Abstract
Objective
We examined whether plasma β-amyloid (Aβ)42/Aβ40, as measured by a high-precision assay, accurately diagnosed brain amyloidosis using amyloid PET or CSF p-tau181/Aβ42 as reference standards.
Methods
Using an immunoprecipitation and liquid chromatography–mass spectrometry assay, we measured Aβ42/Aβ40 in plasma and CSF samples from 158 mostly cognitively normal individuals that were collected within 18 months of an amyloid PET scan.
Results
Plasma Aβ42/Aβ40 had a high correspondence with amyloid PET status (receiver operating characteristic area under the curve [AUC] 0.88, 95% confidence interval [CI] 0.82–0.93) and CSF p-tau181/Aβ42 (AUC 0.85, 95% CI 0.79–0.92). The combination of plasma Aβ42/Aβ40, age, and APOE ε4 status had a very high correspondence with amyloid PET (AUC 0.94, 95% CI 0.90–0.97). Individuals with a negative amyloid PET scan at baseline and a positive plasma Aβ42/Aβ40 (<0.1218) had a 15-fold greater risk of conversion to amyloid PET-positive compared to individuals with a negative plasma Aβ42/Aβ40 (p = 0.01).
Conclusions
Plasma Aβ42/Aβ40, especially when combined with age and APOE ε4 status, accurately diagnoses brain amyloidosis and can be used to screen cognitively normal individuals for brain amyloidosis. Individuals with a negative amyloid PET scan and positive plasma Aβ42/Aβ40 are at increased risk for converting to amyloid PET-positive. Plasma Aβ42/Aβ40 could be used in prevention trials to screen for individuals likely to be amyloid PET-positive and at risk for Alzheimer disease dementia.
Classification of evidence
This study provides Class II evidence that plasma Aβ42/Aβ40 levels accurately determine amyloid PET status in cognitively normal research participants.
Alzheimer disease (AD) is the most common cause of dementia in older adults.1 A key neuropathologic feature of AD is extracellular amyloid plaques comprising β-amyloid (Aβ) peptides including lengths of 42 and 40 amino acids (Aβ42 and Aβ40, respectively). CSF levels of Aβ42, total tau (t-tau), and phosphorylated tau181 (p-tau) are well-established biomarkers of AD brain pathology,2 but their assessment requires a lumbar puncture. Amyloid PET scans are also well-validated, but use radiation, are costly, and have limited availability.3–6 Earlier AD drug trials recruited participants with a clinical syndrome of AD dementia, but approximately 25% of study participants did not have detectable brain amyloidosis.7 Recent AD drug trials have used CSF biomarkers and amyloid PET to screen potential participants for brain amyloidosis.6,8–10
A blood-based biomarker would enable more rapid and inexpensive screening of potential participants, particularly for prevention trials, where rates of negative amyloid PET scans are approximately 70%.11 Recent reports have demonstrated that high-precision assays for plasma Aβ42/Aβ40 are strongly predictive of brain amyloidosis.12,13 In this study, we evaluate the diagnostic accuracy of an immunoprecipitation mass spectrometry (IPMS) plasma Aβ42/Aβ40 assay for brain amyloidosis. Further, we evaluate the ability of plasma Aβ42/Aβ40 to predict conversion from amyloid PET-negative to amyloid PET-positive and the stability of plasma Aβ42/Aβ40 over time. Finally, we examine how the use of plasma Aβ42/Aβ40 combined with age and APOE ε4 status decreases or eliminates the number of confirmatory tests required to select a cohort with brain amyloidosis.
Methods
Participants
The study cohort represents a convenience sample. Participants enrolled in longitudinal studies of memory and aging at Washington University who underwent plasma collection within 18 months of an amyloid PET scan were considered for inclusion based on plasma availability. Because the IPMS assay used 1.6 mL of plasma, samples were selected for which the biorepository had relatively large amounts of plasma available as determined by the biorepository core leader. Participants of all ages and diagnoses were included, but the biorepository had greater availability of plasma from younger and cognitively normal participants. All participants underwent clinical assessments that included the Clinical Dementia Rating (CDR)14 and Mini-Mental State Examination.15 APOE genotype was obtained from the Knight Alzheimer Disease Research Center (ADRC) Genetics Core.16
Standard protocol approvals, registrations, and patient consents
All procedures were approved by the Washington University Human Research Protection Office, and written informed consent was obtained from each participant.
Plasma and CSF collection and processing
CSF was collected as previously described.17 Participants underwent lumbar puncture at 8 am following overnight fasting. Twenty to thirty milliliters of CSF was collected in a 50-mL polypropylene tube via gravity drip using an atraumatic Sprotte 22-G spinal needle. The tube was inverted gently to disrupt potential gradient effects and centrifuged at low speed to pellet any cellular debris. The CSF was then aliquoted into polypropylene tubes and stored at −80°C. CSF Aβ42, t-tau, and p-tau181 were measured with the corresponding Elecsys immunoassays on the Roche (Basel, Switzerland) cobas e601 analyzer.18
At the same session as CSF collection, blood was drawn into two 10-mL syringes precoated with 0.5 M EDTA, then transferred to two 15-mL polypropylene tubes containing 120 μL 0.5 M EDTA. The samples were kept on wet ice until centrifugation (<2 hours) to separate plasma from blood cells. The plasma was then transferred to a single 50-mL polypropylene tube, gently mixed, aliquoted into polypropylene tubes, and stored at −80°C.
Immunoprecipitation of Aβ38, Aβ40, and Aβ42
Targeted Aβ isoforms (Aβ38, Aβ40, and Aβ42) were simultaneously immunoprecipitated from 1.6 mL of plasma or 0.5 mL of CSF via a monoclonal anti-Aβ mid-domain antibody (HJ5.1, anti-Aβ13-28) conjugated to M-270 Epoxy Dynabeads (Invitrogen, Carlsbad, CA).19 Samples were added to 380 μL of a master mix containing 5.26X protease inhibitor cocktail (Roche), 0.263% (w/v) Tween-20, 2.63X phosphate-buffered saline, and 2.63 M guanidine. Plasma samples were spiked with 20 μL of a solution containing 3.75 pg/μL 12C15N-Aβ38, 25 pg/μL 12C15N-Aβ40, and 2.5 pg/μL 12C15N-Aβ42 (labeled peptides from rPeptide, Athens, GA) in 4:1 0.1% ammonium hydroxide:acetonitrile while CSF samples were spiked with 20 μL of a solution containing 75 pg/μL 12C15N-Aβ38, 500 pg/μL 12C15N-Aβ40, and 50 pg/μL 12C15N-Aβ42 in 4:1 0.1% ammonium hydroxide:acetonitrile. All subsequent immunoprecipitation steps were performed as previously described.12
Liquid chromatography–mass spectrometry
Plasma analyses were performed as previously described.12 CSF analyses were performed on a Waters (Milford, MA) Xevo TQ-S triple quadrupole mass spectrometer interfaced with a Waters nanoAcquity chromatography system. For CSF analyses, extracted digests were reconstituted with 50 µL of 20 nM BSA Digest (Pierce, Appleton, WI) in 10% formic acid/10% acetonitrile. A 4.5 μL aliquot of each reconstituted digest was loaded via direct injection onto a Waters 100 × 0.075 mm Acquity M-class HSS T3 column in 10% acetonitrile/2% dimethyl sulfoxide (DMSO)/0.1% formic acid with a flow rate of 600 nL/min for 12 minutes. After loading, peptides were resolved using an 8-minute linear gradient at 400 nL/min from 10% acetonitrile/2% DMSO/0.1% formic acid to 50% acetonitrile/2% DMSO/0.1% formic acid. The initial gradient was followed by a steeper linear gradient to 65% acetonitrile/2% DMSO/0.1% formic acid over 2 minutes at 400 nL/min. The column was then washed with 95% acetonitrile/2%DMSO/0.1% formic acid for 5 minutes at 400 nL/min. Finally, the column was equilibrated back to initial solvent conditions for 5 minutes at 600 nL/min.
