Abstract
Lactating rats exhibit stable individual differences in pup licking/grooming. We used in vivo voltammetry to monitor changes in extracellular dopamine (DA) in the nucleus accumbens (n. Acc) shell of lactating rats interacting with pups and found that (1) the DA signal increased significantly with pup licking/grooming; (2) the onset of such increases preceded pup licking/grooming; and (3) the magnitude and duration of the increase in the DA signal were significantly correlated with the duration of the licking/grooming bout. In females characterized on the basis of behavioral observations as high-licking/grooming mothers, the magnitude of the increase in the DA signal associated with licking/grooming was significantly greater than in low-licking/grooming dams. Dopamine transporter binding in the n. Acc was increased in low-compared with high-licking/grooming mothers. Injection of the selective DA uptake inhibitor GBR 12909 [1-(2-(Bis-(4-fluorophenyl)methoxy)ethyl)-4-(3 phenypropyl)piperazine dihydrochloride] (5 mg/kg, s.c.) increased the DA signal in the n. Acc and pup licking/grooming in low-licking/grooming mothers to levels comparable with those observed in high-licking/grooming dams. Receptor autoradiographic studies showed elevated levels of D1 and D3 receptors in the n. Acc shell region in high-licking/grooming dams. These results suggest that high- and low-licking/grooming dams differ in mesolimbic dopaminergic activity associated with mother-pup interactions. Such differences may serve as neural substrates for individual differences in the motivational component of maternal behavior.
Keywords: maternal, behavior, dopamine, dopamine receptors, nucleus accumbens, voltammetry
Introduction
Variations in maternal behavior in the rat (Champagne et al., 2003a) are directly related to the development in offspring of individual differences in cognition (Liu et al., 2000), behavioral and endocrine responses to stress (Liu et al., 1997; Caldji et al., 1998), and maternal behavior (Francis et al., 1999; Champagne et al., 2001). Maternal care, notably pup licking/grooming, can thus serve as a mechanism for the transmission of individual differences in gene expression and behavior across generations (Francis et al., 1999; Caldji et al., 2003). Individual differences in pup licking/grooming (LG) are associated with estrogen-oxytocin interactions within brain regions implicated in the onset of maternal behavior (Pedersen et al., 1994; Bridges, 1996; Numan and Sheehan, 1997). Thus, lactating females that exhibit elevated levels of pup LG (i.e., high-LG mothers) show increased expression of estrogen receptor α and estrogen sensitivity in the medial preoptic area (MPOA; Champagne et al., 2003b), where estrogen acts to increase oxytocin receptor binding (Caldwell et al., 1994). Thus, high-LG mothers show increased oxytocin receptor binding (Francis et al., 2000; Champagne et al., 2001).
Mesolimbic dopamine (DA) is a potential downstream target for oxytocinergic regulation and is implicated in the expression of maternal behavior. Oxytocin neurons within the MPOA appear to project directly to the ventral tegmental area (VTA; Numan and Sheehan, 1997), and oxytocin regulates dopamine-mediated behavioral effects of psychostimulants (Sarnyai et al., 1992). Administration of an oxytocin receptor antagonist into the VTA impairs maternal behavior (Pedersen et al., 1994), and oxytocin stimulates dopamine release in some cell groups (Yuan and Pan, 1996), although this has not yet been directly assessed at the level of the VTA. In microdialysis studies, presentation of pups to a lactating dam increases extracellular DA levels (Hansen et al., 1993) and cFos expression in the nucleus accumbens (n. Acc; Fleming et al., 1994). Dopamine-depleting 6-OHDA lesions of the n. Acc or the DA cell bodies in the VTA significantly disrupt maternal behavior (Hansen et al., 1991; Hansen, 1994). Infusions of D1 and D2 receptor antagonists into the n. Acc reduces pup retrieval and LG (Giordano et al., 1990; Hansen et al., 1991; Stern and Taylor, 1991; Keer and Stern, 1999). Interestingly, the n. Acc is broadly implicated in the processes of incentive motivation and mediates appetitive behaviors toward a wide range of “natural” reinforcers (Mitchell and Gratton, 1992; Robbins and Everitt, 1996; Ikemoto and Panksepp, 1999).
Pharmacological, lesion, and targeted gene knockout manipulations have been implemented to elucidate the mechanisms underlying maternal behavior. However, individual differences in maternal care can also be quantified and associated with variations in receptor, hormone, and neurotransmitter levels in relevant brain regions. This approach provides a powerful model for establishing the mechanisms that regulate behavior within the normal range observed in a species. We used in vivo voltammetry to examine DA release in the n. Acc shell of freely moving, lactating low- and high-LG dams interacting with their pups as well as in vitro receptor autoradiography to examine dopamine receptor systems. The results suggest that individual differences in maternal licking/grooming are directly related to variations in the magnitude of the DA signal at the level of the n. Acc.
Materials and Methods
Subjects. The animals used in these studies were outbred Long-Evans hooded rats born in our colony and housed in 46 × 18 × 30 cm Plexiglas cages that permitted a clear view of all activity within the cage. Food and water were provided ad libitum. The colony was maintained on a 12 hr light/dark schedule with lights on at 8 A.M.. The animals underwent routine cage maintenance beginning on day 8 of life but were otherwise unmanipulated. All procedures were performed according to guidelines developed by the Canadian Council on Animal Care and protocols approved by the McGill University Animal Care Committee. At the time of weaning on day 22 of life, the females were housed in same-sex, same-litter groups of two animals per cage. After mating and throughout lactation, adult females were housed singly.
Maternal behavior. Maternal behavior was observed for six 75 min observation periods daily for the first 6 d postpartum by individuals trained to a high level of inter-rater reliability (i.e., >0.90). Observations occurred at three periods during the light (1 A.M. and 1 and 5 P.M.) and two during the dark (6 A.M. and 9 P.M.) phases of the light/dark cycle. Within each observation period, the behavior of each mother was scored every 3 min (25 observations/period × five periods per day = 125 observations per mother per day) for the following behaviors: mother off pups, mother carrying pup, mother licking and grooming any pup, mother nursing pups in either an arched back posture, a “blanket” posture in which the mother lies over the pups, or a passive posture in which the mother is lying either on her back or side while the pups nurse. A detailed description of these behaviors is provided in Myers et al. (1989) (Champagne et al., 2003a).
In vivo electrochemical recordings: electrochemical probes. The voltammetric electrodes each consisted of a bundle of three 30-μm-diameter carbon fibers (Textron Systems, Wilmington, MA) that extended 50-100 μm beyond the sealed tip of a pulled glass capillary (outer diameter, 0.5 mm). The exposed fibers were repeatedly coated (∼8-10 coats) with a 5% solution of Nafion (Aldrich, Milwaukee, WI), a perfluorinated ionomer that promotes the exchange of cations such as DA but impedes the exchange of interfering anionic species such as ascorbic acid (AA) and 3,4-dihydroxyphenylacetic acid (DOPAC). Each electrode was calibrated before implantation to determine its sensitivity to DA and its selectivity for DA compared with AA. Calibrations were performed in 0.1 m PBS, pH 7.4, that contained 250 μm AA to mimic brain extracellular conditions. Only electrodes with a highly linear response (r ≤ 0.997) to increasing concentrations of DA and a nominal DA-to-AA selectivity ratio of >1000:1 were used. Electrodes used in the present study had a mean ± SEM DA-to-AA selectivity ratio of 1619:1 ± 133:1. Equally important, we have shown previously that these Nafion-coated carbon fiber electrodes will retain their sensitivity for DA and their selectivity for DA against AA (and DOPAC) for several days after implantation (Doherty and Gratton, 1997).
Probe implantation. Lactating females were removed from their home cages the day after parturition and prepared for surgery. Pups remained in the home cage under a heat lamp for the duration of the surgery (1 hr) and recovery period (3 hr). The animals were pretreated with atropine sulfate (0.6 mg/kg, i.p.) to reduce bronchial secretions, anesthetized with sodium pentobarbital (60 mg/kg, i.p.), and placed in a stereotaxic apparatus with the incisor bar adjusted to maintain the skull between bregma and lambda horizontal. Electrochemical probes were lowered into the nucleus accumbens shell (Paxinos and Watson, 1986; coordinates: 1.6 mm anterior to bregma, 0.7 mm lateral to the midline, and 7.4 mm ventral to the surface of the cortex). Animals were also each implanted with a Ag/AgCl reference electrode and a stainless steel ground wire in the contralateral and ipsilateral parietal cortices, respectively. Miniature pin connectors soldered to the voltammetric and reference electrodes and ground wire were inserted into a Carleton connector (Ginder Scientific, Ottawa, Ontario, Canada). The assembly was then secured with acrylic dental cement to four stainless steel screws threaded into the cranium. After a 3 hr recovery period, females were returned to their home cages and observed to be alert and to engage in full maternal behavior toward pups (i.e., retrieve pups and initiate a nursing bout).
Electrochemical measurements. Electrochemical recordings were performed using a computer-controlled, high-speed chronoamperometric apparatus (Quanteon, Lexington, KY). An oxidation potential of +0.55 mV (with respect to the reference electrode) was applied to the electrode for 100 msec at a rate of 5 Hz. The oxidation current was digitally integrated during the last 80 msec of each pulse. The sums of every 10 digitized oxidative cycles of the chronoamperometric waveform were automatically converted into equivalent values of DA concentration using the in vitro calibration factor. Values were displayed graphically on a video monitor at 2 sec intervals. The reduction current generated when the potential was returned to resting level (0.0 V for 100 msec) was digitized and summed in the same manner and served as an index to identify the main electroactive species contributing to the stress-induced increases in electrochemical signals. With Nafion-coated electrodes and a sampling rate of 5 Hz, the magnitude of the increase in reduction current elicited by an elevation in DA concentration is typically 60-80% of the corresponding increase in oxidation current [reduction-to-oxidation ratio (red:ox), 0.6-0.8 (Gerhardt et al., 1984, 1989; Gratton et al., 1989; Mitchell and Gratton, 1991, 1992; Pentney and Gratton, 1991; Doherty and Gratton, 1992)]. Previous work also indicates that the oxidation of AA is virtually irreversible (red:ox, 0-0.1), whereas that of DOPAC is almost entirely reversible (red:ox, 1.0); the reduction-to-oxidation ratios for norepinephrine and serotonin (5-HT) are 0.4-0.5 and 0.1-0.3, respectively.
