Abstract
Using a spinal cord slice preparation and patch-clamp recordings from spinal cord dorsal horn neurons, we examined excitatory and inhibitory circuits connecting to lamina V neurons after the activation of afferent central terminal vanilloid receptor-1 (VR1) receptors and P2X receptors. We found that single neurons in lamina V often received excitatory inputs from two chemically defined afferent pathways. One of these pathways was polysynaptic from capsaicin-sensitive afferent terminals. In this pathway the capsaicin-sensitive afferent input first activated interneurons in superficial laminas, and then the excitatory activity was transmitted onto lamina V neurons. The second excitatory input was monosynaptic from αβm-ATP-sensitive/capsaicin-insensitive afferent terminals. Both capsaicin-sensitive and αβm-ATP-sensitive/capsaicin-insensitive pathways also recruited polysynaptic inhibitory inputs to lamina V neurons. Furthermore, we demonstrated that simultaneous activation of both capsaicin-sensitive afferent pathways and αβm-ATP-sensitive/capsaicin-insensitive pathways could generate a temporal summation of excitatory inputs onto single lamina V neurons. These convergent pathways may provide a mechanism of sensory integration for two chemically defined sensory inputs and may have implications in different sensory states.
Keywords: capsaicin; VR1 receptors; ATP; α,β-methylene-ATP; P2X receptors; EPSCs; IPSCs; spinal cord slice preparation
The capsaicin vanilloid receptor-1 receptor (VR1) is a ligand-gated cation channel that functions as a sensor for heat, protons, and endogenous VR1 ligands (Caterina et al., 1997; Kress and Zeilhofer, 1999; Zygmunt et al., 1999; Hwang et al., 2000; Smart et al., 2000). VR1 is expressed on a subpopulation of primary afferent neurons that have small diameter and are potentially nociceptive sensory neurons (Jancso et al., 1977; Holzer, 1991;Caterina et al., 1997; Guo et al., 1999). At peripheral nerve endings VR1 activation may be associated with the generation of burning pain sensation (Szolcsanyi, 1977; Holzer, 1991; Tominaga et al., 1998). VR1 plays an essential role in tissue injury- and inflammation-induced thermal hyperalgesia (Caterina et al., 2000; Davis et al., 2000). In addition to their expression at peripheral nerve endings, VR1 immunoreactivity (VR1-ir) also is found on the central terminals of nociceptive afferent fibers (Guo et al., 1999).
P2X receptors (P2X) are a family of cation channels gated by extracellular ATP (Jahr and Jessell, 1983; Krishtal et al., 1983). To date, seven P2X subunits (P2X1 to P2X7) have been cloned (North and Surprenant, 2000; Khakh et al., 2001), and six (P2X1 to P2X6) are expressed on primary sensory neurons (Collo et al., 1996; Vulchanova et al., 1996, 1997, 1998; Xiang et al., 1998; C. Li et al., 1999; Novakovic et al., 1999). Activation of P2X at peripheral sensory nerve endings may initiate sensory impulses and may be associated with nociceptive and non-nociceptive sensory signals (Burnstock and Wood, 1996; Cook et al., 1997; Sawynok and Reid, 1997; Dowd et al., 1998; Cockayne et al., 2000; Souslova et al., 2000;Tsuda et al., 2000). Immunochemical studies have shown the presence of P2X subunits on the central terminals of primary afferents (Guo et al., 1999) and have shown that presynaptic P2X could modulate sensory synaptic transmission (Gu and MacDermott, 1997; Li et al., 1998).
Dorsal root ganglion (DRG) neurons expressing VR1 usually also express P2X3 receptors (Guo et al., 1999; Ueno et al., 1999; Wood, 2000). Therefore, capsaicin-sensitive afferent fibers are usually ATP-sensitive as well (C. Li et al., 1999; Ueno et al., 1999; Petruska et al., 2000). Based on the immunoreactivity for VR1 and P2X3 (Guo et al., 1999), sensory signals induced by the activation of VR1 or P2X3 should be conveyed primarily to the superficial laminas of the spinal cord. However, many DRG neurons express P2X, but not VR1 (C. Li et al., 1999; Ueno et al., 1999; Petruska et al., 2000). These primary sensory neurons are ATP-sensitive/capsaicin-insensitive (Tsuda et al., 2000). For ATP-sensitive/capsaicin-insensitive afferents, many of their central terminals synapse directly on deep dorsal horn (DH) neurons in lamina V (Nakatsuka and Gu, 2001). Thus, capsaicin-sensitive and ATP-sensitive/capsaicin-insensitive afferents are two chemically distinct populations of sensory fibers.
Within the neuronal network of the DH, synaptic interactions of different sensory inputs may play a role in sensory phenomena such as hyperalgesia and allodynia. The sensory interactions could be involved in capsaicin-induced receptive field expansion and secondary hyperalgesia (Simone et al., 1989a; LaMotte et al., 1991; Pedersen et al., 1996). Here, we examined synaptic pathways of capsaicin-sensitive inputs and αβm-ATP-sensitive/capsaicin-insensitive inputs and their interactions in the DH neuronal network.
MATERIALS AND METHODS
Tissue preparation. Transverse spinal cord slices (500 μm in thickness) were prepared from L5 spinal cords of rats at the postnatal age of 11–21 d (Nakatsuka et al., 2000). Some slices were prepared from adult rats at ages between 7 and 9 weeks as described previously (Yoshimura and Jessell, 1990). Spontaneous EPSCs (sEPSCs), miniature EPSCs (mEPSCs), spontaneous IPSCs (sIPSCs), and miniature IPSCs (mIPSCs) were recorded from slices without dorsal roots attached. Evoked EPSCs (eEPSCs) were recorded from slices with L5 dorsal roots attached. The length of the dorsal roots was in the range of 4–15 mm. In each experiment a spinal cord slice was transferred to a recording chamber and placed on the stage of an upright microscope equipped with an infrared differential interference contrast (IR-DIC) system. The volume of the recording chamber was ∼0.5 ml. Lamina regions were identified with a 10× objective. Recordings from spinal slices of postnatal rats were performed under visual guidance; individual neurons could be identified with a 40× objective. A blind-patch technique was used for recordings in spinal cord slice preparations from adult rats (Nakatsuka et al., 2000). The spinal cord slice was superfused with Krebs' solution flowing at 10 ml/min at room temperature (22°C). The Krebs' solution contained (in mm): 117 NaCl, 3.6 KCl, 2.5 CaCl2, 1.2 MgCl2, 1.2 NaH2PO4, 25 NaHCO3, and 11 glucose; the solution was equilibrated with 95% O2/5% CO2, and the pH of the saturated solution was 7.4.
Patch-clamp recordings. Whole-cell patch-clamp recordings were made from DH neurons with electrodes filled with an internal solution containing (in mm): 135 K+-gluconate, 5 KCl, 0.5 CaCl2, 2 MgCl2, 5 EGTA, and 5 HEPES; the pH of the solution was adjusted to 7.3 with NaOH, and the osmolarity was adjusted to 320 mOsm with sucrose. The resistances of the electrodes were ∼5 MΩ when filled with the internal solution. The junction potentials of the electrodes were not corrected. The access resistance was below 20 MΩ and was not compensated. Signals were amplified and filtered at 2 kHz (Axopatch 200B) and sampled at 5 kHz. When EPSCs were recorded, the cells were held at −60 mV. At this holding potential the outward IPSCs were minimized and usually undetectable. sEPSCs were recorded in the absence of tetrodotoxin (TTX), and mEPSCs in the presence of 0.5 μm TTX, 20 μm bicuculline, and 2 μm strychnine were present in the bath solution in some experiments. When IPSCs were recorded, the cells were held at −10 mV. This holding potential was close to the reversal potential for glutamate receptors. Therefore, the inward EPSCs were minimized and usually undetectable. sIPSCs were recorded in the absence of TTX and mIPSCs in the presence of 0.5 μm TTX. In some experiments for the recordings of sIPSCs and mIPSCs, 20 μm 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) and 50 μmd-(−)-2-amino-5-phosphonopentanoic acid (d-APV) also were present in the bath solution. In experiments designed to record sEPSCs and sIPSCs simultaneously, the cells were held at −45 mV. At this holding potential the sEPSCs were inward currents and the sIPSCs were outward currents. α,β-Methylene-ATP (αβm-ATP; 100 μm), capsaicin (2 μm), and other testing compounds were applied via the bath solution. The intervals for multiple applications of testing compounds were 20 min. Analyses of sEPSCs, mEPSCs, sIPSCs, and mIPSCs were performed as described previously (Gu and MacDermott, 1997) with the commercial software Mini Analysis (Synaptosoft, Decatur, GA;http://www.synaptosoft.com). Cells were assigned to be responsive to the testing compounds when there were >20% increases in the frequency of EPSCs or IPSCs. To record eEPSCs, we applied stimuli (30–120 μA, 0.1 msec for Aδ fiber; 200–800 μA, 0.1 msec for C-fiber) to a dorsal root with a suction electrode, and we judged monosynaptic connection by constant latency of eEPSCs (Nakatsuka et al., 2000). Conduction velocity was calculated on the basis of the latency of eEPSCs and the length of the dorsal root. In experiments to determine action potential firing on postsynaptic neurons, DH neurons in lamina V were under current-clamp configuration.
