Abstract
The primary aim of this study was to evaluate the antitumor efficacy of the bromodomain inhibitor JQ1 in pancreatic ductal adenocarcinoma (PDAC) patient-derived xenograft (tumorgraft) models. A secondary aim of the study was to evaluate whether JQ1 decreases expression of the oncogene c-Myc in PDAC tumors, as has been reported for other tumor types.
We used five PDAC tumorgraft models that retain specific characteristics of tumors of origin to evaluate the antitumor efficacy of JQ1. Tumor-bearing mice were treated with JQ1 (50 mg/kg daily for 21 or 28 days). Expression analyses were performed with tumors harvested from host mice after treatment with JQ1 or vehicle control. An nCounter® PanCancer Pathways Panel (NanoString Technologies, Seattle, WA, USA) of 230 cancer-related genes was used to identify gene products affected by JQ1. Quantitative RT-PCR, immunohistochemistry and immunoblots were done to confirm that changes in RNA expression reflected changes in protein expression. JQ1 inhibited the growth of all five tumorgraft models (P<0.05), each of which harbors a KRAS mutation; but induced no consistent change in expression of c-Myc protein. Expression profiling identified CDC25B, a regulator of cell cycle progression, as one of three RNA species (TIMP3, LMO2 and CDC25B) down-regulated by JQ1 (P<0.05). Inhibition of tumor progression was more closely related to decreased expression of nuclear CDC25B than to changes in c-Myc expression. JQ1 and other agents that inhibit the function of proteins with bromodomains merit further investigation for treating PDAC tumors. Work is ongoing in our laboratory to identify effective drug combinations that include JQ1.
Keywords: pancreatic ductal adenocarcinoma, bromodomain inhibitor, JQ1, patient-derived xenograft models, tumorgraft, c-Myc, CDC25B, expression profiles, NanoString Technologies
INTRODUCTION
Pancreatic ductal adenocarcinoma (PDAC), the most common form of pancreatic cancer (PC), is a highly aggressive malignancy of the exocrine pancreas and accounts for ~40,000 patients deaths annually.1 Among adult solid tumors PC ranks as the fourth leading cause of cancer related deaths in the United States, with an overall 5-year survival of 6%.1, 2 PDAC is distinguished by late clinical presentation, making ~80% of patients ineligible for surgical resection. Patients that do undergo resection have a marginally better 5-year survival of 15-20%.3 Chemotherapy, often combined with radiotherapy, is the primary treatment for most patients presenting with advanced or metastatic disease. The nucleoside analog gemcitabine has been the standard of care for PDAC patients for over a decade, but effective treatment remains challenging.4, 5
Intuitively, profiles of molecular and genetic abnormalities characteristic of a given tumor type would suggest effective drug targeting strategies. Genetic abnormalities that occur frequently in PDAC tumors include mutations that constitutively activate the KRAS oncogene, that silence the tumor suppressor TP53, or that promote cell cycle progression regulated by CDKN2A.6-8 A detailed genomic analysis of 24 primary PDAC tumors showed a set of 12 pathways that were altered in 67-100% of tumors analyzed.9 Those data suggested that the epidermal growth factor receptor (EGFR) pathway likely contributes to progression of PDAC tumors.9 A second commonly dysregulated pathway in PDAC and other solid tumors involves c-Myc, a basic helix-loop-helix leucine zipper transcription factor thought to influence expression of ~15% of the genome responsible for cell growth, proliferation, and apoptosis. c-Myc is amplified in >30% and overexpressed in >40% of PDAC tumors.10 Consistent with these observations, increased expression of c-Myc in a genetically engineered mouse model of pancreatic cancer induces tumor formation.11
Although the importance of c-Myc in cancer progression is well documented, until recently it has been difficult to target this oncogene. Delmore et al determined that the bromodomain and extraterminal (BET) family of proteins regulate c-Myc expression and function.12 BET proteins such as bromodomain containing 4 (BRD4) recruit proteins comprising macromolecular transcription complexes to specific chromatin sites. BET proteins (BRD2, BRD3, BRD4, and BRDT) possess an extraterminal domain and tandem bromodomains that recognize acetylated lysine residues on histones. JQ1 binds to the domain of BET proteins that interacts with histones, thereby competitively inhibiting binding of BET proteins to chromatin and decreasing expression of RNA species dependent on this mechanism of transcription.13 One such RNA species is that encoding c-Myc, as BRD4 is required to recruit p-TEFb to initiate transcription of c-Myc.12 Inhibition of c-Myc expression is thought to be an essential mechanism by which BET inhibitors suppress tumor progression in hematological malignancies.14-16 JQ1 has shown efficacy in preclinical models of a variety of tumor types including multiple myeloma, medulloblastoma, NMC (NUT midline carcinoma), neuroblastoma, and glioblastoma.17-21
In the present study we used five PDAC tumorgraft models, each of which expresses relatively high levels of c-Myc protein, to evaluate the impact of the BET inhibitor JQ1 on tumor growth. We also compared expression of 230 cancer-related genes to identify gene products affected by JQ1. JQ1 suppressed tumor growth in all tumorgraft models. However, we observed no consistent association between inhibition of tumor growth in vivo and decreased c-Myc expression. JQ1 did inhibit expression of CDC25B, a regulator of cell cycle progression.
RESULTS
Clinical characteristics of tumors of origin
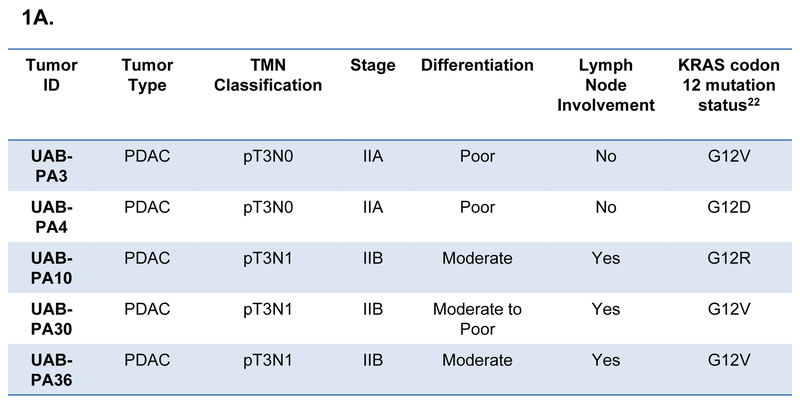
The models used in this study (UAB-PA3, -PA4, -PA10, -PA30 and -PA36) were derived from tumors of patients who underwent surgery as a standard of care at the University of Alabama at Birmingham Hospital.22 Models were derived from tumors of patients with stage II PDAC, with three of the five patients having lymph node involvement at the time of surgical resection (Fig. 1A). All primary tumors had mutations in codon 12 of the KRAS gene. These models were chosen for their readily detectable expression of c-Myc by immunohistochemistry (IHC) (Fig. 1B).
