Abstract
Background:
Recurrent acute (RAP) and chronic pancreatitis (CP) are associated with alcohol consumption and cigarette smoking. Etiology of RAP and CP is complex, and effects of alcohol and smoking may be limited to specific patient subsets. We examined the current prevalence of alcohol use, smoking and their association with RAP and CP in patients evaluated at US referral centers.
Methods:
The North American Pancreatitis Study-2, a multicenter consortium of 20 US centers prospectively enrolled 540 CP, 460 RAP patients and 695 controls from 2000–2006. Using self-reported monthly alcohol consumption during the maximum lifetime drinking period, we classified drinking status as “abstainer”, “light” (≤0.5 drinks/day), “moderate” (females: >0.5–1, males: >0.5–2), “heavy” (females: >1-<5, males: >2-<5), or “very heavy” (≥5, both genders). Smoking was classified as “never”, “past” or “current” and quantified (packs/day, pack years).
Results:
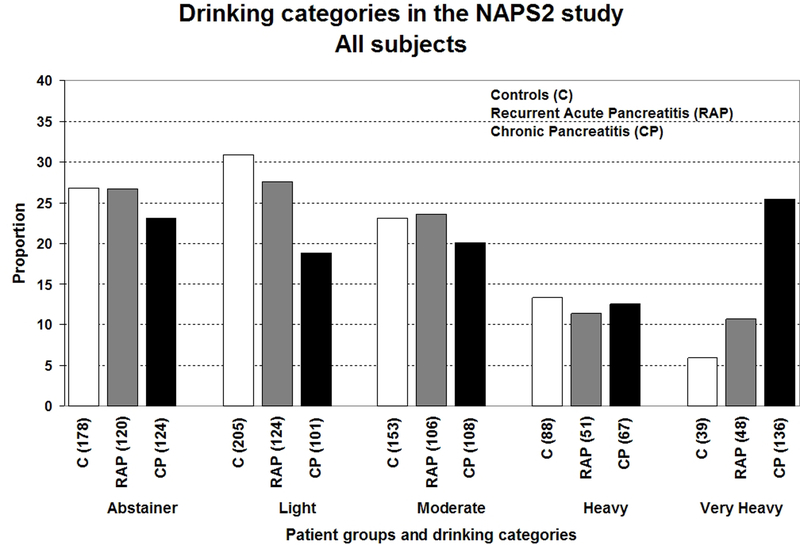
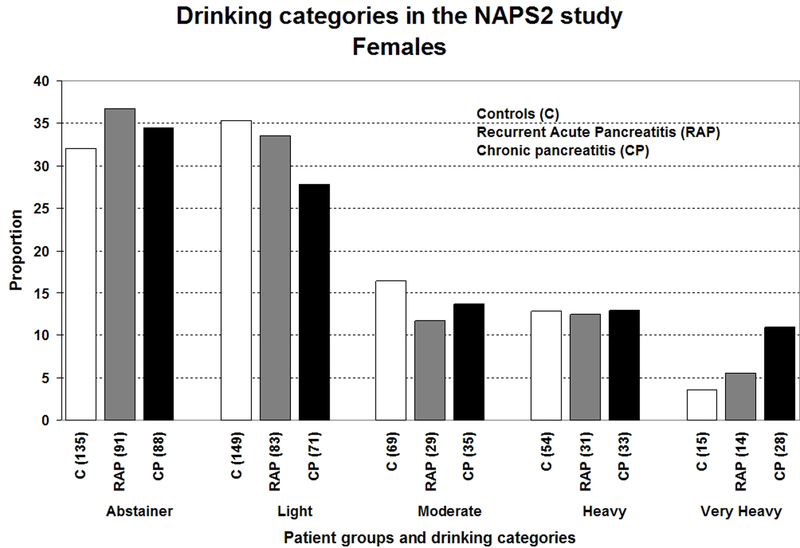
Overall, participants’ average age was 49.7±15.4 years; 87.5% were white and 56.5% were female. Approximately one-fourth of both controls and patients were lifetime abstainers. The prevalence of very heavy drinking among males and females was 38.4% and 11% for CP; 16.9% and 5.5% for RAP; 10% and 3.6% for controls. Compared to abstaining and light drinking, very heavy drinking was significantly associated with CP (OR 3.10; 95% CI 1.87–5.14) after controlling for age, gender, smoking and BMI. Cigarette smoking was an independent, dose-dependent risk factor for CP and RAP.
Conclusions:
Very heavy alcohol consumption and smoking are independent risks for CP. A minority of pancreatitis patients currently seen at US referral centers report very heavy drinking.
Introduction:
Chronic Pancreatitis (CP) is an inflammatory syndrome of the pancreas characterized by progressive parenchymal fibrosis, maldigestion, diabetes mellitus and pain1. Excessive alcohol consumption has been identified as the primary etiologic factor in numerous studies of adult CP2–8, although fewer than 5% of heavy drinkers develop CP9, 10. In 1978, a landmark South African study observed a dose-dependent risk of CP from alcohol consumption11. However, threshold levels and drinking patterns were not evaluated and the study only included men. Other studies have suffered from similar limitations8. Cigarette smoking has been reported to be an independent risk factor for developing CP8, 12–14 and increases its rate of progression15, 16. Recently, genetic factors have been identified which also increase the risk of pancreatitis, independent of alcohol consumption or cigarette smoking17–21. No previous study has evaluated the role of alcohol and smoking in both men and women in recurrent acute pancreatitis (RAP) and CP. Additionally, the current prevalence of alcohol consumption and smoking and their associated risk with RAP and CP in the United States (US) is unknown.
We hypothesized that the etiology of RAP and CP is complex, and that the effects of alcohol and smoking may be limited to specific patient subsets. To further characterize the risk of RAP and CP in US men and women, we prospectively ascertained 1000 pancreatitis patients and 695 controls from 20 centers with expertise in pancreatic diseases and performed adjusted analyses of alcohol consumption and cigarette smoking.
Methods:
Study population:
The North American Pancreatitis Study 2 (NAPS2) prospectively enrolled 1000 affected patients (540 CP, 460 RAP) using strict entry criteria and 695 controls from 20 secondary or tertiary pancreatic care centers across the United States between the years of 2000 and 2006. Spouses and family members comprised 61.7% of controls (34.2% and 27.5%, respectively) while the remaining controls were friends or unrelated to pancreatitis subjects. Detailed study protocol and methods for the NAPS2 study have been published22. RAP was defined by the presence of ≥2 documented episodes of acute pancreatitis, and CP by the presence of changes primarily on imaging studies or histology. All patients meeting these criteria were eligible for inclusion. Each study center obtained approval from its Institutional Review Board or Ethics Committee. Informed consent was obtained from all participants prior to their enrollment.
