Abstract
Objective:
Recent in vivo results have shown prominent tissue sparing effect of radiotherapy with ultra-high dose rates (FLASH) compared to conventional dose rates (CONV). Oxygen depletion has been proposed as the underlying mechanism, but in vitro data to support this have been lacking. The aim of the current study was to compare FLASH to CONV irradiation under different oxygen concentrations in vitro.
Methods:
Prostate cancer cells were irradiated at different oxygen concentrations (relative partial pressure ranging between 1.6 and 20%) with a 10 MeV electron beam at a dose rate of either 600 Gy/s (FLASH) or 14 Gy/min (CONV), using a modified clinical linear accelerator. We evaluated the surviving fraction of cells using clonogenic assays after irradiation with doses ranging from 0 to 25 Gy.
Results:
Under normoxic conditions, no differences between FLASH and CONV irradiation were found. For hypoxic cells (1.6%), the radiation response was similar up to a dose of about 5–10 Gy, above which increased survival was shown for FLASH compared to CONV irradiation. The increased survival was shown to be significant at 18 Gy, and the effect was shown to depend on oxygen concentration.
Conclusion:
The in vitro FLASH effect depends on oxygen concentration. Further studies to characterize and optimize the use of FLASH in order to widen the therapeutic window are indicated.
Advances in knowledge:
This paper shows in vitro evidence for the role of oxygen concentration underlying the difference between FLASH and CONV irradiation.
Background
Studies on FLASH irradiation, delivery of irradiation with ultra-high dose rates, have gained much focus in recent years, as they present in vivo results indicating a widening of the therapeutic window compared to irradiation with conventional dose rates (CONV).1–4 Normal tissues like lung, brain, skin and abdomen have all been shown to have an increased tolerance to FLASH compared to CONV irradiation.5–9 Encouragingly, FLASH irradiation seems to be at least as efficient at treating tumor tissue as CONV irradiation.5,7,10 It has been suggested that this seemingly differential effect is due to differences in tissue oxygenation in tumor and healthy tissues.11,12 However, while the recent promising studies on FLASH irradiation focus on in vivo results, including the report of the first treated patient,13 there is a need for in vitro correlates to enlighten the role of oxygen behind the FLASH effect.3,4
In an irradiated cell culture, the radiochemical depletion of intracellular oxygen is dependent on the delivered dose and the available oxygen.14,15 The re-diffusion of oxygen in a monolayer of cultured cells has been shown to occur on the 10−2 s scale (10% in about 10−3 s, 50% in about 10−2 s, and 90% in about 10−1 s).16 Thus, while the oxygen concentration is only marginally affected during CONV irradiation, a local and transient oxygen depletion may become apparent after a certain dose during FLASH irradiation. Besides local and transient oxygen depletion, radical–radical interaction is another hypothesized reason for the FLASH effect. FLASH irradiation results in a high local radical concentration, leading to radical–radical interactions and less free radicals available to interact with DNA.17 Biological damage produced by X-ray/electron radiation is dominated by indirect action, i.e. free radicals interacting with DNA.18 Furthermore, radical-induced DNA damage can be permanently 'fixed' by molecular oxygen, rendering DNA damage irreparable. Consequently, lower oxygen concentration is recognized to cause radio-resistance.19
The current study aims at investigating the role of oxygen concentration for the FLASH effect in vitro, through a direct comparison of toxicity between FLASH and CONV irradiation at different oxygen concentrations.
Methods and materials
Irradiation
FLASH and CONV 10 MeV electron beam irradiation of cells were performed with a modified Elekta Precise (Elekta AB, Stockholm, Sweden) medical linear accelerator (LINAC), which has been previously described.20 With the gantry at 180° and with the collimators positioned for a 40 × 40 cm2 field at isocenter, the cell flasks were positioned on top of the cross-hair foil. For CONV irradiation, the flat beam profile allowed for flasks to be irradiated in groups of five, and the average dose rate at this distance was 14 Gy/min. For FLASH irradiation, cell flasks were irradiated one-at-a-time, positioned in the central flat region of the beam profile. The dose was delivered with an integer number of pulses, with a nominal dose-per-pulse of 3 Gy, and a pulse repetition frequency of 200 Hz, resulting in an average dose rate of 600 Gy/s.