VR1 immunostaining. Spinal cord slice sections (250 μm in thickness) were obtained in the same way as those sections for electrophysiology recordings. They were put in a 35 mm Petri dish and fixed with 4% paraformaldehyde (PFA; in PBS buffer solution) for 12 hr at 4°C. The slices were transferred into a solution containing 4% PFA and 0.4% Triton X-100 and incubated at 4°C for 2 hr. They were washed three times with PBS and then mounted onto glass slides and allowed to air dry. Then they were encircled with hydrophobic resin (PAP Pen, The Binding Site). Slices were incubated for 1 hr in a solution of 1:30 normal goat serum in PBS with 0.4% Triton X-100 (GS-PBS-T) to block nonspecific antibody binding. Slices were incubated with a polyclonal guinea pig anti-VR1 receptor antibody (1:2000; Neuromics, Minneapolis, MN) overnight at 4°C. After a rinse with 1% goat serum in PBS solution three times, for 20 min each time, the slices were incubated further for 3 hr at room temperature with a secondary antibody. The secondary antibody (1:100 in 1% goat serum PBS solution) was a goat anti-guinea pig IgG conjugated with AlexaFluor 594 (Molecular Probes, Eugene, OR). Slices were washed three more times with 1% goat serum in PBS solution. After a glycerol-based anti-photobleach medium was applied, the slices were covered with coverslips. Sections were viewed by a fluorescent microscope (OlympusIX-70), and images were captured with a digital camera.
αβ-Methylene-ATP, pyridoxalphosphate-6-azophenyl-2′,4′-disulfonic acid (PPADS), capsaicin, bicuculline, strychnine, and capsazepine were purchased from Sigma (St. Louis, MO). CNQX, d-APV, and TTX were purchased from Tocris Cookson (St. Louis, MO). Unless otherwise indicated, the data represent the mean ± SEM. Paired Student'st tests were used for statistical comparison, and significance was considered at the p < 0.05 level.
RESULTS
Spreading excitatory activity of capsaicin-sensitive inputs to deep laminas
In the spinal cord slice preparation, the central terminals of primary afferent fibers remain in the slice sections and are functionally intact for synaptic transmission, although the dorsal roots are removed (P. Li et al., 1999; Nakatsuka et al., 2000). In this study synaptic activity in the lamina V neurons was examined. When capsaicin (2 μm) was bath applied for 1 min, there was a large increase in the sEPSC frequency in all 18 neurons that were recorded (Fig. 1A). The sEPSC frequency was 382 ± 55% of control (n = 18; p < 0.05) after capsaicin application. The effects of capsaicin lasted for >2 min. Capsaicin-induced increases of sEPSC frequency (495 ± 81% of control, n = 6) were abolished in the presence of the VR1 receptor antagonist 10 μm capsazepine (101 ± 4%,n = 6; Fig. 1B). CNQX (10 μm), a non-NMDA receptor antagonist, completely blocked sEPSCs (n = 6; Fig. 1C). When similar experiments were performed and recordings were made from neurons in lamina III–IV, four neurons of seven recordings also showed increases in sEPSC frequency. The remaining three cells showed few changes after capsaicin application (data not shown). Thus, capsaicin-sensitive inputs can transmit excitatory activity to deep laminas, especially to lamina V.
Fig. 1.
Capsaicin-induced excitatory synaptic activity in lamina V neurons. A, Capsaicin produced a large increase in sEPSC frequency in lamina V neurons. Two sample traces (left) show sEPSCs recorded from a lamina V neuron before (Control) and after the application of 2 μm capsaicin for 1 min (Cap). The time course of the changes in sEPSC frequency is shown in the histogram (middle). The graph on the right shows the pooled results from 18 lamina V neurons. The capsaicin effect on each cell was obtained from a time course histogram of sEPSC frequency and is the averaged response in 30 sec around the peak value.B, VR1 receptor antagonist capsazepine (10 μm) abolished the effects of capsaicin. Three sample traces (left) show sEPSCs before (Control) and after the application of 2 μm capsaicin (Cap) and after the application of 2 μm capsaicin in the presence of 10 μm capsazepine (Cap/Capz). The time course of sEPSC frequency is shown in the histogram (right). Similar results were obtained in five other neurons. Capsazepine was preapplied for 10 min. C, The sEPSCs were blocked completely by 20 μm CNQX (n = 6).D, Capsaicin produced little effect on mEPSC frequency in lamina V neurons. Experiments were performed in the presence of 0.5 μm TTX. Two sets of sample traces show mEPSCs before (Control/TTX) and after the application of 2 μm capsaicin (Cap/TTX). The graph on the right shows the pooled results from 21 cells. In all of the experiments capsaicin was applied for 1 min. The intervals for multiple applications of testing compounds were 20 min in all of the experiments.
TTX has been used widely to block active interneuronal transmission; any polysynaptic transmission between DH neurons should be blocked by 0.5 μm TTX (Gu et al., 1996; Gu and MacDermott, 1997). In sharp contrast to the above capsaicin effects in the absence of TTX, in the presence of 0.5 μm TTX the capsaicin did not produce any increase in the frequency of mEPSCs in 20 of 21 cells that were recorded in lamina V (Fig. 1D). These results strongly suggest that most capsaicin-sensitive inputs to lamina V neurons are transmitted polysynaptically and that few capsaicin-sensitive terminals synapse monosynaptically on lamina V neurons.
To determine whether, in the absence of TTX, capsaicin-induced excitatory synaptic activity in lamina V was via interneurons in the superficial laminas, we removed superficial laminas (Fig.2A, left) and then performed experiments in the same way as those shown in Figure1A. Under this condition only a few neurons (3 of 15 cells; Fig. 2A, right) in lamina V showed increases of sEPSC frequency by 2 μm capsaicin. This result is in sharp contrast to the recordings of sEPSCs from intact slices in the absence of TTX. Figure 2B is a summary comparing the percentage of lamina V neurons responsive to capsaicin applications in the three different experimental conditions that have been described above.
Fig. 2.
Removal of superficial laminas abolishes the capsaicin-induced activity in lamina V neurons. A, The photomicrograph (left) shows a spinal cord slice with one side of the superficial laminas removed. A part of a patch electrode is seen also, and the electrode tip is inside the tissue ∼70 μm from the surface. The location of the electrode tip is in lamina V. The graph on the right shows sEPSC frequency recorded from lamina V neurons in such preparations after a 1 min application of 2 μm capsaicin (n = 15). B, A summary comparing the percentage of lamina V neurons that had increased sEPSCs by capsaicin under three conditions: the intact spinal cord slice in the absence of TTX (Intact/no TTX; n = 18), the intact spinal cord slice in the presence of TTX (Intact/TTX; n = 21), and the spinal cord slice with one side of the superficial laminas removed and in the absence of TTX (No superficial laminae/no TTX;n = 15). Data are from Figures1A,D, 2A.
To determine whether, in the superficial laminas, capsaicin-sensitive central terminals and DH neurons were connected monosynaptically, we determined the effects of capsaicin on mEPSCs in lamina II neurons in the presence of 0.5 μm TTX. After the application of 2 μm capsaicin, 14 of 16 cells showed increases in the mEPSC frequency (Fig. 3A,B); the overall changes of mEPSC frequency were 460 ± 60% of control (n = 16; p < 0.05). This result provided electrophysiological evidence that capsaicin-sensitive central terminals synapse monosynaptically on superficial DH neurons. Consistent with this conclusion, immunostaining with an antibody against VR1 receptors showed that VR1-ir was distributed mainly in the superficial laminas (laminas I and II; Fig. 3C). The results from Figures 1 to 3C support a major capsaicin-sensitive pathway that relays excitatory activity to lamina V neurons (Fig.3D).
Fig. 3.