Analysis of mass spectrometry data
Peptides derived from human Aβ contained amino acids with the naturally occurring 14Nitrogen (14N) isotope, while peptides derived from the exogenous Aβ spiked into samples as a standard contained amino acids that were uniformly labeled with the 15Nitrogen (15N) isotope. The precursor/product ion pairs utilized were chosen as previously described12,19 and the derived integrated peak areas were analyzed using the Skyline software package.20 For each isotopomer (14N or 15N) of the Aβ isoforms (Aβ38, Aβ40, or Aβ42), integrated peak areas for selected product ions were summed. The Aβ42 concentration was calculated as follows: the sum of the integrated peak areas for the product ions derived from the 14N isotopomer for Aβ42 divided by the sum of the integrated peak areas for the product ions derived from the 15N isotopomer for Aβ42 multiplied by the concentration of spiked Aβ42 15N internal standard. The Aβ40 concentration was calculated with the same approach. The final Aβ42/Aβ40 ratio was obtained by dividing the calculated Aβ42 concentration by the calculated Aβ40 concentration.
All mass spectrometry and quality control analyses were performed prior to sample unblinding. Values that failed quality control were not used if they did not meet threshold criteria for sample preparation (missing/mishandled samples), signal intensity, chromatographic properties (peak width/shape), coefficient of variation (technical replicates), and mass spectral noise. Three percent of plasma and CSF samples failed the quality control (QC) protocol and were dropped from the study.
Plasma was collected from a cognitively normal young individual and an older individual known to have brain amyloidosis for use as high and low QC calibrators, respectively. The high and low QC calibrators, along with intermediate mixes of the high and low QC calibrators, were run with every batch of plasma samples. Raw plasma Aβ42/Aβ40 ratio values were normalized to the QC calibrators using linear regression to minimize batch-to-batch variability (see appendix e-1, doi.org/10.5061/dryad.hr45320). This normalization was planned a priori because of batch effects that were observed in unpublished experiments. Although high and low QC calibrators were also run with CSF samples, no significant batch-to-batch variability was noted and therefore no normalization was performed.
Amyloid PET imaging
Amyloid PET was used as the primary reference standard for amyloidosis because it is a well-established biomarker that is widely used in clinical trials for assessment of brain amyloid burden.5,6,8 Participants underwent a dynamic scan with either 11C Pittsburgh compound B (PiB) or 18F AV45. PiB PET imaging was performed with a Siemens 962 HR + ECAT PET or Biograph mCT scanner (Siemens/CTI, Knoxville, KY). AV45 PET imaging was performed with a Siemens Biograph mMR scanner (Siemens/CTI). Structural MRI using magnetization-prepared rapid gradient echo T1-weighted images was acquired at 3T and processed using FreeSurfer 5.321 (freesurfer.net/) to derive cortical and subcortical regions of interest.22 Regional data from the 40- to 60-minutes postinjection window for PiB and the 50- to 70-minutes window for AV45 were converted to standardized uptake value ratios (SUVRs) using cerebellar gray as a reference and partial volume-corrected using a regional spread function approach.23 Values from the left and right lateral orbitofrontal, medial orbitofrontal, precuneus, rostral middle frontal, superior frontal, superior temporal, and middle temporal cortices were averaged together to represent a mean cortical SUVR. Amyloid PET positivity was defined a priori with the established cutoffs of >1.42 for PiB24 and >1.22 for AV45.25 Amyloid PET Centiloid was used to combine PiB and AV45 data on a similar scale.26,27
Statistical analyses
Characteristics of amyloid PET-positive and PET-negative groups were compared using Student t tests for continuous variables and χ2 tests or Fisher exact tests for categorical variables. Receiver operating characteristic (ROC) analyses were performed to evaluate the ability of either plasma or CSF Aβ42/Aβ40 to diagnose amyloid PET status and were implemented with PROC LOGISTIC. Positive percent agreement (PPA) was defined as the percent of amyloid PET-positive individuals who were positive by a given plasma or CSF Aβ42/Aβ40 value. Negative percent agreement (NPA) was defined as the percent of amyloid PET-negative individuals who were negative by a given plasma or CSF Aβ42/Aβ40 value. The Youden Index for each potential plasma or CSF Aβ42/Aβ40 value was calculated as the PPA plus the NPA minus 1 and the value with the maximum Youden Index was selected as the cutoff value. For high areas under the curve (AUCs) (>0.90), we used the Wilson28 score interval for calculation of confidence intervals (CIs).
Because amyloid PET Centiloid values were not normally distributed, Spearman correlations were used to evaluate the relationship between amyloid PET Centiloid and plasma or CSF Aβ42/Aβ40 values. Analysis of covariance with plasma or CSF Aβ42/Aβ40 as the outcome variable and centered age (age = the mean age for the cohort of 63.7 years), APOE ε4 status, and sex as predictors were implemented with PROC GLM. Models predicting last amyloid PET status for initially amyloid PET-negative individuals using baseline plasma or CSF Aβ42/Aβ40 status and follow-up time were implemented in PROC LOGISTIC with exact estimates because there were relatively few amyloid PET converters.29 For individuals with more than one plasma sample, the intraindividual annual rate of change was computed and group differences were compared with one-way analysis of variance (ANOVA) and Tukey multiple comparisons tests.
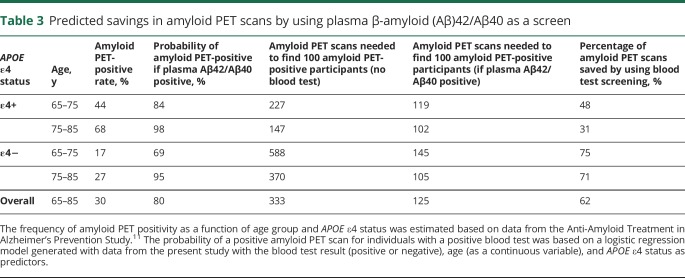
For calculation of predicted savings in number of amyloid PET scans by screening with plasma Aβ42/Aβ40, the frequency of amyloid PET positivity as a function of age group and APOE ε4 status was estimated based on data from the Anti-Amyloid Treatment in Alzheimer's (A4) Prevention Study.11 The calculations assume that 35% of participants were APOE ε4 carriers, 76% were age 56–75 years old, and 24% were 75–85 years old. The probability of a positive amyloid PET scan for individuals with a positive blood test was based on a logistic regression model generated with data from the present study with the blood test result (positive or negative), age (as a continuous variable), and APOE ε4 status as predictors.
Statistical analyses were conducted using SAS 9.4 (SAS Institute Inc., Cary, NC). Plots were created with GraphPad Prism version 7.04 (GraphPad Software, La Jolla, CA). Heat maps were generated with the R ggplot2 package. A p value <0.05 was considered statistically significant.
Data availability statement
Data in the study will be deposited in the Washington University Knight ADRC dataset, which will be shared by request from any qualified investigator upon approval by the Knight ADRC data request committee.
Results
Participants
A total of 210 plasma samples from 158 individuals were analyzed (see table 1 for participant characteristics) by IPMS. The SD for measurements of both Aβ42 and Aβ40 was 1 pg/mL and the coefficient of variation (CV) for the plasma assay was 5% for Aβ42, 0.6% for Aβ40, and 4% for Aβ42/Aβ40 (see appendix e-2, doi.org/10.5061/dryad.hr45320). A total of 186 available CSF samples collected the same day as plasma from 145 individuals were assayed for Aβ42/Aβ40 by IPMS. Data on CSF Aβ42, t-tau, and p-tau, as measured by Elecsys immunoassays, were available for 152 individuals.