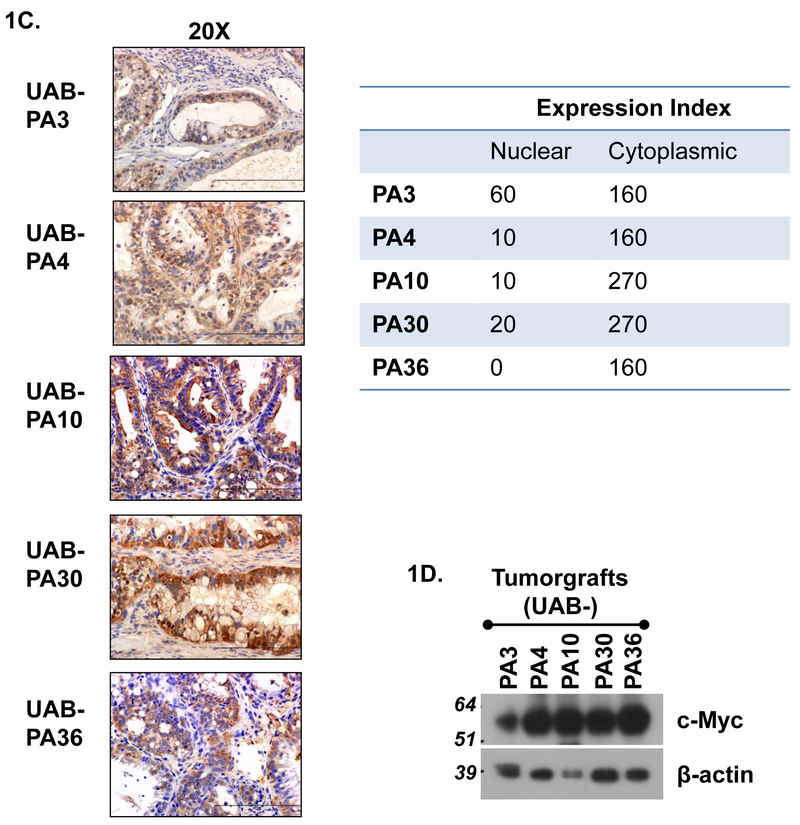
Figure 1. Characteristics of tumors from which PDAC models were derived.
A) Clinical characteristics of patients from whom specimens were acquired. B) IHC analysis and expression indices of c-Myc expression by five primary tumors and normal pancreatic tissue. C) IHC analysis and expression indices of c-Myc expression of tumorgrafts (F2 generation) derived from tumors shown in Panel B. Expression index = expression intensity (+1, +2, +3) x % cells positive for c-Myc expression. D) Immunoblot analysis of c-Myc protein of tumorgraft specimens shown in 1C.
PDAC primary tumors express higher levels of c-Myc than normal pancreas.
IHC analysis of FFPE tissues demonstrated minimal-to-high (1+ to 3+) nuclear expression and intermediate-to-high (2+ to 3+) cytoplasmic expression of c-Myc protein in all primary tumors of origin (Fig. 1B). Normal pancreatic (NP) tissue had foci of predominantly cytoplasmic c-Myc in both ductal and acinar tissue. Overall, c-Myc expression was relatively low in NP (expression index 80) compared to primary tumors (expression indices 180-270). IHC analyses demonstrated similar levels of c-Myc in tumorgraft models and their tumors of origin, with the exception of UAB-PA4 tumors which expressed less nuclear c-Myc protein than the tumor from which it was derived.(Fig. 1C). UAB-PA4 also had a lower ratio of nuclear:cytoplasmic (N:C) c-Myc protein, but the impact of this change in ratio of cell phenotype is unclear. Immunoblot analyses confirmed a readily detectable level of c-Myc protein in all models (Fig. 1D). Because the cytotoxicity of JQ1 has been reported to depend on down-regulation of c-Myc,19,23 we next evaluated the effect of JQ1 on tumor cell morphology, tumor growth in vivo and on c-Myc expression in these tumors.
JQ1 suppressed tumor growth in vivo in preclinical models of PDAC.
To evaluate the efficacy of JQ1, we administered a well-tolerated regimen of JQ1 (50 mg/kg daily x 28 or 21 days) to mice bearing PDAC tumorgrafts. JQ1 inhibited tumor growth by 40-62% compared to vehicle control (VC) in all five independently derived models (Fig. 2A). Model UAB-PA36 was the least responsive to JQ1 treatment with a difference in tumor volume being observed only on the final day of treatment. Time to detection of a JQ1-induced decrease in tumor volume ranged from day 14 (UAB-PA4) to day 28 (UAB-PA36).
Figure 2. JQ1 inhibited tumor growth and expression of the cell proliferation marker Ki67 in PDAC tumor cells.
A) Average volumes of tumors in mice treated with JQ1 (50 mg/kg) or vehicle (VC) daily for 28 days (UAB-PA3, -PA10, -PA30, -PA36; N=10 tumors) or 21 days (UAB-PA4; N=8 tumors). B) Tumor tissue was harvested and embedded in paraffin after the termination of treatment, and IHC done to detect the cell proliferation marker Ki67. 10 random high power fields (HPF = 400x) were taken and positive nuclei were counted and divided by the total number of nuclei. Two independent experiments were done. C) Expression indices for Ki-67 immunostaining of tumor tissue harvested after completion of therapy. *P<0.05; **P<0.01; ***P<0.001.
We corroborated the effect of JQ1 on tumor volume by immunostaining for the proliferation marker Ki67. Proliferation index was defined as percent of nuclei with detectable Ki67 expression, using photomicrographs imaged using Photoshop CS4 (Figs. 2B, 2C). Ki67 staining of UAB-PA3, -PA4, -PA10, and -PA30 tumors was decreased in JQ1- compared to VC-treated mice, indicating that fewer tumor cells were traversing the cell cycle in the JQ1-treated animals. Consistent with the minimal effect of JQ1 on UAB-PA36 tumors, no difference was seen in the proliferation index of tumors from treated versus control mice bearing this tumor. The data demonstrate that JQ1 inhibited the growth of five PDAC tumor models and decreased the proliferation index in four of the five models of independent origin. The fifth model, UAB-PA36, showed no decrease in proliferation index and was the least sensitive to the cytotoxicity of JQ1 in vivo.
JQ1 induced apoptosis in a subset of models.
Tumors were harvested 24 or 48 hours after termination of treatment, and their histology compared using FFPE sections stained with H&E (Fig. 3A). JQ1 increased the number of apoptotic (white arrows) and necrotic (yellow arrows) tumor cells in the UAB-PA10 and -PA30 models, each of which was responsive to JQ1. JQ1 also induced a slight reduction in peritumoral stroma of tumorgraft models UAB-PA3, -PA4, and -PA10 (white circles) (Fig. 3A), and an increase in stromal hyalinization in UAB-PA3, -PA10 and -PA30 (green circles). Consistent with minimal decrease in tumor growth, UAB-PA36 tumors from JQ1-treated mice had decreases in nuclear atypia and increases in N:C ratio, but no apparent increase in apoptotic or necrotic tumor cells. Of interest, JQ1 appeared to induce tumor cell differentiation in UAB-PA4 tumors (Fig. 3A, 40x image, right panel), with tumor cells having more well preserved nuclear polarity, less nuclear polymorphism and stratification, and a lower N:C ratio than tumor cells from VC-treated mice. This observation is consistent with a previous report showing that JQ1 induced squamous cell differentiation of 797NUT midline carcinoma (NMC) cells.19 However, UAB-PA4 was the only model that showed differentiation after JQ1 treatment. As JQ1 has been reported to exert its antitumor effect by suppressing c-Myc expression in hematological and solid tumors 15, 17, 19, 24-28 we next examined whether suppression of tumor growth was coincident with decreased expression of c-Myc in PDAC tumorgrafts.
