Abstract
The AMPA receptor, ubiquitous in brain, is termed “ionotropic” because it gates an ion channel directly. We found that an AMPA receptor can also modulate a G-protein to gate an ion channel indirectly. Glutamate applied to a retinal ganglion cell briefly suppresses the inward current through a cGMP-gated channel. AMPA and kainate also suppress the current, an effect that is blocked both by their general antagonist CNQX and also by the relatively specific AMPA receptor antagonist GYKI-52466. Neither NMDA nor agonists of metabotropic glutamate receptors are effective. The AMPA-induced suppression of the cGMP-gated current is blocked when the patch pipette includes GDP-β-S, whereas the suppression is irreversible when the pipette contains GTP-γ-S. This suggests a G-protein mediator, and, consistent with this, pertussis toxin blocks the current suppression. Nitric oxide (NO) donors induce the current suppressed by AMPA, and phosphodiesterase inhibitors prevent the suppression. Apparently, the AMPA receptor can exhibit a “metabotropic” activity that allows it to antagonize excitation evoked by NO.
Keywords: AMPA, glutamate, ionotropic, metabotropic, G-protein, retina, rat
Glutamate, the major excitatory transmitter in brain, activates a class of receptors termed “ionotropic” because they directly gate ion channels (Nakanishi, 1992; Seeburg, 1993; Hille, 1994; Riedel, 1996; Pass, 1998). Recently, an ionotropic glutamate receptor of the subclass that binds AMPA was discovered in cortical homogenates to also have a “metabotropic” function: it activates a G-protein to suppress adenylyl cyclase (Wang et al., 1997). We wondered whether the AMPA receptor has other metabotropic functions and whether these might include indirect gating of ion channels. We thought to probe for a metabotropic effect in a system suited for subsequent investigation of its role in neural integration.
We chose the retinal ganglion cell because the effects of stimulating its dendritic AMPA receptors can be investigated in a slice preparation (Aizenman et al., 1988; Mittman et al., 1990; Cohen et al., 1994;Leinders-Zufall et al., 1994; Taylor et al., 1995; Zhang et al., 1995;Coleman and Miller, 1998; Matsui et al., 1998) and because the role in neural integration could then be investigated in the intact retinain vitro. Certain ganglion cells express a cGMP-gated channel that causes an inward current when nitric oxide (NO) stimulates guanylyl cyclase to raise [cGMP] (Ahmad et al., 1994). The natural source of NO is probably a class of amacrine cell that stains intensely for NADPH diaphorase (NO synthase) (Sandell, 1985; Sager, 1986). Reasoning that a current stimulated by one signal (NO) ought to be antagonized by another, we tested the AMPA receptor and discovered that in retinal ganglion cells it can activate a G-protein to suppress the cGMP-gated current.
MATERIALS AND METHODS
Preparation and recording. Slices from adult rat retina were cut at 200 μm (Werblin, 1978) and viewed on a Zeiss (Oberkochen, Germany) upright microscope with differential interference contrast optics (×40 water-immersion objective). Ganglion cells were identified in the slice by their position and size. Membrane currents were recorded in the whole-cell configuration (Hamill et al., 1981) using a patch-clamp amplifier (Axopatch 200A; Axon Instruments, Foster City, CA) linked to a computer. The voltage-clamp procedures were controlled by the pClamp software (Axon Instruments). Data were low-pass filtered (four-pole Bessel type) with a cutoff frequency of 5 kHz and then digitized at 10 kHz by an analog-to-digital interface. All experiments were performed at room temperature (23–25°C).
Solutions and drugs. The control Ringer’s solution contained (in mm): NaCl, 135; KCl, 5; CaCl2, 1; MgCl2, 1; HEPES, 10; and glucose, 10. The solution was adjusted with NaOH to pH 7.4 and bubbled with oxygen. CoCl2 (1 mm), picrotoxin (100 μm), and strychnine (1 μm) were also added to block synaptic transmission. The recording pipette contained (in mm): CsCl, 140; CaCl2, 1; EGTA or BAPTA, 5; HEPES, 10; and Mg-ATP, 2. The solution was adjusted with CsOH to pH 7.4. Pipette resistance was ∼7 MΩ.
Test substances were applied through the bath [8-bromo-cGMP, 8-bromo-cAMP, 8-p-chlorophenylthio-cGMP, CNQX, GYKI-52466, α-methyl-4-caboxyphenylglycine (MCPG), α-cyclopropyl-4-phosphonophenylglycine (CPPG), 1-methyl-3-isobutylxanthine (IBMX), zaprinast, methylene blue, or sodium nitroprusside], via pressure ejection for 1 sec from a “puffer” pipette (glutamate, AMPA, kainate, NMDA,l-2-amino-4-phosphonobutyrate [l-AP-4], or 1S,3R-1-aminocyclopentane-trans-1,3-dicarboxylic acid [trans-(1S,3R)-ACPD]), or via the patch pipette (cGMP, GTP-γ-S, GDP-β-S, pertussis toxin, or cholera toxin). MCPG and CPPG were purchased from Tocris Cookson (Ballwin, MO). Other chemicals were from Sigma (St. Louis, MO).
