Highlights
* Review of behavioral and neuroimaging literature on biological motion processing in autism. * Discussion of social brain mechanisms and their disruption in autism. * Presentation of neuroendophenotypes of autism from an fMRI task of biological motion perception. * Exploration of the diagnostic utility of brain responses to biological motion.
Keywords: Autism, Biological motion, Neuroendophenotypes, Diagnostic utility
Abstract
Disruptions in the visual perception of biological motion are emerging as a hallmark of autism spectrum disorder (ASD), consistent with the pathognomonic social deficits of this neurodevelopmental disorder. Accumulating evidence suggests an early and marked divergence in ASD from the typical developmental tuning of brain regions to process social information. In this review, we discuss a relatively recent yet substantial literature of behavioral and neuroimaging studies that consistently indicates impairments in biological motion perception in ASD. We then illustrate the fundamental disruption in this form of social perception in autism, drawing connections between a genetic liability to develop autism and disrupted associated brain mechanisms, as we describe neuroendophenotypes of autism derived from an fMRI study of biological motion perception in children with autism and their unaffected siblings. Finally, we demonstrate the diagnostic utility of brain responses to biological motion. With the ability to measure brain function in the first year of life comes the potential to chart the development of disrupted biological motion processing in ASD and to specify the gene–brain–behavior interactions shaping this atypical trajectory. We propose that a comprehensive understanding of the development of impaired responses to biological motion in ASD can inform future diagnosis and treatment approaches.
1. Introduction
As inherently social creatures, we typically code human movement as something ‘special’. As such, modern social vision theories hold that the human visual system analyzes people differently than objects (Adams et al., 2010). Typical observers exhibit robust sensitivity to the movements of those around us (e.g., Blake and Shiffrar, 2007); such biological motion cues include eye movements, facial expressions and full body gestures and actions. Disruptions in the visual perception of biological motion are emerging as a hallmark of autism spectrum disorder (ASD), consistent with the pathognomonic social deficits of this neurodevelopmental disorder. Indeed, individuals with impaired social function, such as children and adults with autism spectrum disorder (ASD), exhibit impaired visual sensitivity to (e.g., Blake et al., 2003, Kaiser et al., 2010a; Table 1) and atypical brain mechanisms (e.g., Kaiser et al., 2010b, Freitag et al., 2008; Table 2) for processing the actions of other people. Studies of this disruption highlight and inform our understanding of a core aspect of this neurodevelopmental disorder, namely qualitative social impairments. Evidence from behavioral and neuroimaging studies is accumulating to indicate that individuals with ASD exhibit early and marked divergence from the typical tuning to process social information. Here, we consider the rapidly expanding literature on biological motion perception in ASD and suggest that the continued pursuit of a comprehensive understanding of the developmental trajectory of this disruption, as well as a specification of the gene–brain–behavior interactions that shape this atypical trajectory, has great potential to inform future diagnostic and treatment approaches. We address five key questions in this review: (1) What is the relationship between the visual perception of biological motion and the broader domain of social perception? (2) What are the neuroanatomical substrates of social perception? (3) What is the behavioral evidence for disrupted biological motion perception in autism? (4) What are the neural mechanisms underlying disrupted biological motion perception in autism? And (5) what is the diagnostic utility of neuroimaging studies of biological motion perception in autism?
Table 1.
Summary of psychophysical studies of biological motion perception with point-light stimuli. Group Diff = group differences, where (+) indicates a finding of distinct performance between individuals with and without ASD and (−) indicates equivalent performance between groups.
| Article | Motion | ASD participants | Results | Group Diff |
|---|---|---|---|---|
| Moore et al. (1997) | Human body and objects | CA: 11–19 | Disrupted emotion perception n.s.: action perception |
+, − |
| Blake et al. (2003) | Human body actions | CA: 8–10 | Decreased sensitivity to coherent human motion | + |
| Klin et al. (2003) | Human body actions with sounds | Mean CA: 2 | No preference for canonical human motion | + |
| Hubert et al. (2007) | Human body and objects | CA: 15–34 | Disrupted emotion perception n.s.: action perception |
+, − |
| Parron et al. (2008) | Human body and objects | CA: 7–18 | Disrupted emotion perception n.s.: action perception |
+, − |
| Klin and Jones (2008) | Human body actions with sounds | 15 month old infant | No preference for canonical human motion | + |
| Klin et al. (2009) | Human body actions with sounds | Mean CA: 2 | No preference for canonical human motion | + |
| Atkinson (2009) | Human body actions and emotions | Mean CA: 31 | Disrupted action and emotion recognition | + |
| Murphy et al. (2009) | Human gait | Mean CA: 26 | n.s.: both groups faster RT for normal vs. scrambled walkers | − |
| Kaiser et al. (2010a) | Human body and objects | Mean CA: 20 | No elevated sensitivity to human motion | + |
| Saygin et al. (2010) | Human gait and objects | Mean CA: 34 | n.s.: perceptual thresholds between groups | − |
| Koldewyn et al. (2010) | Human gait, coherent form and motion | Mean CA: 15 | Disrupted biological motion perception | + |
| Annaz et al. (2010) | Human gait, coherent form and motion | Mean CA: 8 | Disrupted biological motion perception | + |
Table 2.
Summary of fMRI studies of biological motion perception with point-light stimuli. IPL = inferior parietal lobule, vlPFC = ventrolateral prefrontal cortex, pSTS = posterior superior temporal sulcus, vmPFC = ventromedial prefrontal cortex, and FG = fusiform gyri.
| Article | Motion | ASD participants | Results |
|---|---|---|---|
| Herrington et al. (2007) | Human gait | Mean CA: 28 | Hypoactivation in superior temporal gyrus, angular gyrus |
| Freitag et al. (2008) | Human gait | Mean CA: 18 | Atypical response in superior temporal areas, insulae, IPL |
| Kaiser et al. (2010b) | Human body actions | CA: 4–17 | Hypoactivation in vlPFC, amygdala, pSTS, vmPFC, FG |
2. What is the relationship between the perception of biological motion and the broader domain of social perception?
Social interaction and communication relies upon the accurate perception and interpretation of other people's actions. As such, humans have evolved highly efficient systems for social perception to survive and thrive in the social world. Social perception refers to the initial stages in the processing of biological motion cues (e.g., eye, face and whole body movements) that culminate in the accurate analysis of the psychological states, motives, and intentions of other individuals (Allison et al., 2000). Studies of the visual perception of biological motion commonly use point-light stimuli (for review see Blake and Shiffrar, 2007) which are created by attaching markers or point-lights to a person's body and head and then recording that person's movements so that only the point-lights are visible (Johansson, 1973). The resultant displays isolate motion information, while limiting form information, as they are best recognized as human when the dots are in motion. These stimuli are easily manipulated (i.e. inverted, scrambled, masked) for experimental purposes resulting in an expansive literature which shows that typical observers can readily detect considerable social information from point-light displays including the actor's identity (Jokisch et al., 2006, Loula et al., 2005), gender (Kozlowski and Cutting, 1977, Pollick et al., 2005), actions (Dittrich, 1993, Poizner et al., 1981), emotion (Atkinson et al., 2004, Clarke et al., 2005, Chouchourelou et al., 2006, Dittrich et al., 1996, Pollick et al., 2001), intentions (Runeson and Frykholm, 1983, Sebanz and Shiffrar, 2009), vulnerability (Gunns et al., 2002), and potential reproductive fitness (Brown et al., 2005). The social tuning of the typical observer is evident in their enhanced behavioral sensitivity to point-light displays of biological motion relative to animal (Pinto and Shiffrar, 2009) or object (Kaiser et al., 2010a) motion. It is further revealed in the enhanced neural sensitivity of systems involved in processing biological motion to point-light displays of human actions relative to those of a dog or tractor (Kaiser et al., 2010c).