Monosynaptic connections between capsaicin-sensitive afferent terminals and superficial lamina neurons.A, Capsaicin produced a large increase in mEPSC frequency in lamina II neurons. Two sample traces show mEPSCs before (Control) and after the application of 2 μm capsaicin in the presence of 0.5 μm TTX.B, Pooled results show that 2 μm capsaicin produced increases in mEPSC frequency in most lamina II neurons that were tested (14 of 16 cells). C, Fluorescent microscopic image of VR1 receptor immunoreactivity in a spinal cord section. Strong VR1-ir was observed in superficial laminas (n = 8).D, A summary of the proposed capsaicin-sensitive pathway based on the results from Figures 1-3C.
αβ-Methylene-ATP-sensitive/capsaicin-insensitive inputs to lamina V neurons
A previous study in our laboratory (Nakatsuka and Gu, 2001) has indicated that many afferent central terminals to lamina V neurons express αβm-ATP-sensitive P2X receptors and that these terminals appear to be capsaicin-insensitive. The monosynaptic connection of these terminals to lamina V neurons and their capsaicin sensitivity were confirmed in the present study. As shown in Figure4, 100 μm αβm-ATP increased mEPSC frequency in a lamina V neuron in the presence of 0.5 μm TTX. The effect lasted for >3 min with a 1 min αβm-ATP application (Fig. 4C), suggesting that a nondesensitizing αβm-ATP-sensitive type of P2X receptor mediated the responses. Of 28 lamina V neurons that were recorded, 27 cells showed such a response to 100 μmαβm-ATP. The overall changes of mEPSC frequency were 391 ± 56% of control (n = 28; p < 0.05). The αβm-ATP-induced increases of mEPSC frequency were abolished (n = 6; Fig. 4A) in the presence of the P2X receptor antagonist PPADS (10 μm). CNQX (20 μm) completely blocked mEPSCs in the cells for which 100 μm αβm-ATP increased mEPSC frequency (Fig. 4B; n = 6). Because few capsaicin-sensitive terminals monosynaptically connected with lamina V neurons (Fig. 1D), most αβm-ATP-sensitive terminals that synapse directly on lamina V neurons should be αβm-ATP-sensitive/capsaicin-insensitive terminals. This was confirmed by testing the effects of both capsaicin and αβm-ATP on mEPSC frequency in the same lamina V neurons in the presence of 0.5 μm TTX (Fig. 4C). As demonstrated in Figure 4C, capsaicin did not produce any change in mEPSC frequency in a cell for which αβm-ATP produced a large increase in mEPSC frequency (see also Fig.5A,B). Figure4D schematically illustrates the major monosynaptic αβm-ATP-sensitive/capsaicin-insensitive pathway to lamina V neurons, based on the effects of αβm-ATP and capsaicin on mEPSC frequency recorded from lamina V neurons (see also Nakatsuka and Gu, 2001).
Fig. 4.
Monosynaptic connections between αβm-ATP-sensitive terminals and lamina V neurons. A, Activation of αβm-ATP-sensitive P2X receptors produced increases in mEPSC frequency in lamina V neurons. Three sets of traces show mEPSCs before (Control; left) and after the application of 100 μm αβm-ATP (middle) and after the application of 100 μm αβm-ATP in the presence of 10 μm PPADS (right). The mEPSC frequency became 391 ± 56% of control (n = 28) after the application of 100 μm αβm-ATP. The effects of αβm-ATP were abolished completely in the presence of 10 μm PPADS (n = 6). B, The sEPSCs were blocked completely by 20 μm CNQX (n = 6). C, The histogram shows that 100 μm αβm-ATP produced an increase in mEPSC frequency in a lamina V neuron and that 2 μm capsaicin had no effect in the same neuron. Similar results were observed in 15 other cells. D, The diagram illustrates the presence of an αβm-ATP-sensitive/capsaicin-insensitive pathway to lamina V neurons (see also Nakatsuka and Gu, 2001).
Fig. 5.
Synaptic convergence of excitatory inputs from capsaicin-sensitive and αβm-ATP-sensitive/capsaicin-insensitive pathways. A, The histogram shows the effects of capsaicin and αβm-ATP on the frequency of mEPSCs and sEPSCs in the same neuron. Capsaicin (2 μm) did not induce any change in mEPSC frequency. In the same neuron αβm-ATP (100 μm) induced an increase in mEPSC frequency. After the washout of TTX both capsaicin and αβm-ATP increased the sEPSC frequency. B, Summary of the changes in the frequency and amplitude of mEPSCs in the experiments represented inA (n = 16). Only αβm-ATP induced increases of mEPSC frequency in lamina V neurons. C, Summary of the changes in the frequency and amplitude of sEPSCs in the experiments represented in A (n = 14). sEPSC frequency was increased by capsaicin and by αβm-ATP in the absence of TTX. The data represent the mean ± SEM; *p < 0.05, paired Student's ttests.
Synaptic convergence of excitatory inputs from capsaicin-sensitive and αβm-ATP-sensitive/capsaicin-insensitive pathways
To determine whether excitatory inputs from capsaicin-sensitive terminals and αβm-ATP-sensitive/capsaicin-insensitive terminals converged onto single lamina V neurons, we recorded mEPSCs and sEPSCs from the same neurons after the sequential applications of capsaicin and αβm-ATP. As shown in Figure 5A, αβm-ATP (100 μm), but not capsaicin (2 μm), produced increases of mEPSC frequency in the presence of 0.5 μm TTX. However, in the same neuron in the absence of TTX, not only αβm-ATP but also capsaicin produced large increases of sEPSC frequency (Fig.5A). Figure 5B summarizes the changes in the frequency of mEPSCs after the sequential applications of capsaicin and αβm-ATP in the same neurons in the presence of TTX. Capsaicin (2 μm) did not have any effect on mEPSC frequency (n = 16). However, mEPSC frequency was increased to 447 ± 106% of control (n = 16; p< 0.05; Fig. 5B) by 100 μmαβm-ATP in the same neurons. After the washout of TTX the effects of capsaicin (2 μm) and αβm-ATP (100 μm) were determined in 14 of the above 16 cells. The results are summarized in Figure 5C. In contrast to mEPSCs, after the washout of TTX the sEPSC frequency was increased substantially by capsaicin in all 14 cells that were tested. The overall changes were 465 ± 81% of control (n = 14; p < 0.05; Fig. 5C). Similar to the effects of αβm-ATP on mEPSCs, αβm-ATP also produced increases of sEPSC frequency (408 ± 68% of control, n = 14; p < 0.05). In addition to the changes in sEPSC frequency, there was also a slight but significant increase in the sEPSC amplitude by capsaicin and αβm-ATP (Fig. 5C). These results indicate the convergence of two excitatory inputs from capsaicin-sensitive and αβm-ATP-sensitive/capsaicin-insensitive pathways to lamina V neurons (see Fig. 10).
Fig. 10.
Schematic illustration of a proposed synaptic convergence of capsaicin-sensitive and αβm-ATP-sensitive/capsaicin-insensitive sensory pathways onto lamina V neurons. The diagram shows two excitatory sensory pathways. One is from αβm-ATP-sensitive/capsaicin-insensitive afferent central terminals. These terminals make monosynaptic excitatory synapses onto lamina V neurons (1). The other pathway is from capsaicin-sensitive afferent central terminals. These terminals first synapse onto interneurons in superficial lamina (2). Then the excitatory inputs are relayed to lamina V neurons (3). The site 1synapse is sensitive to αβm-ATP but insensitive to capsaicin. The site 2 synapse is sensitive to capsaicin. Because most capsaicin-sensitive afferent neurons express rapidly desensitizing P2X receptors (C. Li et al.; 1999; Ueno et al., 1999; Nakatsuka and Gu, 2001), capsaicin-sensitive terminals also may be sensitive to αβm-ATP, i.e., capsaicin-sensitive/αβm-ATP-sensitive terminals. The diagram also shows the recruitment of inhibitory pathways mediated by GABAergic/glycinergic interneurons (light gray). Other pathways may be present and are not shown in the diagram.
Young rats were used in the above experiments (ages between 11 and 21 d). We also performed experiments in spinal cord slices obtained from adult rats (ages between 7 and 9 weeks) to determine whether similar results could be obtained in mature animals. In six lamina V cells that were tested in the presence 0.5 μmTTX, 100 μm αβm-ATP increased mEPSC frequency in all six cells (170 ± 13% of control; p < 0.05; data not shown). Of these cells, four cells were tested with 2 μm capsaicin, and none of them showed increases of mEPSC frequency (104 ± 5% of control). However, when TTX was not present, 2 μm capsaicin increased sEPSC frequency (283 ± 102% of control, n = 4;p < 0.05; data not shown). These results are similar to those obtained in young rats.