RESULTS
Recording from ganglion cells in the slice preparation of adult rat retina, we confirmed the observations of Ahmad et al. (1994) that certain ganglion cells express a cGMP-gated current. When a membrane-permeant analog of cGMP (8-bromo-cGMP) was bath-applied at 1 mm to a ganglion cell voltage-clamped at −50 mV, there was a sustained inward current (217 ± 6 pA; mean ± SEM) (Fig.1a). This current disappeared in normal Ringer’s solution. The same current was obtained with another membrane-permeant analog of cGMP [1 mm8-p-chlorophenylthio-cGMP, which strongly resists hydrolysis by phosphodiesterase (PDE)] in the bath and also when the recording pipette contained cGMP (Fig. 1d). A permeant analog of cAMP was ineffective (n = 4). The cGMP-gated current was observed in approximately half of the cells (37 of 73).
Fig. 1.
Stimulation of an AMPA receptor reduced a sustained inward current caused by cGMP. a,b, Puffer application of 100 μm glutamate for 1 sec (thin arrows) or 100 μm AMPA induced a fast transient inward current. Superfusion with 1 mm 8-bromo-cGMP (bars) activated a sustained inward current that was reduced (thick arrows) by puffer application of glutamate or AMPA. c, Application of 100 μm AMPA failed to reduce the 8-bromo-cGMP-induced sustained current in the solution containing 100 μm CNQX.d, Intracellular dialysis with 1 mm cGMP caused a slowly developing inward current that was also reduced (thick arrow) by puffer application of 100 μm AMPA. Whole-cell recording established att = 0. e, Normalized responses to AMPA application plotted from b and d on a faster scale. All cells were held at −50 mV.
When the sustained cGMP-gated current was induced by 8-bromo-cGMP, a puff of 100 μm glutamate evoked a biphasic response (Fig.1a). First, there was a transient inward current, as expected for direct gating of an ionotropic receptor; then, there was a brief reduction of the sustained inward current (41 ± 7%;n = 5) (Fig. 1a). The same biphasic response was evoked by AMPA (10–100 μm) (Fig. 1b) and by the AMPA receptor agonist kainate (10 μm;n = 3). The amplitude of the second phase (reduced inward current) was similar for 100 μm AMPA and 10 μm kainate (38 ± 6 and 34 ± 11%, respectively, with 8-bromo-cGMP). The amplitude of the second phase was also similar for 100 μm by AMPA and 100 μmglutamate (54 ± 7 and 55 ± 8%, respectively, with cGMP). However, when the sustained inward current was induced by 8-p-chlorophenylthio-cGMP, 100 μm AMPA did not reduce the second phase of the response (3 ± 1%;n = 3). The reduction by AMPA of the inward current was faster (Fig. 1e, thick arrow) when the sustained current was induced by cGMP rather than by 8-bromo-cGMP. The latency to maximal reduction after the AMPA puff was 4 ± 2 sec for cGMP and 11 ± 3 sec for 8-bromo-cGMP.
To identify which type of glutamate receptor reduced the inward current, we applied various agonists and antagonists of the ionotropic receptors. CNQX (100 μm), an antagonist of both AMPA and kainate subtypes, diminished the effect of AMPA (n = 4) (Fig. 1c). Furthermore, GYKI-52466 (100 μm), a specific antagonist of the AMPA receptor (Donevan and Rogawski, 1993), also diminished the effect of kainate (n = 3). Therefore, kainate receptors are probably not involved. The AMPA effects observed here, both the conventional transient inward current and the novel reduction of the sustained inward current, seem to desensitize rather little, as reported by others for AMPA responses of bipolar and ganglion cells in mammalian retina (Cohen et al., 1994;Sasaki and Kaneko, 1996). Finally, NMDA, applied as a 100 μm puff, evoked a monophasic inward current but did not reduce the inward current (n = 4) (Fig.2a). Thus, whatever causes this brief reduction of the inward current, it is apparently triggered specifically by the AMPA receptor.
Fig. 2.
The sustained inward current caused by cGMP was unaffected by NMDA and mGluR agonists. a, Application of 100 μm NMDA for 1 sec from a puffer pipette (arrows) failed to reduce the 8-bromo-cGMP-induced sustained current. b, Application of 100 μml-AP-4 (arrows) had no effect on a cell before or during superfusion of 1 mm8-bromo-cGMP. All cells were held at −50 mV.