Most of the above studies involved only adult participants but recent compelling evidence suggests that sensitivity to biological motion is present very early in life. Although the typical tuning to biological motion is honed through experience and develops throughout an individual's lifetime (Carter and Pelphrey, 2006, Giese and Poggio, 2003, Jastorff et al., 2009), it seems that typically developing human infants arrive into the world predisposed to attend to biological motion. For instance, Simion et al. (2008) found that 2-day-old infants, with no experience seeing hens, preferentially attended to upright point-light hen motion compared to random motion or inverted hen motion. These results indicate that infants possess an extremely early sensitivity to biological motion (see also Fox and McDaniel, 1982, Moore et al., 2007, Bertenthal and Pinto, 1994). It is unclear, though, whether a tuning to human motion is present from birth. Moreover, sensitivity to biological motion is seen in a variety of species including domestic cats (Blake, 1993), monkeys (Oram and Perrett, 1996), pigeons (Dittrich et al., 1998, Omori and Watanabe, 1996) and chicks (Vallortigara et al., 2005). Another example of an early preference for biological motion, that is independent of visual experience, comes from newly hatched chicks that have been reared in the dark (Vallortigara et al., 2005). Taken together, these studies suggest that highly efficient, early emerging, evolutionary conserved systems support biological motion perception. Notably, specialized processing of biological motion supports successful functioning in the social world as point-light displays of human movement elicit social behavior in typically developing 12-month-old infants (Yoon and Johnson, 2009) and prime perception and action in typically developed adults (Orban de Xivry et al., 2010). While evidence seems to be accumulating for an early sensitivity to biological motion that is tightly linked to social behavior, further studies are needed to confirm and fully describe the specificity of such a preference (see Méary et al., 2007) and connection between social behavior and social perception.
3. What are the neuroanatomical substrates of social perception?
A significant body of behavioral and brain research with non-human primates and humans supports the hypothesis that the human mind has evolved specialized mechanisms for perceiving and reasoning about social exchanges (Cosmides, 1989). Originally described by Brothers (1990), the “social brain” supports social perception thereby allowing us to solve problems of great adaptive significance. Consistent with Brothers’ influential model of the social brain, neuroimaging studies with humans have revealed a brain system comprised of several different neuroanatomical structures involved in social perception and social cognition including, but not limited to, the superior temporal sulcus, amygdala, orbital frontal cortex, and fusiform gyrus (e.g., Adolphs, 1999, Allison et al., 2000, Frith and Frith, 1999, Frith and Frith, 2010, Puce and Perrett, 2003). The superior temporal sulcus region, particularly the posterior aspect in the right hemisphere, analyzes biological motion cues to detect, interpret and predict the actions and intentions of others (e.g., Bonda et al., 1996, Pelphrey et al., 2005a, Pelphrey et al., 2005b, Saxe et al., 2004). The orbital frontal cortex, situated at the base of the frontal lobes, is involved in social reward, such as viewing attractive faces, and general reward processes (e.g., O’Doherty, 2004, Rolls, 2000, Rolls, 2009). The fusiform gyrus, located in the ventral occipitotemporal cortex, is involved in the identification and recognition of faces (e.g., Kanwisher et al., 1997, Puce et al., 1996). Additionally, the amygdala, a complex structure that is highly interconnected with cortical (including the superior temporal sulcus and fusiform gyrus) and other subcortical brain structures, supports the recognition of emotional states of others through analysis of facial expressions (e.g., Morris et al., 1996), the evaluation of personal space (Kennedy et al., 2009), and multiple aspects of the experience and regulation of emotion (e.g., Davis and Whalen, 2001, LeDoux, 2000). A comprehensive understanding of the interconnection of these and other neuroanatomical structures is essential to fully characterizing social brain function. Notably, all of these brain regions work in concert with each other to support distinct and overlapping aspects of social perception (e.g., Adolphs, 1999, Baron-Cohen, 1995, Frith and Frith, 2010, Heberlein et al., 2004, Pessoa and Adolphs, 2010, Puce and Perrett, 2003).
A vast literature has demonstrated the engagement of the social brain in response to point-light displays of biological motion (for review see Blake and Shiffrar, 2007, Giese and Poggio, 2003). Central to our discussion, the right posterior superior temporal sulcus plays a critical role in the perception of biological motion as evidenced by studies of lesion patients and transcranial magnetic stimulation (Grossman et al., 2005, Saygin, 2007). (Note that we acknowledge the role of several brain regions in the perception of biological motion, but focus our discussion mostly on the superior temporal sulcus region as it is strongly implicated in ASD.) Initial neuroimaging studies in our laboratory helped to establish the specificity this brain region's responses to biological motion (Pelphrey et al., 2003). In an fMRI study of typically developing young adults, we compared the response from the posterior superior temporal sulcus to four different types of motion conveyed via animated virtual-reality characters (Pelphrey et al., 2003). Participants viewed biological motion of a walking robot or human. They also viewed a nonmeaningful but complex nonbiological motion in the form of a disjointed mechanical figure as well as a complex, meaningful, and nameable nonbiological motion involving the movements of a grandfather clock. We reasoned that a region selectively responsive to biological motion should respond strongly to both the man and the robot walking, but not respond to the mechanical figure or the grandfather clock. We found strong and equivalent activity in the right posterior superior temporal sulcus to the human and robot walking and very little activity to the moving clock and the mechanical figure (Fig. 1A). This pattern of results was very different from that observed in the nearby motion-responsive visual area called MT or V5 (Zeki et al., 1991), which responded robustly to all four of our stimulus conditions (Fig. 1B). We concluded that the posterior superior temporal sulcus selectively processes biological motion. This finding led us to begin to view the posterior superior temporal sulcus as one component of the neural system supporting social perception, via its identification and representation of observed human actions as compared to the movements of other objects.
Fig. 1.
Activation within the right posterior superior temporal sulcus (A) and area MT (B) in response to biological motions and mechanical motions. There is a heightened response to the biological motion, conveyed by a walking person and a walking robot, in the right posterior superior temporal sulcus whereas the various biological and nonbiological motion stimuli elicit an equivalent response in area MT.
In further studies, we and others have shown that the posterior superior temporal sulcus region codes for the context of biological motion and is involved in analyzing the intentions of moving stimuli (e.g., Castelli et al., 2000, Pelphrey et al., 2005a, Pelphrey et al., 2005b). For example, we found that the right posterior superior temporal sulcus exhibits a differential response to biological motion that is congruent or incongruent with the actor's intention as exhibited by their facial expressions (Vander Wyk et al., 2009). These studies highlight the role of the posterior superior temporal sulcus in the visual analysis of biological motion from simple differentiation of human and mechanical motion to more complex computations such as those involved in intention understanding. These processes of action understanding are necessary first steps to function in the social world that support higher level processing such as mentalizing (see Frith and Frith, 1999). There is growing evidence that the superior temporal sulcus codes actions at a basic and abstract level and is especially influenced by the context of an action (for thoughtful discussion see Pyles and Grossman, 2011). Based on evidence from single-unit recordings in monkeys (Perrett et al., 1985) and imaging studies with humans (Grossman et al., 2010), some researchers argue that cells within the superior temporal sulcus encode actions from a basic level of body kinematics to more abstract properties, such as those seen in anthropomorphized moving shapes. In a comparison of neural activation during the perception of Heider and Simmel-like displays and point-light displays of human action, Gobbini et al. (2007) (see also Pyles, 2009) reported a high degree of overlap in the posterior superior temporal sulcus during the perception of both types of displays suggesting a role for this region in representing perceived actions and the implied intentions of those actions. The behavioral and neural results reviewed above indicate that action interpretation, for human motion in particular and social action in general, is typically encoded by the superior temporal sulcus. Importantly, although our discussion has focused on the STS region, this brain area is part of a network involved in action understanding that includes frontal regions (e.g., medial prefrontal cortex; Amodio and Frith, 2006) and limbic regions (e.g., amygdala; Bonda et al., 1996).
4. What is the behavioral evidence for disrupted biological motion perception in autism?
Studies of the visual perception of biological motion provide a window into social dysfunction in ASD, a fundamentally social disorder that is currently diagnosed based on qualitative impairments in social function and communication accompanied by rigid or stereotyped behaviors. We have previously elaborated upon a model of ASD that implicates an early and initial failure to develop the specialized brain mechanisms for social perception and cognition which results in abnormal development and the phenotypic expression of ASD (Pelphrey et al., 2011). Here, in support of this model, we review selected behavioral and brain imaging studies of biological motion perception in ASD to reflect the current knowledge base and suggest a way forward for translational social neuroscience research.