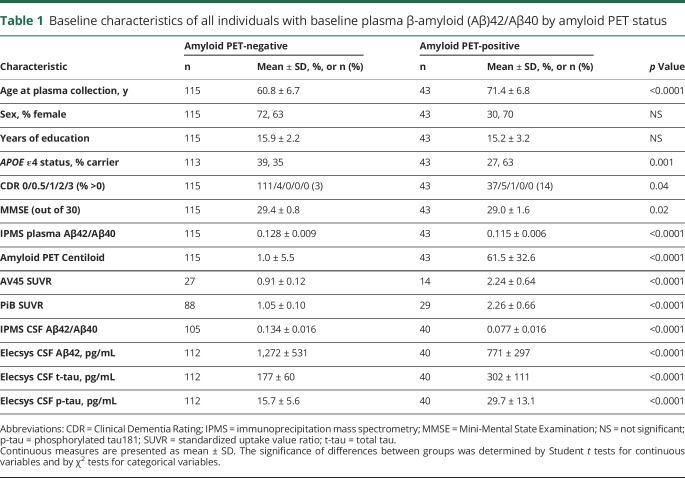
Table 1.
Baseline characteristics of all individuals with baseline plasma β-amyloid (Aβ)42/Aβ40 by amyloid PET status
An amyloid PET scan performed within 18 months of the baseline plasma sample was negative for 115 individuals and positive for 43 individuals. The average interval between the plasma collection and the amyloid PET scan was 0.26 ± 0.35 years (mean ± SD) with a range of 0–1.5 years. The baseline age range extended from 46.1 to 86.9 years. Compared to amyloid PET-negative individuals, individuals who were amyloid PET-positive were older (71.4 ± 6.8 vs 60.8 ± 6.7 years, p < 0.0001), were more likely to carry an APOE ε4 allele (63% vs 35%, p = 0.001), were more likely to have cognitive impairment as demonstrated by a CDR greater than 0 (14% vs 3%, p = 0.04), and had lower CSF Aβ42 and higher CSF t-tau and p-tau (p < 0.0001) by Elecsys immunoassays.
Correspondence of baseline plasma and CSF Aβ42/Aβ40 to baseline amyloid PET
Individuals with a positive amyloid PET at baseline had a significantly lower baseline plasma Aβ42/Aβ40 compared to individuals with a negative amyloid PET at baseline (0.115 ± 0.006 vs 0.128 ± 0.009, p < 0.0001) (figure 1A). ROC analysis demonstrated that baseline plasma Aβ42/Aβ40 was a good predictor of baseline amyloid PET status, with an AUC of 0.88 (95% CI 0.82–0.93) (figure 1C). The cohort represented a wide age range, but the performance of the assay was similar in a subcohort of individuals (n = 101) older than 60 years (AUC 0.87, 95% CI 0.80–0.94). A plasma Aβ42/Aβ40 cutoff of <0.1218 was considered positive and had the maximum Youden Index with a PPA of 0.88 (95% CI 0.75–0.96) and an NPA of 0.76 (95% CI 0.67–0.83) with amyloid PET status (figure 1C). Baseline plasma Aβ42/Aβ40 was inversely correlated with amyloid PET on the continuous Centiloid scale (figure 1E), with a Spearman ρ of −0.55 (95% CI −0.65 to −0.43). Baseline plasma Aβ40 was weakly correlated with amyloid PET Centiloid (Spearman ρ of 0.29, 95% CI 0.13–0.43), while plasma Aβ42 was not significantly correlated with amyloid PET Centiloid (figure e-1, A and B, doi.org/10.5061/dryad.hr45320).
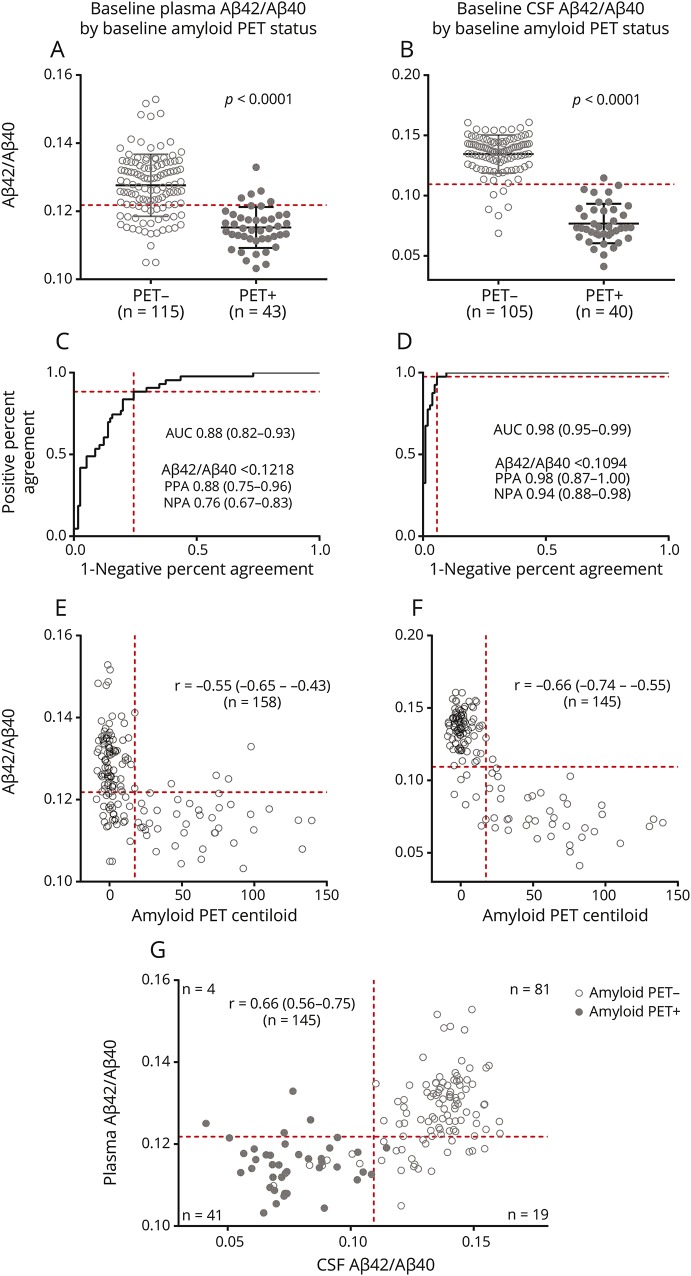
Figure 1. Correspondence of baseline plasma and CSF β-amyloid (Aβ)42/Aβ40 with baseline amyloid PET.
Baseline (A) plasma and (B) CSF Aβ42/Aβ40 were decreased in baseline amyloid PET-positive individuals. Receiver operating characteristic analyses demonstrate that baseline plasma (C) and CSF (D) Aβ42/Aβ40 were predictive of baseline amyloid PET status. The area under the curve is noted with 95% confidence intervals (CIs). For the cutoffs listed, the positive percent agreement and negative percent agreement is provided with 95% CIs. Baseline (E) plasma and (F) CSF Aβ42/Aβ40 were inversely correlated with baseline amyloid PET binding as measured on the Centiloid scale. (G) Baseline plasma and CSF Aβ42/Aβ40 were correlated. The Spearman ρ (r) is noted with 95% CIs for (E–G). Dashed red lines depict cutoffs for plasma or CSF Aβ42/Aβ40 based on the maximum Youden Index (A–G) or, for amyloid PET Centiloid, the established cutoff for amyloid PET positivity (E and F).