Testing procedure. Electrochemical recordings began 2 d after surgery. Immediately before each recording session, the in vitro calibration factor for the animal's electrode (the slope of the function relating increases in oxidation current to increases in DA concentration) was entered in the data acquisition software. Dams were placed in a sound-attenuating recording chamber containing bedding material and connected to the chronoamperometric instrument by a shielded cable and a low-impedance commutator (Airflyte, Bayonne, NJ). A preamplifier configured as a current-to-voltage converter (gain, 1 × 108) was connected directly onto the animal's head assembly to minimize electrical interference. Once the dams were connected to the chronoamperometric instrument, the pups were introduced into the recording chamber, and electrochemical recordings were allowed to stabilize for 60 min, after which monitoring of mother-pup interactions began. Maternal behaviors were monitored continuously for 4-6 hr on postpartum days 4 and 5 by a trained observer who noted the onset and duration of all behaviors on an observation record form. The time of onset of each behavior, particularly an LG bout, was also noted to correlate the behavior with the electrochemical signal record. An LG bout was defined as a continuous period of licking/grooming that was not interrupted by a pause of >5 sec in duration.
The changes in DA signal associated with bouts of LG were characterized pharmacologically in a small group of high- and low-LG dams (n = 3 per group). These animals were prepared for electrochemical recordings as described above. The dams were placed in the recording chamber followed by the pups, and the electrochemical recordings were allowed to stabilize for 2 hr. One hour into the stabilization period, dams were injected with either a selective DA uptake inhibitor [1-(2-(Bis-(4-fluorophenyl)methoxy)ethyl)-4-(3 phenylpropyl)piperazine dihydrochloride (GBR 12909); 5 mg/kg, s.c.], oraD2 and D3 receptor agonist (quinpirole, 0.5 mg/kg, s.c.) or an equivalent volume of vehicle (saline, 1 ml/kg, s.c.). Doses of GRB 12909 and quiniprole were selected on the basis of previous reports indicating that these doses were capable of enhancing and inhibiting, respectively, central dopaminergic activity using in vivo voltammetry (Doherty and Gratton, 1997, 1999). Starting 1 hr after injection, maternal behaviors and changes in DA signal were monitored continuously for 2 hr. Each dam received the three drug treatments in different orders on consecutive test days.
Histology. At the conclusion of the experiment, animals were deeply anesthetized with sodium pentobarbital (75 mg/kg, i.p.) and transcardially perfused with 0.9% saline followed by 10% formalin. The brains were stored in 10% formalin and subsequently cryoprotected in a 30% sucrose-formalin solution before being sliced. Electrode placements were confirmed from 40 μm thionin-stained coronal sections.
Electrochemical data. Because of the inherent differences in the sensitivity of Nafion-coated electrodes, the changes in oxidation current recorded with different electrodes (i.e., in different animals) cannot be assumed to be equivalent. Thus, valid comparisons are possible only if the sensitivity of each electrode is calibrated against a standard and the electrochemical data are expressed as standard equivalent values. Because DA was used as the standard to calibrate electrode sensitivity, in vivo changes in oxidation current are expressed as nanomolar equivalent values of DA concentration. Data are presented as changes in electrochemical signal (nanomolar DA equivalent) relative to the signal level 60 min after dams were placed in the recording chamber. The electrochemical signal level at this time is referred to as “baseline” and was given a value of 0 nm. Therefore, a value of 0 nm is not meant to correspond to the absolute concentration of extracellular DA; rather, the electrochemical data reflect relative changes in DA signal associated with bouts of licking/grooming. Group comparisons are based on the peak DA signal increases during each LG bout as well as on the DA signal amplitude taken at the onset and the end of each bout of LG.
Receptor binding assays. Lactating high- and low-LG females were killed through rapid decapitation on postpartum day 6. Brains were extracted, placed briefly in isopentane, and kept at -80°C until processed. Brains were sectioned in the coronal plane at 16 μm, and sections were thaw-mounted onto poly-l-lysine-coated slides that were stored at -80°C until the assay was performed.
D1 receptor binding. Coronal, slide-mounted sections were placed in preincubation buffer (120 mm NaCl, 5 mm KCl, 0.1% ascorbic acid, and 50 mm Tris-HCl, pH 7.4) for 20 min at room temperature and then incubated for 60 min at room temperature with 2 nm [3H](R)-(+)-7-chloro-8-hydroxy-3-methyl-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepine hydrochloride (SCH23390) (80 Ci/mmol; NEN Boston MA) in 120 mm NaCl, 5 mm KCl, 0.1% asorbic acid, and 50 mm Tris-HCl (pH 7.4). Ketanserine (30 mm) was added to the buffer to block 5-HT2 receptors. Non-specific binding was determined by adding 1 μm butaclamol. Sections were washed in ice cold buffer (3 × 5 min), distilled H2O(3 × 5 min), slides were left to dry overnight and then exposed to Biomax film for 6 weeks.
D2 receptor binding. Coronal, slide-mounted sections were placed in pre-incubation buffer (120 mm NaCl, 5 mm KCl, 0.1% asorbic acid, 50 mm Tris-HCl (pH 7.4)) for 20 min at RT and then incubated for 60 min at RT with 5.7 nm [3H]Raclopride (80 Ci/mmol; PerkinElmer Life Sciences, Norwalk, CT) in 120 mm NaCl, 5 mm KCl, 0.1% asorbic acid, and 50 mm Tris-HCl, pH 7.4. Nonspecific binding was determined by adding 1 μm butaclamol. Sections were washed in ice-cold buffer (three times for 5 min) and distilled H2O (three times for 5 min), and slides were left to dry overnight and then exposed to Biomax film for 3 weeks.
D3 receptor binding. Coronal slide-mounted sections were placed in preincubation buffer (120 mm NaCl, 0.1% asorbic acid, 300 μm GTP, and 50 mm Tris-HCl, pH 7.4) for 30 min at room temperature and then incubated for 120 min at room temperature with 2 nm [3H]7-hydroxy-N,N-di-n-propyl-2-aminotetralin (150 Ci/mmol; PerkinElmer Life Sciences) in 120 mm NaCl, 0.1% asorbic acid, 300 μm GTP, and 50 mm Tris-HCl, pH 7.4. 1,3-Di-o-tolylguanidine (5 μm) was added to the incubation buffer to block σ sites. Nonspecific binding was determined by adding 1 μm dopamine. Sections were washed in ice-cold Tris-HCl, pH 7.4 (two times for 10 min) and distilled H2O (two times for 5 min), and slides were left to dry overnight and then exposed to Biomax film for 10 weeks.
Dopamine transporter binding. Coronal slide-mounted sections were placed in preincubation buffer (120 mm NaCl and 50 mm Tris-HCl, pH 7.4) for 20 min at room temperature and then incubated for 60 min at room temperature with 10 nm [3H] 1-(2-Diphenylmethoxyethyl)-4-(3-phenylpropyl)piperazine dihydrochloride (GBR 12935) (40 Ci/mmol; PerkinElmer Life Sciences) in 120 mm NaCl and 50 mm Tris-HCl, pH 7.4. Nonspecific binding was determined by adding 1 μm N-[1-(2-benzo[b]thiophenyl)cyclohexyl]piperidine. Sections were washed in ice cold Tris-HCl, pH 7.4 (four times for 5 min) and distilled H2O (two times for 5 min), and slides were left to dry overnight and then exposed to Biomax film for 4 weeks.
Results
Characterization of low- and high-LG dams
The frequency of maternal LG across a large (n = 100-200) number of mothers is normally distributed (Champagne et al., 2003a). Hence, high- and low-LG mothers represent two ends of a continuum rather than distinct populations. To define these populations for the current studies, we observed the maternal behavior in cohorts of mothers, ranging from 30 to 40 dams with their pups, and devised the group mean and SD for each behavior over the first 6 d of life as previously described (Champagne et al., 2003a). High-LG mothers were defined as females whose frequency scores for LG were >1 SD above the mean. Low-LG mothers were defined as females whose frequency scores for were >1 SD below the mean. The mean ± SEM licking/grooming score for the low-LG dams used in these studies was 7.4 ± 0.2%, and for high-LG dams it was 14.7 ± 0.4%. These statistics correspond closely to those obtained with larger populations (Champagne et al., 2003a). Licking/grooming behavior has been shown to be a highly stable trait in lactating rats (Champagne et al., 2003a). As such, these characterized females were remated and classified as high- or low-LG on the basis of their behavior to their previous litter.
Low- and high-LG lactating females (n = 8 per group) were implanted with voltammetric probes on day 1 postpartum. Four animals (two from each group) were excluded from the analysis because the chronamperometric recordings indicated that the probes were not functioning properly. The remaining six lowand six high-LG dams were included in the behavioral and electrochemical analysis. Approximately 6 hr of chronoamperometric recording and behavioral observation were conducted for each animal.