A growing number of behavioral studies have examined the visual perception of biological motion by observers with ASD. This literature points to a clear impairment in biological motion processing but provides a hazy picture of the exact nature of the deficits (see Table 1). Studies utilizing preferential looking paradigms suggest an early disruption in biological motion processing as toddlers with ASD preferentially attend to the non-social audiovisual synchronies in point-light displays of human action (Klin et al., 2009). This early inattention to biological motion may contribute to or result in diminished perceptual sensitivity in childhood. The majority of studies with children and adults report atypical performance on a variety of tasks including descriptive, direction discrimination and detection tasks. When asked to verbally describe point-light displays of biological motion, children and adults with ASD exhibit impairments in emotion perception but intact action perception (Hubert et al., 2007, Moore et al., 1997, Parron et al., 2008). While such findings may outline strengths and weaknesses in the biological motion processing profile of observers with ASD, these results might equally reflect differences in verbal abilities and/or experience between the groups. A recent paper by Atkinson (2009) described disrupted action and emotion recognition in a group of adults with ASD. In contrast to the previous studies that found specific disruptions in emotion processing, the groups in Atkinson's study were matched on age, gender and cognitive ability (IQ), suggesting that peripheral factors may explain the group differences reported in the prior studies. Indeed, several laboratories have reported deficits in biological motion processing in individuals with ASD (Blake et al., 2003, Kaiser et al., 2010a).
Recently, studies have addressed the question of whether the disrupted visual sensitivities described above are specific to human motion or to biological motion in general. Given findings of disrupted global motion processing in ASD, it is possible that the differences reported above may reflect deficits in motion processing in general rather than in biological motion per se (for review see Kaiser and Shiffrar, 2009). In a series of psychophysical tasks, members of our group compared the visual sensitivity to human, animal and object motion in individuals with ASD and matched-controls (Kaiser et al., 2010a, Kaiser and Shiffrar, 2011). These studies revealed a consistent difference between these two groups. While typically developing children and adults exhibited enhanced sensitivity to the presence of human and animal motion, individuals with ASD demonstrated equivalent sensitivity to all three types of motion. Furthermore, we found that autistic traits correlated with human motion detection but not animal or object motion (Kaiser and Shiffrar, 2011). Such results highlight the direct connection between real-world social abilities and social perception. Consistent with this work, Koldewyn et al. (2010) conducted rigorous psychophysical tasks with relatively large groups of adolescents with and without ASD to determine whether biological motion or coherent motion perception is disrupted in ASD. While they initially found group differences on coherent motion and biological motion tasks, when the authors accounted for performance IQ, a specific deficit in biological motion processing emerged whereas the ASD group no longer exhibited deficits in coherent motion perception. In contrast to this work, some recent studies with adult participants have reported typical performance on psychophysical biological motion tasks (Murphy et al., 2009, Saygin et al., 2010). The participants in these studies had mean ages of 26 and 34 respectively, which may account for the findings of typical performance (see also Cook et al., 2009). Of course, it is unlikely that recent findings of typical performance translate to normative social function in these adults with ASD. As evident in Table 1, findings of normal performance on biological motion perception tasks are more likely in adult than child populations possibly indicative of developmental improvements (and/or alternative strategies) in social perception in adults with ASD and emphasizing the potential for plasticity in this domain. Taken together, the behavioral studies of biological motion processing in ASD indicate that although some basic processes may be spared in some individuals (e.g. action recognition, direction discrimination, thresholds), the overall visual sensitivity to human motion, per se, is markedly disrupted in ASD.
5. What neural mechanisms underlie disrupted biological motion perception in autism?
Neuroimaging studies of biological motion perception reveal a clearer picture of dysfunction in ASD. Here we focus on functional magnetic resonance imaging (fMRI) research. Three studies have examined the brain responses to biological motion in point-light displays of biological motion. Initial fMRI studies reported disrupted brain mechanisms in adolescent and young adult participants relative to age and IQ-matched controls (Table 2). These studies compliment work from our group and others that describes an early derailment of social brain mechanisms in children with ASD during social perception including viewing eye movements (Pelphrey et al., 2005a, Pelphrey et al., 2005b, Pelphrey and Carter, 2008), the attribution of intentions to moving people (Pelphrey et al., 2011), perception of moving geometric shapes that evoke attributions of intention (Castelli et al., 2002), and human speech perception (Boddaert et al., 2003, Gervais et al., 2004). To date, neuroimaging studies of the visual perception of point-light displays of human motion in ASD each report differential brain responses accompanied by aspects of typical behavioral performance on direction discrimination (Herrington et al., 2007) and coherence discrimination (Freitag et al., 2008) tasks. Notably, we found a similar pattern of disrupted brain mechanisms with intact behavior in a cohort of children with and without ASD during our fMRI biological motion task (described in greater detail below).
The field has not yet discovered a consistent neurochemical, neurophysiological, or neuroanatomical abnormality that can be directly used to inform diagnosis of ASD. As illustrated in Table 1, Table 2, a substantial literature has emerged to characterize disrupted biological motion perception in individuals with ASD. However, a great deal of work is needed to fully describe the developmental trajectory and nature of this disruption. The studies reviewed above provide a snapshot of the behavioral or brain response to biological motion in ASD. While such work informs our current understanding of disrupted social perception, developmental studies – including longitudinal studies of infants, toddlers and children with and without ASD, as well as those at increased risk for being identified with ASD – are critical to provide insights into the underlying components of this developmental disorder. These studies could also contribute to the identification of developmental brain endophenotypes to facilitate genetic studies.
Neuroimaging studies of the perception of point-light displays of biological motion had focused on adolescent and adult participants until our group recently extended this work to children as young as 4 years of age (Table 2). In this fMRI study, we identified common and distinct brain mechanisms for biological motion perception in children with ASD and their unaffected siblings (US) relative to typically developing (TD) children without a first- or second-degree relative with ASD (Kaiser et al., 2010b). The unique three group design allowed us to identify three types of neural signatures of autism: (1) “state regions,” dysfunction in brain mechanisms unique to the children with ASD; (2) “trait regions,” disrupted neural circuitry shared by the US and children with ASD; and, (3) “compensatory activity,” the recruitment of additional brain areas during a social perception task by the US. As illustrated in Fig. 2, conjunction analyses of the differential activation to biological motion relative to scrambled motion identified state activity (red color map) localized to the left ventrolateral prefrontal cortex, right amygdala, right posterior superior temporal sulcus, ventromedial prefrontal cortex, and bilateral fusiform gyri. Trait activity (blue color map) was localized to bilateral fusiform gyrus, left dorsolateral prefrontal cortex, and right inferior temporal gyrus. Compensatory activity (green color map) was localized to the right posterior superior temporal sulcus and the ventromedial prefrontal cortex.
Fig. 2.
Neural signatures of autism revealed by conjunction analyses of the biological motion > scrambled motion contrasts. State activity (TD > ASD ∩ US > ASD; red map) was localized to the left ventrolateral prefrontal cortex, right amygdala, right posterior superior temporal sulcus, ventromedial prefrontal cortex, and bilateral fusiform gyri. Trait activity (TD > ASD ∩ TD > US; blue map) was localized to the bilateral fusiform gyri, left dorsolateral prefrontal cortex, and right inferior temporal gyrus. Compensatory activity (US > TD ∩ US > ASD; green map) was localized to the right posterior superior temporal sulcus and ventromedial prefrontal cortex. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article.)
Although disorder heterogeneity is an important challenge for ASD research, the neural response to biologically-relevant social stimuli clearly differentiated children with and without the disorder, reflecting the robust nature of the typical response to such stimuli and the centrality of social deficits and associated brain dysfunction in ASD (Pelphrey et al., 2011). The state regions characterize the nature of disruption in social brain circuitry in young children with ASD, extending work with older populations (Freitag et al., 2008, Herrington et al., 2007). Future studies should aim to establish key factors resulting in atypical activity in the state regions in individuals with ASD. For example, aberrant gaze behavior may contribute to differential response to the biological motion stimuli in children with ASD; although initial eye-tracking analyses indicate equivalent time spent viewing both coherent and scrambled biological motion stimuli by children with and without ASD in a new group of subjects (Kaiser et al., 2011). It is interesting to note that whereas studies of adults primarily localized hypoactivation in ASD groups to posterior superior temporal regions, our state regions included several nodes of the social brain: the right posterior superior temporal sulcus and bilateral fusiform gyri as well as frontal areas and limbic structures including ventromedial prefrontal cortex, left ventrolateral prefrontal cortex, and the right amygdala. This work increases our ability to chart the atypical development of disrupted brain mechanisms for biological motion perception into early childhood. Yet, the participants in our study were only as young as 4 years of age. Since ASD emerges in the first year of life, the hypoactivation in the state regions could reflect the result of living with ASD. The trait activity cannot be explained in this way.