Figure 3. JQ1 induced no consistent change in mRNA or protein expression in the five PDAC models used.
A) Histological analysis of H&E stained sections from tumors from mice treated with JQ1 or vehicle control (VC). Photomicrographs in the left panels were acquired at 10x magnification. Images of UAB-PA4 in the right panels were acquired at 40x magnification. B) qRT-PCR analyses of c-Myc mRNA expression. Analyses were done using ΖΖCT method, and results were normalized to GAPDH. C) Immunoblot analyses detected no differences in expression of c-Myc in tumors from mice treated with JQ1 compared to mice treated with VC. Human normal pancreas (NP) was used as a control. D, E) IHC analyses and expression indices of c-Myc and BRD4. *P<0.05; **P<0.01; ***P<0.001.
JQ1 inhibited expression of c-Myc RNA, but had no consistent effect on c-Myc protein.
We evaluated c-Myc expression in tumorgrafts exposed to JQ1 compared to vehicle control by expression array (NanoString), qRT-PCR, and immunoblot. Firstly, array results failed to demonstrate a difference in c-Myc RNA levels in tumors from JQ1 compared to VC treated mice (data not shown). Secondly, a qRT-PCR method29 and human-specific c-Myc primer set showed that JQ1-treated tumors expressed ~17-30% less c-Myc RNA than VC-treated tumors (Fig. 3B), values that were regarded as minimal but that were statistically significant in four of the five models. The discrepancy between expression array data and qRT-PCR data was attributed to the marginal decrease, if any, in c-Myc RNA induced by JQ1. Thirdly, to determine whether the putative decrease in c-Myc RNA reflected decreased protein expression, we assessed c-Myc by immunoblot and immunohistochemistry. Immunoblots detected no effect of exposure to JQ1 on c-Myc protein, and increased expression of this oncogene in tumorgrafts compared to normal pancreas (Fig. 3C). IHC detected a decrease (~1.8-fold) in c-Myc protein only in one model (UAB-PA10) (Figs. 3D, 3E). The other four models showed <10% nuclear immunoreactivity and expression indices of 100-200 with moderate staining intensity of tumors from both JQ1- and VC-treated mice. We concluded that JQ1 had little effect on levels of c-Myc protein in these PDAC models. Further, although JQ1 is thought to inhibit cell growth by inhibiting BRD4 function rather than expression, we verified that BRD4 protein levels were also unaffected by exposure to JQ1. IHC data detected no consistent changes in level of expression or subcellular localization of c-Myc or BRD4 protein (Figs. 3D, 3E).
The minimal decrease in RNA encoding c-Myc suggested by qRT-PCR data was not seen in the NanoString assay or by immunoblots, as mentioned above. However, to rule out an effect of JQ1 on c-Myc function, we evaluated the effect of JQ1 on transcriptional targets of c-Myc, and found that only one of nineteen gene products that have been reported to be transcriptional targets of c-Myc was decreased in all five models, following exposure to JQ1 (Fig. 4A). This observation suggests that c-Myc function was unaffected and is unlikely to be critical to the cytotoxic mechanism of JQ1 in PDAC tumors. Of note, the list of putative transcriptional targets of c-Myc in Fig. 4A was compiled from multiple references that demonstrate the likelihood of regulation by c-Myc in various types of cells and tissues. 30-48 The genes listed do not necessarily represent transcriptional targets of c-Myc in PDAC tumors. The single exception to this observation may be CDC25B, and is discussed below. The regulatory region of CDC25B has been reported to have a single Myc/Max binding site,47 and is regarded as a potential c-Myc transcriptional target.
Figure 4. Expression analysis using NanoString technologies identified nine genes altered by JQ1 treatment in all five tumorgraft models.
A) Genes that are reported to be transcriptional targets of c-Myc, and their range of expression among the given tumorgraft models used. B) Heat map of the ranked global expression values of the nine candidate genes was generated from statistical analysis of NanoString expression profiling. C) Table of the nine genes that are commonly up- or down-regulated in all five models, after exposure to JQ1.
The data suggest that inhibition of growth of PDAC tumors may be independent of down-regulation of c-Myc, as has been suggested by findings using ex vivo systems, higher doses of JQ1 (100-500 nM, 2.5 μM), and lung adenocarcinoma and glioblastoma cells.21, 49 To identify alternative gene products whose up- or down-regulation might be affected by BRD4 inhibition and that might contribute to the mechanism of cytotoxicity of JQ1, we ranked the NanoString data to identify the 30 genes whose expression was decreased to the greatest extent, following exposure to JQ1. We also investigated the effect of exposure of JQ1 on CDC25B expression.
JQ1 altered the expression of nine gene products in all five models.
The 30 gene products most-affected by JQ1, as evaluated by NanoString, are listed in Supplementary data (Table. S1). The values in the Table represent the average change among the five models, but do not necessarily reflect similar changes in each of the five models. A Partek Genomic Suite program identified six RNA species commonly up-regulated and three RNA species commonly down-regulated in the five models (Figs. 4B, 4C). Fig. 4B shows a heatmap of these nine genes (blue = down-regulation; red = up-regulation, details in Methods). Exposure to JQ1 increased expression of PTEN, CASP2, MSH6, BRAF, NOTCH11, ERCC4 by 1.1- to 1.8-fold (P = 0.026, 0.028, 0.033, 0.035, 0.036, and 0.048, respectively), and decreased expression of LMO2, TIMP3, and CDC25B by 1.3- to 1.4-fold (P = 0.026, 0.032, and 0.048, respectively) (Fig. 4C). The change in expression of each of these RNAs was less than 2-fold, and none appears in the list of most-affected genes. The potential involvement of CDC25B was investigated further based on the following considerations. Heatmap results indicated a uniform down-regulation of CDC25B in all five PDAC tumorgraft models. CDC25B has been reported to be an oncogene 50 and to be overexpressed in PDAC tumors,51 and targeted CDC25B inhibitors inhibit the growth of PC cell lines.51 CDC25B (cell division cycle 25B) belongs to a family of cell cycle phosphatases (CDC25A, CDC25B, and CDC25C) that regulate cell cycle progression by dephosphorylation of cyclin dependent kinases (CDK), thereby activating CDK/cylin complexes and stimulating cell proliferation. CDC25B accumulates in the nucleus during the G2 phase of the cell cycle to initiate entry into mitosis.52,53
Expression of CDC25B, a member of the CDC25 phosphatase family.
qRT-PCR analyses confirmed a JQ1-dependent reduction in expression of CDC25B RNA in three models (Fig. 5A; p<0.05); but as was seen with c-Myc expression the degree of decrease was less than 2-fold. Immunoblot data demonstrated that JQ1 decreased CDC25B protein expression by 33% (UAB-PA3) to 99% (UAB-PA4) in the four tumorgraft models most sensitive to JQ1 (Fig. 5B). Expression indices demonstrated that exposure to JQ1 decreased expression of nuclear CDC25B from 33% (UAB-PA10) to 83% (UAB-PA4) (Fig. 5C). No decrease in CDC25B protein was observed in the model least sensitive to JQ1 (UAB-PA36).