Licking/grooming behavior
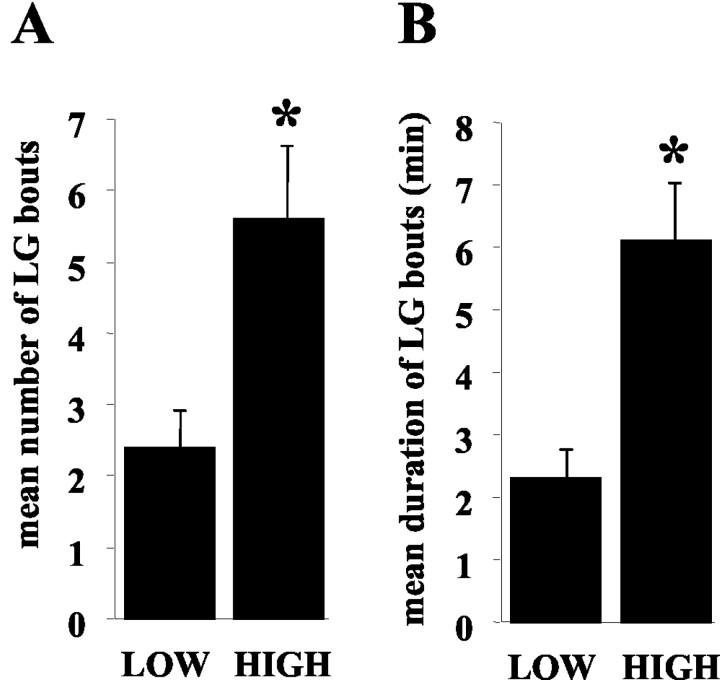
During the observation sessions (6 hr/dam), a total of 47 licking/grooming bouts were observed. A “bout” represented a relatively continuous period of pup licking/grooming. The duration and frequency of LG bouts differed significantly between high- and low-LG dams (Fig. 1). Overall, 29 bouts were observed in high-LG females (mean, 5.6 ± 1.0 bouts per female), and 18 bouts were observed in the low-LG females (mean, 2.4 ± 0.5 bouts per female). The durations of LG bouts ranged from 50 sec to 15 min in high-LG dams (mean, 6.1 ± 0.9 min/dam) and 50 sec to 5 min in low-LG dams (mean, 2.3 ± 0.5 min/dam). High- and low-LG dams differed both in the number of LG bouts [t(10) = 2.8; p < 0.05] and the duration of observed LG bouts [t(10) = 3.9; p < 0.01].
Figure 1.
Mean ± SEM number of LG bouts (A) and duration (in minutes) of LG bouts (B) observed during the 6 hr testing sessions with high- and low-LG dams. High-LG dams engaged in more LG bouts (p < 0.05) and longer-duration LG bouts (p < 0.01) compared with low-LG dams.
Licking/grooming-associated changes in DA signal in lowand high-LG dams
The location of the electrode placement for the dams used in the voltammetry studies is presented in Figure 2. Only animals with histologically confirmed electrode placement in the n. Acc shell were included in the data analysis (n = 6 per group). Chronoamperometic recordings were analyzed for all 47 LG bouts observed. Representative examples of the changes in DA signals recorded during bouts of LG are shown in Figure 3A. Typically, an increase in signal was observed while the dam was in a nursing posture and just before the onset of the bout of LG. The DA signal continued to increase after the onset of the licking/grooming bout, remained elevated for a varying period (which was highly correlated with the duration of the bout; see below), and then decreased before the termination of the LG bout. What is important to note is that the increase in the DA signal preceded the onset of the LG bout. The average number of seconds between the onset of the rise in dopamine and the onset of licking/grooming was 37.4 ± 7.0 sec, ranging from 5 to 230 sec.
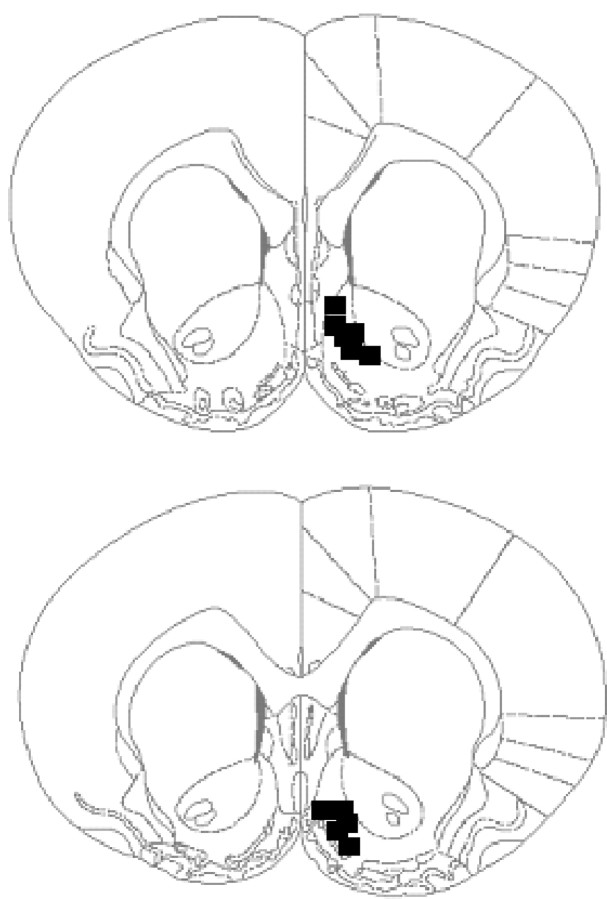
Figure 2.
Schematic illustration of the sites of electrochemical probe placement of the six high- and six low-LG dams included in the electrochemical and behavioral analysis. All animals included in the analysis had confirmed probe placement in the nucleus accumbens shell.
Figure 3.
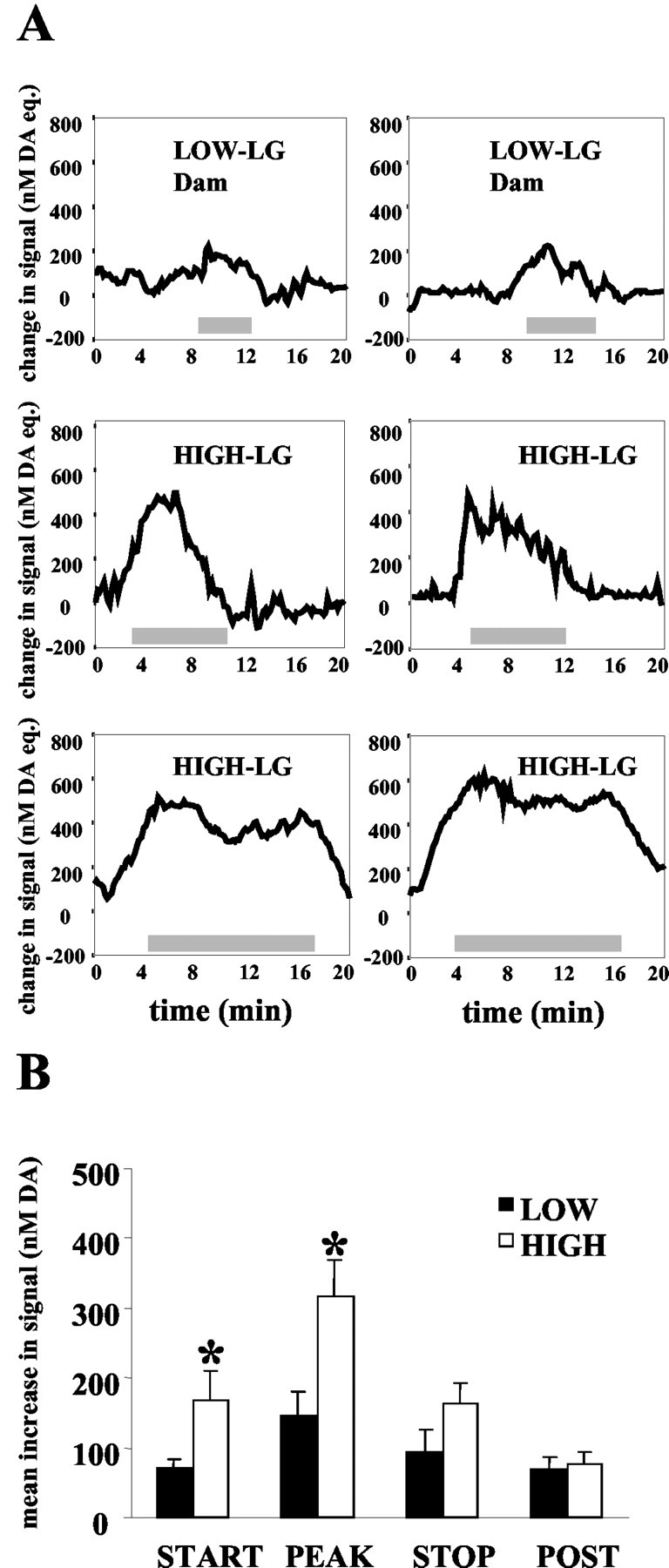
A, Representative illustrations of the change in nanomolar DA equivalent signal in the nucleus accumbens shell of high- and low-LG dams during bouts of licking/grooming. The gray bar indicates the duration of the LG bout (in minutes). B, Mean ± SEM increase in nanomolar DA equivalent signal associated with licking/grooming bouts in high- and low-LG dams. High-LG dams had higher increases in DA signal at the start of the LG bout (p < 0.05), and peak increases during the bout were also elevated in high-LG compared with low-LG dams (p < 0.01). No group differences in DA signal were observed at the end of the LG bout or 30 sec after the termination of the LG bout.
For purposes of statistical comparisons, the amplitude of DA signal increases were determined at (1) the onset of an LG bout, (2) during the LG bout (the peak increase in DA during the bout), (3) at the end of the LG bout, and (4) 30 sec after the LG bout (Fig. 3B). Repeated measures ANOVA of the averaged amplitude of DA signal increases at these time points revealed a main effect of maternal LG status [F(1,10) = 6.91; p < 0.05], a main effect of time [F(3,30) = 13.7; p < 0.001], and a significant LG status × time interaction [F(3,30) = 3.5; p < 0.05]. Post hoc analyses (Tukey's honestly significant difference) indicated that the amplitude of signal increases recorded at the onset of LG bouts was significantly greater in high-LG dams than in low-LG dams (p < 0.05). This was true also of the peak DA signal increases recorded during bouts of LG (p < 0.01). No group differences in DA signal level were observed at the end of the bout or 30 sec after the bout.
Duration of DA signal increases in relation to LG bout duration
The duration of the increase in DA signal associated with LG was also compared between low- and high-LG dams. Analysis indicated that the duration of the increase in the DA signal was significantly longer in high-LG dams (mean. 5.6 ± 1.2 min/dam) compared with low-LG dams (mean, 2.7 ± 0.5 min/dam) during licking/grooming bouts [t(10) = 2.14; p < 0.05; Figure 4A]. Correlation analysis between duration of DA signal increase and duration of the licking/grooming bout indicated a highly significant linear correlation (r = 0.98; p < 0.001), which was present in both high-LG dams (r = 0.99; p < 0.001) and low-LG dams (r = 0.86; p < 0.001; Fig. 4B).