Trait regions likely reflect the genetic vulnerability to develop ASD, as these are areas of dysfunction in US and children with ASD. Thus, these regions represent a potential neuroendophenotype, and therefore provide a novel means of overcoming genomic complexity and disorder heterogeneity in identifying genetic liability for the disorder. In line with Gottesman and Gould's definition of endophenotypes (2003), trait activations in the left dorsolateral prefrontal cortex, right inferior temporal gyrus, and bilateral fusiform gyri, emerged during a social perception task relating to a core deficit in ASD and were shared between affected individuals (ASD) and first-degree relatives (US). It is important to note that we explicitly ruled out the broad autism phenotype in the US. This limits the possibility that the brain function in this group reflects the developmental outcome of living with autistic-traits. This finding presents a rich opportunity for future studies to apply this quantitative endophenotype to inform genome-wide association studies to identify candidate genetic mechanisms and associated pathophysiological pathways.
The finding of compensatory activity, areas of enhanced activity unique to the US, suggests that the genetic risk for ASD can result in both disruptions in brain mechanisms for processing biological motion (trait activity) and related recovery from this risk in the form of additional neural involvement for normative social function. Notably, the compensatory activity was localized to the ventromedial prefrontal cortex and the right posterior superior temporal sulcus, regions that support social perception and social cognition (e.g. Adolphs, 1999, Allison et al., 2000, Puce and Perrett, 2003). Future studies are needed to characterize the origins of the compensatory activity. We posit that the engagement of additional brain mechanisms represents the result of a developmental process, likely influenced by genetic and environmental factors. Since the magnitude of activation in these regions did not correlate with age in the original sample and, thus, is present by 4 years of age, this process likely occurs very early in development. It is possible that compensatory activity reflects protective genetic factors in addition to the genetic liability in US exhibited by trait activity. It will be important for future studies to characterize the genetic contributions to variability in activation levels within these regions. In addition, research is needed to compare activity in these regions in US participants with and without the broad autism phenotype (BAP; Hurley et al., 2007), to determine the function and etiology of this brain response to biological motion. The most striking implication of the finding of compensatory activity is that these regions could serve as targets for intervention and, with further study, lead to a better understanding of the mechanisms through which successful treatments function.
6. What is the diagnostic utility of neuroimaging studies of biological motion perception in autism?
Can neuroimaging studies of the brain mechanisms for biological motion perception aid in diagnosing ASD? To evaluate the potential diagnostic utility of the neural signatures we described earlier, we conducted Receiver Operating Characteristic (ROC) analyses on the “discovery cohort” described above (see Fig. 3; Kaiser et al., 2010b) and a “replication cohort” comprised of a new set of children with and without ASD matched on chronological age and cognitive ability (Elliott, 1990; see Table 3 for characterization of the replication cohort). An ROC analysis is a graphical plot of the true positive rate (i.e., sensitivity) vs. the false positive rate (i.e., 1 − specificity or 1 − true negative rate), for a binary classifier system as its discrimination threshold is varied. Our analyses focused on the positive classification of ASD. For each classification procedure, we conducted a discriminant analysis with diagnosis as a grouping variable (e.g., ASD vs. TD). In the case of state and trait regions, all of the regions were entered together. For all analyses, the prior probabilities were computed from group sizes. The results from the discriminant analyses are reported in Table 4. Each ROC curve was created using the probability of membership in the relevant group (e.g. ASD) as the test variable and the actual group membership as the state variable. The area under the curve (AUC) of each ROC analysis serves as a measure of the probability the classification system ranks a person with ASD (chosen at random) higher than a randomly chosen individual without ASD with larger values indicating superior performance in model testing. An AUC of 0.5 corresponds to chance performance. The ROC graphs are shown in Fig. 3. These graphs aid in visualizing and evaluating classifiers based on their performance (Fawcett, 2006) and provide an analysis of the behavior of a diagnostic system (Swets, 1988). Of course, we do not propose that an ASD diagnosis should be determined based solely on neuroimaging data. However, the results of the ROC analyses described below illustrate the remarkable classification abilities of imaging data from a short fMRI scan of viewing point-light displays of biological motion.
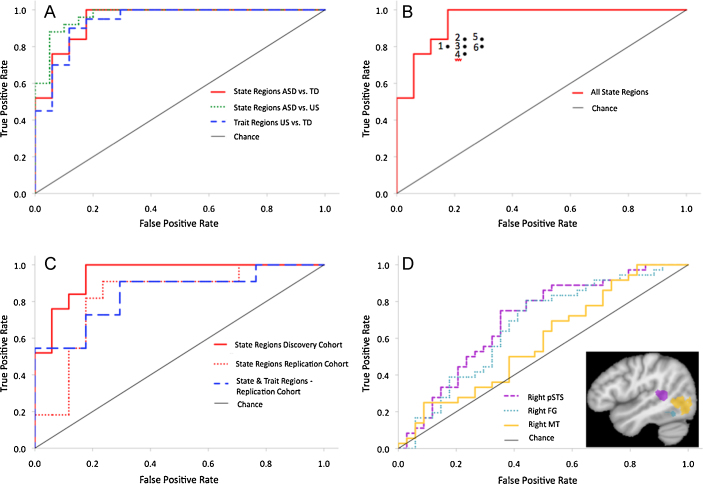
Fig. 3.
ROC graphs for the state and trait regions as discrete classifiers (A) and individual points (B) of each state region including the (1) ventromedial prefrontal cortex, (2) right posterior superior temporal sulcus, (3) left ventrolateral prefrontal cortex, (4) right fusiform gyrus, (5) left fusiform gyrus, and (6) right amygdala in the discovery cohort. (C) ROC graphs for state and trait regions based on discriminant analyses for classifying ASD vs. TD in the discovery and replication cohort. (D) ROC graphs for biological motion ROIs in typical adults as discrete classifiers in the discovery and replication cohort combined.
Table 3.
Characterization of the replication cohort. Means are reported for each item in the table with standard deviations in parentheses, unless otherwise indicated. TD and ASD groups were matched on overall cognitive ability as measured by the DAS-II Global Composite Ability (DAS-II GCA) and chronological age. Autism Diagnostic Observation Schedule (ADOS; Lord et al., 2000) scores, using the ADOS Algorithm for DSM-IV/ICD-10 Autism Diagnosis, are reported for the RRB (Restricted and Repetitive Behaviors) and SA (Social Affect) subscales, as well as the total score for all participants who received Module 3 (n = 10).
| Replication cohort | ASD | TD |
|---|---|---|
| N (m:f) | 11 (8:3) | 17 (12:5) |
| Mean age | 11.8 (3.1) | 11.7 (2.6) |
| DAS-II GCA | 91.9 (18.1) | 104.0 (18.2) |
| ADOS-RRB | 3.1 (2.2) | – |
| ADOS-SA | 11.1 (4.8) | – |
| ADOS-total | 14.2 (6.2) | – |
Table 4.
Regions and cohort(s), area under the curve, asymptotic significance and % correctly classified for each of the ROC analyses (shown in Fig. 3).
| Regions | Cohort | Area under curve | Asymptotic significancea | Correctly classified (%) |
|---|---|---|---|---|
| State | Discoveryb | .948 | .000 | 96.0 |
| State | Discoveryc | .968 | .000 | 92.0 |
| Trait | Discoveryb | .938 | .000 | 90.0 |
| State R pSTS | Discoveryb | .899 | .000 | 75.0 |
| State vmPFC | Discoveryb | .831 | .000 | 75.0 |
| State R FG | Discoveryb | .842 | .000 | 75.0 |
| State L FG | Discoveryb | .861 | .001 | 72.2 |
| State L vlPFC | Discoveryb | .861 | .000 | 75.0 |
| State R amygdala | Discoveryb | .807 | .001 | 72.2 |
| Adult R pSTS | Discovery and replicationb | .697 | .005 | 75.0 |
| Adult R FG | Discovery and replicationb | .666 | .017 | 69.4 |
| Adult R MT | Discovery and replicationb | .584 | .226 | 55.6 |
| Adult All | Discovery and replicationb | .724 | .001 | 72.2 |
| State | Replicationb | .824 | .004 | 54.5 |
| State and trait | Replicationb | .845 | .002 | 72.7 |
Null hypothesis: true area = .5.
