and process for 1 hr. Note that 9EG7 inhibits process outgrowth on fibronectin but not laminin.
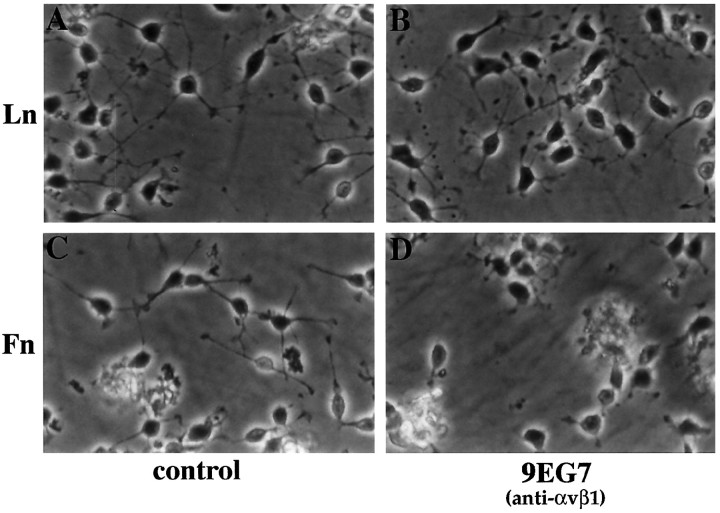
Migration assays with mouse oligodendrocyte precursor cells were carried out on the AGM substrate. Unlike the experiments with rat cells, which were performed in serum-free conditions, the experiments with mouse cells were carried out in the presence of 1% horse serum to promote survival of mouse oligodendrocyte precursor cells. As shown in Figure 11, the anti-β1 antiserum inhibited the migration of mouse oligodendrocyte precursor cells on AGM, as it had on rat, reducing migration after 2 d to 33.13 ± 10.4% of the control (p < 0.01). The anti-αvβ1 monoclonal antibody 9EG7 also inhibited migration, reducing migration to 58.4 ± 17.6% of the control (p < 0.05). In contrast, the anti-α6 blocking antibody GoH3 did not reduce the extent of migration. As before, no changes in morphology were seen in association with these changes in migration, although the mouse oligodendrocyte precursors grown under these conditions do not show the characteristic bipolar morphology seen in the rat cells, being rather less polarized and with a greater number of short processes (Fig. 11).
Fig. 11.
The effect of integrin inhibitors on oligodendrocyte precursor migration on AGM. Mouse oligodendrocyte precursor cell migration away from agarose drops (prepared as described in Materials and Methods) after 2 d on AGM was measured under control conditions (A) or in the presence of anti-β1 antiserum (B), the anti-α6 GoH3 monoclonal antibody (C), or the anti-αvβ1 9EG7 monoclonal antibody (D). Note that cell migration was inhibited by the anti-β1 antiserum and the anti-αvβ1 9EG7 monoclonal antibody but not by the anti-α6 GoH3 antibody.
αvβ1 but not α6β1 promotes oligodendrocyte precursor migration on a laminin/fibronectin/vitronectin composite substrate
The results with the anti-αvβ1 9EG7 and anti-α6 GoH3 monoclonal antibodies suggest that the αvβ1 but not the α6β1 integrin plays the dominant role in promoting oligodendrocyte precursor migration on AGM. This result might be expected, however, if the α6β1 ligand laminin was either masked or not present in the AGM. To address this issue, experiments were carried out both on laminin substrates (which promote adhesion, outgrowth, and migration, as described above) and on a composite ECM substrate coated with a solution containing 10 μg/ml of laminin, fibronectin, and vitronectin. As shown in Figure 12, the anti-α6 monoclonal antibody GoH3 did not significantly reduce oligodendrocyte precursor migration on either laminin substrates or the composite matrix of fibronectin, laminin, and vitronectin (p > 0.05). In contrast, anti-β1 antiserum reduced the extent of migration on the composite substrate to 46.03 ± 1.98% of the control (p < 0.001). This result shows that α6β1 is not playing a significant role in oligodendrocyte precursor migration, even when the α6β1 ligand laminin is present within the substrate.
Fig. 12.