Drinking categories:
Study participants completed a questionnaire with assistance from a trained study coordinator that included detailed information on alcohol consumption and smoking. Alcohol questions assessed use in terms of quantity, duration, patterns of use and relationship with pancreatitis exacerbations as described in detail in an earlier publication22. “Ever drinking” was defined as a lifetime alcohol intake of >20 drinks. We stratified subjects into five drinking categories by average reported alcohol consumption in a month during the maximum lifetime drinking period (average number of drinks consumed on a drinking day and number of days per month this amount was consumed) using definitions similar to the National Health Interview Survey (NHIS)23. Drinking categories included “abstainers” (no alcohol use or <20 drinks in lifetime), “light drinkers” (≤0.5 drinks/day or ≤3 drinks/week), “moderate drinkers” (>0.5–1 drinks/day or 4–7 drinks/week for females; >0.5–2 drinks/day or 4–14 drinks/week for males), “heavy drinkers” (>1 - <5 drinks/day or 8–34 drinks/week for females; >2 - <5 drinks/day or 15–34 drinks/week for males), and “very heavy drinkers” (≥5 drinks/day or ≥35 drinks/week for both genders). Drinking category was assigned directly to 1507 subjects (88.9%) while in 141 subjects (8.3%) it could be assigned after review of available responses on quantity, frequency, drinking patterns and responses to TWEAK questions (described below). Drinking category could not be assigned to 47 subjects (2.8%)22. Information on average daily drinking and duration was used to determine alcohol exposure (in kilograms) during the maximum lifetime drinking period.
Drinking patterns:
As previously described22, subjects also provided the following information for each drinking phase of their life, beginning with the age of drinking onset: age phase started, pattern of drinking, age pattern changed and average number of drinks consumed per day during each phase. The drinking patterns included: 1 - abstinent; 2 - occasionally (<15 drinks/month on average – no binges); 3 - weekend drinker (up to 6 drinks/day for up to 2 days/week); 4 - moderate drinking (15 drinks/month up to two drinks/day); 5 - heavier drinking (>2 drinks/day); 6 - binge drinking (at least 3 days in a row of heavy drinking of >6 drinks/day). The total duration of lifetime alcohol consumption and the proportion of time spent at different drinking levels were calculated using information on drinking patterns and associated ages.
At-Risk drinking:
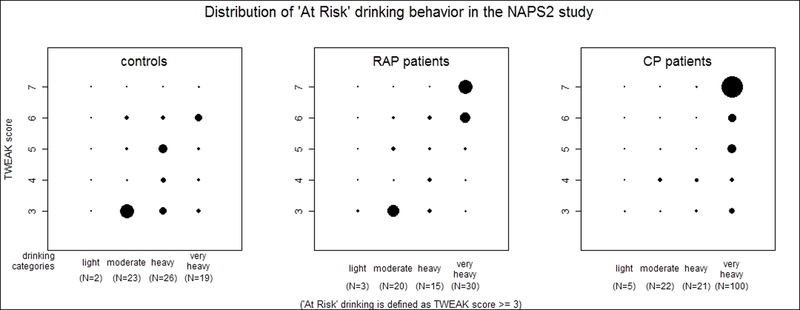
Ever drinkers also completed the TWEAK questionnaire (hold version)24, 25 to characterize At-Risk drinking habits. TWEAK is an acronym for the five questions used in this questionnaire: “T”olerance, “W”orry, “E”ye-opener, “A”mnesia, and “K”-ut down. Based on the responses, a composite TWEAK score (range 0–7) was generated for each individual24. Detailed information on the questions and derivation of TWEAK score is provided in the methods paper22. A reference period was used for pancreatitis patients (“in the months before getting pancreatitis”) but not for controls. At-Risk drinking was defined as being a score of ≥3.
Cigarette smoking:
Smoking status was classified as “never” (smoked < 100 cigarettes in lifetime) or “ever” (smoked >100 cigarettes). Ever smokers were categorized as “past” or “current” smokers. Amount of smoking was categorized as <1 or ≥1 pack per day (ppd)22 and quantified as pack years (number; <12, 12–35, >35).
Statistics:
Descriptive analyses are presented as proportions for categorical data and as mean ± standard deviation (SD) or median and interquartile range (IQR) for continuous data. Comparisons of continuous variables between controls and pancreatitis patients were performed using one-way analysis of variance (ANOVA) and Kruskal-Wallis test where applicable. If significant results were observed, appropriate two group comparisons were performed using student t-test or Mann-Whitney U test. Categorical data were compared using the chi-squared test. The distribution of subjects in different drinking categories and amount of smoking was compared using the Cochran-Armitage test. The correlation between the drinking categories and TWEAK scores was tested using the Spearman correlation coefficient.
Multivariable logistic regression was used to assess associations of alcohol consumption and cigarette smoking with pancreatitis after controlling for potential confounding variables (age, gender, and current or maximum body mass index [BMI]). Data was evaluated for collinearity and interactions. For CP, we also performed stratified regression analyses for males and females; abstainers and ever drinkers; never and ever smokers; whites and blacks. Age was assessed as a continuous variable; all other variables in the models were categorical variables, including alcohol (drinking categories), smoking (never or ever; never, <1, ≥1 ppd; never, <12, 12–35, >35 pack years), BMI (≤25, >25-≤30, >30) and gender. We used abstainers and light drinkers as the reference category for alcohol consumption. Regression models were evaluated by the goodness of fit χ2 test. Two-sided p-values <0.05 were considered significant. Data analysis was performed using the R Project software (www.r-project.org) and SPSS version 16 (SPSS Inc., Chicago, Ill., USA).
Results:
Overall, the mean age for the NAPS2 cohort was 49.7±15.4 yrs, 87.5% were White and 56.5% were female. Control and CP patients were older than RAP patients, and a greater proportion of controls were female (Table 1). Drinking behavior differed by gender and pancreatitis type. Heavy or very heavy drinking was reported by a much greater proportion of CP patients than controls or RAP patients. While approximately half of males with CP were heavy or very heavy drinkers, close to two-thirds of females with CP were self-reported abstainers or light drinkers. The demographics and distribution of drinking and smoking categories were generally similar across the top ten centers that recruited over 80% of all participants.