ASD vs. TD.
ASD vs. US.
As illustrated in Fig. 3A, the state regions exhibit exquisite sensitivity (high true positive rate) and specificity (low false positive rate) in distinguishing individuals with ASD from TD children and US, respectively. Further, the trait regions – a neuroendophenotype of ASD – reliably differentiated US from TD children. This finding is particularly exciting given the defining criteria of the trait regions, brain areas of dysfunction that are, on average, common to US and children with ASD. Whereas state regions may represent the result of living with ASD, and associated experience-dependent neurodevelopmental processes, the trait regions reflect a genetic risk for the disorder. The trait regions were able to identify US at a true positive rate of 90% and false positive rate of 12%. This classification performance highlights the potential to identify genetic risk for ASD in families without a child with a diagnosis of ASD as the brain response to biological motion in the trait regions represents a biomarker of liability for autism.
To determine the predictive power of the individual state regions as discrete classifiers, we conducted additional ROC analyses of the neural signatures in the discovery cohort. Activation in each state region – including the ventromedial prefrontal cortex, the right posterior superior temporal sulcus, the left ventrolateral prefrontal cortex, and bilateral fusiform gyri – provided high sensitivity (all above 76%) and specificity (all above 71%) in classifying children with ASD (Fig. 3B). Nonetheless, the combination of all of the state regions resulted in a superior classification, with a sensitivity and specificity of 85% and 88%, respectively, demonstrating the importance this network of social brain regions, and a neural systems level (Frith and Frith, 2001), in distinguishing children with ASD from typically developing children. Taken together, the ROC analyses conducted on the discovery cohort supplement our discovery of the state and trait regions by revealing high sensitivity and specificity of these regions in distinguishing children with and without ASD.
While the aforementioned ROC analyses confirm the strength of brain responses to biological motion in predicting ASD diagnosis in the discovery cohort, these results must be interpreted with restraint given that the state and trait regions were defined by group contrasts with this very set of participants. Although this approach provides a validation of our previously reported empirical findings (Kaiser et al., 2010b) and comparable analyses have been used in other neuroimaging studies of ASD (e.g. Ecker et al., 2010), a true test of the predictive power of the neural signatures requires that such analyses are conducted on additional groups of subjects (Stevenson and Kellett, 2010). Thus, to determine the predictive power of the neural signatures in a new group of participants, we conducted additional ROC analyses using the state and trait regions in the replication cohort. The replication cohort consisted of 28 children with (n = 11; 3 females) and without ASD (n = 17; 5 females) matched on chronological age (7–15 years) and cognitive ability. This new group of participants completed the same fMRI procedure and data was analyzed following the same parameters as in our previous study (for details see Kaiser et al., 2010a, Kaiser et al., 2010b). Results from this replication cohort illustrate the predictive power of the fMRI findings described above. Activation within state and trait regions provided a highly sensitive and specific classification of ASD (Fig. 3C). The state regions were able to identify individuals with ASD at 81.8% sensitivity and 82.4% specificity with a significant area under the curve (AUC) of .824. The ROC analysis for the state and trait regions combined revealed a somewhat larger AUC, .845. The substantial predictive utility of the state and trait regions in a new group of participants verifies the strength of the neural signatures and provides support for the interpretation of the trait regions as a robust neuroendophenotype of ASD.
We conducted a final set of ROC analyses using activity within biological motion processing regions from a group of eight typical adults (including three females, with a mean age of 24.7 years, ranging in age from 20 to 34?) to classify ASD in the discovery and replication cohorts combined. Regions of differential response to Biological relative to Scrambled motion were identified at an RFX, q < 01, k > 12. In the adults, these regions, including the right posterior superior temporal sulcus, right fusiform gyrus, and right MT, exhibited differential activation to biological motion relative to scrambled motion on the task described above (Kaiser et al., 2010b). As shown in the ROC charts in Fig. 3D, the right posterior superior temporal sulcus and right fusiform gyrus had moderate sensitivity (both 75%) and specificity (both above 62%) in classifying children with ASD. The prediction rate utilizing the response from area MT was essentially at chance. This pattern of results supports an understanding of ASD as a disorder characterized by specific disruption of social brain regions rather than generalized brain dysfunction. We were recently given a unique opportunity to test the predictive validity of our neural signatures in a special case where a US – from a new cohort of siblings not discussed above – received a diagnosis of ASD some time after participating in our fMRI study. Our discriminant analysis using the brain activity within the state regions classified this individual as having ASD. This case study exemplifies the potential for the neural signatures to assist in identifying individuals at-risk for ASD and reveals the power of brain responses to biological motion as a window into the social deficits and associated neural atypicalities in ASD.
7. Conclusions and future directions
In sum, we addressed five critical questions in this review: (1) What is the relationship between the visual perception of biological motion and the broader domain of social perception? (2) What are the neuroanatomical substrates of social perception? (3) What is the behavioral evidence for disrupted biological motion perception in autism? (4) What are the neural mechanisms underlying disrupted biological motion perception in autism? And (5) what is the diagnostic utility of neuroimaging studies of biological motion perception in autism? Our discussion aimed to establish disruptions in the visual perception of biological motion as a hallmark of ASD, which may serve as a channel to the pathognomonic social deficits of the disorder, and to describe the potential diagnostic utility of disrupted brain mechanisms for social perception.
It is our hope that the ROC analyses described above will set the stage for future translational social neuroscience research. Future studies are needed to determine whether the activity in the state and trait regions can be used to correctly classify children with ASD from other neurodevelopmental disorders. For example, we are currently conducting similar studies with comparison samples including children with obsessive-compulsive disorder. Critically, the most impactful findings may emerge from the application of our approach to younger children. For instance, it is possible that classification based on functional near infrared-spectroscopy data comparing cortical brain responses to biological motion in a similarly defined set of regions could aid in diagnostic assessments of ASD in the first years of life. Several research groups are using near infrared-spectroscopy to study early brain responses to biological motion (e.g., Ichikawa et al., 2010, Lloyd-Fox et al., 2010) and implicate the posterior superior temporal sulcus and other regions in this process. The critical studies will include tracking the development of brain mechanisms for siblings of individuals with ASD and determining whether they eventually receive a diagnosis. Tracking these individuals could lead to the discovery of the timing and nature of disruptions in social brain development (see also Pelphrey et al., 2011). The extension of our trait measures of brain mechanisms for the visual perception of biological motion in infant siblings has the potential to function as biomarkers for autism and the compensatory mechanisms can inform early intervention in infant siblings as measures of success. Such work holds great promise to inform our understanding of the development of neural dysfunction in ASD.
Acknowledgements
This work was supported by a grant from The Simons Foundation. The authors thank Uta Frith and four anonymous reviewers for helpful comments on an earlier version of this manuscript.