Dosimetry
Dosimetry for FLASH and CONV irradiation was performed using dose rate independent21 Gafchromic EBT3 film (Ashland Specialty Ingredients G.P., Bridgewater, NJ) positioned at the bottom inside of a cell flask, above which a 2 mm thick sheet of polystyrene was positioned in order to mimic the cell’s location in the flask below 2 mm of media. The flask with the film was placed at the irradiation position and irradiated before and after each cell experiment. In conjunction with the film measurements, online dosimeter measurements were performed in order to enable verification of the dose delivery during the subsequent cell irradiations. For CONV irradiation, the on-board monitor (transmission) chamber was used to verify the dose delivery during the cell irradiations. Unfortunately, the monitor chamber was not useful for FLASH irradiation due to the extreme drop in ion collection efficiency for measurement in high dose-per-pulse beams.22 Instead, a Farmer-type ionization chamber placed at a specific position in the ceiling of the treatment room was used to verify the dose delivery during the FLASH irradiations. Due to the increased distance to the chamber, the dose-per-pulse decreased sufficiently to eliminate the problem of poor ion collection efficiency, thus making it useful as an online dosimeter during FLASH irradiation. For each separate experiment, the readings of the online dosimeters were correlated to the dose rate independent film measurements.
Clonogenic assay
The prostate cancer cell line DU145 was acquired from LGC Standards Ltd. (Teddington, UK). Cells were grown in monolayers in RPMI 1640 media with 10% fetal bovine serum and 1% penicillin–streptomycin, at 37°C in a humidified atmosphere with 5% CO2. Mycoplasma-tests were performed regularly. For clonogenic assays, cells were plated in an appropriate cell number in Falcon T12.5 flasks (Thermo-Fisher Scientific TM, Waltham, MA) and allowed to adhere over night. The flasks were placed in a hypoxic chamber as described below and transported to the irradiation device with sealed caps and irradiated within 10 min. The FLASH and CONV-flasks were prepared identically at the same occasion, and irradiated within minutes apart. Care was taken to ascertain a distinct medium volume of 2.50 ml per flask, since varying medium volume could potentially influence the results. After irradiation, the cap was untightened and cells were incubated under normoxic conditions in 37°C for 13–15 days for colonies to form. Flasks were then stained and analysed as previously described.23 Colonies containing more than 50 cells were regarded as survivors.
Varying oxygen concentrations
To test the effect of oxygen concentration, a hypoxia chamber (InVivo2 Hypoxia Work Station 400, Baker Ruskinn Technology Ltd, Bridgend, UK) was used to lower the oxygen concentration in the flasks. The partial oxygen pressure () was set to resemble varying levels of hypoxia and physoxia (set points 1, 2, 4, and 8 %), compared to normoxia (20%).24 The actual oxygen concentration in the medium at the time of irradiation was measured with an oxygen electrode (Oxygen Meter pHenomenal ®, OX 4100 h, VWR). Flasks were placed in the hypoxia chamber 1 h prior to irradiation. The duration of hypoxia–incubation was chosen to allow the cells and surrounding medium to equilibrate, but keeping the incubation-time as low as possible to minimize the induction of biological hypoxia responses.
Statistical considerations
Experiments were conducted at 12 different occasions, with duplicate-quintuplicate flasks for each dose level and oxygen concentration. All data was analyzed in RStudio v. 1.0.136 (RStudio Team (2015). RStudio, Inc., Boston, MA, URL http://www.rstudio.com/). The parameters of the linear–quadratic model25 were fitted to the survival data using a non-linear least-square method (‘lsqnonlin’ in Matlab). The Wilcoxon rank-sum test was used to calculate statistical differences, with a chosen significance level of 5% (α = 0.05).