Recruitment of inhibitory pathways
Both capsaicin-sensitive and αβm-ATP-sensitive/capsaicin-insensitive inputs also recruited inhibitory inputs onto lamina V neurons (Fig.6). The recruitment of inhibitory inputs was determined by measuring sIPSCs when lamina V neurons were held at −10 mV. The inhibitory inputs produced outward postsynaptic currents under this condition (Fig. 6A). The frequency of sIPSCs was increased after the application of 2 μm capsaicin in all 12 cells that were recorded (Fig. 6A,C). In the same neurons the frequency of sIPSCs also was increased after the application of 100 μm αβm-ATP (Fig. 6B,C). The sIPSCs were blocked completely in the presence of 20 μm bicuculline plus 2 μm strychnine (Fig. 6A,B), indicating that the sIPSCs were mediated by GABAergic/glycinergic inputs from DH inhibitory interneurons. Figure 6C summarizes the changes in the frequency and amplitude of sIPSCs after the sequential applications of 2 μm capsaicin and 100 μm αβm-ATP in the same neurons. sIPSC frequency was 388 ± 51% of control (n = 12;p < 0.05) after capsaicin application and 441 ± 83% of control (n = 12; p < 0.05) after αβm-ATP application. sIPSC amplitude also was increased slightly after the applications of αβm-ATP and capsaicin. When experiments were performed in the presence of 0.5 μm TTX to block active interneuronal transmission (Fig. 6D), both mIPSC frequency and amplitude were not changed after the application of 2 μm capsaicin (n = 8) or 100 μm αβm-ATP (n = 8). These results indicate that both capsaicin-sensitive and αβm-ATP-sensitive/capsaicin-insensitive pathways recruit inhibitory synaptic circuitry and that the recruited inhibitory inputs can converge onto the same lamina V neurons.
Fig. 6.
Recruitment of inhibitory circuitry after the activation of central terminal VR1 receptors or P2X receptors.A, Capsaicin induced an increase in sIPSC frequency. Three sets of sample traces show sIPSCs recorded from a lamina V neuron in control (left), after the application 2 μm capsaicin alone (middle), and after the application of 2 μm capsaicin in the presence of both 20 μm bicuculline and 2 μm strychnine (right). B, The αβm-ATP induced an increase of sIPSC frequency. Experiments were similar to those inA except that 100 μm αβm-ATP was tested. The recordings in A and B were performed on the same neuron. C, Summary of the increases in sIPSC frequency induced by 2 μm capsaicin or 100 μm αβm-ATP in the same neurons (filled bars; n = 12). The changes of sIPSC amplitude are shown also (open bars;n = 12). The experiments were performed in the absence of TTX. D, Capsaicin and αβm-ATP had no effect on mIPSCs. Of the 12 cells tested inC, eight cells also were tested in the presence of 0.5 μm TTX. The frequency (filled bars) and amplitude (open bars) of mEPSCs were not affected by 2 μm capsaicin or 100 μm αβm-ATP (n = 8). In all recordings the cells were held at −10 mV. The data represent the mean ± SEM; *p < 0.05; paired Student's t tests.
Synaptic convergence of excitatory and inhibitory inputs to the same lamina V neurons after the activation of capsaicin-sensitive and αβm-ATP-sensitive/capsaicin-insensitive pathways
To determine whether both excitatory and inhibitory inputs converge on the same lamina V neurons, we recorded sEPSCs and sIPSCs simultaneously, and we examined the effects of capsaicin as well as αβm-ATP in the same lamina V neurons. The simultaneous recordings of sEPSCs and sIPSCs were performed with cells held at −45 mV. At this holding potential the glutamatergic sEPSCs were inward currents and GABAergic/glycinergic sIPSCs were outward currents (Fig.7A). As shown by the sample traces in Figure 7A, the increases in the frequency of both sEPSCs and sIPSCs were observed clearly under this simultaneous recording condition after the applications of αβm-ATP (100 μm) and capsaicin (2 μm). The sEPSC frequency increased to 347 ± 97% of control (p < 0.05; n= 6), and the sIPSC frequency increased to 447 ± 186% of control (p < 0.05; n = 6) after the application of 2 μm capsaicin (Fig.7B). In the same neurons αβm-ATP (100 μm) increased the sEPSC frequency to 363 ± 68% of control (p < 0.05; n= 6) and increased the sIPSC frequency to 372 ± 107% of control (p < 0.05; n = 6; Fig.7B). Based on the results from Figures 1 to 7, a schematic diagram in Figure 10 summarizes the major pathways involved in the excitatory and inhibitory convergence of capsaicin-sensitive and αβm-ATP-sensitive/capsaicin-insensitive pathways.
Fig. 7.
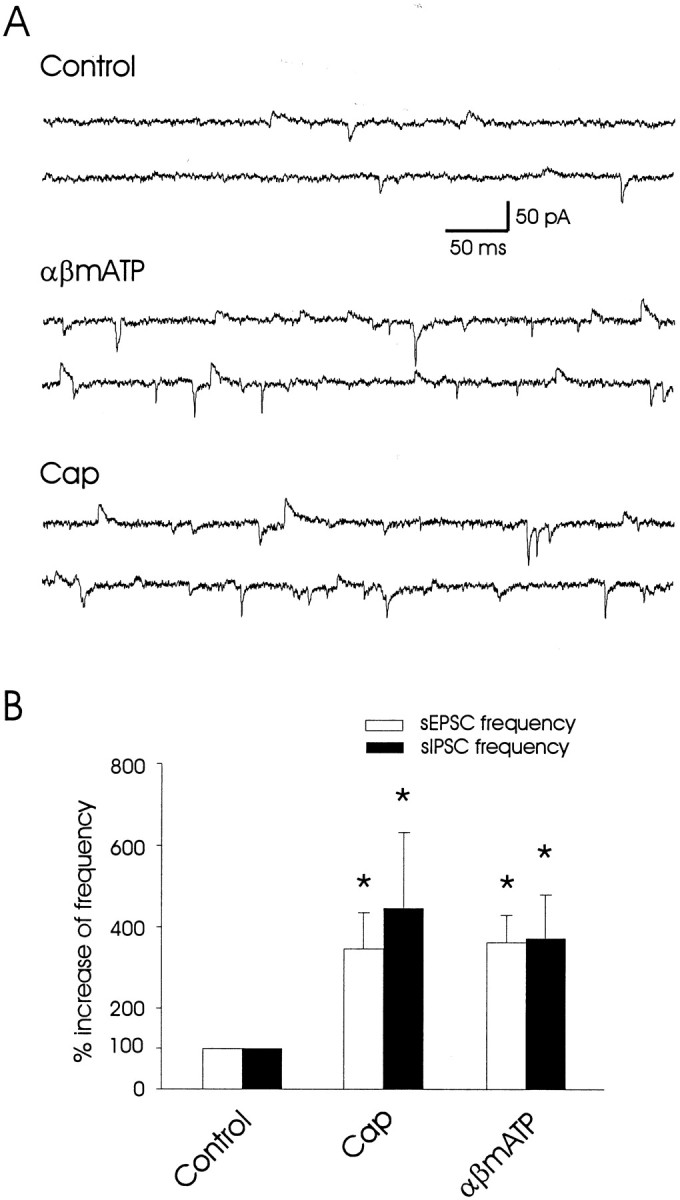
Synaptic convergence of both excitatory and inhibitory inputs on the same lamina V neurons. A, Sample traces show simultaneous recordings of both sEPSCs and sIPSCs in a lamina V neuron in normal bath solution (Control), after the application of 100 μm αβm-ATP, and after the application of 2 μm capsaicin in a lamina V neuron. The cell was voltage clamped at −45 mV. At this holding potential both the sEPSCs (inward currents) and sIPSCs (outward currents) could be detected simultaneously. B, A summary shows the increases of both sEPSC frequency and sIPSC frequency in experiments (n = 6) as represented in A. The data represent the mean ± SEM; *p < 0.05, paired Student's t test.
