Abstract
New neurons continue to be born in the ventricular zone (VZ) of the lateral ventricles in the brain of adult birds. On the basis of serial section reconstruction and electron microscopy, we determined that the VZ of the adult canary brain is composed of three main cell types (A, B, and E). Type A cells were never found in contact with the ventricle and had microtubule-rich processes typical of young migrating neurons. Type B cells were organized as a pseudostratified epithelium, all contacted the ventricle, and most had a characteristic single cilium. Type E cells, also in contact with ventricle, were ultrastructurally similar to the mammalian multiciliated ependymal cells. After six injections of [3H]-thymidine (1 every 12 hr), Types A and B cells were found labeled. Type E cells were never [3H]-thymidine labeled. One to two hours after a single injection of [3H]-thymidine, all labeled cells corresponded to Type B cells. At survivals of 5, 24, and 74 hr after [3H]-thymidine injection, the proportion of labeled Type B cells decreased and that of Type A cells increased, indicating that Type B cells were the primary precursors. Most [3H]-labeled nuclei at 1–2 hr after [3H]-thymidine injection were separated from the ventricular cavity, but most of the mitotic cells were adjacent to the ventricle. This observation and measurements of the distance between labeled nuclei and the ventricular surface at 1, 5, 7, and 11 hr after [3H]-thymidine injection indicate that Type B cell nuclei move toward the ventricle to divide. This work reveals the architecture of the VZ in an adult vertebrate brain, identifies the primary precursor of new neurons, and describes nuclear translocation of these precursors during the cell cycle.
Keywords: neurogenesis, stem cells, neuronal migration, mitosis, ependyma, songbirds, neuroblasts, cilium
Most neurons in the CNS of vertebrates are born in the ventricular zone (VZ) (Boulder Committee, 1970), a pseudostratified columnar epithelium adjacent to the brain ventricles. During brain development cells replicate their DNA in the deeper (basal) VZ and move to the apical VZ to undergo mitosis adjacent to the ventricular surface (Sauer, 1935; Sidman et al., 1959; Hinds and Ruffett, 1971; Fujita, 1960; Takahashi et al., 1993; Chenn and McConnell, 1995). This to-and-fro movement of the nuclei is commonly known as interkinetic or intermitotic migration (Jacobson, 1991). Neurons are thought to be derived from these interkinetic cells (Chenn and McConnell, 1995; Takahashi et al., 1996, 1997).
It is generally believed that the VZ disappears as brain histogenesis comes to an end (The Boulder Committee, 1970; Jacobson, 1991) and is transformed into the ependymal layer, which is composed of cells that do not divide (Bruni et al., 1985; Sarnat, 1995). Instead, ependymal cells function as the epithelial barrier between the cerebrospinal fluid and the brain parenchyma and in the circulation of cerebrospinal fluid (Bruni et al., 1985; Peters et al., 1991; Sarnat, 1995). Thus, the function of the walls of the brain ventricles is considered different in embryos and adults. This view, however, does not take into consideration that neuronal precursors and neurogenesis persist in or close to the walls of the lateral ventricles of many adult vertebrates (for review, see Easter, 1983; Lopez-Garcia, 1993; Nottebohm and Alvarez-Buylla, 1993; Gould and Cameron, 1996). These precursors are of interest not only for their role in the adult brain, but also because of their potential use in therapeutic neuronal replacement (Kirschenbaum et al., 1994).
One of the best-studied systems of adult neuronal production is that of the canary brain (Goldman and Nottebohm, 1983; Paton and Nottebohm, 1984; Nottebohm, 1985; Alvarez-Buylla and Nottebohm, 1988). In adult canaries, and presumably in other birds (Nottebohm, 1985; Ling et al., 1997), neurons are born in the walls of the lateral ventricle. Neuronal differentiation begins soon after mitosis (Barami et al., 1995) and within the next few days the young neurons engage in a long migration to reach most areas of the telencephalon (Alvarez-Buylla and Nottebohm, 1988). Neurogenesis in canaries becomes restricted to the telencephalon around hatching (Alvarez-Buylla et al., 1994). In the canary high vocal center, and probably other regions of the telencephalon, older neurons are continuously replaced with new ones (Nottebohm and Alvarez-Buylla, 1993) in a process thought to be related to plasticity (Nottebohm, 1985; Alvarez-Buylla et al., 1990a).
Proliferating cells in the juvenile and adult canary brain are largely restricted to the walls of the lateral ventricles. Within this wall, proliferation occurs at higher rates in regions of the lateral wall of the lateral ventricle that are denominated “hot spots,” in which a large number of new neurons are born (Alvarez-Buylla et al., 1990b). Unlike the developing brain, proliferation in the VZ of adult canaries is not associated with growth or with the production of parenchymal glioblasts (Alvarez-Buylla and Nottebohm, 1988). Little is known, however, about the proliferating cells in the VZ of adult canaries or of how this germinal layer is organized. It is also of interest to understand how ependymal functions and neurogenesis may be segregated in the walls of the brain ventricles in an adult brain.
Here we have determined the cell types and three-dimensional organization of the VZ in the adult canary brain. We identify the neuronal precursors and show that these cells undergo interkinetic migration within the VZ. This is the first study to identify the primary precursors and to show the interkinetic migration of these cells in the adult vertebrate brain.
MATERIALS AND METHODS
One-year-old female Wasserschlager canaries (Serinus canaria) were used for all experiments. Birds were bred and maintained indoors at the yearly New York State photoperiod. Seeds and water were available ad libitum. Before perfusion with saline or fixatives, birds were deeply anesthetized with 0.5 mg of pentobarbital (Nembutal) injected into the pectoral muscle. All animal procedures were in accordance with institutional guidelines approved by The Rockefeller University.
Electron microscopy (EM)
The brains were fixed by intracardial perfusion with 0.9% saline followed by 50 ml of 2% paraformaldehyde and 2.5% glutaraldehyde in 0.1 m phosphate buffer (PB), pH 7.4, followed by overnight (4°C) immersion in the same fixative. Frontal vibratome sections (100 μm thick) at the level of the anterior commissure were collected serially and post-fixed in 2% osmium tetroxide for 1 hr. After dehydration in ascending concentrations of ethanol, sections were transferred to propylene oxide, impregnated overnight with TABB 812 resin (Marivac Limited, Halifax, Canada) or araldite (Durcupan; Fluka, Buchs, Switzerland), and flat-embedded. Small fragments (∼0.5 × 1 mm) of the lateral wall of the lateral ventricle (see Fig. 1) were cut and reembedded, and the VZ was cut transversely on a Reichert ultramicrotome. Sections were collected on mesh grids or on single-hole Formvar-coated grids. Ultrathin sections (60 nm) were stained with lead citrate and uranyl acetate, and observed in a Jeol 100CX EM or Philips CM-10. Twenty birds were used for the ultrastructural reconstructions and cell classification studies.
Fig. 1.

A, Hemisection of the adult canary brain at the level of the anterior commissure (AC). The lateral ventricle is indicated by dots, and the approximate locations of regions shown in B–D are indicated by black rectangles. Regions studied in detail are in the dorsal and ventral neurogenic hot spots (Alvarez-Buylla et al., 1990b). H, Hyperstriatum; Hp, hippocampus; N, neostriatum; LPO, lobus parolfactorius; Ot, optic tectum. B, Region of the dorsal neurogenic hot spot with [3H]-thymidine-labeled cells (arrows) in the lateral wall facing hyperstriatum. The ventricle in this region is collapsed and the dorsal wall, facing Hp, has no labeled cells. This animal received six injections of [3H]-thymidine at 12 hr intervals and was killed 1 hr after the last injection. The germinal epithelium is composed of a single layer of flattened cells. C, Region of high proliferation in the ventral hot spot of the same animal as inB. The VZ in this region is composed of multiple layers, and many labeled cells (some indicated by arrows) are found in all layers. Few cilia are observed in regions of high proliferation. D, In contrast, regions with many cilia (arrows), such as this one ventral to C, contain fewer labeled cells. E, Electron micrograph of the transition zone between heavily ciliated and relatively unciliated epithelium showing the three major cell types in the VZ of the ventral hotspot: heavily ciliated VZ composed of Type E cells (e) or ependymal cells on theleft, VZ composed of Type B cells (b) on the right, and two Type A cells (a) deeper in the epithelium. Notice the characteristic ultrastructure of the different cell types (see text for description of corresponding ultrastructures). V, Ventricle lumen. This micrograph is from a region betweenC and D.
[3H]-thymidine autoradiography in 2-μm-thick sections
Experiment A. Two adult canaries received six injections of [3H]-thymidine every 12 hr (100 μl per injection of [3H]-thymidine, 6.7 Ci/mm; New England Nuclear). Birds were killed 1 hr after the last injection, and the brains were fixed and processed for electron microscopy as described above. Semithin sections (2 μm thick) were cut and placed on slides. Slides were then covered with autoradiographic emulsion (NTB2, Kodak) and incubated for 1 month at 4°C. Autoradiograms were developed for 3 min in D19 developer (Kodak) at 17°C. Sections were observed stained either with Giemsa or toluidine blue or unstained under Nomarski illumination (Nikon); [3H]-labeled cells were photographed in these semithin sections at several magnifications. A cell was considered labeled if its nucleus was overlaid by six or more autoradiographic grains and the same cell was labeled in an adjacent section. Semithin sections were then reembedded, detached from the glass slides, and sectioned for EM examination. Labeled cells under the EM were identified on the basis of the light microscope photomicrographs. A total of 66 cells were studied.