References
- Adams R.B., Ambady N., Nakayama K., Shimojo S. Oxford University Press; Oxford: 2010. The Science of Social Vision. [Google Scholar]
- Adolphs R. Social cognition and the human brain. Trends Cogn. Sci. 1999;3:469–479. doi: 10.1016/s1364-6613(99)01399-6. [DOI] [PubMed] [Google Scholar]
- Allison T., Puce A., McCarthy G. Social perception from visual cues: roles of the STS region. Trends Cogn. Sci. 2000;4:267–278. doi: 10.1016/s1364-6613(00)01501-1. [DOI] [PubMed] [Google Scholar]
- Amodio D.M., Frith C.D. Meeting of minds: the medial frontal cortex and social cognition. Nat. Rev. Neurosci. 2006;7:268–277. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- Annaz D., Remington A., Milne E., Coleman M., Campbell R., Thomas M., Swettenham J. Atypical development of motion processing trajectories in children with autism. Dev. Sci. 2010 doi: 10.1111/j.1467-7687.2009.00939.x. (published online on December 28, 2009) [DOI] [PubMed] [Google Scholar]
- Atkinson A.P., Dittrich W.H., Gemmell A.J., Young A.W. Emotion perception from dynamic and static body expressions in point-light and full-light displays. Perception. 2004;33:717–746. doi: 10.1068/p5096. [DOI] [PubMed] [Google Scholar]
- Atkinson A.P. Impaired recognition of emotions from body movements is associated with elevated emotion coherence thresholds in autism spectrum disorders. Neuropsychologia. 2009;47:3023–3029. doi: 10.1016/j.neuropsychologia.2009.05.019. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S. Bradford, MIT Press; Cambridge, MA: 1995. Mindblindness: An Essay on Autism and Theory of Mind. [Google Scholar]
- Bertenthal B.I., Pinto J. Global processing of biological motions. Psychol. Sci. 1994;5:221–225. [Google Scholar]
- Blake R. Cats perceive biological motion. Psychol. Sci. 1993;4:54–57. [Google Scholar]
- Blake R., Shiffrar M. Perception of human motion. Annu. Rev. Psychol. 2007;58:47–73. doi: 10.1146/annurev.psych.57.102904.190152. [DOI] [PubMed] [Google Scholar]
- Blake R., Turner L.M., Smoski M.J., Pozdol S.L., Stone W.L. Visual recognition of biological motion is impaired in children with autism. Psychol. Sci. 2003;14:151–157. doi: 10.1111/1467-9280.01434. [DOI] [PubMed] [Google Scholar]
- Boddaert N., Belin P., Chabane N., Poline J.-B., Barthélémy C., Mouren-Simeoni M.-C., Brunelle F., Samson Y., Zilbovicius M. Perception of complex sounds: abnormal pattern of cortical activation in autism. Am. J. Psychiatry. 2003;160:2057–2060. doi: 10.1176/appi.ajp.160.11.2057. [DOI] [PubMed] [Google Scholar]
- Bonda E., Petrides M., Ostry D., Evans A. Specific involvement of human parietal systems and the amygdala in the perception of biological motion. J. Neurosci. 1996;16:3737–3744. doi: 10.1523/JNEUROSCI.16-11-03737.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brothers L. The social brain: a project for integrating primate behavior and neurophysiology in a new domain. In: Cacioppo J.T., editor. Foundations in Social Neuroscience. Massachusetts Institute of Technology; Boston: 1990. pp. 27–51. [Google Scholar]
- Brown W.M., Cronk L., Grochow K., Jacobson A., Liu C.K., Popovic Z., Trivers R. Dance reveals symmetry especially in young men. Nature. 2005;438:48–50. doi: 10.1038/nature04344. [DOI] [PubMed] [Google Scholar]
- Carter E.J., Pelphrey K.A. School-aged children exhibit domain-specific responses to biological motion. Social Neurosci. 2006;1:396–411. doi: 10.1080/17470910601041382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castelli F., Frith C., Happé F., Frith U. Autism, Asperger syndrome and brain mechanisms for the attribution of mental states to animated shapes. Brain. 2002;125:1839–1849. doi: 10.1093/brain/awf189. [DOI] [PubMed] [Google Scholar]
- Castelli F., Happé F., Frith U., Frith C. Movement and mind: a functional imaging study of perception and interpretation of complex intentional movement patterns. Neuroimage. 2000;12:314–325. doi: 10.1006/nimg.2000.0612. [DOI] [PubMed] [Google Scholar]
- Chouchourelou A., Matsuka T., Harber K., Shiffrar M. The visual analysis of emotional actions. Soc. Neurosci. 2006;1:63–74. doi: 10.1080/17470910600630599. [DOI] [PubMed] [Google Scholar]
- Clarke T.J., Bradshaw M.F., Field D.T., Hampson S.E., Rose D. The perception of emotion from body movement in point-light displays of interpersonal dialogue. Perception. 2005;34:1171–1180. doi: 10.1068/p5203. [DOI] [PubMed] [Google Scholar]
- Cook J., Saygin A.P., Swain R., Blakemore S. Reduced sensitivity to minimum-jerk biological motion in autism spectrum conditions. Neuropsychologia. 2009;47:3275–3278. doi: 10.1016/j.neuropsychologia.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosmides L. The logic of social exchange: has natural selection shaped how humans reason? Studies with the Wason selection task. Cognition. 1989;31:187–276. doi: 10.1016/0010-0277(89)90023-1. [DOI] [PubMed] [Google Scholar]
- Davis M., Whalen P.J. The amygdale: vigilance and emotion. Mol. Psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- Dittrich W.H. Action categories and the perception of biological motion. Perception. 1993;22:15–22. doi: 10.1068/p220015. [DOI] [PubMed] [Google Scholar]
- Dittrich W.H., Troscianko T., Lea S.E., Morgan D. Perception of emotion from dynamic point-light displays represented in dance. Perception. 1996;25:727–738. doi: 10.1068/p250727. [DOI] [PubMed] [Google Scholar]
- Dittrich W.H., Lea S.E.G., Barrett J., Gurr P.R. Categorization of natural movements by pigeons: visual concept discrimination and biological motion. J. Exp. Anal. Behav. 1998;70:281–299. doi: 10.1901/jeab.1998.70-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ecker C., Rocha-Rego V., Johnston P., Mourao-Miranda J., Marquand A., Daly E.M., Brammer M.J., Murphy C., Murphy D.G. Investigating the predictive value of whole-brain structural MR scans in autism: a pattern classification approach. Neuroimage. 2010;49:44–56. doi: 10.1016/j.neuroimage.2009.08.024. [DOI] [PubMed] [Google Scholar]
- Elliott C.D. Psychological Corp.; San Antonio, TX: 1990. Differential Ability Scales (DAS) [Google Scholar]
- Fawcett T. An introduction to ROC analysis. Pattern Recogn. Lett. 2006;27:861–874. [Google Scholar]
- Fox R., McDaniel C. The perception of biological motion by human infants. Science. 1982;218:486–487. doi: 10.1126/science.7123249. [DOI] [PubMed] [Google Scholar]
- Freitag C.M., Konrad C., Haberlen M., Kleser C., Gontard A., Reith W., Troje N.F., Krick C. Perception of biological motion in autism spectrum disorders. Neuropsychologia. 2008;46:1480–1494. doi: 10.1016/j.neuropsychologia.2007.12.025. [DOI] [PubMed] [Google Scholar]
- Frith U., Frith C. The biological basis of social interaction. Curr. Dir. Psychol. Sci. 2001;10:5151–5155. [Google Scholar]
- Frith C.D., Frith U. Interacting minds-a biological basis. Science. 1999;286:1692–1695. doi: 10.1126/science.286.5445.1692. [DOI] [PubMed] [Google Scholar]
- Frith U., Frith C.D. The social brain: allowing humans to boldly go where no other species has been. Phil. Trans. R. Soc. B. 2010;365:165–176. doi: 10.1098/rstb.2009.0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gervais H., Belin P., Boddaert N., Leboyer M., Coez A., Sfaello I., Barthelemy C., Brunelle F., Samson Y., Zilbovicius M. Abnormal cortical voice processing in autism. Nat. Neurosci. 2004;7:801–802. doi: 10.1038/nn1291. [DOI] [PubMed] [Google Scholar]
- Giese M., Poggio Neural mechanisms for the recognition of biological movements. Nat. Rev. Neurosci. 2003;4:179–192. doi: 10.1038/nrn1057. [DOI] [PubMed] [Google Scholar]
- Gobbini M.I., Koralek A.C., Bryan R.E., Montgomery K.J., Haxby J.V. Two takes on the social brain: a comparison of theory of mind tasks. J Cog. Neurosci. 2007;19:1803–1814. doi: 10.1162/jocn.2007.19.11.1803. [DOI] [PubMed] [Google Scholar]