The effect of αvβ1 and α6β1 inhibition on oligodendrocyte precursor migration over ECM substrates. The extent of mouse oligodendrocyte precursor cell migration away from agarose drops (prepared as described in Materials and Methods) after 2 d on either laminin or a composite laminin/fibronectin/vitronectin substrate was measured in the presence of the anti-α6 GoH3 monoclonal antibody or anti-β1 antiserum. The extent of migration is presented as a percentage of migration observed under control conditions, with no antibody present. Note that the anti-α6 GoH3 antibody had no significant inhibitory effect on either laminin or the composite substrate (p > 0.05), whereas the anti-β1 antiserum significantly inhibited migration on the composite substrate (p < 0.001).
DISCUSSION
In this study we have used the Varani migration assay to investigate the role of ECM/integrin interactions in oligodendrocyte precursor migration. Two major conclusions have emerged. First, the purified ECM substrates laminin, fibronectin, and vitronectin, as well as an ECM secreted by astrocytes, are effective at promoting cell migration, whereas collagen is not. Second, oligodendrocyte precursor migration on AGM is inhibited by blocking αvβ1 but not α6β1, suggesting a dominant role for the αvβ1 integrin in the migration process.
The agarose drop assay as a method of investigating oligodendrocyte precursor migration on ECM substrates
The mechanisms regulating the migration of oligodendrocyte precursor cells has been addressed by in vitro studies using both time-lapse microscopy (Small et al., 1987; Noble et al., 1988;Kiernan and ffrench-Constant, 1993) and chemotactic chamber assays (Armstrong et al., 1990; Frost et al., 1996). In the current study we have used a novel method based on the Varani agarose drop assay. In the presence of PDGF, oligodendrocyte precursor cells at the periphery of the drop start to migrate away within hours and continue their migration for at least 7 d. Unlike time lapse, this method permits the simultaneous analysis of different experimental conditions. It also has advantages over the use of the chemotaxis chamber, including the ability to monitor cell migration at intermediate time points, to change experimental conditions within an experiment, and to examine cell morphology during the experiment. A potential problem is that these experiments continue for several days, and the extent of cell dispersal from the drop will reflect both cell migration and proliferation. Time-lapse analysis, however, shows that there is consistent migration of precursors at all time points examined. Furthermore, migration of precursors still occurs in the presence of the mitotic inhibitor aphidicholin, showing that cell division is not a major factor in the extent of dispersal in the assay.
Involvement of ECM and integrins in oligodendrocyte precursor migration
The ECM molecules laminin, fibronectin, and vitronectin, and a complex ECM mixture derived from astrocytes (AGM) all promote cell migration. These results are in agreement with parallel experiments performed in our laboratory showing that both fibronectin and merosin, a member of the laminin family, promote oligodendrocyte precursor migration within a modified chemotaxis chamber assay (Frost et al., 1996). Our findings also show that integrins are involved in oligodendrocyte precursor migration on these ECM substrates. The anti-ECMR antibody, which recognizes all oligodendroglial integrins, blocked cell migration. Having shown previously that oligodendroglial cells express α6β1 and several αv integrins, we targeted these specific integrins with RGD peptides and function-blocking antibodies. The RGD peptides and anti-β1 antiserum both reduced migration on AGM to ∼50% of the control value. As argued in the results, the RGD effect pointed to the involvement of αvβ1 integrin in migration. This was confirmed directly by using two monoclonal antibodies: G0H3, specific to α6β1 (Sonnenberg et al., 1987), and 9EG7, which we find is specific for the αvβ1 integrin expressed by oligodendrocyte precursors, despite the fact that they also express other β1 integrins. The specificity of this antibody for a subset of expressed β1 integrins was shown on lymphocytes in the original paper describing 9EG7 (Lenter et al., 1993), and we have also observed this selectivity on fibroblasts, where 9EG7 immunoprecipitated only α1β1, despite the expression of higher levels of α5β1 (R. Milner and C. ffrench-Constant, unpublished observations). As such, 9EG7 represents a reporter antibody that recognizes a subset of the β1 integrins expressed on different cell types, presumably reflecting different conformations of these integrins relating to activation state (Lenter et al., 1993).