Experiment B. Sixteen adult canaries received a single 100 μl injection of [3H]-thymidine (6.7 Ci/mm; New England Nuclear) into the pectoral muscle. Groups of birds were killed 1–2, 5, 24, and 74 hr after injection, and their brains were processed for autoradiography and EM as in Experiment A (see Table 2).
Table 2.
Types of [3H]-labeled cells at different survivals
| Survival time (hr) | Birds (n) | Total cells | A cells | B cells | Mitosis | Picnotic | Contact |
|---|---|---|---|---|---|---|---|
| 1–2 | 6 | 27 | 0 | 27 (100%) | 0 | 0 | 6 (22.2%) |
| 5 | 3 | 29 | 2 (6.8%) | 20 (69%) | 6 (20.7%) | 1 (3.5%) | 7 (24.1%) |
| 24 | 3 | 16 | 6 (37.5%) | 8 (50%) | 0 | 2 (12.5%) | 1 (6.3%) |
| 74 | 4 | 26 | 19 (73.1%) | 7 (26.9%) | 0 | 0 | 2 (7.7%) |
Survivals, number of birds, and type of [3H]-labeled cells studied at the four indicated survivals after a single injection of [3H]-thymidine. The number of these cells exposed to the ventricle (contact) and numbers of [3H]-thymidine-labeled mitotic cells are also indicated.
Position of [3H]-thymidine-labeled cells in the VZ
Twenty female canaries received a single 50 μl intramuscular injection of [3H]-thymidine; 1, 3, 5, 7, and 11 hr after injection, groups of 4 birds were killed and perfused with 0.9% saline followed by 3% paraformaldehyde in PB and embedded in polyethylene glycol (Alvarez-Buylla et al., 1987). Frontal sections were cut at 6 μm intervals on a rotary microtome, mounted on chromalum-coated glass slides, and processed for autoradiography (Alvarez-Buylla and Nottebohm, 1988). The position of 50 labeled VZ cells per bird were mapped under a 63× objective (10× oculars) with the aid of a computer-yoked microscope (Alvarez-Buylla and Vicario, 1988). This analysis was done in the VZ facing lobus parolfactorius (LPO) in four noncontiguous sections per bird spanning the anterior commissure. Cell selection within this region was random, and the person who was counting did not know the group to which each section belonged. For each labeled cell the shortest distance from the center of the nucleus to the surface of the VZ was measured. The dose of [3H]-thymidine was lowered for this experiment because thicker sections were used, resulting in a larger fraction of the labeled nuclei within sections (Clark et al., 1990). In addition, β-particle penetration is greater after polyethylene glycol than in plastic-embedded tissue. Cells were considered labeled if they contained more than six autoradiographic grains overlying their nucleus. This labeling criterion was more than 20 times above background.
Reconstructions
The lateral wall of the lateral ventricle of two canaries was processed for electron microscopy as above. Three different regions of the lateral wall of the lateral ventricle were reconstructed in serial ultrathin sections using the light and electron microscope (Doetsch et al., 1997). Regions examined are equivalent to those shown in black rectangles in Figure 1A. Sections were cut with a diamond knife in the following order: one 1.5 μm semithin section, followed by six ultrathin sections (80–100 nm). Each ∼2 μm unit (semithin + ultrathin sections) corresponded to one level. For each region, this serial sectioning was done for 15 consecutive levels, 10 of which are shown in Figure 4 for the dorsal and 10 for one ventral region. Semithin sections were stained with 1% toluidine blue. Ultrathin sections from each level were placed on Formvar-coated single-slot grids, stained with lead citrate, and photographed under EM. Photo montages of each level were assembled, and individual cells were identified and traced in different colors onto acetates. These acetate drawings were then scanned into a Macintosh computer and converted into vector maps with Adobe Streamline and Macromedia Freehand to generate the maps in Figure 4.
Fig. 4.
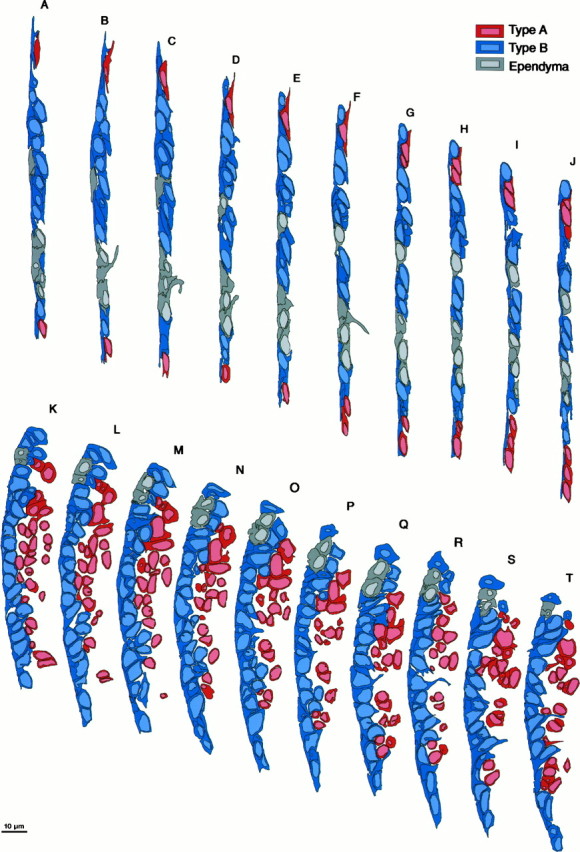
Serial section EM analysis of the dorsal (sectionsA–J, region b of Fig.1A) and ventral (sections K–T, region c in Fig. 1A) part of the lateral wall of the lateral ventricle showing the composition and architecture of the VZ in adult canaries. Each section is separated 2 μm from the next, and the ventricle is to theleft in all drawings. In the dorsal VZ the epithelium is thin and flattened. Clusters of Type A cells are next to Type B cells that form a flattened pseudostratified epithelium. Interspersed among Type B cells are small groups of ependymal cells, each with broad exposure to the ventricle. The basal process of some Type E cells can be observed (sections B and F). In the ventral region (K–T) the VZ is thick and rich in Type B cells, with only small islands of ependymal cells. Type B cells are pseudostratified, and a basal process is observed in some of these cells (sections O, R, andT). Type A cells in this region are more abundant and are loosely organized as clusters at the interface with the underlying parenchyma. Most of the Type A cells have a thin rim of cytoplasm around them; however, some clusters of Type A cells in this region are larger and have more cytoplasm (e.g., sectionM). These Type A cells were distinguished from Type B cells because they had microtubules, a tangential expansion, or a centriole close to the cell nuclei or in a tangential process.
To study the structure of the ventral hot spot VZ (c in Fig. 1A) in more detail, cells in this region were reconstructed in serial thin sections separated by ∼200 nm. For this reconstruction, 150 (gold, ∼100 nm thick) ultrathin sections were collected serially on 10 Formvar-coated single-slot grids. Sections were stained with lead citrate, and one in every three sections was photographed under the EM. Individual cells were identified in prints, and only cells completely contained within the 150 sections were used for the analysis (see Table 3). Distinguishing ultrastructural characteristics were scored for each cell. In ambiguous cases, we went back to the ultrathin series and confirmed the presence of ultrastructural cellular features (indicated in Table 3) under the EM.
Table 3.
Ultrastructural characteristics of the VZ in serial section reconstructions (intervals of 200 nm). Each cell is represented by a row, and each column shows which of the six ultrastructural features scored were present in each section: contact with ventricle, gray block; no contact with ventricle, white block; cillium, dot; centriole, X; radial process, triangle; tangential process, square. Eighty-four cells were contained within the studied sections.

RESULTS
Three major cell types coexist in the VZ of the lateral ventricle
The VZ of adult canaries varies in morphology, thickness, and cell arrangement. We concentrated our analysis on the lateral wall of the lateral ventricle at the level of the anterior commissure, a region of high proliferation in the adult canary brain (Fig.1A) (Alvarez-Buylla et al., 1990b). This VZ varies from a one- to two-cell-thick epithelium in its dorsal and lateral extent (Fig. 1B), to a thicker epithelium in its ventral part facing LPO where the ventral neurogenic hot spot is localized (Fig. 1C). The ventral hot spot is three to seven cells thick and is the region of the adult canary brain with the highest cell proliferation. Cells in the dorsal VZ were more flattened and elongated than in the VZ of the ventral hot spot but had similar ultrastructural features. Three main cell types were distinguished ultrastructurally in both ventral and dorsal aspects of the lateral wall of the lateral ventricle (Figs. 1E,2). We will refer to these as Types A, B, and E for ependymal cells (see below). The distinguishing characteristics of these cell types are summarized in Table1.