- Gottesman I.I., Gould T.D. The endophenotype concept in psychiatry: etymology and strategic intentions. Am. J. Psychiatry. 2003;160:636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- Grossman E.D., Jardine N.L., Pyles J. fMR-adaptation reveals invariant coding of biological motion on the human STS. Front. Hum. Neurosci. 2010;4:15. doi: 10.3389/neuro.09.015.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman E.D., Battelli L., Pascual-Leone A. Repetitive TMS over posterior STS disrupts perception of biological motion. Vision Research. 2005;45:2847–2853. doi: 10.1016/j.visres.2005.05.027. [DOI] [PubMed] [Google Scholar]
- Gunns R.E., Johnston L., Hudson S.M. Victim selection and kinematics: a point-light investigation of vulnerability to attack. J. Nonverbal Behav. 2002;26:129–158. [Google Scholar]
- Heberlein A.S., Adolphs R., Tranel D., Damasio H. Cortical regions for judgments of emotions and personality traits from point-light walkers. J. Cogn. Neurosci. 2004;16:1143–1158. doi: 10.1162/0898929041920423. [DOI] [PubMed] [Google Scholar]
- Herrington J.D., Baron-Cohen S., Wheelwright S.J., Singh K.D., Bullmore E.T., Brammer M., Williams S.C.R. The role of MT+/V5 during biological motion perception in Asperger syndrome: an fMRI study. Res. Autism Spectr. Disord. 2007;1:14–27. [Google Scholar]
- Hubert B., Wicker B., Moore D.G., Monfardini E., Duverger H., Da Fonseca D., Deruelle C. Brief report recognition of emotional and non-emotional biological motion in individuals with Autistic Spectrum Disorders. J. Autism Dev. Disord. 2007;37:1386–1392. doi: 10.1007/s10803-006-0275-y. [DOI] [PubMed] [Google Scholar]
- Hurley R.S.E., Losh M., Parlier M., Reznick J.S., Piven J. The broad autism phenotype questionnaire. J. Autism Dev. Disord. 2007;37:1679–1690. doi: 10.1007/s10803-006-0299-3. [DOI] [PubMed] [Google Scholar]
- Ichikawa H., Kanazawa S., Yamaguchi M.K., Kakigi R. Infant brain activity while viewing facial movement of point-light displays as measured by near-infrared spectroscopy (NIRS) Neurosci. Lett. 2010;48:90–94. doi: 10.1016/j.neulet.2010.06.086. [DOI] [PubMed] [Google Scholar]
- Jastorff J., Kourtzi Z., Giese M.A. Visual learning shapes the processing of complex movement stimuli in the human brain. J. Neurosci. 2009;29:14026–14038. doi: 10.1523/JNEUROSCI.3070-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson G. Visual perception of biological motion and a model for its analysis. Percept. Psychophys. 1973;14:201–211. [Google Scholar]
- Jokisch D., Daum I., Troje N.F. Self recognition versus recognition of others by biological motion: viewpoint-dependent effects. Perception. 2006;35:911–920. doi: 10.1068/p5540. [DOI] [PubMed] [Google Scholar]
- Kaiser M.D., Delmolino L., Tanaka J.W., Shiffrar M. Comparison of visual sensitivity to human and object motion in Autism Spectrum Disorder. Autism Res. 2010;3:191–195. doi: 10.1002/aur.137. [DOI] [PubMed] [Google Scholar]
- Kaiser M.D., Hudac C.M., Shultz S., Lee S.M., Cheung C., Berken A.M., Deen B., Pitskel N.B., Sugrue D.R., Voos A.C., Saulnier C.A., Ventola Pl., Wolf J.M., Klin A., Vander Wyk B.C., Pelphrey K.A. Neural signatures of autism. Proc. Natl. Acad. Sci. U.S.A. 2010;107:21223–21228. doi: 10.1073/pnas.1010412107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser M.D., Shiffrar M., Pelphrey K.A. Specificity of the pSTS response to human and animal biological motion. Social and Affective Neuroscience Conference; Chicago, Illinois, November 2010 ; 2010. [Google Scholar]
- Kaiser M.D., Eilbott J., Bennett R.B., Sugrue D.R., Pelphrey K.A. International Meeting for Autism Research; San Diego, CA: May 2011. Neural Signatures Predict Autism Diagnosis. [Google Scholar]
- Kaiser M.D., Shiffrar M. The visual perception of motion by observers with autism spectrum disorder: a review and synthesis. Psychon. Bull. Rev. 2009;16:761–777. doi: 10.3758/PBR.16.5.761. [DOI] [PubMed] [Google Scholar]
- Kaiser M.D., Shiffrar M. Variability in the visual perception of human motion as a function of the observer's autistic traits. In: Johnson K., Shiffrar M., editors. Visual Perception of the Human Body in Motion: Findings, Theory, and Practice. Oxford University Press; 2011. [Google Scholar]
- Kanwisher N., McDermott J., Chun M.M. The fusiform face area: a module in human extrastriate cortex specialized for face perception. J. Neurosci. 1997;17:4302–4311. doi: 10.1523/JNEUROSCI.17-11-04302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy D.P., Gläscher J., Tyszka J.M., Adolphs R. Personal space regulation by the human amygdala. Nat. Neurosci. 2009;12:1226–1227. doi: 10.1038/nn.2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klin A., Jones W. Altered face scanning and impaired recognition of biological motion in a 15-month-old infant with autism. Dev. Sci. 2008;11:40–46. doi: 10.1111/j.1467-7687.2007.00608.x. [DOI] [PubMed] [Google Scholar]
- Klin A., Jones W., Schultz R., Volkmar F. The enactive mind, or from actions to cognition: lessons from autism. Philos. Trans. R. Soc. Lond. B: Biol. Sci. 2003;358:345–360. doi: 10.1098/rstb.2002.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klin A., Lin D.J., Gorrindo P., Ramsay G., Jones W. Two-year-olds with autism orient to non-social contingencies rather than biological motion. Nature. 2009;459:257–261. doi: 10.1038/nature07868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koldewyn K., Whitney D., Rivera S.M. The psychophysics of visual motion and global form processing in autism. Brain. 2010;133:599–610. doi: 10.1093/brain/awp272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozlowski L.T., Cutting J.E. Recognizing the sex of a walker from a dynamic point-light display. Percept. Psychophys. 1977;21:575–580. [Google Scholar]
- LeDoux J.E. Emotion circuits in the brain. Annu. Rev. Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Lloyd-Fox S., Blasi A., Everdell N., Elwell C.E., Johnson M.H. Selective cortical mapping of biological motion processing in young infants. J. Cogn. Neurosci. 2010 doi: 10.1162/jocn.2010.21598. [DOI] [PubMed] [Google Scholar]
- Lord C. The Autism Diagnostic Observation Scale-Generic: a standard measure of social and communication deficits associated with the spectrum of autism. J. Autism Dev. Disord. 2000;307:205–223. [PubMed] [Google Scholar]
- Loula F., Prasad S., Harber K., Shiffrar M. Recognizing people from their movement. J. Exp. Psychol. Hum. Percept. Perform. 2005;31:210–220. doi: 10.1037/0096-1523.31.1.210. [DOI] [PubMed] [Google Scholar]
- Méary D., Kitromilides E., Mazens K., Graff C., Gentaz E. Four-day-old human neonates look longer at non-biological motions of a single point-of-light. PLoS ONE. 2007;2:e186. doi: 10.1371/journal.pone.0000186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore D.G., Goodwin J.E., George R., Axelsson E.L., Braddick F.M.B. Infants perceive human point-light displays as solid forms. Cognition. 2007;104:377–396. doi: 10.1016/j.cognition.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Moore D.G., Hobson R.P., Lee A. Components of person perception: an investigation with autistic, non-autistic retarded and typically developing children and adolescents. Br. J. Dev. Psychol. 1997;15:401–423. [Google Scholar]
- Morris J.S., Frith C.D., Perrett D.I., Rowland D., Young A.W., Calder A.J., Dolan R.J. A differential neural response in the human amygdale to fearful and happy facial expressions. Nature. 1996;383:812–815. doi: 10.1038/383812a0. [DOI] [PubMed] [Google Scholar]
- Murphy P., Brady N., Fitzgerald M., Troje N.F. No evidence for impaired perception of biological motion in adults with autistic spectrum disorders. Neuropsychologia. 2009;47:3225–3235. doi: 10.1016/j.neuropsychologia.2009.07.026. [DOI] [PubMed] [Google Scholar]