The observation that α6β1 seems to play only a limited role in migration on AGM raises the question as to why the α6β1 ligand laminin is so effective at promoting precursor migration. One possibility is that the uncharacterized αvβ80k integrin expressed by oligodendrocyte precursors (Milner and ffrench-Constant, 1994) may bind laminin and would not be blocked by GoH3. Alternatively, oligodendrocyte precursor cells may also express nonintegrin laminin receptors.
An interesting conclusion from our results is that blocking oligodendrocyte precursor migration is not associated with any changes in differentiation. This follows from the observation that precursors maintain the bipolar morphology of undifferentiated cells during migration blockade. Moreover, precursor cells released from migration blockade resume migration at the same speed as control cells. Taken together, these experiments show that the signaling pathways regulating migration and differentiation can be separated. This is in contrast to the situation with proliferation and differentiation, because cells cultured in the absence of mitogenic factors differentiate constitutively into oligodendrocytes once they drop out of division (Temple and Raff, 1985, 1986).
A role for the αvβ1 integrin in cell migration
Our finding that αvβ1 is a migration-promoting integrin for oligodendrocyte precursors is consistent with previous studies showing that β1 integrins promote migration in other cell types. This has been shown in the neural crest cell lineage using β1 integrin function-blocking antibodies (Bronner-Fraser, 1986) and in neuronal precursors of the developing tectum using antisense cDNA to reduce levels of β1 (Galileo et al., 1992). More recently, the β1 integrin gene has been knocked out in F9 teratocarcinoma cells and in embryonic stem (ES) cells. The ES cells were then unable to migrate toward a chemotactic source in Boyden chambers (Fassler et al., 1995), whereas β1-deficient F9 teratocarcinoma cells lost their ability to migrate on fibronectin and vitronectin substrates, despite expressing at least two other vitronectin receptors, αvβ3 and αvβ5 (Stephens et al., 1993). As in the F9 cells, our data indicate that αvβ3 is not playing a major role in promoting oligodendrocyte precursor cell migration. This is in contrast to other studies in different cell types supporting a role for αvβ3 in migration both in vitro(Leavesley et al., 1992; Delannet et al., 1994) and during metastatic invasion in vivo (Albelda et al., 1990; Gehlsen et al., 1992; Seftor et al., 1992). To our knowledge, the present study provides the first evidence for a role of αvβ1 in migration.
This work raises the question as to the nature of the αvβ1 ligandin vivo. Several ligands have been described, including fibronectin (Vogel et al., 1990), vitronectin (Bodary and McLean, 1990), and fibrinogen and osteopontin (Liaw et al., 1995). Both fibronectin and vitronectin are present within white matter tracts early in development (Chun and Shatz., 1988; Neugebauer et al., 1991;Sheppard et al., 1991). Although the precise distribution of fibronectin during the process of myelination has not yet been established, recent evidence shows vitronectin to be a candidate ligand for interaction with oligodendroglial integrins during myelination. Immunocytochemical studies in adult rat brain show that vitronectin is expressed within several different myelinated tracts (Einheber et al., 1996). The presence of a known ECM ligand for αvβ1 in myelinated tracts suggests that interactions between oligodendroglial integrins and ECM will occur in vivo, although immunocytochemical studies in earlier developmental stages are required. Based on our cell culture data, it is likely that any such interactions will play important instructive roles in regulating oligodendroglial migration.
Two other possibilities exist for the αvβ1 ligand. First, Cardwell and Rome (1988a) identified an RGD-blockable component within AGM that was not recognized by antibodies against fibronectin or vitronectin, raising the possibility that a novel αvβ1 ligand might be secreted by astrocytes. Second, the ligand in vivo may be a cell-surface molecule rather than an ECM molecule. Other β1 and αv integrins recognize cell surface ligands: α4β1 binds VCAM (Elices et al., 1990), whereas α6β1 binds fertilin (Almeida et al., 1995), a member of the ADAM family of cell-surface molecules (Wolfsberg et al., 1995). More recently, both α5β1 and αvβ3 have been shown to interact with L1 (Ruppert et al., 1995; Montgomery et al., 1996). It is possible, therefore, that αvβ1 binds an unidentified cell-surface molecule within axonal tracts, such as L1, and that this provides a mechanism for guidance of migrating oligodendroglia.