Figure 5. JQ1 inhibited CDC25B RNA and protein expression in PDAC tumograft models.
A) Relative CDC25B mRNA expression in five tumorgraft models was calculated usingΖΖCT methods. GAPDH was used to normalize data. qRT-PCR analysis demonstrated that exposure to JQ1 decreased expression of mRNA encoding CDC25B in three of the five tumorgraft models. B) Immunoblot analysis documented that JQ1 decreased expression of CDC25B protein expression in the four tumorgraft models most sensitive to JQ1. Bar graph depicts quantitation of immunoblot in 5B. C) IHC data showed CDC25B expression of tumors from JQ1 compared to VC treated mice. Expression indices were calculated as described in Methods. *P<0.05; **P<0.01; ***P<0.001.
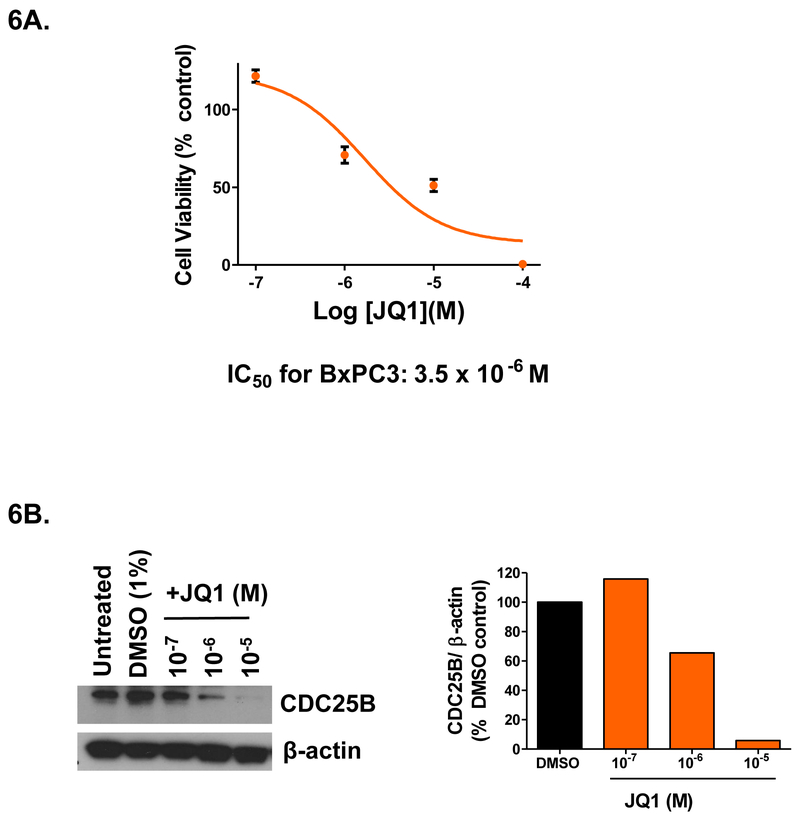
Further, we demonstrated a dose response relationship between concentration of JQ1, CDC25B protein expression, and inhibition of cell growth using an in vitro model. We exposed BxPC3 pancreatic cancer cells to JQ1 to determine the IC50 and to evaluate the level of expression of CDC25B protein over a range of JQ1 concentrations (10−7 to 10−4 M) (Fig. 6). Growth inhibition data (Fig. 6A) demonstrate an IC50 of 3.5 × 10−6 M. Immunoblot data (Fig. 6B) demonstrate a ~34-94% decrease in CDC25B protein at concentrations of JQ1 (M) of 1.0 × 10−6 and 1.0 × 10−5. The data demonstrate a simultaneous inhibition of cell growth and decrease in CDC25B expression.
Figure 6. JQ1 inhibited CDC25B protein expression in BxPC3 pancreatic cancer cells in a dose-dependent manner.
A) Cell viability assays showed IC50 value of 3.5×10−6 M when BxPC3 cells were exposed to a various JQ1 concentrations for 72 hours. B) Immunoblot showed CDC25B protein expression after 10−7-10−5 M JQ1 treatment in BxPC3 cells. Right panel: Bar graph depicting quantitation of the immunoblot shown in 6B.
In summary, data from in vivo models demonstrate that exposure to JQ1 inhibited CDC25B expression in tumor models most sensitive to this agent. In vitro data demonstrated dose-dependent decreases in BxPC3 cell viability and CDC25B protein by JQ1. The data also suggest that decreased expression of c-Myc is unlikely to be a primary mechanism by which JQ1 inhibits PDAC cell growth, as we observed no consistent change in c-Myc RNA or protein expression, or function as assessed by the level of expression of transcriptional targets of this transcription factor. In vivo data demonstrating JQ1-mediated inhibition of tumor progression indicate that bromodomain inhibitors merit further investigation for treatment of PDAC tumors as single agents and in combination.
DISCUSSION
The main goal of this study was to evaluate the efficacy of the bromodomain inhibitor JQ1 in tumorgraft models of pancreatic ductal adenocarcinoma. A secondary goal was to evaluate the likelihood that the mechanism of cytotoxicity of JQ1 in this tumor type depends on down-regulating c-Myc expression, as has been reported.19 The main findings of the study were that a well-tolerated (non-toxic) regimen of JQ1 reduced relative tumor volume by 40-62% in five independently derived models and that JQ1 did not consistently impact expression of c-Myc protein or its transcriptional targets. The data did show a dose-dependent decrease in CDC25B expression in vitro and inhibition of tumorgraft growth by JQ1 in vivo. Our study is unique in its evaluation of the efficacy of JQ1 in PDAC tumors using independently derived tumorgraft models that harbor KRAS mutations and in its demonstration that exposure to JQ1 decreases CDC25B expression in PDAC tumor cells sensitive to this agent.