Fig. 2.
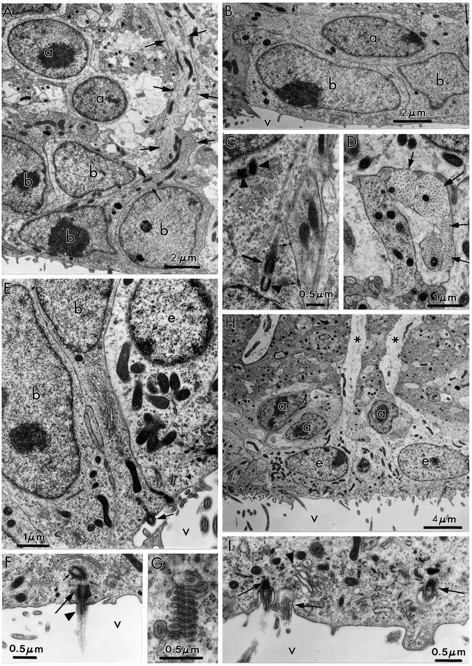
Ultrastructural characteristics of the three main cell types in the adult canary VZ. A, Photomicrograph of Types A and B cells. Type A cells (a) are deeper in the VZ and have very scant cytoplasm. In contrast, Type B cells (b) have a cytoplasm rich in organelles, contact the ventricular lumen (see Results for full description), and frequently show a long basal process (arrows) entering the underlying brain parenchyma. B, Type A (a) cells can be found under Type B (b) or E (not seen here) cells. When cut longitudinally, Type A cells have a spindle shape and tangentially oriented processes (arrows). V, Ventricle. C, A pair of centrioles is observed frequently at the base of Type A (a) cell processes (arrowheads). Some of these centrioles are adjacent to a basal body and an intracellular cilium (large arrow). Processes of Type A cells are linked to neighboring cells by small electron-dense junctional complexes (small arrows). D, Cross section of Type A (a) cell processes (arrows) showing bundles of microtubules. The cell bodies of some of these processes were identified in serial section reconstructions (Table 3, Fig. 3). E, Ultrastructure of apical cytoplasm of Types B (b) and E (e) cells. Both Type B cells contact the ventricle, one of them through a thin process that squeezes between the neighboring B and E cells. Notice the difference in the electron density, number of polyribosomes, and mitochondria between Types B and E cells. Part of the characteristic cilium (arrow) of Type B cells can also be observed in the cell in the middle. V, Ventricle. F, Most apical cytoplasm of Type B cell showing characteristic cilium (arrowhead), basal body (large arrow), and associated centriole (small arrow). In contrast to Type A cells, the centrioles of Type B cells are found close to the ventricular surface; also notice the Golgi apparatus (above the centriole) of Type B cells with flattened sacculae. V, Ventricle. G, Annulate lamellae in the apical cytoplasm of Type B cell. H, Ultrastructure of Type E or ependymal cells (e). Basal processes (asterisks) in Type E cells were electrolucent and rich in intermediate filaments. The luminal surface contains microvilli and clusters of cilia. Three Type A (a) cells are also observed in this micrograph.V, Ventricle. I, Detail of apical cytoplasm in ependymal cells; notice multiple cilia (arrows) and swollen Golgi apparatus sacculae (arrowhead). V, Ventricle.
Table 1.
Morphological characteristics of different cell types in the adult avian VZ
| Type A | Type B | Type E | |
|---|---|---|---|
| Soma position | Basal (no contract with ventricle) | Apical–basal (contact with ventricle) | Apical (contact with ventricle) |
| Processes | Tangential | Radial (electrondense) | Radial (light) |
| Nuclei | Round–spindle | Oval–irregular | Round–oval |
| Chromatin | Lax (clumps) | Lax (occasionally condensed) | Small aggregates |
| Nucleoli | 1–2 (large) | 1–2 (large) | 1–2 (small) |
| Cytoplasm | Scant | Dark | Light (basal)–dark (apical) |
| Smooth endoplasmic reticulum | + | +++ | + |
| Annulate lamellae | No | Yes | No |
| Golgi apparatus | Small | Large (thin saccules) | Small (swollen saccules) |
| Vesicles closely associated with Golgi | ++ | ++++ | + |
| Free ribosomes | ++++ | ++ | ++ |
| Mitochondria | + | +++ (round–small) | +++ (large–elongated) |
| Centriole | Close to nuclei, away from ventricle | Most apical cytoplasm | None detected |
| Cilium | Internalized (8+0), away from ventricle | One–specialized (8+0), contact with ventricle | Many (9+2), contact with ventricle |
| Ciliary rootlets | No | No | Yes |
| Microtubules | ++++ | ++ | + |
| Intermediate filaments | − | ++ | +++ |
| Junctional complex | Small zonulae adherens | Small desmosomes | Large junctional complex |
| Interdigitation | −− | + | ++ |
These characteristics are based on serial section ultrathin reconstruction of the different cell types. +, Few; ++, intermediate; +++, abundant; ++++, extremely abundant.
Type A cells (Figs. 1E, 2A–D; also see Fig. 8) had an undifferentiated appearance; usually just a thin rim of cytoplasm was visible around their nuclei. This scant cytoplasm contained a small Golgi apparatus, few rough endoplasmic reticulum but abundant polyribosomes, and few round to oval mitochondria. Pinocytic vesicles (coated pits) were also common in Type A cells (Fig.8B). The nucleus was round or elongated, with darker chromatin than that of Types B and E cells, and contained one or two large nucleoli. Type A cells were generally found in clusters with other cells of similar morphology between the brain parenchyma and the epithelial layer formed by Type B and E cells (see below). Type A cell processes were most frequently oriented parallel to the ventricular wall (Fig. 2B). These processes were rich in microtubules (Fig. 2D) and established small electron-dense contacts with neighboring cells (Fig. 2C). Frequently, a pair of centrioles was observed in one of these processes close to the nucleus (Fig. 2C; see Fig.8B), and on occasions the centrioles were associated with the basal body of a cilium (Fig. 2C). In contrast to Types A and B cells, this cilium was not on the ventricular surface but was close to the cell body and probably internalized. Type A cells were never observed in contact with the ventricular lumen (see Table 3).
Fig. 8.

Two labeled Type A cells (1 and2; see autoradiography in Nomarski photomicrograph in the inset) in the ventral hot spot facing a nonciliated section of the ventricular wall 24 hr after [3H]-thymidine administration. A, Low-magnification electron micrograph showing the multilayered VZ. Labeled cells 1 and 2 are among other Type A cells (a) and are separated from the surface of the ventricular lumen by Type B cells (b). Notice the tangential orientation of Type A cells and how the process in cell 2(arrows) seems to reach into the underlying parenchyma.B, High-magnification electron micrograph showing ultrastructural characteristics of labeled Type A cell1: a pair of centrioles (arrowheads), abundant polyribosomes, and coated pits (arrows). Notice also the many cross sections of microtubules.
Type B cells (Figs. 1E, 2A,E–G; also see Figs. 5, 6, and 8) were found adjacent to the ventricular lumen, or their nuclei were deeper in the VZ and arranged as a pseudostratified epithelium. The nuclei were oval or irregular in shape and occasionally had small invaginations. Nuclei of Type B cells had prominent nucleoli and lax chromatin, but in some Type B cells chromatin was found in different states of aggregation (see cell 1 in Fig. 5). The apical cytoplasm contained many polyribosomes, occasional stacks of annulate lamellae (Fig. 2G), and a large Golgi apparatus (Fig. 2F). The dictyosomes of Type B cells, unlike those of Type E cells, had many thin, undulated saccules associated with a rich set of coated vesicles (Figs.2F, 3). Mitochondria were also smaller and rounder than those observed in Type E cells (Fig.2E) but more abundant than in Type A cells. Junctional complexes were observed between Type B cells or Type E and B cells close to the luminal surface (Figs.4B,5); however, these junctional complexes were smaller than those observed among Type E cells (Fig.1E). Type B cells had thin and electron-dense basal processes (Fig. 2A), unlike those of Type E cells that were thicker and electrolucent (Fig. 2H). Type B cell basal processes contained microtubules, mitochondria, intermediate filaments, and free ribosomes. In single sections, some Type B cells found deeper in the VZ were apparently not connected to the ventricular lumen. However, three-dimensional reconstructions (see below) revealed that Type B cells had an end foot that reached the ventricle (Figs.2E, 5B). One striking feature of Type B cells was a single specialized cilium without a rootlet but adjacent to the centriole (Figs. 2F, 3). Transverse and longitudinal serial sections through 10 of these cilia showed that they were 3.0–3.5 μm long and 0.2 μm in diameter. They contained eight or nine pairs of microtubules in the periphery and none in the center (i.e., 8 + 0 or 9 + 0 structure). The distal end of the cilium had an electron-dense enlargement of 0.25 μm (Fig. 3A). The plasma membrane was closer to the microtubules compared with the cilia in ependymal cells. The specialized cilia of Type B cells were straight or slightly curved, suggesting that they are relatively rigid.