These observations complement previous work showing a role for growth factors in the control of oligodendrocyte precursor migration (Small et al., 1987; Noble et al., 1988; Armstrong et al., 1990; Kiernan and ffrench-Constant, 1993; Frost et al., 1996). They emphasize that the control of migration in vivo will be regulated by both integrin- and growth factor-mediated signaling pathways and as such add significantly to our understanding of oligodendrocyte precursor cell biology. Our results also suggest a model by which integrins might regulate oligodendrocyte precursor migration. Oligodendroglia lose migratory potential on differentiation, and in this study we have shown that this occurs over 7 d. During the same time scale, there is a loss of αvβ1 from the cell surface and expression of αvβ5 (Milner and ffrench-Constant, 1994). Given that the αvβ1 integrin promotes migration, the developmental switch of αv-associated β subunits may play a key role in regulating the timing of oligodendroglial migration. A prediction of this model is that overexpression of the αvβ1 heterodimer may enhance the migration of oligodendroglial cells in vivo. If so, this might have useful implications for future therapeutic strategies using transplanted oligodendrocyte precursors to repair widespread demyelinated lesions.
Footnotes
This work was funded by the Wellcome Trust. R.M. held a Wellcome prize fellowship, C.S. is a Wellcome Trust senior fellow in basic biomedical science, and C.ff-C. was a Wellcome Trust senior clinical fellow. We are grateful to Drs. J. Salzer and S. Einheber for sharing results before publication.
Correspondence should be addressed to Charles ffrench-Constant, Wellcome/Cancer Research Campaign Institute of Developmental Biology and Cancer, Tennis Court Road, Cambridge CB2 1QR, UK.
REFERENCES
- 1.Adams JC, Watt FM. Regulation of development and differentiation by the extracellular matrix. Development. 1993;117:1183–1198. doi: 10.1242/dev.117.4.1183. [DOI] [PubMed] [Google Scholar]
- 2.Albelda SM, Mette SA, Elder DE, Stewart R, Damjanovich L, Herlyn M, Buck C. Integrin distribution in malignant melanoma: association of the beta 3 subunit with tumour progression. Cancer Res. 1990;50:6757–6764. [PubMed] [Google Scholar]
- 3.Alfandari D, Whittaker CA, DeSimone DW, Darribere T. Integrin αv subunit is expressed on mesodermal cell surfaces during amphibian gastrulation. Dev Biol. 1995;170:249–261. doi: 10.1006/dbio.1995.1212. [DOI] [PubMed] [Google Scholar]
- 4.Almeida EAC, Huovila A-PJ, Sutherland AE, Stephens LE, Calarco PG, Shaw LM, Mercurio AM, Sonnenberg A, Primakoff P, Myles DG, White JM. Mouse egg integrin α6β1 functions as a sperm receptor. Cell. 1995;81:1095–1104. doi: 10.1016/s0092-8674(05)80014-5. [DOI] [PubMed] [Google Scholar]
- 5.Armstrong RC, Harvath L, Dubois-Dalcq ME. Type 1 astrocytes and oligodendrocyte-type 2 astrocyte glial progenitors migrate toward distinct molecules. J Neurosci Res. 1990;27:400–407. doi: 10.1002/jnr.490270319. [DOI] [PubMed] [Google Scholar]
- 6.Bodary SC, McLean JW. The integrin β1 subunit associates with the vitronectin receptor αv subunit to form a novel vitronectin receptor in a human embryonic kidney cell line. J Biol Chem. 1990;265:5938–5941. [PubMed] [Google Scholar]
- 7.Bronner-Fraser M. An antibody to a receptor for fibronectin and laminin perturbs cranial neural crest development in vivo. Dev Biol. 1986;117:528–536. doi: 10.1016/0012-1606(86)90320-9. [DOI] [PubMed] [Google Scholar]