Table 1:
Demographic and selected risk factors among controls, recurrent acute and chronic pancreatitis patients in the NAPS2 cohort
| Variable | All Subjects | Males | Females | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Controls (695) |
RAP (460) |
CP (540) |
Controls (249) (35.8%) |
RAP ¶^ (205) (44.6%) |
CP § (283) (52.4%) |
Controls (446) (64.2%) |
RAP (255) (55.4%) |
CP (257) (47.6%) |
|
|
Age at enrollment (yrs) (mean ± SD) |
52.2 ± 14.5 |
§ 46 ± 15.8 |
§¥ 49.5 ± 15.4 |
53.2 ± 15.3 |
§ 47.4 ± 15.2 |
¶ 49.6 ± 15 |
51.7 ± 14 |
§
♦ 44.9 ± 16.2 |
49.3 ± 16.5 |
| Race (%) White Black Others |
606 (87.4) 41 (5.9) 46 (6.6) |
415 (90.4) 24 (5.2) 20 (4.4) |
¶
♦ 458 (85) 58 (10.8) 23 (4.3) |
220 (88.7) 14 (5.6) 14 (5.6) |
184 (90.2) 10 (4.9) 10 (4.9) |
*
^ 240 (84.8) 33 (11.7) 10 (3.5) |
386 (86.7) 27 (6.1) 32 (7.2) |
231 (90.6) 14 (5.5) 10 (3.9) |
218 (85.2) 25 (9.8) 13 (5.1) |
| Drinking Category (%) Abstainer Light Moderate Heavy Very Heavy |
178 (26.8) 205 (30.9) 153 (23.1) 88 (13.3) 39 (5.9) |
120 (26.7) 124 (27.6) 106 (23.6) 51 (11.4) 48 (10.7) |
§¥ 124 (23.1) 101 (18.8) 108 (20.1) 67 (12.5) 136 (25.4) |
43 (17.8) 56 (23.2) 84 (34.9) 34 (14.1) 24 (10) |
29 (14.4) 41 (20.4) 77 (38.3) 20 (10) 34 (16.9) |
§¥ 36 (12.8) 30 (10.7) 73 (26) 34 (12.1) 108 (38.4) |
135 (32.0) 149 (35.3) 69 (16.4) 54 (12.8) 15 (3.6) |
91 (36.7) 83 (33.5) 29 (11.7) 31 (12.5) 14 (5.6) |
88 (34.5) 71 (27.8) 35 (13.7) 33 (12.9) 28 (11.0) |
| “At-Risk” drinking (%) |
85 (12.2) |
* 77 (16.7) |
§¥ 171 (31.7) |
46 (18.5) |
52 (25.4) |
§¥ 125 (44.2) |
39 (8.7) |
25 (9.8) |
§
♦ 46 (17.9) |
| Smoking (%) Never Ever Past Current |
346 (50.3) 202 (29.4) 140 (20.3) |
204 (44.9) 145 (31.9) 105 (23.1) |
§¥ 153 (28.6) 129 (24.1) 253 (47.3) |
108 (43.9) 96 (39.0) 42 (17.1) |
75 (37.1) 79 (39.1) 48 (23.8) |
§¥ 55 (19.6) 74 (26.4) 151 (53.9) |
238 (53.8) 106 (24.0) 98 (22.2) |
129 (51.2) 66 (26.2) 57 (22.6) |
§
♦ 98 (38.4) 55 (21.6) 102 (40.0) |
| Smoking amount <12 pk yrs (%) 12–35 pk yrs (%) >35 pk yrs (%) |
111 (17.8) 106 (17.0) 61 (9.8) |
71 (17.6) 76 (18.9) 52 (12.9) |
§¥ 76 (16.7) 108 (23.7) 118 (25.9) |
40 (17.9) 48 (21.5) 27 (12.1) |
* 28 (15.5) 45 (24.9) 33 (18.2) |
§¥ 38 (15.9) 60 (25.1) 86 (36.0) |
71 (17.7) 58 (14.5) 34 (8.5%) |
43 (19.4) 31 (14.0) 19 (8.6) |
§¶ 38 (17.6) 48 (22.2) 32 (14.8) |
| Smoking amount <1 PPD (%) ≥1 PPD (%) |
157 (23.5) 164 (24.6) |
¶ 93 (20.9) 149 (33.4) |
§¥ 147 (28.7) 213 (41.5) |
50 (21.1) 79 (33.3) |
* 37 (18.8) 85 (43.1) |
§¥ 73 (27.1) 141 (52.4) |
107 (24.9) 85 (19.8) |
56 (22.5) 64 (25.7) |
§^ 74 (30.3) 72 (29.5) |
| BMI (maximum) Normal-low Overweight Obese |
128 (19.9) 220 (34.2) 295 (45.9) |
89 (21.8) 142 (34.7) 178 (43.5) |
§¶ 132 (28.6) 162 (35.0) 168 (36.4) |
20 (8.8) 99 (43.4) 109 (47.8) |
35 (18.8) 67 (36.0) 84 (45.2) |
§
* 59 (24.5) 95 (39.4) 87 (36.1) |
108 (26.0) 121 (29.2) 186 (44.8) |
54 (24.2) 75 (33.6) 94 (42.2) |
* 73 (33.0) 67 (30.3) 81 (36.7) |
Proportions presented are based on effective numbers.
BMI – Body Mass Index; pk yrs – Pack years of smoking
Comparisons: Controls vs RAP or CP:
<0.05
<0.01
<0.001
RAP vs CP:
<0.05
<0.01
<0.001
Definitions: Drinking categories - Abstainer: no alcohol use or <20 drinks in lifetime, Light drinker: ≤3 drinks/week or ≤0.5 drinks/day, Moderate drinker: 4–7 drinks/week or >0.5–1 drinks/day for females; 4–14 drinks/week or >0.5–2 drinks/day for males, Heavy drinker: 8–34 drinks/week or >1 - <5 drinks/day for females; 15–34 drinks/week or >2 -<5 drinks/day for males, and, Very heavy drinker: ≥35 drinks/week or ≥5 drinks/day for both genders.
At-Risk drinking was defined by a TWEAK score of ≥3.
BMI categories – Normal/low: ≤25; overweight: >25-≤30; obese: >30.
Missing information: Race (controls −2, RAP-1, CP-1), Drinking categories (controls - 32, RAP - 11, CP - 1), Smoking status (controls - 7, RAP - 6, CP - 5), pack years of smoking: (controls - 71, RAP - 57, CP - 85); packs per day of smoking (controls - 28, RAP - 14, CP - 27); maximum BMI (controls - 52, RAP - 51, CP - 78).
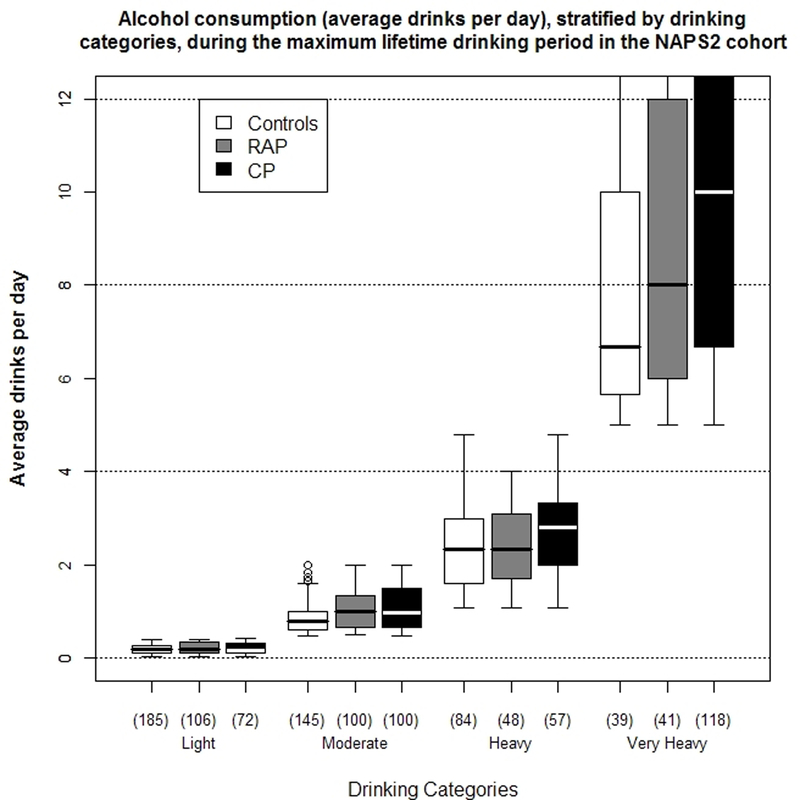
Figures 1a–1c illustrates the distribution of drinking categories for controls and pancreatitis patients. Alcohol exposure (average drinks/day [Figure 2a], duration and amount of alcohol consumed during the maximum lifetime drinking period), the proportion of lifetime drinking spent at higher drinking levels (i.e. levels 5 or 6) increased from light to very heavy drinkers for both controls and pancreatitis patients.