No published studies have evaluated the antitumor effects of JQ1 in tumorgraft models of pancreatic cancer. The literature does report the efficacy of JQ1 for a variety of malignant cell types including lung cancer, glioblastoma, medulloblastoma, neuroblastoma, and hematological malignancies 18, 20, 21, 24, 26, 49 Model systems used range from cell lines and ex vivo systems to transgenic mice 21, 28, 49 Relevant to this study, Sahai et al demonstrated that JQ1 and BRD4 knockdown suppressed proliferation of pancreatic tumor cells in an in vitro three-dimensional collagen model.55 Mechanistically, JQ1 competitively inhibits the BET proteins such as BRD4 from binding to acetylated lysine residues of histones, thereby preventing the association of transcriptional complexes with chromatin.12, 19
Multiple other studies suggest that a critical mechanism by which JQ1 inhibits tumor cell growth is its effect on c-Myc expression through BET protein inhibition.13, 19 Data for this hypothesis have been generated primarily with hematologic tumor cells or NUT midline carcinoma with NUT-BRD4/BRD3 fusion protein. However, other mechanisms showing c-Myc-independent cytotoxicity have been implicated in some solid tumor models. Lockwood et al reported that a subset of lung adenocarcinoma cell lines was sensitive to JQ1 and that this sensitivity did not correlate with c-Myc down-regulation.54 The authors identified the transcription factor FOS-like antigen 1 (FOSL1) which was down-regulated by JQ1 treatment and BRD4 knockdown.54 Additionally, Fowler et al demonstrated that JQ1 did not inhibit c-Myc expression in all tumor types.28 The authors proposed c-Myc expression might be regulated by multiple transcriptional and epigenetic mechanisms.28
Our data suggest the involvement of CDC25B in pancreatic tumor cells. Although JQ1 is considered to be a targeted agent for the BET proteins, BRD4 in particular is a component of a transcription process that affects multiple RNA species, including c-Myc. c-Myc, in turn, is a transcription factor that regulates expression of a myriad of gene products. Taken together the data suggest that the efficacy of JQ1 may result from effects on multiple cellular proteins and processes downstream of BRD4. Of interest, particularly in light of the numerous gene products dependent on BRD4-associated transcription, is the observation that JQ1 is well tolerated at doses up to 100mg/kg in murine models.13, 56
With respect to our observation that CDC25B is one of the proteins affected by JQ1, we note that Guo et al showed that two targeted CDC25B inhibitors (NSC663284 and NSC668394) inhibited the growth of pancreatic cancer cells in vitro, by inducing a G2/M cell cycle arrest.51 This work demonstrated that pharmacologic inhibition of CDC25B was sufficient to inhibit cell cycle progression of PDAC tumor cells. Additionally, Yu et al reported that knock down of CDC25B in renal cell carcinoma cells was associated with reduced migratory and invasive potential, characteristics of a less malignant phenotype.57 Our observations with in vivo models are consistent with published in vitro and in vivo findings, and emphasize the potential utility of treating PDAC tumors with BET or CDC25B inhibitors. The data suggest that decreased CDC25B expression, either as a potential downstream target of c-Myc47 or by an as yet unknown mechanism, likely contributes to PDAC tumor growth inhibition.
Regarding the effect of JQ1 on PDAC tumor growth, our data demonstrate the inhibitory effect of this BRD4 inhibitor on subcutaneous tumors derived from primary human PDAC tumors. This preclinical model is restricted to the use of early passage tumors, and each of the five models used retains specific genetic and molecular characteristics of the primary tumors from which they were derived. We propose that this model system has relevance in evaluating the efficacy of novel agents for the treatment of PDAC. An alternative preclinical model of orthotopic PDAC will be used in the future to compare the effect of JQ1 on the metastatic potential and pattern of tumor progression of PDAC tumors, as orthotopic models as described by Hoffman and colleagues58-64 etain metastatic properties of PDAC tumors that are not accurately assessed using subcutaneous models.
In conclusion, JQ1 suppressed the growth of five independently derived PDAC tumorgraft models, and decreased expression of CDC25B.In vitro data indicated a dose-response relationship between concentration of JQ1 and decrease in CDC25B expression. We propose that JQ1 merits further evaluation for efficacy in treating PDAC tumors, alone and in combination with other agents.
MATERIALS AND METHODS
Ethics statement
This study included human subjects and vertebrate animals. All protocols and procedures were approved by the University of Alabama at Birmingham Institutional Review Board (IRB) or the University of Alabama at Birmingham Institutional Animal Care and Use Committee (IACUC).
Patient-derived xenograft (tumorgraft) models
Female SCID CB 17−/− mice, 4-6 weeks old (Taconic Farms, Newtown, MA, USA) were housed under barrier conditions with a 12-hour light/dark cycle and ad libitum access to food and water. Methods for development, transplantation, and characterization of tumorgrafts generated directly from surgical specimens were reported previously.22
Treatment of tumor-bearing mice
We prepared JQ1 according to published procedures.19 When transplanted tumors reached ~100-200 mm3 in size, we randomized mice (8 or 10 tumors/group), and administered 50 mg/kg JQ1 or vehicle control (VC) intraperitoneally (i.p.) daily for 21 (UAB-PA4) or 28 (UAB-PA3, -PA10, -PA30, and -PA36) days. We measured tumor size with Vernier calipers (Fowler/Slyvac, Newton, MA, USA) twice weekly, and calculated tumor volume assuming a perfect sphere and the equation v = (π/6)*d3. Twenty-four (UAB-PA3, -PA4, -PA30, -PA36) or forty-eight (UAB-PA10) hours after completion of treatment, mice were euthanized; and tumors were fixed in formalin and embedded in paraffin (FFPE), or frozen in liquid nitrogen and stored at -80°C.
Immunoblots
Frozen specimens were homogenized using a liquid nitrogen-cooled biopulverizer (MidSci, St. Louis, MO, USA) and placed in cell lysis buffer (Cell Signaling Technology, Danvers, MA, USA) containing protease inhibitors (Complete Mini, Roche Diagnostics, Indianapolis, IN, USA). SDS-PAGE analysis of lysates was done using standard methods. Primary antibodies were: anti-human c-Myc (AHO0062; Life Technologies, Grand Island, NY, USA), CDC25B (ab70927; abcam, Cambridge, MA, USA or Cell Signaling; 9525), and β-actin (A5316; Sigma-Aldrich, St. Louis, MO, USA). Blots were quantitated using Adobe Photoshop CS5 (San Jose, CA, USA) or Image Studio Lite (Li-Cor Biotechnology; Lincoln, NE, USA).
NanoString nCounter® RNA expression analysis
Total RNA was isolated from frozen specimens by Trizol-choloroform. RNA samples (400 ng) were analyzed in the UAB Nanostring Laboratory http://www.uab.edu/medicine/radonc/en/nanostring) using an nCounter® Analysis System (NanoString Technologies, Seattle, WA, USA.65 Briefly, two human sequence-specific capture probes comprised of a human sequence-specific oligonucleotide plus a short common sequence linked to biotin were constructed. RNA + capture probe + fluorescence-labeled reporter probes complementary to capture probes were hybridized under conditions facilitating formation of triplexes (mRNA + capture probe + reporter probe). Excess probe and partial complexes were removed by affinity purification. Probe/mRNA complexes were loaded into streptavidin coated cartridges, to immobilize triplexes on the cartridge. Current applied to the cartridge aligned and extended the complexes to facilitate imaging. An nCounter Digital analyzer acquired digital images of the fluorescence-labeled target mRNA affixed to the cartridge using a CCD camera and microscope objective. Digital images of 230 cancer related genes (Cancer Reference Panel) were analyzed to detect unique “barcodes” corresponding to the gene of interest. Expression level was quantitated by comparison with six reference genes.
Data analysis was conducted with nSolver v1.1 software (NanoString Technologies, Seattle, WA, USA), after normalizing for variations in binding efficiency, hybridization, and purification, using spiked-in positive controls and normalizing background values using negative control probes. Data are reported as fold-difference in expression between JQ1 and VC treated tumors.