To test whether a metabotropic glutamate receptor (mGluR) might reduce the inward current, we applied mGluR agonists. These includedl-AP-4 (n = 4) (Fig. 2b) and ACPD (n = 3). Although these compounds evoke large currents in ON bipolar cells (Nawy and Jahr, 1990; Shiells and Falk, 1990; Yamashita and Wässle, 1991; Tian and Slaughter, 1994) at the concentration used (100 μm), they did not affect the inward cGMP-gated current. Furthermore, mGluR antagonists MCPG and CPPG, both applied at 300 μm, did not affect the AMPA-evoked reduction of the steady inward current (n = 4). We conclude that the reduction by AMPA of the sustained inward current is not caused by activation of an mGluR.
If the reduction by AMPA of the sustained inward current results from closing the cGMP-gated channel, both the reduction and the sustained current should have the same reversal potential. The reduction by AMPA of the sustained inward current was outward at negative potentials and reversed at 0 mV (Fig. 3b). Similarly, the sustained current induced by 8-bromo-cGMP was inward at negative potentials and also reversed at approximately 0 mV (Fig.3b). Similar values were obtained from four cells (3 ± 4 mV), consistent with a previous report (Ahmad et al., 1994). Thus, both the cGMP-gated current and its AMPA-induced reduction reversed at the same membrane potential (n = 4) (Fig.3c), suggesting that AMPA suppresses the cGMP-gated current. If so, AMPA should also suppress the sustained cGMP-gated current induced by the NO donor, sodium nitroprusside, and we confirmed this (n = 4) (Fig. 4).
Fig. 3.
AMPA-induced reduction of current reversed at the same potential as the cGMP-gated current. a, Brief current evoked by a puff of 100 μm AMPA was inward at negative potentials and reversed at 0 mV. b, Same cell in Ringer’s solution containing 1 mm 8-bromo-cGMP. Slow transient current evoked by AMPA was outward at negative potentials and reversed at 0 mV. c, I–V curves of the steady and reduced cGMP-gated currents. Both currents reversed at 2 mV. These experiments suggest that AMPA actually suppresses the cGMP-gated current.
Fig. 4.
Superfusion with 100 μm sodium nitroprusside (SNP) activated a sustained inward current reduced by puffer application of 100 μm AMPA for 1 sec (arrows). A cell was held at −50 mV.
To investigate how the AMPA receptor suppresses the cGMP-gated current, we considered a role for Ca2+ as a second messenger (Kaupp and Koch, 1992; Nakanishi, 1992; Hille, 1994; Koutalos and Yau, 1996). If Ca2+ entering through the AMPA receptor was involved, removing extracellular Ca2+ should abolish the suppression. However, with 5 mm EGTA or BAPTA in a recording pipette, AMPA puffed onto a ganglion cell in Ca2+-free Ringer’s solution evoked the usual suppression of the cGMP-gated current (n = 6) (Fig.5a). AMPA (100 μm) reduced the current by 41 ± 7%, which was similar to that in control Ringer’s solution (38 ± 6%). The latency (12 ± 4 sec) to maximal current reduction after the AMPA puff was also similar to the control (11 ± 3 sec). When all permeant cations were removed from the medium, 8-bromo-cGMP induced no inward current at negative potentials; however, it did induce a sustained outward current at positive potentials, and this current was suppressed by AMPA. Thus, neither Ca2+ nor Na+ influx is required for the suppression, and we sought another mechanism.
Fig. 5.
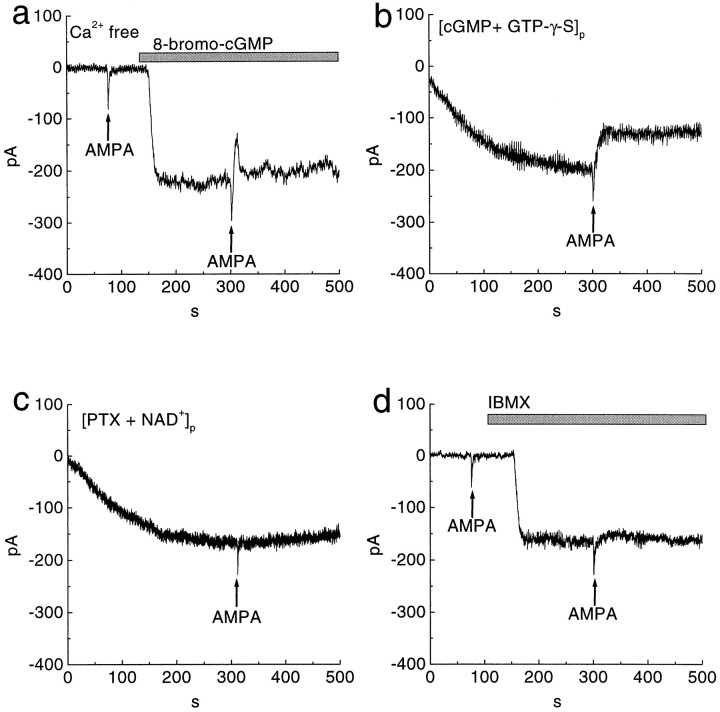
The suppression by AMPA of the cGMP-gated current is mediated by a G-protein that activates PDE. a, Application of 100 μm AMPA (arrows) suppressed the 8-bromo-cGMP-induced sustained current in the Ca2+-free solution. The recording pipette contained 5 mm EGTA. b, Dialysis of a cell with 1 mm cGMP and 100 μm GTP-γ-S produced an inward current. An AMPA puff (arrow) evoked a brief inward current and suppressed the sustained inward current irreversibly. Whole-cell recording established at t= 0. c, Dialysis of a cell with pertussis toxin (PTX; 1 μg/ml) and NAD+ (1 mm) also produced an inward current. An AMPA puff (arrow) evoked a brief inward current but failed to suppress the sustained inward current. d, Superfusion with 100 μm IBMX (bar) activated an inward current. An AMPA puff (arrows) also failed to suppress the inward current induced by IBMX. All cells were held at −50 mV.