Figure 1.
Distribution of drinking categories based on self-reported alcohol consumption during the maximum lifetime drinking period among controls, recurrent acute (RAP) or chronic pancreatitis (CP) patients in the NAPS2 study- a) all study participants; b) males; c) females. Refer to methods section for definitions of drinking categories.
Figure 2.
a) Distribution of self-reported alcohol consumption (average drinks per day) among controls, recurrent acute (RAP) and chronic pancreatitis (CP) patients in the NAPS2 study stratified by drinking categories. Data is presented as median and IQR. The whiskers represent data within 1.5x IQR above and below the 25th and 75th percentiles and open circles represent outliers. Refer to methods section for definitions of drinking categories.
2b) Distribution of “At-Risk” drinking behavior among controls, recurrent acute (RAP) and chronic pancreatitis (CP) patients in the NAPS2 study. Controls and pancreatitis patients with a TWEAK score <3 and subjects who were assigned a drinking category are not shown in the figure.
Very heavy drinkers formed a distinct subgroup of controls and pancreatitis patients. Alcohol exposure during the maximum lifetime drinking period in this subgroup was much higher than among the other drinking categories. These individuals also spent a higher proportion of their life engaging in heavier or binge drinking. An overlap for alcohol exposure with very heavy drinkers was observed for 10–20% of moderate and 15–30% of heavy drinkers.
Overall, the lifetime drinking duration for controls (median 29 years; IQR 17, 38) and CP patients (median 27; IQR 17, 37) was higher than RAP patients (median 22, IQR 9, 32). Binge drinking during any life period was reported by 4.6% of controls, 7.3% of RAP and 14.8% of CP patients who provided responses to questions on drinking categories. Among pancreatitis patients, the majority of binge drinkers were either heavy (30% of RAP, 8% of CP) or very heavy (65% of RAP, 88% of CP) drinkers. The proportion of time spent binge drinking by very heavy drinkers increased from controls to RAP and CP. At-risk drinking was more common for CP patients than RAP patients or controls, among both men and women (Table 1). A strong correlation was observed between TWEAK score and drinking categories for the entire cohort (r = 0.69, p<0.001), as well as among the individual groups (Figure 2b).
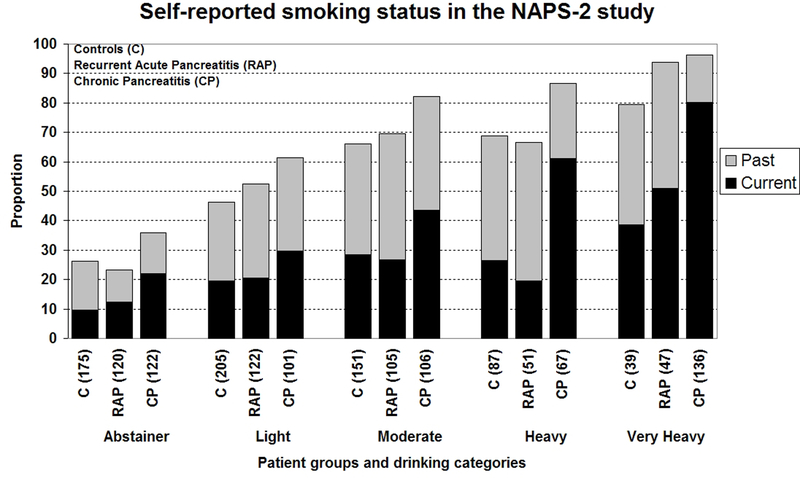
Cigarette smoking was more prevalent among CP patients compared to controls and RAP patients (Table 1). The prevalence and amount of smoking (Figure 3a, b) increased linearly with the level of drinking in controls and pancreatitis patients. Almost two-thirds of very heavy drinkers and 51.6% heavy drinkers with CP reported smoking ≥1 ppd compared to 13.9% abstainers and 34.3% light drinkers. The amount of smoking was significantly higher in CP patients (26.6 pack years; IQR 12, 46) compared to RAP (19.5; IQR 7.9, 36.1, p=0.001) and controls (16.2; IQR 6, 32.7). CP patients also had significantly longer smoking duration (median 30.5 years; IQR 19.7, 39) than controls (21.9; IQR 11.4, 33.1; p<0.001) and RAP patients (22.7; IQR 12, 32; p<0.001). Duration of smoking for CP patients was significantly longer than controls for all drinking levels except abstainers and light drinkers, and from RAP patients who were light or heavy drinkers.
Figure 3.
Distribution of self reported smoking among controls, recurrent acute (RAP) or chronic pancreatitis (CP) patients in the NAPS2 study stratified by drinking categories - a) smoking status presented as past or current; b) amount of smoking is presented as pack years of smoking. The proportions are based on effective numbers. Never smokers account for the proportions not reflected in the graphs.
Association of alcohol and smoking with RAP and CP:
Results for multivariable logistic regression analyses for RAP and CP are displayed in Table 2. In adjusted models, increasing age appeared to have a negative association with RAP (odds ratio [OR] for 10 year increase in age - 0.72, 95% confidence interval [CI] 0.65–0.79, p<0.001) and CP (OR for 10 year increase in age - 0.80, 95% CI 0.73–0.89, p<0.001). Compared to controls, RAP (OR 1.61, 95% CI 1.19–2.18, p=0.002) and CP patients (OR 1.53, 95% CI 1.13–2.07, p=0.006) were more often male. While heavy smoking was positively associated with RAP, alcohol consumption was not associated with RAP at any level. Compared to abstainers and light drinkers, after controlling for age, gender, BMI and smoking, very heavy alcohol consumption was significantly associated with CP (OR 3.10, 95% CI 1.87–5.14). The association between smoking and CP was dose-dependent.