CSF p-tau/Aβ42 as measured by the Elecsys platform was chosen as an alternative reference standard for brain amyloidosis because this measure has the highest correspondence with amyloid PET of the established CSF biomarkers and better distinguishes amyloid PET status than Aβ42 alone (figure e-2, doi.org/10.5061/dryad.hr45320).18,30 For plasma Aβ42/Aβ40, the AUC was 0.85 (95% CI 0.79–0.92) for a CSF Elecsys p-tau/Aβ42 cutoff of 0.019818 and 0.85 (95% CI 0.78–0.92) for a cutoff of 0.0220.30
As expected, baseline CSF Aβ42/Aβ40 as measured by IPMS was lower in individuals with a positive amyloid PET at baseline (figure 1B). The concordance between CSF Aβ42/Aβ40 and amyloid PET was nearly perfect (figure 1D), with an AUC of 0.98 (95% CI 0.95–0.99). A CSF Aβ42/Aβ40 cutoff of <0.1094 was considered positive and had the maximum Youden Index with a PPA of 0.98 (95% CI 0.87–1.0) and an NPA of 0.94 (95% CI 0.88–0.98). Baseline CSF Aβ42/Aβ40 was inversely correlated with amyloid PET Centiloid (figure 1F), with a Spearman ρ of −0.66 (95% CI −0.74 to −0.55). Similar inverse correlations between plasma and CSF Aβ42/Aβ40 and amyloid PET were obtained when the 2 tracers used, PiB and AV45, were evaluated separately (figure e-3, doi.org/10.5061/dryad.hr45320).
Baseline plasma and CSF Aβ42/Aβ40 were correlated (Spearman ρ of 0.66, 95% CI 0.56–0.75) (figure 1G). Using the cutoffs described herein, plasma and CSF Aβ42/Aβ40 had concordant predictions for amyloid status in 122 of 145 individuals (84%). All individuals with both a high (negative) CSF and plasma Aβ42/Aβ40 were amyloid PET-negative (n = 81). A total of 35 of 41 individuals (85%) with both a low (positive) plasma and CSF Aβ42/Aβ40 were amyloid PET-positive, and 6 (15%) were PET-negative. A total of 18 of 19 individuals (95%) with a positive plasma Aβ42/Aβ40 but negative CSF Aβ42/Aβ40 were amyloid PET-negative. Four individuals with a negative plasma Aβ42/Aβ40 but positive CSF Aβ42/Aβ40 were amyloid PET-positive. Plasma Aβ42 and Aβ40 were not individually significantly correlated with CSF Aβ42 and Aβ40, respectively (figure e-1, C and D, doi.org/10.5061/dryad.hr45320).
Relationship between plasma or CSF Aβ42/Aβ40 and age, APOE ε4 status, and sex
Baseline plasma Aβ42/Aβ40 was lower with older age (p < 0.0001) and was lower in APOE ε4 carriers (p < 0.0001) and men (p = 0.002) (figure 2A and table 2). Each decade of age, APOE ε4 carrier status, and male sex was associated with lower plasma Aβ42/Aβ40 levels by ∼0.005 (for comparison, the difference between plasma Aβ42/Aβ40 in amyloid PET-positive and PET-negative individuals was ∼0.012). In models for baseline plasma Aβ42/Aβ40, there was no significant interaction between age and APOE ε4 status, age, and sex, or APOE ε4 status and sex. Baseline CSF Aβ42/Aβ40 was lower with older age and was lower in APOE ε4 carriers (both p < 0.0001) (figure 2B and table 2). In contrast to plasma Aβ42/Aβ40, CSF Aβ42/Aβ40 did not vary by sex.
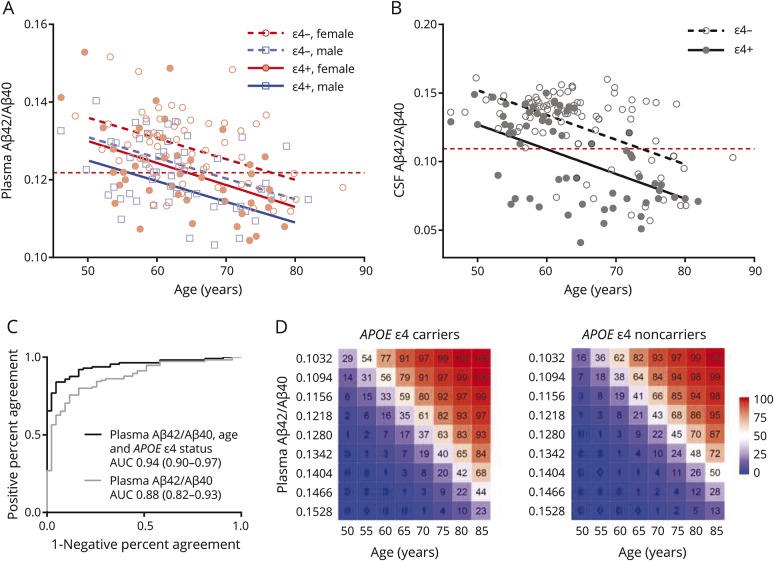
Figure 2. Relationship of age, APOE ε4 status, and sex with baseline plasma and CSF β-amyloid (Aβ)42/Aβ40.
(A) Baseline plasma Aβ42/Aβ40 was lower with older age and was lower in APOE ε4 carriers and men. (B) Baseline CSF Aβ42/Aβ40 was lower with older age and was lower in APOE ε4 carriers. Horizontal dashed red lines depict cutoffs for plasma or CSF Aβ42/Aβ40. Sloped lines represent the estimated Aβ42/Aβ40 as a function of age for the cross-sectional groups. (C) Receiver operating characteristic analysis demonstrated a trend towards a higher area under the curve (AUC) for prediction of amyloid PET status when age and APOE ε4 status were included in the model. The AUC is noted with 95% confidence intervals. (D) The combination of plasma Aβ42/Aβ40, age, and APOE ε4 status was used to predict the likelihood of amyloid PET positivity.
Table 2.
Relationship between plasma or CSF β-amyloid (Aβ)42/Aβ40 and age, APOE ε4 status, and sex

Adding age and APOE ε4 status to a model for prediction of amyloid PET status by plasma Aβ42/Aβ40 improved the AUC from 0.88 (95% CI 0.82–0.93) to 0.94 (95% CI 0.90–0.97) (figure 2C). Both age (p < 0.001) and APOE ε4 status (p < 0.03) were significant predictors in this model. When added to the model, sex was not a significant predictor, likely because the model already correctly classified nearly all participants and sex did not improve classification of the remaining few discordant cases. The combination of plasma Aβ42/Aβ40, age, and APOE ε4 status were used to predict the likelihood of amyloid PET positivity (figure 2D).
Prediction of amyloid PET conversion
A subcohort of 100 individuals underwent at least 1 amyloid PET scan >1.5 years following their baseline plasma sample (for subcohort characteristics, see table e-1, doi.org/10.5061/dryad.hr45320). For all individuals in this subcohort, the average interval between the baseline plasma collection and last amyloid PET scan was 3.9 ± 1.4 years with a range of 1.9–9.0 years. A logistic regression model that included follow-up time from plasma collection to the last amyloid PET scan found that plasma Aβ42/Aβ40 was a good predictor of amyloid PET status at the last amyloid PET scan (AUC 0.88, 95% CI 0.81–0.95). A total of 94 of the 100 individuals in the subcohort with longitudinal amyloid PET data also had matched CSF samples that underwent analysis by IPMS. A similar model found that CSF Aβ42/Aβ40 was an excellent predictor of amyloid PET status at the last amyloid PET scan (AUC 0.96, 95% CI 0.92–0.98).