We considered a role for a G-protein, possibly the Gifamily (Gi or Go), because AMPA can stimulate Gi (Wang et al., 1997) and because the inner plexiform and ganglion cell layers are immunoreactive for Gi/o (Terashima et al., 1987; Vardi et al., 1993; Oguni et al., 1996). To test for a G-protein activity, we used 1 mmcGMP in the patch pipette to cause a sustained inward current (as in Fig. 1d) but also included 100 μm GTP-γ-S. An AMPA puff induced the expected inward transient, followed by suppression of the sustained current. However, when the AMPA dissipated, the sustained current was not restored; rather, the suppression became essentially irreversible (n = 4) (Fig. 5b). This would be expected if an activated G-protein had bound GTP-γ-S because this GTP analog does not hydrolyze. In a separate experiment with 1 mm cGMP in the pipette, we used 500 μm GDP-β-S to competitively block G-protein activation (Nawy and Jahr, 1990). This produced a sustained inward current, which AMPA failed to suppress (n = 3).
Evidence that the G-protein species might be Gi/o came from experiments with pertussis toxin. Pertussis toxin ADP ribosylates Gi/o (Milligan, 1988), thereby blocking its downstream effect. Dialyzing a cell with pertussis toxin and NAD+ (omitting cGMP) produced a sustained inward current, which AMPA also failed to suppress (n = 5) (Fig. 5c). When NAD+ was omitted, pertussis toxin alone failed to produce the effect (n = 3), suggesting that ADP-ribosylation by pertussis toxin of Gi/o induced the inward current. This effect of pertussis toxin is consistent with previous results with ON bipolar cell (Shiells and Falk, 1992). Cholera toxin, which ADP ribosylates Gs, caused no inward current until 8-bromo-cGMP was added, and then the usual suppression was observed (n = 3). Because the cGMP-gated current seems to be suppressed by activating Gi/o, we considered possible intermediate effectors.
PDE seemed a natural candidate, by analogy with photoreceptors and certain bipolar cells in which a G-protein activates PDE to suppress a cGMP-gated current (Yau and Baylor, 1989; Kaupp and Koch, 1992; Lamb and Pugh, 1992; Koutalos and Yau, 1996). Furthermore, as noted, suppression by AMPA of the inward current was faster when the sustained current was induced by cGMP rather than by 8-bromo-cGMP (Fig.1e). This would be expected from a PDE mechanism because the brominated analog hydrolyzes slowly (Wei et al., 1998). The amplitude of the current suppression was approximately similar for both compounds. Although this might seem contradictory, the sluggish hydrolysis of PDE of 8-bromo-cGMP may be counterbalanced by its sixfold higher affinity for 8-bromo versus native cGMP (Wei et al., 1998). These considerations led us to try inhibitors of PDE.
We found, as first reported by Ahmad et al. (1994), that the PDE inhibitor IBMX induced a sustained inward current in a ganglion cell that expresses the cGMP-gated channel (n = 4) (Fig.5d). This implies that the cell also expresses an active PDE that regulates intracellular cGMP. With IBMX in the bath, an AMPA puff failed to suppress the inward current (n = 4) (Fig.5d). We obtained the same result using a selective inhibitor of cGMP phosphodiesterase, zaprinast (n = 3). Presumably AMPA was ineffective because Gi/o could not activate PDE. The suppression by AMPA of the cGMP-gated current was observed when, to the bath containing 8-bromo-cGMP, we added 1 mm methylene blue, which completely inhibits guanylyl cyclase (n = 4) (Danziger et al., 1993; Fratelli et al., 1995; Stuart-Smith et al., 1998). This result suggests that the suppression does not involve modulation of the cyclase.