Results
Surviving fraction at different doses
The clonogenic assays show that the surviving fraction (SF) of cells after irradiation depends on delivered dose, oxygen concentration as well as dose rate (Figure 1). There was no statistically significant difference between FLASH and CONV for the SF-curves under normoxic conditions. However, for hypoxic conditions (measured relative partial oxygen pressure of 1.6%) the curves seem to separate at 5–10 Gy, with an apparent difference above 15 Gy, and a highly significant effect at 18 Gy (p < 0.001). According to previously published data, the difference is expected to disappear and the SF-curves to converge again at anoxic conditions (relative partial oxygen pressure of 0%).26,27 To fit the data to the LQ-model, we assumed the FLASH effect to be reflected in the β-term, in analogy to previous models with dose-rate dependence for the β-term.28,29 Therefore, when fitting the linear–quadratic model, the α-parameter was kept fixed while the β-parameter was constrained to be the same for FLASH and CONV at normoxic (equal to ) as well as anoxic conditions (equal to ). This was achieved by setting , where is either FLASH or CONV, and is a power-law interpolation parameter going from zero to one as the relative partial oxygen pressure is going from 0 to 20%. The resulting values of the fitting parameters were =0.2329 Gy−1, =0.0025 Gy−2, 0.0194 Gy−2, =0.7162, and =0.4464.
Figure 1.
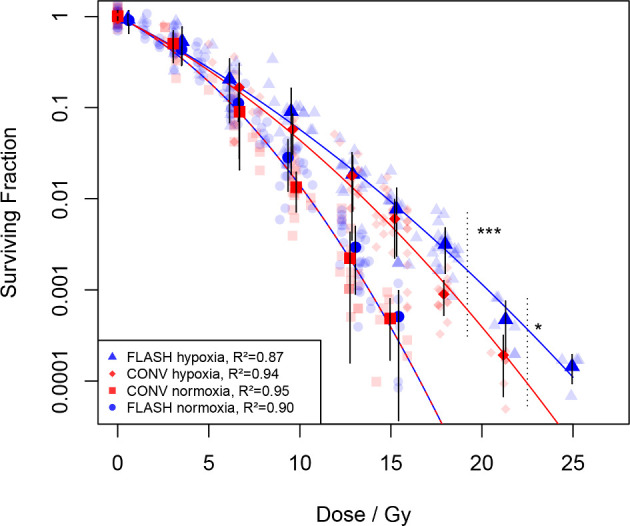
Surviving fraction of cells at different doses, under normoxic (20% oxygen concentration) and hypoxic (1.6% oxygen concentration) conditions, for FLASH [circles (normoxia) and triangles (hypoxia)] and conventional dose rates [CONV, squares (normoxia) and diamonds (hypoxia)], determined by clonogenic assays. Bold markers denote the average surviving fraction, with error bars representing one standard deviation, plotted against the average of the actual dose for each dose group (nominally multiples of 3 Gy). Curves represent data fitted to the LQ-model as described under Results, with corresponding R2—values shown in the legend. Data from 10 separate experiments, in total 393 flasks with individually determined irradiation dose. ***p < 0.001 using Wilcoxon rank sum-test comparing FLASH vs CONV for data points within 18 ± 0.5 Gy, and *p < 0.05 at 21 ± 0.5 Gy. LQ, linear–quadratic.
SF at different oxygen concentrations
A dose level of 18 Gy was chosen for further studies with varying oxygen concentrations. At the lowest oxygen concentration (relative partial oxygen pressure of 1.6%) in our set-up, there was a large sparing effect for FLASH compared to CONV (Figure 2). This is also illustrated in Figure 3., where the surviving fraction of cells is shown as a function of the measured values for relative partial oxygen pressure. The relative sparing effect of FLASH shown in Figure 3. (i.e. the ratio between the surviving fractions for FLASH and CONV) was calculated based on the fitted linear–quadratic model. In Figure 4., all experimental data for the different dose and oxygen levels are shown together with the linear–quadratic fit, including the anoxic limit (i.e. the SF-curve at ) as estimated by the linear–quadratic fit.