- 8.Cardwell MC, Rome LH. Evidence that an RGD-dependent receptor mediates the binding of oligodendrocytes to a novel ligand in a glial-derived matrix. J Cell Biol. 1988a;107:1541–1549. doi: 10.1083/jcb.107.4.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cardwell MC, Rome LH. RGD-containing peptides inhibit the synthesis of myelin-like membrane by cultured oligodendrocytes. J Cell Biol. 1988b;107:1551–1559. doi: 10.1083/jcb.107.4.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheresh DA, Spiro RC. Biosynthetic and functional properties of an Arg-Gly-Asp-directed receptor involved in human melanoma cell adhesion to vitronectin, fibrinogen and Von Willebrand factor. J Biol Chem. 1987;262:17703–17711. [PubMed] [Google Scholar]
- 11.Cheresh DA, Smith JW, Cooper HM, Quaranta V. A novel vitronectin receptor integrin (αvβx) is responsible for distinct adhesive properties of carcinoma cells. Cell. 1989;57:59–69. doi: 10.1016/0092-8674(89)90172-4. [DOI] [PubMed] [Google Scholar]
- 12.Chun JJM, Shatz CJ. A fibronectin-like molecule is present in the developing cat cerebral cortex and is correlated with subplate neurons. J Cell Biol. 1988;106:857–872. doi: 10.1083/jcb.106.3.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Damsky CH, Knudsen KA, Buck CA. Integral membrane glycoproteins related to cell-substratum adhesion in mammalian cells. J Cell Biochem. 1982;18:1–13. doi: 10.1002/jcb.1982.240180102. [DOI] [PubMed] [Google Scholar]
- 14.Delannet M, Martin F, Bossy B, Cheresh DA, Reichardt LF. Specific roles of the αvβ1, αvβ3 and αvβ5 integrins in avian neural crest cell adhesion and migration on vitronectin. Development. 1994;120:2687–2702. doi: 10.1242/dev.120.9.2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diamond MS, Springer TA. The dynamic regulation of integrin adhesiveness. Curr Biol. 1994;4:506–517. doi: 10.1016/s0960-9822(00)00111-1. [DOI] [PubMed] [Google Scholar]
- 16.Einheber S, Milner TA, Preissner KT, Salzer JL (1996) Vitronectin is associated with myelin sheaths in the adult rat brain. Soc Neurosci Abstr, in press.
- 17.Elices MJ, Osborn L, Takada Y, Crouse C, Luhowskyj S, Hemler ME, Lobb RR. VCAM-1 on activated endothelium interacts with the leucocyte integrin VLA-4 at a site distinct from the VLA-4/fibronectin binding site. Cell. 1990;60:577–584. doi: 10.1016/0092-8674(90)90661-w. [DOI] [PubMed] [Google Scholar]
- 18.Fassler R, Pfaff M, Murphy J, Noegel AA, Johansson S, Timpl R, Albrecht R. Lack of β1 integrin gene in embryonic stem cells affects morphology, adhesion and migration but not integration into the inner cell mass of blastocysts. J Cell Biol. 1995;128:979–988. doi: 10.1083/jcb.128.5.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frost E, Kiernan BW, Faissner A, ffrench-Constant C (1996) Regulation of oligodendrocyte precursor migration by extracellular matrix: evidence for substrate-specific inhibition of migration by tenascin-C. Dev Neurosci, in press. [DOI] [PubMed]
- 20.Galileo DS, Majors J, Horwitz AF, Sanes JR. Retrovirally introduced antisense integrin RNA inhibits neuroblast migration in vivo. Neuron. 1992;9:1117–1131. doi: 10.1016/0896-6273(92)90070-t. [DOI] [PubMed] [Google Scholar]
- 21.Gehlsen KR, Davis GE, Sriramarao CA. Integrin expression in human melanoma cells with differing invasive and metastatic properties. Clin Exp Metastasis. 1992;10:111–120. doi: 10.1007/BF00114587. [DOI] [PubMed] [Google Scholar]
- 22.Hall DE, Reichardt LF, Crowley E, Holley B, Moezzi H, Sonnenberg A, Damsky CH. The α1β1 and α6β1 integrin heterodimers mediate cell attachment to distinct sites on laminin. J Cell Biol. 1990;110:2175–2184. doi: 10.1083/jcb.110.6.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Helfrich MH, Nesbitt SA, Dorey EL, Horton MA. Rat osteoclasts adhere to a wide range of RGD (Arg-Gly-Asp) peptide-containing proteins, including the bone sialoproteins and fibronectin via a β3 integrin. J Bone Miner Res. 1992a;7:335–343. doi: 10.1002/jbmr.5650070314. [DOI] [PubMed] [Google Scholar]