Fig. 5.

Labeled Type B cell (1) 1 hr after [3H]-thymidine injection.Inset shows labeled cell in Giemsa-stained semithin section. A, Notice how the labeled cell (1) extends a process toward the ventricle and a second process in the opposite direction around an adjacent Type B cell. B, This same cell (1) at higher magnification in a different ultrathin section reaches the ventricular lumen (large arrow), squeezing between processes of other Type B cells. This process contains small junctional complexes with neighboring cells (small arrows) close to its apical end. Notice the characteristic cilium of the neighboring Type B cell (arrowhead).
Fig. 6.

Pair (possibly daughter cells) of [3H]-labeled Type B cells in the dorsal VZ 5 hr after [3H]-thymidine injection.Inset shows the light photomicrograph of a semithin section stained with Giemsa. Notice how cell 2 is exposed to the ventricular lumen (between arrows). The apical borders of this cell are joined by zonulae adherens junctions (arrowheads) to neighboring cells. The dotted line indicates the position of the ventricle. Notice a dark intraventricular cell, possibly a microglia (m).
Fig. 3.
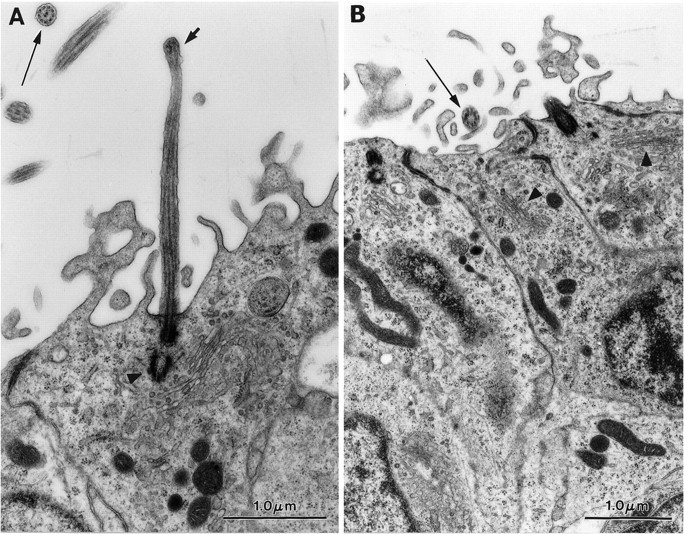
Specialized cilium in Type B cells.A, Longitudinal section of one of these cilia (short arrow). In the top left corner is a cross section of a Type E cell cilium (arrow). Notice the centriole at the base of cilium (arrowhead).B, Cross section (arrow) of a Type B cell cilium showing an 8+0 microtubule structure. Thearrowheads point to the Golgi apparatus of two Type B cells.
Type E cells had the structure typical of ciliated ependymal cells (Del Brio et al., 1991; Peters et al., 1991). The nuclear morphology was very similar among these cells, with multiple chromatin aggregates and one or two small nucleoli over a pale nucleoplasm (Figs.1E, 2E,H,I). The apical cytoplasm was denser than the basal cytoplasm and contained abundant basal bodies, ciliary rootlets, large spherical or elongated mitochondria, some clusters of free ribosomes, and a few short endoplasmic reticulum cisternae. Multiple small Golgi apparatus with swollen saccules were observed between the nucleus and the apical surface. This luminal face had microvilli and multiple cilia. These cilia were 15–20 μm long and had a 9 + 2 microtubule structure. Ependymal cells were cuboidal to columnar in the ventral VZ and flattened in the dorsal VZ. Lateral processes of ependymal cells were heavily interdigitated and joined by large junctional complexes close to the ventricular lumen (Fig. 1E). The basal processes of Type E cells were electrolucent and very rich in intermediate filaments and mitochondria and could be followed for up to 50–100 μm in some cells.
In addition to these three cell types, the VZ of adult canaries contained tanycytes, microglial cells, and endothelial cells. Capillaries were particularly abundant in the ventral proliferative hot spot. The cytoplasm of tanycytes was electron-dense with abundant dense-core vesicles and was packed with large elongated mitochondria (not shown). Tanycytes were more abundant in the medial wall of the lateral ventricle, facing the hippocampus. The nucleus of tanycytes was small and irregularly shaped, with large clusters of heterochromatin. These cells had a mitochondria-rich basal process penetrating the adjacent brain parenchyma that occasionally ended on neighboring blood vessels. These cells were clearly different from the Types B and E cells, with characteristics similar to those described previously for mammalian tanycytes (Millhouse, 1972; Cupedo and de Weerd, 1985; Rafols and Goshgarian, 1985; Del Brio et al., 1991; Peters et al., 1991). Their unique highly differentiated morphology supports our previous suggestion, based on light microscopic examination, that tanycytes in birds are different from radial cells (Alvarez-Buylla et al., 1987).
Serial section analysis of the avian VZ
To understand how the neurogenic VZ of adult canaries was organized, we serially sectioned three different regions of the lateral wall of the lateral ventricle, equivalent to those shown in black rectangles in Figure 1A. One section every 2 μm was photographed under the EM and studied in detail. Individual cells in multiple sections were identified by numbers, and their contours were digitized in different colors (see Materials and Methods): ependymal cells, gray; Type B cells, blue; and Type A cells, red. This analysis is illustrated in Figure 4A–J for the dorsal region (b in Fig. 1A) and in Figure4K–T for the ventral region (c in Fig. 1A). The difference in organization and composition of distinct VZ regions was evident in these reconstructions. The dorsal region (equivalent tob in Fig. 1A) contained alternating ependymal and Type B cells, with a few flattened Type A cells between the epithelium and underlying parenchyma. Type A cells were found in small clusters usually associated with Type B cells. Although the VZ in this dorsal region was flattened, the pseudostratified organization of clusters of Type B cells could be discerned. In contrast, ependymal cells formed a single layer of heavily interdigitated cells.
In the ventral hot spot (equivalent to c in Fig.1A) the VZ was largely composed of Type B cells with small islands of ependymal cells (Fig. 4K–T). Type B cells were pseudostratified, and most reached the ventricle. However, it was not possible by studying one section every 2 μm to determine whether all Type B cells reached the ventricle (see below). The contours of Type B cells changed sharply from one section to the next, suggesting a very irregular morphology. Tangentially arranged elongated Type A cells were observed in the interface of the VZ and the underlying brain. Type A cells in this region were loosely organized into clusters associated with ependymal or Type B cells or both. In the region just ventral to this hot spot (equivalent tod in Fig. 1A), the VZ changed again (not shown). Here the VZ was largely composed of ependymal cells with only small islands of Type B cells. Interestingly, Type A cells were also common in this region. They were small and spindle-shaped, and the majority seemed to be cut transversely in our frontal sections.
[3H]-thymidine labeling indicates that Type B cells are the primary precursors
We treated birds with six injections of [3H]-thymidine, one every 12 hr, and killed them 1 hr after the last injection. This procedure resulted in many labeled cells, as identified in autoradiograms at the light microscope level (Fig. 1B,C). Of 66 [3H]-labeled cells then analyzed at the electron microscope, 25 were Type A, 38 were Type B, and 3 were in mitosis. No labeled ependymal cells were found. Although this result indicated that ependymal cells were not actively proliferating, we could not determine whether both Type A and Type B cells were dividing, or whether one cell type gave rise to the other. We therefore treated birds with a single [3H]-thymidine injection and fixed the brains 1, 5, 24, and 74 hr later. This nucleoside remains in the circulation for <2 hr after a single injection (Alvarez-Buylla et al., 1990b). A single [3H]-thymidine injection resulted in many fewer labeled cells than after six injections. As above, labeled cells were first identified in 2 μm sections with the light microscope and then studied at the EM level. In total we identified 98 [3H]-labeled cells from the different survival groups under the EM (Table 2). Again, no labeled ependymal cells were detected.
One to two hours after [3H]-thymidine injection, labeled cells had the morphology of Type B cells (Table 2, Fig. 5). At this time labeled nuclei were generally separated from the surface of the ventricle by other cell bodies or processes of neighboring cells; however, 6 of the 27 cells studied at 1–2 hr had a thin extension that reached the ventricular surface (Fig. 5), but none of these cells had broad contacts with the ventricle. It was not possible to determine whether other labeled Type B cells contacted the ventricle. No labeled mitotic cells were encountered in the 1–2 hr survival group, but clusters of heterochromatin (Fig. 5) were noticed in some labeled Type B cells, which suggests that these cells were preparing for mitosis. With increasing survival time, the proportion of labeled Type B cells decreased, whereas that of Type A cells increased (Table 2). By 74 hr, of the 26 cells identified at the EM, only 7 (26.9%) corresponded to Type B cells. Very few of the labeled cells identified at 24 and 74 hr after [3H]-thymidine administration were found to contact the ventricle (Table 2). In contrast, 7 of 29 [3H]-labeled cells identified at the EM at the 5 hr survival contacted the cerebrospinal fluid. These cells were exposed to the ventricle (Fig. 6), and six of them were in mitosis (Table 2, Fig.7).