DISCUSSION
In ganglion cells that express the cGMP-gated channel, AMPA acts in two ways: (1) transiently opens a cation channel; and (2) activates a G-protein that, via PDE, reduces [cGMP] to suppress a cGMP-gated current (Fig. 6). This second effect of AMPA cannot be attributed to activation of a genuine mGluR receptor because agonists and antagonists of mGluRs were ineffective. We feel confident that a PDE is involved because the effect was abolished by inhibitors of PDE and also by the analog of cGMP, which strongly resists hydrolysis. In short, this AMPA receptor seems to exhibit both ionotropic and metabotropic activities. This conclusion fits biochemical evidence that an AMPA receptor in cortex can activate a G-protein to modulate cAMP (Wang et al., 1997) and that a kainate receptor in hippocampus can activate a G-protein to modulate yet another biochemical cascade (phospholipase C; Rodriguez-Moreno and Lerma, 1998). Thus, metabotropic effects of ionotropic glutamate receptors may prove to be both widespread and diverse.
Fig. 6.
Model explains how the AMPA receptor in retinal ganglion cells might suppress the cGMP-gated current. NO released from amacrine cells raises [cGMP] (Sagar, 1986), inducing the cGMP-gated current (Ahmad et al., 1994). Ganglion cells express Gi/o-coupled metabotropic receptors (shown by aquestion mark), such as GABAB (Zhang et al., 1997) and D2-dopamine receptor (Djamgoz and Wagner, 1992). Unidentified link is shown with a broken arrow.GLU, Glutamate; R, G-protein-coupled receptor; G, Gi or Goprotein.
Given that the suppression by AMPA of the cGMP-gated current acts via a non-NMDA ionotropic glutamate receptor, can the family be identified? Probably, it is the AMPA family (GluR1–4) because of the following: (1) ganglion cells express mRNA for these subunits most strongly (Hamassaki-Britto et al., 1993); (2) the concentrations of AMPA (10–100 μm) were insufficient to activate native kainate receptors (Lerma et al., 1993; Clarke et al., 1997; Huettner, 1997); and (3) the effect was blocked by GYKI-52466, which is relatively specific for AMPA receptors. Which particular AMPA subunits (GluR1–4) suppress the cGMP-gated current cannot be determined until their specific agonists and antagonists become available.
AMPA receptors on CNS neurons desensitize rapidly (Colquhoun et al., 1992; Raman and Trussell, 1992; Seeburg, 1993). Therefore, it surprised us that, as in previous reports on mammalian retina (Cohen et al., 1994; Sasaki and Kaneko, 1996), puff-applied AMPA causes little obvious desensitization. However, the time course of our puff application is slow compared with the rapid-flow method (Colquhoun et al., 1992; Raman and Trussell, 1992) used to study desensitization. If our ganglion cells desensitize in milliseconds, we would not see the effect. Another possibility is that ganglion cells might express an alternatively spliced “flip” module of the AMPA receptor, which desensitizes more slowly than a receptor containing both “flip” and “flop” modules (Sommer et al., 1990; Seeburg, 1993). Finally, a heteromer of AMPA subunits (GluR1–4) and kainate subunits (GluR5–7 and KA1/2) might resist desensitization.
How the AMPA receptor couples to the G-protein remains to be established. Seven transmembrane receptors, including the mGluRs, contain a cytoplasmic tail with a conserved amino acid sequence that binds the G-protein (Nakanishi, 1992; Hille, 1994; Riedel, 1996; Pass, 1998). However, the AMPA receptor is assembled from hetero-oligomers that lack such a sequence (Sommer et al., 1990; Nakanishi, 1992;Seeburg, 1993; Pass, 1998), so the AMPA receptor probably does not affect the G-protein directly. Because there are now three examples in which AMPA and kainate receptor families use a G-protein to trigger a biochemical cascade (adenylyl cyclase, Wang et al., 1997; phospholipase C, Rodriguez-Moreno and Lerma, 1998; PDE, present study), it will be important to identify the coupling mechanism.
Approximately half of the recorded ganglion cells expressed the cGMP-gated current, and all of these showed the AMPA-induced suppression. We identified ganglion cells in the slice by their location (ganglion cell layer) and large size (compared with displaced amacrine cells) but did not study their morphology. It will now be interesting to determine which type(s) of ganglion cell express this mechanism and to learn its role in visual processing. Possibly, NO amacrine cells increase the cGMP-gated inward current of a ganglion cell and thus enhance spiking. In a preliminary experiment, we indeed observed that 8-bromo-cGMP had this effect. Glutamate from bipolar cells can suppress the cGMP-gated current to briefly curtail these extra spikes. Thus, the metabotropic action of the AMPA receptor might contribute to suppress excitation from the NO amacrine cells. Naturally, there are other possibilities, but this one emphasizes what may be a general mechanism in many brain regions: that glutamate can antagonize a key excitatory effect of NO.