- O’Doherty J.P. Reward representations and reward-related learning in the human brain: insights from neuroimaging. Curr. Opin. Neurobiol. 2004;14:769–776. doi: 10.1016/j.conb.2004.10.016. [DOI] [PubMed] [Google Scholar]
- Omori E., Watanabe S. Discrimination of Johansson's stimuli in pigeons. Int. J. Comp. Psychol. 1996;9:92. [Google Scholar]
- Oram M.W., Perrett D.I. Integration of form and motion in the anterior superior temporal polysensory area (STPa) of the macaque monkey. J. Neurophysiol. 1996;76:109–129. doi: 10.1152/jn.1996.76.1.109. [DOI] [PubMed] [Google Scholar]
- Orban de Xivry J.J., Coppe S., Lefèvre P., Missal M. Biological motion drives perception and action. J. Vis. 2010;10:1–11. doi: 10.1167/10.2.6. [DOI] [PubMed] [Google Scholar]
- Parron C., Da Fonseca D., Santos A., Moore D.G., Monfardini E., Deruelle C. Recognition of biological motion in children with autistic spectrum disorders. Autism. 2008;12:261–274. doi: 10.1177/1362361307089520. [DOI] [PubMed] [Google Scholar]
- Pelphrey K.A., Carter E.J. Charting the typical and atypical development of the social brain. Dev. Psychopathol. 2008;20:1081–1102. doi: 10.1017/S0954579408000515. [DOI] [PubMed] [Google Scholar]
- Pelphrey K.A., Morris J.P., Michelich C.R., Allison T., McCarthy G. Functional anatomy of biological motion perception in posterior temporal cortex: an fMRI study of eye, mouth, and hand movements. Cereb. Cortex. 2005;15:1866–1876. doi: 10.1093/cercor/bhi064. [DOI] [PubMed] [Google Scholar]
- Pelphrey K.A., Morris J.P., McCarthy G. Neural basis of eye gaze processing deficits in autism. Brain. 2005;128:1038–1048. doi: 10.1093/brain/awh404. [DOI] [PubMed] [Google Scholar]
- Pelphrey K.A., Shultz S., Hudac C.M., Vander Wyk B. Research review: constraining heterogeneity: the social brain and its development in autism spectrum disorder. J. Child Psychol. Psychiatry. 2011 doi: 10.1111/j.1469-7610.2010.02349.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelphrey K.A., Mitchell T.V., McKeown M.J. Brain activity evoked by the perception of human walking: controlling for meaningful coherent motion. J. Neurosci. 2003;23:6819–6825. doi: 10.1523/JNEUROSCI.23-17-06819.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrett D.I., Smith P.A.J., Potter D.D., Mistlin A.J., Head A.S., Milner A.D., Jeeves M.A. Visual cells in the temporal cortex sensitive to face view and gaze direction. Proc. R. Soc. Lon. 1985;223:293–317. doi: 10.1098/rspb.1985.0003. [DOI] [PubMed] [Google Scholar]
- Pessoa L., Adolphs R. Emotion processing and the amygdala: from a ‘low road’ to ‘many roads’ of evaluating biological significance. Nat. Rev. Neurosci. 2010;11(11):773–783. doi: 10.1038/nrn2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto J., Shiffrar M. The visual perception of human and animal motion in point-light displays. Soc. Neurosci. 2009;4:332–346. doi: 10.1080/17470910902826820. [DOI] [PubMed] [Google Scholar]
- Poizner H., Bellugi U., Lutes-Driscoll V. Perception of American sign language in dynamic point-light displays. J. Exp. Psychol. Hum. Percept. Perform. 1981;7:430–440. doi: 10.1037//0096-1523.7.2.430. [DOI] [PubMed] [Google Scholar]
- Pollick F.E., Paterson H.M., Bruderlin A., Sanford A.J. Perceiving affect from arm movement. Cognition. 2001;82:B51–B61. doi: 10.1016/s0010-0277(01)00147-0. [DOI] [PubMed] [Google Scholar]
- Pollick F.E., Kay J.W., Heim K., Stringer R. Gender recognition from point-light walkers. J. Exp. Psychol. Hum. Percept. Perform. 2005;31:1247–1265. doi: 10.1037/0096-1523.31.6.1247. [DOI] [PubMed] [Google Scholar]
- Puce A., Allison T., Asgari M., Gore J.C., McCarthy G. Differential sensitivity of human visual cortex to faces, letterstrings, and textures: a functional magnetic resonance imaging study. J. Neurosci. 1996;16:5205–5215. doi: 10.1523/JNEUROSCI.16-16-05205.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puce A., Perrett D. Electrophysiology and brain imaging of biological motion. Philos. Trans. R. Soc. Lond. B: Biol. Sci. 2003;358:435–445. doi: 10.1098/rstb.2002.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyles, J.A., 2009. Neural mechanisms of dynamic object perception. Ph.D. Dissertation, University of California, Irvine, United States – California. Retrieved November 3, 2009, from Dissertations & Theses, A&I (Publication No. AAT 3355913).
- Pyles J.A., Grossman E.D. Neural mechanisms for biological motion and animacy. In: Johnson K., Shiffrar M., editors. Perception of the Human Body in Motion: Findings, Theory and Practice. Oxford University Press; New York: 2011. [Google Scholar]
- Rolls E.T. The orbitofrontal cortex and reward. Cereb. Cortex. 2000;10:284–294. doi: 10.1093/cercor/10.3.284. [DOI] [PubMed] [Google Scholar]
- Rolls E.T. Functional neuroimaging of umami taste: what makes umami pleasant? Am. J. Clin. Nutr. 2009;90:804–813. doi: 10.3945/ajcn.2009.27462R. [DOI] [PubMed] [Google Scholar]
- Runeson S., Frykholm G. Kinematic specification of dynamics as an informational basis for person-and-action perception: expectation, gender recognition, and deceptive intention. J. Exp. Psychol. Gen. 1983;112:585–615. [Google Scholar]
- Saxe R., Xiao D.K., Kovacs G., Perrett D.I., Kanwisher N. A region of right posterior superior temporal sulcus responds to observed intentional actions. Neuropsychologia. 2004;42:1435–1446. doi: 10.1016/j.neuropsychologia.2004.04.015. [DOI] [PubMed] [Google Scholar]
- Saygin A.P. Superior temporal and premotor brain areas necessary for biological motion perception. Brain. 2007;130:2452–2461. doi: 10.1093/brain/awm162. [DOI] [PubMed] [Google Scholar]
- Saygin A.P., Cook J., Blakemore S. Unaffected perceptual thresholds for biological and non-biological form-from-motion perception in autism spectrum conditions. PLoS ONE. 2010;5:e13491. doi: 10.1371/journal.pone.0013491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebanz N., Shiffrar M. Detecting deception in a bluffing body: the role of expertise. Psychon. Bull. Rev. 2009;16:170–175. doi: 10.3758/PBR.16.1.170. [DOI] [PubMed] [Google Scholar]
- Simion F., Regolin L., Bulf H. A predisposition for biological motion in the newborn baby. Proc. Natl. Acad. Sci. U.S.A. 2008;15:809–813. doi: 10.1073/pnas.0707021105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson J.L., Kellett K.A. Can magnetic resonance imaging aid diagnosis of the autism spectrum? J. Neurosci. 2010;30:16763–16765. doi: 10.1523/JNEUROSCI.4946-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swets J. Measuring the accuracy of diagnostic systems. Science. 1988;240:1285–1293. doi: 10.1126/science.3287615. [DOI] [PubMed] [Google Scholar]
- Vallortigara G., Regolin L., Marconato F. Visually inexperienced chicks exhibit spontaneous preference for biological motion patterns. PLoS Biol. 2005;3:1312–1316. doi: 10.1371/journal.pbio.0030208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vander Wyk B.C., Hudac C.M., Carter E.J., Sobel D.M., Pelphrey K.A. Action understanding in the superior temporal sulcus region. Psychol. Sci. 2009;20:771–777. doi: 10.1111/j.1467-9280.2009.02359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon J.M.D., Johnson S.C. Biological motion displays elicit social behavior in 12-month-olds. Child Dev. 2009;80:1069–1075. doi: 10.1111/j.1467-8624.2009.01317.x. [DOI] [PubMed] [Google Scholar]
- Zeki S., Watson J.D., Lueck C.J., Friston K.J., Kennard C., Frackowiak R.S. A direct demonstration of functional specialization in human visual cortex. J. Neurosci. 1991;11:641–649. doi: 10.1523/JNEUROSCI.11-03-00641.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]