Fig. 7.

Labeled mitotic cell 5 hr after [3H]-thymidine administration.Inset shows semithin section stained with Giemsa.A, Low-magnification electron micrograph of this cell closely associated with two processes (arrows): one rich in intermediate filaments, probably from an ependymal cell projecting into underlying parenchyma, the other electron-dense, probably from a Type B cell. These processes do not seem to originate in the dividing cell but from closely apposed cells. The exposure of this mitotic cell to the ventricular lumen is indicated by arrowheads.B, At higher magnification, abundant pinocytic vesicles (arrows) and microvilli (arrowheads) but no cilia are seen in the exposed surface of the dividing cell. Notice how this dividing cell establishes junctional complexes (open arrows) with neighboring cells.
In contrast to the earlier survival points, many of the labeled cells at 24 and 74 hr corresponded to Type A cells (Table 2, Fig.8). At 24 hr 37.5% of the labeled cells corresponded to Type A cells, and this proportion increased to 73.1% at the 74 hr survival. [3H]-labeled cells at these longer survivals were frequently found in clusters (Figs. 8,9), which suggests that some precursors divided several times and gave rise to multiple Type A cells. In the dorsal extent of the VZ where the epithelium is thinner, [3H]-labeled cells were found in groups of no more than two cells. In contrast, we found as many as six [3H]-labeled cells in one cluster in the ventral hot spot. In two clusters of labeled cells in the ventral hot spot, we found three [3H]-labeled pyknotic cells (one at 5.5 hr and two at 24 hr survivals) (Fig. 9). These cells had the ultrastructure of cells undergoing apoptosis, with a light cytoplasm, a highly condensed chromatin, and a wrinkled or broken nuclear envelope. Given the overall low numbers of pyknotic cells observed in the VZ, it was remarkable that these dying cells were labeled by [3H]-thymidine. In two other instances dying cells were found within one or two cell diameters of a mitotic cell. These results suggest that some of the progeny of dividing cells die within the VZ. This is consistent with recent evidence from the developing mammalian brain, indicating that a significant fraction of newly formed cells in the VZ and SVZ die (Thomaidou et al., 1997) before leaving this germinal layer. Programmed cell death may serve to eliminate cells carrying mutations or inappropriate differentiation programs, or it may adjust the number of cells within the proliferative pool. We could not determine whether dying cells corresponded to Type A or Type B cells.
Fig. 9.

Cluster of labeled cells in the ventral hot spot 24 hr after [3H]-thymidine administration. Theinset shows the autoradiogram of the semithin section photographed with Nomarski optics. Two labeled cells (4and 5) in this group are undergoing apoptosis.
Mitoses
In all the material analyzed, 18 mitotic cells (labeled and unlabeled) were encountered, 15 of which had chromosomes very close to the ventricle. These cells were frequently exposed to the lumen (Fig.7), some of them with a large area. Because the ultrastructure of cells was altered during mitosis, we could not identify these cells by type; however, in the ventral VZ, mitotic cells were found in stretches of the VZ containing Type B cells but never in regions where the majority of the neuroepithelium is formed by ependymal cells. Mitotic cells had short, finger-like cytoplasmic extensions to the ventricular lumen, but no cilia (Fig. 7). The orientation of the mitotic aster in all of these cells was parallel or slightly angled with respect to the wall of the ventricle, but never perpendicular to this wall. This suggested that daughter cells in the VZ of adult canaries split tangentially (or close to tangentially) to the ventricular surface. Six [3H]-labeled mitotic cells were encountered 5 hr after [3H]-thymidine (Table 2, Fig. 7). These labeled mitoses were all exposed to the ventricular lumen. Three mitotic cells encountered during serial section reconstruction (Table3) were also exposed to the ventricle, but no cilium or basal processes were observed on these cells. This suggested that the cilium in Type B cells and probably the basal extension were retracted during mitosis. A similar conclusion has been drawn from serial section analysis in the embryonic mammalian neuroepithelium (Stensaas and Stensaas, 1968).
The position of [3H]-labeled VZ nuclei changes with increasing survival
Two of the above observations suggest that Type B cells change position as the cell cycle progresses. (1) The majority of [3H]-labeled nuclei at 1–2, 24, and 74 hr were at a distance from the ventricle. In contrast, a significant number of the [3H]-labeled cells were adjacent to the ventricle 5 hr after [3H]-thymidine; (2) most of the mitotic cells encountered were in contact with the ventricular cavity.
These observations suggest that cells move toward the VZ to undergo mitosis. To provide further support for this hypothesis, we measured the distance from the center of the labeled nuclei to the surface of the ventricle in birds that survived 1, 3, 5, 7, or 11 hr after receiving a single injection of [3H]-thymidine. We did this analysis in the ventral neurogenic hotspot where the thickness of the VZ allowed us to reliably measure changes in the position of labeled cells under the light microscope at different survival times after [3H]-thymidine administration.
Three and five hours after [3H]-thymidine injection, the distribution of [3H]-labeled cells had shifted toward the ventricle as compared with 1 or 7 and 11 hr survivals (Fig. 10). Nuclei moved from an average distance of 6.5 μm at 1 hr to 5.6 and 5.9 μm at 3 and 5 hr, respectively. By 7 and 11 hr, labeled nuclei had moved away from the ventricle to 6.5 and 6.7 μm, respectively. These values are probably an underestimate of the true displacements of nuclei or cells, because they correspond to averages of a nonsynchronous population of dividing cells. To obtain a more accurate representation of this interkinetic migration we determined the average distance from the center of the [3H]-labeled nuclei to the ventricular surface for the 20 cells closest to the ventricle for each bird in the different survival groups. As shown in Figure 11, 3 and 5hr after [3H]-thymidine injection, cells are significantly closer to the ventricle than at the other survival times studied. Consistent with the ultrastructural findings, these experiments indicate that proliferating Type B cells in the adult avian VZ are changing position as they go through the different phases of the cell cycle.
Fig. 10.
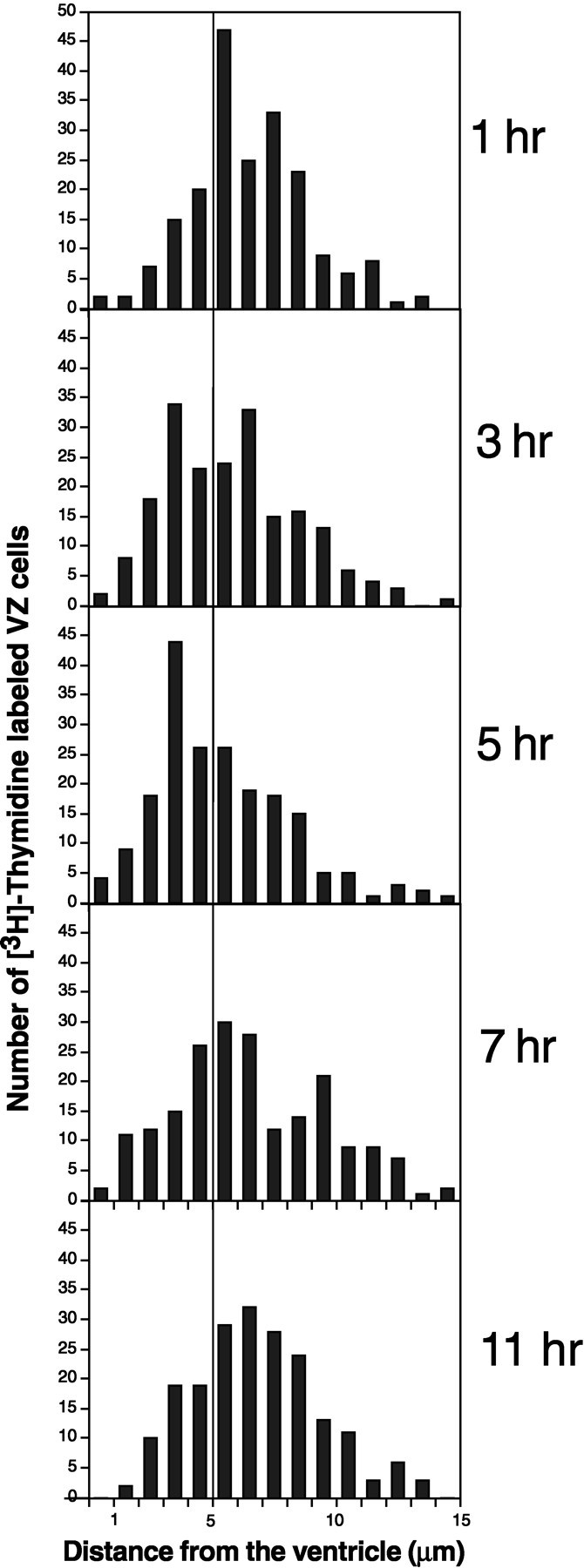
Histograms of distance from the center of [3H]-labeled cells to the surface of the ventricle in the ventral hot spot of adult canaries. Each histogram compounds 200 cells counted in four birds (50 cells/bird). A vertical line at 5 μm distance aids in the comparison of distributions. Three and five hours after [3H]-thymidine injection more cells are <5 μm from the ventricle as compared with after 1, 7, and 11 hr, when most of the cells are to the right of the line.