Table 2:
Multivariable logistic regression analyses showing associations between alcohol consumption and smoking with Recurrent Acute and Chronic Pancreatitis in the NAPS2 study
| Variable Sample size* |
Recurrent Acute Pancreatitis Controls – 555 (79.8%) RAP – 364 (79.1%) |
Chronic Pancreatitis Controls – 555 (79.8%) CP – 416 (77%) |
||
|---|---|---|---|---|
| Odds Ratio (95% CI) | p-value | Odds Ratio (95% CI) | p-value | |
|
Alcohol Use: Abstainer or Light (reference) Moderate Heavy Very Heavy |
1 0.82 (0.57 – 1.18) 0.70 (0.45 – 1.10) 1.16 (0.64 – 2.09) |
0.29 0.12 0.62 |
1 0.81 (0.56 – 1.18) 0.83 (0.54 – 1.29) 3.10 (1.87 – 5.14) |
0.27 0.41 <0.001 |
|
Smoking Status: Never smoker (reference) <12 pack years 12–35 pack years >35 pack years |
1 0.96 (0.65 – 1.41) 1.44 (0.97 – 2.13) 1.91 (1.17 – 3.11) |
0.83 0.07 0.01 |
1 1.34 (0.90 – 2.01) 2.15 (1.46 – 3.17) 4.59 (2.91 – 7.25) |
0.14 <0.001 <0.001 |
|
Body Mass Index (maximum): Normal or low (reference) Overweight Obese |
1 0.88 (0.60 – 1.31) 0.91 (0.63 – 1.32) |
0.53 0.63 |
1 0.70 (0.48 – 1.01) 0.51 (0.36 – 0.74) |
0.06 <0.001 |
Effective sample sizes; Models shown are also adjusted for age and gender; smoking status: Pack years – number of pack years of smoking; BMI: Normal: ≤25; overweight: >25 and ≤30; obese - >30.
Models using other smoking variables (ever vs. never; never vs. past vs. current; packs per day), and current BMI had larger sample sizes for all groups and showed similar associations.
Stratified models for CP are presented in Table 3. Very heavy drinking was significantly associated with CP in males, ever smokers, Whites and Blacks. A dose-dependent relationship between smoking and CP was noted for males, females, ever drinkers and Whites. When we tested for interaction between drinking categories and smoking (never/ever or amount), a non-significant main effect was seen for the interaction term. However, a trend for interactive relationship between drinking and smoking was observed in stratified models. Among ever smokers, the odds of a CP patient to be a very heavy drinker were almost 5 times as compared to an abstainer or light drinker (Table 3). In stratified models for drinking categories, after controlling for age, gender and maximum BMI, the odds ratios for heavy smoking associated with CP increased with the level of drinking (Table 4). Among heavy or very heavy drinkers, the odds of a CP patient to be a heavy smoker (>35 pack years) were 13 times as compared to a never smoker. Similar observation was noted when smoking was used in the models as ever vs. never or as packs per day (data not shown).
Table 3:
Multivariable logistic regression analyses showing associations between alcohol consumption and smoking with chronic pancreatitis patients compared to controls in the NAPS2 cohort in stratified models
| Variable | Odds Ratio (95% Confidence Interval) | |||||||
|---|---|---|---|---|---|---|---|---|
| Males | Females | Ever drinkers | Never Drinkers (Abstainers) | Ever Smokers | Never Smokers | Whites | Blacks | |
| Sample sizes >> |
Controls 197 (79.1%) CP 219 (77.3%) |
Controls 358 (80.2%) CP 197 (76.7%) |
Controls 431 (83.3%) CP 324 (77.8%) |
Controls 145 (81.4%) CP 92 (74.2%) |
Controls 307 (88%) CP 325 (84%) |
Controls 302 (87%) CP 153 (132%) |
Controls 485 (80%) CP 353 (77.1%) |
Controls 31 (75.6%) CP 46 (79.3%) |
|
Alcohol Use: Abstainer/Light (Reference) Moderate Heavy Very Heavy |
1 0.93 (0.53–1.64) 0.91 (0.44–1.88) 4.56 (2.22–9.36) $ |
1 0.78 (0.45 – 1.33) 0.86 (0.49 – 1.50) 1.95 (0.90 – 4.24) |
- | - |
1 1.00 (0.64 – 1.56) 1.06 (0.65 – 1.73) 4.69 (2.76 – 7.97)$ |
1 0.72 (0.38 – 1.35) 0.63 (0.28 – 1.41) 1.37 (0.37 – 5.08) |
1 0.78 (0.53 – 1.17) 0.76 (0.48 – 1.20) 2.51 (1.46 – 4.34)$ |
1 2.43 (0.32 – 18.49) 2.15 (0.23 – 19.78) 20.86 (1.38 – 316.13)* |
|
Smoking: Never smoker (Reference) <12 pack yrs 12–35 pack yrs >35 pack yrs |
1 1.50 (0.78 – 2.91) 2.52 (1.35 – 4.70)# 8.34 (4.13 – 16.84)$ |
1 1.27 (0.76 – 2.11) 2.08 (1.26 – 3.44)# 2.63 (1.39 – 5.01)# |
1 2.04 (1.31 – 3.17)# 3.29 (2.18 – 5.08)$ 8.07 (4.97 – 13.08)$ |
1 0.81 (0.31 – 2.15) 3.26 (1.23 – 8.67)* 2.35 (0.71 – 7.78) |
- | - |
1 1.21 (0.79 – 1.85) 2.04 (1.35 – 3.10)# 4.71 (2.92 – 7.58)$ |
1 1.20 (0.26 – 15.32) 6.20 (0.83 – 46.06) 4.26 (0.25 – 75.13) |
|
Body Mass Index (maximum): Normal or low (Reference) Overweight Obese |
1 0.48 (0.24 – 0.94)* 0.39 (0.20 – 0.76)$ |
1 0.88 (0.55 – 1.41) 0.59 (0.38 – 0.92)* |
1 0.70 (0.46 – 1.06) 0.45 (0.30 – 0.68)$ |
1 0.56 (0.25 – 1.23) 0.75 (0.36 – 1.56) |
1 0.51 (0.32 – 0.81)# 0.32 (0.20 – 0.50)$ |
1 1.07 (0.61 – 1.89) 0.85 (0.49 – 1.48) |
1 0.79 (0.53 – 1.19) 0.64 (0.43 – 0.95)* |
1 0.17 (0.03 – 1.16) 0.14 (0.02 – 0.95)* |
Models shown are also adjusted for age and gender; smoking status: Pack yrs – number of pack years; BMI: Normal: ≤25; overweight: >25 and ≤30; obese - >30; P-value:
<0.05
<0.01
<=0.001
Models using other smoking variables (ever vs. never; never vs. past vs. current; packs per day), and current BMI had larger sample sizes for all groups and showed similar associations.
For never drinkers (abstainers), the model using smoking as ever vs. never showed association for ever smoking with CP as OR 1.91 (95% CI 1.11 – 3.31).