Temporal summation in lamina V neurons
Because capsaicin-sensitive and αβm-ATP-sensitive/capsaicin-insensitive pathways displayed convergent excitatory and inhibitory activities onto lamina V neurons, we determined whether the presence of two excitatory inputs could overcome the inhibitory influence, resulting in hyperactivity in lamina V neurons. Under current-clamp configuration the occurrence of action potential spikes was measured after the applications of 2 μm capsaicin alone, 100 μm αβm-ATP alone, or both of them together. Some action potential spikes were observed for brief periods when capsaicin or αβm-ATP was applied separately (Fig. 8A,B). However, when capsaicin and αβm-ATP were coapplied, a larger increase in spike frequency occurred, lasting up to 10 min after a 2 min application of capsaicin plus αβm-ATP (n = 4; Fig. 8A,B). These results suggest that the convergent excitatory inputs from capsaicin-sensitive and αβm-ATP-sensitive/capsaicin-insensitive pathways may produce a nonlinear summation and long-lasting enhancement of lamina V activity.
Fig. 8.

Temporal summation and action potential spikes after simultaneous activation of both capsaicin-sensitive and αβm-ATP-sensitive/capsaicin-insensitive pathways. A, Three traces show the action potential spikes after the application of 2 μm capsaicin alone (top), 100 μm αβm-ATP alone (middle), and both of them together (bottom). B, Time course of action potential spikes after αβm-ATP alone, capsaicin alone, and both of them together (n = 4). Resting membrane potentials were −68 ± 7 mV. Action potential spikes are truncated to show some sEPSPs. Each data point inB represents the number of action potentials in a period of 30 sec.
Dorsal root stimulation-evoked synaptic convergence of sensory inputs from capsaicin-sensitive and αβm-ATP-sensitive/capsaicin-insensitive afferent fibers
Dorsal root stimulation evoked monosynaptic A-fiber eEPSCs with or without polysynaptic eEPSCs in lamina V neurons. The occurrence of polysynaptic eEPSCs often depended on stimulation intensity. As shown in Figure 9, at Aδ-fiber stimulation intensity (100 μA, 0.1 msec) a lamina V neuron showed only monosynaptic Aδ-like eEPSCs (Fig. 9A). When stimulation intensity increased to C-fiber intensity (500 μA, 0.1 msec) (Nakatsuka et al., 2000), dorsal root stimulation resulted in monosynaptic eEPSCs followed by polysynaptic eEPSCs in the same lamina V neuron (Fig. 9B). Similar results were obtained in 16 other cells. At C-fiber stimulation the conduction velocity for the initial monosynaptic eEPSCs was 2.8 ± 0.2 m/sec (2.0–5.1 m/sec;n = 17), within the Aδ-fiber conduction range. The subsequent polysynaptic eEPSCs were separated either completely (Fig.9B) or partially (Fig. 9C) from the monosynaptic Aδ-like eEPSCs, depending on the length of the dorsal roots.
Fig. 9.
Evoked monosynaptic and polysynaptic excitatory inputs to lamina V neurons and their sensitivity to capsaicin.A, Four sample traces, aligned at the time of each stimulus, represent eEPSCs (indicated by I) obtained in a lamina V neuron after dorsal root stimulation (4 stimuli) at Aδ-fiber stimulation intensity (∼100 μA, 0.1 msec). Note that there is no change in the latency of eEPSCs, indicating monosynaptic transmission. B, eEPSCs were elicited from the same neuron in A after dorsal root stimulation (4 stimuli) at C-fiber stimulus intensity (∼500 μA, 0.1 msec). Two groups of eEPSCs were observed. In the first group (indicated byI), eEPSCs occurred with the same latency as those shown in A, and the afferent conduction velocity was 5.3 m/sec. In the second group (indicated byII), eEPSCs had a longer latency.Arrows point to the changes in the latency of the group II eEPSCs, indicating polysynaptic transmission. The apparent conduction velocity for these polysynaptic inputs was ≤0.6 m/sec. Conduction velocity is calculated on the basis of eEPSC latency and the length of the attached dorsal root. In the spinal cord slice used inA and B, the attached root was extra long (15 mm). C, D, An example shows monosynaptic Aδ-like eEPSCs (I) and polysynaptic eEPSCs (II) recorded from a lamina V neuron in normal bath solution (C) and after the bath application of 2 μm capsaicin for 10 min (D). The afferent conduction velocity was 2.1 m/sec for the monosynaptic inputs that generated monosynaptic Aδ-like eEPSCs. The apparent conduction velocity was ≤0.46 m/sec for the polysynaptic inputs. The length of the dorsal root in this experiment was 4 mm.E, A summary of the results (n = 4) from experiments represented in C and D. The amplitude of polysynaptic eEPSCs was determined after subtracting the extrapolated decay component of the previous monosynaptic eEPSCs.
The monosynaptic Aδ-like inputs and the polysynaptic inputs to the same lamina V neurons were examined further for their sensitivity to capsaicin. Dorsal root stimulation was applied at C-fiber stimulus intensity to elicit monosynaptic Aδ-like eEPSCs and polysynaptic eEPSCs first (Fig. 9C), and then the effects of 2 μm capsaicin on these eEPSCs were determined (Fig. 9D). In four lamina V neurons that were tested, the monosynaptic Aδ-like eEPSCs were not affected significantly by capsaicin (224 ± 35 pA in control vs 229 ± 32 pA in the presence of capsaicin; Fig. 9C–E). However, the polysynaptic eEPSCs were sensitive to capsaicin in all four cells that were tested (Fig. 9C–E); the average amplitude of polysynaptic eEPSCs was decreased significantly from 156 ± 45 pA in the control condition to 24 ± 10 pA in the presence of 2 μm capsaicin. Together with our recent study that showed αβm-ATP sensitivity of monosynaptic Aδ-like eEPSCs in almost all lamina V neurons that were recorded (Nakatsuka and Gu, 2001), these results suggest that sensory impulses elicited at high stimulation intensity may result in the convergence of synaptic inputs from capsaicin-sensitive and αβm-ATP-sensitive/capsaicin-insensitive afferent fibers to lamina V neurons.
DISCUSSION
We have studied putative synaptic circuits involved in the transmission of capsaicin-sensitive and αβm-ATP-sensitive/capsaicin-insensitive inputs in the spinal cord by activation of central terminal VR1 receptors and αβm-ATP-sensitive P2X receptors. Capsaicin-sensitive inputs initially are transmitted from primary afferent terminals to the superficial laminas. The excitatory activity of capsaicin-sensitive inputs is transformed further to both excitatory and inhibitory signals in the dorsal horn and is spread into lamina V neurons. Furthermore, polysynaptic capsaicin-sensitive inputs converge with monosynaptic αβm-ATP-sensitive/capsaicin-insensitive inputs onto lamina V neurons (Fig. 10). This convergence produces temporal summation in a single lamina V neuron.
Spreading excitatory activity of capsaicin-sensitive inputs from the superficial laminas to lamina V neurons
VR1-ir has been observed mainly in the superficial laminas (see also Tominaga et al., 1998; Guo et al., 1999). In the present study the mEPSC frequency was increased when recordings were made from lamina II neurons. Because mEPSCs were measured in the presence of TTX, the active interneuronal transmission among DH neurons was prevented (Gu et al., 1996). Therefore, monosynaptic connections between capsaicin-sensitive fibers and lamina II neurons were revealed electrophysiologically. In contrast to the recordings in lamina II, few lamina V neurons showed increased mEPSC frequency by capsaicin. This indicates that few capsaicin-sensitive afferent fibers have monosynaptic connections with lamina V neurons. However, when sEPSCs were examined in the absence of TTX, capsaicin produced an increase in sEPSC frequency. This result suggests that capsaicin-sensitive inputs could be transmitted to lamina V polysynaptically. The spread of capsaicin-sensitive activity to deep laminas was mediated by interneurons in the superficial laminas. This is supported further by the results that capsaicin-induced increases of sEPSCs in lamina V neurons were abolished after the removal of the superficial laminas. These results indicate that there are extensive neuronal interactions between functionally distinct spinal cord laminar regions. It has been assumed that neurons in the superficial laminas interact with cells in the deep DH. Consistent with this assumption, anatomical evidence showed synaptic-like contacts between superficial and deep DH neurons (Ritz and Greenspan, 1985; Light and Kavookjian, 1988). However, this assumption has been questioned because of the lack of strong electrophysiological evidence (Willis and Coggeshall, 1991). Our results indicate that superficial and deep laminas, the two functionally distinct sensory regions in the DH, have extensive signal transmission when VR1 receptors on primary afferent fibers are activated.
The VR1 receptor has been shown to be a sensor for nociceptive heat stimuli (Caterina et al., 1997, 2000; Davis et al., 2000). The widespread neuronal activity after the activation of VR1 receptors suggests that capsaicin-sensitive inputs have profound effects on spinal cord neuronal activity not only in superficial laminas but also in deep laminas. The widespread neuronal activity after the activation of VR1 receptors may be a mechanism for the development of secondary hyperalgesia after intradermal capsaicin injections or burning injury (Simone et al., 1989b; LaMotte et al., 1991; Pedersen et al., 1996) and for VR-1 receptor-mediated thermal allodynia after tissue inflammation (Caterina et al., 2000; Davis et al., 2000).