Figure 2.
Surviving fraction of cells under different relative partial oxygen pressure after irradiation with 18 Gy, with either FLASH or conventional dose rates (CONV). Panels show the results from three separate experiments (box: quartiles; band: median; whiskers: highest/lowest point if ≤1.5 IQR; outlier:>1.5 IQR). Results from statistical comparison (Wilcoxon rank-sum test) between FLASH and CONV revealed highly significant (***, p < 0.001) difference for relative partial oxygen pressure of 1.6%, significant (*, p < 0.05) for 2.7 and 4.4%, and no significant differences for either 8.3% or 20%. IQR, interquartile range.
Figure 3.

The surviving fraction of cells irradiated with 18 Gy as a function of relative partial oxygen pressure for FLASH (circles) and CONV (squares) irradiations, together with the linear quadratic fit (solid lines). Error bars indicate the range of data from the three separate experiments, bold markers the average surviving fraction, and asterisks indicate data below our detection limit. The relative sparing effect of FLASH (i.e. the ratio between the surviving fractions for FLASH and CONV), as calculated from the fitted curves, is also shown, with its lines becoming dashed where the model extrapolates the data.
Figure 4.

Summary of all experimental data for the different doses and oxygen levels (i.e. from Figures 1 and 2) together with the linear-quadratic fit, both for FLASH (triangles, circles and dashed lines) and CONV (diamonds, squares, and solid lines), including the model’s prediction of the anoxic limit at a relative partial oxygen pressure of 0% (top solid line), as well as the prediction at 0.01 and 0.1% relative partial oxygen pressures. The assumption of an anoxic limit is based on previously published data.26,27 Markers denote the average surviving fraction, plotted against the average of the actual dose for each dose group (nominally multiples of 3 Gy).
Discussion
In the current paper, we provide in vitro evidence for the role of oxygen concentration underlying the biological difference between FLASH and CONV irradiation. In normoxic conditions, the SF-curves for FLASH and CONV overlap, in agreement with previous work by others.27,30–32 However, in hypoxia (relative partial oxygen pressure of 1.6%), a FLASH-sparing effect starts at 5–10 Gy, is apparent at ≥15 Gy, and significant at 18 Gy. Previous publications have shown that for oxygen concentrations of 0.4%, 5–10 Gy is sufficient to deplete the cellular oxygen at ultra-high dose rates.12 So, for our levels of oxygen concentration, we expect total oxygen depletion to occur at doses higher than the values used in this study, i.e. any “breaking” of the survival curve should happen above the doses used in our study. However, any transient lowering of the partial oxygen pressure due to high dose rate irradiation would still be expected to increase the cells' radioresistance.