Table 4:
Multivariable logistic regression analyses showing trends for the association of smoking with Chronic Pancreatitis in models stratified by drinking categories*
|
Sample size |
Drink Category | |||
|---|---|---|---|---|
| Abstainer | Light | Moderate | Heavy or Very Heavy | |
| Controls 145 (81.4%) CP 92 (74.2%) |
Controls 174 (84.9%) CP 79 (78.2%) |
Controls 132 (86.3%) CP 89 (82.4%) |
Controls 104 (81.9%) CP 156 (76.8%) |
|
|
Smoking: Never <12 pk yrs 12–35 pk yrs >35 pk yrs |
1 0.81 (0.31 – 2.15) 3.26 (1.23 – 8.67)* 2.35 (0.71 – 7.78) |
1 1.51 (0.71 – 3.21) 2.31 (1.10 – 4.85)* 3.27 (1.26 – 8.49)* |
1 1.87 (0.82 – 4.28) 2.17 (0.96 – 4.90) 7.59 (2.93 – 19.63)$ |
1 3.13 (1.25 – 7.88)* 4.47 (1.93 – 10.34)$ 13.41 (5.23 – 34.4)$ |
Pk yrs: pack years; Models are adjusted for age, gender and maximum body mass index; p-value:
- <0.05
- <0.01
- <0.001
No significant main effect was seen for the interaction term between drinking categories and smoking categories in the model containing all drinking categories. Stratified analyses were performed to assess for trends.
Separate models for heavy and very heavy drinkers showed stronger association for smoking (higher Odds Ratios) for very heavy drinkers but the confidence intervals were large reflecting small sample size.
Discussion:
In the largest U.S. study of risk factors for RAP and CP to date, we observed a smaller than expected relationship of alcohol consumption and pancreatitis. We confirmed the results of past studies that have noted a significant association of very heavy alcohol consumption and cigarette smoking with CP. Our study is the first to demonstrate that a significant increase in the risk of CP occurs only above a threshold of ≥5 alcohol drinks per day. Contrary to previous studies2, 4–8, only 38.4% of males and 11% of females with CP were very heavy drinkers. Although heavy smokers tended to be heavy drinkers, smoking itself was a significant risk factor for pancreatitis.
Accurate quantification of alcohol consumption and its relationship to disease risk is a challenge in any observational study. We used drinking category definitions similar to the US general population survey (NHIS)23 and the recommendations of other government agencies26, although we divided the NHIS heavier drinking category into heavy and very heavy to perform more precise comparisons. Self reported alcohol intake during the maximum lifetime drinking period served as primary data to create drinking categories. This criterion was chosen for subject classification, rather than total lifetime alcohol consumption, to reflect the time of highest alcohol-associated risk. Furthermore, use of lifetime alcohol consumption criteria would classify older individuals with small amounts of daily alcohol consumption over many years in the same risk category as younger individuals who consumed alcohol more heavily for a few years (e.g. 100 kg alcohol can be consumed in 11.6 years at 2 drinks/day or in only 4.6 years at 5 drinks/day).
During the maximum lifetime drinking period, only about one-fourth of CP patients reported alcohol consumption in amounts typically associated with CP. To avoid underestimation of “heavy drinking”, we analyzed our data using several cut-offs. Including subjects in the moderate and heavy drinking categories whose alcohol exposure during the maximum lifetime drinking period overlaps with very heavy drinkers increases the proportion of very heavy drinkers to 9.8% of controls, 13.8% of RAP and 31.7% of CP patients. Using a lower threshold for heavy drinking (i.e. >14 drinks/week for males and >7 drinks/week for females), the proportion of heavy or very heavy drinkers among CP patients would have been 37.9% (50.5% males, 23.9% females). Using presence of either an overlap of alcohol exposure during the maximum lifetime drinking period or At-Risk or Binge drinking, the proportion of very heavy drinkers increases to 17.4% among controls, 21.7% among RAP patients and 40.2% (58.3% males, 20.2% females) among participants with CP. Using only average number of drinks on drinking days and omitting frequency increases the proportion of subjects drinking ≥5 drinks on a drinking day to 41.6% (57.2% males, 19.7% females) for CP patients. However, such criteria will classify an infrequent drinker who consumes ≥5 drinks on a drinking day also as a “very heavy drinker”, and classify a large proportion of controls (24.1% overall, 39.1% males, 15.5% females) as very heavy drinkers. Regardless of classification, the proportion of pancreatitis that can be attributed to heavy alcohol intake in our study is much lower than reported in many studies of CP2, 4–8.
Our observation of a lower prevalence of heavy drinking among CP patients is not completely unexpected. Most of the previous large studies of CP evaluated subjects between 1960s-80s (compared to 2000–2006 for NAPS2) when alcohol was the predominate recognized etiological factor for CP2, 5–7. There is recent, growing knowledge of other causes of CP, including genetic mutations in PRSS1, CFTR, SPINK1 and chymotrypsin C genes17–21. Therefore, subjects without an obvious etiology might be more likely to be referred to expert centers for evaluation (referral bias). Our overall lower rates of heavy drinking may also have been attributed to our study’s greater proportion of females compared to other studies2, 4–8. Lastly, NAPS2 used imaging evidence on ERCP or CT as the primary enrollment criteria, while many previous studies of CP were conducted prior to routine, clinical use of CT or MRI, when diagnosis relied on less sensitive methodology, such as the presence of steatorrhea, diabetes mellitus and pancreatic calcifications on abdominal x-ray - which are commonly observed in alcoholic men who are heavy smokers.
We found the threshold for association between alcohol use and CP to be ≥5 drinks/day. The wide spectrum of reported smoking and drinking habits in this US population allowed us to evaluate the role of alcohol and smoking in stratified analyses. Although ours is the largest study of its kind to date, the current study was not adequately powered for analysis of all important subgroups (e.g.: small proportion of - very drinkers among females, very heavy drinkers among non-smokers, non-smokers among very heavy drinkers, small number of Blacks). However, our results provide important trends that should be addressed in future studies. Although there were a limited number of Blacks in the NAPS2 study, the previously reported association between alcohol and CP in Blacks was identified, suggesting racial differences in susceptibility to alcoholic pancreatitis14, 27.
Our results confirm the association between smoking and CP8, 12, 16, 28, and demonstrated that this effect is dose-dependent. A lack of significance for the interaction term between drinking and smoking was likely due to coexistent heavy drinking and smoking habits, and a low prevalence of very heavy drinking among non-smokers and heavy smoking at lower levels of drinking. However, a trend for synergist effect of increasing tobacco plus alcohol use observed in this study is expected based on several observations. In our study, the primary diagnostic criteria for CP was the presence of pancreatic fibrosis. Although the biology of fibrosis is complex, it is now clear that pancreatic stellate cell is responsible for deposition of most of the extracellular fibrotic proteins, including collagen I and II, fibronectin and other matrix proteins29–31. Stellate cells are transformed from inactive to active states and proliferation in the context of injury, free radicals, proinflammatory cytokines, and other factors. The deposition of matrix proteins is driven by additional factors including the anti-inflammatory cytokine transforming growth factor ß1 (TGF-ß1) and various growth factors29–32. Both alcohol and components of tobacco (e.g. carbon monoxide) are known to have significant effects on the immune system33, 34, but the synergistic mechanisms that lead to fibrosis in CP in humans have not yet been determined. An inverse association was observed between BMI and CP. Plausible hypotheses for this association include weight loss from malabsorption or fear of eating secondary to pain5, 35; additionally, CP patients were often smokers, and cigarette smoking is associated with lower weight and BMI36.