αβ-Methylene-ATP-sensitive/capsaicin-insensitive inputs to lamina V neurons
The increase of mEPSC frequency by αβm-ATP in lamina V neurons indicates the presence of αβm-ATP-sensitive P2X receptors on the presynaptic terminals to lamina V neurons. We have demonstrated previously that αβm-ATP-sensitive terminals on lamina V neurons were derived mainly from Aδ fibers and that activation of P2X receptors on those terminals not only increased spontaneous glutamate release from those central terminals but also potentiated the evoked EPSCs (Nakatsuka and Gu, 2001). The present work, together with our previous results (Nakatsuka and Gu, 2001), also indicates that αβm-ATP-sensitive terminals to lamina V neurons are capsaicin-insensitive. The presence of ATP-sensitive/capsaicin-insensitive sensory neurons has been shown previously in acutely dissociated DRG neurons (C. Li et al., 1999; Ueno et al., 1999; Petruska et al., 2000; Tsuda et al., 2000). It remains to be shown whether αβm-ATP-sensitive/capsaicin-insensitive afferent fibers represent all or part of ATP-sensitive/capsaicin-insensitive afferent fibers. Functionally, it has been shown that P2X receptors on αβm-ATP-sensitive/capsaicin-insensitive neurons play a role in mechanical allodynia (Tsuda et al., 2000).
Synaptic convergence and summation of capsaicin-sensitive input and αβm-ATP-sensitive/capsaicin-insensitive input onto lamina V neurons
We have found that the two chemically distinct sensory inputs, capsaicin-sensitive and αβm-ATP-sensitive/capsaicin-insensitive inputs, synaptically converge onto lamina V neurons. Lamina V neurons of the spinal cord DH are known to receive a variety of sensory inputs, including nociceptive and non-nociceptive inputs (Willis and Coggeshall, 1991; Woolf, 1994). Sensory convergence has been observed in many lamina V neurons after electrical or physical stimuli (Mendell, 1966; Wagman and Price, 1969; McMahon and Morrison, 1982; Takahashi and Yokota, 1983; Alarcon and Cervero, 1990). Our study shows that two chemically defined sensory inputs synaptically converge onto lamina V neurons. The demonstration of the synaptic convergence of capsaicin-sensitive input and the αβm-ATP-sensitive/capsaicin-insensitive input should help us to understand further the functional roles of VR1 receptors and a subtype of P2X receptors in sensory transmission.
The synaptic convergence shown here includes both excitatory and inhibitory pathways. The recruitment of inhibitory inputs during the activation of capsaicin-sensitive and αβm-ATP-sensitive/capsaicin-insensitive pathways may provide a negative feedback mechanism. The recruitment of inhibitory pathways after the application of αβm-ATP was less likely because of the direct activation of P2X receptors on DH interneurons. Previously, two studies showed that ATP increased the release of GABA and glycine from a subpopulation of DH neurons (Hugel and Schlichter, 2000; Rhee et al., 2000). However, in the same studies the effects were not produced by αβm-ATP, indicating the absence of αβm-ATP-sensitive P2X receptors on inhibitory DH interneurons in postnatal rats. Consistently, we did not find significant changes of mIPSC frequency by αβm-ATP. Thus, the increase of sIPSC frequency by αβm-ATP is attributable to the recruitment of inhibitory DH interneurons (Fig.7).
Convergent excitatory inputs may overcome inhibitory controls and produce a nonlinear temporal summation, resulting in long-lasting increases of action potential firing in lamina V neurons (Fig. 8). Another possible contribution to the increased activity in lamina V during the coapplication of αβm-ATP and capsaicin (Fig.8A,B) is the synergistic effect of αβm-ATP and capsaicin on capsaicin-sensitive terminals. However, capsaicin-sensitive DRG neurons usually only express the rapidly desensitizing P2X receptors (Guo et al., 1999; C. Li et al., 1999; Petruska et al., 2000; Nakatsuka and Gu, 2001), and these receptors only have transient synaptic effects when activated (Labrakakis et al., 2000). Therefore, it is less likely that αβm-ATP action on capsaicin-sensitive terminals plays a significant role in the long-lasting temporal summation. However, long-lasting synaptic effects of αβm-ATP have been observed for the αβm-ATP-sensitive/capsaicin-insensitive terminals to lamina V neurons (Fig. 4C; see also Nakatsuka and Gu, 2001). Thus the long-lasting increases of action potential firing in lamina V neurons after the coapplication of αβm-ATP and capsaicin (Fig. 8) should represent mainly the temporal summation of excitatory inputs from capsaicin-sensitive and αβm-ATP-sensitive/capsaicin-insensitive inputs.
Dorsal root stimulation-evoked synaptic convergence of sensory inputs from capsaicin-sensitive and αβm-ATP-sensitive/capsaicin-insensitive afferent fibers
We have shown that monosynaptic Aδ-like eEPSCs and polysynaptic eEPSCs converge on the same lamina V neurons after dorsal root stimulation at C-fiber intensity. By testing their capsaicin sensitivity, we have shown that the monosynaptic Aδ-like inputs are insensitive to capsaicin, but most polysynaptic afferent inputs are sensitive to capsaicin (Fig. 9). These results extend our findings on the basis of the recordings of sEPSCs and mEPSCs. Together with our previous findings that the evoked monosynaptic Aδ-like eEPSCs were sensitive to αβm-ATP (Nakatsuka and Gu, 2001), our results suggest that sensory impulses carried by capsaicin-sensitive fibers and αβm-ATP-sensitive/capsaicin-insensitive fibers can converge on the same lamina V neurons.
Capsaicin-sensitive polysynaptic inputs were evident by the suppression of polysynaptic eEPSCs in the presence of capsaicin. Consistently, monosynaptic capsaicin-sensitive eEPSCs in lamina II were blocked by capsaicin (Yang et al., 1999), potentially because of conduction block (Urban and Dray, 1993; Yang et al., 1999). We found that polysynaptic eEPSCs were not abolished completely by capsaicin. This may suggest that some capsaicin-insensitive inputs also converge polysynaptically on lamina V neurons. The lack of capsaicin sensitivity for monosynaptic Aδ-like eEPSCs was observed in all four cells that were tested in lamina V, suggesting that Aδ-afferent fibers to these neurons were capsaicin-insensitive. However, our results do not exclude the presence of capsaicin-sensitive Aδ-fibers (Urban and Dray, 1993; Ringkamp et al., 2001). Capsaicin-sensitive fibers appear to be rare in lamina V, based on VR1-ir and our mEPSC experiments. Thus, capsaicin-sensitive Aδ-fibers, if present in a large number, may be located mainly in the superficial laminas.
Implications
Simultaneous activation of different sensory pathways by endogenous ligands for VR1 receptors and P2X receptors may be common during tissue injury, inflammation, and other pathological conditions because chemical mediators such as protons, ATP, and ADP may be released under these conditions. Thus the putative excitatory summation of capsaicin-sensitive and αβm-ATP-sensitive/capsaicin-insensitive inputs may occur, which may lead to the development of hyperactivity in deep DH neurons. However, convergent inhibitory inputs may play an important role in preventing such hyperactivity. The balance among these excitatory and inhibitory circuits may have important physiological function. A shift of the balance may represent state-dependent sensory processing.
Footnotes
This work was supported by National Institutes of Health Grant NS38254 (J.G.G), by Office of Naval Research Grant N00014-1-0188 (J.G.G), and by a Human Frontier Science Program grant (M.Y). We thank A. MacDermott, S. Siegelbaum, D. Price, B. Cooper, and R. Yezierski for providing thoughtful comments on this manuscript. We appreciate J. X. Ling for general assistance during this work.
Correspondence should be addressed to Jianguo G. Gu, McKnight Brain Institute of the University of Florida, University of Florida, Box 100416, Gainesville, FL 32610. E-mail: jgu@dental.ufl.edu.