Histological analysis: hematoxylin and eosin (H&E) staining
Fresh tumor tissue was placed for 24 hours in 10% neutral buffered formalin (Fisher Scientific, Suwanee, GA, USA) immediately after harvest, and then embedded in paraffin. Thin sections (5 μm) were prepared by the Comparative Pathology Laboratory at UAB (Birmingham, AL, USA). H&E staining was performed as previously described.22
Immunohistochemistry (IHC)
Slides containing thin sections of tumor specimens were baked at 60°C, deparaffinized in xylene, and rehydrated using a decreasing ethanol gradient. After antigen retrieval, endogenous peroxidase activity was blocked with 3% hydrogen peroxide in methanol. Non-specific protein binding was minimized by incubation in 10% horse serum (Vector Laboratories, Burlington, CA, USA), and primary antibody binding was accomplished by overnight incubation. Antibody binding was detected using IMMpress secondary reagent (Vector Laboratories, Burlington, CA, USA) and DAB High Contrast chromogen (Scytek Laboratories, Logan, UT, USA). Tissues were counterstained with Harris Hematoxylin (Fisher Scientific, Suwanee, GA, USA). Photomicrographs were taken using an Olympus BH-2 microscope with DP70 camera and DPS-BSW v3.1 software (Center Valley, PA, USA). Proliferation Indices66: 10 random high power fields (HPF = 400x) were taken, and positive nuclei counted and divided by the total number of nuclei in two independent experiments. Expression index = % of positive cells x staining intensity (1+ 2+ or 3+).67
Real-time RT-PCR (qRT-PCR)
Total RNA (2 μg) was used as the template for cDNA synthesis, using the Superscript® III synthesis kit (Invitrogen, Carlsbad, CA, USA). 2x SsoFast™ EvaGreen® cocktail (Bio-Rad Laboratories, Hercules, CA, USA) was diluted with nuclease free water and combined with cDNA and appropriate primers. c-MYC Forward: 5’-CGACTCTGAGGAGGAACAAG-3’, c-MYC Reverse: 5’GTGATCCAGACTCTGACCTT-3’, GAPDH Forward: 5’AACATCATCCCTGCTTCCAC-3’, GAPDH Reverse: 5’-GACCACCTGGTCCTCAGTGT-3’; CDC25B Forward: 5’- GTGATCCCAGGTGACGACAT-3’; CDC25B Reverse: 5’-TTTGATTCCACACCCTCGCAC-3’. Reaction conditions were: denaturation at 94°C for 1 min; 40 cycles of 15 sec at 94°C, 10 sec at 50°C; extension at 72°C for 10 sec. Reactions were done using a CFX96 System and analyzed using Bio-Rad CFX manager software v1.5 (Bio-Rad Laboratories, Hercules, CA, USA).
In vitro cell viability assays
Cell viability assays were done using standard methods.68 Briefly, cells were plated in 96-well plates at 1.5 × 103 cells per well. After 24 hours, cells were treated with either DMSO (1%) or 10 fold serial dilutions of JQ1 for 72 hours. Cell viability was assessed by standard alamarBlue (Fisher Scientific, Suwanee, GA, USA) protocols. Fluorescence was measured at 590 nm using a microplate reader (Molecular Devices, LLC. Sunnyvale, CA, USA) to calculate IC50s (GraphPad Prism software).
Bioinformatics
Raw values from NanoString were normalized using Partek Genomic Suite (PGS, St. Louis, MI, USA) to house-keeping genes prior to paired group comparison (JQ1 versus vehicle control). The log2-transformed fold-difference between JQ1-treated and VC-treated mice was averaged across all 5 models for each gene and expression ranked from lowest to highest. Data were imported into GENE-E (version 3.0.214; Broad Institute, Cambridge, MA, USA) to generate a heatmap. The color intensity on the heat map reflects global expression with a minimum (25%) in blue and a maximum (75%) in red. Cluster analysis, using one-minus Pearson correlation of rows only and distance for the clustering, was calculated using an average linkage algorithm.69
Statistics
Tumor volumes were compared by Two-Way-ANOVA and all values are presented as mean ± SEM. RNA expression (a minimum of three independent experiments for qRT-PCR) was compared by 2-tailed t test using GraphPad Prism 5.0 software (San Diego, CA, USA). P < 0.05 was considered significant.
Supplementary Material
Table S1. A list of 30 down-regulated genes by JQ1 in five tumorgraft models.
ACKNOWLEDGMENTS
The authors thank Drs. Donald J. Buchsbaum, William E. Grizzle, Mary-Ann Bjornsti, and Christopher Klug for their insightful guidance and helpful discussions. This research was supported in part by UAB/UMN SPORE in pancreatic cancer (P50 CA101955).
Support: This research was supported in part by UAB/UMN SPORE in pancreatic cancer (P50 CA101955).
ABBREVIATIONS
- PDAC
pancreatic ductal adenocarcinoma
- PDX
patient-derived xenograft
- BET
bromodomain and extraterminal
Footnotes
CONFLICT OF INTEREST
James E. Bradner and DFCI founded Tensha Therapeutics to develop derivatives of JQ1 and similar molecules for cancer therapy.
REFERENCES
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin 2014; 64: 9–29. [DOI] [PubMed] [Google Scholar]
- 2.American Cancer Society. Cancer Facts and Figures 2014. American Cancer Society; 2014: Atlanta. [Google Scholar]
- 3.Gillen S, Schuster T, Meyer Zum Buschenfelde C, Friess H, Kleeff J. Preoperative/neoadjuvant therapy in pancreatic cancer: a systematic review and meta-analysis of response and resection percentages. PLoS Med 2010; 7: e1000267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burris H, Storniolo AM. Assessing clinical benefit in the treatment of pancreas cancer: gemcitabine compared to 5-fluorouracil. Eur J Cancer 1997; 33 Suppl 1: S18–22. [DOI] [PubMed] [Google Scholar]
- 5.Burris HA 3rd, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol 1997; 15: 2403–2413. [DOI] [PubMed] [Google Scholar]
- 6.Bryant KL, Mancias JD, Kimmelman AC, Der CJ. KRAS: feeding pancreatic cancer proliferation. Trends Biochem Sci 2014; 39: 91–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morton JP, Timpson P, Karim SA, Ridgway RA, Athineos D, Doyle B et al. Mutant p53 drives metastasis and overcomes growth arrest/senescence in pancreatic cancer. Proc Natl Acad Sci U S A 2010; 107: 246–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rozenblum E, Schutte M, Goggins M, Hahn SA, Panzer S, Zahurak M et al. Tumor-suppressive pathways in pancreatic carcinoma. Cancer Res 1997; 57: 1731–1734. [PubMed] [Google Scholar]
- 9.Jones S, Zhang X, Parsons DW, Lin JC, Leary RJ, Angenendt P et al. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science 2008; 321: 1801–1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schleger C, Verbeke C, Hildenbrand R, Zentgraf H, Bleyl U. c-MYC activation in primary and metastatic ductal adenocarcinoma of the pancreas: incidence, mechanisms, and clinical significance. Mod Pathol 2002; 15: 462–469. [DOI] [PubMed] [Google Scholar]