Potential limitations for generalizability of our results could be the choice of controls, under-reporting of alcohol consumption by study subjects, misclassification of subjects into drinking categories, higher proportion of females, and participation of expert centers for patient enrollment. The choice of spouses, relatives or friends as controls who may have shared drinking and smoking habits with affected participants could have introduced a conservative bias and lowered the odds ratios for alcohol use and smoking with pancreatitis. The prevalence of heavy or very heavy drinkers among controls was higher than in the general population23. However, this finding was likely due to our use of a different reference period for creation of drinking categories (“maximum lifetime drinking period” rather than “past 12 months”)23. Analysis of current drinking in our controls demonstrated that the distribution of heavier drinking was generally similar to the US general population23.
Self-reported alcohol consumption has been shown to be a reliable and valid measure of an individual’s alcohol consumption when compared to other measures (in-person interviews, collateral reports/sources) in research settings37, 38. The validity of drinking category assignments in our study was confirmed by the significant correlation between the drinking categories with self-reported alcohol exposure, drinking patterns and answers to the TWEAK questionnaire24, 25. The sensitivity of detecting At-Risk drinking in CP patients in our study (using very heavy drinking as the reference) was slightly lower (87%) and specificity higher (86%) compared to previously reported results of studies of known alcoholics24.
Although our study included a higher proportion of females (47.6% of CP), 35% of patients in the largest US study on idiopathic and alcoholic CP were females5. We performed stratified analyses for the prevalence and associations of alcohol and smoking. Therefore, a lower prevalence of very heavy drinking in our cohort cannot be explained solely on the basis of a higher proportion of females in the NAPS2 cohort. Our study population was enrolled from secondary or tertiary centers with expertise in pancreatic diseases. A lower proportion of very heavy drinkers and a higher proportion of females in our study highlight the need for future studies to determine the current distribution of demographics and risk factors in the US at a population level.
While our study confirms very heavy alcohol consumption and cigarette smoking as independent predictors of CP, it also demonstrates that the great majority of CP subjects seen at US referral centers are not “alcoholics” and that many, in fact, are abstainers. Our results indicate that patients with CP can be stratified into three distinct categories: those with no or minimal alcohol intake where alcohol is unrelated to disease, very heavy drinkers where alcohol and smoking may play a dominant role, and moderate or heavy drinkers where alcohol could be an etiologic cofactor with smoking, other environmental or genetic variables.
In conclusion, only very heavy alcohol consumption and cigarette smoking are significant independent risk factors for CP. Risk for CP from alcohol consumption occurs above a threshold level while risk due to smoking is dose-dependent. Drinking levels in subjects with RAP are similar to controls. Only a minority of patients with RAP and CP patients currently seen at secondary or tertiary US centers could be categorized as very heavy drinkers.
Acknowledgements:
The following centers also contributed patients to this study: Simon K. Lo MD, Department of Medicine, Cedars-Sinai Medical Center, University of California, Los Angeles; Mark T. DeMeo MD, Department of Medicine, Rush University Medical Center, Chicago, IL; William M. Steinberg MD, Washington Hospital Center, Washington DC; Michael L. Kochman MD, Department of Medicine, University of Pennsylvania, Philadelphia, PA; Babak Etemad MD, Department of Gastroenterology and Hepatology, Ochsner Medical Center, New Orleans, LA; Christopher E. Forsmark MD, Department of Medicine, University of Florida, Gainesville, FL. The authors thank Emil Bauer and Pat Schuetz for data entry and data management.
This research was supported by DK061451 (DCW), the National Pancreas Foundation (DCW), Robert and Vicki Hall, and Andrew and Michelle Aloe.
This study was presented at the Digestive Disorders Week May 17–22, 2008 in San Diego, CA, USA and printed in abstract form in Gastroenterology 2008;134(4):A225.
The authors thank Albert B. Lowenfels, MD, Professor of Surgery, New York Medical College, Valhalla NY for reviewing the manuscript and providing helpful comments; and Elizabeth D. Kennard, PhD, Epidemiology Data Center, University of Pittsburgh for reviewing the manuscript, reviewing and providing input in statistical analyses.
Dhiraj Yadav, Michael O’Connell and David C Whitcomb had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Potential conflict of interest relevant to this manuscript: None
References:
- 1.Etemad B, Whitcomb DC. Chronic pancreatitis: diagnosis, classification, and new genetic developments. Gastroenterology 2001;120:682–707. [DOI] [PubMed] [Google Scholar]
- 2.Ammann RW, Akovbiantz A, Largiader F, Schueler G. Course and outcome of chronic pancreatitis. Longitudinal study of a mixed medical-surgical series of 245 patients. Gastroenterology 1984;86:820–828. [PubMed] [Google Scholar]
- 3.Andersen BN, Pedersen NT, Scheel J, Worning H. Incidence of alcoholic chronic pancreatitis in Copenhagen. Scand J Gastroenterol 1982;17:247–52. [DOI] [PubMed] [Google Scholar]
- 4.Lankisch PG, Assmus C, Maisonneuve P, Lowenfels AB. Epidemiology of pancreatic diseases in Luneburg County. A study in a defined german population. Pancreatology 2002;2:469–477. [DOI] [PubMed] [Google Scholar]
- 5.Layer P, Yamamoto H, Kalthoff L, Clain JE, Bakken LJ, DiMagno EP. The different courses of early- and late-onset idiopathic and alcoholic chronic pancreatitis. Gastroenterology 1994;107:1481–1487. [DOI] [PubMed] [Google Scholar]
- 6.Marks IN, Bank S, Louw JH. Chronic pancreatitis in the Western Cape. Digestion 1973;9:447–53. [DOI] [PubMed] [Google Scholar]
- 7.Robles-Diaz G, Vargas F, Uscanga L, Fernandez-del Castillo C. Chronic pancreatitis in Mexico City. Pancreas 1990;5:479–483. [DOI] [PubMed] [Google Scholar]