Fig. 11.
Average distances between the center of the nuclei and the ventricular surface of the 20 [3H]-labeled cells closest to the ventricle in the ventral hot spot of adult canaries. Each bar is the average of four birds. At 3 and 5 hr survivals, a significant (p = 0.02; Mann–WhitneyU) reduction in this distance was observed compared with 1 or 11 hr survivals. Averages for 1, 7, and 11 hr were not significantly different. One-factor ANOVA also showed significant differences (p < 0.05) between 1 hr and 3 or 5 hr survivals. Likewise, 3 and 5 hr survivals were significantly different from 11 hr survivals. The dashed lineindicates the average VZ cell radius perpendicular to the ventricle in the ventral hot spot.
Type B cells maintain an end foot on the ventricle
The above results indicate that Type B cells divide and that their position shifts during the cell cycle from the basal to the apical VZ. This movement could be attributable to the migration of the cell and the formation of a process that reaches the ventricle, or to the migration of the nucleus within a preformed process. The irregular shape of Type B cells made the identification of the apical and basal processes in single sections, or reconstructions of sections separated by 2 μm, difficult or impossible. To determine whether Type B cells maintain an end foot on the ventricle and to determine the three-dimensional morphology of all cell types in more detail, we reconstructed part of the ventral hot spot (the region rich in Type B cells), analyzing 1 out of every 2 thin sections (a separation of ∼200 nm between sections). Eighty-four cells were completely contained within the 42 ultrathin sections that were analyzed (Table3). Of these, 33 corresponded to Type A cells, 44 to Type B cells, 3 to mitoses, and 4 to Type E cells. All Type B cells were directly in contact with or had an end foot on the ventricle. Notice (Table 3) that the number of sections in which Type B cells contacted the ventricle was variable; cells close to the lumen had a large area exposed (e.g., cells 27 and 28), whereas those far from the ventricle (e.g., cell 44) had a thin end foot on the ventricle. Except for four cells, all Type B cells had the characteristic cilium with an associated centriole (Fig.2F). Also the majority of Type B cells had a basal process present in one or several sections. There was no apparent correlation between the presence of the cilium in a section and the presence of a basal process. The cilium was also not necessarily centered in the exposed surface of a Type B cell, but could appear to one side of the cell (e.g., cell 25). This variability in structure most likely reflects the dynamic nature of Type B cells. The structure of Type B cells indicates that interkinetic movements are probably caused by the migration of the nuclei within the cell. The three mitotic cells encountered in this reconstruction were exposed to the ventricle.
The contact with the ventricular lumen was evident in sections transecting an ependymal cell (Table 3). This indicates that ependymal cells, unlike Type B cells, are widely exposed to the ventricle and have a more homogeneous morphology. In every section in which Type E cells contacted the ventricle, multiple cilia were observed. The morphology of ependymal cells was regular, cuboidal, and not pseudostratified (Figs. 2E, 3).
The serial section analysis confirmed that Type A cells had a very different structure and orientation than Types B and E cells. Of the 33 Type A cells identified, none had contact with the ventricle (Table 3). Instead, the majority of these cells had tangential extensions that correspond to the microtubule-rich processes described previously (Fig. 2B,D). Unlike that in Type B cells, the centriole in Type A cells was far from the ventricular surface and generally was associated with one of the tangential processes. These tangential processes were woven between basal processes from Type B or E cells. It was therefore difficult to reconstruct all of these processes, and this may account for the Type A cells in which a centriole could not be found.
DISCUSSION
Type B cells are the primary precursors
Proliferation in the adult avian VZ is related to neurogenesis. In adult canaries, cell division is largely restricted to the walls of the lateral ventricles that face regions of the telencephalon that receive new neurons. Within these walls, neurogenesis is most prominent in proliferative hot spots where the majority of migrating neurons arise. Our EM and [3H]-thymidine analysis showed that the primary proliferating cells within the hot spots corresponded to Type B cells. Among Type B cells, mitotic figures, annulate lamellae, and different stages of chromatin aggregation were observed. These are all characteristics of actively dividing cells. In addition, Type B cells had ultrastructural characteristics of undifferentiated cells similar to those in the embryonic neuroepithelium (Hinds and Ruffett, 1971;Levitt et al., 1981). Type B cells shared the ventricular surface with ependymal cells, but multiple ultrastructural features distinguished them from these cells. Type B cells were also different from Type A cells, tanycytes, and microglial cells. Together, these observations indicate that Type B cells are the primary neuronal precursors in the adult canary VZ.
Among the characteristics of Type B cells, of most interest was the atypical cilium associated with a centriole in their apical cytoplasm. The three-dimensional reconstruction (Table 3) indicated that most Type B cells had this cilium. A similar single cilium has been observed in the developing neuroepithelium (Sotelo and Trujillo-Cenóz, 1958;Stensaas and Stensaas, 1968; Hinds and Ruffett, 1971). This specialized cilium with its associated centriole may function as an organizing center for the cytoskeleton, perhaps related to the interkinetic movement of these cells.
Radial cells divide (Misson et al., 1988; Alvarez-Buylla et al., 1990b;Gray and Sanes, 1992; Goldman et al., 1996) and may serve as neuronal precursors. Consistent with this hypothesis, Type B cells had a basal process. However, we also observed that ependymal cells (which do not divide) maintained an intermediate filament-rich basal process. We do not know how far the processes of Types B and E cells reach or whether one or both of these processes serve as guides for young neurons (Alvarez-Buylla and Nottebohm, 1988). We also could not determine which of these cells expressed the form of vimentin detected by antibody 40E-C, the marker that originally revealed radial cells in the adult avian brain (Alvarez-Buylla et al., 1987). Thus, this study raises the possibility that multiple cell types in the adult avian VZ have a radial cell morphology.
Type A cells
With increasing survival after [3H]-thymidine administration, the number of labeled Type B cells decreased, but that of Type A cells increased. Unlike Type B cells, these smaller cells did not contact the VZ, and their processes were oriented parallel to the surface of the ventricle. Type A cells were clustered between the VZ and the underlying parenchyma. Barami et al. (1995) describes cells in a similar position in the adult avian VZ that express the neuronal marker Hu. On the basis of the morphology and position of the centriole (Barami et al., 1995, their Fig. 5D), these Hu-positive cells correspond to Type A cells. In addition, the morphology of Type A cells is very similar to that of young migrating neurons in the adult mammalian subventricular zone (Lois et al., 1996; Doetsch et al., 1997) and of young migrating neurons farther out from the VZ of adult canaries (our unpublished observation). These observations suggest that Type A cells are young neurons and that these cells are probably derived from Type B cells. Consistent with this interpretation, in some Type A cells a structure similar to the atypical cilium of Type B cells was observed (Fig. 2C); however, the cilium of Type A cells was internalized in the cytoplasm. An intracytoplasmic cilium has also been described in fully differentiated, newly formed neurons in the adult canary brain (Goldman and Nottebohm, 1983). This cilium may reflect the epithelial origin of newly formed neurons from Type B cells. As early as 5 hr after [3H]-thymidine injection, some Type A cells were detected, which suggests a rapid transition of Type B to Type A cells. This observation is consistent with work indicating that some young neurons in the avian VZ begin differentiation soon after [3H]-thymidine incorporation (Barami et al., 1995).
We do not know why Type A cells accumulate at the interface of the VZ and the underlying parenchyma. It has been observed before that very few young migrating neurons emerge from the walls of the lateral ventricle sooner than 3 d after [3H]-thymidine treatment (Alvarez-Buylla and Nottebohm, 1988). Type A cells may remain in this interface region in preparation for the subsequent long intraparenchymal journey. A switch from the cell adhesion molecule N-cadherin to Ng-CAM takes place at this time and may allow the young neurons to delaminate and escape the neurogenic epithelium (Barami et al., 1994).
Interkinetic migration in the adult avian brain
Interkinetic movements are well known in the early neuroepithelium (Sauer, 1935; Fujita, 1960; Takahashi et al., 1993; Chenn and McConnell, 1995) but have not been described before in the adult brain. Our results indicate that Type B cells displayed “to and fro” movements between the deep layers of the VZ and the luminal surface during the cell cycle. Three observations support the interkinetic migration of Type B cells. (1) The majority of [3H]-labeled nuclei identified at 1, 24, or 74 hr after [3H]-thymidine injection were separated from the ventricular surface, but most of the cells observed in mitosis and several of the [3H]-labeled cells at the 5 hr survival were directly exposed to the ventricle. (2) The distance from the center of labeled cells to the surface of the ventricle decreased 3–5 hr after [3H]-thymidine injection and increased again at longer survivals; (3) Type B cells had processes of variable diameters that contacted the ventricular surface, which suggests extension or retraction of the cell from that position.