REFERENCES
- 1.Alarcon G, Cervero F. The effects of electrical stimulation of A and C visceral afferent fibres on the excitability of viscerosomatic neurons in the thoracic spinal cord of the cat. Brain Res. 1990;509:24–30. doi: 10.1016/0006-8993(90)90304-t. [DOI] [PubMed] [Google Scholar]
- 2.Burnstock G, Wood JN. Purinergic receptors: their role in nociception and primary afferent neurotransmission. Curr Opin Neurobiol. 1996;6:526–532. doi: 10.1016/s0959-4388(96)80060-2. [DOI] [PubMed] [Google Scholar]
- 3.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 4.Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, Julius D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- 5.Cockayne DA, Hamilton SG, Zhu QM, Dunn PM, Zhong Y, Novakovic S, Malmberg AB, Cain G, Berson A, Kassotakis L, Hedley L, Lachnit WG, Burnstock G, McMahon SB, Ford AP. Urinary bladder hyporeflexia and reduced pain-related behaviour in P2X3-deficient mice. Nature. 2000;407:1011–1015. doi: 10.1038/35039519. [DOI] [PubMed] [Google Scholar]
- 6.Collo G, North RA, Kawashima E, Merlo-Pich E, Neidhart S, Surprenant A, Buell G. Cloning of P2X5 and P2X6 receptors and the distribution and properties of an extended family of ATP-gated ion channels. J Neurosci. 1996;16:2495–2507. doi: 10.1523/JNEUROSCI.16-08-02495.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cook SP, Vulchanova L, Hargreaves KM, Elde R, McCleskey EW. Distinct ATP receptors on pain-sensing and stretch-sensing neurons. Nature. 1997;387:505–508. doi: 10.1038/387505a0. [DOI] [PubMed] [Google Scholar]
- 8.Davis JB, Gray J, Gunthorpe MJ, Hatcher JP, Davey PT, Overend P, Harries MH, Latcham J, Clapham C, Atkinson K, Hughes SA, Rance K, Grau E, Harper AJ, Pugh PL, Rogers DC, Bingham S, Randall A, Sheardown SA. Vanilloid receptor-1 is essential for inflammatory thermal hyperalgesia. Nature. 2000;405:183–187. doi: 10.1038/35012076. [DOI] [PubMed] [Google Scholar]
- 9.Dowd E, McQueen DS, Chessell IP, Humphrey PP. P2X receptor-mediated excitation of nociceptive afferents in the normal and arthritic rat knee joint. Br J Pharmacol. 1998;125:341–346. doi: 10.1038/sj.bjp.0702080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gu JG, MacDermott AB. Activation of ATP P2X receptors elicits glutamate release from sensory neuron synapses. Nature. 1997;389:749–753. doi: 10.1038/39639. [DOI] [PubMed] [Google Scholar]
- 11.Gu JG, Albuquerque C, Lee CJ, MacDermott AB. Synaptic strengthening through activation of Ca2+-permeable AMPA receptors. Nature. 1996;381:793–796. doi: 10.1038/381793a0. [DOI] [PubMed] [Google Scholar]
- 12.Guo A, Vulchanova L, Wang J, Li X, Elde R. Immunocytochemical localization of the vanilloid receptor 1 (VR1): relationship to neuropeptides, the P2X3 purinoceptor, and IB4 binding sites. Eur J Neurosci. 1999;11:946–958. doi: 10.1046/j.1460-9568.1999.00503.x. [DOI] [PubMed] [Google Scholar]
- 13.Holzer P. Capsaicin: cellular targets, mechanisms of action, and selectivity for thin sensory neurons. Pharmacol Rev. 1991;43:143–201. [PubMed] [Google Scholar]
- 14.Hugel S, Schlichter R. Presynaptic P2X receptors facilitate inhibitory GABAergic transmission between cultured rat spinal cord dorsal horn neurons. J Neurosci. 2000;20:2121–2130. doi: 10.1523/JNEUROSCI.20-06-02121.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hwang SW, Cho H, Kwak J, Lee SY, Kang CJ, Jung J, Cho S, Min KH, Suh YG, Kim D, Oh U. Direct activation of capsaicin receptors by products of lipoxygenases: endogenous capsaicin-like substances. Proc Natl Acad Sci USA. 2000;97:6155–6160. doi: 10.1073/pnas.97.11.6155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jahr CE, Jessell TM. ATP excites a subpopulation of rat dorsal horn neurons. Nature. 1983;304:730–733. doi: 10.1038/304730a0. [DOI] [PubMed] [Google Scholar]
- 17.Jancso G, Kiraly E, Jancso-Gabor A. Pharmacologically induced selective degeneration of chemosensitive primary sensory neurons. Nature. 1977;270:741–743. doi: 10.1038/270741a0. [DOI] [PubMed] [Google Scholar]
- 18.Khakh BS, Burnstock G, Kennedy C, King BF, North RA, Seguela P, Voigt M, Humphrey PP. International Union of Pharmacology. XXIV. Current status of the nomenclature and properties of P2X receptors and their subunits. Pharmacol Rev. 2001;53:107–118. [PubMed] [Google Scholar]
- 19.Kress M, Zeilhofer HU. Capsaicin, protons, and heat: new excitement about nociceptors. Trends Pharmacol Sci. 1999;20:112–118. doi: 10.1016/s0165-6147(99)01294-8. [DOI] [PubMed] [Google Scholar]
- 20.Krishtal OA, Marchenko SM, Pidoplichko VI. Receptor for ATP in the membrane of mammalian sensory neurons. Neurosci Lett. 1983;35:41–45. doi: 10.1016/0304-3940(83)90524-4. [DOI] [PubMed] [Google Scholar]
- 21.Labrakakis C, Gerstner E, MacDermott AB. Adenosine triphosphate-evoked currents in cultured dorsal root ganglion neurons obtained from rat embryos: desensitization kinetics and modulation of glutamate release. Neuroscience. 2000;101:1117–1126. doi: 10.1016/s0306-4522(00)00373-0. [DOI] [PubMed] [Google Scholar]
- 22.LaMotte RH, Shain CN, Simone DA, Tsai E-F. Neurogenic hyperalgesia: psychophysical studies of underlying mechanisms. J Neurophysiol. 1991;66:190–211. doi: 10.1152/jn.1991.66.1.190. [DOI] [PubMed] [Google Scholar]
- 23.Li C, Peoples RW, Lanthorn TH, Li ZW, Weight FF. Distinct ATP-activated currents in different types of neurons dissociated from rat dorsal root ganglion. Neurosci Lett. 1999;263:57–60. doi: 10.1016/s0304-3940(99)00114-7. [DOI] [PubMed] [Google Scholar]
- 24.Li P, Calejesan AA, Zhuo M. ATP P2X receptors and sensory synaptic transmission between primary afferent fibers and spinal dorsal horn neurons in rats. J Neurophysiol. 1998;80:3356–3360. doi: 10.1152/jn.1998.80.6.3356. [DOI] [PubMed] [Google Scholar]
- 25.Li P, Wilding TJ, Kim SJ, Calejesan AA, Huettner JE, Zhuo M. Kainate receptor-mediated sensory synaptic transmission in mammalian spinal cord. Nature. 1999;397:161–164. doi: 10.1038/16469. [DOI] [PubMed] [Google Scholar]
- 26.Light AR, Kavookjian AM. Morphology and ultrastructure of physiologically identified substantia gelatinosa (lamina II) neurons with axons that terminate in deeper dorsal horn laminae (III–V). J Comp Neurol. 1988;267:172–189. doi: 10.1002/cne.902670203. [DOI] [PubMed] [Google Scholar]
- 27.McMahon SB, Morrison JF. Two group of spinal interneurones that respond to stimulation of the abdominal viscera of the cat. J Physiol (Lond) 1982;322:21–34. doi: 10.1113/jphysiol.1982.sp014019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mendell LM. Physiological properties of unmyelinated fiber projection to the spinal cord. Exp Neurol. 1966;16:316–332. doi: 10.1016/0014-4886(66)90068-9. [DOI] [PubMed] [Google Scholar]
- 29.Nakatsuka T, Gu JG. ATP P2X receptor-mediated enhancement of glutamate release and evoked excitatory postsynaptic currents in dorsal horn neurons of the rat spinal cord. J Neurosci. 2001;21:6522–6531. doi: 10.1523/JNEUROSCI.21-17-06522.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nakatsuka T, Ataka T, Kumamoto E, Tamaki T, Yoshimura M. Alteration in synaptic inputs through C-afferent fibers to substantia gelatinosa neurons of the rat spinal dorsal horn during postnatal development. Neuroscience. 2000;99:549–556. doi: 10.1016/s0306-4522(00)00224-4. [DOI] [PubMed] [Google Scholar]