- 11.Lin WC, Rajbhandari N, Liu C, Sakamoto K, Zhang Q, Triplett AA et al. Dormant cancer cells contribute to residual disease in a model of reversible pancreatic cancer. Cancer Res 2013; 73: 1821–1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Delmore JE, Issa GC, Lemieux ME, Rahl PB, Shi J, Jacobs HM et al. BET bromodomain inhibition as a therapeutic strategy to target c-Myc. Cell 2011; 146: 904–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mertz JA, Conery AR, Bryant BM, Sandy P, Balasubramanian S, Mele DA et al. Targeting MYC dependence in cancer by inhibiting BET bromodomains. Proc Natl Acad Sci U S A 2011; 108: 16669–16674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Trabucco SE, Gerstein RM, Evens AM, Bradner JE, Shultz LD, Greiner DL et al. Inhibition of bromodomain proteins for the treatment of human diffuse large B-cell lymphoma. Clin Cancer Res 2015; 21: 113–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Knoechel B, Roderick JE, Williamson KE, Zhu J, Lohr JG, Cotton MJ et al. An epigenetic mechanism of resistance to targeted therapy in T cell acute lymphoblastic leukemia. Nat Genet 2014; 46: 364–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roderick JE, Tesell J, Shultz LD, Brehm MA, Greiner DL, Harris MH et al. c-Myc inhibition prevents leukemia initiation in mice and impairs the growth of relapsed and induction failure pediatric T-ALL cells. Blood 2014; 123: 1040–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Loven J, Hoke HA, Lin CY, Lau A, Orlando DA, Vakoc CR et al. Selective inhibition of tumor oncogenes by disruption of super-enhancers. Cell 2013; 153: 320–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bandopadhayay P, Bergthold G, Nguyen B, Schubert S, Gholamin S, Tang Y et al. BET bromodomain inhibition of MYC-amplified medulloblastoma. Clin Cancer Res 2014; 20: 912–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Filippakopoulos P, Qi J, Picaud S, Shen Y, Smith WB, Fedorov O et al. Selective inhibition of BET bromodomains. Nature 2010; 468: 1067–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Puissant A, Frumm SM, Alexe G, Bassil CF, Qi J, Chanthery YH et al. Targeting MYCN in neuroblastoma by BET bromodomain inhibition. Cancer Discov 2013; 3: 308–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheng Z, Gong Y, Ma Y, Lu K, Lu X, Pierce LA et al. Inhibition of BET bromodomain targets genetically diverse glioblastoma. Clin Cancer Res 2013; 19: 1748–1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garcia PL, Council LN, Christein JD, Arnoletti JP, Heslin MJ, Gamblin TL et al. Development and histopathological characterization of tumorgraft models of pancreatic ductal adenocarcinoma. PLoS One 2013; 8: e78183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ott CJ, Kopp N, Bird L, Paranal RM, Qi J, Bowman T et al. BET bromodomain inhibition targets both c-Myc and IL7R in high-risk acute lymphoblastic leukemia. Blood 2012; 120: 2843–2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Da Costa D, Agathanggelou A, Perry T, Weston V, Petermann E, Zlatanou A et al. BET inhibition as a single or combined therapeutic approach in primary paediatric B-precursor acute lymphoblastic leukaemia. Blood Cancer J 2013; 3: e126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feng Q, Zhang Z, Shea MJ, Creighton CJ, Coarfa C, Hilsenbeck SG et al. An epigenomic approach to therapy for tamoxifen-resistant breast cancer. Cell Res 2014; 24: 809–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Loosveld M, Castellano R, Gon S, Goubard A, Crouzet T, Pouyet L et al. Therapeutic targeting of c-Myc in T-cell acute lymphoblastic leukemia, T-ALL. Oncotarget 2014; 5: 3168–3172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Asangani IA, Dommeti VL, Wang X, Malik R, Cieslik M, Yang R et al. Therapeutic targeting of BET bromodomain proteins in castration-resistant prostate cancer. Nature 2014; 510: 278–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fowler T, Ghatak P, Price DH, Conaway R, Conaway J, Chiang CM et al. Regulation of MYC expression and differential JQ1 sensitivity in cancer cells. PLoS One 2014; 9: e87003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001; 25: 402–408. [DOI] [PubMed] [Google Scholar]
- 30.Guerra L, Albihn A, Tronnersjo S, Yan Q, Guidi R, Stenerlow B et al. Myc is required for activation of the ATM-dependent checkpoints in response to DNA damage. PLoS One 2010; 5: e8924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chandra S, Priyadarshini R, Madhavan V, Tikoo S, Hussain M, Mudgal R et al. Enhancement of c-Myc degradation by BLM helicase leads to delayed tumor initiation. J Cell Sci 2013; 126: 3782–3795. [DOI] [PubMed] [Google Scholar]
- 32.Chen Y, Xu J, Borowicz S, Collins C, Huo D, Olopade OI. c-Myc activates BRCA1 gene expression through distal promoter elements in breast cancer cells. BMC Cancer 2011; 11: 246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li LH, Nerlov C, Prendergast G, MacGregor D, Ziff EB. c-Myc represses transcription in vivo by a novel mechanism dependent on the initiator element and Myc box II. EMBO J 1994; 13: 4070–4079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang WJ, Wu SP, Liu JB, Shi YS, Huang X, Zhang QB et al. MYC regulation of CHK1 and CHK2 promotes radioresistance in a stem cell-like population of nasopharyngeal carcinoma cells. Cancer Res 2013; 73: 1219–1231. [DOI] [PubMed] [Google Scholar]
- 35.Schuldiner O, Benvenisty N. A DNA microarray screen for genes involved in c-MYC and N-MYC oncogenesis in human tumors. Oncogene 2001; 20: 4984–4994. [DOI] [PubMed] [Google Scholar]
- 36.Menssen A, Hermeking H. Characterization of the c-MYC-regulated transcriptome by SAGE: identification and analysis of c-MYC target genes. Proc Natl Acad Sci U S A 2002; 99: 6274–6279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Koshiji M, To KK, Hammer S, Kumamoto K, Harris AL, Modrich P et al. HIF-1alpha induces genetic instability by transcriptionally downregulating MutSalpha expression. Mol Cell 2005; 17: 793–803. [DOI] [PubMed] [Google Scholar]
- 38.Li Z, Van Calcar S, Qu C, Cavenee WK, Zhang MQ, Ren B. A global transcriptional regulatory role for c-Myc in Burkitt’s lymphoma cells. Proc Natl Acad Sci U S A 2003; 100: 8164–8169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sibani S, Price GB, Zannis-Hadjopoulos M. Decreased origin usage and initiation of DNA replication in haploinsufficient HCT116 Ku80+/− cells. J Cell Sci 2005; 118: 3247–3261. [DOI] [PubMed] [Google Scholar]
- 40.Philipp A, Schneider A, Vasrik I, Finke K, Xiong Y, Beach D et al. Repression of cyclin D1: a novel function of MYC. Mol Cell Biol 1994; 14: 4032–4043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gartel AL, Ye X, Goufman E, Shianov P, Hay N, Najmabadi F et al. Myc represses the p21(WAF1/CIP1) promoter and interacts with Sp1/Sp3. Proc Natl Acad Sci U S A 2001; 98: 4510–4515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Seoane J, Le HV, Massague J. Myc suppression of the p21(Cip1) Cdk inhibitor influences the outcome of the p53 response to DNA damage. Nature 2002; 419: 729–734. [DOI] [PubMed] [Google Scholar]