The three-dimensional reconstructions indicate that all Type B cells maintain contact with the ventricle, which suggests that this interkinetic migration is attributable to the displacement of the nucleus and cytoplasm within the processes of Type B cells and not to the formation of a process and migration of entire cells. Interkinetic migration in the adult avian brain is therefore similar to that described during development. However, unlike the embryonic VZ, the adult avian VZ is thin and compact. For this reason, the nuclear displacements in the adult VZ are small compared with those in the embryo. The function of intermitotic nuclear migration is not known. The present results indicate that intermitotic migration continues even in the adult VZ, where the epithelium has become compacted and intermixed with ependymal cells. This process probably plays an important role during proliferation or early neuronal differentiation. The cerebrospinal fluid may contain factors that are required for mitosis or early stages of differentiation. Interestingly, most of the mitotic cells in this study were exposed to the cerebrospinal fluid and had endocytic vesicles (Fig. 6) on their surface, which suggests internalization of substances present in the ventricular lumen.
The position of the cell with respect to the ventricular cavity may also allow segregation of factors within the cytoplasm that are important for cell differentiation (Chenn and McConnell, 1995). Work inDrosophila (Doe, 1996) and the developing mammalian brain (Chenn and McConnell, 1995; Zhong et al., 1996) suggests that early stages in neuronal differentiation may occur by the segregation of transcription factors during mitosis. The plane of mitosis may be related to this segregation process (Chenn and McConnell, 1995). The spindles of mitotic cells studied here were oriented mainly tangentially (parallel) to the ventricle. The large number of Type A cells observed at longer survival times indicates that many of these mitoses must have generated daughter cells undergoing differentiation. Although this observation suggests that tangential mitosis of precursors in the adult avian brain VZ generates differentiated progeny, we cannot exclude the possibility that small angular variations in the plane of mitosis occurred, resulting in the differential segregation of signals within the cytoplasm of these cells.
Neurogenesis versus ependymal function
Ependymal cells in canaries showed morphology similar to the simple ependymal cells described previously in mammals (Tennyson and Pappas, 1962; Peters et al., 1991). Canary ependymal cells were not labeled by multiple injections of [3H]-thymidine or at any of the survival times studied after a single injection of this nucleoside. Our observations are consistent with the notion that ependymal cells do not proliferate (Sarnat, 1995). This suggests that ependymal cells are not the population of proliferating cells that gives rise to new neurons. Because no labeled ependymal cells were observed at longer survivals after single or multiple [3H]-thymidine injections, we also infer that under normal conditions the progeny of Type B cells do not differentiate into ependymal cells.
Ependymal cells shared the walls of the lateral ventricle with Type B cells. As discussed above, Type B cells are organized and behave similarly to those of the embryonic neuroepithelium. Clearly the adult germinal epithelium retained the functional and anatomical characteristics of a VZ. The fact that ependymal cells were also present in the same epithelium with Type B cells suggests that ependymal functions and neurogenesis can coexist. Our results indicate that these two functions are segregated among two different kinds of cells within the same epithelial layer.
Our study concentrated on the VZ of the lateral wall of the lateral ventricle at the level of the anterior commissure. Our ultrastructural analysis included three distinct regions of this wall. Differences in cell composition and cell types may occur at other rostrocaudal levels of the telencephalon not included in this study. Preliminary observations indicate that the VZ in other regions of the telencephalon also contained the three main cell types described here (not shown). The criteria for cell identification established by the present study should aid future work in determining regional variation of the VZ in the developing and adult avian brain. In particular, the characteristic multiple cilia in ependymal cells versus a single short cilium characteristic of Type B cells may allow the identification (e.g., by scanning EM) of patches of neuronal stem cells in the walls of the brain ventricles.
Conclusion
The present study has determined the architecture of the adult canary VZ (summarized in Fig. 12) and has identified the primary precursors as Type B cells (blue). These cells are organized as a pseudostratified epithelium and maintain an end foot on the ventricular surface. Similar to the neuroepithelial cells in the embryo, Type B cells moved toward the ventricle to undergo mitosis. Type B cells gave rise to Type A cells (red) that move away from the ventricular surface and become oriented parallel to this wall. These cells seemed to correspond to young migrating neurons. Ependymal cells (gray) shared the ventricular surface with Type B cells but did not divide. Our results revealed the dynamic nature of the VZ and have provided the morphological basis for identification of neuronal precursors in the VZ of an adult vertebrate brain.
Fig. 12.
Schematic summary of results. Three main cell types comprise the VZ of adult canaries: Type A (red), Type B (blue), and ependymal cells (gray). Ependymal cells are multiciliated and do not divide. Type B cells are pseudostratified, and all contact the ventricle that has a single short cilium on this lumen. These cells are the primary precursors. Their nuclei migrate from basal to apical VZ to round up and divide adjacent to the ventricular cavity (bottom). Type A cells do not contact the ventricle, are oriented largely parallel to the ventricular surface, and have characteristics of young migrating neurons. Results suggest that Type A cells are derived from Type B cells and may move tangentially to the VZ before radial migration.
Footnotes
This work was supported by National Institutes of Health Grants HD32116 and NS 24478. We thank Jose G. Baltazar and Marie Therese Merchant for technical assistance, and Fernando Nottebohm for providing the birds for this study. We are also grateful to Fiona Doetsch, Steven Goldman, and S. Rasika for their comments on this manuscript.
A.A.-B. and J.M. G.-V. contributed equally to this work.
Correspondence should be addressed to Dr. Alvarez-Buylla, The Rockefeller University Field Research Center, Tyrrel Road, Millbrook, New York 12545.
REFERENCES
- 1.Alvarez-Buylla A, Nottebohm F. Migration of young neurons in adult avian brain. Nature. 1988;335:353–354. doi: 10.1038/335353a0. [DOI] [PubMed] [Google Scholar]
- 2.Alvarez-Buylla A, Vicario DS. Simple microcomputer system for mapping tissue sections with the light microscope. J Neurosci Methods. 1988;25:165–173. doi: 10.1016/0165-0270(88)90155-0. [DOI] [PubMed] [Google Scholar]
- 3.Alvarez-Buylla A, Buskirk DR, Nottebohm F. Monoclonal antibody reveals radial glia in adult avian brain. J Comp Neurol. 1987;264:159–170. doi: 10.1002/cne.902640203. [DOI] [PubMed] [Google Scholar]
- 4.Alvarez-Buylla A, Kirn JR, Nottebohm F. Birth of projection neurons in adult avian brain may be related to perceptual or motor learning. Science. 1990a;249:1444–1446. doi: 10.1126/science.1698312. [DOI] [PubMed] [Google Scholar]
- 5.Alvarez-Buylla A, Theelen M, Nottebohm F. Proliferation “hot spots” in adult avian ventricular zone reveal radial cell division. Neuron. 1990b;5:101–109. doi: 10.1016/0896-6273(90)90038-h. [DOI] [PubMed] [Google Scholar]
- 6.Alvarez-Buylla A, Ling C-Y Ling, Yu WS. Contribution of neurons born during embryonic juvenile and adult life to the brain of adult canaries: regional specificity and delayed birth of neurons in the song control nuclei. J Comp Neurol. 1994;347:233–248. doi: 10.1002/cne.903470207. [DOI] [PubMed] [Google Scholar]
- 7.Barami K, Kirschenbaum B, Lemmon V, Goldman SA. N-cadherin and Ng-CAM/8D9 are involved serially in the migration of newly generated neurons into the adult songbird brain. Neuron. 1994;13:567–582. doi: 10.1016/0896-6273(94)90026-4. [DOI] [PubMed] [Google Scholar]