In the subcohort with longitudinal amyloid PET data, 74 were amyloid PET-negative at baseline; 8 converted to amyloid PET-positive over the follow-up period while 66 remained amyloid PET-negative. The amyloid PET converters had lower baseline plasma Aβ42/Aβ40 than the individuals who remained amyloid PET-negative (0.117 ± 0.008 vs 0.128 ± 0.009, respectively, p < 0.01 by Student t test; see table e-1, doi.org/10.5061/dryad.hr45320, and figure 3, A and C). A logistic regression model that included follow-up time demonstrated that amyloid PET-negative individuals with a positive plasma Aβ42/Aβ40 (<0.1218) had a 15-fold increased risk of conversion to amyloid PET-positive compared to individuals with a negative plasma Aβ42/Aβ40 (p = 0.01 by exact test, figure 3E). Sixty-eight of the 74 individuals with longitudinal amyloid PET data who were amyloid PET-negative at baseline had matched CSF samples with Aβ42/Aβ40 by IPMS. The amyloid PET converters with CSF data (n = 7) had a lower baseline CSF Aβ42/Aβ40 compared to the 61 individuals who remained amyloid PET-negative (0.110 ± 0.014 vs 0.136 ± 0.016, respectively, p < 0.001 by Student t test; see table e-1, doi.org/10.5061/dryad.hr45320, and figure 3, B and D). Amyloid PET-negative individuals with positive CSF Aβ42/Aβ40 (<0.1094) had a 21-fold increased risk of conversion to amyloid PET-positive compared to individuals with a negative plasma Aβ42/Aβ40 (p = 0.03 by exact test, figure 3F).
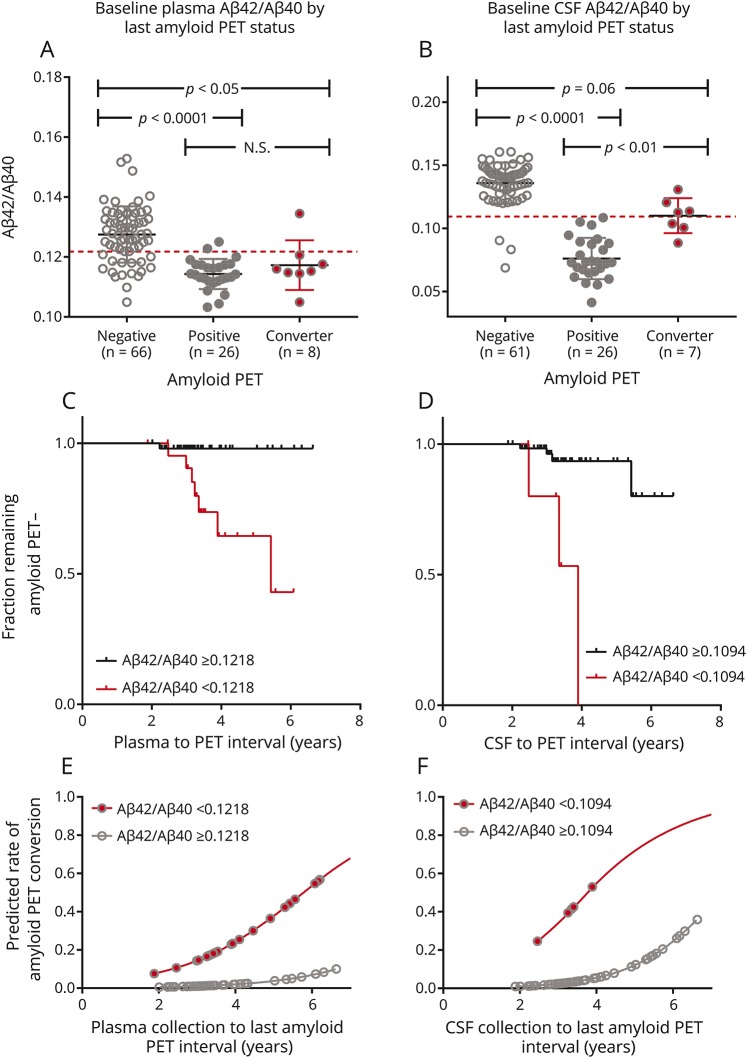
Figure 3. Baseline plasma and CSF β-amyloid (Aβ)42/Aβ40 predict amyloid PET status conversion.
(A) Individuals who were amyloid PET-negative at baseline and converted to amyloid PET-positive over the follow-up period had lower baseline plasma Aβ42/Aβ40 than individuals who remained amyloid PET-negative. (B) There was also a trend towards lower baseline CSF Aβ42/Aβ40 in amyloid PET converters vs nonconverters. Dashed red lines depict cutoffs for plasma or CSF Aβ42/Aβ40. A one-way analysis of variance was significant for both A and B at p < 0.0001 and the results of Tukey multiple comparison tests are shown in the plots. The fraction of individuals remaining amyloid PET-negative by plasma or CSF Aβ42/Aβ40 status is depicted (C, D). For individuals who remained amyloid PET-negative, the tick marks represent the time of the last negative amyloid PET scan. For individuals who converted to amyloid PET-positive, the tick marks represent the time of the first positive amyloid PET scan. Individuals who were amyloid PET-negative at baseline with a positive plasma Aβ42/Aβ40 had a 15-fold greater risk of conversion to amyloid PET-positive compared to individuals with a negative plasma Aβ42/Aβ40, p = 0.01 (E). Individuals who were amyloid PET-negative at baseline with a positive CSF Aβ42/Aβ40 had a 21-fold greater risk of conversion to amyloid PET-positive compared to individuals with a negative CSF Aβ42/Aβ40, p = 0.03 (F). For E and F, the prediction model was truncated at 7 years.
Amyloid PET converters had a significantly higher baseline amyloid PET Centiloid value compared to individuals who remained amyloid PET-negative (6.9 ± 4.7 vs −0.5 ± 4.0, p < 0.0001), suggesting amyloid PET converters had below-threshold brain amyloidosis. One individual classified as an amyloid PET converter with negative plasma and CSF Aβ42/Aβ40 at both the first and last time points had Elecsys CSF biomarkers that were inconsistent with brain amyloidosis (at the last time point CSF Aβ42 was 1,434 pg/mL, t-tau was 193 pg/mL, and p-tau was 17.5 pg/mL), suggesting the last amyloid PET scan may have been false-positive.
Longitudinal change in plasma and CSF Aβ42/Aβ40
A subcohort of 50 individuals had longitudinal plasma Aβ42/Aβ40 collected within 18 months of a longitudinal amyloid PET scan (figure 4; see table e-2, doi.org/10.5061/dryad.hr45320, for participant characteristics), allowing examination of intraindividual rate of change in plasma Aβ42/Aβ40. For all participants in this subcohort, the average interval between the first and last plasma collections was 3.6 ± 1.2 years with a range of 1.9–7.1 years. Thirty-nine of these individuals also had CSF samples that were analyzed for Aβ42/Aβ40 by IPMS. The intraindividual rate of change for each participant was estimated. There was a significant decline in both plasma (−0.0011/y) and CSF Aβ42/Aβ40 (−0.0023/y) over time (p < 0.001 and p < 0.0001 by 1-sample t test, respectively). There was no difference in the rate of change of plasma Aβ42/Aβ40 by amyloid PET group (one-way ANOVA was not significant; figure 4C). However, amyloid PET converters had a faster decline in CSF Aβ42/Aβ40 compared to individuals who were amyloid PET-positive both at baseline and the last amyloid PET scan (p < 0.05 for one-way ANOVA, p < 0.05 for Tukey post hoc test; figure 4D).
Figure 4. Longitudinal change in plasma and CSF β-amyloid (Aβ)42/Aβ40.