- 31.North RA, Surprenant A. Pharmacology of cloned P2X receptors. Annu Rev Pharmacol Toxicol. 2000;40:563–580. doi: 10.1146/annurev.pharmtox.40.1.563. [DOI] [PubMed] [Google Scholar]
- 32.Novakovic SD, Kassotakis LC, Oglesby IB, Smith JA, Eglen RM, Ford AP, Hunter JC. Immunocytochemical localization of P2X3 purinoceptors in sensory neurons in naive rats and following neuropathic injury. Pain. 1999;80:273–282. doi: 10.1016/s0304-3959(98)00225-5. [DOI] [PubMed] [Google Scholar]
- 33.Pedersen JL, Crawford ME, Dahl JB, Brennum J, Kehlet H. Effect of preemptive nerve block on inflammation and hyperalgesia after human thermal injury. Anesthesiology. 1996;84:1020–1026. doi: 10.1097/00000542-199605000-00002. [DOI] [PubMed] [Google Scholar]
- 34.Petruska JC, Cooper BY, Johnson RD, Gu JG. Distribution patterns of different P2X receptor phenotypes in acutely dissociated dorsal root ganglion neurons of adult rats. Exp Brain Res. 2000;134:126–132. doi: 10.1007/s002210000414. [DOI] [PubMed] [Google Scholar]
- 35.Rhee JS, Wang ZM, Nabekura J, Inoue K, Akaike N. ATP facilitates spontaneous glycinergic IPSC frequency at dissociated rat dorsal horn interneuron synapses. J Physiol (Lond) 2000;524:471–483. doi: 10.1111/j.1469-7793.2000.t01-1-00471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ringkamp M, Peng YB, Wu G, Hartke TV, Campbell JN, Meyer RA. Capsaicin responses in heat-sensitive and heat-insensitive A-fiber nociceptors. J Neurosci. 2001;21:4460–4468. doi: 10.1523/JNEUROSCI.21-12-04460.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ritz LA, Greenspan JD. Morphological features of lamina V neurons receiving nociceptive input in cat sacrocaudal spinal cord. J Comp Neurol. 1985;238:440–452. doi: 10.1002/cne.902380408. [DOI] [PubMed] [Google Scholar]
- 38.Sawynok J, Reid A. Peripheral adenosine 5′-triphosphate enhances nociception in the formalin test via activation of a purinergic P2X receptor. Eur J Pharmacol. 1997;330:115–121. doi: 10.1016/s0014-2999(97)01001-7. [DOI] [PubMed] [Google Scholar]
- 39.Simone DA, Baumann TK, Collins JG, LaMotte RH. Sensitization of cat dorsal horn neurons to innocuous mechanical stimulation after intradermal injection of capsaicin. Brain Res. 1989a;486:185–189. doi: 10.1016/0006-8993(89)91293-6. [DOI] [PubMed] [Google Scholar]
- 40.Simone DA, Baumann TK, LaMotte RH. Dose-dependent pain and mechanical hyperalgesia in humans after intradermal injection of capsaicin. Pain. 1989b;38:99–107. doi: 10.1016/0304-3959(89)90079-1. [DOI] [PubMed] [Google Scholar]
- 41.Smart D, Gunthorpe MJ, Jerman JC, Nasir S, Gray J, Muir AI, Chambers JK, Randall AD, Davis JB. The endogenous lipid anandamide is a full agonist at the human vanilloid receptor (hVR1). Br J Pharmacol. 2000;129:227–230. doi: 10.1038/sj.bjp.0703050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Souslova V, Cesare P, Ding Y, Akopian AN, Stanfa L, Suzuki R, Carpenter K, Dickenson A, Boyce S, Hill R, Nebenuis-Oosthuizen D, Smith AJ, Kidd EJ, Wood JN. Warm-coding deficits and aberrant inflammatory pain in mice lacking P2X3 receptors. Nature. 2000;407:1015–1017. doi: 10.1038/35039526. [DOI] [PubMed] [Google Scholar]
- 43.Szolcsanyi J. A pharmacological approach to elucidation of the role of different nerve fibres and receptor endings in mediation of pain. J Physiol (Paris) 1977;73:251–259. [PubMed] [Google Scholar]
- 44.Takahashi M, Yokota T. Convergence of cardiac and cutaneous afferents onto neurons in the dorsal horn of the spinal cord in the cat. Neurosci Lett. 1983;38:251–256. doi: 10.1016/0304-3940(83)90377-4. [DOI] [PubMed] [Google Scholar]
- 45.Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, Raumann BE, Basbaum AI, Julius D. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 1998;21:531–543. doi: 10.1016/s0896-6273(00)80564-4. [DOI] [PubMed] [Google Scholar]
- 46.Tsuda M, Koizumi S, Kita A, Shigemoto Y, Ueno S, Inoue K. Mechanical allodynia caused by intraplantar injection of P2X receptor agonist in rats: involvement of heteromeric P2X2/3 receptor signaling in capsaicin-insensitive primary afferent neurons. J Neurosci. 2000;20:1–5. doi: 10.1523/JNEUROSCI.20-15-j0007.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ueno S, Tsuda M, Iwanaga T, Inoue K. Cell type-specific ATP-activated responses in rat dorsal root ganglion neurons. Br J Pharmacol. 1999;126:429–436. doi: 10.1038/sj.bjp.0702319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Urban L, Dray A. Actions of capsaicin on mouse dorsal root ganglion cells in vitro. Neurosci Lett. 1993;157:187–190. doi: 10.1016/0304-3940(93)90733-2. [DOI] [PubMed] [Google Scholar]
- 49.Vulchanova L, Arvidsson U, Riedl M, Wang J, Buell G, Surprenant A, North RA, Elde R. Differential distribution of two ATP-gated channels (P2X receptors) determined by immunocytochemistry. Proc Natl Acad Sci USA. 1996;93:8063–8067. doi: 10.1073/pnas.93.15.8063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vulchanova L, Riedl MS, Shuster SJ, Buell G, Surprenant A, North RA, Elde R. Immunohistochemical study of the P2X2 and P2X3 receptor subunits in rat and monkey sensory neurons and their central terminals. Neuropharmacology. 1997;36:1229–1242. doi: 10.1016/s0028-3908(97)00126-3. [DOI] [PubMed] [Google Scholar]
- 51.Vulchanova L, Riedl MS, Shuster SJ, Stone LS, Hargreaves KM, Buell G, Surprenant A, North RA, Elde R. P2X3 is expressed by DRG neurons that terminate in inner lamina II. Eur J Neurosci. 1998;10:3470–3478. doi: 10.1046/j.1460-9568.1998.00355.x. [DOI] [PubMed] [Google Scholar]
- 52.Wagman IH, Price DD. Reponses of dorsal horn cells of M. mulatta to cutaneous and sural A and C fiber stimuli. J Neurophysiol. 1969;32:803–817. doi: 10.1152/jn.1969.32.6.803. [DOI] [PubMed] [Google Scholar]
- 53.Willis WD, Jr, Coggeshall RE. Sensory mechanisms of the spinal cord, pp 79–151. Plenum; New York: 1991. [Google Scholar]
- 54.Wood JN. II. Genetic approaches to pain therapy. Am J Physiol Gastrointest Liver Physiol. 2000;278:G507–G512. doi: 10.1152/ajpgi.2000.278.4.G507. [DOI] [PubMed] [Google Scholar]
- 55.Woolf CJ. The dorsal horn: state-dependent sensory processing and the generation of pain. In: Wall PD, Melzack R, editors. Textbook of pain, 3rd Ed. Churchill-Livingstone; Edinburgh: 1994. pp. 101–112. [Google Scholar]
- 56.Xiang Z, Bo X, Burnstock G. Localization of ATP-gated P2X receptor immunoreactivity in rat sensory and sympathetic ganglia. Neurosci Lett. 1998;256:105–108. doi: 10.1016/s0304-3940(98)00774-5. [DOI] [PubMed] [Google Scholar]
- 57.Yang K, Kumamoto E, Furue H, Li YQ, Yoshimura M. Action of capsaicin on dorsal root-evoked synaptic transmission to substantia gelatinosa neurons in adult rat spinal cord slices. Brain Res. 1999;830:268–273. doi: 10.1016/s0006-8993(99)01408-0. [DOI] [PubMed] [Google Scholar]
- 58.Yoshimura M, Jessell TM. Amino acid-mediated EPSPs at primary afferent synapses with substantia gelatinosa neurones in the rat spinal cord. J Physiol (Lond) 1990;430:315–335. doi: 10.1113/jphysiol.1990.sp018293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zygmunt PM, Petersson J, Andersson DA, Chuang H, Sorgard M, Di Marzo V, Julius D, Hogestatt ED. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature. 1999;400:452–457. doi: 10.1038/22761. [DOI] [PubMed] [Google Scholar]