Footnotes
This work was supported by National Institutes of Health Grant EY00828. We thank N. Vardi, S. Zigmond, P. Liebman, D. Manning, S. Nawy, and A. Kaneko for comments; R. Smith, M. Freed, L. Haarsma, J. Demb, and J. Tanaka for technical advice; and S. Watanabe for technical advice on the slice preparation.
Correspondence should be addressed to Dr. Fusao Kawai, c/o Dr. Peter Sterling, 123 Anatomy/Chemistry Building, Department of Neuroscience, School of Medicine, University of Pennsylvania, Philadelphia, PA 19104-6058.
REFERENCES
- 1.Ahmad I, Leinders-Zufall T, Kocsis JD, Shepherd GM, Zufall F, Barnstable CJ. Retinal ganglion cells express a cGMP-gated cation conductance activatable by nitric oxide donors. Neuron. 1994;12:155–165. doi: 10.1016/0896-6273(94)90160-0. [DOI] [PubMed] [Google Scholar]
- 2.Aizenman E, Frosch MP, Lipton SA. Responses mediated by excitatory amino acid receptors in solitary retinal ganglion cells from rat. J Physiol (Lond) 1988;396:75–91. doi: 10.1113/jphysiol.1988.sp016951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clarke VR, Ballyk BA, Hoo KH, Mandelzys A, Pellizzari A, Bath CP, Thomas J, Sharpe EF, Davies CH, Ornstein PL, Schoepp DD, Kamboj RK, Collingridge GL, Lodge D, Bleakman D. A hippocampal GluR5 kainate receptor regulating inhibitory synaptic transmission. Nature. 1997;389:599–603. doi: 10.1038/39315. [DOI] [PubMed] [Google Scholar]
- 4.Cohen ED, Zhou ZJ, Fain GL. Ligand-gated currents of α and β ganglion cells in the cat retinal slice. J Neurophysiol. 1994;72:1260–1269. doi: 10.1152/jn.1994.72.3.1260. [DOI] [PubMed] [Google Scholar]
- 5.Coleman PA, Miller RF. Do N-methyl-d-aspartate receptors mediate synaptic responses in the mudpuppy retina? J Neurosci. 1998;8:4728–4733. doi: 10.1523/JNEUROSCI.08-12-04728.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Colquhoun D, Jonas P, Sakmann B. Action of brief pulses of glutamate on AMPA/kainate receptors in patch from different neurons of rat hippocampal slices. J Physiol (Lond) 1992;458:261–287. doi: 10.1113/jphysiol.1992.sp019417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Danziger RS, Star RA, Matsumoto S, Coca-Prados M, DeSantis L, Pang IH. Characterization of soluble guanylyl cyclase in transformed human non-pigmented epithelial cells. Biochem Biophys Res Commun. 1993;195:958–962. doi: 10.1006/bbrc.1993.2137. [DOI] [PubMed] [Google Scholar]
- 8.Djamgoz MB, Wagner HJ. Localization and function of dopamine in the adult vertebrate retina. Neurochem Int. 1992;20:139–191. doi: 10.1016/0197-0186(92)90166-o. [DOI] [PubMed] [Google Scholar]
- 9.Donevan SD, Rogawski MA. GYKI-52466, a 2,3-benzodiazepine, is a highly selective, noncompetitive antagonist of AMPA/kainate receptor responses. Neuron. 1993;10:51–59. doi: 10.1016/0896-6273(93)90241-i. [DOI] [PubMed] [Google Scholar]
- 10.Fratelli M, Delgado R, Zinetti M, Galli G, Rolland Y, Ghezzi Chlorpromazine inhibits nitric oxide-mediated increase in intracellular cGMP in a mouse teratocarcinoma cell line. Inflamm Res. 1995;44:287–290. doi: 10.1007/BF02032570. [DOI] [PubMed] [Google Scholar]
- 11.Hamassaki-Britto DE, Hermans-Borgmeyer I, Heinemann S, Hughes TE. Expression of glutamate receptor genes in the mammalian retina: the localization of GluR1 to GluR7 mRNAs. J Neurosci. 1993;13:1888–1898. doi: 10.1523/JNEUROSCI.13-05-01888.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high resolution current recording from cells and cell-free membrane patches. Pflügers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- 13.Hille B. Modulation of ion-channel function by G-protein-coupled receptors. Trends Neurosci. 1994;17:531–536. doi: 10.1016/0166-2236(94)90157-0. [DOI] [PubMed] [Google Scholar]
- 14.Huettner JE. Functional properties of kainate receptors. In: Monaghan DT, Wenthold RJ, editors. The ionotropic glutamate receptors. Humana; Totowa, NJ: 1997. pp. 265–283. [Google Scholar]