Both (A) plasma and (B) CSF Aβ42/Aβ40 declined within individuals over time. Thin lines connect values within an individual. The bolded rates are the average rates of change for the entire longitudinal cohort and are represented by thick black lines. Dashed red lines depict cutoffs for plasma or CSF Aβ42/Aβ40 based on the analyses shown in figure 1. The rates of change for plasma and CSF Aβ42/Aβ40 for each individual were determined by linear regression and the slopes were plotted. One-sample t tests were used to determine whether the rates of change were significantly different from zero. The rate of change for plasma Aβ42/Aβ40 did not vary significantly by amyloid PET group (C). Amyloid PET converters had a faster decline in CSF Aβ42/Aβ40 compared to individuals who were amyloid PET-positive at both first and last time points (D). Dashed red lines depict a slope of zero (no change). Dotted lines are the average rate of change by for the entire longitudinal cohort.
Utility of plasma Aβ42/Aβ40 as a screening test
The value of using plasma Aβ42/Aβ40 to screen for individuals with a high risk of brain amyloidosis was evaluated (table 3). The frequency of amyloid PET positivity as a function of age group and APOE ε4 status was based on data from the A4 Prevention Study, which included cognitively normal individuals aged 65–85 years.11 The probability of a positive amyloid PET scan for individuals with a positive blood test was based on a logistic regression model generated with data from the present study. By screening individuals with a positive plasma Aβ42/Aβ40, fewer confirmatory amyloid PET scans would be required to obtain a cohort of 100 individuals with a positive amyloid PET scan. The percentage of amyloid PET scans saved by first screening participant with plasma Aβ42/Aβ40 was highest in APOE ε4 noncarriers and younger individuals. For a cohort similar to A4, screening participants with plasma Aβ42/Aβ40 could reduce the number of amyloid PET scans required by approximately 62%.
Table 3.
Predicted savings in amyloid PET scans by using plasma β-amyloid (Aβ)42/Aβ40 as a screen
Discussion
This study provides Class II evidence that plasma Aβ42/Aβ40, as measured by an immunoprecipitation and liquid chromatography–mass spectrometry assay, accurately diagnoses brain amyloidosis.31 It has previously been shown that individuals with brain amyloidosis experience a decline in cognitive performance and a high rate of progression to AD dementia.32–34 In this study cohort, which comprised almost exclusively cognitively normal individuals (94% with a CDR = 0), we found good performance of plasma Aβ42/Aβ40 for detection of brain amyloidosis (ROC AUC 0.88), suggesting that plasma Aβ42/Aβ40 may be used as a screening tool for those at risk of AD dementia. Moreover, we found that individuals with a positive plasma Aβ42/Aβ40 but negative amyloid PET scan have a 15-fold higher risk of converting to amyloid PET-positive (p = 0.01). The sensitivity of the plasma Aβ42/Aβ40 assay to amyloid PET-negative individuals who convert to amyloid PET-positive suggests that plasma Aβ42/Aβ40 becomes positive earlier than the established amyloid PET threshold used for this study. Therefore, a positive plasma Aβ42/Aβ40 with a negative amyloid PET scan may represent early amyloidosis rather than a false-positive result in some individuals. In addition, we found that plasma and CSF Aβ42/Aβ40 declined within individuals over time, likely reflecting accumulation of brain amyloid in some participants. Overall, our results demonstrate that plasma Aβ42/Aβ40, as measured by a high-precision assay, could accurately detect brain amyloidosis in AD prevention drug trials that recruit cognitively normal research participants.
Many studies over the last 2 decades have evaluated plasma Aβ42 as a biomarker for AD, typically using immunoassays with relatively high variance and uncertain specificity, and overall found poor and inconsistent performance.35 The ratio of plasma Aβ42 to Aβ40, as measured by high-precision assays, has previously been shown by our group and others to have a high correspondence to brain amyloidosis.12,13 Plasma Aβ42/Aβ40 may have higher concordance with amyloidosis than plasma Aβ42 and Aβ40 separately because this ratio may normalize for preanalytical variability36 or differences in Aβ levels related to circadian rhythms37 or other biological variation not related to brain amyloidosis.
The study cohort included many more participants than our previous study12 and found that the difference in plasma Aβ42/Aβ40 between amyloid PET-positive and PET-negative individuals was small (0.128 ± 0.009 vs 0.115 ± 0.006, ∼11%), but highly significant when measured with the IPMS assay, which has a CV for plasma Aβ42/Aβ40 of 4% (the CV was 5% for Aβ42% and 0.6% for Aβ40). This small difference in plasma Aβ42/Aβ40 would likely not be reliably measured by standard plate-based ELISAs, which have a CV for plasma Aβ42 and Aβ40 ranging from ∼6% to 24%.38 The high accuracy of the IPMS assay in detecting brain amyloidosis is likely due to the high precision of mass spectrometry as an assay platform including the direct measurement of multiple specific Aβ species. Also, measuring both Aβ42 and Aβ40 in the same sample at the same time may reduce the variability introduced by measuring analytes with 2 separate assays. These factors may also explain why CSF Aβ42/Aβ40 as measured by a similar IPMS assay had such exceptionally high concordance with amyloid PET (AUC 0.98) in this study.
We found that plasma Aβ42/Aβ40 levels were significantly associated with age, APOE ε4 status, and sex. Recent studies using lower precision assays have found that plasma Aβ42/Aβ40 as measured by ELISA was associated with age and APOE ε4 status39 and that models including age and APOE ε4 status better predict amyloid status,40 but it is unclear whether these studies examined the relationship between sex and plasma Aβ42/Aβ40 levels. Interestingly, CSF Aβ42/Aβ40 levels were modulated by age and APOE ε4 status, but not sex. This dissociation suggests that plasma and CSF Aβ42/Aβ40 levels may be influenced by different factors. Other studies have explored factors that may modify plasma Aβ42/Aβ40,39,41,42 but further studies using high-precision Aβ42/Aβ40 assays and larger cohorts are needed to clearly define these factors. Knowledge of factors that modify plasma Aβ42/Aβ40 can be used to improve models for prediction of brain amyloidosis. We found that a model for prediction of amyloid PET status including plasma Aβ42/Aβ40, age, and APOE ε4 status reached an AUC of 0.94. Current CSF biomarker tests exhibit approximately this level of correspondence with amyloid PET,18,30 suggesting that plasma Aβ42/Aβ40, especially when combined with other factors, may be accurate enough for clinical use at some point.
The most immediate use of the plasma Aβ42/Aβ40 assay is screening for brain amyloidosis in potential participants for AD drug trials. Age and APOE ε4 status could be used to improve the accuracy of the screen. If the plasma Aβ42/Aβ40 screen were positive, then a confirmatory test such as amyloid PET or CSF biomarkers could be performed, depending on the needs of the study. The plasma Aβ42/Aβ40 screen would significantly reduce the number of confirmatory tests required to select a cohort of research participants with brain amyloidosis, especially in the case of prevention trials, which recruit cognitively normal individuals who have a relatively low rate of brain amyloidosis. We estimate that for a prevention trial similar to A4,11 prescreening with plasma Aβ42/Aβ40 would reduce the number of amyloid PET scans required by 62%, resulting in substantially reduced time and costs for recruitment. If the plasma Aβ42/Aβ40 test combined with age and APOE ε4 status continues to demonstrate very high accuracy in diagnosis of brain amyloidosis (AUC of ∼0.95), a single blood test including plasma Aβ42/Aβ40 and APOE genotype may be used for study inclusion without a need for confirmatory amyloid PET or CSF. We expect that even after correction for covariates, a small number of individuals will have false-positive or false-negative results based on plasma Aβ42/Aβ40 caused by variations in preanalytical conditions, imprecision in the assay, or biological variation. It is possible that amyloid PET or CSF biomarkers are slightly more accurate in detection of brain amyloidosis than plasma Aβ42/Aβ40. Future studies with the gold standard of AD neuropathology are needed to determine the true correspondence of brain amyloidosis with plasma Aβ42/Aβ40, CSF Aβ42/Aβ40, and amyloid PET. It remains to be determined whether testing with plasma Aβ42/Aβ40 alone vs plasma Aβ42/Aβ40 followed by confirmatory testing with amyloid PET or CSF biomarkers results in clinically significant differences, especially considering the additional burdens and costs associated with the confirmatory tests.