Figure 4.
A, Mean ± SEM duration (in minutes) of the increase in nanomolar DA equivalent signal during LG bouts in high- and low-LG dams. The duration of increase in DA signal was significantly longer in high-LG compared with low-LG dams (p < 0.05). B, Scattergram illustrating the correlation between duration of LG bout and duration of the corresponding increases in DA signal. A highly significant positive correlation was found between the duration of LG and the duration of the DA increase (r = 0.98; p < 0.001).
Magnitude of DA signal increases in relation to LG bout duration
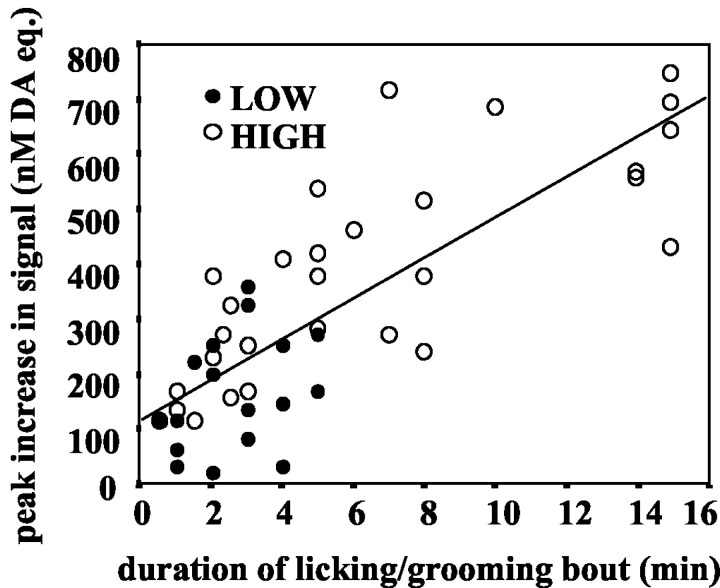
We also examined the relation between the duration of individual bouts of LG and the magnitude of DA signal increase recorded in n. Acc. All 47 bouts were included in the analysis. Pearson analysis indicated a significant positive correlation coefficient between the duration (minutes) of LG bouts and both the magnitude of DA signal increases at the onset of LG (r = 0.53; p < 0.001) and the peak signal increases during LG bouts (r = 0.80; p < 0.001; Fig. 5). Peak DA signal was correlated with LG bout duration in both high-LG (r = 0.80; p < 0.001) and low-LG (r = 0.41; p < 0.05) mothers.
Figure 5.
Scattergram illustrating the correlation between duration (in minutes) of a licking/grooming bout and the magnitude of the peak change in nanomolar DA equivalent signal associated with the bout (r = 0.80; p < 0.001).
Pharmacological characterization of LG-associated changes in DA signal
During the observation sessions after drug administration, it became apparent that the quinpirole treatment was having a dramatic behavioral effect on the females. Quinpirole-treated dams displayed pronounced behavioral impairments, failing to build a nest, retrieve pups, and assume a nursing posture over the pups, thus precluding the possibility of observing LG-associated changes in the DA signal. As such, the behavioral analysis was limited to the saline and GBR 12909 treatment sessions (Fig. 6).
Figure 6.
A, Mean ± SEM time (in minutes) spent licking/grooming by high- and low-LG dams (n = 3 per group) given subcutaneous injection of saline or GBR 12909 (5 mg/kg). GBR 12909 abolished group differences in licking/grooming. Treatment with GBR 12909 resulted in increased LG in low-LG dams (p < 0.01). B, Mean ± SEM change in nanomolar DA equivalent signal in the n. Acc of high- and low-LG dams during LG bouts after drug or saline treatment. Injection of GBR 12909 1 hr before the observation session resulted in an increase in DA signal (p < 0.05) compared with saline treatment.
Statistical analysis (ANOVA) of frequency of licking/grooming during the postinjection observation session indicated a significant group × treatment interaction [F(1,8) = 6.01; p < 0.05]. This effect was independent of the order in which the drugs were administered. Post hoc analysis revealed that high-LG dams engaged in more LG compared with low-LG dams after saline injection (p < 0.05); however, GBR 12909 abolished this group difference. Post hoc analyses indicated that, relative to the vehicle control, GBR 12909 enhanced LG in low-LG females (p < 0.01). The DA signal associated with these licking/grooming bouts also differed as a function of treatment. Two-way ANOVA of peak dopamine release during the LG bouts observed indicated a significant group × treatment interaction [F(1,13) = 5.5; p < 0.05]. The peak DA signal associated with LG bouts was higher in high-LG compared with low-LG dams after saline injection (p < 0.05); however, as with the behavioral difference, GBR 12909 abolished this group difference. Relative to the vehicle control, GBR 12909 increased the DA signal in low-LG females (p < 0.05).
Previous studies (Hansen et al., 1993) showed that mother-pup separation and reunion are associated with an increased dopamine signal in the n. Acc. This procedure thus provided a method for illustrating that the electrochemical signal measured by our voltammetric probes could be dampened or enhanced with appropriate pharmacological manipulations and thus provided a validation of the dopamine recording. In this way, we were not dependent on the natural occurrence of licking/grooming behavior to make this determination. Pups were removed from the testing apparatus and placed in a standard cage containing bedding material for 1 min at room temperature and then returned to the testing apparatus. Pups were placed in a pile outside the nest when they were returned to the apparatus. Saline-treated females, both high- and low-LG, approached pups when they were returned to the apparatus, sniffed the pups, and then began retrieving the pups and placing them in the nest within the 2 min period after return of the pups. Quinpirole-treated females also approached and sniffed the pups within the 2 min period after the pups were returned but did not proceed to retrieve the pups to the nest. GBR-treated females, like saline-treated females, proceeded to sniff and retrieve the pups when they were returned to the apparatus. Both GBR- and saline-treated females were observed to lick pups and assume a nursing posture over them within the 10 min period after the 1 min separation, whereas in the quinpirole-treated females, no licking/grooming of pups was observed, and females did not crouch over pups. As can be seen in Figure 7, A and B, this simple manipulation elicited reproducible DA signal increases in both high- and low-LG nontreated dams, the peak amplitude of which was greater in the high-LG mothers. Figure 8 summarizes the effects of GBR 12909 and quinpirole on the DA signal associated with brief mother-pup separation. Statistical analysis revealed a main effect of treatment on DA signal in the n. Acc [F(2,8) = 131.9; p < 0.001]. Post hoc analysis indicated that, in both high- and low-LG dams, GBR 12909 significantly potentiated the peak DA signal increases that occurred after the removal of pups (p < 0.05), whereas quinpirole produced the opposite effect (p < 0.05). Representative examples of the changes in the DA signal observed during mother-pup separation and reunion after the different drug treatment conditions are presented in Figure 8. The results obtained with GBR12909 and quinpirole provide further confirmation that the increases in electrochemical signals reported here are attributable primarily to elevations in extracellular DA levels. The potentiating effect of GBR 12909, a selective DA uptake blocker (Andersen, 1989), is consistent with those reported previously (Doherty and Gratton, 1996), as well as with evidence from other sources showing that DA release in n. Acc is subject to high-affinity reuptake via the DA transporter (Cass and Gerhardt, 1995). Quinpirole, a D2 and D3 receptor agonist, potently attenuated DA signal increases associated with the removal and handling of pups. At the low dose used here, this effect of quinpirole is most likely attributable to the preferential activation of terminal and somatodendritic autoreceptors, which provide negative feedback control over DA release and DA cell firing (Galloway et al., 1986; Talmaciu et al., 1986; Starke et al., 1989; Moghaddam and Bunney, 1990).
Figure 7.

Changes in nanomolar DA equivalent signal in a high-LG dam and a low-LG dam (nontreated) associated with the brief (1 min) removal of pups from the testing apparatus. The first arrow (open bar) indicates the time of removal of pups, and the second arrow (filled bar) indicates the time of return of pups to the testing apparatus.
Figure 8.