Earlier in vitro studies with bacteria irradiated at FLASH dose rates found a sparing effect.33,34 In mammalian cells, studies using lower oxygen pressures provided evidence for FLASH-sparing,17,35 whereas others could not reproduce the finding for oxygenated mammalian cells.26,27,31,36 It has been shown that the sparing effect of FLASH depends on the intracellular oxygen concentration,31,36–39 but these studies lack a direct comparison with CONV. Under anoxic conditions, direct comparisons between FLASH and CONV revealed no significant differences.26,27 Based on the hypothesis of FLASH-induced depletion of oxygen as well as radical–radical interaction, it is congruent that totally anoxic cells are equally sensitive to FLASH as to CONV. Theoretical modelling of the FLASH effect supports these hypotheses and fits well to our data, although it is suggested by Pratx et al that a separation only occurs between relative partial oxygen pressures of above 0% and below 5%.40,41 Ideally, an experiment including totally anoxic cells should be conducted. Unfortunately, we were not able to decrease the relative partial oxygen pressure below 1.6% using our current set-up. However, the resulting intracellular oxygen concentrations, which resulted in significant differences in survival for cells exposed to FLASH compared to CONV irradiation, are similar to what is found in normal tissue.24
The in vivo results for FLASH irradiation are promising, showing a widening of the therapeutic window.1–4 These and our in vitro results indicate that after a certain dose, cells starts to behave as if they were hypoxic when exposed to FLASH irradiation. A local and transient radiochemical oxygen depletion is a leading theory in being responsible for the FLASH effect together with radical–radical interaction.11,12,17 With our set-up, we see a clear FLASH effect which depends on oxygen concentration (Figures 1 and 2). The ratio of surviving fraction between FLASH and CONV (i.e. the relative sparing effect of FLASH) peaks at a factor of 2.5 for oxygen concentrations of 1–5%, similar to previously published in vitro data8 and then drops towards a factor of 1 for higher and lower oxygen concentrations (Figures 3 and 4), according to our linear–quadratic fit to our and previously published data.26,27 Although the current in vitro results would explain part of the FLASH-sparing effects seen in vivo5–9, the tissue responses in vivo may be more complicated to derive, and could be influenced by various factors such as inflammation, immune response, and varying hypoxia. The current type of phenomenological modelling is limited to the measured data (which in our case is limited by relying on other groups’ data at 0% oxygen and not having any data showing the drop in ratio between oxygen concentrations of 0–1.6%) and does not describe the underlying radiobiological mechanisms of the observed effect. For that, dedicated mechanistic modelling studies are needed. However, with our set-up, we could now start to perform such detailed studies where beam parameters (dose rate, dose-per-pulse, pulse repetition frequency) are varied to further study oxygen kinetics and its importance for the FLASH effect. Furthermore, FLASH irradiation in combination with different pharmaceuticals (e.g. radical scavengers) could be tested with this set-up. With the shown importance of oxygen and the notion that many tumors are hypoxic, it may be possible to widen the therapeutic window. However, there is also a possible risk that under some circumstances (irradiation dose, relative hypoxic state in tumor and normal tissue), the therapeutic window might narrow, such that FLASH spares the tumor but not the surrounding tissue. Hence, theoretical modelling of the FLASH effect will play an important role to predict clinically relevant scenarios.
Before the FLASH technique can be transferred in an optimized way to the clinic, the radiobiological mechanisms behind the effect needs to elucidated. In this study, we were able to show that the FLASH effect can be clearly quantified in vitro, a set-up needed to specify the mechanisms involved. We also show that the FLASH effect is highly dependent on the intracellular oxygen concentration, indicating the importance of the depletion and rediffusion of oxygen and/or radical–radical interaction for the FLASH effect.
Footnotes
Acknowledgement: The authors wish to thank professor Mattias Belting, Oncology and Pathology, Kampradlab, Lund University, Lund, Sweden for valuable discussions and for providing us time in the hypoxia chamber, and Dr. Stephen McMahon, Queen’s University, Belfast, UK for fruitful conversations.
Funding: Governmental research funding (ST-ALF). Berta Kamprad Foundation. John and Augusta Persson's Foundation.
Contributor Information
Gabriel Adrian, Email: gabriel.adrian@med.lu.se.
Elise Konradsson, Email: elise.konradsson@med.lu.se.
Michael Lempart, Email: michael.lempart@googlemail.com.
Sven Bäck, Email: Sven.Back@skane.se.
Crister Ceberg, Email: crister.ceberg@med.lu.se.
Kristoffer Petersson, Email: kristoffer.petersson@med.lu.se.