- 15.Kaupp UB, Koch K-W. Role of cGMP and Ca2+ in vertebrate photoreceptor excitation and adaptation. Annu Rev Neurosci. 1992;54:153–175. doi: 10.1146/annurev.ph.54.030192.001101. [DOI] [PubMed] [Google Scholar]
- 16.Koutalos Y, Yau K-W. Regulation of sensitivity in vertebrate rod photoreceptors by calcium. Trends Neurosci. 1996;19:73–81. doi: 10.1016/0166-2236(96)89624-x. [DOI] [PubMed] [Google Scholar]
- 17.Lamb TD, Pugh EN., Jr G-protein cascades: gain and kinetics. Trends Neurosci. 1992;15:291–298. doi: 10.1016/0166-2236(92)90079-n. [DOI] [PubMed] [Google Scholar]
- 18.Leinders-Zufall T, Rand MN, Waxman SG, Kocsis JD. Differential role of two Ca2+-permeable non-NMDA glutamate channels in rat retinal ganglion cell: kainate-induced cytoplasmic and nuclear Ca2+ signals. J Neurophysiol. 1994;72:2503–2516. doi: 10.1152/jn.1994.72.5.2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lerma J, Paternain AV, Naranjo JR, Mellstrom B. Functional kainate selective glutamate receptor in cultured hippocampal neurons. Proc Natl Acad Sci USA. 1993;90:11688–11692. doi: 10.1073/pnas.90.24.11688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matsui K, Hosoi N, Tachibana M. Excitatory synaptic transmission in the inner retina: paired recording of bipolar cells and neurons of the ganglion cell layer. J Neurosci. 1998;18:4500–4510. doi: 10.1523/JNEUROSCI.18-12-04500.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Milligan G. Techniques used in the identification and analysis of function of pertussis toxin-sensitive guanine nucleotide binding proteins. Biochem J. 1988;255:1–13. doi: 10.1042/bj2550001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mittman S, Taylor WR, Copenhagen DR. Concomitant activation of two types of glutamate receptor mediates excitation of salamander retinal ganglion cells. J Physiol (Lond) 1990;428:175–197. doi: 10.1113/jphysiol.1990.sp018206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakanishi S. Molecular diversity of glutamate receptors and implications for brain function. Science. 1992;258:597–603. doi: 10.1126/science.1329206. [DOI] [PubMed] [Google Scholar]
- 24.Nawy S, Jahr CE. Suppression by glutamate of cGMP-activated conductance in retinal bipolar cells. Nature. 1990;346:269–271. doi: 10.1038/346269a0. [DOI] [PubMed] [Google Scholar]
- 25.Oguni M, Shinohara H, Asano T, Kato K, Setogawa T. Ontogeny of GTP-binding proteins, Gi and G(o), in rat retina. Histochem Cell Biol. 1996;106:235–240. doi: 10.1007/BF02484406. [DOI] [PubMed] [Google Scholar]
- 26.Pass Y. The macro- and microarchitectures of the ligand-binding domain of glutamate receptors. Trends Neurosci. 1998;21:117–125. doi: 10.1016/s0166-2236(97)01184-3. [DOI] [PubMed] [Google Scholar]
- 27.Raman IM, Trussell LO. The kinetics of the response to glutamate and kainate in neurons of the avian cochlear nucleus. Neuron. 1992;9:173–186. doi: 10.1016/0896-6273(92)90232-3. [DOI] [PubMed] [Google Scholar]
- 28.Riedel G. Function of metabotropic glutamate receptors in learning and memory. Trends Neurosci. 1996;19:219–224. doi: 10.1016/0166-2236(96)20012-8. [DOI] [PubMed] [Google Scholar]
- 29.Rodriguez-Moreno A, Lerma J. Kainate receptor modulation of GABA release involves a metabotropic function. Neuron. 1998;20:1211–1218. doi: 10.1016/s0896-6273(00)80501-2. [DOI] [PubMed] [Google Scholar]
- 30.Sagar S. NADPH-diaphorase histochemistry in the rabbit retina. Brain Res. 1986;373:153–158. doi: 10.1016/0006-8993(86)90325-2. [DOI] [PubMed] [Google Scholar]
- 31.Sandell JH. NADPH diaphorase cells in the mammalian inner retina. J Comp Neurol. 1985;238:466–472. doi: 10.1002/cne.902380410. [DOI] [PubMed] [Google Scholar]
- 32.Sasaki T, Kaneko A. l-glutamate-induced responses in OFF-type bipolar cells of the cat retina. Vision Res. 1996;36:787–795. doi: 10.1016/0042-6989(95)00176-x. [DOI] [PubMed] [Google Scholar]