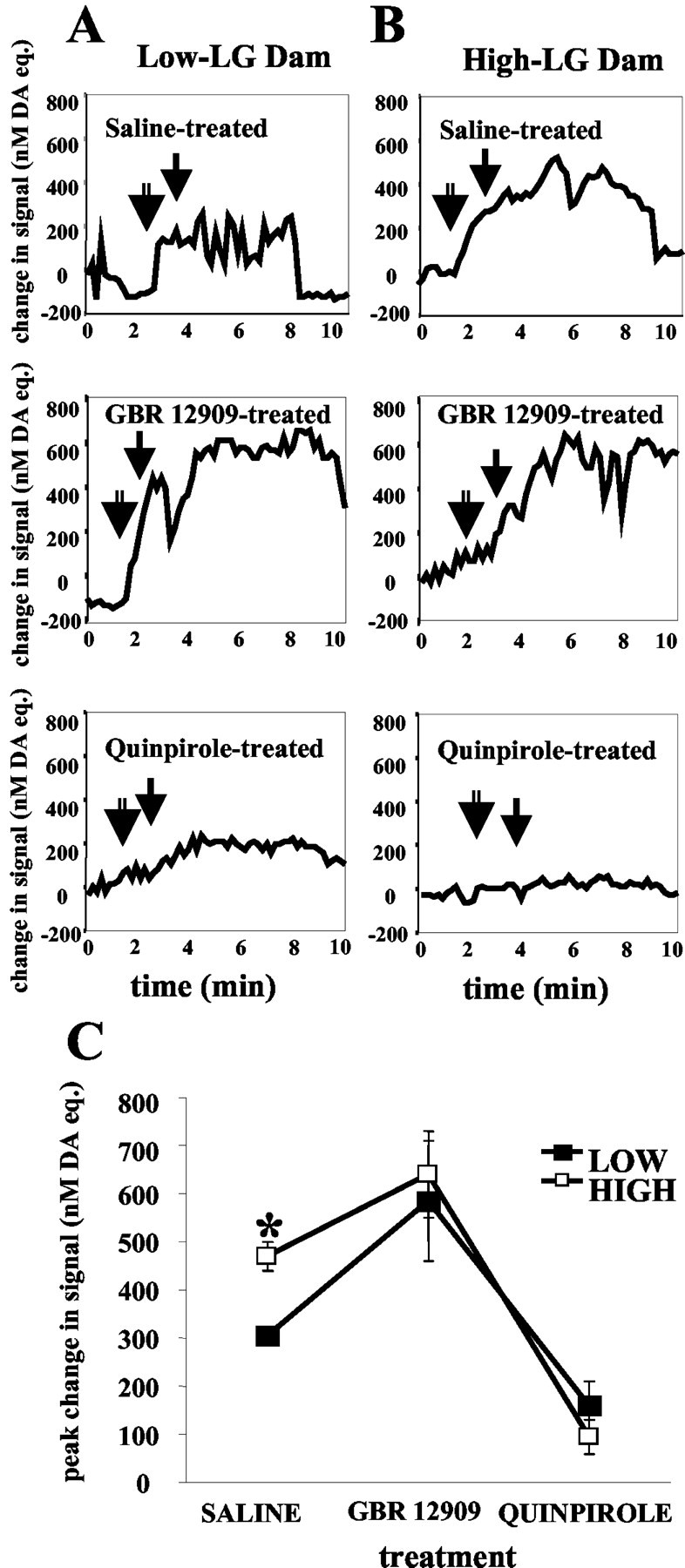
Changes in nanomolar DA equivalent signal in a high-LG dam (A) and a low-LG dam (B) after the brief (1 min) removal of pups from the testing apparatus. The first arrow (open bar) indicates the time of removal of pups, and the second arrow (filled bar) indicates time of return of pups to the testing apparatus. C, Mean ± SEM change in nanomolar DA equivalent signal in the n. Acc of high- and low-LG dams after the brief (1 min) removal of pups from the testing apparatus. Injection of GBR 12909 2 hr before testing resulted in an increase in DA signal (p < 0.05), whereas injection of quinpirole resulted in a decrease in DA signal (p < 0.05) compared with saline treatment.
Dopamine receptor and dopamine transporter binding in the nucleus accumbens
Dopamine receptor binding (D1-D3) was measured in both the shell and core regions of the n. Acc of lactating day 6 postpartum low- and high-LG dams (n = 5 per group; Fig. 9). Two-way ANOVA (region × group) of D1 receptor binding indicated a main effect of group [F(1,8) = 16.65; p < 0.05]. Analysis of D3 receptor binding indicated a main effect of region [F(1,8) = 16.65; p < 0.001], a main effect of group [F(1,8) = 15.78; p < 0.001], and a significant group × region interaction [F(1,8) = 13.242; p < 0.01]. Post hoc analyses indicated that high-LG dams showed higher levels of D1 (p < 0.05) and D3 (p < 0.001) receptor binding in the n. Acc shell compared with low-LG dams. No group differences were found in D1-D3 binding in the n. Acc core, and no group differences were found in D2 binding in the n. Acc shell region.
Figure 9.
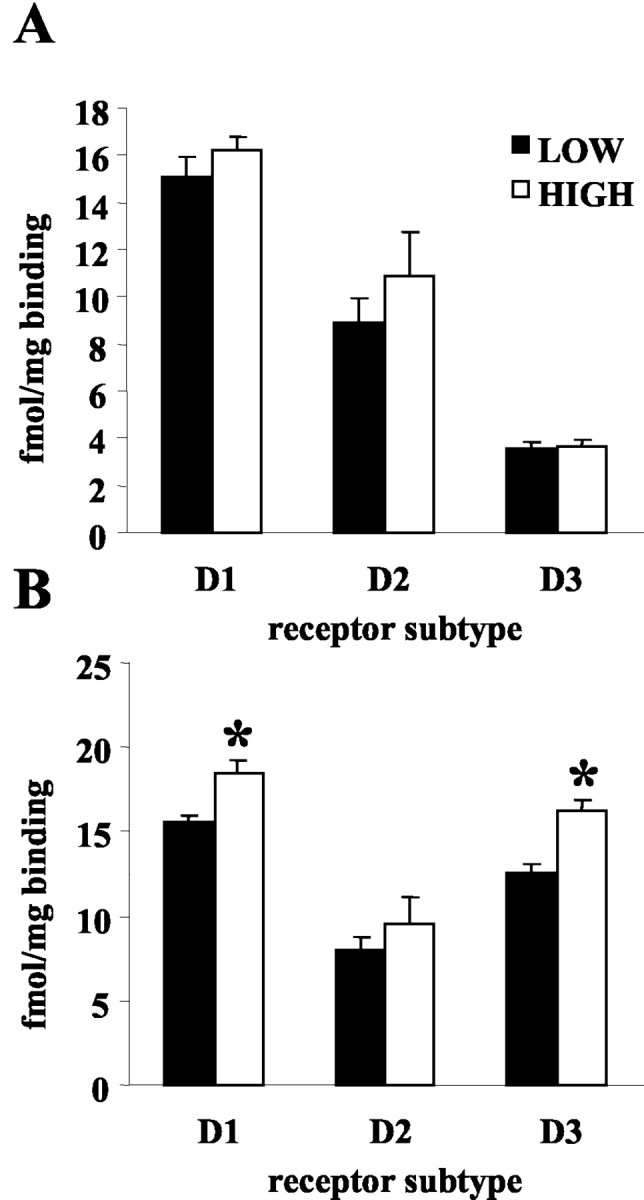
Mean ± SEM DA receptor binding (femtomoles per milligram) in the nucleus accumbens core (A) and shell (B) of high- and low-LG lactating dams. D1 (p < 0.05) and D3 (p < 0.001) receptor binding was elevated in the n. Acc shell of high-compared with low-LG dams. No group differences were found in DA receptor binding in the n. Acc core region. Overall, D3 receptor binding was elevated in the shell compared with the core (p < 0.001).
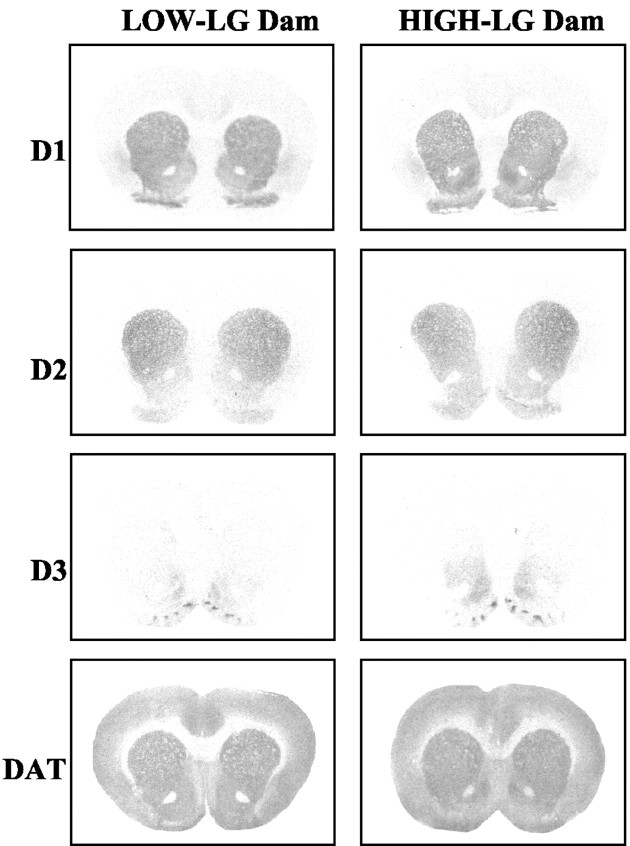
DA transporter (DAT) binding was compared in both the n. Acc shell and core of the same low and high-LG dams included in the receptor binding analyses. Two-way (group × region) ANOVA indicated a main effect of group [F(1,17) = 9.35; p < 0.01] and a significant group × region interaction [F(1,17) = 5.3; p < 0.05]. Post hoc analyses indicated that lactating low-LG dams had higher levels of DA transporter in the n. Acc shell compared with high-LG dams (p < 0.01). No significant group differences were found in the n. Acc core region. Representative photomicrographs of D1-D3 and DAT binding are presented in Figure 10.
Figure 10.
Representative photomicrographs of DA receptor and transporter binding in the nucleus accumbens of high- and low-LG lactating dams.
Discussion
Differences in maternal LG were reflected in the magnitude and duration of increases in DA signal at the level of the n. Acc. Maternal LG of pups is associated with an increased DA signal in the n. Acc shell; this increase precedes the onset of LG, and both the duration and the magnitude of the increase in the DA signal in the n. Acc shell predict the duration of LG bouts. The DA signal in the n. Acc was significantly elevated immediately before and during an LG bout in high-compared with low-LG mothers.
Mother-pup interactions in the rat are associated with increases in cFos-positive cells in the VTA (Lin et al., 1998) and n. Acc (Lonstein et al., 1998). Electrolytic (Galfori and Le Moal, 1979; Numan and Smith, 1984) or 6-hydroxydopamine (Hansen et al., 1991, 1993) lesions of the VTA or the n. Acc greatly disrupt maternal behavior. Infusion of the mixed D1-D2 receptor antagonist flupenthixol into the n. Acc significantly reduces pup LG (Keer and Stern, 1999).