- 24.Helfrich MH, Nesbitt SA, Horton MA. Integrins on rat osteoclasts: characterization of two monoclonal antibodies (F4 and F11) to rat β3. J Bone Miner Res. 1992b;7:345–351. doi: 10.1002/jbmr.5650070315. [DOI] [PubMed] [Google Scholar]
- 25.Hemler ME. VLA proteins in the integrin family: structures, functions, and their role on leucocytes. Annu Rev Immunol. 1990;8:365–400. doi: 10.1146/annurev.iy.08.040190.002053. [DOI] [PubMed] [Google Scholar]
- 26.Hirsch E, Gullberg D, Balzac F, Altruda F, Silengo L, Tarone G. αv integrin subunit is predominantly located in nervous tissue and skeletal muscle during mouse development. Dev Dynamics. 1994;20:108–120. doi: 10.1002/aja.1002010203. [DOI] [PubMed] [Google Scholar]
- 27.Hynes RO. Integrins: versatility, modulation and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- 28.Hynes RO, Lander AD. Contact and adhesive specificities in the associations, migrations, and targeting of cells and axons. Cell. 1992;68:303–322. doi: 10.1016/0092-8674(92)90472-o. [DOI] [PubMed] [Google Scholar]
- 29.Kiernan BW, ffrench-Constant C (1993) Oligodendrocyte precursor (O-2A progenitor cell) migration: a model system for the study of cell migration in the developing central nervous system. Development [Suppl]:219–225. [PubMed]
- 30.Knudsen KA, Rao PE, Damsky CH, Buck CA. Membrane glycoproteins involved in cell-substratum adhesion. Proc Natl Acad Sci USA. 1981;78:6071–6073. doi: 10.1073/pnas.78.10.6071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koivunen E, Gay DA, Ruoslahti E. Selection of peptides binding to the α5β1 integrin from phage display library. J Biol Chem. 1993;268:20205–20210. [PubMed] [Google Scholar]
- 32.Lachapelle F, Gumpel M, Baulac M, Jacque C, Duc P, Baumann N. Transplantation of CNS fragments into the brain of shiverer mutant mice: extensive myelination by implanted oligodendrocytes. I. Immunohistochemical studies. Dev Neurosci. 1984;6:325–334. doi: 10.1159/000112359. [DOI] [PubMed] [Google Scholar]
- 33.Lachapelle F, Duhamel-Clerin E, Gansmüller A, Baron-Van Erercooren A, Villarroya H, Gumpel M. Transplanted transgenically marked oligodendrocytes survive, migrate and myelinate in the normal mouse brain as they do in the shiverer mouse brain. Eur J Neurosci. 1994;6:814–824. doi: 10.1111/j.1460-9568.1994.tb00992.x. [DOI] [PubMed] [Google Scholar]
- 34.Leavesley DI, Ferguson GD, Wayner EA, Cheresh DA. Requirement of the integrin β3 subunit for carcinoma cell spreading or migration on vitronectin and fibrinogen. J Cell Biol. 1992;117:1101–1107. doi: 10.1083/jcb.117.5.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lenter M, Uhlig H, Hamann A, Jeno P, Imnof B, Vestweber D. A monoclonal antibody against an activation epitope on mouse integrin chain β1 blocks adhesion of lymphocytes to the endothelial integrin α6β1. Proc Natl Acad Sci USA. 1993;90:9051–9055. doi: 10.1073/pnas.90.19.9051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Levison SW, Goldman JE. Both oligodendrocytes and astrocytes develop from progenitors in the subventricular zone of postnatal rat forebrain. Neuron. 1993;10:201–212. doi: 10.1016/0896-6273(93)90311-e. [DOI] [PubMed] [Google Scholar]