REFERENCES
- 1.Symonds P, Jones GDD. Flash radiotherapy: the next technological advance in radiation therapy? Clin Oncol 2019; 31: 405–6. doi: 10.1016/j.clon.2019.05.011 [DOI] [PubMed] [Google Scholar]
- 2.Al-Hallaq H, Cao M, Kruse J, Klein E. Cured in a flash: reducing normal tissue toxicities using Ultra-High-Dose rates. Int J Radiat Oncol Biol Phys 2019; 104: 257–60. doi: 10.1016/j.ijrobp.2019.01.093 [DOI] [PubMed] [Google Scholar]
- 3.Vozenin M-C, Hendry JH, Limoli CL. Biological benefits of ultra-high dose rate flash radiotherapy: sleeping Beauty Awoken. Clin Oncol 2019; 31: 407–15. doi: 10.1016/j.clon.2019.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Durante M, Brauer-Krisch E, Hill M. Faster and safer? flash ultra-high dose rate in radiotherapy. Br J Radiol 2017; 54: 20170628. doi: 10.1259/bjr.20170628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Favaudon V, Caplier L, Monceau V, Pouzoulet F, Sayarath M, Fouillade C, et al. Ultrahigh dose-rate flash irradiation increases the differential response between normal and tumor tissue in mice. Sci Transl Med 2014; 6: 245ra93. doi: 10.1126/scitranslmed.3008973 [DOI] [PubMed] [Google Scholar]
- 6.Montay-Gruel P, Petersson K, Jaccard M, Boivin G, Germond J-F, Petit B, et al. Irradiation in a flash: unique sparing of memory in mice after whole brain irradiation with dose rates above 100Gy/s. Radiother Oncol 2017; 124: 365–9. doi: 10.1016/j.radonc.2017.05.003 [DOI] [PubMed] [Google Scholar]
- 7.Vozenin M-C, De Fornel P, Petersson K, Favaudon V, Jaccard M, Germond J-F, et al. The advantage of flash radiotherapy confirmed in mini-pig and Cat-cancer patients. Clin Cancer Res 2019; 25: 35–42. doi: 10.1158/1078-0432.CCR-17-3375 [DOI] [PubMed] [Google Scholar]
- 8.Montay-Gruel P, Acharya MM, Petersson K, Alikhani L, Yakkala C, Allen BD, et al. Long-Term neurocognitive benefits of flash radiotherapy driven by reduced reactive oxygen species. Proc Natl Acad Sci U S A 2019; 116: 10943–51. doi: 10.1073/pnas.1901777116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Loo BW, Schuler E, Lartey FM, Rafat M, King GJ, Trovati S, et al. P003) delivery of ultra-rapid flash radiation therapy and demonstration of normal tissue sparing after abdominal irradiation of mice. Int J Radiat Oncol Biol Phys 2017; 98: E16. doi: 10.1016/j.ijrobp.2017.02.101 [DOI] [Google Scholar]
- 10.Bourhis J, Montay-Gruel P, Gonçalves Jorge P, Bailat C, Petit B, Ollivier J, et al. Clinical translation of flash radiotherapy: why and how? Radiother Oncol 2019; 139: 11–17. doi: 10.1016/j.radonc.2019.04.008 [DOI] [PubMed] [Google Scholar]
- 11.Hall EJ. Radiation dose-rate: a factor of importance in radiobiology and radiotherapy. Br J Radiol 1972; 45: 81–97. doi: 10.1259/0007-1285-45-530-81 [DOI] [PubMed] [Google Scholar]
- 12.Wilson P, Jones B, Yokoi T, Hill M, Vojnovic B. Revisiting the ultra-high dose rate effect: implications for charged particle radiotherapy using protons and light ions. Br J Radiol 2012; 85: e933–9. doi: 10.1259/bjr/17827549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bourhis J, Sozzi WJ, Jorge PG, Gaide O, Bailat C, Duclos F, et al. Treatment of a first patient with FLASH-radiotherapy. Radiotherapy and Oncology 2019; 139: 18–22. doi: 10.1016/j.radonc.2019.06.019 [DOI] [PubMed] [Google Scholar]