We confirmed previous findings (Hansen et al., 1993) of increased accumbens DA levels during periods of mother-pup interactions using microdialysis and sampling over an entire nest bout (i.e., 20 min). The advantage conferred by chronoamperometric recording is the temporal resolution with sampling every 2 sec. With this level of resolution, it was possible to demonstrate that increases in DA release precede the onset of LG bouts (Fig. 3). This finding is consistent with previous work using chronoamperometric recording to measure DA activity elicited by food reinforcement (Kiyatkin and Gratton, 1994; Richardson and Gratton, 1996). Rats trained to lever press for food show increases in n. Acc DA before food delivery. These findings suggest that increases in n. Acc DA activity precede the consummatory phase of the behavior and are part of the incentive phase of reward-mediated behaviors. The mesolimbic DA system, including the VTA-to-n. Acc projection, is known to mediate the influence of incentive motivational states on behavior (Robbins and Everitt, 1996), including responses to naturally rewarding stimuli such as food (Kiyatkin and Gratton, 1994; Richardson and Gratton, 1996) and sexual partners (Mitchell and Gratton, 1992). Pups are a strong appetitive stimulus for the lactating rat. Postpartum females will lever press to obtain access to pups, and this response is decreased significantly with lesions to either the MPOA or the n. Acc (Lee et al., 2002).
The increased DA signal associated with pup handling and removal from the nest is consistent with previous studies using this manipulation (although typically longer in duration) to reinstate maternal behavior (Hansen, 1994). This procedure was associated with DA signal in the n. Acc shell comparable in magnitude with that associated with longer bouts of LG (Figs. 3, 7). The neonatal handling paradigm, in which a brief period (i.e., 5-15 min) of mother-pup separation is repeated over several consecutive days (Levine, 1957; Denenberg and Karas, 1959), is associated with dramatic increases in pup LG (Lee and Williams, 1974; Liu et al., 1997). Note, however, that the brief separation is associated with handling of the pups, among other disruptions, and the procedures here are not sufficient to determine the precise stimulus conditions that increase the DA signal. Nevertheless, the brief separation used here is typical of pup handling manipulations; thus, it is tempting to speculate that the increased DA levels associated with mother-pup separation and reunion mediate the dramatic increase in pup-directed behavior. Interestingly, mild tail shock produces a substantial increase in DA levels in the n. Acc shell (Di Chiara et al., 1999) and stimulates maternal behavior in the rat in a “dose-dependent” manner (Szechtman et al. 1977). The n. Acc. is reciprocally connected with many regions that are known to be directly involved in maternal behavior, including the preoptic area, bed nucleus of the stria terminalis, and periaqueductal gray (Lonstein and Stern, 1997; Numan and Sheehan, 1997). The n. Acc. also projects to the ventral pallidum, which is crucial for multiple oral behaviors, and dopamine receptor blockade in the n. Acc. significantly reduces pup licking/grooming (Keer and Stern, 1999; Stern and Lonstein, 2001).
Individual differences in maternal LG were associated with differences in DAT binding in the n. Acc shell. The DAT is a reuptake site that serves as a major source of clearance for DA from the synapse in the n. Acc (Wightman et al., 1988; Cass et al., 1993; Zahniser et al., 1999). Increased levels of DAT are commonly associated with reduced extracellular DA levels in the n. Acc (Baumann et al., 2002). Blockade of the DAT site with GBR 12909, a highly selective DAT blocker (Andersen, 1989), resulted in an increase in DA signal in the n. Acc and an enhanced level of pup LG in the low-LG mothers (Fig. 6). After the brief removal of pups, GBR 12909 eliminated the differences in the DA signal between high- and low-LG mothers (Fig. 8). These findings suggest that the increased DAT levels in the n. Acc shell could serve as a mechanism for the differences in the DA signal in the n. Acc and maternal behavior between high- and low-LG mothers.
The differences in the DA signal in the n. Acc shell between high- and low-LG dams were also associated with differences in relevant receptor sites. Both D1 and D2 receptors in the n. Acc shell are associated with appetitive responses (Wolterink et al., 1993; Ikemoto et al., 1997) and subject to variation with changes in reproductive state (Bakowska and Morrell, 1995). The D1 antagonist SCH23390 and D2 antagonist clebopride impair maternal LG after parturition (Byrnes et al., 2002). Likewise, mixed D1-D2 antagonists infused directly into the n. Acc shell reduce maternal LG and pup retrieval (Keer and Stern, 1999). D3 receptor antagonism has not been studied in relation to maternal behavior; however, like D1, this subtype is implicated in behavioral sensitization and reward processes (Parsons et al., 1996; Pilla et al., 1999; Vorel et al., 2002). Thus, the elevated levels of D1 and D3 receptors in high-LG dams may enhance the postsynaptic response to DA, contributing to the observed differences in maternal LG.
The functional distinction between the role of the n. Acc shell and core is apparent in studies of stress responsivity (Kalivas and Duffy, 1995; Chretien et al., 1998), food reinforcement (Sokolowski et al., 1998), sexual behavior (Jenkins and Becker, 2001; Lopez and Ettenberg, 2002), and maternal behavior (Keer and Stern, 1999; Li and Fleming, 2003). The n. Acc shell receives both direct and indirect connections from the MPOA via the VTA (Simon et al., 1979; Numan and Smith, 1984; Zahm and Heimer, 1993). Knife cuts severing the lateral projections from the MPOA to the VTA seriously disrupt maternal behavior (Numan and Smith, 1984). There appears to be an oxytocinergic projection from the MPOA to the VTA (Pedersen et al., 1994; Numan and Sheehan 1997), and infusion of an oxytocin receptor antagonist into the VTA disrupts maternal behavior (Pedersen et al. 1994). Estrogen increases levels of the oxytocin receptor in multiple brain regions (Breton et al., 1995; Breton and Zingg, 1997), including the MPOA (Patchev et al., 1993), an effect mediated through interactions with the estrogen receptor α subtype (Young et al., 1998). Because estrogen receptors are not colocalized with oxytocin immunoreactivity with the MPOA (Cruttwell et al., 1995), the estrogenic regulation of oxytocin activity is likely trans-synaptic. Nevertheless, infusion of an oxytocin antisera or receptor antagonism blocks the effect of estrogen on maternal behavior (Fahrbach et al., 1985; Pedersen et al. 1985). High-LG females show increased estrogen receptor α expression and higher levels of oxytocin receptor binding in the MPOA by comparison with low-LG dams (Francis et al., 2000; Champagne et al., 2001, 2003b) and intracerebroventricular infusion of a selective oxytocin receptor antagonist reduces LG in high-LG dams to levels comparable with those of low-LG dams.
Oxytocin stimulates DA release within the tuberoinfundibular system (Yuan and Pan, 1996). Oxytocin also regulates behavioral responses to psychostimulant drugs, which are often mediated by increased DA release (Kovacs et al., 1998; Sarnyai, 1998). Oxytocin-induced self-grooming behavior, which is blocked by oxytocin receptor antagonist administration to the VTA, has also been found to be blocked by 6-OHDA lesions of the nucleus accumbens and by administration of a dopamine receptor antagonist (Drago et al., 1986; Stivers et al., 1988). We propose that oxytocin receptors in the MPOA activate an oxytocinergic MPOA-VTA projection, increasing the release of DA from VTA neurons projecting to the n. Acc. These effects are further enhanced by differences in levels of DAT within the n. Acc shell as well as by postsynaptic differences in D1 and D3 receptor levels. Moreover, DA can also serve to activate estrogen receptors (Smith et al., 1993; Gangolli et al., 1997) and oxytocin release (Clarke et al., 1979; Moos and Richard, 1979; Crowley et al., 1991), further driving the differences in oxytocin receptor levels and maternal behavior.
Footnotes
This work was supported by National Institute of Mental Health Grant MH060381 and a grant from the Canadian Institutes for Health Research (CIHR). F.A.C. received a CIHR graduate fellowship. M.J.M. received a CIHR senior investigator award and a distinguished scientist award from the National Alliance for Research in Schizophrenia and Affective Disorders. A.G. is a senior scientist of the Fonds de la Recherché en Santé du Québec.
Correspondence should be addressed to Michael J. Meaney, Douglas Hospital Research Centre, 6875 LaSalle Boulevard, Montreal, Quebec H4H 1R3, Canada. E-mail: michael.meaney@mcgill.ca.