- 8.Talamini G, Bassi C, Falconi M et al. Alcohol and smoking as risk factors in chronic pancreatitis and pancreatic cancer. Digestive diseases and sciences 1999;44:1303–1311. [DOI] [PubMed] [Google Scholar]
- 9.Lankisch PG, Lowenfels AB, Maisonneuve P. What is the risk of alcoholic pancreatitis in heavy drinkers? Pancreas 2002;25:411–2. [DOI] [PubMed] [Google Scholar]
- 10.Yadav D, Eigenbrodt ML, Briggs MJ, Williams DK, Wiseman EJ. Pancreatitis: prevalence and risk factors among male veterans in a detoxification program. Pancreas 2007;34:390–8. [DOI] [PubMed] [Google Scholar]
- 11.Durbec JP, Sarles H. Multicenter survey of the etiology of pancreatic diseases. Relationship between the relative risk of developing chronic pancreaitis and alcohol, protein and lipid consumption. Digestion 1978;18:337–50. [DOI] [PubMed] [Google Scholar]
- 12.Bourliere M, Barthet M, Berthezene P, Durbec JP, Sarles H. Is tobacco a risk factor for chronic pancreatitis and alcoholic cirrhosis? Gut 1991;32:1392–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin Y, Tamakoshi A, Hayakawa T, Ogawa M, Ohno Y, Research Committee on Intractable Pancreatic D. Associations of alcohol drinking and nutrient intake with chronic pancreatitis: findings from a case-control study in Japan. The American Journal of Gastroenterology 2001;96:2622–2627. [DOI] [PubMed] [Google Scholar]
- 14.Lowenfels AB, Zwemer FL, Jhangiani S, Pitchumoni CS. Pancreatitis in a native American Indian population. Pancreas 1987;2:694–697. [DOI] [PubMed] [Google Scholar]
- 15.Maisonneuve P, Lowenfels AB, Mullhaupt B et al. Cigarette smoking accelerates progression of alcoholic chronic pancreatitis. Gut 2005;54:510–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Imoto M, DiMagno EP. Cigarette smoking increases the risk of pancreatic calcification in late-onset but not early-onset idiopathic chronic pancreatitis. Pancreas 2000;21:115–119. [DOI] [PubMed] [Google Scholar]
- 17.Whitcomb DC, Gorry MC, Preston RA et al. Hereditary pancreatitis is caused by a mutation in the cationic trypsinogen gene. Nature genetics 1996;14:141–145. [DOI] [PubMed] [Google Scholar]
- 18.Witt H, Luck W, Hennies HC et al. Mutations in the gene encoding the serine protease inhibitor, Kazal type 1 are associated with chronic pancreatitis. Nat Genet 2000;25:213–6. [DOI] [PubMed] [Google Scholar]
- 19.Sharer N, Schwarz M, Malone G et al. Mutations of the cystic fibrosis gene in patients with chronic pancreatitis. N Engl J Med 1998;339:645–52. [DOI] [PubMed] [Google Scholar]
- 20.Cohn JA, Friedman KJ, Noone PG, Knowles MR, Silverman LM, Jowell PS. Relation between mutations of the cystic fibrosis gene and idiopathic pancreatitis. N Engl J Med 1998;339:653–8. [DOI] [PubMed] [Google Scholar]
- 21.Rosendahl J, Witt H, Szmola R et al. Chymotrypsin C (CTRC) variants that diminish activity or secretion are associated with chronic pancreatitis. Nat Genet 2008;40:78–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Whitcomb DC, Yadav D, Adam S et al. Multicenter Approach to Recurrent Acute and Chronic Pancreatitis in the United States: The North American Pancreatitis Study 2 (NAPS2). Pancreatology 2008;8:520–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.National Center of Health Statistics, Health, United States, 2006. With Chartbook on Trends in the Health of Americans Hyattesville, MD: 2006. [PubMed] [Google Scholar]
- 24.Chan AW, Pristach EA, Welte JW, Russell M. Use of the TWEAK test in screening for alcoholism/heavy drinking in three populations. Alcohol Clin Exp Res 1993;17:1188–92. [DOI] [PubMed] [Google Scholar]
- 25.Russell M, Martier SS, Sokol RJ et al. Screening for pregnancy risk-drinking. Alcohol Clin Exp Res 1994;18:1156–61. [DOI] [PubMed] [Google Scholar]
- 26.Dufour MC. What is moderate drinking? Defining “Drinks” and Drinking Levels. Alcohol Research & Health 1999;23(1):5–14. [PMC free article] [PubMed] [Google Scholar]
- 27.Lowenfels AB, Maisonneuve P, Grover H et al. Racial factors and the risk of chronic pancreatitis. The American Journal of Gastroenterology 1999;94:790–794. [DOI] [PubMed] [Google Scholar]
- 28.Lin Y, Tamakoshi A, Hayakawa T, Ogawa M, Ohno Y. Cigarette smoking as a risk factor for chronic pancreatitis: a case-control study in Japan. Research Committee on Intractable Pancreatic Diseases. Pancreas 2000;21:109–114. [DOI] [PubMed] [Google Scholar]
- 29.Apte MV, Pirola RC, Wilson JS. Battle-scarred pancreas: role of alcohol and pancreatic stellate cells in pancreatic fibrosis. J Gastroenterol Hepatol 2006;21 Suppl 3:S97–S101. [DOI] [PubMed] [Google Scholar]
- 30.Bachem MG, Zhou Z, Zhou S, Siech M. Role of stellate cells in pancreatic fibrogenesis associated with acute and chronic pancreatitis. J Gastroenterol Hepatol 2006;21 Suppl 3:S92–6. [DOI] [PubMed] [Google Scholar]
- 31.Omary MB, Lugea A, Lowe AW, Pandol SJ. The pancreatic stellate cell: a star on the rise in pancreatic diseases. J Clin Invest 2007;117:50–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wittel UA, Pandey KK, Andrianifahanana M et al. Chronic pancreatic inflammation induced by environmental tobacco smoke inhalation in rats. Am J Gastroenterol 2006;101:148–59. [DOI] [PubMed] [Google Scholar]
- 33.Hoetzel A, Dolinay T, Schmidt R, Choi AM, Ryter SW. Carbon monoxide in sepsis. Antioxid Redox Signal 2007;9:2013–26. [DOI] [PubMed] [Google Scholar]
- 34.Deng X, Wang L, Elm MS et al. Chronic alcohol consumption accelerates fibrosis in response to cerulein-induced pancreatitis in rats. American Journal of Pathology 2005;166:93–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ammann RW, Muellhaupt B. The natural history of pain in alcoholic chronic pancreatitis. Gastroenterology 1999;116:1132–1140. [DOI] [PubMed] [Google Scholar]
- 36.Rasky E, Stronegger WJ, Freidl W. The relationship between body weight and patterns of smoking in women and men. Int J Epidemiol 1996;25:1208–12. [DOI] [PubMed] [Google Scholar]
- 37.Del Boca FK, Darkes J. The validity of self-reports of alcohol consumption: state of the science and challenges for research. Addiction 2003;98 Suppl 2:1–12. [DOI] [PubMed] [Google Scholar]
- 38.Connors GJ, Maisto SA. Drinking reports from collateral individuals. Addiction 2003;98 Suppl 2:21–9. [DOI] [PubMed] [Google Scholar]