This study fits into phases 2 and 3 (out of 5 phases) for validation of plasma Aβ42/Aβ40 as a biomarker for AD, as it evaluates the ability of plasma Aβ42/Aβ40 to detect early AD (in most of the study participants, asymptomatic brain amyloidosis) and explores the effects of covariates on plasma Aβ42/Aβ40 levels.43 A limitation of this study is that we used amyloid PET and CSF biomarkers as the reference standard for brain amyloidosis, rather than the true gold standard of neuropathology, because most of the study participants are still alive. A limitation of all assays for plasma Aβ42 and Aβ40 is that a certified reference standard does not currently exist that could be used to standardize absolute values. Finally, an important limitation of this study is that the cohort was designed to evaluate the correspondence of plasma Aβ42/Aβ40 with brain amyloidosis, not symptomatic AD, and was not powered to evaluate the relationship between plasma Aβ42/Aβ40 and cognitive impairment. More comprehensive studies are currently underway to further validate this assay in multiple large international cohorts, including cohorts that will evaluate the relationship between plasma Aβ42/Aβ40 and symptomatic AD, which will help to assess the clinical utility of this assay. If further validated, this assay will accelerate progress towards an effective therapy for AD by decreasing the time, cost, and risk of drug trials, and one day enable a blood test in the clinic to identify patients who could benefit from disease-modifying treatment.
Acknowledgment
The authors thank the research volunteers who participated in the studies from which these data were obtained and their families; and the Clinical, Biomarker, and Imaging Cores at the Knight Alzheimer's Disease Research Center for participant evaluation and sample and data collection.
Glossary
- A4 Prevention Study
Anti-Amyloid Treatment in Alzheimer's Prevention Study
- Aβ
β-amyloid
- AD
Alzheimer disease
- ANOVA
analysis of variance
- AUC
area under the curve
- CDR
Clinical Dementia Rating
- CI
confidence interval
- CV
coefficient of variation
- DMSO
dimethyl sulfoxide
- IPMS
immunoprecipitation mass spectrometry
- NPA
negative percent agreement
- p-tau
phosphorylated tau181
- PiB
Pittsburgh compound B
- PPA
positive percent agreement
- QC
quality control
- ROC
receiver operating characteristic
- SUVR
standardized uptake value ratio
Appendix. Authors

Footnotes
Study funding
This study was supported by Anonymous Foundation (R.J. Bateman, PI), Alzheimer's Association Zenith grant (R.J. Bateman, PI), and National Institute on Aging grants NIH R56AG061900 (R.J. Bateman, PI), P01AG026276, P01AG03991, and P50AG05681 (J.C. Morris, PI). Additional support for imaging was provided by the Barnes Jewish Hospital Foundation and NIH P30NS098577, R01EB009352, and UL1TR000448. For Florbetapir F18 (AV45) imaging, doses and partial financial support were provided by Eli Lilly/Avid Radiopharmaceuticals. S.E. Schindler is supported by K23AG053426. B.A. Gordon is supported by K01AG053474 and the Barnes Jewish Hospital Foundation Willman Scholar Fund.
Disclosure
S. Schindler has an immediate family member who recently owned stock in Eli Lilly. J. Bollinger has submitted the US provisional patent application “Plasma Based Methods for Detecting CNS Amyloid Deposition” as a coinventor. V. Ovod has submitted the US provisional patent application “Plasma Based Methods for Detecting CNS Amyloid Deposition” as a coinventor. K. Mawuenyega receives royalty income based on technology (methods for simultaneously measuring the in vivo metabolism of 2 or more isoforms of a biomolecule, and blood plasma assay) licensed by Washington University to C2N Diagnostics. He has submitted the US provisional patent application “Plasma Based Methods for Detecting CNS Amyloid Deposition” as a coinventor. Y. Li and B. Gordon report no disclosures relevant to the manuscript. D. Holtzman cofounded and is on the scientific advisory board of C2N Diagnostics. Washington University and Dr. Holtzman have equity ownership interest in C2N Diagnostics and receive royalty income based on technology (stable isotope labeling kinetics and blood plasma assay) licensed by Washington University to C2N Diagnostics. He receives income from C2N Diagnostics for serving on the scientific advisory board. He is on the scientific advisory board of Denali and Proclara. His laboratory receives research support from AbbVie, Denali, and C2N Diagnostics. Neither J. Morris nor his family owns stock or has equity interest (outside of mutual funds or other externally directed accounts) in any pharmaceutical or biotechnology company. Dr. Morris is currently participating in clinical trials of antidementia drugs from Eli Lilly and Company and Biogen. He is funded by NIH grants #P50AG005681, P01AG003991, P01AG026276, and UF1AG032438. T. Benzinger receives research support from Avid Radiopharmaceuticals, Eli Lilly, and Cerveau. She has or is currently participating in clinical trials of sponsored by Janssen, Eli Lilly, Pfizer, Biogen, and Roche. She has received travel support from the American Society for Neuroradiology, the Alzheimer's Association, and the People's Republic of China. C. Xiong reports no disclosures relevant to the manuscript. A. Fagan has received research funding from Biogen, Fujirebio, and Roche Diagnostics. She is a member of the scientific advisory boards for Roche, Genentech, and AbbVie and consults for Araclon/Griffols and DiamiR. R. Bateman cofounded C2N Diagnostics. Washington University and Dr. Bateman have equity ownership interest in C2N Diagnostics and receive royalty income based on technology (stable isotope labeling kinetics and blood plasma assay) licensed by Washington University to C2N Diagnostics. He receives income from C2N Diagnostics for serving on the scientific advisory board. Washington University, with Dr. Bateman as coinventor, has submitted the US provisional patent application “Plasma Based Methods for Detecting CNS Amyloid Deposition.” He consults for Roche, Genentech, AbbVie, Pfizer, Boehringer-Ingelheim, and Merck. Go to Neurology.org/N for full disclosures.