- 14.Weiss H, Epp ER, Heslin JM, Ling CC, Santomasso A. Oxygen depletion in cells irradiated at ultra-high dose-rates and at conventional dose-rates. Int J Radiat Biol Relat Stud Phys Chem Med 1974; 26: 17–29. doi: 10.1080/09553007414550901 [DOI] [PubMed] [Google Scholar]
- 15.Spitz DR, Buettner GR, Petronek MS, St-Aubin JJ, Flynn RT, Waldron TJ, et al. An integrated physico-chemical approach for explaining the differential impact of flash versus conventional dose rate irradiation on cancer and normal tissue responses. Radiother Oncol 2019; 139: 23–7. doi: 10.1016/j.radonc.2019.03.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ling CC, Michaels HB, Epp ER, Peterson EC. Oxygen diffusion into mammalian cells following ultrahigh dose rate irradiation and lifetime estimates of oxygen-sensitive species. Radiat Res 1978; 76: 522–32. doi: 10.2307/3574801 [DOI] [PubMed] [Google Scholar]
- 17.Berry RJ, Hall EJ, Forster DW, Storr TH, Goodman MJ. Survival of mammalian cells exposed to X rays at ultra-high dose-rates. Br J Radiol 1969; 42: 102–7. doi: 10.1259/0007-1285-42-494-102 [DOI] [PubMed] [Google Scholar]
- 18.Hall EJ, Giaccia AJ. Radiobiology for the Radiologist. 7th ed Philadelphia: Lippincott Williams & Wilkins; 2012. [Google Scholar]
- 19.Robert Grimes D, Partridge M. A mechanistic investigation of the oxygen fixation hypothesis and oxygen enhancement ratio. Biomed. Phys. Eng. Express 2015; 1: 045209. doi: 10.1088/2057-1976/1/4/045209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lempart M, Blad B, Adrian G, Bäck S, Knöös T, Ceberg C, et al. Modifying a clinical linear accelerator for delivery of ultra-high dose rate irradiation. Radiotherapy and Oncology 2019; 139: 40–5. doi: 10.1016/j.radonc.2019.01.031 [DOI] [PubMed] [Google Scholar]
- 21.Jaccard M, Petersson K, Buchillier T, Germond J-F, Durán MT, Vozenin M-C, et al. High dose-per-pulse electron beam dosimetry: usability and dose-rate independence of EBT3 Gafchromic films. Med Phys 2017; 44: 725–35. doi: 10.1002/mp.12066 [DOI] [PubMed] [Google Scholar]
- 22.Petersson K, Jaccard M, Germond J-F, Buchillier T, Bochud F, Bourhis J, et al. High dose-per-pulse electron beam dosimetry - A model to correct for the ion recombination in the Advanced Markus ionization chamber. Med Phys 2017; 44: 1157–67. doi: 10.1002/mp.12111 [DOI] [PubMed] [Google Scholar]
- 23.Adrian G, Ceberg C, Carneiro A, Ekblad L. Rescue effect inherited in colony formation assays affects radiation response. Radiat Res 2018; 189: 44–52. doi: 10.1667/RR14842.1 [DOI] [PubMed] [Google Scholar]
- 24.McKeown SR, normoxia D. physoxia and hypoxia in tumours - Implications for treatment response. Br J Radiol 2014; 87: 1–12. doi: 10.1259/bjr.20130676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McMahon SJ. The linear quadratic model: usage, interpretation and challenges. Phys. Med. Biol. 2018; 64: 01TR01. doi: 10.1088/1361-6560/aaf26a [DOI] [PubMed] [Google Scholar]
- 26.Zackrisson BU, Nyström UH, Ostbergh P. Biological response in vitro to pulsed high dose rate electrons from a clinical accelerator. Acta Oncol 1991; 30: 747–51. doi: 10.3109/02841869109092451 [DOI] [PubMed] [Google Scholar]
- 27.Cygler J, Klassen NV, Ross CK, Bichay TJ, Raaphorst GP. The survival of aerobic and anoxic human glioma and melanoma cells after irradiation at ultrahigh and clinical dose rates. Radiat Res 1994; 140: 79–84. doi: 10.2307/3578571 [DOI] [PubMed] [Google Scholar]