- 37.Liaw L, Skinner MP, Raines EW, Ross R, Cheresh DA, Schwartz MA, Giachelli CM. The adhesive and migratory effects of osteopontin are mediated via distinct cell surface integrins. Role of alpha v beta 3 in smooth muscle cell migration to osteopontin in vitro. J Clin Invest. 1995;95:713–724. doi: 10.1172/JCI117718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Malek-Hedayat S, Rome LH. Expression of a β1-related integrin by oligodendroglia in primary culture: evidence for a functional role in myelination. J Cell Biol. 1994;124:1039–1046. doi: 10.1083/jcb.124.6.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McCarthy KD, De Vellis J. Preparation of separate astroglial and oligodendroglia cell cultures from rat cerebral tissue. J Cell Biol. 1980;85:890–902. doi: 10.1083/jcb.85.3.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McKinnon RD, Smith C, Behar T, Smith T, Dubois-Dalcq M. Distinct effects of bFGF and PDGF on oligodendrocyte progenitor cells. Glia. 1993;7:245–254. doi: 10.1002/glia.440070308. [DOI] [PubMed] [Google Scholar]
- 41.Milner R, ffrench-Constant C. A developmental analysis of oligodendroglial integrins in primary cells: changes in αv-associated β subunits during differentiation. Development. 1994;120:3497–3506. doi: 10.1242/dev.120.12.3497. [DOI] [PubMed] [Google Scholar]
- 42.Montgomery AMP, Becker JC, Sui C-H, Lemmon VP, Cheresh DA, Pancook JD, Zhao X, Reisfeld RA. Human cell adhesion molecule L1 and rat homologue NILE are ligands for integrin αvβ3. J Cell Biol. 1996;132:475–485. doi: 10.1083/jcb.132.3.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Neugebauer KM, Emmett CJ, Venstrom KA, Reichardt LF. Vitronectin and thrombospondin promote retinal neurite outgrowth: developmental regulation and role of integrins. Neuron. 1991;6:345–358. doi: 10.1016/0896-6273(91)90244-t. [DOI] [PubMed] [Google Scholar]
- 44.Noble M, Murray K, Stroobant P, Waterfield M, Riddle P. Platelet-derived growth factor promotes division and motility and inhibits premature differentiation of the oligodendrocyte type-2-astrocyte progenitor cell. Nature. 1988;333:560–565. doi: 10.1038/333560a0. [DOI] [PubMed] [Google Scholar]
- 45.Paterson JA, Privat A, Ling EA, Leblond C. Investigation of glial cells in semithin sections. III. Transformation of subependymal cells into glial cells, as shown by radioautography after 3H-thymidine injection into the lateral ventricle of the brain of young rats. J Comp Neurol. 1973;149:83–102. doi: 10.1002/cne.901490106. [DOI] [PubMed] [Google Scholar]
- 46.Raff MC MR, Fields KL, Lisak RP, Dorfman SH, Silberberg DH, Gregson NA, Leibowitz S, Kennedy MC. Galactocerebroside is a specific cell-surface antigenic marker for oligodendrocytes in culture. Nature. 1978;274:813–816. [PubMed] [Google Scholar]
- 47.Raff MC, Lillien LE, Richardson WD, Burne JF, Noble MD. Platelet-derived growth factor from astrocytes drives the clock that times oligodendrocyte development in culture. Nature. 1988;333:562–565. doi: 10.1038/333562a0. [DOI] [PubMed] [Google Scholar]
- 48.Rakic P. Mode of cell migration to the superficial layers of fetal monkey neocortex. J Comp Neurol. 1972;145:61–84. doi: 10.1002/cne.901450105. [DOI] [PubMed] [Google Scholar]
- 49.Ranscht B, Clapshaw PA, Price J, Noble M, Seifert W. Development of oligodendrocytes and Schwann cells studied with a monoclonal antibody against galactocerebroside. Proc Natl Acad Sci USA. 1982;79:2709–2713. doi: 10.1073/pnas.79.8.2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ruoslahti E. Integrins. J Clin Invest. 