Copyright © 2004 Society for Neuroscience 0270-6474/04/244113-11$15.00/0
References
- Andersen PH (1989) The dopamine inhibitor GBR 12909: selectivity and molecular mechanism of action. Eur J Pharmacol 166: 493-504. [DOI] [PubMed] [Google Scholar]
- Bakowska JC, Morrell JI (1995) Quantitative autoradiographic analysis of D1 and D2 dopamine receptors in rat brain in early and late pregnancy. Brain Res 703: 191-200. [DOI] [PubMed] [Google Scholar]
- Baumann MH, Ayestas MA, Sharpe LG, Lewis DB, Rice KC, Rothman RB (2002) Persistent antagonism of methamphetamine-induced dopamine release in rats pretreated with GBR12909 decanoate. J Pharmacol Exp Ther 301: 1190-1197. [DOI] [PubMed] [Google Scholar]
- Breton C, Zingg HH (1997) Expression and region-specific regulation of the oxytocin receptor gene in rat brain. Endocrinology 138: 1857-1862. [DOI] [PubMed] [Google Scholar]
- Breton C, Pechoux C, Morel G, Zingg HH (1995) Oxytocin receptor messenger ribonucleic acid: characterization, regulation, and cellular localization in the rat pituitary gland. Endocrinology 136: 2928-2936. [DOI] [PubMed] [Google Scholar]
- Bridges RS (1996) Biochemical basis of parental behavior in the rat. Adv Study Behav 25: 215-242. [Google Scholar]
- Byrnes EM, Rigero BA, Bridges RS (2002) Dopamine antagonists during parturition disrupt maternal care and the retention of maternal behavior in rats. Pharmacol Biochem Behav 73: 869-875. [DOI] [PubMed] [Google Scholar]
- Caldji C, Tannenbaum B, Sharma S, Francis D, Plotsky P, Meaney MJ (1998) Maternal care during infancy regulates the development of neural systems mediating the expression of fearfulness in the rat. Proc Natl Acad Sci USA 95: 5335-5340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldji C, Diorio J, Meaney MJ (2003) Variations in maternal care alter GABAA receptor subunit expression in brain regions associated with fear. Neuropsychopharmacology 28: 1950-1959. [DOI] [PubMed] [Google Scholar]
- Caldwell JD, Walker CH, Pedersen CA, Barakat AS, Mason GA (1994) Estrogen increases affinity of oxytocin receptors in the medial preoptic area-anterior hypothalamus. Peptides 15: 1079-1084. [DOI] [PubMed] [Google Scholar]
- Cass WA, Gerhardt GA (1995) In vivo assessment of dopamine uptake in rat medial prefrontal cortex: comparison with dorsal striatum and nucleus accumbens. J Neurochem 65: 201-207. [DOI] [PubMed] [Google Scholar]
- Cass WA, Zahniser NR, Flach KA, Gerhardt GA (1993) Clearance of exogenous dopamine in rat dorsal striatum and nucleus accumbens: role of metabolism and effects of locally applied uptake inhibitors. J Neurochem 61: 2269-2278. [DOI] [PubMed] [Google Scholar]
- Champagne F, Diorio J, Sharma S, Meaney MJ (2001) Naturally occurring variations in maternal behavior in the rat are associated with differences in estrogen-inducible central oxytocin receptors. Proc Natl Acad Sci USA 98: 12736-12741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne F, Francis DD, Mar A, Meaney MJ (2003a) Naturally-occurring variations in maternal care in the rat as a mediating influence for the effects of environment on the development of individual differences in stress reactivity. Physiol Behav 79: 359-371. [DOI] [PubMed] [Google Scholar]
- Champagne FA, Weaver ICG, Diorio J, Sharma S, Meaney MJ (2003b) Natural variations in maternal care are associated with estrogen receptor alpha expression and estrogen sensitivity in the MPOA. Endocrinology 144: 4720-4724. [DOI] [PubMed] [Google Scholar]
- Chretien P, Pomerleau F, Gratton A (1998) Regional differences in the acute ventral striatal dopamine response to repeated stress. Soc Neurosci Abstr 24: 484. [Google Scholar]
- Clarke G, Lincoln DW, Merrick LP (1979) Dopaminergic control of oxytocin release in lactating rats. J Endocrinol 83: 409-420. [DOI] [PubMed] [Google Scholar]
- Crowley WR, Parker SL, Armstrong WE, Wang W, Grosvenor CE (1991) Excitatory and inhibitory dopaminergic regulation of oxytocin secretion in the lactating rat: evidence for respective mediation by D-1 and D-2 dopamine receptor subtypes. Neuroendocrinology 53: 493-502. [DOI] [PubMed] [Google Scholar]
- Cruttwell CJ, Herbison AE, Bicknell RJ (1995) Differential cellular localization of oestrogen receptor immunoreactivity and oxytocin mRNA and immunoreactivity in the rat preoptic area. Neurosci Lett 200: 89-92. [DOI] [PubMed] [Google Scholar]
- Denenberg VH, Karas GG (1959) Effects of differential infantile handling upon weight gain and mortality in the rat and mouse. Science 130: 629-630. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Loddo P, Tanda G (1999) Reciprocal changes in prefrontal and limbic dopamine responsiveness to aversive and rewarding stimuli after chronic mild stress: implications for the psychobiology of depression. Biol Psychiatry 46: 1624-1633. [DOI] [PubMed] [Google Scholar]
- Doherty MD, Gratton A (1992) High-speed chronoamperometric measurements of mesolimbic and nigrostriatal dopamine release associated with repeated daily stress. Brain Res 586: 295-302. [DOI] [PubMed] [Google Scholar]
- Doherty MD, Gratton A (1996) Medial prefrontal cortical D1 receptor modulation of the meso-accumbens dopamine response to stress: an electrochemical study in freely-behaving rats. Brain Res 715: 86-97. [DOI] [PubMed] [Google Scholar]
- Doherty MD, Gratton A (1997) NMDA receptors in nucleus accumbens modulate stress-induced dopamine release in nucleus accumbens and ventral tegmental area. Synapse 26: 225-234. [DOI] [PubMed] [Google Scholar]
- Doherty MD, Gratton A (1999) Effects of medial prefrontal cortical injections of GABA receptor agonists and antagonists on the local and nucleus accumbens dopamine responses to stress. Synapse 32: 288-300. [DOI] [PubMed] [Google Scholar]
- Drago F, Caldwell JD, Pedersen CA, Continella G, Scapagnini U, Prange Jr AJ (1986) Dopamine neurotransmission in the nucleus accumbens may be involved in oxytocin-enhanced grooming behavior of the rat. Pharmacol Biochem Behav 24: 1185-1188. [DOI] [PubMed] [Google Scholar]
- Fahrbach SE, Morrell JI, Pfaff DW (1985) Possible role for endogenous oxytocin in estrogen-facilitated maternal behavior in rats. Neuroendocrinology 40: 526-532. [DOI] [PubMed] [Google Scholar]
- Fleming AS, Suh EJ, Korsmit M, Rusak B (1994) Activation of Fos-like immunoreactivity in the medial preoptic area and limbic structures by maternal and social interactions in rats. Behav Neurosci 108: 724-734. [DOI] [PubMed] [Google Scholar]
- Francis DD, Diorio J, Liu D, Meaney MJ (1999) Non-genomic transmission across generations of maternal behavior and stress responses in the rat. Science 286: 1155-1158. [DOI] [PubMed] [Google Scholar]
- Francis DD, Champagne F, Meaney MJ (2000) Variations in maternal behaviour are associated with differences in oxytocin receptor levels in the rat. J Neuroendocrinol 12: 1145-1148. [DOI] [PubMed] [Google Scholar]
- Galfori O, Le Moal M (1979) Disruption of maternal behavior and appearance of cannibalism after ventral tegmental lesions. Physiol Behav 23: 317-323. [DOI] [PubMed] [Google Scholar]
- Galloway MP, Wolf ME, Roth RH (1986) Regulation of dopamine synthesis in the medial prefrontal cortex is mediated by release modulating autoreceptors: studies in vivo. J Pharmacol Exp Ther 236: 689-698. [PubMed] [Google Scholar]
- Gangolli EA, Conneely OM, O'Malley BW (1997) Neurotransmitters activate the human estrogen receptor in a neuroblastoma cell line. J Steroid Biochem Mol Biol 61: 1-9. [DOI] [PubMed] [Google Scholar]
- Gerhardt GA, Oke AF, Nagy G, Moghaddam B, Adams RN (1984) Nafion-coated electrodes with high selectivity for CNS electrochemistry. Brain Res 290: 390-395. [DOI] [PubMed] [Google Scholar]
- Gerhardt GA, Friedemann M, Brodie MS, Vickroy TW, Gratton AP, Hoffer BJ, Rose GM (1989) The effects of cholecystokinin (CCK-8) on dopamine-containing nerve terminals in the caudate nucleus and nucleus accumbens of the anesthetized rat: an in vivo electrochemical study. Brain Res 499: 157-163. [DOI] [PubMed] [Google Scholar]
- Giordano B, Johnson AE, Rosenblatt JS (1990) Haloperidol-induced disruption of retrieval behavior and reversal with apomorphine in lactating rats. Physiol Behav 48: 211-214. [DOI] [PubMed] [Google Scholar]
- Gratton A, Hoffer BJ, Gerhardt GA (1989) In vivo electrochemical studies of monoamine release in the medial prefrontal cortex of the rat. Neuroscience 29: 57-64. [DOI] [PubMed] [Google Scholar]
- Hansen S (1994) Maternal behavior of female rats with 6-OHDA lesions in the ventral striatum: characterization of the pup retrieval deficit. Physiol Behav 55: 615-620. [DOI] [PubMed] [Google Scholar]
- Hansen S, Harthon C, Wallin E, Lofberg L, Svensson K (1991) The effects of 6-OHDA-induced dopamine depletions in the ventral or dorsal striatum on maternal and sexual behavior in the female rat. Pharmacol Biochem Behav 39: 71-77. [DOI] [PubMed] [Google Scholar]
- Hansen S, Bergvall AH, Nyiredi S (1993) Interaction with pups enhances dopamine release in the ventral striatum of maternal rats: a microdialysis study. Pharmacol Biochem Behav 45: 673-676. [DOI] [PubMed] [Google Scholar]
- Ikemoto S, Panksepp J (1999) The role of nucleus accumbens dopamine in motivated behavior: a unifying interpretation with special reference to reward seeking. Brain Res Rev 31: 6-41. [DOI] [PubMed] [Google Scholar]
- Ikemoto S, Glazier BS, Murphy JM, McBride WJ (1997) Role of dopamine D1 and D2 receptors in the nucleus accumbens in mediating reward. J Neurosci 17: 8580-8587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins WJ, Becker JB (2001) Role of the striatum and nucleus accumbens in paced copulatory behavior in the female rat. Behav Brain Res 121: 119-128. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Duffy P (1995) Selective activation of dopamine transmission in the shell of the nucleus accumbens by stress. Brain Res 675: 325-328. [DOI] [PubMed] [Google Scholar]