References
- 1.Hebert LE, Weuve J, Scherr PA, Evans DA. Alzheimer disease in the United States (2010-2050) estimated using the 2010 census. Neurology 2013;80:1778–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blennow K, Hampel H, Weiner M, Zetterberg H. Cerebrospinal fluid and plasma biomarkers in Alzheimer disease. Nat Rev Neurol 2010;6:131–144. [DOI] [PubMed] [Google Scholar]
- 3.Witte MM, Foster NL, Fleisher AS, et al. Clinical use of amyloid-positron emission tomography neuroimaging: practical and bioethical considerations. Alzheimer's Demen 2015;1:358–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O'Brien JT, Herholz K. Amyloid imaging for dementia in clinical practice. BMC Med 2015;13:163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klunk WE, Engler H, Nordberg A, et al. Imaging brain amyloid in Alzheimer's disease with Pittsburgh compound-B. Ann Neurol 2004;55:306–319. [DOI] [PubMed] [Google Scholar]
- 6.Mattsson N, Carrillo MC, Dean RA, et al. Revolutionizing Alzheimer's disease and clinical trials through biomarkers. Alzheimer's Demen 2015;1:412–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karran E, Hardy J. Antiamyloid therapy for Alzheimer's disease: are we on the right road? N Engl J Med 2014;370:377–378. [DOI] [PubMed] [Google Scholar]
- 8.Sperling RA, Rentz DM, Johnson KA, et al. The A4 study: stopping AD before symptoms begin? Sci translational Med 2014;6:228fs213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carrillo MC, Brashear HR, Logovinsky V, et al. Can we prevent Alzheimer's disease? Secondary “prevention” trials in Alzheimer's disease. Alzheimer's Dement 2013;9:123–131 e121. [DOI] [PubMed] [Google Scholar]
- 10.Honig LS, Vellas B, Woodward M, et al. Trial of solanezumab for mild dementia due to Alzheimer's disease. N Engl J Med 2018;378:321–330. [DOI] [PubMed] [Google Scholar]
- 11.Sperling RA, Donohue M, Raman R, et al. The Anti-Amyloid Treatment in Asymptomatic Alzheimer's Disease (A4) study: report of screening data results. Alzheimer's Dement 2018;14:P215–P216. [Google Scholar]
- 12.Ovod V, Ramsey KN, Mawuenyega KG, et al. Amyloid beta concentrations and stable isotope labeling kinetics of human plasma specific to central nervous system amyloidosis. Alzheimer's Dement 2017;13:841–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakamura A, Kaneko N, Villemagne VL, et al. High performance plasma amyloid-beta biomarkers for Alzheimer's disease. Nature 2018;554:249–254. [DOI] [PubMed] [Google Scholar]
- 14.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology 1993;43:2412–2414. [DOI] [PubMed] [Google Scholar]
- 15.Folstein MF, Folstein SE, McHugh PR. Mini-mental state: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–198. [DOI] [PubMed] [Google Scholar]
- 16.Pastor P, Roe CM, Villegas A, et al. Apolipoprotein Eepsilon4 modifies Alzheimer's disease onset in an E280A PS1 kindred. Ann Neurol 2003;54:163–169. [DOI] [PubMed] [Google Scholar]
- 17.Fagan AM, Mintun MA, Mach RH, et al. Inverse relation between in vivo amyloid imaging load and cerebrospinal fluid Abeta42 in humans. Ann Neurol 2006;59:512–519. [DOI] [PubMed] [Google Scholar]
- 18.Schindler SE, Gray JD, Gordon BA, et al. Cerebrospinal fluid biomarkers measured by Elecsys assays compared to amyloid imaging. Alzheimer's Dement 2018;14:1460–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mawuenyega KG, Kasten T, Sigurdson W, Bateman RJ. Amyloid-beta isoform metabolism quantitation by stable isotope-labeled kinetics. Anal Biochem 2013;440:56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pino LK, Searle BC, Bollinger JG, Nunn B, MacLean B, MacCoss MJ. The Skyline ecosystem: informatics for quantitative mass spectrometry proteomics. Mass Spectrom Rev Epub 2017 Jul 9. [DOI] [PMC free article] [PubMed]
- 21.Fischl B, van der Kouwe A, Destrieux C, et al. Automatically parcellating the human cerebral cortex. Cereb Cortex 2004;14:11–22. [DOI] [PubMed] [Google Scholar]
- 22.Su Y, D'Angelo GM, Vlassenko AG, et al. Quantitative analysis of PiB-PET with FreeSurfer ROIs. PLoS One 2013;8:e73377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Su Y, Blazey TM, Snyder AZ, et al. Partial volume correction in quantitative amyloid imaging. Neuroimage 2015;107:55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vlassenko AG, McCue L, Jasielec MS, et al. Imaging and cerebrospinal fluid biomarkers in early preclinical Alzheimer disease. Ann Neurol 2016;80:379–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mishra S, Gordon BA, Su Y, et al. AV-1451 PET imaging of tau pathology in preclinical Alzheimer disease: defining a summary measure. Neuroimage 2017;161:171–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klunk WE, Koeppe RA, Price JC, et al. The Centiloid Project: standardizing quantitative amyloid plaque estimation by PET. Alzheimer's Dement 2015;11:1–15 e11-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Su Y, Flores S, Hornbeck RC, et al. Utilizing the Centiloid scale in cross-sectional and longitudinal PiB PET studies. Neuroimage Clin 2018;19:406–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wilson E. Probable inference, the law of succession, and statistical inference. J Am Stat Assoc 1927;22:209–212. [Google Scholar]
- 29.Hirji K, Mehta C, Patel N. Computing distributions for exact logistic regression. J Am Stat Assoc 1987;82:1110–1117. [Google Scholar]
- 30.Hansson O, Seibyl J, Stomrud E, et al. CSF biomarkers of Alzheimer's disease concord with amyloid-beta PET and predict clinical progression: a study of fully automated immunoassays in BioFINDER and ADNI cohorts. Alzheimer's Dement 2018;14:1470–1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gross RA, Johnston KC. Levels of evidence: taking neurology to the next level. Neurology 2009;72:8–10. [DOI] [PubMed] [Google Scholar]
- 32.Vos SJ, Xiong C, Visser PJ, et al. Preclinical Alzheimer's disease and its outcome: a longitudinal cohort study. Lancet Neurol 2013;12:957–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Donohue MC, Sperling RA, Petersen R, Sun CK, Weiner MW, Aisen PS. Association between elevated brain amyloid and subsequent cognitive decline among cognitively normal persons. JAMA 2017;317:2305–2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morris JC, Roe CM, Grant EA, et al. Pittsburgh compound B imaging and prediction of progression from cognitive normality to symptomatic Alzheimer disease. Arch Neurol 2009;66:1469–1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Olsson B, Lautner R, Andreasson U, et al. CSF and blood biomarkers for the diagnosis of Alzheimer's disease: a systematic review and meta-analysis. Lancet Neurol 2016;15:673–684. [DOI] [PubMed] [Google Scholar]
- 36.Willemse E, van Uffelen K, Brix B, Engelborghs S, Vanderstichele H, Teunissen C. How to handle adsorption of cerebrospinal fluid amyloid beta (1-42) in laboratory practice? Identifying problematic handlings and resolving the issue by use of the Abeta42/Abeta40 ratio. Alzheimer's Dement 2017;13:885–892. [DOI] [PubMed] [Google Scholar]
- 37.Bateman RJ, Wen G, Morris JC, Holtzman DM. Fluctuations of CSF amyloid-beta levels: implications for a diagnostic and therapeutic biomarker. Neurology 2007;68:666–669. [DOI] [PubMed] [Google Scholar]
- 38.Okereke OI, Xia W, Irizarry MC, et al. Performance characteristics of plasma amyloid-beta 40 and 42 assays. J Alzheimers Dis 2009;16:277–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nakamura T, Kawarabayashi T, Seino Y, et al. Aging and APOE-epsilon4 are determinative factors of plasma Abeta42 levels. Ann Clin Transl Neurol 2018;5:1184–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Verberk IMW, Slot RE, Verfaillie SCJ, et al. Plasma amyloid as prescreener for the earliest Alzheimer pathological changes. Ann Neurol 2018;84:648–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Toledo JB, Vanderstichele H, Figurski M, et al. Factors affecting Abeta plasma levels and their utility as biomarkers in ADNI. Acta Neuropathol 2011;122:401–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Toledo JB, Shaw LM, Trojanowski JQ. Plasma amyloid beta measurements: a desired but elusive Alzheimer's disease biomarker. Alzheimers Res Ther 2013;5:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Frisoni GB, Boccardi M, Barkhof F, et al. Strategic roadmap for an early diagnosis of Alzheimer's disease based on biomarkers. Lancet Neurol 2017;16:661–676. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data in the study will be deposited in the Washington University Knight ADRC dataset, which will be shared by request from any qualified investigator upon approval by the Knight ADRC data request committee.