- 28.Thames HD. An ‘Incomplete-repair’ Model for Survival after Fractionated and Continuous Irradiations. Int J Radiat Biol Relat Stud Phys Chem Med 1985; 47: 319–39. doi: 10.1080/09553008514550461 [DOI] [PubMed] [Google Scholar]
- 29.Brenner DJ. The Linear-Quadratic model is an appropriate methodology for determining Isoeffective doses at large doses per fraction. Semin Radiat Oncol 2008; 18: 234–9. doi: 10.1016/j.semradonc.2008.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prempree T, Michelsen A, Merz T. The repair time of chromosome breaks induced by pulsed x-rays of ultra-high dose-rate. Int J Radiat Biol Relat Stud Phys Chem Med 1969; 15: 571–4. doi: 10.1080/09553006914550871 [DOI] [PubMed] [Google Scholar]
- 31.Nias AHW, Swallow AJ, Keene JP, Hodgson BW. Survival of HeLa cells from 10 nanosecond pulses of electrons. Int J Radiat Biol Relat Stud Phys Chem Med 1970; 17: 595–8. doi: 10.1080/09553007014550751 [DOI] [PubMed] [Google Scholar]
- 32.Buonanno M, Grilj V, Brenner DJ. Biological effects in normal cells exposed to flash dose rate protons. Radiotherapy and Oncology 2019; 139: 51–5. doi: 10.1016/j.radonc.2019.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.DEWEY DL, BOAG JW. Modification of the oxygen effect when bacteria are given large pulses of radiation. Nature 1959; 183: 1450–1. doi: 10.1038/1831450a0 [DOI] [PubMed] [Google Scholar]
- 34.Epp ER, Weiss H, Santomasso A. The oxygen effect in bacterial cells irradiated with high-intensity pulsed electrons. Radiat Res 1968; 34: 320–5. doi: 10.2307/3572557 [DOI] [PubMed] [Google Scholar]
- 35.Town CD. Effect of high dose rates on survival of mammalian cells. Nature 1967; 215: 847–8. doi: 10.1038/215847a0 [DOI] [PubMed] [Google Scholar]
- 36.Berry RJ, Stedeford JBH. Reproductive survival of mammalian cells after irradiation at ultra-high dose-rates: further observations and their importance for radiotherapy. Br J Radiol 1972; 45: 171–7. doi: 10.1259/0007-1285-45-531-171 [DOI] [PubMed] [Google Scholar]
- 37.Nias AHW, Swallow AJ, Keene JP, Hodgson BW. Effects of pulses of radiation on the survival of mammalian cells. Br J Radiol 1969; 42: 553. doi: 10.1259/0007-1285-42-499-553-b [DOI] [PubMed] [Google Scholar]
- 38.Epp ER, Weiss H, Djordjevic B, Santomasso A. The radiosensitivity of cultured mammalian cells exposed to single high intensity pulses of electrons in various concentrations of oxygen. Radiat Res 1972; 52: 324–32. doi: 10.2307/3573572 [DOI] [PubMed] [Google Scholar]
- 39.Michaels HB, Epp ER, Ling CC, Peterson EC. Oxygen sensitization of CHO cells at ultrahigh dose rates: prelude to oxygen diffusion studies. Radiat Res 1978; 76: 510–21. doi: 10.2307/3574800 [DOI] [PubMed] [Google Scholar]
- 40.Pratx G, Kapp DS. Ultra-High-Dose-Rate flash irradiation may spare hypoxic stem cell niches in normal tissues. Int J Radiat Oncol Biol Phys 2019; 105: 190–2. doi: 10.1016/j.ijrobp.2019.05.030 [DOI] [PubMed] [Google Scholar]
- 41.Pratx G, Kapp DS. A computational model of radiolytic oxygen depletion during flash irradiation and its effect on the oxygen enhancement ratio. Phys. Med. Biol. 2019; 64: 185005. doi: 10.1088/1361-6560/ab3769 [DOI] [PubMed] [Google Scholar]