- 43.Kulkarni MV, Franklin DS. N-Myc is a downstream target of RET signaling and is required for transcriptional regulation of p18(Ink4c) by the transforming mutant RET(C634R). Mol Oncol 2011; 5: 24–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Qi Y, Gregory MA, Li Z, Brousal JP, West K, Hann SR. p19ARF directly and differentially controls the functions of c-Myc independently of p53. Nature 2004; 431: 712–717. [DOI] [PubMed] [Google Scholar]
- 45.Hermeking H, Rago C, Schuhmacher M, Li Q, Barrett JF, Obaya AJ et al. Identification of CDK4 as a target of c-MYC. Proc Natl Acad Sci U S A 2000; 97: 2229–2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Born TL, Frost JA, Schonthal A, Prendergast GC, Feramisco JR. c-Myc cooperates with activated Ras to induce the cdc2 promoter. Mol Cell Biol 1994; 14: 5710–5718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Galaktionov K, Chen X, Beach D. Cdc25 cell-cycle phosphatase as a target of c-myc. Nature 1996; 382: 511–517. [DOI] [PubMed] [Google Scholar]
- 48.Matsumura I, Tanaka H, Kanakura Y. E2F1 and c-Myc in cell growth and death. Cell Cycle 2003; 2: 333–338. [PubMed] [Google Scholar]
- 49.Shimamura T, Chen Z, Soucheray M, Carretero J, Kikuchi E, Tchaicha JH et al. Efficacy of BET bromodomain inhibition in Kras-mutant non-small cell lung cancer. Clin Cancer Res 2013; 19: 6183–6192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Galaktionov K, Lee AK, Eckstein J, Draetta G, Meckler J, Loda M et al. CDC25 phosphatases as potential human oncogenes. Science 1995; 269: 1575–1577. [DOI] [PubMed] [Google Scholar]
- 51.Guo J, Kleeff J, Li J, Ding J, Hammer J, Zhao Y et al. Expression and functional significance of CDC25B in human pancreatic ductal adenocarcinoma. Oncogene 2004; 23: 71–81. [DOI] [PubMed] [Google Scholar]
- 52.Lindqvist A, Kallstrom H, Karlsson Rosenthal C. Characterisation of Cdc25B localisation and nuclear export during the cell cycle and in response to stress. J Cell Sci 2004; 117: 4979–4990. [DOI] [PubMed] [Google Scholar]
- 53.Gabrielli BG, De Souza CP, Tonks ID, Clark JM, Hayward NK, Ellem KA. Cytoplasmic accumulation of cdc25B phosphatase in mitosis triggers centrosomal microtubule nucleation in HeLa cells. J Cell Sci 1996; 109 ( Pt 5): 1081–1093. [DOI] [PubMed] [Google Scholar]
- 54.Lockwood WW, Zejnullahu K, Bradner JE, Varmus H. Sensitivity of human lung adenocarcinoma cell lines to targeted inhibition of BET epigenetic signaling proteins. Proc Natl Acad Sci U S A 2012; 109: 19408–19413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sahai V, Kumar K, Knab LM, Chow CR, Raza SS, Bentrem DJ et al. BET bromodomain inhibitors block growth of pancreatic cancer cells in three-dimensional collagen. Mol Cancer Ther 2014; 13: 1907–1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Matzuk MM, McKeown MR, Filippakopoulos P, Li Q, Ma L, Agno JE et al. Small-molecule inhibition of BRDT for male contraception. Cell 2012; 150: 673–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yu XY, Zhang Z, Zhang GJ, Guo KF, Kong CZ. Knockdown of Cdc25B in renal cell carcinoma is associated with decreased malignant features. Asian Pac J Cancer Prev 2012; 13: 931–935. [DOI] [PubMed] [Google Scholar]
- 58.Fu X, Guadagni F, Hoffman RM. A metastatic nude-mouse model of human pancreatic cancer constructed orthotopically with histologically intact patient specimens. Proc Natl Acad Sci U S A 1992; 89: 5645–5649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Suetsugu A, Katz M, Fleming J, Truty M, Thomas R, Moriwaki H et al. Multi-color palette of fluorescent proteins for imaging the tumor microenvironment of orthotopic tumorgraft mouse models of clinical pancreatic cancer specimens. J Cell Biochem 2012; 113: 2290–2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Suetsugu A, Katz M, Fleming J, Truty M, Thomas R, Saji S et al. Imageable fluorescent metastasis resulting in transgenic GFP mice orthotopically implanted with human-patient primary pancreatic cancer specimens. Anticancer Res 2012; 32: 1175–1180. [PubMed] [Google Scholar]
- 61.Suetsugu A, Katz M, Fleming J, Truty M, Thomas R, Saji S et al. Non-invasive fluorescent-protein imaging of orthotopic pancreatic-cancer-patient tumorgraft progression in nude mice. Anticancer Res 2012; 32: 3063–3067. [PubMed] [Google Scholar]
- 62.Hiroshima Y, Zhao M, Maawy A, Zhang Y, Katz MH, Fleming JB et al. Efficacy of Salmonella typhimurium A1-R versus chemotherapy on a pancreatic cancer patient-derived orthotopic xenograft (PDOX). J Cell Biochem 2014; 115: 1254–1261. [DOI] [PubMed] [Google Scholar]
- 63.Hiroshima Y, Maawy A, Zhang Y, Murakami T, Momiyama M, Mori R et al. Metastatic recurrence in a pancreatic cancer patient derived orthotopic xenograft (PDOX) nude mouse model is inhibited by neoadjuvant chemotherapy in combination with fluorescence-guided surgery with an anti-CA 19-9-conjugated fluorophore. PLoS One 2014; 9: e114310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hiroshima Y, Zhang Y, Murakami T, Maawy A, Miwa S, Yamamoto M et al. Efficacy of tumor-targeting Salmonella typhimurium A1-R in combination with anti-angiogenesis therapy on a pancreatic cancer patient-derived orthotopic xenograft (PDOX) and cell line mouse models. Oncotarget 2014; 5: 12346–12357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Geiss GK, Bumgarner RE, Birditt B, Dahl T, Dowidar N, Dunaway DL et al. Direct multiplexed measurement of gene expression with color-coded probe pairs. Nat Biotechnol 2008; 26: 317–325. [DOI] [PubMed] [Google Scholar]
- 66.Gerdes J, Lemke H, Baisch H, Wacker HH, Schwab U, Stein H. Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J Immunol 1984; 133: 1710–1715. [PubMed] [Google Scholar]
- 67.Briasoulis E, Tsokos M, Fountzilas G, Bafaloukos D, Kosmidis P, Samantas E et al. Bcl2 and p53 protein expression in metastatic carcinoma of unknown primary origin: biological and clinical implications. A Hellenic Co-operative Oncology Group study. Anticancer Res 1998; 18: 1907–1914. [PubMed] [Google Scholar]
- 68.O'Brien J, Wilson I, Orton T, Pognan F. Investigation of the Alamar Blue (resazurin) fluorescent dye for the assessment of mammalian cell cytotoxicity. Eur J Biochem 2000; 267: 5421–5426. [DOI] [PubMed] [Google Scholar]
- 69.Eisen MB, Spellman PT, Brown PO, Botstein D. Cluster analysis and display of genome-wide expression patterns. Proc Natl Acad Sci U S A 1998; 95: 14863–14868. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. A list of 30 down-regulated genes by JQ1 in five tumorgraft models.