- 33.Seeburg PH. The molecular biology of mammalian glutamate receptor channels. Trends Neurosci. 1993;16:359–365. doi: 10.1016/0166-2236(93)90093-2. [DOI] [PubMed] [Google Scholar]
- 34.Shiells RA, Falk G. The glutamate receptor of rod bipolar cells are linked to a cyclic GMP cascade via a G-protein. Proc R Soc Lond B Biol Sci. 1990;242:91–94. doi: 10.1098/rspb.1990.0109. [DOI] [PubMed] [Google Scholar]
- 35.Shiells RA, Falk G. The glutamate-receptor linked cGMP cascade of retinal on-bipolar cell is pertussis and cholera toxin-sensitive. Proc R Soc Lond B Biol Sci. 1992;247:17–20. doi: 10.1098/rspb.1992.0003. [DOI] [PubMed] [Google Scholar]
- 36.Sommer B, Keinanen K, Verdoorn TA, Wisden W, Burnashev N, Herb A, Kohler M, Takagi T, Sakmann B, Seeburg PH. Flip and flop: a cell-specific functional switch in glutamate-operated channels of the CNS. Science. 1990;249:1580–1585. doi: 10.1126/science.1699275. [DOI] [PubMed] [Google Scholar]
- 37.Stuart-Smith K, Warner DO, Jones KA. The role of cGMP in the relaxation to nitric oxide donors in airway smooth muscle. Eur J Pharmacol. 1998;341:225–233. doi: 10.1016/s0014-2999(97)01455-6. [DOI] [PubMed] [Google Scholar]
- 38.Taylor WR, Chen E, Copenhagen DR. Characterization of spontaneous excitatory synaptic currents in salamander retinal ganglion cells. J Physiol (Lond) 1995;486:207–221. doi: 10.1113/jphysiol.1995.sp020803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Terashima T, Katada T, Oinuma M, Inoue Y, Ui M. Immunohistochemical localization of guanine nucleotide-binding protein in rat retina. Brain Res. 1987;410:97–100. doi: 10.1016/s0006-8993(87)80026-4. [DOI] [PubMed] [Google Scholar]
- 40.Tian N, Slaughter MM. Pharmacological similarity between the retinal APB receptor and the family of metabotropic glutamate receptors. J Neurophysiol. 1994;71:2258–2268. doi: 10.1152/jn.1994.71.6.2258. [DOI] [PubMed] [Google Scholar]
- 41.Vardi N, Matesic DF, Manning DR, Liebman PA, Sterling P. Identification of a G-protein in depolarizing rod bipolar cells. Vis Neurosci. 1993;10:473–478. doi: 10.1017/s0952523800004697. [DOI] [PubMed] [Google Scholar]
- 42.Wang Y, Small DL, Stanimirovic DB, Morley P, Durkin JP. AMPA receptor-mediated regulation of a Gi-protein in cortical neurons. Nature. 1997;389:502–504. doi: 10.1038/39062. [DOI] [PubMed] [Google Scholar]
- 43.Wei J-Y, Cohen ED, Genieser H-G, Barnstable CJ. Substituted cGMP analogs can act as selective agonists of the rod photoreceptor cGMP-gated cation channel. J Mol Neurosci. 1998;10:53–64. doi: 10.1007/BF02737085. [DOI] [PubMed] [Google Scholar]
- 44.Werblin FS. Transmission along and between rods in the tiger salamander retina. J Physiol (Lond) 1978;280:449–470. doi: 10.1113/jphysiol.1978.sp012394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yamashita M, Wässle H. Response of rod bipolar cells isolated from the rat retina to the glutamate agonist 2-amino-4-phosphonobutyric acid (APB). J Neurosci. 1991;11:2372–2382. doi: 10.1523/JNEUROSCI.11-08-02372.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yau K-W, Baylor DA. Cyclic GMP-activated conductance of retinal photoreceptor cells. Annu Rev Neurosci. 1989;12:289–327. doi: 10.1146/annurev.ne.12.030189.001445. [DOI] [PubMed] [Google Scholar]
- 47.Zhang D, Sucher NJ, Lipton SA. Co-expression of AMPA/kainate receptor-operated channels with high and low Ca2+ permeability in single rat retinal ganglion cells. Neuroscience. 1995;67:177–188. doi: 10.1016/0306-4522(94)00627-h. [DOI] [PubMed] [Google Scholar]
- 48.Zhang J, Shen W, Slaughter MM. Two metabotropic γ-aminobutyric acid receptors differentially modulated calcium currents in retinal ganglion cells. J Gen Physiol. 1997;110:45–58. doi: 10.1085/jgp.110.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]