- Keer SE, Stern JM (1999) Dopamine receptor blockade in the nucleus accumbens inhibits maternal retrieval and licking, but enhances nursing behavior in lactating rats. Physiol Behav 67: 659-669. [DOI] [PubMed] [Google Scholar]
- Kiyatkin EA, Gratton A (1994) Electrochemical monitoring of extracellular dopamine in nucleus accumbens of rats lever-pressing for food. Brain Res 652: 225-234. [DOI] [PubMed] [Google Scholar]
- Kovacs GL, Sarnyai Z, Szabo G (1998) Oxytocin and addiction: a review. Psychoneuroendocrinology 23: 945-962. [DOI] [PubMed] [Google Scholar]
- Lee A, Clancy S, Fleming AS (2002) Mother rats bar-press for pups: effects of lesions of the mpoa and limbic sites on maternal behavior and operant responding for pup-reinforcement. Behav Brain Res 108: 215-231. [DOI] [PubMed] [Google Scholar]
- Lee MHS, Williams DI (1974) Changes in licking behaviour of rat mother following handling of young. Anim Behav 22: 679-681. [Google Scholar]
- Levine S (1957) Infantile experience and resistance to psychological stress. Science 126: 405-406. [DOI] [PubMed] [Google Scholar]
- Li M, Fleming AS (2003) Differential involvement of nucleus accumbens shell and core subregions in maternal memory in postpartum female rats. Behav Neurosci 117: 426-445. [DOI] [PubMed] [Google Scholar]
- Lin S-H, Miyata S, Weng W, Matsunaga W, Ichikawa J, Furuya K, Nakashima T, Kiyohara T (1998) Comparison of the expression of two immediate early gene proteins, FosB and Fos, in the rat preoptic area, hypothalamus and brainstem during pregnancy, parturition and lactation. Neursci Res 32: 333-341. [DOI] [PubMed] [Google Scholar]
- Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D, Freedman A, Sharma S, Pearson D, Plotsky PM, Meaney MJ (1997) Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science 277: 1659-1662. [DOI] [PubMed] [Google Scholar]
- Liu D, Diorio J, Day JC, Francis DD, Meaney MJ (2000) Maternal care, hippocampal synaptogenesis and cognitive development in rats. Nat Neurosci 3: 799-806. [DOI] [PubMed] [Google Scholar]
- Lonstein JS, Stern JM (1997) Somatosensory contributions to c-fos activation within the caudal periaqueductal gray of lactating rats: effects of perioral, rooting, and suckling stimuli from pups. Horm Behav 32: 155-166. [DOI] [PubMed] [Google Scholar]
- Lonstein JS, Simmons DA, Swann JM, Stern JM (1998) Forebrain expression of cFos due to active maternal behavior in lactating rats. Neuroscience 82: 267-228. [DOI] [PubMed] [Google Scholar]
- Lopez HH, Ettenberg A (2002) Sexually conditioned incentives: attenuation of motivational impact during dopamine receptor antagonism. Pharmacol Biochem Behav 72: 65-72. [DOI] [PubMed] [Google Scholar]
- Mitchell JB, Gratton A (1991) Opioid modulation and sensitization of dopamine release elicited by sexually relevant stimuli: a high speed chronoamperometric study in freely behaving rats. Brain Res 551: 20-27. [DOI] [PubMed] [Google Scholar]
- Mitchell JB, Gratton A (1992) Partial dopamine depletion of the prefrontal cortex leads to enhanced mesolimbic dopamine release elicited by repeated exposure to naturally reinforcing stimuli. J Neurosci 12: 3609-3618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghaddam B, Bunney BS (1990) Acute effects of typical and atypical antipsychotic drugs on the release of dopamine from prefrontal cortex, nucleus accumbens, and striatum of the rat: an in vivo microdialysis study. J Neurochem 54: 1755-1760. [DOI] [PubMed] [Google Scholar]
- Moos F, Richard P (1979) Effects of dopaminergic antagonist and agonist on oxytocin release induced by various stimuli. Neuroendocrinology 28: 138-144. [DOI] [PubMed] [Google Scholar]
- Myers MM, Brunelli SA, Squire JM, Shindeldecker RD, Hofer MA (1989) Maternal behavior of SHR rats and its relationship to offspring blood pressures. Dev Psychobiol 22: 29-53. [DOI] [PubMed] [Google Scholar]
- Numan M, Sheehan TP (1997) Neuroanatomical circuitry for mammalian maternal behavior. Ann NY Acad Sci 807: 101-125. [DOI] [PubMed] [Google Scholar]
- Numan M, Smith HG (1984) Maternal behavior in rats: evidence for the involvement of preoptic projections to the ventral tegmental area. Behav Neurosci 98: 712-727. [DOI] [PubMed] [Google Scholar]
- Parsons LH, Caine SB, Sokoloff P, Schwartz JC, Koob GF, Weiss F (1996) Neurochemical evidence that postsynaptic nucleus accumbens D3 receptor stimulation enhances cocaine reinforcement. J Neurochem 67: 1078-1089. [DOI] [PubMed] [Google Scholar]
- Patchev VK, Schlosser SF, Hassan AH, Almeida OF (1993) Oxytocin binding sites in rat limbic and hypothalamic structures: site-specific modulation by adrenal and gonadal steroids. Neuroscience 57: 537-543. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C (1986) The rat brain in stereotaxic coordinates. Sydney: Academy. [DOI] [PubMed]
- Pedersen CA, Caldwell JD, Johnson MF, Fort SA, Prange AJ Jr (1985) Oxytocin antiserum delays onset of ovarian steroid-induced maternal behavior. Neuropeptides 6: 175-182. [DOI] [PubMed] [Google Scholar]
- Pedersen CA, Caldwell JD, Walker C, Ayers G, Mason GA (1994) Oxytocin activates the postpartum onset of rat maternal behavior in the ventral tegmental and medial preoptic areas. Behav Neurosci 108: 1163-1171. [DOI] [PubMed] [Google Scholar]
- Pentney RJ, Gratton A (1991) Effects of local delta and mu opioid receptor activation on basal and stimulated dopamine release in striatum and nucleus accumbens of rat: an in vivo electrochemical study. Neuroscience 45: 95-102. [DOI] [PubMed] [Google Scholar]
- Pilla M, Perachon S, Sautel F, Garrido F, Mann A, Wermuth CG, Schwartz JC, Everitt BJ, Sokoloff P (1999) Selective inhibition of cocaine-seeking behaviour by a partial dopamine D3 receptor agonist. Nature 400: 371-375. [DOI] [PubMed] [Google Scholar]
- Richardson NR, Gratton A (1996) Behavior-relevant changes in nucleus accumbens dopamine transmission elicited by food reinforcement: an electrochemical study in rat. J Neurosci 16: 8160-8169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins TW, Everitt BJ (1996) Neurobehavioural mechanisms of reward and motivation. Curr Opin Neuobiol 6: 228-236. [DOI] [PubMed] [Google Scholar]
- Sarnyai Z (1998) Oxytocin and neuroadaptation to cocaine. Prog Brain Res 119: 449-466. [DOI] [PubMed] [Google Scholar]
- Sarnyai Z, Biro E, Babarczy E, Vecsernyes M, Laczi F, Szabo G, Krivan M, Kovacs GL, Telegdy G (1992) Oxytocin modulates behavioural adaptation to repeated treatment with cocaine in rats. Neuropharmacology 31: 593-598. [DOI] [PubMed] [Google Scholar]
- Simon H, Le Moal M, Calas A (1979) Efferents and afferents of the ventral tegmental-A10 region studied after local injection of [3H]leucine and horseradish peroxidase. Brain Res 178: 17-40. [DOI] [PubMed] [Google Scholar]
- Smith CL, Conneely OM, O'Malley BW (1993) Modulation of the ligand-independent activation of the human estrogen receptor by hormone and antihormone. Proc Natl Acad Sci USA 90: 6120-6124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolowski JD, Conlan AN, Salamone JD (1998) A microdialysis study of nucleus accumbens core and shell dopamine during operant responding in the rat. Neuroscience 86: 1001-1009. [DOI] [PubMed] [Google Scholar]
- Starke K, Gothert M, Kilbinger H (1989) Modulation of neurotransmitter release by presynaptic autoreceptors. Physiol Rev 69: 864-989. [DOI] [PubMed] [Google Scholar]
- Stern JM, Lonstein JS (2001) Neural mediation of nursing and related maternal behaviors. Prog Brain Res 133: 263-278. [DOI] [PubMed] [Google Scholar]
- Stern JM, Taylor LA (1991) Haloperidol inhibits maternal retrieval and licking, but facilitates nursing behavior and milk ejection in lactating rats. J Neuroendocrinol 3: 591-596. [DOI] [PubMed] [Google Scholar]
- Stivers JA, Kaltwasser MT, Hill PS, Hruby VJ, Crawley JN (1988) Ventral tegmental oxytocin induces grooming. Peptides 9[Suppl 1]: 223-231. [DOI] [PubMed] [Google Scholar]
- Szechtman H, Siegel HI, Rosemblatt JS, Komisaruk BR (1977) Tail-pinch facilitates onset of maternal behavior in rats. Physiol Behav 19: 807-809. [DOI] [PubMed] [Google Scholar]
- Talmaciu RK, Hoffmann IS, Cubeddu LX (1986) Dopamine autoreceptors modulate dopamine release from the prefrontal cortex. J Neurochem 47: 865-870. [DOI] [PubMed] [Google Scholar]
- Vorel SR, Ashby Jr CR, Paul M, Liu X, Hayes R, Hagan JJ, Middlemiss DN, Stemp G, Gardner EL (2002) Dopamine D3 receptor antagonism inhibits cocaine-seeking and cocaine-enhanced brain reward in rats. J Neurosci 22: 9595-9603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wightman RM, Amatore C, Engstrom RC, Hale PD, Kristensen EW, Kuhr WG, May LJ (1988) Real-time characterization of dopamine overflow and uptake in the rat striatum. Neuroscience 25: 513-523. [DOI] [PubMed] [Google Scholar]
- Wolterink G, Phillips G, Cador M, Donselaar-Wolterink I, Robbins TW, Everitt BJ (1993) Relative roles of ventral striatal D1 and D2 dopamine receptors in responding with conditioned reinforcement. Psychopharmacology 110: 355-364. [DOI] [PubMed] [Google Scholar]
- Young LJ, Wang Z, Donaldson R, Rissman EF (1998) Estrogen receptor alpha is essential for induction of oxytocin receptor by estrogen. Neuro-Report 9: 933-936. [DOI] [PubMed] [Google Scholar]
- Yuan ZF, Pan JT (1996) Stimulatory effect of central oxytocin on tuberoinfundibular dopaminergic neuron activity and inhibition on prolactin secretion: neurochemical and electrophysiological studies. Endocrinology 137: 4120-4125. [DOI] [PubMed] [Google Scholar]
- Zahm DS, Heimer L (1993) Specificity in the efferent projections of the nucleus accumbens in the rat: comparison of the rostral pole projection patterns with those of the core and shell. J Comp Neurol 327: 220-232. [DOI] [PubMed] [Google Scholar]
- Zahniser NR, Larson GA, Gerhardt GA (1999) In vivo dopamine clearance rate in rat striatum: regulation by extracellular dopamine concentration and dopamine transporter inhibitors. J Pharmacol Exp Ther 289: 266-277. [PubMed] [Google Scholar]