- 8.Barami K, Iversen K, Furneaux H, Goldman SA. Hu protein as an early marker of neuronal phenotypic differentiation by subependymal zone cells of the adult songbird forebrain. J Neurobiol. 1995;28:82–101. doi: 10.1002/neu.480280108. [DOI] [PubMed] [Google Scholar]
- 9.Boulder Committee. Embryonic vertebrate central nervous system: revised terminology. Anat Rec. 1970;166:257–262. doi: 10.1002/ar.1091660214. [DOI] [PubMed] [Google Scholar]
- 10.Bruni JE, Del Bigio MR, Clattenburg RE. Ependyma: normal and pathological. A review of the literature. Brain Res Rev. 1985;9:1–19. doi: 10.1016/0165-0173(85)90016-5. [DOI] [PubMed] [Google Scholar]
- 11.Chenn A, McConnell SK. Cleavage orientation and the asymmetric inheritance of Notch1 immunoreactivity in mammalian neurogenesis. Cell. 1995;82:631–641. doi: 10.1016/0092-8674(95)90035-7. [DOI] [PubMed] [Google Scholar]
- 12.Clark SJ, Cynx J, Alvarez-Buylla A, O’Loughlin B, Nottebohm F. On variables that affect estimates of the true sizes and densities of radioactively labeled cell nuclei. J Comp Neurol. 1990;301:114–122. doi: 10.1002/cne.903010111. [DOI] [PubMed] [Google Scholar]
- 13.Cupedo RNJ, de Weerd H. Tanycytes in the medial habenular nucleus of the rat. Anat Embryol. 1985;172:7–10. doi: 10.1007/BF00318938. [DOI] [PubMed] [Google Scholar]
- 14.Del Brio MA, Riera P, García JM, Alvarez-Uría M. Cell types of the third ventricle wall of the rabbit (Oryctolagus cuniculus). J Submicrosc Cytol Pathol. 1991;23:147–157. [PubMed] [Google Scholar]
- 15.Doe CQ. Asymmetric cell division and neurogenesis. Curr Opin Genet Dev. 1996;6:562–566. doi: 10.1016/s0959-437x(96)80084-0. [DOI] [PubMed] [Google Scholar]
- 16.Doetsch F, Garcia-Verdugo JM, Alvarez-Buylla A. Cellular composition and three-dimensional organization of the subventricular germinal zone in the adult mammalian brain. J Neurosci. 1997;17:5046–5061. doi: 10.1523/JNEUROSCI.17-13-05046.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Easter SS. Postnatal neurogenesis and changing connections. Trends Neurosci. 1983;6:53–56. [Google Scholar]
- 18.Fujita S. Mitotic pattern and histogenesis of the central nervous system. Nature. 1960;185:702–703. doi: 10.1038/185702a0. [DOI] [PubMed] [Google Scholar]
- 19.Goldman SA, Nottebohm F. Neuronal production, migration, and differentiation in a vocal control nucleus of the adult female canary brain. Proc Natl Acad Sci USA. 1983;80:2390–2394. doi: 10.1073/pnas.80.8.2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goldman SA, Zukhar A, Barami K, Mikawa T, Niedzwiecki D. Ependymal/subependymal zone cells of postnatal and adult songbird brain generate both neurons and nonneuronal siblings in vitro and in vivo. J Neurobiol. 1996;30:505–520. doi: 10.1002/(SICI)1097-4695(199608)30:4<505::AID-NEU6>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 21.Gould E, Cameron HA. Regulation of neuronal birth, migration and death in the rat dentate gyrus. Dev Neurosci. 1996;18:22–35. doi: 10.1159/000111392. [DOI] [PubMed] [Google Scholar]
- 22.Gray GE, Sanes JR. Lineage of radial glia in the chicken optic tectum. Development. 1992;114:271–283. doi: 10.1242/dev.114.1.271. [DOI] [PubMed] [Google Scholar]
- 23.Hinds JW, Ruffett TL. Cell proliferation in the neural tube: an electron microscopic and golgi analysis in the mouse cerebral vesicle. Z Zellforsch. 1971;115:226–264. doi: 10.1007/BF00391127. [DOI] [PubMed] [Google Scholar]
- 24.Jacobson M. Developmental neurobiology. Plenum; New York: 1991. [Google Scholar]
- 25.Kirschenbaum B, Nedergaard M, Preuss A, Barami K, Fraser RAR, Goldman SA. In vitro neuronal production and differentiation by precursor cells derived from the adult human forebrain. Cereb Cortex. 1994;6:576–589. doi: 10.1093/cercor/4.6.576. [DOI] [PubMed] [Google Scholar]
- 26.Levitt PR, Cooper ML, Rakic P. Coexistence of neuronal and glial precursor cells in the cerebral ventricular zone of the fetal monkey: an ultrastructural immunoperoxidase analysis. J Neurosci. 1981;1:27–39. doi: 10.1523/JNEUROSCI.01-01-00027.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ling C, Zuo M, Alvarez-Buylla A, Cheng M-F. Neurogenesis in juvenile and adult ring doves. J Comp Neurol. 1997;379:300–312. [PubMed] [Google Scholar]
- 28.Lois C, Garcia-Verdugo JM, Alvarez-Buylla A. Chain migration of neuronal precursors. Science. 1996;271:978–981. doi: 10.1126/science.271.5251.978. [DOI] [PubMed] [Google Scholar]
- 29.Lopez-Garcia C. Postnatal neurogenesis and regeneration in the lizard cerebral cortex. In: Cuello C, editor. Neuronal cell death and repair. Elsevier; Amsterdam: 1993. pp. 237–246. [Google Scholar]
- 30.Millhouse OE. Light and electron microscopic studies of the ventricular wall. Z Zellforsch. 1972;127:149–174. doi: 10.1007/BF00306799. [DOI] [PubMed] [Google Scholar]
- 31.Misson JP, Edwards MA, Yamamoto M, Caviness VS., Jr Mitotic cycling of radial glial cells of the fetal murine cerebral wall: a combined autoradiographic and immunohistochemical study. Dev Brain Res. 1988;38:183–190. doi: 10.1016/0165-3806(88)90043-0. [DOI] [PubMed] [Google Scholar]
- 32.Nottebohm F. Neuronal replacement in adulthood. In: Nottebohm F, editor. Hope for a new neurology, Vol 457. New York Academy of Sciences; New York: 1985. pp. 143–161. [DOI] [PubMed] [Google Scholar]
- 33.Nottebohm F, Alvarez-Buylla A. Neurogenesis and neuronal replacement in adult birds. In: Cuello AC, editor. Neuronal cell death and repair. Elsevier; Amsterdam: 1993. pp. 227–236. [Google Scholar]
- 34.Paton JA, Nottebohm F. Neurons generated in the adult brain are recruited into functional circuits. Science. 1984;225:1046–1048. doi: 10.1126/science.6474166. [DOI] [PubMed] [Google Scholar]
- 35.Peters E, Palay SL, Webster H de F. The fine structure of the nervous system: neurons and their supporting cells. Oxford UP; New York: 1991. [Google Scholar]
- 36.Rafols JA, Goshgarian HG. Spinal tanycytes in the adult rat: a correlative golgi gold-toning study. Anat Rec. 1985;211:75–86. doi: 10.1002/ar.1092110112. [DOI] [PubMed] [Google Scholar]
- 37.Sarnat HB. Ependymal reactions to injury. A review. J Neuropathol Exp Neurol. 1995;54:1–15. doi: 10.1097/00005072-199501000-00001. [DOI] [PubMed] [Google Scholar]
- 38.Sauer FC. Mitosis in the neural tube. J Comp Neurol. 1935;62:377–405. [Google Scholar]
- 39.Sidman RL, Miale IL, Feder N. Cell proliferation and migration in the primitive ependymal zone: an autoradiographic study of histogenesis in the nervous system. Exp Neurol. 1959;1:322–333. doi: 10.1016/0014-4886(59)90024-x. [DOI] [PubMed] [Google Scholar]
- 40.Sotelo JR, Trujillo-Cenóz O. Electron microscope study of the development of ciliary components of the neural epithelium of the chick embryo. Z Zellforsch. 1958;49:1–12. doi: 10.1007/BF00335059. [DOI] [PubMed] [Google Scholar]
- 41.Stensaas LJ, Stensaas S. An electron microscopy study in the matrix and intermediate laminae of the cerebral hemisphere of the 45 mm rabbit embryo. Z Zellforsch. 1968;91:341–365. doi: 10.1007/BF00440763. [DOI] [PubMed] [Google Scholar]
- 42.Takahashi T, Nowakowski RS, Caviness VS., Jr Cell cycle parameters and patterns of nuclear movement in the neocortical proliferative zone of the fetal mouse. J Neurosci. 1993;13:820–833. doi: 10.1523/JNEUROSCI.13-02-00820.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Takahashi T, Nowakowski RS, Caviness VS., Jr The leaving or Q fraction of the murine cerebral proliferative epithelium: a general model of neocortical neuronogenesis. J Neurosci. 1996;16:6183–6196. doi: 10.1523/JNEUROSCI.16-19-06183.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Takahashi T, Nowakowski RS, Caviness VS., Jr The mathematics of neocortical neuronogenesis. Dev Neurosci. 1997;19:17–22. doi: 10.1159/000111179. [DOI] [PubMed] [Google Scholar]
- 45.Tennyson VM, Pappas GD. An electron microscope study of ependymal cells of the fetal, early postnatal and adult rabbit. Z Zellforsch. 1962;56:595–618. doi: 10.1007/BF00540584. [DOI] [PubMed] [Google Scholar]
- 46.Thomaidou D, Mione MC, Cavanagh JFR, Parnavelas JG. Apoptosis and its relation to the cell cycle in the developing cerebral cortex. J Neurosci. 1997;17:1075–1085. doi: 10.1523/JNEUROSCI.17-03-01075.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhong WM, Feder JN, Jiang MM, Jan LY, Jan YN. Asymmetric localization of a mammalian numb homolog during mouse cortical neurogenesis. Neuron. 1996;17:43–53. doi: 10.1016/s0896-6273(00)80279-2. [DOI] [PubMed] [Google Scholar]