1991;87:1–5. doi: 10.1172/JCI114957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ruppert M, Aigner S, Hubbe M, Yagita H, Altevogt P. The L1 adhesion molecule is a cellular ligand for VLA-5. J Cell Biol. 1995;131:1881–1891. doi: 10.1083/jcb.131.6.1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Seftor RE, Seftor EA, Gehlsen KR, Stetler-Syevenson WG, Brown PD, Ruoslahti E, Hendrix MJ. Role of the alpha v beta 3 integrin in human melanoma cell invasion. Proc Natl Acad Sci USA. 1992;89:1557–1561. doi: 10.1073/pnas.89.5.1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shaw CE, Milner R, Compston DAS, ffrench-Constant C. Analysis of integrin expression on oligodendrocytes during axo-glial interaction by using rat–mouse xenocultures. J Neurosci. 1996;16:1163–1172. doi: 10.1523/JNEUROSCI.16-03-01163.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sheppard AM, Hamilton SK, Pearlman AL. Changes in the distribution of extracellular matrix components accompany early morphogenetic events of mammalian cortical development. J Neurosci. 1991;11:3928–3942. doi: 10.1523/JNEUROSCI.11-12-03928.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Small RK, Riddle P, Noble M. Evidence for migration of oligodendrocyte-type-2 astrocyte progenitor cells into the developing rat optic nerve. Nature. 1987;328:155–157. doi: 10.1038/328155a0. [DOI] [PubMed] [Google Scholar]
- 56.Sonnenberg A, Janssen H, Hogervorst F, Calafat J, Hilgers J. A complex of platelet glycoproteins Ic and IIa identified by a rat monoclonal antibody. J Biol Chem. 1987;262:10376–10383. [PubMed] [Google Scholar]
- 57.Stephens LE, Sonne JE, Fitzgerald ML, Damsky CH. Targeted deletion of β1 integrins in F9 embryonal carcinoma cells affects morphological differentiation but not tissue-specific gene expression. J Cell Biol. 1993;123:1607–1620. doi: 10.1083/jcb.123.6.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Streuli CH, Bailey N, Bissell MJ. Control of mammary epithelial differentiation: basement membrane induces tissue-specific gene expression in the absence of cell-cell interaction and morphological polarity. J Cell Biol. 1991;115:1383–1395. doi: 10.1083/jcb.115.5.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Suzuki S, Argraves WS, Pytela R, Arai H, Krusius T, Pierschbacher MD, Ruoslahti E. cDNA and amino acid sequences of the cell adhesion protein receptor recognizing vitronectin reveal a transmembrane domain and homologies with other adhesion protein receptors. Proc Natl Acad Sci USA. 1986;83:8614–8618. doi: 10.1073/pnas.83.22.8614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tawil N, Wilson P, Carbonetto S. Expression and distribution of functional integrins in rat CNS glia. J Neurosci Res. 1994;39:436–477. doi: 10.1002/jnr.490390411. [DOI] [PubMed] [Google Scholar]
- 61.Temple S, Raff MC. Differentiation of a bipotential glial progenitor cell in single cell microwell. Nature. 1985;313:223–225. doi: 10.1038/313223a0. [DOI] [PubMed] [Google Scholar]
- 62.Temple S, Raff MC. Clonal analysis of oligodendrocyte development in culture: evidence for a developmental clock that counts cell division. Cell. 1986;44:773–779. doi: 10.1016/0092-8674(86)90843-3. [DOI] [PubMed] [Google Scholar]
- 63.Varani J, Orr W, Ward PA. A comparison of the migration patterns of normal and malignant cells in two assay systems. Am J Pathol. 1978;90:159–172. [PMC free article] [PubMed] [Google Scholar]
- 64.Vogel B, Tarone G, Giancotti F, Gailit J, Rouslahti E. A novel fibronectin receptor with an unexpected subunit composition (αvβ1). J Biol Chem. 1990;265:5934–5937. [PubMed] [Google Scholar]
- 65.Wolfsberg TG, Straight PD, Gerena RL, Huovila A-PJ, Primakoff P, Myles DG, White JM. ADAM, a widely distributed and developmentally regulated gene family encoding membrane proteins with a disintegrin and metalloprotease domain. Dev Biol. 1995;169:378–383. doi: 10.1006/dbio.1995.1152. [DOI] [PubMed] [Google Scholar]